Abstract
Severe acute respiratory coronavirus 2 (SARS‐CoV‐2) has been associated with neurological complications, including acute encephalopathy. To better understand the neuropathogenesis of this acute encephalopathy, we describe a series of patients with coronavirus disease 2019 (COVID‐19) encephalopathy, highlighting its phenomenology and its neurobiological features. On May 10, 2020, 707 patients infected by SARS‐CoV‐2 were hospitalized at the Geneva University Hospitals; 31 (4.4%) consecutive patients with an acute encephalopathy (64.6 ± 12.1 years; 6.5% female) were included in this series, after exclusion of comorbid neurological conditions, such as stroke or meningitis. The severity of the COVID‐19 encephalopathy was divided into severe and mild based on the Richmond Agitation Sedation Scale (RASS): severe cases (n = 14, 45.2%) were defined on a RASS < −3 at worst presentation. The severe form of this so‐called COVID‐19 encephalopathy presented more often a headache. The severity of the pneumonia was not associated with the severity of the COVID‐19 encephalopathy: 28 of 31 (90%) patients did develop an acute respiratory distress syndrome, without any difference between groups (p = .665). Magnetic resonance imaging abnormalities were found in 92.0% (23 of 25 patients) with an intracranial vessel gadolinium enhancement in 85.0% (17 of 20 patients), while an increased cerebrospinal fluid/serum quotient of albumin suggestive of blood‐brain barrier disruption was reported in 85.7% (6 of 7 patients). Reverse transcription‐polymerase chain reaction for SARS‐CoV‐2 was negative for all patients in the cerebrospinal fluid. Although different pathophysiological mechanisms may contribute to this acute encephalopathy, our findings suggest the hypothesis of disturbed brain homeostasis and vascular dysfunction consistent with a SARS‐CoV‐2‐induced endotheliitis.
Keywords: COVID‐19, encephalopathy, MRI, vasculitis
1. INTRODUCTION
Coronavirus disease‐2019 (COVID‐19) has been associated with an increased prevalence of acute encephalopathy 1 referred to as COVID‐19 encephalopathy. By definition, 2 its clinical and radiological spectrum is heterogeneous. 1 , 3 , 4 The severe acute respiratory coronavirus 2 (SARS‐CoV‐2) may enter into the brain via a hematogenous or a direct trans‐nasal route through the olfactory nerve. 5 The physiopathology of this encephalopathy is poorly understood: external factors including intubation and sedative medication, or isolation imposed by the social distancing strategy, as well as specific SARS‐CoV‐2 related factors may contribute to the encephalopathy. 3
Early recognition of this acute condition, especially in the intensive care unit (ICU), is key as it has been associated with increased hospital length of stay and higher mortality. 6 We recently reported five patients with a severe form of COVID‐19 encephalopathy clinically responsive to steroid suggestive of an inflammatory‐mediated mechanism. 7 To better understand its neuropathogenesis, we describe a series of patients with COVID‐19 encephalopathy, highlighting its phenomenology and its neurobiological features.
2. MATERIALS AND METHODS
2.1. Study population
On May 10, 2020, seventy‐four days into the COVID‐19 outbreak in Geneva (Switzerland), 707 patients infected by SARS‐CoV‐2 were hospitalized at the Geneva University Hospitals. Among them, 31 (4.4%) were evaluated by a neurologist with a final diagnosis of COVID‐19 encephalopathy: 3 in the ICU, 22 in the intermediate care units, and 6 in the standard care unit. SARS‐CoV‐2 infection was documented by a positive SARS‐CoV‐2 reverse transcription‐polymerase chain reaction (RT‐PCR) assay from a nasopharyngeal swab at the time of the hospitalization. COVID‐19 encephalopathy was defined by a rapidly developing (less than 4 weeks) pathobiological process in the brain leading to delirium, decreased level of consciousness or coma. 2 Here, we focused on our series on patients with delirium or subsyndromal delirium (according to the definition of the consensus statement 2 ) at the time of the neurological assessment without etiology, after appropriate delirium screening and exclusion of classical medical etiologies, such as electrolyte disturbances, infection, drug or alcohol toxicity and/or withdrawal, metabolic disorders, low perfusion state or acute central nervous system conditions, such as stroke or meningitis (Figure 1). We also excluded patients with meningeal enhancement or presence of meningism. The severity of the COVID‐19 encephalopathy was divided into severe and mild based on the Richmond Agitation Sedation Scale (RASS): severe cases were defined on a RASS < −3 at worst presentation (meaning deep sedation – no response to voice but possible movement or eye‐opening to physical stimulation). Following our inclusion criteria, all patients at the time of the neurological evaluation were not comatose but presented a delirium or a subsyndromal delirium that was quantified by the confusion assessment method (CAM).
Figure 1.
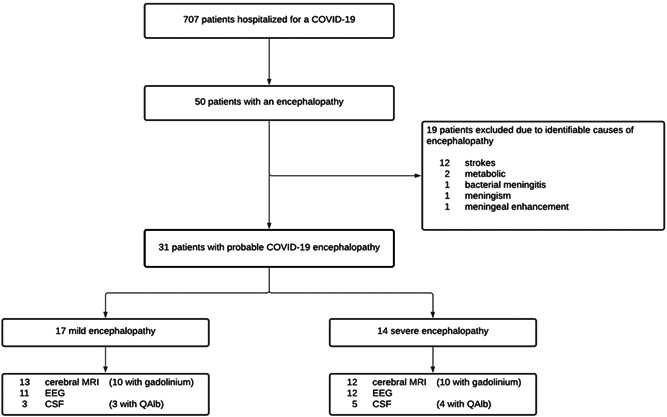
Flow chart. COVID‐19, coronavirus disease 2019; CSF, cerebrospinal fluid; EEG, electroencephalogram; MRI, magnetic resonance imaging; QAlb, quotient of albumin
2.2. Paraclinical evaluation: Magnetic resonance imaging (MRI), electroencephalogram (EEG), and cerebrospinal fluid (CSF)
Electronic medical records, MRI, EEG, blood, and CSF samples were based upon retrospective chart review and descriptive assessment of the patients during their hospitalization. All clinical and neurological manifestations were reviewed and confirmed by two trained neurologists. A major disagreement between the two neurologists was resolved by the consultation with a third neurologist. MRI (available for 25 patients—12 in the severe COVID‐19 encephalopathy group and 13 in the mild group) was acquired on a clinical scanner equipped with a head coil. In addition to a standard scanning neuro head protocol, a post‐contrast fat‐saturated T1‐weighted black blood VISTA was performed in 20 patients (10 patients included in the severe COVID‐19 encephalopathy group and 10 in the mild group): MRI was performed on a 1.5 T Philips system equipped with a head coil. The 3D VISTA fat suppressed VISTA (TE, 17 ms; TR, 400 ms; 1.2 mm thickness, 1024 × 1024) sequence was performed in the coronal plane before and after iv administration of contrast material (Gadovist, Bayer, Berlin). Axial reconstructions were done in both instances. During contrast administration, a 3D angiogram (TE: 1.98 ms, TR: 5.6 ms, 1.10‐mm thick slices) of the carotids was additionally performed as well as post‐contrast T1 axial images (TE, 2.46 ms; TR, 262 ms; 5‐mm thick slices) over the brain. Suspected inflammation of vessel walls was diagnosed when contrast enhancement of the intracranial vessel wall was concentric and homogeneous: vessel wall enhancement greater than 50% of the circumference. 8 All MRIs were reviewed by two board‐certified neuroradiologists. A standard video‐EEG in accordance with the international 10–20 system was recorded in 23 patients (12 patients included in the severe COVID‐19 encephalopathy group and 11 in the mild group). CSF spinal tap was performed in 8 of 31 patients. The study was approved by the institutional review board of the Geneva University Hospitals (protocol #2020‐01206—approved May 25, 2020).
2.3. Statistical analysis
Baseline characteristics were summarized using means and standard deviations or frequencies and percentages, as appropriate. The normality of data distribution was checked using the Shapiro‐Francia test. Between‐group comparisons (severe vs. mild COVID‐19 encephalopathy) were performed using unpaired t test, Mann‐Whitney U test or Fisher exact test, as appropriate. We performed stepwise forward logistic regression models to identify which combination of neurological symptoms was associated with severe COVID‐19 encephalopathy. The proportion of the variance explained by the models was estimated by the pseudo‐R 2. All analyses were conducted using SPSS version 25 (SPSS Inc.).
3. RESULTS
Clinical characteristics of the 31 patients were compared in Table 1 between the 14 patients with a severe form of COVID‐19 encephalopathy and the 17 with a mild form.
Table 1.
Clinical characteristics of patients with COVID‐19 encephalopathy at admission
| Total (n = 31) | Severe (n = 14) | Mild (n = 17) | P valuea | |
|---|---|---|---|---|
| Age (years) | 64.6 ± 12.1 | 65.6 ± 7.8 | 63.8 ± 15.0 | .680 |
| Gender (female), n (%) | 2 (6.5) | 2 (14.3) | 0 (0) | .195 |
| Education, mean (0–3) | 2.30 ± 0.73 | 2.00 ± 0.82 | 2.46 ± 0.66 | .226 |
| Duration before improvement (days) | 12.1 ± 11.3 | 9.3 ± 5.8 | 14.3 ± 14.2 | .212 |
| Duration of COVID‐19 symptoms before SACRE onset (days) | 20.9 ± 8.1 | 22.1 ± 6.4 | 19.8 ± 9.3 | .421 |
| ICU (days) (n = 29) | 16.2 ± 7.1 | 18.7 ± 5.1 | 14.1 ± 7.9 | .057 |
| Intubation (days) (n = 28) | 13.2 ± 6.2 | 16.1 ± 4.8 | 10.8 ± 6.4 | .014 |
| Length of stay (days) | 61.4 ± 36.0 | 54.6 ± 19.6 | 66.9 ± 45.3 | .325 |
| Comorbidities, n (%) | ||||
| Smokingb | 3 (10.0) | 2 (15.4) | 1 (5.9) | .565 |
| Cardiovascular risk factorc (0–4) | 22 (71.0) | 10 (71.4) | 12 (70.6) | 1.000 |
| Body Mass Index (kg/m2) | 28.7 ± 6.1 | 28.9 ± 5.1 | 28.5 ± 6.7 | .873 |
| Chronic cardiac diseased | 8 (25.8) | 4 (28.6) | 4 (23.5) | 1.000 |
| Pulmonary diseasese | 7 (22.6) | 3 (21.4) | 4 (23.5) | 1.000 |
| Dementiab, f | 3 (10.0) | 0 (0.0) | 3 (17.6) | .238 |
| Alcohol dependence | 2 (6.5) | 2 (14.3) | 0 (0.0) | .195 |
| Symptoms at admission, n (%) | ||||
| Dyspnea | 22 (71.0) | 11 (78.6) | 11 (64.7) | .456 |
| Chest paing | 1 (3.8) | 1 (10.0) | 0 (0.0) | .385 |
| Coughh | 21 (72.4) | 9 (69.2) | 12 (75.0) | 1.000 |
| Fever | 29 (93.5) | 12 (85.7) | 17 (100.0) | .196 |
| Fatigue | 28 (96.6) | 13 (100.0) | 15 (93.8) | 1.000 |
| Myalgia | 9 (40.9) | 5 (55.6) | 4 (30.8) | .384 |
| Diarrhea | 4 (18.2) | 1 (12.5) | 3 (21.4) | 1.000 |
| Disposition | ||||
| Modified Rankin Scale | 2.42 ± 1.40 | 2.3 ± 1.1 | 2.5 ± 1.6 | .628 |
Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit.
p value: t test or Fisher exact test.
n = 30 patients.
Cardiovascular risk factors: hypertension; diabetes, dyslipidemia, obstructive sleep apnea.
Chronic cardiac disease: coronary artery disease or congestive heart failure.
Pulmonary diseases: Chronic obstructive pulmonary disease or interstitial lung disease.
Dementia: chronic neurodegenerative disease or vascular dementia.
n = 26 patients.
n = 29 patients.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The mean age of patients was 64.6 ± 12.1 years with a vast majority of males (93.5%). All females with COVID‐19 encephalopathy were severely affected. COVID‐19 encephalopathy was present at admission before other symptoms in 6% of patients and started 20.9 ± 8.1 days after COVID‐19 symptom onset. The mean duration before clinical improvement of COVID‐19 encephalopathy was 12.1 ± 11.3 days. The most common symptoms at admission were fatigue (96.6%), fever (93.5%), and cough (72.4%).
The severity of the pneumonia was not associated with severity of the COVID‐19 encephalopathy: 28 of 31 (90%) patients did develop an acute respiratory distress syndrome; the mean O2 request (FiO2) at worst presentation (or right before intubation) was 67.3 ± 15.9%; 28 of 31 (90%) patients were intubated; finally, sepsis was reported in 7 of 31 (22.6%) patients.
The length of stay in the ICU (16.2 ± 7.1 days) and the length of hospital stay (61.4 ± 36.0 days) were similar between the severe and the mild groups. However, the duration of intubation was longer in the severe group in comparison to the mild group. The modified Rankin scale at discharge was 2.42 ± 1.40. 26 of 31 (84%) did not recover and presented a worse mRS at discharge than premorbid. No patients included in the current series died during their hospitalization.
Neurological symptoms and signs are presented in Table 2.
Table 2.
Neurological evaluation and brain imaging
| Total (n = 31) | Severe (n = 14) | Mild (n = 17) | p valuea | |
|---|---|---|---|---|
| Neurological symptoms during hospitalization, n (%) | ||||
| Inattention | 26 (83.9) | 14 (100.0) | 12 (70.6) | .048 |
| Obnubilationb | 17 (63.0) | 9 (81.8) | 8 (50.0) | .124 |
| Disorganized Thinking | 20 (76.9) | 10 (90.9) | 10 (66.7) | .197 |
| Perseverationc | 22 (91.7) | 9 (90.0) | 13 (92.9) | 1.000 |
| Fluctuationd | 26 (86.7) | 12 (85.7) | 14 (87.5) | 1.000 |
| Agitation | 16 (51.6) | 8 (57.1) | 8 (47.1) | .722 |
| Hyposmiae | 4 (36.4) | 1 (33.3) | 3 (37.5) | 1.000 |
| Dizzinessf | 1 (8.3) | 0 (0.0) | 1 (10.0) | 1.000 |
| Hypoacousiae | 1 (9.1) | 0 (0.0) | 1 (11.1) | 1.000 |
| Headachec | 7 (29.2) | 6 (60.0) | 1 (7.1) | .009 |
| Neurological signs at neurological evaluation, n (%) | ||||
| CAM (total score) | 2.32 ± 1.28 | 2.78 ± 0.70 | 1.94 ± 1.51 | .052 |
| RASS (total score) | −0.77 ± 1.91 | −2.00 ± 1.61 | 0.24 ± 1.52 | <.001 |
| Focal neurological signsd | 8 (26.7) | 3 (23.1) | 5 (29.4) | 1.000 |
| Corticospinal tract signsg | 10 (35.7) | 7 (53.8) | 3 (20.0) | .114 |
| Sensory deficith | 3 (12.0) | 1 (10.0) | 2 (13.3) | 1.000 |
| Cranial nerve deficitd | 4 (13.3) | 2 (15.4) | 2 (11.8) | 1.000 |
| MRI (n = 25), n (%) | ||||
| MRI abnormalities | 23 (92.0) | 11 (91.7) | 12 (92.3) | 1.000 |
| Any vessels enhancementsi | 17 (85.0) | 9 (90.0) | 8 (80.0) | 1.000 |
| Microbleeds | 11 (44.0) | 6 (50.0) | 5 (38.5) | .695 |
| EEG (n = 23), n (%) | ||||
| EEG abnormalities | 17 (73.9) | 9 (75.0) | 8 (72.7) | 1.000 |
| Focal or generalized slowing (delta or theta activities) | 17 (73.9) | 9 (75.0) | 8 (72.7) | 1.000 |
| Focal seizures | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
Abbreviations: CAM, confusion assessment method; EEG, electroencephalogram; MRI, magnetic resonance imaging; RASS, Richmond Agitation Sedation Scale.
p value: t test or Fisher exact test.
n = 27 patients.
n = 24 patients.
n = 30 patients.
n = 11 patients.
n = 12 patients.
n = 28 patients.
n = 25 patients.
n = 20 patients.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Patients with severe versus mild COVID‐19 encephalopathy presented more often a headache (60.0% vs. 7.1%; p = .005); headache appears before the onset of encephalopathy in 6 of 7 patients (86%). Using a stepwise forward logistic regression model, headache was the only neurological symptom selected by the model with a 12 times risk of developing a severe COVID‐19 encephalopathy (OR = 12.0; 95% CI [1.2–117.4]; p = .033), explaining 15% of the variability of the severity. Patients with severe versus mild COVID‐19 encephalopathy tended to present more often corticospinal tract signs at neurological examination (53.8% vs. 20.0%; p = .062). The mean CAM score at neurological assessment was 2.32 ± 1.28. COVID‐19 encephalopathy severity (RASS total score at the time of the neurological assessment) was associated with the duration of intubation (r = −0.443; p = .013).
MRI abnormalities (Table 2) were reported in 92.0% (23 of 25 patients) with COVID‐19 encephalopathy. Noteworthily, intracranial vessel gadolinium enhancement was observed in 85.0% of patients (17 of 20). The vast majority of the vessel enhancement was found on vertebral arteries without sign of stenosis or downstream ischemia (Figure 2 and supplementary materials). Intracranial vessel gadolinium enhancement was confirmed by two board‐certified neuroradiologists. Furthermore, inflammatory atheromatous plaques, as a potential cause of such intracranial vessel enhancement, were excluded by angio‐MR, angio‐CT or echo‐doppler. Cerebral microbleeds were reported in 11 of 25 patients (44%): the mean number of microbleeds by patients was 16.2 ± 28.8; lobar (superficial) distribution of microbleeds was reported in 2 of 11 patients (18.2%), deep in 3 of 11 patients (27.3%), and 6 of 11 patients (54.5%) had a mixed (combination of lobar and deep) distribution. There were no differences in term of MRI abnormalities between severe and mild COVID‐19 encephalopathy. Finally, we did not report any T2 or FLAIR hyperintensities in the medial temporal lobe in any patient. No ictal discharge was reported at electroencephalogram (EEG), while EEG slowing was noticed in 73.9% (17 of 23 patients). Serum concentration of C‐reactive protein was 57.7 ± 53.8 mg/L at the time of the neurological assessment for the all samples; the group with sign of intracranial gadolinium vessel enhancement presented an increased concentration of C‐reactive protein in comparison to those without gadolinium enhancement (63.6 ± 54.6 vs. 6.9 ± 9.5 mg/L; p = .012) (Figure 3). We also measured serum concentration of interleukin‐6 (IL‐6) in a subsample of patients with post‐contrast fat saturated T1‐weighted black blood VISTA sequence: 14 in the group with intracranial gadolinium vessel enhancement and 3 in the group without intracranial gadolinium vessel enhancement. Although IL‐6 was higher in the group of patients with signs of intracranial gadolinium vessel enhancement than in the group without signs of intracranial gadolinium vessel enhancement, the difference was not significant (766.2 ± 799.7 vs. 161.7 ± 60.7 pg/ml, respectively; p‐value = .432). CSF white blood cell count was normal in 8 of 8 patients, whereas CSF/serum quotient of albumin (QAlb—measured in 7 of 8 patients) was increased in 85.7% (mean QAlb = 11.6 ± 5.5). RT‐PCR for SARS‐CoV‐2 was negative for all patients in the CSF (measured in 7 of 8).
Figure 2.
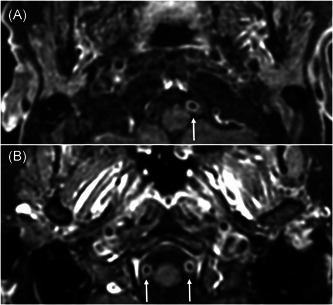
Post‐contrast fat saturated axial T1‐weighted black Blood VISTA images in two patients with COVID‐19 encephalopathy (TE, 17 ms; TR, 400 ms, image thickness, 1.5 mm). The upper image (A) in a patient shows circumferential enhancement in the wall of the left vertebral artery (arrow: V4 segment), and the lower image (B) shows a bilateral enhancement of the vessel walls of the V4 segment (arrows) in another patient. COVID‐19, coronavirus disease 2019; TE, echo time; TR, repetition time
Figure 3.
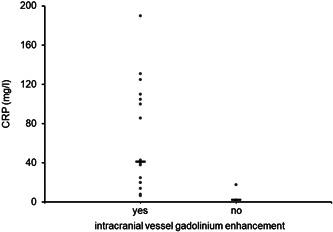
C‐reactive protein (CRP) titer according to the presence (yes) or the absence (no) of gadolinium enhancement in intracranial arteries. The 17 patients with gadolinium enhancement in intracranial arteries present an increased CRP titer in comparison to the three patients without gadolinium enhancement in intracranial arteries (p = .012)
Among the 31 patients, 2 patients were treated by high‐dose steroid (methylprednisolone 0.5 g/d iv for 5 days) due to a prolonged delirium without any improvement. Following steroid initiation, both patients presented a clinical improvement with resolution of delirium.
4. DISCUSSION
We report on a series of patients with COVID‐19 encephalopathy, including clinical and neurobiological features. The prevalence of COVID‐19 encephalopathy in our series is certainly underestimated, 9 as patients hospitalized for SARS‐CoV‐2 infection were not systematically screened by a neurologist, but evaluated only when the referral physicians (internists or intensivists) asked for a neurological consult; patients with subtle signs may have been not identified by the referral physicians.
In this consecutive series of COVID‐19 encephalopathy, (i) clinical findings (high prevalence of headache in severe patients), (ii) neuroradiological signs (high proportion of gadolinium enhancement in large intracranial arteries) and (iii) biological features (increase CSF QAlb suggestive of blood‐brain barrier disruption) may suggest among other mechanisms a pathophysiological mechanism related to an inflammation of the vessel wall for COVID‐19 encephalopathy development – the endothelial hypothesis. Although the comparison is limited, as our current series does not include patients with stroke, such endothelial dysfunction has been demonstrated on a brain biopsy of a patient with temporal hematoma and subarachnoid hemorrhage. 10 Non‐convulsive status or infraclinic seizures were ruled out by routine EEG that were consistent with the recent description of EEG findings in critically ill patients with COVID‐19. 11 A hypoxemia‐induced encephalopathy may an alternative hypothesis, as the majority of our cohort has been intubated (28 of 31) due to the severity of pneumonia and an acute respiratory distress syndrome. However, the severity of the COVID‐19 encephalopathy was not associated with the severity of pneumonia. An immunopathogenic mechanism related to COVID‐19 has been proposed in other neurological complications of the SARS‐CoV‐2 infection, for example in cases of Guillain‐Barre syndrome 12 or of limbic encephalitis. 13 However, absence of white matter lesion and absence of meningeal or parenchymal gadolinium enhancement on MRI, and absence of pleocytosis in the CSF are not in favor of such hypothesis. The elevation of C‐reactive protein in patients with COVID‐19 encephalopathy with intracranial gadolinium vessel enhancement may also suggest an inflammatory mediated mechanism for this COVID‐19 encephalopathy. In addition, whether serum concentration of IL‐6, one of the pro‐inflammatory cytokines involved in the so‐called cytokine storm, was higher in the group of patients with contrast enhancement of intracranial vessels in comparison to those without enhancement, this difference was not significant. Finally, the use of sedative‐hypnotic and anticholinergic agents in critical ill COVID‐19 patients, unstable comorbidities, or social isolation are many factors that possibly contribute to delirium in COVID‐19. 3 Furthermore, the CSF biological findings—increased CSF QAlb, CSF white blood cell count within normal range, and the absence of direct proof of SARS‐CoV‐2 in the CSF—are in favor of an indirect (or inflammatory) effect of SARS‐CoV‐2 for explaining this encephalopathy. This hypothesis supports the rationale of a steroid‐responsive encephalopathy, as recently reported by our group 7 and others. 14 It is of note that the role of steroids in the clinical improvement of our patients and others 7 , 14 needs to be confirmed in future prospective studies, as the majority of our patients spontaneously recovered from encephalopathy without steroids.
Although the neuropathogenesis of COVID‐19 encephalopathy is still unknown, this observation suggests the hypothesis of disturbed brain homeostasis and vascular dysfunction consistent with the recent description of a SARS‐CoV‐2‐induced endotheliitis in autopsy. 15 However, we should acknowledge that in the current series, we did not have any histological evidence of endotheliitis or vasculitis in other organs, as reported by others. 10 , 15 Finally, the patients included in this cohort did not have any clinical evidence of vasculitis in other organs, explaining why we did not perform a standardized screening for autoimmune vasculitis.
Others and our group 16 , 17 suggested that the phenomenon of “happy or silent hypoxemia”—hypoxemia without dyspnea—reported in many patients with severe COVID‐19 pneumonia could be the consequence of inappropriate cortical processing of interoceptive information from the respiratory system. Dyspnea perception involves the activation of various cortical regions, especially the insula, 18 and the presence of COVID‐19 encephalopathy may interfere with such a physiological complex mechanism of dyspnea perception. In the current series, the prevalence of dyspnea was similar between severe and mild encephalopathy. Therefore, we are not able to support the hypothesis suggesting that severe COVID‐19 encephalopathy may be associated with the phenomenon of “happy or silent hypoxemia.” Future studies should investigate this hypothesis by quantifying the severity of the dyspnea with appropriate questionnaires.
This retrospective study has some limitations. Although we followed strict inclusion criteria for COVID‐19 encephalopathy (delirium or subsyndromal delirium without cause), only subgroups of patients underwent a different evaluation: MRI in 81%, EEG in 74%, and CSF analysis in 26% of patients. Although we could not definitively rule out encephalitis in the 23 patients without CSF analyses, the clinical decision to exclude the CSF analyses was done after a diagnostic conference involving neurologists and internal medicine physicians based on the follow‐up and common medical knowledge at the time of the pandemic, namely that SARS‐CoV‐2 rarely produces encephalitis. 1 An MRI and an EEG were not available for 3 patients; however, the clinical presentation (delirium at a distance of pneumonia) and follow‐up of these patients were highly suggestive of COVID‐19 encephalopathy. Finally, we should acknowledge that none of the patients died during their hospitalization; this may suggest that the patients with the most severe COVID‐19 pneumonia (those who eventually died) were not referred for a neurological consult and consecutively not included in this series; that would restrict the generalization of the study findings to patients with COVID‐19 encephalopathy, who survive during their hospitalization.
5. CONCLUSIONS
At this time of the pandemic, recognition of COVID‐19 encephalopathy and appropriate treatment are needed, as the long‐term neuropsychiatric consequences of this encephalopathy are not yet established, and that the evolution of the pandemic depends on various unknown scenarios.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conception and design of the study: Frédéric Assal, Patrice H. Lalive, and Gilles Allali. Acquisition and analysis of data: Marjolaine Uginet, Gautier Breville, Frédéric Assal, Karl‐Olof Lövblad, Maria Isabel Vargas, Jérôme Pugin, Jacques Serratrice, Patrice H. Lalive, and Gilles Allali. Drafting the manuscript: Gilles Allali and all authors critically revised the drafted manuscript and approved the submitted manuscript.
ETHICS APPROVAL STATEMENT
The study was approved by the institutional review board of the Geneva University Hospitals (protocol #2020‐01206 – approved May 25, 2020).
Supporting information
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
This study has been performed in the Geneva University Hospitals. This study was funded by a donor from the Private Foundation of the Geneva University Hospitals.
Uginet M, Breville G, Assal F, et al. COVID‐19 encephalopathy: Clinical and neurobiological features. J Med Virol. 2021;93:4374‐4381. 10.1002/jmv.26973
Marjolaine Uginet and Gautier Breville have contributed equally to this study.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Koralnik IJ, Tyler KL. COVID‐19: a global threat to the nervous system. Ann Neurol. 2020;88:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slooter AJC, Otte WM, Devlin JW, et al. Updated nomenclature of delirium and acute encephalopathy: statement of ten societies. Intensive Care Med. 2020;46:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kotfis K, Williams Roberson S, Wilson JE, Dabrowski W, Pun BT, Ely EW. COVID‐19: ICU delirium management during SARS‐CoV‐2 pandemic. Crit Care. 2020;24:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in patients with COVID‐19: A retrospective multicenter study. Neurology. 2020;95:e1868‐e1882. [DOI] [PubMed] [Google Scholar]
- 5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92:552‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753‐1762. [DOI] [PubMed] [Google Scholar]
- 7. Pugin D, Vargas MI, Thieffry C, et al. COVID‐19‐related encephalopathy responsive to high doses glucocorticoids. Neurology. 2020;95:543‐546. [DOI] [PubMed] [Google Scholar]
- 8. Lindenholz A, van der Kolk AG, Zwanenburg JJM, Hendrikse J. The use and pitfalls of intracranial vessel wall imaging: how we do it. Radiology. 2018;286:12‐28. [DOI] [PubMed] [Google Scholar]
- 9. Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy‐associated morbidity in COVID‐19 patients. Ann Clin Transl Neurol. 2020;7:2221‐2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hernández‐Fernández F, Sandoval Valencia H, Barbella‐Aponte RA, et al. Cerebrovascular disease in patients with COVID‐19: neuroimaging, histological and clinical description. Brain. 2020;143:3089‐3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vespignani H, Colas D, Lavin BS, et al. Report of EEG finding on critically ill patients with COVID‐19. Ann Neurol. 2020;88:626‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filosto M, Cotti Piccinelli S, Gazzina S, et al. Guillain‐Barre syndrome and COVID‐19: an observational multicentre study from two Italian hotspot regions. J Neurol Neurosurg Psychiatry. 2020:jnnp‐2020‐324837. [DOI] [PubMed] [Google Scholar]
- 13. Zambreanu L, Lightbody S, Bhandari M, et al. A case of limbic encephalitis associated with asymptomatic COVID‐19 infection. J Neurol Neurosurg Psychiatry. 2020;91:1229‐1230. [DOI] [PubMed] [Google Scholar]
- 14. Pilotto A, Odolini S, Stefano Masciocchi S, et al. Steroid‐responsive encephalitis in COVID‐19 disease. Ann Neurol. 2020;88(2):423‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allali G, Marti C, Grosgurin O, Morelot‐Panzini C, Similowski T, Adler D. Dyspnea: the vanished warning symptom of COVID‐19 pneumonia. J Med Virol. 2020;92:2272‐2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Couzin‐Frankel J. The mystery of the pandemic's 'happy hypoxia'. Science. 2020;368:455‐456. [DOI] [PubMed] [Google Scholar]
- 18. Burki NK, Lee LY. Mechanisms of dyspnea. Chest. 2010;138:1196‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


