Abstract
The outbreak of Coronavirus disease 2019 (COVID‐19) has caused a global health crisis. Nevertheless, no antiviral treatment has yet been proven effective for treating COVID‐19 and symptomatic supportive cares have been the most common treatment. Therefore, the present study was designed to evaluate the effects of propolis and Hyoscyamus niger L. extract in patients with COVID‐19. This randomized clinical trial was conducted on 50 cases referred to Akhavan and Sepehri Clinics, Kashan university of medical sciences, Iran. Subjects were divided into two groups (intervention and placebo). This syrup (containing 1.6 mg of methanolic extract along with 450 mg of propolis per 10 mL) was administered three times a day to each patient for 6 days. The clinical symptoms of COVID‐19 such as: dry cough, shortness of breath, sore throat, chest pain, fever, dizziness, headache, abdominal pain, and diarrhea were reduced with propolis plus Hyoscyamus niger L. extract than the placebo group. However, the administration of syrup was not effective in the control of nausea and vomiting. In conclusion, syrup containing propolis and Hyoscyamus niger L. extract had beneficial effects in ameliorating the signs and symptoms of COVID‐19 disease, in comparison with placebo groups.
Keywords: COVID‐19, Hyoscyamus niger L., propolis
1. INTRODUCTION
The latest threat to global health is the ongoing outbreak of the respiratory disease that was recently given the name COVID‐19 (Acter et al., 2020). COVID‐19 was recognized in December 2019 and shown to be caused by a novel coronavirus, which is structurally related to the virus that causes severe acute respiratory syndrome (SARS) (Fauci, Lane, & Redfield, 2020). People with COVID‐19 have had different symptoms ranging from mild symptoms to severe illness. Patient with fever, cough, difficulty breathing, fatigue, loss of taste or smell, sore throat, and diarrhea may have COVID‐19 (Ali & Alharbi, 2020). If the patient is showing serious dyspnea, respiratory frequency ≥30/min, blood oxygen saturation ≤93%, and/or lung infiltrates >50% within 24–48 has to refer to emergency medical care immediately (Mehta et al., 2020). With the development of COVID‐19 globally and the declaration of the COVID‐19 outbreak as a Public Health Emergency of International Concern by the World Health Organization (Wu, Leung, & Leung, 2020), there is an urgent and rapid need for rapid diagnostics, vaccines, and therapeutics to detect, and prevent this disease. Today, the common focus has been on the development of new therapeutics, including antivirals and vaccines (Pang et al., 2020).
Ayurvedic and Unani medicine is based on non‐toxic plant materials and devoid of serious side effects. Herbal extracts have been used for their antiviral properties and more tolerable side effects since ancient times (Kim et al., 2010; Lin, Hsu, & Lin, 2014). Hyoscyamus niger has a very long history of use as a medicinal herb, and was used extensively as a sedative and pain killer. Also, this plant was utilized for the treatment of abdominal colic and pain due to worm infestation, spasmodic cough, asthma, and pulmonary infections (Al‐Snafi, 2018; Younis et al., 2018). Steroid glycosides such as hyoscyamoside are important compounds in the methanolic seed extract of Hyoscyamus niger L. (Jesus, Martins, Gallardo, & Silvestre, 2016). Hyoscyamoside is a flavonoid with potential antioxidant activity, and is a phytoprogesterone that could rejuvenate and repair lung tissue and improve asthma symptoms (Michalak et al., 2020; Zhang, Zhang, Luo, & Kong, 2013).
Propolis (bee glue) is a complex resinous mixture produced by honeybees (Apis mellifera) used by humans since ancient times for its medicinal properties. Propolis acts as a barrier to prevent yeasts, molds, viruses, and bacteria from infecting tissue (Bachevski, Damevska, Simeonovski, & Dimova, 2020). Quercetin is a flavonol present in propolis, and its derivatives were found to inhibit the protease enzymes from the SARS‐CoV‐1 and MERS‐CoV viruses in vitro (Rocha et al., 2013). In addition, quercetin can modulate the cellular unfolded protein response (UPR). Because coronaviruses can utilize the UPR to complete their entire replication cycle, quercetin might have anticoronavirus activity through its modulation of this pathway (Polansky & Lori, 2020). A promising pharmacological approach for the treatment of COVID‐19 is to target the downstream effectors, such as p21‐activated kinases (PAKs). Caffeic acid phenethyl ester (CAPE), an important constituent of propolis, has been shown to down‐regulate RAC (a signaling protein present in human cells), therefore acting as a RAC/CDC42‐activated kinase 1 (PAK1) inhibitor. These data support the idea that CAPE could be useful to inhibit or prevent coronavirus‐induced fibrosis in the lungs (Bachevski et al., 2020; Maruta & He, 2020).
To our best knowledge, studies are rare to demonstrate the effects of propolis and Hyoscyamus niger L. extract on clinical symptoms in patients with COVID‐19. Therefore, due to reports about the beneficial effects of propolis and Hyoscyamus niger L. methanolic extract containing steroidal glycosides, we tried to evaluate the therapeutic effects of propolis and Hyoscyamus niger L. extract on clinical symptoms in patients with COVID‐19.
2. MATERIAL AND METHODS
2.1. Trial design
This study was registered in the Iranian website: http://www.irct.ir: IRCT20200516047462N1. The participants were recruited from May 7 to June 19, 2020, Akhavan and Sepehri Clinics, Kashan university of medical sciences, Iran. Then, the trial was conducted among 50 subjects with acute respiratory syndrome suspected to COVID‐19, aged 18–75 years. This trial was conducted with informed consent was signed by all patients. The research ethics committee of Kashan University of Medical Sciences (KAUMS) approved the study protocol.
2.2. Inclusion criteria
Patients who, in addition to acute respiratory disease (ARD), have at least 2 of the following criteria: Respiratory Rate > 30, 85% < PO2 < 93%, pulmonary chest CT infiltration, and clinical judgment of a specialist physician for COVID‐19. The included subjects were male and female aged 18–65 years with the personal desire to enter the study (Ai et al., 2020).
2.3. Exclusion criteria
Pregnancy and lactation; a history of allergy to the components of the plant product; any serious concomitant cardiovascular disease; diabetes mellitus; any condition in the patient considered by the physician's judgment to prevent the continuation of therapeutic intervention; history of side effects after consuming any plant product components; nausea, vomiting, or food intolerance; resistant hypoxemia to treatment; decreased level of consciousness; hemodynamic instability.
2.4. Study design
Subjects were randomly divided into two groups, the treatment group (receiving the product plus routine medication, n = 25) and the placebo group (receiving placebo plus routine medication, n = 25). Propolis was first dissolved in isopropanol, but the final preparation contained less than 0.5% isopropyl alcohol. The extract of Hyoscyamus niger L. seeds was prepared using a Soxhlet extractor device. First, 60% aqueous methanol was used to extract the phenols. To remove the alcohol, the solution was rotary evaporated at 60°C, first methanol was evaporated at 50 to 55°C and then water was removed from the extract. The extract was obtained by freeze‐drying as a dry powder that could be dissolved in distilled water (8 g of dry powder can be dissolved in 10 mL of distilled water). Finally, 1% hydroalcoholic solution (isopropyl alcohol) of propolis was added to the aqueous solution. Extract (Küpeli Akkol et al., 2020; Reza, Mohammad, Golnaz, & Gholamreza, 2009). The 10 mL of syrup (containing 1.6 mg of methanolic of Hyoscyamus niger L extract plus 450 mg of propolis) was administered three times a day to each patient for 6 days. The active ingredients were dissolved in simple syrup BP (British Pharmacopeia) and this simple syrup was also used as a placebo.
To record clinical manifestation, we selected a combination of signs and symptoms according to the clinical trial guidelines of the Ministry of Health of Iran and covid‐19 related references. After all, the symptoms were graded according to the following categories: without symptoms (zero), mild symptoms without the need for drug intervention (Score 1), Moderate symptoms requiring drug intervention and limitation of over‐activity (Score 2), Severe symptoms and limitation of the patient's initial activities (Score 3), Life‐threatening and emergency complications (Score 4), Death (Score 5) (Jeong et al., 2020; Struyf et al., 2020).
Randomization and allocation were concealed to the subjects and researchers until the final analyses were completed.
2.5. Randomization
A randomized clinical trial with a control parallel group is performed on 50 patients. Subjects were divided into two groups (intervention and placebo) by method of permuted blocked randomization with 4 and 6 blocks. Neither the patients nor the physician know which subjects are in the intervention or placebo groups.
2.6. Statistical analysis
The Kolmogorov–Smirnov test was done to evaluate the normality of the data. We used the Chi‐square test, Independent sample t‐test, Wilcoxon Test, and Mann–Whitney test to analyze the data, using SPSS‐18 (SPSS Inc., Chicago, IL). p < .05 was considered as statistically significant.
TABLE 1.
General characteristics of the study participants a
| Placebo group (n = 25) | Intervention group (n = 25) | p b | |
|---|---|---|---|
| Age (years) | 43.54 ± 13.86 | 41.48 ± 13.77 | T = 0.52; p = .60 |
| Gender (%) | |||
| Male | 53.21 | 48.68 | Χ b = 0.32; p = .57 c |
| Female | 46.79 | 51.32 | |
| Education (%) | |||
| Elementary | 6.1 | 5.9 | |
| Intermediate | 3.4 | 3.6 | |
| Diploma | 2.9 | 2.1 | Χ b = 0.54; p = .97 c |
| College | 14.3 | 16.7 | |
| Bachelor of Science (BSc) | 21.7 | 22.3 | |
| Marital status (%) | |||
| Permanent marriage | 30.4 | 42.6 | |
| Single | 15.5 | 11.5 | Χ b = 2.10; p = .15 c |
| Widow/divorced | 0 | 0 | |
| Job (%) | |||
| Unemployed | 12.7 | 10.3 | |
| Employed | 16.0 | 23.0 | Χ b = 2.56; p = .28 c |
| Others | 22.4 | 15.6 |
Data are mean ± SDs.
Obtained from independent t‐test.
Obtained from Pearson Chi‐square test.
3. RESULTS
Our data showed that there was no significant difference between placebo and intervention groups at the beginning of the trial (Table 1). A significant difference was observed in dry cough scores between intervention and placebo groups after 2 days of treatment (p < .05). This difference was more significant on day 4 and day 6 (p < .001, p < .01, respectively) (Figure 1a).
FIGURE 1.
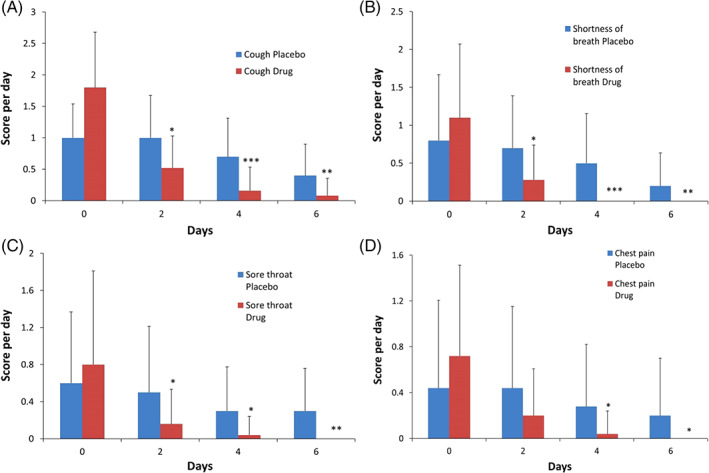
(a–d) The evaluation of Hyoscyamus niger L. methanolic extract with propolis in patients with COVID‐19 symptoms such as: (a) cough, (b) shortness of breath, (c) sore throat, and (d) chest pain. These symptoms were evaluated in two group (drug, n = 25 and placebo, n = 25) in 0, 2, 4 and 6 days after treatment. The values were given as the mean ± SD with * (p < .05), ** (p < .01), *** (p < .001) indicated statistically significant differences between two groups [Colour figure can be viewed at wileyonlinelibrary.com]
The shortness of breath score was shown in Figure 1b. This score was significantly lower in the intervention group compared to the placebo group on day 2, 4, and 6 (p < .05, p < .001, p < .01, respectively) (Figure 1b). Our data indicated that the sore throat was significantly reduced on day 2, 4, and 6 in the intervention group compared to the placebo group (p < .05, p < .05, p < .01, respectively) (Figure 1c). In addition, the intervention group showed significantly less chest pain compared to placebo on day 4 and 6 (p < .05) (Figure 1d).
At each visit, the subjects were asked about the side effects. The results of these symptoms are presented in Table 2. No significant differences were indicated between the two groups in terms of fever symptoms on day 0. With the administration of syrup, fever decreased significantly in the intervention group in day 2 (p < .05).
TABLE 2.
The assessment of drug on other symptoms in patient with COVID‐19
| Days parameters | 0 | 2 | 4 | 6 | |
|---|---|---|---|---|---|
| Fever | Placebo | 0.2 ± 0.4 | 0.2 ± 0.4 | 0 | 0 |
| Drug | 0.32 ± 0.5 | 0 * | 0 | 0 | |
| Effect size | 0.25 | ||||
| Headache | Placebo | 0.6 ± 0.7 | 0.52 ± 0.7 | 0.48 ± 0.6 | 0.2 ± 0.4 |
| Drug | 1 ± 1 | 0.04 ± 0.2 | 0 | 0 | |
| Effect size | 0.39 | 0.37 | 0.15 | ||
| Muscular pain | Placebo | 0.8 ± 0.9 | 0.72 ± 0.8 | 0.56 ± 0.7 | 0.4 ± 0.6 |
| Drug | 1.24 ± 1.2 | 0.44 ± 0.5 | 0.08 ± 0.3 ** | 0 ** | |
| Effect size | 0.32 | 0.38 | 0.26 | ||
| Diarrhea | Placebo | 0.16 ± 0.5 | 0.16 ± 0.5 | 0.08 ± 0.3 | 0.08 ± 0.3 |
| Drug | 0.08 ± 0.3 | 0 | 0 | 0 | |
| Effect size | 0.81 | 0.32 | 0.32 | ||
| Runny nose | Placebo | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.16 ± 0.4 | 0.08 ± 0.3 |
| Drug | 0.12 ± 0.3 | 0* | 0* | 0 | |
| Effect size | 0.15 | 0.90 | 0.32 | ||
| Sore throat and larynx | Placebo | 0.5 ± 0.77 | 0.5 ± 0.71 | 0.3 ± 0.56 | 0.16 ± 0.47 |
| Drug | 0.7 ± 0.9 | 0.24 ± 0.52* | 0.04 ± 0.2* | 0 | |
| Effect size | 0.30 | 0.20 | 0.71 | ||
| Fatigue | Placebo | 1 ± 1.04 | 1 ± 0.93 | 0.88 ± 0.83 | 0.68 ± 0.8 |
| Drug | 1.9 ± 1.04 | 0.68 ± 0.63 | 0.4 ± 0.5* | 0.08 ± 0.28*** | |
| Effect size | 52% | 39% | 38% | ||
| Anorexia | Placebo | 0.7 ± 0.75 | 0.6 ± 0.71 | 0.36 ± 0.49 | 0.24 ± 0.44 |
| Drug | 0.6 ± 0.82 | 0.04 ± 0.2 | 0 | 0 | |
| Effect size | 30% | 24% | 14% | ||
| Trembling | Placebo | 0.28 ± 0.54 | 0.16 ± 0.37 | 0.04 ± 0.2 | 0.04 ± 0.2 |
| Drug | 0.4 ± 0.71 | 0 | 0 | 0 | |
| Effect size | 0.14 | 0.42 | 0.42 | ||
| Nausea | Placebo | 0.12 ± 0.33 | 0.12 ± 0.33 | 0.08 ± 0.28 | 0.04 ± 0.2 |
| Drug | 0.44 ± 0.58 | 0 | 0 | 0 | |
| Effect size | 0.12 | 0.66 | 0.50 | ||
| Vomit | Placebo | 0 | 0 | 0 | 0 |
| Drug | 0.04 ± 0.2 | 0 | 0 | 0 | |
| Effect size | |||||
| Dizziness | Placebo | 0.28 ± 0.54 | 0.16 ± 0.37 | 0.2 ± 0.4 | 0.04 ± 0.2 |
| Drug | 0.28 ± 0.54 | 0.04 ± 0.2 | 0* | 0 | |
| Effect size | 0.08 | 0.17 | 0.30 | ||
| Abdominal pain | Placebo | 0.52 ± 0.71 | 0.4 ± 0.57 | 0.32 ± 0.55 | 0.2 ± 0.41 |
| Drug | 0.32 ± 0.63 | 0*** | 0** | 0** | |
| Effect size | 0.22 | 0.14 | 0.91 | ||
Note: The treated group (n = 25) showed a significant positive trend in symptoms, with a noticeable reduction in fever, muscular pain, runny nose, sore throat and larynx, fatigue, dizziness, and abdominal pain. The values were given as the mean ± SD with *(p < .05), **(p < .01), ***(p < .001) indicated statistically significant differences between two groups in different days.
The results also revealed that there were significant decreases between two groups in terms of muscular pain and fatigue on day 4 and 6 (p < .01, p < .001). Also, we observed that runny nose and sore throat or larynx symptoms were reduced significantly in the intervention group compared to placebo group on day 2 and 4 (p < .05). Dizziness was less in intervention group on day 4 (p < .05). There was also a significant difference in terms of abdominal pain in intervention compared to placebo group on day 2, 4, and 6 (p < .001, p < .01) (Table 2). However, our data did not show any difference in symptoms of headache, diarrhea, anorexia, trembling, nausea, or vomiting between the two groups after 6 days (Table 2).
4. DISCUSSION
The results of this study showed that the symptoms of COVID‐19 including: cough, shortness of breath, sore throat, and chest pain were reduced by administration of Hyoscyamus niger L. extract plus propolis, and that this decreasing trend was more pronounced with the increased number of days of treatment. COVID‐19 is a newly emerged infectious respiratory disease with only mild signs in the early stages of infection. However, in a considerable number of subjects, the symptoms deteriorate rapidly and manifest as chest tightness, respiratory distress, and shortness of breath (Xu et al., 2020).
A randomized clinical trial was conducted in China to evaluate the efficacy and side effects of herbal medicines for the treatment of COVID‐19 in 855 subjects. In this study, the combined treatment of different herbal medicines has been compared with Western medicines. Their results showed that combination therapy significantly improved the overall symptoms such as: dry cough, sore throat, fever, and fatigue (Ang, Song, Lee, & Lee, 2020). Herbal extracts were demonstrated to reduce the inflammatory cascade triggered by NF‐kB, a pathway that has been implicated in the respiratory distress caused by SARS‐CoV (Srivastava, Mistry, & Sharma, 2015; Vellingiri et al., 2020). Other studies have also reported the antiviral properties of herbal extracts against bronchitis, and showed plants such as: Justicia adhatoda, Hyoscyamus niger L., and Verbascum thapsus reduced infections caused by influenza viruses. The molecular mechanism by which these plants target influenza virus should be further studied to understand if they interfere with any pathways overlapping between influenza viruses and SARS‐CoV‐2. Hyoscyamus niger L. was demonstrated to be a bronchodilator and, inhibitory effects on the Ca2+ channel. Hyoscyamus niger L. extract could be used to target the orf3a Ca2+channels that trigger various downstream pathways involved in viral infections (Gilani et al., 2008).
Administration of an aqueous extract of propolis for 2 months to asthmatic patients showed that propolis could be used in the treatment of patients with mild to moderate lung disease. Propolis increases the rate of expiratory circulation. It is associated with a higher vital capacity and reduces inflammatory factors such as Tumor necrosis factor‐alpha, interleukin‐6, 8, and 10 in humans (Khayyal et al., 2003). Findings by Gabriele Carra Forte et al. showed that propolis could act as a natural antioxidant in obese adults with asthma, and significantly increased FEV1 (Forced Expiratory Volume in First Second) and improved respiratory status and blood oxygen volume (Forte et al., 2018). Ophori and Wemabu (2010) showed that propolis extract demonstrated an in vitro antimicrobial activity against Klebsiella pneumoniae, Haemophilus influenzae, Streptococcus pneumoniae, Streptococcus pyogenes, and Moraxella catarrhalis. Their findings suggested that propolis is a very effective antimicrobial agent for the management and treatment of upper respiratory tract infections caused by bacterial species (Ophori & Wemabu, 2010).
Our data showed that symptoms of COVID‐19 including: fever, muscular pain, runny nose, sore throat or larynx, fatigue, abdominal pain, and dizziness were decreased in the intervention group compared to the placebo group at different days. The effects of propolis contained in royal jelly and honey were shown by El‐Aidy in 2014 in asthmatic patients. A laboratory study in an asthmatic mouse model showed that propolis was effective in treating asthma, and was able to reduce the level of blood leukocytes such as neutrophils, lymphocytes, eosinophils, and monocytes to a greater degree compared to the other two tested products, and had a similar therapeutic effect in bronchial tissue as dexamethasone This was due to the high antioxidant content of propolis, such as polyphenols (El‐Aidy et al., 2015). Considering the preliminary results we obtained showing the efficacy of this natural product in COVID‐19 treatment, we suggest that further clinical trials, should be carried out as soon as possible. The syrup significantly improved almost all the symptoms, especially headache, dizziness, cough, shortness of breath, fever, and fatigue in the outpatients included in the study. This product showed no drug interactions, and a side effect was seen only in one patient with hot flashes. The limitations of this study include the period until onset of symptoms of the disease was not controlled, and the speed of its progression could be variable. Moreover, it is uncertain if the treatment would be effective in hospitalized patients. The strengths of this study were the effectiveness and safety of the product, as well as the good compliance of the patients in taking the medicine.
5. CONCLUSION
At present, there are no specific antiviral agents, and vaccines against SARS‐CoV2 infection are only just becoming available. Identifying methods able to decrease or prevent viral adhesion and colonization, or which have the ability to inactivate pathogens could be useful. Moreover, natural products could increase the immune response in order to fight against the COVID‐19 disease. Therefore, we suggest that Hyoscyamus niger L. methanolic extract plus propolis may represent a low‐cost naturally‐derived treatment option. These herbal compounds can alleviate or eliminate the symptoms of COVID‐19, and may used as a treatment for COVID‐19 in the future. Of course, future studies should focus on the mechanisms of action of Hyoscyamus niger L. and propolis and its related ingredients, either alone or combined with conventional therapy for COVID‐19 patients. The data contained in this preliminary trial is not sufficient to reach any appropriate conclusions, and larger controlled trials should be conducted to confirm the effectiveness and lack of side effects in COVID‐19 patients.
AUTHORS' CONTRIBUTIONS
MK and HRB contributed in design, conception, and statistical analysis. MK, MN, SPK, AN, AGH, MS, and HRB contributed in data collection and manuscript drafting.
CONFLICTS OF INTEREST
None.
ETHICS STATEMENT
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments. The patients/participants provided their written informed consent to participate in this study.
ACKNOWLEDGMENTS
It has been registered with the IRCT20200516047462N1 registration code in the Iranian Center for Clinical Trials, and was supported by a grant from the Vice‐chancellor for Research, KAUMS, and Iran. We are thankful of all patients who participated in this project.
Kosari M, Noureddini M, Khamechi SP, et al. The effect of propolis plus Hyoscyamus niger L. methanolic extract on clinical symptoms in patients with acute respiratory syndrome suspected to COVID‐19: A clinical trial. Phytotherapy Research. 2021;35:4000–4006. 10.1002/ptr.7116
Funding information Kashan University of Medical Sciences
REFERENCES
- Acter, T. , Uddin, N. , Das, J. , Akhter, A. , Choudhury, T. R. , & Kim, S. (2020). Evolution of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) as coronavirus disease 2019 (COVID‐19) pandemic: A global health emergency. Science of the Total Environment, 730, 138996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai, T. , Yang, Z. , Hou, H. , Zhan, C. , Chen, C. , Lv, W. , … Xia, L. (2020). Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: A report of 1014 cases. Radiology, 296, E32–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, I. , & Alharbi, O. M. (2020). COVID‐19: Disease, management, treatment, and social impact. Science of the Total Environment, 728, 138861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Snafi, A. E. (2018). Therapeutic importance of Hyoscyamus species grown in Iraq (Hyoscyamus albus, Hyoscyamus niger L. and Hyoscyamus reticulates)—A review. IOSR Journal of Pharmacy, 8, 18–32. [Google Scholar]
- Ang, L. , Song, E. , Lee, H. W. , & Lee, M. S. (2020). Herbal medicine for the treatment of coronavirus disease 2019 (COVID‐19): A systematic review and meta‐analysis of randomized controlled trials. Journal of Clinical Medicine, 9, 1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevski, D. , Damevska, K. , Simeonovski, V. , & Dimova, M. (2020). Back to the basics: Propolis and COVID‐19. Dermatologic Therapy, 33, e13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Aidy, W. K. , Ebeid, A. A. , Sallam, A. E.‐R. M. , Muhammad, I. E. , Abbas, A. T. , Kamal, M. , & Sohrab, S. S. (2015). Evaluation of propolis, honey, and royal jelly in amelioration of peripheral blood leukocytes and lung inflammation in mouse conalbumin‐induced asthma model. Saudi Journal of Biological Sciences, 22, 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci, A. S. , Lane, H. C. , & Redfield, R. R. (2020). Covid‐19—Navigating the uncharted. New England Journal of Medicine, 382, 1268–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte, G. C. , da Silva, D. T. R. , Hennemann, M. L. , Sarmento, R. A. , Almeida, J. C. , & de Tarso Roth Dalcin, P. (2018). Diet effects in the asthma treatment: A systematic review. Critical Reviews in Food Science and Nutrition, 58, 1878–1887. [DOI] [PubMed] [Google Scholar]
- Gilani, A. H. , Khan, A.u. , Raoof, M. , Ghayur, M. N. , Siddiqui, B. S. , Vohra, W. , & Begum, S. (2008). Gastrointestinal, selective airways and urinary bladder relaxant effects of Hyoscyamus niger L. are mediated through dual blockade of muscarinic receptors and Ca2+ channels. Fundamental & Clinical Pharmacology, 22, 87–99. [DOI] [PubMed] [Google Scholar]
- Küpeli Akkol, E. , Ilhan, M. , Kozan, E. , Gürağaç Dereli, F. T. , Sak, M. , & Sobarzo‐Sánchez, E. (2020). Insecticidal activity of hyoscyamus niger l. on lucilia sericata causing myiasis. Plants (Basel), 9(5), 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, H. , Lee, J. , Kim, J. , Choen, S. , Sohn, K. M. , Kim, Y.‐S. , & Kiem, S. (2020). Self‐assessment questionnaire for efficient and safe evaluation of patients with mild COVID‐19. Infection & Chemotherapy, 52, 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesus, M. , Martins, A. P. , Gallardo, E. , & Silvestre, S. (2016). Diosgenin: Recent highlights on pharmacology and analytical methodology. Journal of Analytical Methods in Chemistry, 2016, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayyal, M. , El‐Ghazaly, M. , El‐Khatib, A. , Hatem, A. , De Vries, P. , El‐Shafei, S. , & Khattab, M. (2003). A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundamental & Clinical Pharmacology, 17, 93–102. [DOI] [PubMed] [Google Scholar]
- Kim, H.‐Y. , Eo, E.‐Y. , Park, H. , Kim, Y.‐C. , Park, S. , Shin, H.‐J. , & Kim, K. (2010). Medicinal herbal extracts of Sophorae radix, Acanthopanacis cortex, Sanguisorbae radix and Torilis fructus inhibit coronavirus replication in vitro . Antiviral Therapy, 15, 697–709. [DOI] [PubMed] [Google Scholar]
- Lin, L.‐T. , Hsu, W.‐C. , & Lin, C.‐C. (2014). Antiviral natural products and herbal medicines. Journal of Traditional and Complementary Medicine, 4, 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta, H. , & He, H. (2020). PAK1‐blockers: Potential therapeutics against COVID‐19. Medicine in Drug Discovery, 6, 100039–100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, P. , McAuley, D. F. , Brown, M. , Sanchez, E. , Tattersall, R. S. , Manson, J. J. , & Collaboration, H. A. S. (2020). COVID‐19: Consider cytokine storm syndromes and immunosuppression. Lancet (London, England), 395, 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak, O. , Krzeczyński, P. , Cieślak, M. , Cmoch, P. , Cybulski, M. , Królewska‐Golińska, K. , … Ostrowska, K. (2020). Synthesis and anti‐tumour, immunomodulating activity of diosgenin and tigogenin conjugates. The Journal of Steroid Biochemistry and Molecular Biology, 198, 105573. [DOI] [PubMed] [Google Scholar]
- Ophori, E. , & Wemabu, E. (2010). Antimicrobial activity of propolis extract on bacteria isolated from nasopharynx of patients with upper respiratory tract infection admitted to Central Hospital, Benin City, Nigeria. African Journal of Microbiology Research, 4, 1719–1723. [Google Scholar]
- Pang, J. , Wang, M. X. , Ang, I. Y. H. , Tan, S. H. X. , Lewis, R. F. , Chen, J. I.‐P. , … Yang, Q. (2020). Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019‐nCoV): A systematic review. Journal of Clinical Medicine, 9, 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky, H. , & Lori, G. (2020). Coronavirus (COVID‐19), first indication of efficacy of Gene‐Eden‐VIR/Novirin in SARS‐CoV‐2 infections. International Journal of Antimicrobial Agents, 55, 105971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza, H. M. , Mohammad, H. , Golnaz, E. , & Gholamreza, S. (2009). Effect of methanolic extract of Hyoscymus niger L. on the seizure induced by picritoxin in mice. Pakistan Journal of Pharmaceutical Sciences, 22, 308–312. [PubMed] [Google Scholar]
- Rocha, B. A. , Bueno, P. C. P. , Vaz, M. M.d. O. L. L. , Nascimento, A. P. , Ferreira, N. U. , Moreno, G.d. P. , … Campos, J. C. L. (2013). Evaluation of a propolis water extract using a reliable RP‐HPLC methodology and in vitro and in vivo efficacy and safety characterisation. Evidence‐based Complementary and Alternative Medicine, 2013, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, R. A. K. , Mistry, S. , & Sharma, S. (2015). A novel anti‐inflammatory natural product from Sphaeranthus indicus inhibits expression of VCAM1 and ICAM1, and slows atherosclerosis progression independent of lipid changes. Nutrition and Metabolism, 12, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyf, T. , Deeks, J. J. , Dinnes, J. , Takwoingi, Y. , Davenport, C. , Leeflang, M. M. , … Dittrich, S. (2020). Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID‐19 disease. Cochrane Database of Systematic Reviews, 7, 1–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellingiri, B. , Jayaramayya, K. , Iyer, M. , Narayanasamy, A. , Govindasamy, V. , Giridharan, B. , … Ganesan, H. (2020). COVID‐19: A promising cure for the global panic. Science of the Total Environment, 725, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. T. , Leung, K. , & Leung, G. M. (2020). Nowcasting and forecasting the potential domestic and international spread of the 2019‐nCoV outbreak originating in Wuhan, China: A modelling study. The Lancet, 395, 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Han, M. , Li, T. , Sun, W. , Wang, D. , Fu, B. , … Li, X. (2020). Effective treatment of severe COVID‐19 patients with tocilizumab. Proceedings of the National Academy of Sciences, 117, 10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis, W. , Asif, H. , Sharif, A. , Riaz, H. , Bukhari, I. A. , & Assiri, A. M. (2018). Traditional medicinal plants used for respiratory disorders in Pakistan: A review of the ethno‐medicinal and pharmacological evidence. Chinese Medicine, 13, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Zhang, W. , Luo, J. , & Kong, L. (2013). A new steroidal glycoside from the seeds of Hyoscyamus niger L. Natural Product Research, 27, 1971–1974. [DOI] [PubMed] [Google Scholar]


