Abstract
Introduction
Recruitment of Covid‐19 convalescent plasma (CCP) donors may present as a challenge due to inexperience and differences in donor profile as compared to whole blood donation. Present study highlights the deterrents to recruiting CCP donors at a hospital based blood centre.
Materials and methods
Potential CCP donors were contacted individually by telephone and a group approach through camp organisers from May to July 2020. Recruitment challenges were noted and deferrals of these recruited donors during screening and medical examination was obtained and analysed.
Results
Total 1165 potential CCP donors were contacted. Around 47% donors were lost due to challenges related to information storage and retrieval. Fear of health, family pressure, and fear of a new procedure were major reason (27.2%) for unwillingness to donate. The main reasons for deferral among potential donors were multiparity (38%) and being overage/underage (31.6%). Finally, 468 donors were recruited including 408 by individual approach and 60 by a group approach. From these absence of detectable COVID‐19 antibodies were found in 15.4%. Few donors (9.0%) were deferred as they had not completed 28 days post recovery.
Conclusion
The process of CCP donor recruitment differs from that of whole blood donation and requires an individualised approach with involvement of clinicians in the initial phases of the pandemic. A group approach targeting specific organisations could be adopted for a successful CCP collection program. There is a need to relook into some aspects of donor selection such as consideration of multiparous female donors and overage/underage donors after reviewing scientific evidence.
Keywords: COVID‐19 convalescent plasma (CCP) donors, deferrals, deterrents, donor motivation, donor recruitment
1. INTRODUCTION
SARS‐CoV‐2 originated in Wuhan, China and subsequently spread globally with WHO declaring it as a pandemic. Its rapid spread and variable but higher mortality (2%–12%) and lack of any definitive cure lead to the trial of various treatment options. 1 The experience of use of convalescent plasma and its limited benefits in other diseases such as H1N1 influenza pandemic in 2009–2010, SARS‐CoV‐1 epidemic in 2003, MERS‐CoV epidemic in 2012 and Ebola virus 2 , 3 , 4 , 5 disease, projected its use as one of the promising investigational therapies for COVID‐19 disease. Though the effectiveness of COVID‐19 convalescent plasma (CCP) was unknown, but its safety profile and potential benefit led to its use as an off label therapy globally including India. 6
India has a decentralised blood collection system with >3000 centres majority of which are hospital based blood centres. All blood centres operate independently with a provision to transport blood and blood components to other blood centres in case of shortage. Blood collection using cell separators is limited to only a few centres across major cities with plateletpheresis being the most common procedure. During the COVID‐19 pandemic, there was a surge in demand of CCP across major cities in India as well, and blood centres were faced with a challenge to convert COVID‐19 recovered patients into potential CCP donors. The limited number of recovered COVID‐19 patients being pursued by multiple blood centres in the same city made the task of recruiting CCP donors challenging. The lack of donor selection guidelines for CCP donation added to the difficulties in recruiting these donors. The donors were selected following the screening criterion as per Drugs and Cosmetic Act & rule 1945, India and the interim guidance for donor selection in‐view of COVID‐19 pandemic given by National Blood transfusion council which were specific to whole blood donation. 7 A COVID‐19 recovered patient was considered to be eligible for CCP donation when he has completed 28 days post discharge from COVID‐19 treating facility or has completed 28‐days in‐home isolation after being reverse transcriptase‐polymerase chain reaction positive and remained asymptomatic during this period. 8 Our experience may highlight some of the differences and challenges in recruiting these plasma donors in comparison to whole blood and platelet donors. This would also help formulate strategies to approach these donors and ease the process of plasma donation in pandemic situations in future. The present report is thus aimed to analyse the challenges in recruitment of potential convalescent plasma donors as well as to understand the reasons for deferral of plasma donors who were willing to donate convalescent plasma.
2. MATERIAL AND METHODS
2.1. Study setting
The study involved retrospective collection and analysis of data related to recruitment of CCP donor in a blood centre of tertiary care hospital in Northern India. The blood centre started CCP donor recruitment and collection from May 2020 in view of the progressing pandemic. Ethical clearance was obtained from Institutional Ethics Committee before collection and analysis of donor data.
2.2. CCP donor recruitment process
A list of lab‐confirmed COVID‐19 patients treated and discharged from our hospital along with their contact details was requested from the team responsible for treatment of COVID patients. Patients who had completed 28 days post discharge were identified from the list and were contacted by two staff nurses who are trained to recruit blood donors. On making a contact, the blood centre staff introduced themselves and asked the potential donors a convenient time to talk. If the donor was willing to talk, the staff enquired about their health status and assessed their eligibility to donate. Then they were informed about the need and importance of CCP plasma and its potential application in treatment of COVID‐19 patients and were asked for their willingness to donate. If they agreed to donate, they were explained the procedure of plasma collection in brief and the approximate time required for donation. All queries were resolved and basic information like age and parity (in case of female donors) was enquired telephonically before confirming the appointment at blood centre. They were then given an appointment at a suitable time as per their convenience for pre donation screening and health check‐up at the blood centre.
If the donor was unwilling to donate, the reason for their unwillingness was asked and documented verbatim by the recruiter. Various deterrent and deferral reasons were coded from A to J and also divided into subcategories by one of the authors (Table 1).
TABLE 1.
Coding of deterrents and deferral reasons
| Code | Deterrent/deferral reasons |
|---|---|
| A | Not responded to phone calls |
| B | Duration of recovery <28 days |
| C | Previous donation <3 months |
| D | Multiparous women |
| E | Donor out of town |
| F | Donor in containment zone |
| G | Donor unwilling to donate |
| G1: Fear of health | |
| G2: Fear of procedure | |
| G3: Family pressure | |
| G4: Other commitment | |
| G5: No reason given | |
| H | Donor not showed up to blood centre |
| I | Donor deferred due to other medical reasons |
| J | COVID‐19 antibodies not detected in donor |
These coding were reassessed and verified by another author. In case the potential donor could not be reached through telecommunication due to network related issues or phone being switched off or busy at the time, one more attempt was made to contact them after a period of 2 days. Only two attempts were made, and a negative response was documented. Information related to donors with an incorrect contact details were also documented.
On the day of appointment, the donor was asked to fill up a donor history questionnaire followed by a physical examination. An ethylene diamine tetraacetic acid sample was drawn from the donor and the donor was screened for the presence of SARS‐COV‐2 IgG antibodies using Architect i1000SR immunoassay analyser in addition to a complete blood count, Transfusion transmitted infection markers, blood group, antibody screen for unexpected red cell antibodies and total serum protein. Plasmapheresis procedure was performed on donors who fulfilled the eligibility criteria. If the donor was ineligible the reason for deferral were documented and coded. Donors were also recruited by a group approach where blood donation camp organisers and other big organisations were contacted to motivate their recovered personnel to donate CCP. Any voluntary and replacement CCP donors who reported directly to the blood centre were also screened and the same post‐recruitment procedure as described above was followed.
2.3. Study plan
The present study included collation and retrospective analysis of the information collected at the time of recruitment of potential CCP donors focusing on various deterrents as well as the reasons for deferral of plasma donors. The data related to these parameters was collected from the records, and entered into a spreadsheet (Excel, Microsoft office 365). Descriptive statistics was applied to describe the different parameters.
3. RESULTS
3.1. Selection of potentially recruitable CCP donors
During the study period, attempt to recruit CCP donors from a total of 1165 recovered COVID‐19 patients was done using telephonic communication. Three‐fourth (870/1165) of these recovered patients were reachable and one fourth (295/1165) of the patients could not be contacted due to various reasons Figure 1. Of the 74.6% donors who could be reached, 21.7% (253/1165) has their residence in other cities/states and had already left the city thus making them unsuitable to donate due to the lockdown. Among the remaining 617 potentially recruitable CCP donors 61.5% (380) were males and 38.4% (237) were females.
FIGURE 1.
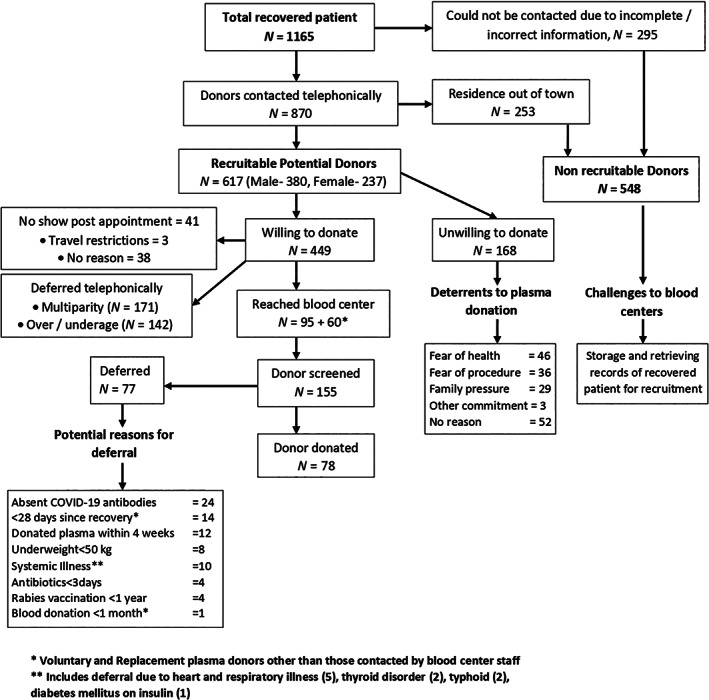
Flow showing process of recruitment of Covid‐19 convalescent plasma donors with challenges, deterrents and reasons for deferral
Deterrents to CCP donation among study subjects
Among the 617 recruitable donors, 27.2% (168/617) expressed their unwillingness to donate plasma out of which 11 were females and 157 were males. Majority of these (30.9%, 52/168) did not gave any specific reason for refusal while 27.4% (46/168) expressed fear of health and weakness as a reason to not donate plasma. 17.3% (29/168) had family pressure to not donate and 2.9% (5/168) had already committed to someone else for plasma donation hence they refused (Figure 1).
Remaining 72.8% (449/617) donors were willing to donate plasma and were given an appointment for health check‐up and screening for their eligibility. However, 9.1% (41/449) of these donors did not show up for their appointment. Of these, three donors could not come due to the lockdown restrictions in their areas.
Reasons for donor deferral of CCP donors
The blood centre in total screened 468 potential plasma donors. These included 408 willing donors who reached the blood centre other than those who were screened and deferred telephonically due to either multiparity or age. These also included 60 voluntary and replacement donors who presented at the blood centre directly through group approach.
Of the total telephonically contacted willing donors 38% (171/449) and 31.6% (142/449) donors were deferred due to multiparity and overage (>60 years)/underage (<18 years) respectively.
Finally, 155 donors were screened with donor history questionnaire and out of them 34.1% (53/155) donors were deferred. The major reasons for deferral at this level included donors who have not completed 28 days post‐discharge or end of home isolation (9.0% [14/155]) and underweight (5.1% [08/155]). These were the donors who showed up directly at the blood centre as voluntary and replacement donors other than contacted by blood bank staff. A proportion of these potential plasma donors (15.4%, 24/155) were deferred due to absence of COVID‐19 antibodies after antibody testing on the day of presentation (Figure 1).
DISCUSSION
With the start of COVID‐19 pandemic a number of potential therapies were explored globally. One such therapy included CCP which was collected from the patients who have recovered from the illness and were supposed to be having protective antibodies against the illness. With publication of various reports describing benefit of using CCP in patients, the demand for the CCP increased exponentially. 9 , 10 However, the availability of the product was limited in the initial phases of the pandemic. Blood collection centres across the world were faced with an uphill task to motivate and recruit potential CCP donors to meet the increasing demand. Present study highlights the experience of a hospital‐based blood centre from a resource limited country in recruiting potential plasma donors.
We observed that the process of motivation and recruitment of a CCP donor differs from that of a whole blood donor in terms of the available pool where entire population between the age group 18–65 years is eligible to donate in the latter, while CCP donor pool was limited to individuals between 18–60 years of age who have just recovered from a serious illness. In a country like ours, where the awareness of voluntary blood donation is limited, motivating recovered patients to donate is a big challenge especially in a pandemic situation and this demands adopting different strategies. Attempts to motivate potential CCP donors during the current pandemic were done by advertising the need and eligibility using print, electronic and social media though various government channels. We however suggest a more personalised approach wherein clinical staff involved in their care could be involved in recruitment. This could be a more practical approach in a country like ours where blood banking is decentralised with each hospital having its own blood collection and distribution. This is in contrast to the recruitment of CCP donors done during EBOLA outbreak in 2014 where “plasma mobile” systems were used for collection and peer educators were appointed to motivate and recruit Ebola virus disease survivors under supervision of medical doctors. 11
We also observed that there was an apprehension among potential CCP donors regarding their health due to procedure. This emphasises the importance of adopting different strategy to motivate and recruit these donors when general public is facing a taboo of COVID‐19 and is living in fear of getting infected while coming to donate in a hospital set up. 12 , 13 This calls for an approach where, in addition to motivating donors for CCP donation, efforts should also be made to inform them of the safety of the procedure. Such donors could also be motivated to donate whole blood instead of donating using apheresis as reported by Wong et al 14 but this approach would limit their chances of repeat donation to once every 3 months as per our country regulations.
While mass communication strategies or blood donation drives along with motivating factors, according to voluntary functions inventory 15 namely ‘Value’, ‘Social’, ’Esteem’ and ‘Understanding’, have been our usual approach for recruiting whole blood donors, a targeted approach need to be adopted for CCP donors. We suggest that the process of motivation should begin at the time when the patient is recovering especially towards the time of discharge in collaboration with the clinical staff, directly involved with patient care in order to build confidence of the potential donor and gain their trust. In addition, COVID‐19 survivors who have already donated CCP would prove to be more efficient recruiters.
It is also evident from the present study that around 50% potential donors were lost due to either inaccurate contact information or a residence out of town similar to CCP recruitment attempt by Wong et al. 14 It may be challenging to maintain the patient information of all the patients to be contacted at a later date especially when the patient needs to be isolated in separate facilities. More than one mode of communication should be registered to avoid loss of donors due to inaccurate/incorrect information. Accurate and complete information of a recovered patient using various tools like a web‐based survey will also facilitate triaging of these patients into eligible, soon to be eligible and ineligible donors and then coordinating them to appropriate blood centres near their residential location. 16 However, success of such approaches will need to be tested in technologically limited, resource poor countries. Explaining the eligibility criteria to donors during telecommunication may be a much more efficient way of recruiting CCP donors in countries like ours but would demand more time and manpower.
We also observed that there was a significant time duration (28 days in case of COVID‐19) after discharge before a patient can donate and this also resulted in loss of donors in the absence of follow‐up. Blood centres should develop mechanisms to contact these potential donors at specified intervals right after discharge so that the motivation to donate CCP is reinforced. This could also be achieved by reducing the eligibility to 14 days specifically for CCP donation as opposed to 28 days as is being followed by some European and American countries 17 when the donors are still motivated. This will also ensure that donation is done in a time period when antibody levels are sufficient. Moreover, unwilling donors on the 15th day can be counselled and motivated and contacted again on the 28th day thereby increasing chances of recruitment. In addition, a discharge advice by the clinician to follow‐up with the blood centre after a specified time (e.g. 1 week after discharge) will help in retaining more donors. Facility of online registration and providing non‐monetary incentives like masks, sanitizers and transport facility to these donors may also provide some confidence to these donors towards blood centres. This will also prevent black marketing of this precious product as was being reported from various countries. 18
In initial phase of the pandemic lack of clarity regarding selection of CCP donors posed a major challenge. Deferral of multiparous female donors, which is not done routinely for whole blood donors, was also added to the already existing guidelines specifically for CCP donations. The rationale behind this was the evidence that plasma from multiparous female donors was earlier reported to be associated with TRALI due to the presence of anti‐HLA and anti‐HNA antibodies. 19 While this may not affect the whole blood donor pool in our country where only 3%–10% of blood donation is contributed by female blood, 20 but it had significant repercussions for recruitment of CCP donors where multiparous female donors formed around 40% of our recruitable donors and 72% of these willing female donors were deferred due to multiparity. Considering that less than one third of multiparous female donors have been shown to have anti‐HLA or anti‐HNA, these recommendations need a relook especially with such limited availability of CCP donors. While a number of countries have relaxed donor selection guidelines for blood donation in view of the decreased blood supply due to pandemic, such relaxation could be considered for CCP donor selection too. We lost around 10% of donors who were between age groups 60–65 years and were eligible to donate blood but not CCP as CCP donations could only be collected till 60 years of age in our country.
As the pandemic progressed and the number of eligible donors increased, we changed our recruitment strategy and approached various organisations where employees were working throughout, and chances of recruiting donors were good. We could conduct an in‐house voluntary plasma donation camp with around 25 donors donating plasma on a single day. Group approach seems to be another way to recruit plasma donors during the pandemic. However, this approach may be adopted once the pandemic has progressed with sufficient number of recovered donors and may not work in the initial phases.
1. Limitations
Our approach to recruit CCP donors was limited by the fact that due to limited trained manpower and time we could contact the donors only once. We could neither follow the donors who initially showed their willingness to donate CCP but did not reported to us on the scheduled day of appointment nor were able to ascertain the reasons for their no‐show.
4. CONCLUSION
Donor selection for a new component using a less known technique could be a challenging task especially in countries like ours where awareness for voluntary donation is less and the donor pool itself is limited. However, an individualised approach with involvement of clinical staff could be a feasible approach in the initial phases of the pandemic that is to be conveyed with utmost sensitivity and good will in order to gain their trust and altruistic affection for the ones in need. A group approach in later phases targeting specific organisations could be adopted. A pandemic poses a unique situation, and a flexible evolving system could be a key to a successful CCP collection program.
CONFLICT OF INTEREST
The authors have no competing interests.
AUTHOR CONTRIBUTION
Conceptualization of study, initial search of the literature, review the searched literature preparation of initial draft, analysis of the data and final preparation of manuscript: Yashaswi Dhiman. Conceptualization of study and final review of manuscript: Poonam Coshic. Conceptualization of study, analysis of data and review of manuscript: Hem Chandra Pandey. Data collection, preparation of initial draft, data analysis and bibliography: Basanta Khatiwada. Coding reassessment and donor recruitment and data collection: Jasmeet Singh. Donor recruitment and data collection: Vikas Mehta and Sanjay Gupta.
Dhiman Y, Coshic P, Pandey HC, et al. Deterrents in recruitment of COVID‐19 convalescent plasma donors: Experience from a hospital‐based blood centre in India. Transfusion Medicine. 2021;31:149–154. 10.1111/tme.12768
REFERENCES
- 1. Yuan J, Li M, Lv G, Lu ZK. Monitoring transmissibility and mortality of COVID‐19 in Europe. Int J Infect Dis. 2020;95:311‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza a (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soo YO, Cheng Y, Wong R, et al. Retrospective comparison of convalescent plasma with continuing high‐dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arabi Y, Balkhy H, Hajeer AH, et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dodd LE, Follmann D, Proschan M, et al. A meta‐analysis of clinical studies conducted during the West Africa Ebola virus disease outbreak confirms the need for randomized control groups. Sci Transl Med. 2019; 27;11(520). 10.1126/scitranslmed.aaw1049. [DOI] [PubMed] [Google Scholar]
- 6. Clinical Management Protocol: COVID‐19 Government of India Ministry of Health and Family Welfare Directorate General of Health Services (EMR Division). 2020. .
- 7. Government of India . Ministry of Health and Family Welfare. Department of Health. The Drugs and Cosmetics Act, 1940 and the Drugs and Cosmetics Rules, 1945, as amended up to 30th June 2005. Schedule F. Part XIIB http://www.cdsco.nic.in/forms/contentpage1.aspx?lid=1888: 2019. p. 268–94.
- 8. Second Interim. National guidance to Blood Transfusion services in India in light of COVID‐19 Pandemic. India NBTCMoHaFWGo . 2020. p.12.
- 9. Ahn JY, Sohn Y, Lee SH, et al. Use of convalescent plasma therapy in two COVID‐19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35(14):e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Psaltopoulou T, Sergentanis TN, Pappa V, et al. The emerging role of convalescent plasma in the treatment of COVID‐19. Hemasphere. 2020;4(3):e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ronse M, Marí Sáez A, Gryseels C, et al. What motivates Ebola survivors to donate plasma during an emergency clinical trial? The case of Ebola‐Tx in Guinea. PLoS Negl Trop Dis. 2018;12(10):e0006885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar S, Azim D, Nasim S, Hashmi SH. Dwindling blood reserves: an ominous downside of COVID‐19 pandemic. Transfus Apher Sci. 2020;59(5):102818. 10.1016/j.transci.2020.102818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sahu KK, Raturi M, Siddiqui AD, Cerny J. "because every drop counts": blood donation during the COVID‐19 pandemic. Transfus Clin Biol. 2020;27(3):105‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong HK, Lee CK, Hung IF, et al. Practical limitations of convalescent plasma collection: a case scenario in pandemic preparation for influenza A (H1N1) infection. Transfusion. 2010;50(9):1967‐1971. [DOI] [PubMed] [Google Scholar]
- 15. Clary EG, Snyder M, Ridge R. Volunteers' motivations: a functional strategy for the recruitment, placement, and retention of volunteers. Nonprofit Manag Leadersh. 1992;2(4):333‐350. [DOI] [PubMed] [Google Scholar]
- 16. Andersen KJ, Klassen SA, Larson KF, et al. Recruitment strategy for potential COVID‐19 convalescent plasma donors. Mayo Clin Proc. 2020;95(11):2343‐2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bloch EM, Goel R, Wendel S, et al. Guidance for the procurement of COVID‐19 convalescent plasma: differences between high and low‐middle income countries. Vox Sang. 2021;116(1):18‐35. 10.1111/vox.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid‐19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Porretti L, Cattaneo A, Coluccio E, et al. Implementation and outcomes of a transfusion‐related acute lung injury surveillance programme and study of HLA/HNA alloimmunisation in blood donors. Blood Transfus. 2012;10(3):351‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma DC, Jain A, Woike P, et al. Female contribution in blood donation and alternatives: female contribution in blood donation and alternatives: fact & factual. Int Blood Res Rev. 2016;5(4):1‐8. [Google Scholar]


