Abstract
Objective
COVID‐19 is a novel infectious disease with a broad spectrum of clinical severity. Patients with systemic vasculitis have an increased risk of serious infections and may be at risk of severe outcomes following COVID‐19. We undertook this study to establish the risk factors for severe COVID‐19 outcomes in these patients, including the impact of immunosuppressive therapies.
Methods
A multicenter cohort was developed through the participation of centers affiliated with national UK and Ireland vasculitis registries. Clinical characteristics and outcomes are described. Logistic regression was used to evaluate associations between potential risk factors and a severe COVID‐19 outcome, defined as a requirement for advanced oxygen therapy, a requirement for invasive ventilation, or death.
Results
The cohort included 65 patients with systemic vasculitis who developed COVID‐19 (median age 70 years, 49% women), of whom 25 patients (38%) experienced a severe outcome. Most patients (55 of 65 [85%]) had antineutrophil cytoplasmic antibody–associated vasculitis (AAV). Almost all patients required hospitalization (59 of 65 [91%]), 7 patients (11%) were admitted to intensive care, and 18 patients (28%) died. Background glucocorticoid therapy was associated with severe outcomes (adjusted odds ratio [OR] 3.7 [95% confidence interval 1.1–14.9]; P = 0.047), as was comorbid respiratory disease (adjusted OR 7.5 [95% confidence interval 1.9–38.2]; P = 0.006). Vasculitis disease activity and nonglucocorticoid immunosuppressive therapy were not associated with severe outcomes.
Conclusion
In patients with systemic vasculitis, glucocorticoid use at presentation and comorbid respiratory disease were associated with severe outcomes in COVID‐19. These data can inform clinical decision‐making relating to the risk of severe COVID‐19 in this vulnerable patient group.
INTRODUCTION
COVID‐19 is a novel, multisystem infectious disease caused by SARS–CoV‐2. COVID‐19 is associated with a broad spectrum of clinical severity (1), ranging from asymptomatic disease to severe respiratory failure and death. In March 2020, the World Health Organization (WHO) characterized COVID‐19 as a global pandemic (2), prompting considerable concerns from health care systems worldwide about their resilience to manage this threat. Critical care service capacity was, and remains, a global priority.
Systemic vasculitis is a rare, multisystem autoimmune disorder. Compared to other rheumatic musculoskeletal diseases, it may result in major organ dysfunction and is therefore typically managed with more potent immunosuppressive therapy and higher doses of glucocorticoids in order to induce and maintain disease remission. While this therapeutic approach successfully manages vasculitis activity, glucocorticoid exposure contributes, in part, to the increased risk of infection in these patients (3, 4). Thus, although risk factors associated with poor outcomes from COVID‐19 in this population are unknown, there is an assumption that these patients are at a high risk.
The RECOVERY trial has demonstrated a benefit of moderate‐dose glucocorticoids in hospitalized general population patients with COVID‐19 who require supplemental oxygen or mechanical ventilation, but showed potential harm when used in milder disease (5). Given these paradoxical effects, it is unknown whether chronic background glucocorticoid exposure makes patients more susceptible to severe COVID‐19 infection or whether it might be protective.
We report the results of a coordinated binational effort to identify the predictors of a severe outcome in the largest reported cohort of systemic vasculitis patients infected with COVID‐19.
PATIENTS AND METHODS
Study design
A registry‐based multicenter cohort was designed to facilitate rapid real‐world data capture. Centers affiliated with the UK and Ireland Vasculitis Registry (UKIVAS; www.ukivas.org/) and the Irish Rare Kidney Disease Registry (RKD; www.tcd.ie/medicine/thkc/research/rare.php) were invited to contribute. UKIVAS covers 89 sites; RKD covers 8 sites across Ireland. Participating centers represent both secondary and tertiary centers across the UK and Ireland, resulting in a broad population sampling frame. A vasculitis‐focused COVID‐19 case report form was developed, underpinned by standardized BioPortal ontologies (e.g., SNOMED CT) (6) and interoperable with other emerging data sets, such as the COVID‐19 Global Rheumatology Alliance (GRA) (7), thereby facilitating future international data linkage as the COVID‐19 pandemic progresses. This enables compatibility of these data with the principles of the global GO‐FAIR initiative (8). Additional modules of the UKIVAS and RKD web apps were developed to support data capture.
Study population
Patients were eligible for case submission if they had a diagnosis of systemic vasculitis and COVID‐19 (laboratory, radiologic, or clinical). The diagnosis of vasculitis was determined by the local specialist clinician, according to the International Chapel Hill Consensus Conference nomenclature of vasculitides (9). Recruitment commenced on March 28, 2020 and is ongoing. For this analysis, the final case was submitted on July 31, 2020. The population sampling frame consisted of individuals under the clinical care of sites associated with the UKIVAS and RKD registries. At the end of July 2020, there were 795 patients in the RKD registry and ~7,400 patients in the UKIVAS registry, with 4 patients enrolled in both. In the UK, the Health Research Authority decision tool determined that ethics approval was not required, and the local sponsor confirmed the project as a service evaluation (R&D reference no. GN20RH165). RKD registry ethics approval was originally granted by the Tallaght University Hospital/St. James's Hospital Joint Research Ethics Committee (reference no. 2019‐08 List 29 [07]). All RKD participants provided informed consent. Additional approvals were not required.
Study variables
Variables included potential predictors of severe outcomes. The selection of predictive variables was informed by emerging risk factors for COVID‐19 disease severity in other populations (10, 11). These included age, sex, ethnicity, smoking status, comorbid conditions, immunosuppressive treatment for vasculitis, and vasculitis disease activity. Among the comorbid conditions, respiratory disease refers to non–vasculitis‐related lower respiratory tract disease, though it is possible that some patients had coexistent vasculitis‐related respiratory disease. Intravenous immunosuppressive therapy was considered to be “current” if the assessing clinician determined that the therapy was likely to exert a biologic effect at the time of COVID‐19 diagnosis; specific definitions were not provided.
Vasculitis disease activity was determined according to global clinician assessment. Outcome data included complications, such as acute kidney injury (AKI), respiratory failure and vasopressor requirement, and death. To enable interoperability, the standardized outcomes (grade range 1–8) from the COVID‐19 GRA case reporting form were used (see Supplementary Table 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41728/abstract) (10). A severe outcome was defined as a composite of requirement for advanced oxygen therapy (such as noninvasive ventilation or high‐flow oxygen device), requirement for invasive ventilation, or death. Dates of hospital and intensive care unit (ICU) admission and discharge were collected in order to derive length of stay. Other variables were collected to characterize the clinical features of COVID‐19 in patients with vasculitis. These included symptoms, laboratory tests, and radiology results. Reporting clinicians were asked to indicate which of these variables contributed to diagnosis.
Statistical analysis
Continuous variables are described as the median and interquartile range (IQR). Categorical variables are described as the number and percentage of patients. Associations between various explanatory variables and the odds of severe outcomes were determined. Unadjusted and age/sex‐adjusted logistic regression models were individually calculated for each explanatory variable and reported as odds ratios (ORs), P values, and 95% confidence intervals (95% CIs). The adjusted ORs for age and sex were derived from a single logistic regression model which included age and sex only. When a potential interaction could account for a positive finding, logistic regression modeling incorporating the explanatory and interacting variables was used. Sensitivity analyses were performed in the event that any effects may have been different in an important subgroup. Missing data were acknowledged in the relevant tables. P values less than 0.05 were considered significant. R (version 4.0.2) was used for data analysis with packages including tidyverse and finalfit.
RESULTS
In total, 65 patients with an established diagnosis of systemic vasculitis who developed COVID‐19 were registered. Fifty‐eight patients were submitted to the UKIVAS registry, and 7 were submitted to the RKD registry, with no duplicate submissions.
Baseline characteristics
The median age was 70 years (IQR 55–76 years) and 49% of the patients were female (Table 1). The majority of patients (55 of 65 [85%]) had antineutrophil cytoplasm antibody–associated vasculitis (AAV): of these, 24 patients (44%) had granulomatosis with polyangiitis, 25 patients (45%) had microscopic polyangiitis, and 6 patients (11%) had eosinophilic granulomatosis with polyangiitis. The characteristics of AAV patients were broadly similar to those of the full cohort (data not shown). Thirty‐two patients (49%) were assessed as having concurrent active disease. Fifteen patients (23%) were suspected to have contracted COVID‐19 through a health care setting; of these, 9 patients (60%) had some degree of active vasculitis. In one case, COVID‐19 was considered to have increased disease activity. For the remaining patients, COVID‐19 was not perceived to have altered or induced disease activity.
Table 1.
Baseline characteristics of the study patients (n = 65)*
| Characteristic | Value | Characteristic | Value |
|---|---|---|---|
| Age, median (IQR) years | 70 (55–76) | Vasculitis, active disease | 32 (49.2) |
| Female sex | 32 (49.2) | Vasculitis disease duration, median (IQR) years | 2.2 (0.76–6.8) |
| Ethnicity | Current immunosuppressive therapy | ||
| Asian | 7 (10.8) | Any immunosuppressive therapy | 56 (86.2) |
| Black | 1 (1.5) | Any immunosuppressive therapy and GCs | 43 (66.2) |
| White | 46 (70.8) | Azathioprine | 12 (18.5) |
| Not stated | 6 (9.2) | GCs (any dose) | 45 (69.2) |
| Missing data | 5 (7.7) | Prednisone 1.0–5.0 mg/day | 19 (29.2) |
| Smoking status | Prednisone ≥5.1 mg/day | 26 (40.0) | |
| Current | 3 (4.6) | Unknown/missing data | 2 (3.1) |
| Former | 15 (23.1) | CYC | 10 (15.4) |
| Never | 26 (40.0) | HCQ | 4 (6.2) |
| Unknown/missing data | 21 (32.3) | IVIG | 1 (1.5) |
| Comorbidities | MMF | 11 (16.9) | |
| Vasculitis† | Rituximab | 22 (33.8) | |
| GPA (or PR3 AAV) | 24 (36.9) | Tacrolimus | 4 (6.2) |
| MPA (or MPO AAV) | 25 (38.5) | Other medications | |
| EGPA | 6 (9.2) | ACE inhibitors | 9 (13.8) |
| LVV | 2 (3.1) | ARB | 8 (12.3) |
| Behçet’s disease | 1 (1.5) | NSAIDs | 2 (3.1) |
| PAN | 1 (1.5) | Unknown/missing data | 5 (7.7) |
| Other | 5 (7.7) | Laboratory tests, median (IQR) | |
| Unknown/missing data | 1 (1.5) | Creatinine, μmoles/liter¶ | 127 (69–204) |
| Diabetes | 13 (20.0) | CRP, mg/liter | 99 (44–149) |
| Hypertension | 25 (38.5) | Lymphocytes, ×109/liter | 0.7 (0.4–0.9) |
| CVD | 17 (26.2) | Method used for COVID‐19 diagnosis | |
| Respiratory disease‡ | 13 (20.0) | PCR | 47 (72.3) |
| Renal disease | 30 (46.2) | Radiologic | 3 (4.6) |
| End‐stage kidney disease§ | Symptoms only | 3 (4.6) | |
| Yes | 17 (26.2) | Unknown/missing data | 12 (18.5) |
| No | 46 (70.8) | ||
| Unknown/missing data | 2 (3.1) | ||
| Organ transplant | 3 (4.6) |
Except where indicated otherwise, values are the number (%) of patients. IQR = interquartile range; GPA = granulomatosis with polyangiitis; PR3 = proteinase 3; AAV = antineutrophil cytoplasmic antibody–associated vasculitis; MPA = microscopic polyangiitis; MPO = myeloperoxidase; EGPA = eosinophilic granulomatosis with polyangiitis; LVV = large vessel vasculitis; PAN = polyarteritis nodosa; CVD = cardiovascular disease; GCs = glucocorticoids; CYC = cyclophosphamide; HCQ = hydroxychloroquine; IVIG = intravenous immunoglobulin; MMF = mycophenolate mofetil; ACE = angiotensin‐converting enzyme; ARB = angiotensin II receptor blocker; NSAIDs = nonsteroidal antiinflammatory drugs; CRP = C‐reactive protein; PCR = polymerase chain reaction.
Other vasculitis diagnoses include IgA vasculitis, leukocytoclastic vasculitis, and unspecified vasculitis.
Refers to non–vasculitis‐related lower respiratory tract disease, though it is possible that some patients had coexistent vasculitis‐related respiratory disease.
Includes 13 patients receiving hemodialysis, 3 kidney transplant recipients, and 1 patient with sustained stage 5 chronic kidney disease.
Excludes patients receiving hemodialysis.
Forty‐seven patients (72%) were diagnosed by polymerase chain reaction (PCR) testing, 3 patients (5%) did not have PCR‐confirmed disease but had radiologic evidence of disease, and 3 patients (5%) were diagnosed by clinical presentation only (Table 1). Data on diagnostic testing were unknown/missing for 12 patients (19%). The characteristics of those diagnosed by PCR testing were broadly similar to the full cohort (data not shown).
Most patients were receiving background glucocorticoids (45 of 65 [69%]) at the time of COVID‐19 diagnosis. Nineteen patients (29%) were receiving the equivalent of ≤5 mg prednisone, and 26 patients (40%) were receiving >5 mg. For those receiving background glucocorticoids, the median dose was the equivalent of 7.5 mg prednisone daily (IQR 5–25 mg). Twenty‐two patients (34%) and 10 patients (15%) had recently been treated with rituximab and cyclophosphamide, respectively (Table 1).
Symptom frequency
The most common symptoms among the included patients were dyspnea (41 of 65 [63%]), fever (38 of 65 [58%]), and cough (37 of 65 [57%]) (Figure 1). Full data on symptoms were missing for 2 patients. Hemoptysis occurred in 3 patients (5%), and epistaxis occurred in 3 individuals (5%); of these, 1 patient had both epistaxis and hemoptysis. Of note, some of these patients had ongoing disease activity prior to COVID‐19 diagnosis.
Figure 1.
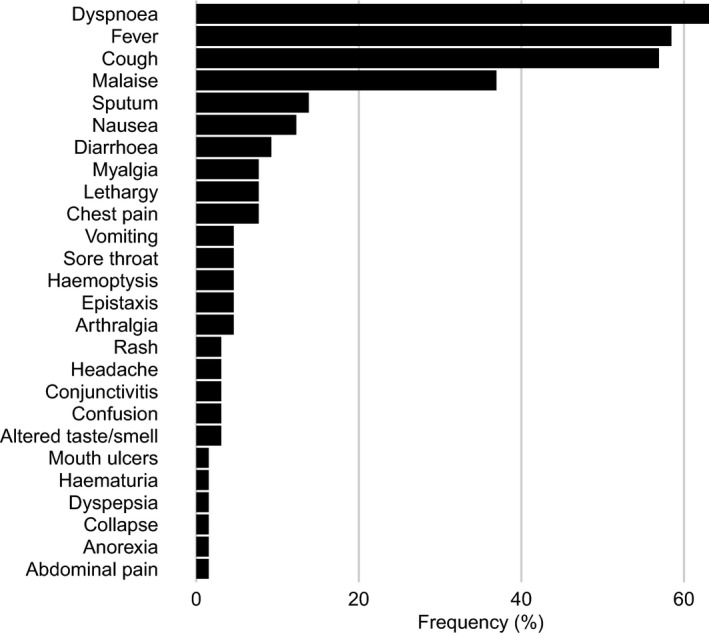
Frequency of patient symptoms at initial presentation.
Complication frequency
Respiratory failure was the most commonly reported complication among patients (35 of 65 [54%]), followed by AKI (12 of 65 [18%]) and secondary infection (10 of 65 [15%]) (Figure 2). Full data on complications were missing for 10 patients.
Figure 2.
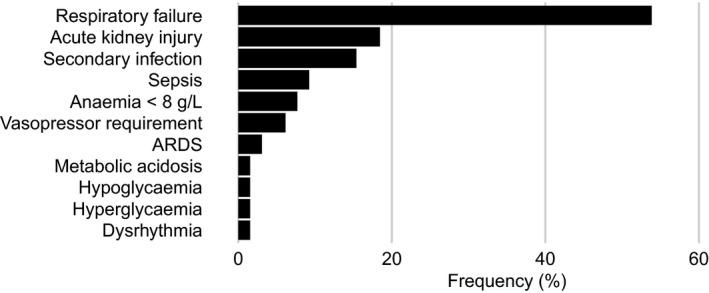
Frequency of patient complications. ARDS = acute respiratory distress syndrome.
Clinical outcomes
Almost all patients required hospitalization (59 of 65 [91%]); 7 patients (11%) were admitted to an ICU, and 18 patients (28%) died (Table 2). The median length of hospital stay for discharged patients was 11 days (IQR 5–27 days). A severe outcome was experienced by 25 of 65 patients (38%).
Table 2.
COVID‐19 disease outcomes in the study patients (n = 65)*
| Hospitalization | 59 (90.8) |
| ICU admission | |
| Yes | 7 (10.8) |
| No | 49 (75.4) |
| Unknown/missing data | 9 (13.8) |
| Graded outcome (grade no.) | |
| Not hospitalized, no limitations on activities (1) | 2 (3.1) |
| Not hospitalized, limitation on activities (2) | 3 (4.6) |
| Hospitalized, not requiring supplemental oxygen (3) | 9 (13.8) |
| Hospitalized, requiring supplemental oxygen (4) | 25 (38.5) |
| Hospitalized, on noninvasive ventilation or high‐flow oxygen devices (5) | 4 (6.2) |
| Hospitalized, on invasive mechanical ventilation or ECMO (6) | 3 (4.6) |
| Death (7) | 18 (27.7) |
| Unknown/missing data (8) | 1 (1.5) |
| Length of hospital stay, median (IQR) days | 11 (5–27) |
| Length of hospital stay, unknown/missing data | 40 (61.5) |
Except where indicated otherwise, values are the number (%) of patients. ICU = intensive care unit; ECMO = extracorporeal membrane oxygenation.
Predictors of severe outcomes
Patients with comorbid respiratory disease were more likely to experience a severe outcome than those without (adjusted OR 7.5 [95% CI 1.9–38.2]; P = 0.006), as were those who had been receiving glucocorticoids (adjusted OR 3.7 [95% CI 1.1–14.9]; P = 0.047) (Table 3). Glucocorticoid exposure remained a poor prognostic indicator even after adjusting for vasculitis disease activity (data not shown). A sensitivity analysis including only patients with a confirmed PCR diagnosis (n = 47) was performed, which demonstrated effect sizes consistent with these findings; this was statistically significant for comorbid respiratory disease but not for glucocorticoid exposure (data not shown). A sensitivity analysis was also performed for individuals with AAV (n = 55) with a similar result. There was insufficient power to assess the association between glucocorticoid dose and poor outcome. Similarly, there was insufficient power to assess for differences between common nonglucocorticoid immunosuppressive agents. Associations were not demonstrated for any demographics, other comorbid conditions, vasculitis diagnosis, vasculitis disease activity, or nonglucocorticoid immunosuppressant medication.
Table 3.
Unadjusted and adjusted ORs for potential risk factors and association with severe outcomes*
|
No. of severe outcomes/ no. of cases (%) |
Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|
| Female | 13/26 (50) | 1.04 (0.51–2.10) | 1.05 (0.52–2.13) |
| Age | – | 1.01 (0.98–1.05) | 1.01 (0.98–1.05) |
| Vasculitis diagnosis | |||
| GPA (referent: not GPA) | 12/24 (50) | 1.71 (0.62–4.81) | 2.19 (0.68–7.63) |
| MPA (referent: not MPA) | 7/25 (28) | 0.53 (0.17–1.52) | 0.43 (0.13–1.36) |
| Comorbidities (referent: individual comorbidity not present) | |||
| Hypertension | 12/25 (48) | 1.46 (0.71–3.04) | 1.39 (0.64–3.04) |
| CVD | 8/17 (47) | 1.32 (0.59–2.93) | 1.08 (0.52–2.23) |
| Respiratory disease | 10/13 (77) | 7.50 (1.99–36.94) | 7.53 (1.93–38.22)† |
| Diabetes | 6/13 (46) | 1.25 (0.51–2.99) | 1.20 (0.48–2.92) |
| Renal disease | 12/30 (40) | 1.00 (0.49–2.03) | 1.05 (0.52–2.14) |
| End‐stage kidney disease | 6/17 (35) | 0.85 (0.25–2.65) | 0.77 (0.22–2.48) |
| Smoking status | |||
| Ever smoker (referent: never) | 9/18 (50) | 2.25 (0.65–8.05) | 2.33 (0.62–9.28) |
| Immunosuppressive therapy | |||
| Any immunosuppressive therapy (referent: not receiving immunosuppressive therapy) | 24/55 (44) | 3.10 (0.70–21.79) | 3.66 (0.77–27.29) |
| GCs (referent: no prednisone) | |||
| Prednisone (any dose) | 22/45 (49) | 3.35 (1.02–13.2) | 3.66 (1.09–14.9)‡ |
| Prednisone 1.0–5.0 mg/day | 10/19 (53) | 3.89 (0.98–17.93) | 3.76 (0.91–18.02) |
| Prednisone ≥5.1 mg/day | 12/26 (46) | 3.00 (0.82–12.86) | 3.32 (0.86–15.35) |
| Other immunosuppressive therapy | |||
| Azathioprine (referent: not receiving azathioprine) | 6/12 (50) | 1.65 (0.46–5.97) | 1.57 (0.42–5.85) |
| CYC (referent: not receiving CYC) | 5/10 (50) | 1.62 (0.41–6.48) | 1.83 (0.44–7.76) |
| Rituximab (referent: not receiving rituximab) | 9/22 (41) | 1.06 (0.36–3.01) | 1.25 (0.40–3.90) |
A severe outcome was defined as a composite of requirement for advanced oxygen therapy (such as noninvasive ventilation or high‐flow oxygen device), requirement for invasive ventilation, or death. A separate logistic regression model including sex and age as a continuous variable was calculated for each explanatory variable. The adjusted models for age and sex were derived from a single logistic regression model which included sex and age as a continuous variable. OR = odds ratio; 95% CI = 95% confidence interval (see Table 1 for other definitions).
P < 0.00645. Respiratory disease refers to non–vasculitis‐related lower respiratory tract disease, though it is possible that some patients had coexistent vasculitis‐related respiratory disease.
P < 0.047.
DISCUSSION
This multicenter study includes the largest cohort of patients with systemic vasculitis who have developed COVID‐19 to date. It identifies comorbid respiratory disease and background glucocorticoid therapy as significant predictors of a severe outcome, defined as a need for advanced oxygen therapy or invasive ventilation, or death. Routinely used nonglucocorticoid immunosuppressants, such as rituximab and cyclophosphamide, were not associated with a severe outcome, nor was vasculitis disease activity.
Glucocorticoids have pleotropic immunologic effects and are generally considered risk factors for infections (12). Glucocorticoids at high doses have been associated with prolonged viral shedding, with a similar effect being observed in other coronaviruses (13, 14). That glucocorticoids are associated with worse COVID‐19 disease outcomes is consistent with findings from across the rheumatic autoimmune spectrum, as demonstrated by the COVID‐19 GRA study (10). Our point estimate for the association of any glucocorticoid dose with severe outcomes (OR 3.7 [95% CI 1.09–14.9]) was comparable to that of the COVID‐19 GRA evaluation of steroids equivalent to ≥10 mg prednisone per day (OR 2.05 [95% CI 1.06–3.96). The confidence interval for this finding is relatively wide, and thus, it remains to be determined if there is a dose threshold at which risk commences.
The association between glucocorticoids and severe outcomes may appear to conflict with findings from the RECOVERY trial (5). This trial demonstrated that low‐dose dexamethasone had a substantial survival benefit in patients hospitalized with COVID‐19. However, the groups that benefited in RECOVERY were those requiring supplemental oxygen, with the greatest benefit derived in those requiring mechanical ventilation. In fact, the point estimate for patients not requiring oxygen suggested that glucocorticoids could be associated with increased mortality, though this was not a statistically significant finding (5). Therefore, prior to requiring oxygen, it may be that glucocorticoids are deleterious, as observed in this and other studies of autoimmune disease (15). Our finding that comorbid respiratory disease (most commonly chronic obstructive pulmonary disease, asthma, and interstitial lung disease) was associated with severe disease outcomes was consistent with a recent general population meta‐analysis (16). Among some patients with chronic lung disease, enhanced respiratory tract angiotensin‐converting enzyme 2 expression, the principal binding site for COVID‐19 cell entry, is a possible explanation for this association (10). Consistent with findings from the COVID‐19 GRA study, we did not find an association of adverse disease outcomes with other immunosuppressive agents (17). This is reassuring, as current guidance emphasizes the importance of maintaining immunosuppressive therapy among uninfected patients due to concerns of destabilizing disease control (18).
This cohort represents a severely affected group, as reflected by the very high proportion of hospitalization (91%). The mortality rate of 28% is similar to that reported in the largest UK study of hospitalized patients carried out by the International Severe Acute Respiratory and emerging Infections Consortium (ISARIC) WHO Clinical Characterisation Protocol UK (CCP‐UK) group, in which 26% of patients died (18). Age, sex, and symptom distribution were also broadly similar to the ISARIC WHO CCP‐UK study. The most common symptoms, in that cohort and ours, were those that have been the most prominent in the case definition: breathlessness, fever, and cough. Both active pulmonary vasculitis and COVID‐19 are recognized causes of hemoptysis (18, 19). In the ISARIC WHO CCP‐UK group, 3.5% of patients experienced this symptom, compared to 3 patients (4.8%) in our cohort. None were thought to have diffuse alveolar hemorrhage, as assessed by the responding clinician. Differentiating COVID‐19 from active pulmonary vasculitis remains a challenge, and indeed these presentations may coexist. Ensuring that these diagnoses are considered when a patient with vasculitis presents with hemoptysis remains of high importance.
A large proportion of patients (49%) had concurrent active vasculitis. A considerable proportion of these patients were believed to have contracted COVID‐19 from a health care setting. Notably, the onset of vasculitis disease activity preceded COVID‐19 infection in almost all cases. However, it is unknown whether COVID‐19 may trigger vasculitis activity in the longer term. The emergence of pediatric multisystem inflammatory syndrome temporally associated with SARS–CoV‐2, a condition bearing strong similarities to Kawasaki disease, is a potential example of SARS–CoV‐2 triggering autoimmune vasculitis, though the pathogenesis is not currently understood (20). We did not find evidence of vasculitis being triggered in the short term. Longitudinal studies will seek to address this question. The prevalence of active disease in this cohort was higher than expected—previous cross‐sectional UK studies have shown disease activity prevalence at ~20% (21). Although disease activity was not associated with worse outcomes, it may be that patients with unstable disease are more vulnerable to contracting COVID‐19. Physician or patient‐led reduction in immunosuppressive treatment, in a bid to limit the impact of COVID‐19, is another potential reason accounting for a high proportion of disease activity. Due to the study design, our data are limited in the extent to which they can answer this question.
Ascertainment bias is likely to have affected this study. Given that most patients were hospitalized, it is likely that patients with milder disease were not identified. The number of PCR‐diagnosed COVID‐19 patients in our study as a proportion of the combined UKIVAS and RKD populations was similar to the proportion of UK cases relative to the UK population for a comparable time period (22). However, due to the study design, it is not possible to derive incidence rates. Patients with vasculitis may have been more likely to contract COVID‐19 due to risk factors such as immunosuppressive therapy and the need to attend health care facilities; therefore, ascertainment bias remains possible.
There was a high preponderance of small vessel vasculitis (SVV) in this study compared to giant cell arteritis (GCA). Although it is a more common condition, patients with GCA are typically older and may have adopted stricter self‐isolation restrictions (according to national guidance). In addition, SVV may have predominated due to a disproportionate number of renal departments, compared to rheumatology centers, represented in the UKIVAS registry. Due to a large majority of patients in this study having SVV, the extent to which its findings can be generalized to other vasculitides is limited. Other limitations of this study include a relative lack of power. As a result, our analyses may not detect some clinically significant effects, and, conversely, the risk of spurious findings is higher. Similarly, due to the limited number of events, our ability to control for multiple potential confounding factors was limited. Due to the heterogeneous immunobiology, phenotypes, and management approaches of systemic vasculitis, it is possible that individual disorders may incur different risks; due to insufficient power, we were limited in the extent to which this could be examined.
This study is the first to describe a cohort of vasculitis patients with COVID‐19. The clinical presentation of COVID‐19 was similar to descriptions in large series of patients without autoimmune disease. Glucocorticoids were associated with an increased risk of severe outcomes, but other immunosuppressants were not. Patients with autoimmune disease have been considered vulnerable during the COVID‐19 pandemic, and many governments have instructed that they adhere to exceptional social isolation restrictions. While patients with systemic vasculitis remain at a higher risk for severe outcomes, these data indicate that some patients may not need to face similar restrictions in the future if other known risk factors are absent. Conversely, patients who are receiving background glucocorticoids or have comorbid respiratory disease should be closely monitored when presenting with COVID‐19, since their risk of progression to a severe state appears to be higher (11). This study primarily describes a cohort of hospitalized patients and is thus more likely to reflect severe COVID‐19 disease. Future work should seek to establish risk factors for severe disease in a wider population. Comparisons with controls who did not contract COVID‐19 would allow for assessment of incidence and risk factors for contracting COVID‐19.
Taken together with findings from other cohorts exposed to immunosuppressant medication, these data could inform future public health guidance for patients with autoimmune disease. These data were designed to be interoperable with other national data sets. Future work should seek to combine international efforts to allow for greater power to assess the factors that impact this potentially vulnerable group.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Rutherford had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Rutherford, Scott, Gray, Barrett, Brix, Dhaun, McAdoo, Jayne, Luqmani, Salama, Little, Basu.
Acquisition of data
Rutherford, Scott, Karabayas, Antonelou, Gray, Barrett, Brix, Dhaun, McAdoo, Geddes, Luqmani, Salama, Little, Basu.
Analysis and interpretation of data
Rutherford, Scott, Gopaluni, Brix, Dhaun, McAdoo, Smith, Geddes, Jayne, Luqmani, Salama, Little, Basu.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors thank the following individuals who contributed data for this project: Jocelyn Berdeprado, Nina Brown, Chee Kay Cheung, Denise De Lord, Oliver Flossman, Venkat Gangaram, Sian Griffin, Alex Hamilton, Philip Ind, Vikki Jubb, Maria King, Sarah Logan, Alice Lorenzi, Ann Morgan, Dan Pugh, Ravindra Rajakariar, Saira Risdale, Michael Robson, Bhrigu Sood, Katie Vinen, and Zoe Wellbelove.
References
- 1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . WHO Director‐General’s opening remarks at the media briefing on COVID‐19–11 March 2020. URL: https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19‐‐‐11‐march‐2020.
- 3. Neumann I. Immunosuppressive and glucocorticoid therapy for the treatment of ANCA‐associated vasculitis [review]. Rheumatology (Oxford) 2020;59:iii60–7. [DOI] [PubMed] [Google Scholar]
- 4. Walsh M, Merkel PA, Peh CA, Szpirt WM, Puéchal X, Fujimoto S, et al. Plasma exchange and glucocorticoids in severe ANCA‐associated vasculitis. N Engl J Med 2020;382:622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al, for the RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Musen MA, Noy NF, Shah NH, Whetzel PL, Chute CG, Story MA, et al. The National Center for Biomedical Ontology. J Am Med Inform Assoc 2012;19:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. COVID‐19 Global Rheumatology Alliance . URL: https://rheum‐covid.org/.
- 8. Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 2016;3:160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 10. Gianfrancesco M, Hyrich KL, Al‐Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The OpenSAFELY Collaborative , Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, et al. OpenSAFELY: factors associated with COVID‐19‐related hospital death in the linked electronic health records of 17 million adult NHS patients [preprint]. medRxiv. May 2020. URL: 10.1101/2020.05.06.20092999. [DOI]
- 12. Cain DW, Cidlowski JA. Immune regulation by glucocorticoids [reivew]. Nat Rev Immunol 2017;17:233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson RM, Vinetz JM. Dexamethasone in the management of covid‐19 [editorial]. BMJ 2020;370:m2648. [DOI] [PubMed] [Google Scholar]
- 14. Li S, Hu Z, Song X. High‐dose but not low‐dose corticosteroids potentially delay viral shedding of patients with COVID‐19. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa829. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanchez‐Ramirez DC, Mackey D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID‐19 outcomes: a systematic review and meta‐analysis. Respir Med 2020;171:106096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith JC, Sausville EL, Girish V, Yuan ML, Vasudevan A, John KM, et al. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS‐CoV‐2 receptor ACE2 in the respiratory tract. Dev Cell 2020;53:514–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Landewé RB, Machado PM, Kroon F, Bijlsma HW, Burmester GR, Carmona L, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS‐CoV‐2. Ann Rheum Dis 2020;79:851–8. [DOI] [PubMed] [Google Scholar]
- 18. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, et al. ANCA‐associated vasculitis [review]. Nat Rev Dis Primers 2020;6:71. [DOI] [PubMed] [Google Scholar]
- 20. Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS‐CoV‐2 mimicking Kawasaki disease (Kawa‐COVID‐19): a multicentre cohort. Ann Rheum Dis 2020;79:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Basu N, McClean A, Harper L, Amft EN, Dhaun N, Luqmani RA, et al. The characterisation and determinants of quality of life in ANCA associated vasculitis. Ann Rheum Dis 2014;73:207–11. [DOI] [PubMed] [Google Scholar]
- 22. Gov.UK Coronavirus (COVID‐19) in the UK. URL: https://coronavirus.data.gov.uk/details/cases.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


