Abstract
Background
Emerging evidence suggests an association between COVID‐19 and acute pulmonary embolism (APE).
Aims
To assess the prevalence of APE in patients hospitalised for non‐critical COVID‐19 who presented clinical deterioration, and to investigate the association of clinical and biochemical variables with a confirmed diagnosis of APE in these subjects.
Methods
All consecutive patients admitted to the internal medicine department of a general hospital with a diagnosis of non‐critical COVID‐19, who performed a computer tomography pulmonary angiography (CTPA) for respiratory deterioration in April 2020, were included in this retrospective cohort study.
Results
Study populations: 41 subjects, median (interquartile range) age: 71.7 (63–76) years, CPTA confirmed APE = 8 (19.51%, 95% confidence interval (CI): 8.82–34.87%). Among patients with and without APE, no significant differences were found with regards symptoms, comorbidities, treatment, Wells score and outcomes. The optimal cut‐off value of d‐dimer for predicting APE was 2454 ng/mL, sensitivity (95% CI): 63 (24–91), specificity: 73 (54–87), positive predictive value: 36 (13–65), negative predictive value: 89 (71–98) and AUC: 0.62 (0.38–0.85). The standard and age‐adjusted d‐dimer cut‐offs, and the Wells score ≥2 did not associate with confirmed APE, albeit a cut‐off value of d‐dimer = 2454 ng/mL showed an relative risk: 3.21; 95% CI: 0.92–13.97; P = 0.073. Heparin at anticoagulant doses was used in 70.73% of patients before performing CTPA.
Conclusion
Among patients presenting pulmonary deterioration after hospitalisation for non‐critical COVID‐19, the prevalence of APE is high. Traditional diagnostic tools to identify high APE pre‐test probability patients do not seem to be clinically useful. These results support the use of a high index of suspicion for performing CTPA to exclude or confirm APE as the most appropriate diagnostic approach in this clinical setting.
Keywords: COVID‐19, venous thromboembolism, pulmonary embolism, d‐dimer, CT angiography
Introduction
Coronavirus disease (COVID‐19) is an illness caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The first cases of COVID‐19 were reported in the city of Wuhan, China, in December 2019, and in the following weeks the infection spread across China and in other countries. 1 , 2
The clinical presentation of COVID‐19 can be very varied, and encompasses asymptomatic infection, mild illness affecting the upper respiratory tract, and severe pneumonia, which may cause life‐threatening respiratory failure. 3 The severity of COVID‐19 cases has been classified as mild, moderate, severe, and critical. 4
Emerging evidence suggests that COVID‐19 can be complicated by acute pulmonary embolism (APE), 5 , 6 , 7 and d‐dimer values have been linked to a higher risk of death in patients with COVID‐19. 8
Although data from different studies indicate that the incidence of venous thromboembolism (VTE) in COVID‐19 patients is higher than figures seen in similarly ill hospitalised non‐COVID‐19 subjects, the exact prevalence of APE in COVID‐19 patients is not known, and the diagnostic value of d‐dimer in subjects with acute SARS‐CoV‐2 infection is not clear. 8 The increase in mortality associated with elevated d‐dimer values in these patients may reflect an increased rate of APE, but it also could be a manifestation of disseminated intravascular coagulation or simply a higher level of systemic inflammation. 7 , 9 In addition, most of the published studies evaluating thrombotic complications of COVID‐19 included intensive care unit (ICU) critically ill patients, 10 , 11 , 12 , 13 , 14 and less is known on non‐critical subjects.
The present study aimed to assess the prevalence of APE in patients hospitalised for non‐critical COVID‐19 who presented respiratory deterioration after admission, as well as to investigate the association of d‐dimer and other clinical and biochemical variables with a confirmed diagnosis of APE in these subjects.
Methods
The study was conducted at the Vimercate Hospital, a 500‐bed General Hospital located in Lombardy, northern Italy. From February to May 2020, 712 patients with a confirmed diagnosis of COVID‐19 were hospitalised in our institution. Among them, in April 2020, 218 subjects were admitted to the internal medicine department wards after being evaluated in the emergency department.
In the first months of 2020, different reports suggesting an increasing risk of APE in COVID‐19 patients were published. 6 Thus, in late March, a multidisciplinary group from our hospital issued an internal protocol with some recommendations to prevent and treat thrombotic complications in COVID‐19 patients. The protocol strongly recommended performing a diagnostic CTPA to confirm or rule out APE in COVID‐19 patients admitted to the internal medicine department wards presenting respiratory deterioration after admission, defined by a PaO2/FiO2 ratio reduction of >30%.
Therefore, in the present retrospective cohort study, we included all COVID‐19 patients admitted to the internal medicine department (sub intensive and acute general beds of the internal medicine department wards) who had CTPA examinations performed from 1 April to 31 April for respiratory deterioration after admission, defined by a reduction of ≥30% of the PaO2/FiO2 ratio. The exclusion criteria were: subjects with a story of bleeding diathesis and/or current use of anticoagulant therapy before hospitalisation; age < 18, and critical COVID‐19 infection, defined by any of the following criteria: (i) respiratory failure needing mechanical assistance; (ii) shock; and (iii) ‘extra pulmonary’ organ failure needing ICU.
Electronic charts of all included patients were retrieved for evaluation. Trained study personnel retrospectively recorded relevant clinical, laboratory and treatment data. The diagnosis of COVID‐19 was confirmed by RNA detection of the SARS‐CoV‐2.
Data of the following laboratory test performed 24–48 h before performing CPTA were collected: d‐dimer, international normalised ratio, C‐reactive protein, white blood cell count, lactate dehydrogenase (LDH), alanine transaminase (ALT), aspartate transaminase (AST), creatinine (Cr), arterial partial pressure of carbon dioxide (PaCO2), arterial oxygen partial pressure (PaO2), fraction of inspired oxygen (FiO2). Albumin, Interleukin 6 and Antithrombin III were measured within 24 h of performing CTPA. d‐dimer was measured by using HemosIL d‐Dimer HS, a latex‐enhanced turbidimetric immunoassay from Instrumentation Laboratory, on the fully automated coagulometer ACL TOP analyser. 15 The normal value declared by the producer is <243 ng/mL. 15
Based on a retrospective chart review of clinical symptoms and patient history factors, Wells score simplified version was calculated for each patient, and it was referred to the day when CPTA was performed. One point was given for the presence of each of the following items: (i) previous PE or DVT; (ii) heart rate ≥ 100 b.p.m.; (iii) surgery or immobilisation within the past 4 weeks; (iv) haemoptysis; (v) active cancer; (vi) clinical signs of DVT; and (vii) alternative diagnosis less likely than PE. Patients with <2 points were categorised as PE unlikely and those with ≥2 points were PE likely. 16 Since CTPA was performed in subjects suspected by presenting APE in addition to COVID‐19 as causing respiratory deterioration, the last item of Wells score (alternative diagnosis less likely than PE) was considered present (1 point) in all cases.
Pulmonary embolism was confirmed on the basis of the presence of a filling defect in one or more pulmonary arteries up to sub‐segmental arteries in CTPA, as stated by certified radiologists belonging to the hospital team, at the time of the acquisition of images. Helical CTPA scans were performed on a Brilliance Philips CT scanner (Philips, Cleveland, OH, USA), which included 64‐detector row capability.
This study was conducted in accordance with the amended Declaration of Helsinki. The protocol was approved by the Local institutional review board, that is the Comitato Etico della Provincia Monza e Brianza. Waiver of written informed consent was granted due to the retrospective, observational design.
Statistical methods
Clinical characteristics and laboratory data were summarised by number and percentage for categorical variables and by median and interquartile range for numerical variables, in the whole study group and according to APE confirmed and excluded. To compare the different characteristics among patients with APE confirmed and APE excluded, Fisher exact test was used for categorical variables and Wilcoxon rank rum test was used for numerical variables.
To evaluate the diagnostic accuracy of d‐dimer to predict APE, a receiver operating characteristic (ROC) curve was fitted and the area under the ROC curve (AUC) with pertinent 95% confidence interval (CI) was estimated. Optimal cut‐off was obtained as the d‐dimer value that maximises both the specificity and the sensitivity. The diagnostic performance of different d‐dimer cut‐offs (standard cut‐off: >243 ng/mL, age‐adjusted cut‐off: patients’ age × 5, ROC curve best discriminating value: 2454 ng/mL) and Wells score (standard cut‐off: >2) was evaluated by computing the corresponding values of sensitivity and specificity, positive predictive value, negative predictive value with pertinent 95% CI. Furthermore, four generalised linear regression models with binomial error and link log were fitted: the response was APE and the explanatory variable was a dichotomous variable discriminating patients with d‐dimer value over the different cut‐offs or Wells score over 2. Results were reported as relative risk (RR) with corresponding 95% CI and P‐values.
All analyses were performed using R software version 4.0.0, with packages OptimalCutpoints, pROC and epiR added.
Results
From 1 to 30 April 2020, 41 patients who were admitted to internal medicine department wards and underwent a CTPA because of respiratory deterioration (defined as a PaO2/FiO2 ratio reduction of ≥30%) after admission represent the study population. The median (interquartile range (IQR)) age of the cohort was 71.7 (63–76) years, 30 (73%) were females, the median days (IQR) since onset of symptoms to hospitalisation was 8 (4–12) and the median days (IQR) since onset of symptoms to CTPA was 11 (7–17). By the end of May 2020, in‐hospital mortality of the cohort was 4.88%, with two patients still hospitalised and 90% already discharged. Clinical characteristics, treatment and outcomes of the study population are shown in Table 1.
Table 1.
Clinical characteristics of the study population, treatment during hospitalisation and outcomes
| Characteristics | Total (n = 41) | APE confirmed (n = 8) | APE excluded (n = 33) | P‐value (APE confirmed vs excluded) |
|---|---|---|---|---|
| Age, median (IQR) (years) | 71.7 (63–76.2) | 67 (57.3–74.4) | 72.1 (63.1–76.2) | 0.459 |
| Female, n (%) | 30 (73.17) | 6 (75) | 24 (72.73) | 1.000 |
| Time since onset of symptoms to hospitalisation, median (IQR) (days) | 8 (4–12) | 8.5 (3.5–14.8) | 8 (4–12) | 0.830 |
| Time since hospitalisation to CTPA, median (IQR) (days) | 11 (7–17) | 11 (1.2–13.5) | 11 (8–17) | 0.680 |
| Symptoms, n (%) | ||||
| Fever | 40 (97.56) | 8 (100) | 32 (96.97) | 1.000 |
| Cough | 26 (63.41) | 5 (62.5) | 21 (63.64) | 1.000 |
| Dyspnoea | 30 (73.17) | 4 (50) | 26 (78.79) | 0.178 |
| Chest pain | 6 (14.63) | 3 (37.5) | 3 (9.09) | 0.077 |
| Diarrhoea | 13 (31.71) | 2 (25) | 11 (33.33) | 1.000 |
| Comorbidities, n (%) | ||||
| Hypertension | 29 (70.73) | 4 (50) | 25 (75.76) | 0.202 |
| Diabetes | 11 (26.83) | 2 (25) | 9 (27.27) | 1.000 |
| Chronic heart disease | 9 (21.95) | 1 (12.5) | 8 (24.24) | 0.659 |
| Active cancer | 3 (7.32) | 0 (0) | 3 (9.09) | 1.000 |
| Smoking | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| CCI, median (IQR) | 1 (0–1) | 0 (0–1) | 1 (0–2) | 0.173 |
| Treatment, n (%) | ||||
| Heparin at prophylactic dose before performing CTPA | 4 (9.76) | 0 (0) | 4 (12.12) | 0.569 |
| Heparin at anticoagulant dose before performing CTPA | 29 (70.73) | 5 (62.5) | 24 (72.73) | 0.672 |
| Hydroxychloroquine | 39 (95.12) | 8 (100) | 31 (93.94) | 1.000 |
| Any antiviral therapy | 12 (29.27) | 3 (37.5) | 9 (27.27) | 0.672 |
| Steroids | 33 (80.49) | 7 (87.5) | 26 (78.79) | 1.000 |
| CPAP | 25 (60.98) | 5 (62.5) | 20 (60.61) | 1.000 |
| Wells score within 48 h before CTPA, median (IQR) | 2 (2–2) | 2 (1.8–2) | 2 (2–2) | 0.681 |
| Outcome, n (%) | ||||
| Discharged | 37 (90.24) | 7 (87.5) | 30 (90.91) | 1.000 |
| Still hospitalised | 2 (4.88) | 1 (12.5) | 1 (3.03) | 1.000 |
| In‐hospital mortality | 2 (4.88) | 0 (0) | 2 (6.06) | 1.000 |
APE, acute pulmonary embolism; CCI, Charlson Comorbidities Index; CPAP, continuous positive airway pressure; CTPA, computed tomography pulmonary angiography; IQR, interquartile range.
The most frequent symptoms were fever (98%), dyspnoea (73%) and cough (63%), and more than 70% (29 cases) of subjects were hypertensive. Most patients had been treated empirically with hydroxychloroquine (95%) and steroids (80%), more than 70% with full anticoagulant doses of heparin before performing CTPA, and 61% with continuous positive airway pressure.
Eight out of 41 (19.51%; 95% CI: 8.82–34.87%) patients presented a confirmed APE after performing CTPA. Except for a trend to a higher prevalence of chest pain among subjects with APE (37% vs 9%; P = 0.077), no statistically significant differences in patients with and without APE were found, with regard to symptoms, comorbidities, treatment, Wells score and outcomes (Table 1). Among patients with confirmed APE, a higher median value of white blood cell count (12. 4 vs 8.4 × 109/L; P = 0.007) and a trend for a lower value of alanine aminotransferase were found. The results of laboratory tests in patients with APE confirmed and excluded are shown in Table 2.
Table 2.
Laboratory data in patients with APE confirmed and excluded
| Normal range missing data (n) | All patients (n = 41) | APE confirmed (n = 8) | APE excluded (n = 33) | P‐value (APE confirmed vs excluded) | |
|---|---|---|---|---|---|
| d‐Dimer, median (IQR) (μg/mL) | 0–243 (0) | 1488 (446–4211) | 3236 (1943–4735) | 1056 (446–2634) | 0.316 |
| INR, median (IQR) | 0.8–1.2 (9) | 1.2 (1.1–1.3) | 1.2 (1.1–1.2) | 1.2 (1.1–1.3) | 0.749 |
| Albumin, median (IQR) (g/dL) | 3.7–5.3 (10) | 3.0 (2.9–3.3) | 2.7 (2.4–3.1) | 3.1 (3–3.3) | 0.284 |
| CRP, median (IQR) (mg/dL) | 0.0–8.0 (0) | 51 (18–140) | 67 (40.2–157.8) | 50 (16–135) | 0.439 |
| WBCC, median (IQR) (×109/L) | 4.0–11.0 (0) | 9.1 (7–12.2) | 12.4 (10.4–13.4) | 8.4 (6.4–10.5) | 0.007 |
| LDH, median (IQR) (U/L) | 135–225 (1) | 332 (251.8–419.2) | 330 (249.2–340.8) | 354.5 (251.8–441.8) | 0.437 |
| ALT, median (IQR) (U/L) | 5–43 (1) | 41 (27.8–53.2) | 34.5 (23–39) | 44.5 (28.8–55) | 0.058 |
| AST, median (IQR) (U/L) | 3–45 (0) | 43.5 (30.8–70) | 44 (28–58.2) | 43.5 (30.8–73.2) | 0.488 |
| Cr, median (IQR) (μmol/L) | 0.6–1.2 (0) | 0.9 (0.8–1) | 0.9 (0.8–1.1) | 0.8 (0.8–1) | 0.383 |
| Interleukin 6, median (IQR) (pg/mL) | <7 (15) | 11.9 (7.8–38.7) | 20.8 (10.6–37.8) | 11.6 (7–35.9) | 0.287 |
| Antithrombin III, median (IQR) | 80–120 (19) | 100.5 (90.2–114.2) | 89 (88–103) | 102 (95–115) | 0.456 |
| PaCO2, median (IQR) (mmHg) | 32–45 (0) | 37 (34–42) | 42 (38–43.2) | 36 (34–39) | 0.171 |
| PaO2, median (IQR) (mmHg) | 83–108 (0) | 95 (71–143) | 129 (65.2–146.2) | 91 (71–139) | 0.934 |
| PaO2/FiO2 ratio, median (IQR) | >350 (0) | 123 (93–186) | 161 (127.8–195.8) | 117 (93–174) | 0.633 |
ALT, alanine aminotransferase; APE, acute pulmonary embolism; AST, aspartate transaminase; Cr, creatinine; CRP, C‐reactive protein; FiO2, fraction of inspired oxygen; INR, international normalised ratio; IQR, interquartile range; LDH, lactate dehydrogenase; PaCO2, arterial partial pressure of carbon dioxide; PaO2,arterial oxygen partial pressure; WBCC, white blood cell count.
The median values of d‐dimer in patients with APE confirmed and excluded were 3236 and 1056 μg/mL (P = 0.316). The discriminant ability of d‐dimer on admission to identify confirmed versus non‐confirmed APE showed an AUC of 0.62 (95% CI: 0.38–0.85%) (Fig. 1). The optimal cut‐off obtained by the ROC curve was 2454 ng/mL, with values: sensitivity, 63% (95% CI: 24–91%); specificity, 73% (95% CI: 54–87%); positive predictive value, 36% (95% CI: 13–65%); and negative predictive value, 89% (95% CI: 71–98%). The standard d‐dimer cut‐off (243 ng/mL), for a confirmed diagnosis of APE, showed values: sensitivity (95% CI), 88% (47–99%); specificity, 12% (3–28%); positive predictive value, 1% (8–36%); and predictive negative value, 80% (28–99%). Figures for the age‐adjusted d‐dimer cut‐off (patients’ age × 5) and Wells score ≥ 2 (likely) were 88% (47–99%), 18% (7–35%), 21% (9–38%), 86% (42–99%), and 13% (0.3–53%), 85% (68–95%), 17% (0.42–64%) and 80% (63–92%), respectively. The RR of the optimal d‐dimer cut‐off (2454 ng/mL) for confirmed APE was 3.31 (P = 0.073), whereas the standard and age adjusted d‐dimer cut‐offs and Wells score presented RR (95% CI) of 0.97 (0.23–16.22; P = 0.977), 1.44 (0.32–24.77; P = 0.711) and 0.83 (0.05–3.67; P = 0.851) respectively (Table 3).
Figure 1.
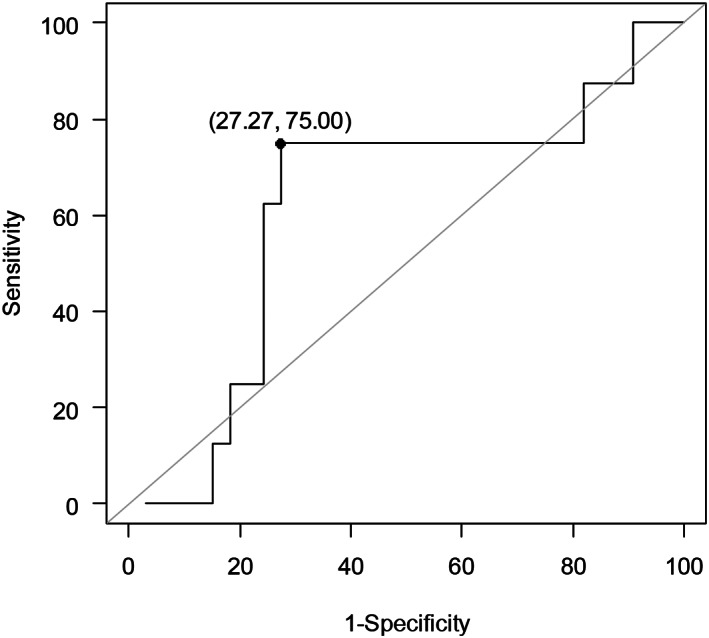
ROC curve to estimate the optimal cut‐off value of d‐dimer for predicting acute pulmonary embolism.
Table 3.
Diagnostic performance of different d‐dimer cut‐offs and Wells score for the diagnosis of APE
| Number of cases (%) with values higher than the cut‐off | RR (95% CI) for the confirmed diagnosis of APE | P‐value | |
|---|---|---|---|
| d‐Dimer (standard cut‐off: 243 ng/mL) | 36 (87.8) | 0.97 (0.23–16.22) | 0.977 |
| d‐Dimer (age adjusted: patients’ age × 5) | 34 (82.93) | 1.44 (0.32–24.77) | 0.711 |
| d‐Dimer (cut‐off: ROC CURVE best discriminating value: 2454 ng/mL) | 14 (34.15) | 3.21 (0.92–13.97) | 0.073 |
| Wells score (≥2: likely) | 6 (14.63) | 0.83 (0.05–3.67) | 0.851 |
APE, acute pulmonary embolism; CI, confidence interval; RR, relative risk.
Discussion
Although emerging data suggest that acutely ill patients with COVID‐19 have an increased risk for APE, the actual prevalence of APE in this clinical setting is not well known. Most information on the thrombotic complications of COVID‐19 derives from studies including critical ill patients and data on non‐critical acutely ill subjects are lacking.
In the present study, we found that among patients who presented respiratory deterioration after being admitted to the internal medicine department with a diagnosis of non‐critical COVID‐19, nearly 20% presented a confirmed APE. In these subjects, the best discriminating cut‐off value of d‐dimer for predicting APE was approximately 10‐fold the standard threshold (2454 ng/mL), showing a trend to be associated with confirmed APE, albeit not statistically significant (RR: 3.21; 95% CI: 0.92–13.97; P = 0.073). The values of d‐dimer, when the standard and age‐adjusted cut‐offs were applied, the simplified Wells score and other laboratory test did not appear to be clinically useful to identify patients with confirmed APE. Moreover, more than 70% have been empirically treated with full anticoagulant doses of heparin before performing CTPA.
Some points are worth discussing. First, the prevalence of APE we found in this clinical setting was high. This finding is even more noticeable when considering that most patients were receiving heparin at anticoagulant doses. As mentioned, most data on prevalence of APE and thrombotic events in general, are from COVID‐19 ICU critical patients. 8 , 10 , 11 , 12 , 13 , 14 , 15 In COVID‐19 patients admitted to the ICU of three Dutch hospitals, Klok et al. found a cumulative incidence of the composite thromboembolic outcome of 31% (95% CI: 20–41%) with APE, representing 81% of all these thrombotic complications (n = 25). 10 The rate of thromboembolic complications, mainly APE, was higher (11.7%) in COVID‐19 patients referred to the ICU from a French hospital, than that observed in a historical control group of non‐COVID‐19 ARDS patients (2.1%). 14 These figures are much higher than the rates of APE observed in non COVID‐19 ICU patients with sepsis or shock receiving guideline‐recommended thromboprophylaxis. 17 In one of the few studies describing thromboembolic events in non‐ICU COVID‐19 patients, 29 out of 91 (31.9%) patients who underwent CTPA presented with APE after admission to the internal medicine department. 18 Moreover, in the meta‐analysis by Malas et al., 19 the overall APE rate was 13% (95% CI: 11–16%): 7% (95% CI: 5–9%) among 16 non‐ICU studies, 19% (95% CI: 14–25%) among 18 ICU studies and 22% (95% CI: 1628%) among postmortem studies. Our study focussed on non‐ICU patients presenting respiratory deterioration after hospitalisation, and our results confirm the finding of a high incidence of APE in this clinical setting. Second, the tools currently used in non‐COVID‐19 patients to estimate the pre‐test probability of APE as part of the diagnostic work‐up (Wells score and standard or age‐adjusted d‐dimer values) seem not to be useful for predicting APE in COVID‐19 patients. These findings are not surprising given these test have been validated in non‐COVID‐19 patients with low‐intermediate risk of APE. This was also true for other laboratory tests, such as arterial blood gas analysis, interleukin‐6, antithrombin III, AST, ALT, LDH and serum creatinine. Our results suggest that a very high d‐dimer cut‐off (approximately 10‐fold the standard threshold) may be associated with a confirmed APE. These data are in keeping with the results of a study reporting that a d‐dimer threshold of 2660 μg/L detected all patients with APE among those hospitalised with COVID‐19. 20 These cut‐off values are much higher than those used to exclude pulmonary embolus in non‐ICU patients. 21 Even though a diagnostic strategy for APE suspicion based on a single variable (d‐dimer) presents evident limitations and guidelines recommend multivariable predicting algorithms for non‐COVID‐19 patients, 21 the diagnostic value of a sharp increase of d‐dimer as marker of increased risk of APE in COVID‐19 patients remains to be established. Some authors have proposed using age‐adjusted d‐dimer cut‐off levels to rule out VTE in COVID‐19 patients. 22 Yet, in our study the age adjusted threshold did not show to be clinically useful. Third, in our study, among acutely ill patients presenting respiratory deterioration after admission to the internal medicine department wards with a diagnosis of COVID‐19 during the so‐called ‘first wave’, most have been treated with full anticoagulant doses of heparin before performing CTAP. The fact that these patients have been considered at a very high risk of having APE probably led physicians to prescribe full anticoagulation instead of prophylaxis with heparin, also because at the time of the study period (April 2020) no clear expert clinical guidance had been issued on this subject. Interestingly, also in the study by Klok et al., 10 it was described that heparin regimens differed between hospitals and the doses increased over time, presumably reflecting an increasing concern on the risk of developing APE in COVID‐19 patients. However, empirical use of anticoagulant doses of heparin may not only be ineffective but also deleterious since it has been well established that high‐dose LMWH administration may be associated with increased incidence of major and fatal bleeding. 23 In fact, pending the results of randomised clinical trials, in patients without a confirmed diagnosis of APE, most authors recommend thromboprophylaxis with LMWH for non‐ICU COVID‐19 patients. 24
Last, in COVID‐19 patients, given the high prevalence of APE, the unavailability of satisfactory tools for estimating pretest probability and the potential high risk of complications associated with the use of empirical anticoagulation, a high index of suspicion for performing CTPA should be strongly recommended in order to exclude or confirm APE. Main contraindications for CTPA are impaired renal function and haemodynamic instability to undergo the test. Our results show that these conditions are relatively rare among non‐ICU COVID‐19 patients.
The main limitations of our study are retrospective and monocentric design and small sample size, with large 95% CI limiting the precision of estimates and the generalisability of results. Yet, we included all consecutive subjects fulfilling the inclusion criteria, to reduce selection bias. Thus, our study population would represent a real‐world sample for severe COVID‐19 patients admitted to internal medicine department wards who presented with respiratory deterioration. Nevertheless, to confirm our findings, larger and multicentre studies are needed. Moreover, our study population was limited to 41 subjects who presented with respiratory deterioration after hospitalisation and thus performing CPAP, from a total of 218 non‐ICU COVID‐19 patients admitted to the internal medicine department wards of our hospital in the study period (April 2020). No data may be provided on the rates of AP in patients who did not present respiratory deterioration, because CPAP was not performed in these subjects.
Conclusions
Among patients hospitalised in the internal medicine department with a diagnosis of non‐critical COVID‐19, in whom a CTPA was performed because of respiratory deterioration after admission, the prevalence of confirmed APE is high (20%). Some validated tools used in the APE diagnostic work‐up of non COVID‐19 patients, such as d‐dimer (standard and age‐adjusted cut‐offs) and Wells score, along with other commonly used laboratory tests, do not seem to be clinically useful to identify patients with confirmed APE.
Most subjects have been treated empirically with full anticoagulant doses of heparin before performing CTPA, even though in many cases CTPA did not confirm the diagnosis of APE. While awaiting additional evidence and the development of new diagnostic and therapeutic algorithms, our results support the use of a high index of suspicion for performing CTPA to exclude or confirm APE as the most appropriate diagnostic approach in this clinical setting.
Funding: None.
Conflict of interest: None.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–42. [DOI] [PubMed] [Google Scholar]
- 2. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID‐19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020; 323: 1545–6. [DOI] [PubMed] [Google Scholar]
- 3. Kho J, Ioannou A, Van den Abbeele K, Mandal AKJ, Missouris CG. Pulmonary embolism in COVID‐19: clinical characteristics and cardiac implications. Am J Emerg Med 2020; 38: 2142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) – China, 2020. CCDC Weekly 2020; 2: 113–22. [PMC free article] [PubMed] [Google Scholar]
- 5. Ehrman RR, Collins J, Harrison N. Prevalence of pulmonary embolism in emergency department patients with suspected COVID‐19: the truth remains unknown. Acad Emerg Med 2020; 27: 1216–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li T, Cheng GS, Pipavath SNJ, Kicska GA, Liu L, Kinahan PE et al. The novel coronavirus disease (COVID‐19) complicated by pulmonary embolism and acute respiratory distress syndrome. J Med Virol 2020; 92: 2205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franco‐Moreno A, Herrera‐Morueco M, Mestre‐Gómez B, Muñoz‐Rivas N, Abad‐Motos A, Salazar‐Chiriboga D et al. Incidence of deep venous thrombosis in patients with COVID‐19 and pulmonary embolism: compression ultrasound COVID study. J Ultrasound Med 2021; 40: 1411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gungor B, Atici A, Baycan OF, Alici G, Ozturk F, Tugrul S et al. Elevated d‐dimer levels on admission are associated with severity and increased risk of mortality in COVID‐19: a systematic review and meta‐analysis. Am J Emerg Med 2021; 39: 173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibañez C, Perdomo J, Calvo A, Ferrando C, Reverter JC, Tassies D et al. High D dimers and low global fibrinolysis coexist in COVID19 patients: what is going on in there? J Thromb Thrombolysis 2020; 51: 308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res 2020; 191: 145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rieder M, Goller I, Jeserich M, Baldus N, Pollmeier L, Wirth L et al. Rate of venous thromboembolism in a prospective all‐comers cohort with COVID‐19. J Thromb Thrombolysis 2020; 50: 558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas W, Varley J, Johnston A, Symington E, Robinson M, Sheares K et al. Thrombotic complications of patients admitted to intensive care with COVID‐19 at a teaching hospital in the United Kingdom. Thromb Res 2020; 191: 76–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost 2020; 18: 1743–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Helms J, Tacquard C, Severac F, Leonard‐Lorant I, Ohana M, Delabranche X et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med 2020; 46: 1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salvagno GL, Lippi G, Montagnana M, Poli G, Giavarina D, Manzato F et al. Performance of the automated and rapid HemosIL d‐dimer HS on the ACL TOP analyzer. Blood Coagul Fibrinolysis 2008; 19: 817–21. [DOI] [PubMed] [Google Scholar]
- 16. Gibson NS, Sohne M, Kruip MJ, Tick LW, Gerdes VE, Bossuyt PM et al. Further validation and simplification of the Wells clinical decision rule in pulmonary embolism. Thromb Haemost 2008; 99: 229–34. [DOI] [PubMed] [Google Scholar]
- 17. Kaplan D, Casper TC, Elliott CG, Men S, Pendleton RC, Kraiss LW et al. VTE incidence and risk factors in patients with severe sepsis and septic shock. Chest 2015; 148: 1224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mestre‐Gómez B, Lorente‐Ramos RM, Rogado J, Franco‐Moreno A, Obispo B, Salazar‐Chiriboga D et al. Incidence of pulmonary embolism in non‐critically ill COVID‐19 patients. Predicting factors for a challenging diagnosis. J Thromb Thrombolysis 2020. 10.1007/s11239-020-02190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID‐19 is high and associated with a higher risk of mortality: a systematic review and meta‐analysis. EClinicalMedicine. 2020; 29: 100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leonard‐Lorant I, Delabranche X, Severac F et al. Acute pulmonary embolism in COVID‐19 patients on CT angiography and relationship to d‐dimer levels. Radiology 2020; 296: E189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vedovati MC, Giustozzi M, Franco L. Beyond the guidelines: novelties, changes and unsolved issues from the 2019 ESC guidelines on pulmonary embolism. Eur J Intern Med 2020; 72: 1–4. [DOI] [PubMed] [Google Scholar]
- 22. Roncon L, Zuin M, Zonzin P. Age‐adjusted d‐dimer cut‐off levels to rule out venous thromboembolism in COVID‐19 patients. Thromb Res 2020; 190: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conti CB, Henchi S, Coppeta GP, Testa S, Grassia R. Bleeding in COVID‐19 severe pneumonia: the other side of abnormal coagulation pattern? Eur J Intern Med 2020; 77: 147–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cattaneo M, Bertinato EM, Birocchi S et al. Pulmonary embolism or pulmonary thrombosis in COVID‐19? Is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thromb Haemost 2020; 120: 1230–2. [DOI] [PMC free article] [PubMed] [Google Scholar]


