Abstract
Identifying the proteins associating with RAS oncoproteins has great potential, not only to elucidate how these mutant proteins are regulated and signal, but also to identify potential therapeutic targets. Here we describe a detailed protocol to employ proximity labeling by the BioID methodology, which has the advantage of capturing weak or transient interactions, to identify in an unbiased manner those proteins within the immediate vicinity of oncogenic RAS proteins.
Keywords: BioID, BirA, proximity labeling, interactome, KRAS, HRAS, NRAS
1. Introduction
The BioID-mediated proximity-labeling approach is an emerging proteomic methodology for unbiased detection of potential protein-protein interactions1. This technology is based on fusing BirA*, the E. coli biotin ligase BirA harboring an R118G mutation, in-frame to a protein-of-interest to covalently affix biotin when cells are treated with exogenous biotin to proteins within ~10 nm, a physiological distance for maintaining protein interactions2,3,4. With the introduction of a new generation of BirA biotin ligases, for example, TurboID and mini TurboID that have a faster biotinylation kinetics and increased efficiency in comparison to BioID suffice for detection of protein-protein interactions5. Biotinylated proteins are then affinity captured with streptavidin and subjected to liquid chromatography coupled to tandem mass spectrometry (LC/MS/MS) to unmask their identity. One of the distinctive feature of BioID in comparison to more traditional approaches, such as coimmunoprecipitation, is detection of weak and transient interactions, as there is no need to maintain a protein complex throughout the enrichment process4. Given these advantages, we employed BioID to identify proteins proximal to each of the oncogenic (G12V) RAS isoforms KRAS(4b), NRAS, and HRAS (Fig. 1). We then benchmarked labelled proteins against a common RAS effector, Raf1, to nominate proteins to the RAS interactomes6. This approach reduced the complexity of the proteome to those proteins most likely to mediate oncogenic RAS function, namely those within the immediate vicinity. The identified interactome can then inform any number of research directions. For example, the small size of the interactome is ideal for generating sgRNA libraries for unbiased screening, as we did to identify KRAS-specific mediators of oncogenic signaling6 or for either in vivo tumor studies or dual-targeting sgRNA libraries that are limited in library size, and so forth. Given the many uses of this approach, we describe here how we performed BioID with oncogenic RAS mutant proteins.
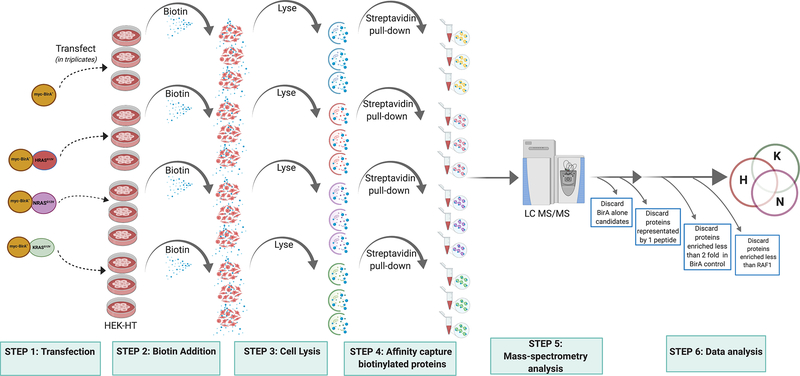
Fig. 1.
A flow-chart showing the overall method to perfom RAS BioID. HEK-HT cells are transfected with myc-BirA*-HRASG12V, myc-BirA*-NRASG12V and myc-BirA*-KRASG12V plasmid constructs in three biological replicates. Exogenous biotin is added to the cells and incubated for 24hrs. Cells are then lysed and subjected to streptavidin affinity capture and eluates are processed for mass-spectrometry analysis for detection of protein interactomes of RAS oncoproteins. All samples are processed in three independent biological replicates until the mass-spectrometry step. The proteomic dataset is analyzed for components that are shared or uniquely identified in all the three RAS isoform interactomes.
2. Materials
Prepare all solutions using nuclease-free water for downstream mass-spectrometry analysis (Invitrogen). Prepare and store all reagents at room temperature (unless indicated otherwise).
pcDNA3.1 myc-BioID vector system (Addgene# 35700).
pBABE-puro vector (Addgene# 1764).
Fugene 6 Transfection Reagent (Promega Corporation).
HEK-HT cells7, although other cell lines can be employed for analysis by optimizing culture conditions.
Biotin Solution: 1 mM biotin (Sigma# B4501) dissolve in serum-free DMEM medium for exogenous addition of biotin to cells. Prepare fresh each time before use.
1X PBS.
Cell Lysis Buffer: 50 mM Tris pH7.5; 500 mM NaCl; 0.2% SDS; Protease inhibitor tablet-EDTA free (Roche); 1 mM EDTA; 1 mM PMSF; 1 mM DTT. Prepare fresh cell lysis buffer before use. PMSF and DTT should be added right before use.
Triton X-100.
Table-top centrifuge.
1.5 ml and 2 ml tubes.
Heat block.
Magnetic Stand.
Rotator Mixer.
Wash Buffer 1: 2% SDS. Prepare fresh before use.
Wash Buffer 2: 0.5% Triton X-100; 1 mM EDTA; 500 mM NaCl; 50 mM Tris pH 7.5. Prepare fresh before use.
Wash Buffer 3: 0.5% IPEGAL-CA630; 1 mM EDTA; 50 mM Tris pH 7.5. Prepare fresh before use.
Dynabeads My One Streptavidin C1 Beads (Thermofisher #65001).
2X Lamelli Sample Buffer.
BSA Blocking buffer: 5% BSA in 1X TBSTX (1X Tris Buffered saline; 0.1% TritonX-100).
Powdered Milk Blocking Buffer: 5% powdered milk in 1X TBST (1X Tris Buffered saline; 0.1% Tween20). Prepare fresh before use.
1X TBSTX (20 mM Tris pH 7.5; 150 mM NaCl; 0.1% w/v TritonX-100).
1X TBST (20 mM Tris pH 7.5; 150 mM NaCl; 0.1% w/v Tween20).
Myc-tag antibody (Cell Signaling Technology #2276).
Pierce High Sensitivity Streptavidin-HRP (ThermoFisher# 21130).
Trifluroacetic acid [TFA].
Acetonitrile LC/MS grade [ACN].
Maximum Recovery LC vials.
Yeast alcohol dehydrogenase (Sigma).
NanoACQUITY UPLC system (Waters) coupled to a QExactive Plus high resolution accurate mass tandem mass spectrometer (ThermoFisher).
Software Packages: Rosetta Elucidator v.4 (Rosetta Biosoftware, Inc.); Swissprot database
3. Methods
3.1. myc-BirA*RAS fusion expression constructs
Myc-BirA*RAS fusion proteins are available at Addgene.
Each of the three RAS transgenes were cloned into the multiple cloning site (BamH1 and EcoRI restriction sites) of myc-BirA* pcDNA3.1 vector system.
A similar cloning strategy can be employed to introduce any desired RAS cDNA into the pcDNA3.1-myc-BirA* expression vector.
3.2. Transfection of myc-BirA*-RASG12V constructs and biotin addition
Split HEK-HT cells one day before transfection (see Note 1). For each condition, a total of three 10 cm tissue culture plates are required to capture the maximum number of interactors with statistical significance and one plate is needed for immunoblot analysis (see Note 2).
At ~80–90% confluency, transfect 6 μg of the desired pcDNA3.1 myc-BirA*-RASG12V construct(s) using 18 μl of the Fugene 6 reagent according to the manufacturer’s protocol. As a control, transfect 6 μg of the pcDNA3.1-myc-BirA* empty vector into a separate plate of cells.
After 24 hours, add 50 μM of 1 mM Biotin Solution, and incubate for another 16–24 hours (see Note 3).
3.3. Lysis of cultured cells for immunoblot analysis
Wash cells with 10 ml 1X PBS three times to remove any excess biotin present in the culture medium.
Add 500 μl of Cell Lysis Buffer to each 10 cm plate and swirl plate for 3 to 5 minutes at room temperature (see Note 4).
Transfer lysed cells from each plate to a pre-chilled 1.5 ml microfuge tube (one tube per plate).
Add 200 μl 2% Triton-X100 and pipette the solution to mix the lysate.
Incubate the mixture by end-to-end rotation for 30 minutes at 4°C.
After 30 minutes of incubation, add equal volume of chilled 50 mM Tris pH 7.5 and mix by repeated pipetting.
Subject the lysate to centrifugation at 12,500 g for 20 minutes at 4°C.
Transfer the lysates to new, pre-chilled 2 ml microfuge tubes and proceed to bead incubation step.
Protein quantification should be performed at this point to ensure equal amounts of total protein are incubated in all of the samples to be tested. A minimum of 1 mg of total protein lysate is required but higher concentrations (5–10 mg) yield better results (see Note 5).
3.4. Streptavidin bead preparation
This step should be performed immediately after the cell lysis step above. Transfer 50 μl of streptavidin magnetic beads in a fresh 1.5 ml microfuge tube for each sample. Incubate the tubes on a magnetic stand for 3 minutes at room temperature and discard the clear solution.
Remove the tubes from the magnetic stand and equilibrate the beads with addition of 1 ml of Cell Lysis Buffer followed by end-to-end rotation for 5 minutes at room temperature.
Place the tubes on the magnetic stand and incubate again for 3 minutes. Discard the lysis buffer and transfer the lysate into a new 2 ml microfuge tubes containing the equilibrated beads.
Incubate the bead-lysate mixture by end-to-end rotation overnight at 4°C.
3.5. Pull down of biotinylated proteins
Perform all steps at room temperature unless indicated otherwise. Incubate tubes on the magnetic stand for 5 minutes. Carefully discard all the lysate without disturbing the beads.
Wash the beads by adding 1 ml of Wash Buffer 1, followed by end-to-end rotation for 5 minutes.
Incubate the mixture on the magnetic stand for 3 minutes, then carefully remove buffer with a micropipette.
Wash the beads by adding 1 ml of Wash Buffer 2, followed by end-to-end rotation for 5 minutes.
Incubate the mixture on the magnetic stand for 3 minutes, then carefully remove buffer with a micropipette.
Wash the beads by adding 1 ml of Wash Buffer 3, followed by end-to-end rotation for 5 minutes.
Incubate the mixture on the magnetic stand for 3 minutes, then carefully remove buffer with a micropipette.
Wash the beads by adding 1 ml of 50 mM Tris pH7.5, followed by end-to-end rotation for 5 minutes.
Incubate the mixture on the magnetic stand for 3 minutes, then carefully remove buffer with a micropipette.
Add 50 μl 2X Lamelli Sample Buffer supplemented with 2 mM biotin to the beads and mix by pipetting the mixture up and down 5 times using a cut 200 μl pipette tip (see Note 6).
Heat the beads at 90°C in a pre-heated heating block for 5 minutes to release the bound proteins from the beads (see Note 7).
Place the tubes on the magnetic stand for 5 minutes and then transfer the eluate to a pre-chilled 1.5 ml microfuge tube and immediately freeze on dry-ice (see Note 10).
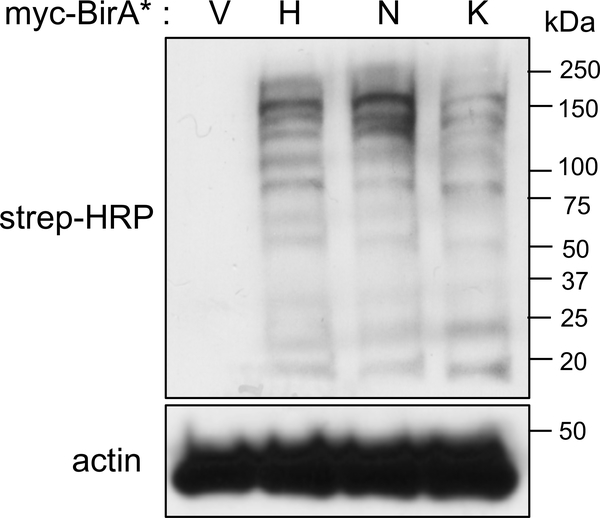
Prior to performing LC/MS/MS analysis, analyze the eluate for biotin labeling (Fig. 2) and protein-expression, as described next (see Note 8, 11).
Fig. 2.
Immunoblot confirming the biotinylation profiles of cells expressing myc-BirA* vector alone (V), myc-BirA*-HRASG12V (H), myc-BirA*-NRASG12V (N), and myc-BirA*-KRASG12V (K) on exogenous addition of biotin that is detected by streptavidin-HRP antibody. Actin is used as a loading control.
3.6. Confirming the biotin- labeling activity of the myc-BirA*-RASG12V fusion protein(s) by immunoblot
Load ~5 to 10 μl of the eluate from one tube per condition into two 10% SDS-polyacrylamide gels, one for detecting biotin-labelled proteins as described here and one for detecting myc-BirA*-RASG12V fusion protein as described next in section 3.6b. Separate proteins by electrophoresis.
Perform protein transfer to a PVDF membrane from both gels using a Turbo Blot system (BioRad) according to the manufacture’s protocol.
Remove and then block one membrane in BSA Blocking Buffer for 30 minutes to 1 hour at room temperature, the other is processed as described next in section 3.7 (see Note 9).
To detect biotin-labeled proteins, incubate the membrane with the Strep-HRP antibody diluted 1:20,000 in BSA Blocking Buffer for 1 hour at room temperature.
Wash the membrane three times in 1X TBST, each for 10 minutes.
Process the membrane for chemiluminescence detection either on X-ray film or by digital acquisition.
3.7. Confirming expression of the myc-BirA*-RASG12V fusion protein(s) by immunoblot
Gel electrophoresis and transfer of proteins are performed as described above.
Incubate the membrane in Powdered Milk Blocking Buffer for 1 hour.
To detect expression of the fusion protein, incubate the membrane with the myc antibody diluted 1:5,000 in Powdered Milk Blocking Buffer for either 1 hour at room temperature or overnight at 4°C.
Wash the membrane three times in 1X TBST, each for 10 minutes.
Process the membrane for chemiluminescence detection either on X-ray film or by digital acquisition.
3.8. Quantitative LC MS/MS analysis
Resolve 50 μl of each sample (namely the three remaining samples per condition) by SDS-PAGE (4–12% Invitrogen NuPAGE; MES buffer) for 3 minutes, followed by fixation and Coomassie staining.
Excise bands and perform in-gel tryptic digestion using standard methodologies (see https://genome.duke.edu/sites/genome.duke.edu/files/In-gelDigestionProtocolrevised_0.pdf).
Dry peptides in a SpeedVac, then reconstitute each sample in 40 μl of 1% TFA/2% ACN containing 25 fmol/μl yeast alcohol dehydrogenase surrogate standard and transferred to Maximum Recovery LC vials. A quality control (QC) pool is prepared by mixing equal volumes of all samples.
Analyze samples using a nanoACQUITY UPLC system coupled to a QExactive Plus high resolution accurate mass tandem mass spectrometer via a nanoelectrospray ionization source.
Import data into Rosetta Elucidator v.4 (Rosetta Biosoftware, Inc.). Align based on the accurate mass and retention time of detected ions (“features”) using PeakTeller algorithm in Elucidator. Calculate relative peptide abundance based on area-under-the-curve (AUC) of the selected ion chromatograms of the aligned features across all runs.
Search MS/MS data against a custom Swissprot database with Homo sapiens taxonomy with additional proteins, including yeast ADH1 and bovine serum albumin, as well as an equal number of reversed-sequence “decoys” for false discovery rate determination (40,546 total entries). Search database and score peptides using the PeptideProphet algorithm, annotate data at a 0.9% peptide false discovery rate.
3.9. Data analysis
In order to assess technical reproducibility, % coefficient of variation (%CV) for each protein is calculated across the four injections of a QC pool interspersed throughout the samples.
To assess biological variability, %CVs are measured for each protein across the individual analyses.
It is possible that differences in expression across treatment groups reflect the biotin capture efficiency or the overall expression level of BirA fusion proteins.
To account for these variables, two additional normalizations of the data are required.
First, to control for the variability in streptavidin pulldown, normalize data to the mean intensity of peptides from endogenously-biotinylated carboxylases (see Note 12).
Second, normalize the data to the levels of BirA* protein across each sample.
However, we found that normalization to BirA* was effective in normalizing the total intensity of Ras proteins (including shared peptides) across the samples, therefore we suggest using the BirA*-normalized data for initial statistical analysis.
As an initial statistical analysis, calculate fold-changes between groups based on the average fold-changes for each comparison (e.g. HRAS versus control, HRAS versus NRAS, etc.) for proteins in which a minimum of 2 peptides are recovered.
An unpaired t-test (for example in Excel) on the log2-transformed data should be performed for each of these comparisons.
The resulting dataset can then be analyzed to identify proteins specific to different RAS isoforms. For example, to select for proteins specifically enriched in HRAS-expressing cells, proteins were filtered to include those quantified by <30% CV across QC pools, had 2-fold greater expression (w/p<0.05) in HRAS versus control, and a 2-fold or greater expression and p<0.05 in both HRAS versus NRAS and HRAS versus KRAS comparisons.
Acknowledgements
This work was supported by R01CA123031 (CMC) and by a Pancreatic Cancer Action Network-NCI Fredrick National Laboratory for Cancer Research KRAS Fellowship and K99CA248495 (H.A.)
Notes
At least 2–3 ×106 cells are required for the analysis.
The experiment should be carried out in triplicates.
The labeling time should be optimized to avoid non-specific binding. We found that labeling for 24 hours yielded the maximal amount of labeling, but this length of time comes at the risk of non-specific labeling. A time-course can be performed to yield optimal labeling conditions dependent on the cell-type.
The lysis step at room temperature allows for better solubilization of proteins from cell membranes allowing for detection of transient or weak interactions.
BCA protein quantification should be performed to load equal amounts of total protein on the streptavidin beads.
Addition of 2 mM Biotin to the SDS loading buffer allows increased recovery of proteins from the streptavidin beads.
Heating samples with intermittent vortexing at high speed results in better recovery of proteins.
Eluates should always be tested to confirm biotin labeling and protein expression.
BSA Blocking Buffer for streptavidin-HRP immunoblots is used instead of Powdered Milk Blocking Buffer, as the latter hinders in binding to the membrane.
Freeze-thawing of eluates for downstream processing should be completely avoided and should be thawed only for final LC/MS/MS analysis.
Other tests of functionality of the fusion protein(s) should be performed as needed beyond confirming biotinylation and expression, such as localization by immunofluorescence, transformation by proliferation assays etc.
A few of the identified hits include the endogenously-biotinylated carboxylases: pyruvate carboxylase; propionyl-CoA carboxylase α-subunit; and β-methylcrotonyl CoA carboxylase α-subunit that are not true interactors of RAS proteins but rather can be used as a surrogate to determine equivalent levels of biotinylation across the samples.
REFERENCES
- 1.Trinkle-Mulcahy L Recent advances in proximity-based labeling methods for interactome mapping. F1000Research 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon K & Beckett D Function of a conserved sequence motif in biotin holoenzyme synthetases. Protein Sci. Publ. Protein Soc 9, 1530–1539 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varnaitė R & MacNeill SA Meet the neighbors: Mapping local protein interactomes by proximity-dependent labeling with BioID. Proteomics 16, 2503–2518 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roux KJ, Kim DI, Raida M & Burke B A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol 196, 801–810 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branon TC et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol 36, 880–887 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adhikari H & Counter CM Interrogating the protein interactomes of RAS isoforms identifies PIP5K1A as a KRAS-specific vulnerability. Nat. Commun 9, 3646 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn WC et al. Creation of human tumour cells with defined genetic elements. Nature 400, 464–468 (1999). [DOI] [PubMed] [Google Scholar]