Abstract
COVID‐19 (SARS‐CoV‐2) causes multiple inflammatory complications, resulting not only in severe lung inflammation but also harm to other organs. Although the current focus is on the management of acute COVID‐19, there is growing concern about long‐term effects of COVID‐19 (Long Covid), such as fibroproliferative changes in the lung, heart and kidney. Therefore, the identification of therapeutic targets not only for the management of acute COVID‐19 but also for preventing Long Covid are needed, and would mitigate against long‐lasting health burden and economic costs, in addition to saving lives. COVID‐19 induces pathological changes via multiple pathways, which could be targeted simultaneously for optimal effect. We discuss the potential pathologic function of increased activity of the endocannabinoid/CB1 receptor system and inducible NO synthase (iNOS). We advocate a polypharmacology approach, wherein a single chemical entity simultaneously interacts with CB1 receptors and iNOS causing inhibition, as a potential therapeutic strategy for COVID‐19‐related health complications.
LINKED ARTICLES
This article is part of a themed issue on The second wave: are we any closer to efficacious pharmacotherapy for COVID 19? (BJP 75th Anniversary). To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v179.10/issuetoc
Keywords: COVID‐19, iNOS inhibitor, peripheral CB1 antagonist, polypharmacology, pulmonary fibrosis, SARS‐CoV‐2
Abbreviations
- ARDS
acute respiratory distress syndrome
- COVID‐19
coronavirus disease 2019
- CT
computed tomography
- SARS‐CoV‐2
severe acute respiratory syndrome‐coronovirus‐2
1. INTRODUCTION
Coronavirus disease (COVID‐19) caused by the SARS‐CoV‐2 virus has emerged as a viral pandemic causing respiratory distress and pulmonary inflammation. Most cases of SARS‐CoV‐2 infection are either asymptomatic or are associated with mild to moderate symptoms followed by a full recovery. However, a substantial percentage of infected individuals develop serious complications requiring intensive care unit hospitalisation and mechanical ventilation via tracheal intubation. In such patients, dyspnoea and hypoxaemia develop shortly after disease initiation that can lead to respiratory failure, acute respiratory distress syndrome (ARDS) and multi‐organ failure.
2. COVID‐19: FROM RESPIRATORY INFECTION TO MULTIPLE METABOLIC HEALTH COMPLICATIONS
Earlier epidemiological surveys have shown that elderly patients or those with underlying chronic conditions such as obesity, chronic kidney disease, heart failure and/or hypertension were more susceptible to severe COVID‐19 infection (Petrilli et al., 2020). In such patients, COVID‐19‐related ARDS may be accompanied by shock, heart failure, acute kidney injury and coagulation dysfunction (Petrilli et al., 2020). As additional features of the viral infection emerge, it appears that respiratory illness alone is no longer the critical factor in COVID‐19 progression, as there are indications that virus‐induced damage to the vascular endothelium underlies the formation of micro‐clots in the lungs and the hypoxia observed in severely ill patients. The blood clots in other organ systems—mainly the brain, kidney and liver and even frostbite‐like swollen wounds on fingers and toes—reflect endothelial impairment as the underlying process (Ackermann et al., 2020). This parallels the ‘cytokine storm’ and leads to a ‘coagulation storm’, ultimately resulting in multi‐organ failure and death in some patient populations (Ackermann et al., 2020). Obesity is known to increase pro‐thrombotic factors and leptin production and hyperleptinaemia in obesity increase thrombotic risk (Konstantinides et al., 2001). Obesity and diabetes are known to be accompanied by metabolic inflammation. Although it has not been definitively established that inflammation is responsible for the increased COVID‐19 morbidity and mortality in obese individuals, a case could be made for normalising obesity‐related hyperleptinaemia as part of therapeutic management strategies in such patients. Additionally, elevated fasting blood glucose upon hospital admission has been highlighted as a predictor of poor prognosis and mortality among COVID‐19 patients even in the absence of diabetes (Carrasco‐Sanchez et al., 2021). Another study provided mechanistic insight on how increased glucose levels alter immune function to favour viral replication, induce monocytic cytokine production and T‐cell dysfunction, which result in lung epithelial damage (Codo et al., 2020). These studies also highlight the importance of maintaining glucose homeostasis during the progression of COVID‐19 infection. Furthermore, blood vessel damage could also explain why pre‐existing conditions like hypertension, obesity, diabetes and heart disease are risk factors for severe complications from SARS‐COV‐2. All of those conditions cause endothelial cell dysfunction which could facilitate hyper‐inflammation in the lungs as a result of the viral attack. Thus, COVID‐19 is not only a respiratory disease but also a metabolic and inflammatory disease, and therefore therapeutic interventions should not be limited to mitigate acute damage caused by SARS‐CoV‐2, but should also aim to resolve many of the pathologies that develop as the viral infection progresses (Figure 1).
FIGURE 1.
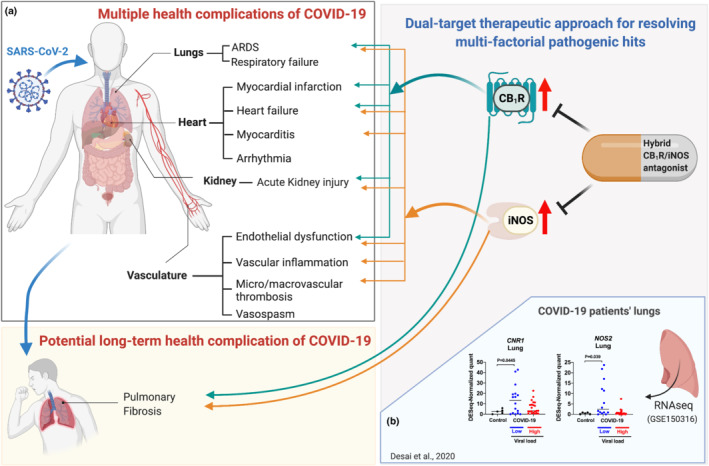
Multi‐target therapeutic strategy for COVID‐19‐related multiple health complications by dual inhibition of CB1 receptors and iNOS. (a) Dual inhibition of CB1 receptors (CB1R) and iNOS may not only attenuate acute COVID‐19 complications such as acute respiratory distress syndrome (ARDS), septicaemia, heart failure, acute kidney injury, endothelial dysfunction and thrombosis but may also mitigate the development of pulmonary fibrosis as a late sequela of COVID‐19. (b) Both CNR1 and NOS2 gene expression are increased in lungs of COVID‐19 patients. Lung expression profiles of CNR1 and NOS2 were retrieved from RNAseq data set (Desai et al., 2020) from five virus‐negative controls, seven COVID cases with low viral load and eight COVID cases with high viral load. Multiple data points obtained from different lung lobes were used for COVID cases considering temporal and spatial heterogeneity of host response to SARS‐CoV‐2 infection. The figure was generated in Biorender.com
3. FINDING CURE FOR ACUTE MANIFESTATIONS OF COVID‐19: FROM SINGLE‐ TO MULTI‐TARGET APPROACHES
Currently, a well justified race is underway globally to test a spectrum of candidate drugs in clinical trials for COVID‐19. Therapeutic approaches involve repurposing earlier approved medications and generic drugs, as well as the use of promising, as yet unapproved, experimental small molecules. Corticosteroids such as dexamethasone and hydrocortisone have shown promise in critically ill patients. Antibody treatments that can contain the ‘cytokine storm’ in COVID‐19, such as convalescent plasma therapy, are also being tested to combat the viral infection and have since gained emergency use authorisation. Operation Warp Speed supports the development and deployment of effective COVID‐19 vaccines in record time. The history of infectious diseases such as HIV‐AIDS suggests that it will take a ‘cocktail’ of drugs, that is, a combination therapy, to minimise the repercussions of the viral infection. Indeed, an approach to simultaneously impact multiple pathologic pathways has been adopted for COVID‐19 (Hung et al., 2020). COVID‐19 patients with underlying chronic diseases are already subjects to polypharmacy. In the present proposal, we advocate the use of a polypharmacology approach wherein a single chemical entity simultaneously engages two distinct targets to improve multiple conditions resulting from COVID‐19 infection. A rational polypharmacology approach can also improve therapeutic efficacy and safety margin and would minimise limitations arising from the use of multiple chemical entities (Proschak et al., 2019), such as differential pharmacokinetics and target organ engagement, possible drug–drug interactions and potential adverse effects in a setting of compromised immune and organ function secondary to the viral attack.
4. POTENTIAL FUTURE HEALTH COMPLICATIONS IN COVID‐19 SURVIVORS: PULMONARY FIBROSIS?
Besides the acute health threat represented by COVID‐19 including ARDS, septicaemia, coagulation dysfunction, kidney dysfunction and pulmonary embolism, there is also growing concern about potential late complications among survivors of an acute COVID‐19 infection. Recently emerging data document, the persistence of symptoms such as fatigue, dyspnoea and chest pain among the survivors of SARS‐CoV‐2 infection 2–3 months following recovery (Carfi et al., 2020). In the case of prior SARS‐CoV epidemics, a substantial proportion of patients developed pulmonary fibrosis within a year of the acute infection (Hui et al., 2005). Based on lung computed tomography (CT) scans of recent COVID‐19 patients and their 1‐year follow up, there is evidence for pulmonary fibrosis development (Picchi et al., 2020; Tale et al., 2020; Ye et al., 2020). Therefore, pulmonary fibrosis could be a late consequence of COVID‐19 (George et al., 2020; Spagnolo et al., 2020; Vasarmidi et al., 2020). Pulmonary fibrosis is a progressive disease, causing significant decline in pulmonary function and mortality within 3–5 years of the initial diagnosis. Currently, idiopathic pulmonary fibrosis is considered a rare disease with an estimated prevalence of 13 to 20 per 100,000 people worldwide. However, in a possible scenario of 20–25% of COVID‐19 survivors developing pulmonary fibrosis, COVID‐19‐related pulmonary fibrosis would become a common disease affecting up to 120 million SARS‐CoV‐2 cases worldwide based on current infection levels. Therefore, it is important to understand the proliferative mechanisms that act as chronic pathological stressors in the lung of COVID‐19 sufferers.
5. INCREASED CB1 RECEPTOR ACTIVITY INVOLVED IN OBESITY, DIABETES, STEATOSIS, DYSLIPIDAEMIA, HYPERLEPTINAEMIA AND SEPTIC SHOCK
Hyper‐inflammation is attributed to the release of pro‐inflammatory cytokines (e.g. IL‐6, IL‐1, IL‐8, TNF‐α), chemokines (MCP‐1, IP‐10) and scaffold molecules (NLRP3 inflammasomes) that may contribute to the rapidly developing systemic organ failure observed in some critically ill COVID‐19 patients. In addition, co‐morbidities like obesity, diabetes and hypertension can exacerbate the negative consequences of the viral attack. Many of the above pathologies involve an overactive endocannabinoid/cannabinoid receptor 1 (CB1) system. Endocannabinoids are bioactive lipid signalling molecules that act via cannabinoid receptors, CB1 and CB2 . These are G‐protein coupled receptor (GPCR) which mediate the effects of the psychoactive ingredients of cannabis. Although CB1 receptors are mainly found in the brain they are also widely distributed in peripheral tissues. Overactivation of the endocannabinoid/CB1 receptor system in peripheral organs contributes to obesity (Ruiz de Azua et al., 2017) and related metabolic disorders including type‐2 diabetes (Jourdan et al., 2013), hepatic steatosis, dyslipidaemia, hyperleptinaemia and leptin resistance (Tam et al., 2010, 2012) and diabetic nephropathy (Jourdan et al., 2014). Conversely, peripheral CB1 receptor antagonism is effective in attenuating obesity and related metabolic complications (Cinar et al., 2020). CB2 receptors are expressed mostly in the periphery, primarily in immune cells, and their activation has anti‐inflammatory properties in multiple inflammatory disorders (Maccarrone et al., 2015). Accordingly, CB2 receptor agonism may have therapeutic benefit in COVID‐19 by limiting inflammatory cytokine release, an effect discussed in recent review articles (Onaivi & Sharma, 2020; Rossi et al., 2020).
6. CB1 RECEPTORS INVOLVED IN INFLAMMATORY DISORDERS: LUNG INFLAMMATION, ARDS, INCREASE VIRAL LOAD, SEPSIS, ACUTE KIDNEY INJURY AND ORGAN FIBROSIS
Recent observations show that endocannabinoids acting via CB1 receptors are induced in certain viral infections and exacerbate viral loads (Reiss, 2010; van der Poorten et al., 2010). In turn, CB1 receptor blockade has been shown to exert anti‐viral effects by repressing viral replication and to protect from or reverse sepsis (Shahidi et al., 2014; Singh et al., 2018). Activation of peripheral CB1 receptors also contributes to septic hypotension whereas CB1 receptor blockade protects against endotoxin‐induced hypotension (Batkai et al., 2001; Varga et al., 1998) and lung injury (Johnson et al., 2015). Additionally, CB1 receptor up‐regulation by potent synthetic cannabinoids is directly linked to hypoxia and acidosis leading to ARDS and respiratory failure (Alon & Saint‐Fleur, 2017). CB1 receptor‐induced lung injury and inflammation are mediated through IL‐1ß, TNF‐α, IL‐6, MCP‐1, IRF5 and NLRP3 signalling (Zawatsky et al., 2020), which lead to pro‐inflammatory and pro‐fibrotic sequelae (Cinar et al., 2017). Furthermore, activating renal and cardiac CB1 receptors induces nephropathy (Jourdan et al., 2018; Udi et al., 2017) and cardiomyopathy (Batkai et al., 2007; Rajesh et al., 2012), respectively. Additionally, use of synthetic cannabinoids results in a high rate of acute kidney injury and cardiac dysfunction (Pacher et al., 2018; Tam, 2016). CB1 receptor activation contributes to organ fibrosis in liver, lung, heart and kidney (Cinar et al., 2020). All this makes CB1 receptors plausible therapeutic targets in metabolic, inflammatory and fibroproliferative disorders.
Indeed, the first‐in‐class, globally acting CB1 antagonist/inverse agonist rimonabant had beneficial effects in metabolic syndrome (Despres et al., 2005) and was consequently approved as an anti‐obesity drug in about 40 countries. However, psychiatric side effects due to blockade of CB1 receptors in the central nervous system resulted in its withdrawal from the clinic and halted the therapeutic development of related brain‐penetrant CB1 antagonists. Increasing evidence in the last decade has shown that non‐brain‐penetrant CB1 antagonists retain the metabolic benefit of globally acting compounds without their adverse behavioural effects and show therapeutic potential in preclinical models of obesity and related metabolic disorders (Cinar et al., 2020).
7. INDUCIBLE NITRIC OXIDE SYNTHASE: INFLAMMATION, ARDS, SEPSIS AND ORGAN FIBROSIS
Another potential therapeutic target that parallels the role of CB1 receptors in many pathologies is inducible nitric oxide synthase (iNOS), an enzyme that catalyses the generation of pro‐inflammatory reactive nitrogen species. Increased expression of iNOS in the lung was detected along with IL‐6 and IL‐8 in ARDS patients following sepsis, suggesting that iNOS along with other pro‐inflammatory mediators produced by alveolar macrophages play an important role in the pathogenesis of acute lung injury (Kobayashi et al., 1998). iNOS expression in the TNF‐α/iNOS pathway was also prominent in malaria infection‐induced ARDS (Galvao‐Filho et al., 2019). iNOS plays a key role in septic shock as mice lacking iNOS have been reported to be resistant to endotoxin‐induced mortality (Vincent et al., 2000). Similarly, iNOS inhibitors have protective effects in various forms of septic shock and inflammation (Southan et al., 1995). The effect of selective iNOS inhibition has been tested in clinical trials for sepsis/endotoxaemia. In humans, proximal tubular injury during systemic inflammation is correlated with up‐regulation of renal iNOS. Hence, the selective inhibition of renal iNOS may resolve sepsis‐induced acute kidney injury (Heemskerk et al., 2009).
Acute lung inflammation and sepsis also lead to cardiovascular complications such as atherosclerotic plaque destabilisation characterised by intraplaque haemorrhage and thrombus (Jaw et al., 2016), which was also the case in COVID‐19 patients (Guzik et al., 2020). iNOS inhibition prevented atherosclerotic plaque destabilisation in lipopolysaccharide (LPS)‐induced acute lung injury (Moritani et al., 2017). Furthermore, inhibition of iNOS attenuates immune complex‐induced vascular injury in rat lungs and skin (Tripathi et al., 2007). iNOS inhibitors also repair myocardial contractility in hearts exposed to pro‐inflammatory cytokines (Xue et al., 2018).
The above suggest that iNOS inhibition could offer therapeutic benefit in COVID‐19‐related acute lung injury, cardiovascular complications such as thrombosis and atherosclerotic plaque destabilisation and sepsis‐induced organ injury such as acute kidney injury.
8. STATUS OF CB1 RECEPTOR AND INOS EXPRESSION IN LUNGS OF COVID‐19 PATIENTS
A recent study reported gene expression profiles in the lungs of patients who had died of COVID‐19 and in five COVID‐19 negative controls, using RNAseq (Desai et al., 2020). Analysis of the data retrieved from the Omnibus (GEO) database, accession number GSE150316, indicated significant increases in CNR1 and NOS2, encoding CB1 receptor and iNOS, respectively, in COVID‐19 lungs with low viral load compared to non‐COVID‐19 controls (see Figure 1). Additionally, iNOS protein expression was also significantly increased in lungs of COVID‐19 patients relative to controls (Chen et al., 2021). The current evidence warrants further investigations of the potential pathologic role of CB1 receptor sand iNOS in COVID‐19 induced lung disease and the therapeutic potential of inhibitors of CB1 receptors and iNOS.
9. DUAL INHIBITION OF PERIPHERAL CB1 RECEPTOR AND INOS FOR ACUTE AND LONG‐TERM HEALTH COMPLICATIONS OF COVID‐19
In view of the findings described above, overactivation of iNOS and increased CB1 receptor signalling may be involved in the acute manifestations of COVID‐19. Although peripheral CB1 receptor antagonism or iNOS inhibition alone may have promise as therapeutic modalities for multiple pathologies associated with COVID‐19, a combined approach may be superior. Combined inhibition of iNOS and CB1 receptors may tame the potentially lethal cytokine storm and severe lung inflammation in COVID‐19 patients. Simultaneous abrogation of CB1 receptor and iNOS pathways may also attenuate clot formation and thrombosis, increasingly seen in severe COVID‐19 infection, septic shock, sepsis‐induced multi‐organ dysfunction such as cardiomyopathy and acute kidney injury, as discussed above.
Beside acute complications, there is a major concern regarding the development of pulmonary fibrosis in some COVID‐19 survivors (Spagnolo et al., 2020), although the true prevalence of pulmonary fibrosis following COVID‐19 will not be established for months or years. Both CB1 receptor and iNOS overactivation promote alveolar inflammation, pulmonary fibrosis initiation and progression (Cinar et al., 2017; Romanska et al., 2002). Simultaneous inhibition of CB1 receptors and iNOS by a hybrid inhibitor resulted in greater anti‐fibrotic effect than did inhibition of either target alone in experimental studies using hybrid CB1 receptor/iNOS inhibitor, MRI‐1867 (Cinar et al., 2017). Whereas dual CB1 receptor/iNOS inhibition may attenuate acute respiratory and metabolic complications of COVID‐19, it remains to be seen whether such treatment also mitigates pulmonary fibrosis development among COVID‐19 survivors? In summary, the combined pharmacological inhibition of CB1 receptors and iNOS has promise for limiting severe organ damage and improving patient outcomes in COVID‐19.
10. CONCLUSION
COVID‐19 leads to serious complications that require early recognition and comprehensive management. Based on the documented involvement of increased CB1 receptor sand iNOS activity in multiple complications of severe COVID‐19 infection, their simultaneous inhibition by a single chemical entity may have considerable therapeutic potential, which needs to be validated in the clinic once a candidate drug becomes available for human use. The strategy outlined can serve as a viable complement to anti‐viral drugs against COVID‐19.
AUTHOR CONTRIBUTIONS
R.C., M.R.I. and G.K. wrote the manuscript.
CONFLICT OF INTEREST
R.C., M.R.I. and G.K. are listed as co‐inventors on US patents covering hybrid CB1 receptor/iNOS antagonists.
ACKNOWLEDGEMENTS
This work was supported by intramural funds from the US National Institute on Alcohol Abuse and Alcoholism.
Cinar, R. , Iyer, M. R. , & Kunos, G. (2022). Dual inhibition of CB1 receptors and iNOS, as a potential novel approach to the pharmacological management of acute and long COVID‐19. British Journal of Pharmacology, 179(10), 2121–2127. 10.1111/bph.15461
Funding information National Institute on Alcohol Abuse and Alcoholism
Contributor Information
Resat Cinar, Email: resat.cinar@nih.gov.
Malliga R. Iyer, Email: malliga.iyer@nih.gov.
DATA AVAILABILITY STATEMENT
No data have been shared.
REFERENCES
- Ackermann, M. , Verleden, S. E. , Kuehnel, M. , Haverich, A. , Welte, T. , Laenger, F. , Vanstapel, A. , Werlein, C. , Stark, H. , Tzankov, A. , Li, W. W. , Li, V. W. , Mentzer, S. J. , & Jonigk, D. (2020). Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID‐19. The New England Journal of Medicine, 383, 120–128. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon, M. H. , & Saint‐Fleur, M. O. (2017). Synthetic cannabinoid induced acute respiratory depression: Case series and literature review. Respiratory Medicine Case Reports, 22, 137–141. 10.1016/j.rmcr.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batkai, S. , Jarai, Z. , Wagner, J. A. , Goparaju, S. K. , Varga, K. , Liu, J. , Wang, L. , Mirshahi, F. , Khanolkar, A. D. , Makriyannis, A. , Urbaschek, R. , Garcia, N. , Sanyal, A. J. , & Kunos, G. (2001). Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nature Medicine, 7, 827–832. 10.1038/89953 [DOI] [PubMed] [Google Scholar]
- Batkai, S. , Mukhopadhyay, P. , Harvey‐White, J. , Kechrid, R. , Pacher, P. , & Kunos, G. (2007). Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. American Journal of Physiology Heart and Circulatory Physiology, 293, H1689–H1695. 10.1152/ajpheart.00538.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfi, A. , Bernabei, R. , Landi, F. , & Gemelli Against C‐P‐ACSG . (2020). Persistent symptoms in patients after acute COVID‐19. JAMA, 324, 603–605. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco‐Sanchez, F. J. , Lopez‐Carmona, M. D. , Martinez‐Marcos, F. J. , Perez‐Belmonte, L. M. , Hidalgo‐Jimenez, A. , Buonaiuto, V. , Fernández, C. S. , Castro, S. J. F. , Luordo, D. , Fontan, P. M. P. , Encinar, J. C. B. , Gamboa, J. O. M. , de la Peña Fernández, A. , Peña, J. D. T. , Solà, J. F. , Lecumberri, J. J. N. , Martínez, F. A. , Espartero, M. E. G. , Ripper, C. J. , … for the SEMI‐COVID‐19 Network . (2021). Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID‐19 regardless of diabetes status: Data from the Spanish SEMI‐COVID‐19 registry. Annals of Medicine, 53, 103–116. 10.1080/07853890.2020.1836566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. J. , Li, K. , Xu, L. , Yu, Y. J. , Wu, B. , He, Y. L. , Zhao, W. E. , Li, D. , Luan, C. X. , Hu, L. , Wang, J. , Ding, J. J. , Yu, Y. F. , Li, J. X. , Tan, Z. M. , Liu, X. F. , Wei, D. , Zhang, Z. H. , Guo, X. J. , … Chen, F. (2021). Novel insight from the first lung transplant of a COVID‐19 patient. European Journal of Clinical Investigation, 51, e13443. 10.1111/eci.13443 [DOI] [PubMed] [Google Scholar]
- Cinar, R. , Gochuico, B. R. , Iyer, M. R. , Jourdan, T. , Yokoyama, T. , Park, J. K. , Coffey, N. J. , Pri‐Chen, H. , Szanda, G. , Liu, Z. , Mackie, K. , Gahl, W. A. , & Kunos, G. (2017). Cannabinoid CB1 receptor overactivity contributes to the pathogenesis of idiopathic pulmonary fibrosis. JCI Insight, 2(8), e92281. 10.1172/jci.insight.92281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinar, R. , Iyer, M. R. , & Kunos, G. (2020). The therapeutic potential of second and third generation CB1R antagonists. Pharmacology & Therapeutics, 208, 107477. 10.1016/j.pharmthera.2020.107477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codo, A. C. , Davanzo, G. G. , Monteiro, L. B. , de Souza, G. F. , Muraro, S. P. , Virgilio‐da‐Silva, J. V. , Prodonoff, J. S. , Carregari, V. C. , de Biagi Junior, C. A. , Crunfli, F. , & Restrepo, J. L. (2020). Elevated glucose levels favor SARS‐CoV‐2 infection and monocyte response through a HIF‐1alpha/glycolysis‐dependent axis. Cell Metabolism, 32, 437–446. 10.1016/j.cmet.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, N. , Neyaz, A. , Szabolcs, A. , Shih, A. R. , Chen, J. H. , Thapar, V. , Nieman, L. T. , Solovyov, A. , Mehta, A. , Lieb, D. J. , Kulkarni, A. S. , Jaicks, C. , Xu, K. H. , Raabe, M. J. , Pinto, C. J. , Juric, D. , Chebib, I. , Colvin, R. B. , Kim, A. Y. , … Deshpande, V. (2020). Temporal and spatial heterogeneity of host response to SARS‐CoV‐2 pulmonary infection. Nature Communications, 11, 6319. 10.1038/s41467-020-20139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres, J. P. , Golay, A. , & Sjostrom, L. (2005). Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. The New England Journal of Medicine, 353, 2121–2134. 10.1056/NEJMoa044537 [DOI] [PubMed] [Google Scholar]
- Galvao‐Filho, B. , de Castro, J. T. , Figueiredo, M. M. , Rosmaninho, C. G. , Antonelli, L. , & Gazzinelli, R. T. (2019). The emergence of pathogenic TNF/iNOS producing dendritic cells (Tip‐DCs) in a malaria model of acute respiratory distress syndrome (ARDS) is dependent on CCR4. Mucosal Immunology, 12, 312–322. 10.1038/s41385-018-0093-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, P. M. , Wells, A. U. , & Jenkins, R. G. (2020). Pulmonary fibrosis and COVID‐19: The potential role for antifibrotic therapy. The Lancet Respiratory Medicine, 8, 807–815. 10.1016/S2213-2600(20)30225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik, T. J. , Mohiddin, S. A. , Dimarco, A. , Patel, V. , Savvatis, K. , Marelli‐Berg, F. M. , Madhur, M. S. , Tomaszewski, M. , Maffia, P. , D'Acquisto, F. , Nicklin, S. A. , Marian, A. J. , Nosalski, R. , Murray, E. C. , Guzik, B. , Berry, C. , Touyz, R. M. , Kreutz, R. , Wang, D. W. , … McInnes, I. B. (2020). COVID‐19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovascular Research, 116, 1666–1687. 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk, S. , Masereeuw, R. , Russel, F. G. , & Pickkers, P. (2009). Selective iNOS inhibition for the treatment of sepsis‐induced acute kidney injury. Nature Reviews. Nephrology, 5, 629–640. 10.1038/nrneph.2009.155 [DOI] [PubMed] [Google Scholar]
- Hui, D. S. , Wong, K. T. , Ko, F. W. , Tam, L. S. , Chan, D. P. , Woo, J. , & Sung, J. J. Y. (2005). The 1‐year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest, 128, 2247–2261. 10.1378/chest.128.4.2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, I. F. , Lung, K. C. , Tso, E. Y. , Liu, R. , Chung, T. W. , Chu, M. Y. , Ng, Y. Y. , Lo, J. , Chan, J. , Tam, A. R. , & Shum, H. P. (2020). Triple combination of interferon beta‐1b, lopinavir‐ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID‐19: An open‐label, randomised, phase 2 trial. Lancet, 395, 1695–1704. 10.1016/S0140-6736(20)31042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaw, J. E. , Tsuruta, M. , Oh, Y. , Schipilow, J. , Hirano, Y. , Ngan, D. A. , Suda, K. , Li, Y. , Oh, J. Y. , Moritani, K. , Tam, S. , Ford, N. , van Eeden, S. , Wright, J. L. , Man, S. F. P. , & Sin, D. D. (2016). Lung exposure to lipopolysaccharide causes atherosclerotic plaque destabilisation. The European Respiratory Journal, 48, 205–215. 10.1183/13993003.00972-2015 [DOI] [PubMed] [Google Scholar]
- Johnson, A. , Neumann, P. H. , Peng, J. , James, J. , Russo, V. , MacDonald, H. , Gertzberg, N. , & Feleder, C. (2015). The intracerebroventricular injection of rimonabant inhibits systemic lipopolysaccharide‐induced lung inflammation. Journal of Neuroimmunology, 286, 16–24. 10.1016/j.jneuroim.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan, T. , Godlewski, G. , Cinar, R. , Bertola, A. , Szanda, G. , Liu, J. , Tam, J. , Han, T. , Mukhopadhyay, B. , Skarulis, M. C. , Ju, C. , Aouadi, M. , Czech, M. P. , & Kunos, G. (2013). Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nature Medicine, 19, 1132–1140. 10.1038/nm.3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan, T. , Park, J. K. , Varga, Z. V. , Paloczi, J. , Coffey, N. J. , Rosenberg, A. Z. , Godlewski, G. , Cinar, R. , Mackie, K. , Pacher, P. , & Kunos, G. (2018). Cannabinoid‐1 receptor deletion in podocytes mitigates both glomerular and tubular dysfunction in a mouse model of diabetic nephropathy. Diabetes, Obesity & Metabolism, 20, 698–708. 10.1111/dom.13150 [DOI] [PubMed] [Google Scholar]
- Jourdan, T. , Szanda, G. , Rosenberg, A. Z. , Tam, J. , Earley, B. J. , Godlewski, G. , Cinar, R. , Liu, Z. , Liu, J. , Ju, C. , Pacher, P. , & Kunos, G. (2014). Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proceedings of the National Academy of Sciences of the United States of America, 111, E5420–E5428. 10.1073/pnas.1419901111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, A. , Hashimoto, S. , Kooguchi, K. , Kitamura, Y. , Onodera, H. , Urata, Y. , & Ashihara, T. (1998). Expression of inducible nitric oxide synthase and inflammatory cytokines in alveolar macrophages of ARDS following sepsis. Chest, 113, 1632–1639. 10.1378/chest.113.6.1632 [DOI] [PubMed] [Google Scholar]
- Konstantinides, S. , Schafer, K. , Koschnick, S. , & Loskutoff, D. J. (2001). Leptin‐dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. The Journal of Clinical Investigation, 108, 1533–1540. 10.1172/JCI13143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone, M. , Bab, I. , Biro, T. , Cabral, G. A. , Dey, S. K. , Di Marzo, V. , Konje, J. C. , Kunos, G. , Mechoulam, R. , Pacher, P. , & Sharkey, K. A. (2015). Endocannabinoid signaling at the periphery: 50 years after THC. Trends in Pharmacological Sciences, 36, 277–296. 10.1016/j.tips.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritani, K. , Jaw, D. , Oh, Y. , Schiplow, J. , Ford, N. , Man, S. P. , & Sin, D. D. (2017). Inhibition of INOS prevents atherosclerotic plaque destabilization related to LPS lung exposure: Results of novel 3D OPT imaging. American Journal of Respiratory and Critical Care Medicine, 195, A5229. [Google Scholar]
- Onaivi, E. S. , & Sharma, V. (2020). Cannabis for COVID‐19: Can cannabinoids quell the cytokine storm? Future Science OA, 6, FSO625. 10.2144/fsoa-2020-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher, P. , Steffens, S. , Hasko, G. , Schindler, T. H. , & Kunos, G. (2018). Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nature Reviews. Cardiology, 15, 151–166. 10.1038/nrcardio.2017.130 [DOI] [PubMed] [Google Scholar]
- Petrilli, C. M. , Jones, S. A. , Yang, J. , Rajagopalan, H. , O'Donnell, L. , Chernyak, Y. , Tobin, K. A. , Cerfolio, R. J. , Francois, F. , & Horwitz, L. I. (2020). Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ, 369, m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchi, G. , Mari, A. , Ricciardi, A. , Carucci, A. C. , Sinatti, G. , Cosimini, B. , di Norcia, M. , Iapadre, N. , Balsano, C. , & Grimaldi, A. (2020). Three cases of COVID‐19 pneumonia in female patients in Italy who had pulmonary fibrosis on follow‐up lung computed tomography imaging. The American Journal of Case Reports, 21, e926921. 10.12659/AJCR.926921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proschak, E. , Stark, H. , & Merk, D. (2019). Polypharmacology by design: A medicinal chemist's perspective on multitargeting compounds. Journal of Medicinal Chemistry, 62, 420–444. 10.1021/acs.jmedchem.8b00760 [DOI] [PubMed] [Google Scholar]
- Rajesh, M. , Batkai, S. , Kechrid, M. , Mukhopadhyay, P. , Lee, W. S. , Horvath, B. , Holovac, E. , Cinar, R. , Liaudet, L. , Mackie, K. , Hasko, G. , & Pacher, P. (2012). Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes, 61, 716–727. 10.2337/db11-0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss, C. S. (2010). Cannabinoids and viral infections. Pharmaceuticals (Basel), 3, 1873–1886. 10.3390/ph3061873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanska, H. M. , Polak, J. M. , Coleman, R. A. , James, R. S. , Harmer, D. W. , Allen, J. C. , & Bishop, A. E. (2002). iNOS gene upregulation is associated with the early proliferative response of human lung fibroblasts to cytokine stimulation. The Journal of Pathology, 197, 372–379. 10.1002/path.1116 [DOI] [PubMed] [Google Scholar]
- Rossi, F. , Tortora, C. , Argenziano, M. , Di Paola, A. , & Punzo, F. (2020). Cannabinoid receptor type 2: A possible target in SARS‐CoV‐2 (CoV‐19) infection? International Journal of Molecular Sciences, 21(11), 3809. 10.3390/ijms21113809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz de Azua, I. , Mancini, G. , Srivastava, R. K. , Rey, A. A. , Cardinal, P. , Tedesco, L. , Zingaretti, C. M. , Sassmann, A. , Quarta, C. , Schwitter, C. , Conrad, A. , Wettschureck, N. , Vemuri, V. K. , Makriyannis, A. , Hartwig, J. , Mendez‐Lago, M. , Bindila, L. , Monory, K. , Giordano, A. , … Lutz, B. (2017). Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. The Journal of Clinical Investigation, 127, 4148–4162. 10.1172/JCI83626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi, M. , Tay, E. S. , Read, S. A. , Ramezani‐Moghadam, M. , Chayama, K. , George, J. , & Douglas, M. W. (2014). Endocannabinoid CB1 antagonists inhibit hepatitis C virus production, providing a novel class of antiviral host‐targeting agents. The Journal of General Virology, 95, 2468–2479. 10.1099/vir.0.067231-0 [DOI] [PubMed] [Google Scholar]
- Singh, P. , Sharma, P. , Nakade, U. P. , Sharma, A. , Gari, M. , Choudhury, S. , Shukla, A. , & Garg, S. K. (2018). Endocannabinoid‐mediated modulation of Gq protein‐coupled receptor mediates vascular hyporeactivity to nor‐adrenaline during polymicrobial sepsis. Pharmacological Reports, 70, 1150–1157. 10.1016/j.pharep.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Southan, G. J. , Szabo, C. , & Thiemermann, C. (1995). Isothioureas: Potent inhibitors of nitric oxide synthases with variable isoform selectivity. British Journal of Pharmacology, 114, 510–516. 10.1111/j.1476-5381.1995.tb13256.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo, P. , Balestro, E. , Aliberti, S. , Cocconcelli, E. , Biondini, D. , Casa, G. D. , Sverzellati, N. , & Maher, T. M. (2020). Pulmonary fibrosis secondary to COVID‐19: A call to arms? The Lancet Respiratory Medicine, 8, 750–752. 10.1016/S2213-2600(20)30222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tale, S. , Ghosh, S. , Meitei, S. P. , Kolli, M. , Garbhapu, A. K. , & Pudi, S. (2020). Post‐COVID‐19 pneumonia pulmonary fibrosis. QJM, 113, 837–838. 10.1093/qjmed/hcaa255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, J. (2016). The emerging role of the endocannabinoid system in the pathogenesis and treatment of kidney diseases. Journal of Basic and Clinical Physiology and Pharmacology, 27, 267–276. 10.1515/jbcpp-2015-0055 [DOI] [PubMed] [Google Scholar]
- Tam, J. , Cinar, R. , Liu, J. , Godlewski, G. , Wesley, D. , Jourdan, T. , Szanda, G. , Mukhopadhyay, B. , Chedester, L. , Liow, J. S. , Innis, R. B. , Cheng, K. , Rice, K. C. , Deschamps, J. R. , Chorvat, R. J. , McElroy, J. F. , & Kunos, G. (2012). Peripheral cannabinoid‐1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metabolism, 16, 167–179. 10.1016/j.cmet.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, J. , Vemuri, V. K. , Liu, J. , Batkai, S. , Mukhopadhyay, B. , Godlewski, G. , Osei‐Hyiaman, D. , Ohnuma, S. , Ambudkar, S. V. , Pickel, J. , & Makriyannis, A. (2010). Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. The Journal of Clinical Investigation, 120, 2953–2966. 10.1172/JCI42551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi, P. , Tripathi, P. , Kashyap, L. , & Singh, V. (2007). The role of nitric oxide in inflammatory reactions. FEMS Immunology and Medical Microbiology, 51, 443–452. 10.1007/s10787-007-0013-x [DOI] [PubMed] [Google Scholar]
- Udi, S. , Hinden, L. , Earley, B. , Drori, A. , Reuveni, N. , Hadar, R. , Cinar, R. , Nemirovski, A. , & Tam, J. (2017). Proximal tubular cannabinoid‐1 receptor regulates obesity‐induced CKD. Journal of the American Society of Nephrology, 28, 3518–3532. 10.1681/ASN.2016101085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poorten, D. , Shahidi, M. , Tay, E. , Sesha, J. , Tran, K. , McLeod, D. , Milliken, J. S. , Ho, V. , Hebbard, L. W. , Douglas, M. W. , & George, J. (2010). Hepatitis C virus induces the cannabinoid receptor 1. PLoS ONE, 5(9), e12841. 10.1371/journal.pone.0012841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga, K. , Wagner, J. A. , Bridgen, D. T. , & Kunos, G. (1998). Platelet‐ and macrophage‐derived endogenous cannabinoids are involved in endotoxin‐induced hypotension. The FASEB Journal, 12, 1035–1044. 10.1096/fasebj.12.11.1035 [DOI] [PubMed] [Google Scholar]
- Vasarmidi, E. , Tsitoura, E. , Spandidos, D. A. , Tzanakis, N. , & Antoniou, K. M. (2020). Pulmonary fibrosis in the aftermath of the COVID‐19 era (review). Experimental and Therapeutic Medicine, 20, 2557–2560. 10.3892/etm.2020.8980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent, J. L. , Zhang, H. , Szabo, C. , & Preiser, J. C. (2000). Effects of nitric oxide in septic shock. American Journal of Respiratory and Critical Care Medicine, 161, 1781–1785. 10.1164/ajrccm.161.6.9812004 [DOI] [PubMed] [Google Scholar]
- Xue, Q. , Yan, Y. , Zhang, R. , & Xiong, H. (2018). Regulation of iNOS on immune cells and its role in diseases. International Journal of Molecular Sciences, 19(12), 3805. 10.3390/ijms19123805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Z. , Zhang, Y. , Wang, Y. , Huang, Z. , & Song, B. (2020). Chest CT manifestations of new coronavirus disease 2019 (COVID‐19): A pictorial review. European Radiology, 30, 4381–4389. 10.1007/s00330-020-06801-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawatsky, C. N. , Abdalla, J. , & Cinar, R. (2020). Synthetic cannabinoids induce acute lung inflammation via cannabinoid receptor 1 activation. ERJ Open Research, 6, 121–2020. 10.1183/23120541.00121-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data have been shared.


