Abstract
The current COVID‐19 pandemic is a global concern. The recent introduction of vaccines has provided a reason for hope, but new problems, such as vaccine hesitancy, have arisen. One of the most important of these issues is the safety of vaccines for pregnant women. In this article, we collected worldwide indications for vaccination, including women who are pregnant or who wish to become pregnant, and reports of adverse reactions to COVID‐19 vaccination. The Japan Society of Obstetrics and Gynecology and the Japanese Society of Infectious Diseases in Obstetrics and Gynecology have published recommendations for the vaccination of pregnant women with a COVID‐19 vaccine. The guidelines are as follows: (1) pregnant women should not be excluded from vaccination; (2) informed consent should be obtained before vaccination; (3) healthcare workers and pregnant women with complications such as diabetes, hypertension, and obesity should be vaccinated preferentially; (4) vaccination should be avoided until 12 weeks of gestation during organogenesis; (5) spouse and family members should be vaccinated actively; and (6) nursing mothers are not particularly affected. This policy has been adopted in government guidelines. Additional efforts should be made to protect pregnant women from infection and severe illness with COVID‐19 by eliminating vaccine hesitancy.
Keywords: adverse effects, COVID‐19, pregnancy, vaccine
Introduction
Vaccination is the most effective way to control the COVID‐19 pandemic. Its success relies on the development of safe and effective vaccines as well as public acceptance. Technical innovation of vaccine development in the past century and understanding of the pathophysiology of COVID‐19 enabled clinical use of anti‐SARS‐CoV‐2 vaccines within 6 months of the onset of the COVID‐19 epidemic. 1 , 2
To date, three vaccines are administered worldwide. Among them, the Pfizer and Moderna mRNA vaccines are approximately 95% effective in preventing the development of COVID‐19 in adults 16 or 18 years of age and older. 3 , 4 , 5 AstraZeneca's chimpanzee adenovirus vector vaccine has a protective effect (VE) of approximately 60%–90% against the development of COVID‐19 in adults 18 years of age and older. 6 , 7 No serious adverse events have been identified so far for any of these vaccines.
However, whether these vaccines are effective in preventing infection in others, that is, whether herd immunity can be acquired, is still unknown. At the very least, the benefits to the individual who receives the vaccination are becoming clear. Priority for vaccination should be given to medical staff and to people who are at risk of severe or life‐threatening illness due to COVID‐19. Of course, the possibility of future reports of serious adverse events or antibody‐dependent enhancement (ADE) of infection must be carefully considered. Nevertheless, when the risk of unknown serious adverse events, which is expected to be less than one in tens of thousands, is weighed against the risk of contracting COVID‐19 in Japan, where the infection is spreading, the benefits of vaccination far outweigh the risks.
Pregnancy and the possible risk of COVID‐19
In Japan, we are collecting information on the severity and clinical courses of patients contracting COVID‐19 during pregnancy. Although the general prognosis, including mortality rates, is not changed in age‐matched nonpregnant female patients in Japan, 8 there have been several case reports of rapid progression to respiratory failure that required ventilator or extracorporeal membrane oxygenation (ECMO) management in the third trimester. In the United States, the Centers for Disease Control and Prevention (CDC) and the American Journal of Obstetrics and Gynecology (AJOG) categorize pregnancy as a risk factor for severe disease. In addition, COVID‐19 infection is an independent risk factor for premature birth and cesarean section due to fetal distress and maternal indications. In addition, there are restrictions on the choice of therapeutics during pregnancy. Recently, reported new variant viruses spreading from the UK and South Africa are believed to be highly contagious and can easily infect young people of childbearing age. 9
Our recommendations
The Japan Society for Infectious Diseases in Obstetrics and Gynecology (JSIDOG) and Japan Society of Obstetrics and Gynecology (JSOG) make the following proposals in the current situation on January 27, 2021. 10
The safety of the COVID‐19 vaccine in pregnant women, especially regarding mid‐ to long‐term adverse reactions and fetal and neonatal safety, is currently unknown.
Considering the current situation of the pandemic, we advocate that pregnant women should not be excluded from vaccination.
Before vaccination, pregnant women should be fully informed that the long‐term adverse reactions are unknown and that the safety of the vaccine for the fetus and the child is not yet established.
The safety of the vaccine for the fetus and the baby should be fully explained before vaccination.
Inoculation should be performed after obtaining consent, and 30 minutes of in‐hospital observation after vaccination is recommended.
Vaccination should be avoided during organogenesis (up to 12 weeks of pregnancy).
Vaccination should be performed at an obstetrics and gynecology facility to check fetal heart movement before and after administration.
For the following people, vaccination is recommended. Pregnant healthcare workers who are at high risk of infection and those with underlying diseases, such as obesity and diabetes, may be at risk of severe disease.
Vaccination should be considered for partners of pregnant women to prevent infection in the home.
Those who wish to become pregnant should be vaccinated before becoming pregnant, if possible. Long‐term contraception is not required after vaccination because anti‐SARS‐CoV‐2 vaccines are not live vaccines.
As each patient has a different background, they are strongly recommended to consult their obstetrician if they have any questions.
Safety of anti‐SARS‐CoV‐2 vaccines
In the United States, the Pfizer vaccine was approved by the Food and Drug Administration (FDA) on December 11, 2020, and vaccination began rapidly on the December 14. Twenty‐one cases of anaphylactic reactions after Pfizer vaccination among 1 893 360 doses have been reported to the US Post‐Vaccination Adverse Event Reporting System (VAERS). The median age of the 21 patients was 40 years, ranging from 27 to 60 years, and 19 of the 21 individuals (90%) were female. Fifteen of the 21 cases occurred within 15 min of vaccination; the median time course was 13 min, ranging from 2 to 150 min. Seventeen of the 21 patients had a history of allergy, and seven of them had a history of anaphylactic reaction. Eighteen of the 21 patients started treatment with intramuscular adrenaline, and one patient started treatment with subcutaneous adrenaline. Three of the 21 patients were admitted to the intensive care unit (ICU), one was admitted to the general hospital ward, and 17 were treated in the emergency room. At the time of this report, 20 of the patients had recovered and returned to home. 11
For the Moderna vaccine, almost identical results have been reported. A total of 10 cases of anaphylactic reactions after the Moderna vaccination were reported to the VAERS. During this period, 4 041 396 doses of the Moderna vaccine were administered as the first dose. The median age of the 10 patients was 47 years, with a range of 31–63 years. All 10 patients (100%) were female. Nine of the 10 cases occurred within 15 min of vaccination; the median time course was 7.5 min, with a range of 1–45 min. Nine of the 10 patients had a history of allergy, and five of them had a history of an anaphylactic reaction. All 10 patients were started on intramuscular adrenaline. Five of the 10 patients were admitted to the ICU (four of them were intubated), one was admitted to the general hospital ward, and four were treated only in the emergency room. As of the time of this report, eight of these patients had recovered and returned home.
Types of vaccines
In the present battle against COVID‐19, humanity has an entirely new weapon, RNA vaccines. 12 Conventional vaccines have been divided into three types: live vaccines, in which viruses or bacteria are live but weakened; inactivated vaccines, in which these pathogens are killed; and component vaccines, in which the antigens are purified from these pathogens or chemically synthesized. All three types of anti‐COVID‐19 vaccines currently in use worldwide are based on new molecular biological technologies. The Pfizer‐BioNTech and Moderna vaccines contain mRNA encoding viral spike proteins encased in lipid nanoparticles, while the AstraZeneca vaccine contains fragments of SARS‐CoV‐2 cDNA in an adenovirus vector 13 that does not replicate in the human body. When both are injected intramuscularly, macrophages and dendritic cells locally take them up and translate them into proteins, which are digested into peptides and finally presented as antigens. Antigenic viral peptides are presented with class I or class II MHC molecules, which are recognized by helper T (Th) cells to induce B cells to produce neutralizing antibodies and cytotoxic T cells to destroy infected cells.
Humoral immune responses to SARS‐CoV‐2 are mediated by neutralizing antibodies directed to viral surface glycoproteins, such as the spike glycoprotein and the nucleocapsid proteins. 5 However, not every antibody has neutralizing activity, and some of them may enhance viral infection. To solve this problem, mRNA encoding a spike protein that binds to the receptor ACE2 was chemically synthesized and encapsulated in lipid nanoparticles to create an RNA vaccine. Although COVID‐19 was the first disease for which an RNA vaccine was clinically applied to humans, the vaccine development has been a long process with a background of technological innovation. Both AstraZeneca's adenovirus vector vaccine and the Pfizer/BioNTech, Moderna mRNA vaccines evoke cellular immune responses mediated by Th1 cells and CD8+ cytotoxic T cells that recognize digested viral antigens presented on class I MHC molecules (Figure 1). In contrast, the induction of regulatory T cells has not been reported to date in vaccine‐induced immune responses. 14
Figure 1.
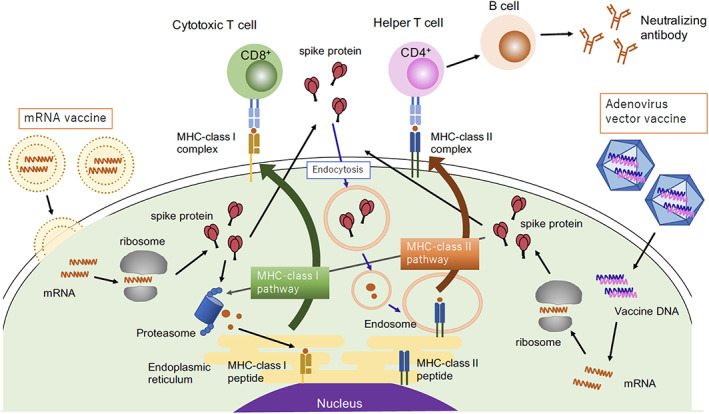
How SARS‐CoV‐2 vaccines are processed in antigen‐presenting cells and recognized by immune cells
Other types of anti‐COVID‐19 vaccines, including inactivated viruses (SinoVac and SinoPharm), adenovirus serotypes 5 and 26 (Sputnik), and DNA vaccines (AnGes), are now in use or phase I/II clinical trials. 5
How many doses of the COVID‐19 vaccine do I need to receive?
Both the Pfizer and Modena vaccines are recommended to be inoculated with doses 21 days apart. Although a single dose induces neutralizing antibodies at some level, effective induction is achieved after a second vaccination. 15 For sufficient prime‐boost effects, the same vaccine must be administered twice. If the vaccine recipient claims any adverse effects, including severe allergic reactions or intolerable pain after the first inoculation, he or she could try two doses of another vaccine.
Test dose administration
If vaccine recipients have a history of anaphylaxis, they might have a higher frequency of anaphylaxis after anti‐SARS‐CoV‐2 vaccine administration. The CDC recommends test dose administration of 0.1 ml under careful observation. After 30 min or more of observation, asymptomatic recipients can be inoculated with a full dose of the vaccine. 16
How long are the vaccines effective?
At this time, it is not known how long the vaccine will be effective in preventing viral infection or whether it will be effective against the new mutant strains. It has been reported that the number of memory B cells does not decrease even when antibody titers decrease and that antibodies cross‐reactive to mutated viruses are produced by hypermutation in memory B cells, but clinical efficacy remains obscure. If novel strains become resistant to previous vaccinations, additional inoculation with newly designed vaccines is possible to induce additional neutralization effects. In addition, mRNA can be manufactured against novel strains based on their sequences without induction of anti‐vector antibodies. In contrast, in the cases of adenovirus (or other virus) vector vaccines, additional vaccinations are limited due to the production of antibodies against the vector itself.
Genomic incorporation of mRNA vaccines
Some people worry that injected mRNA will be incorporated into the genome, but in principle, this is never probable. mRNA needs reverse transcriptase to be incorporated into genomic DNA; however, human cells do not express reverse transcriptase. The placenta expresses several endogenous retroviruses, but they express no active reverse transcriptase. 17 If vaccinated people are infected with retroviruses such as human immunodeficiency virus (HIV) and human T‐lymphotropic virus (HTLV), it is possible that exogenous mRNA can be reverse transcribed by these retroviruses, but there are no reports of SARS‐COV‐2 being reverse transcribed into genomes in HIV‐infected patients with COVID‐19. There have been many cases of human and animal models of retroviral infections. However, there have been no reports of the integration of RNA sequences from other viruses or abundant host mRNAs into their genomes. The FACT check states that mRNAs are not incorporated into DNA, and mRNA integration is a malicious hoax.
Vaccine‐induced infertility
Another famous rumor is that the vaccine not only targets the coronavirus spike protein as designed but also causes infertility by producing antibodies reacting with the trophoblastic protein syncytin‐1. Because the viral protein and the placental protein are structurally similar, antibodies to the coronavirus are thought to inhibit the development of the placenta and subsequently cause infertility. However, this description is completely incorrect. As reported by Lu‐Culligan and Iwasaki, no molecular similarities were observed between coronavirus spike proteins and placental syncytin‐1. 18 Furthermore, sera obtained from women with COVID‐19 did not react with syncytin‐1 or other placental antigens. Clinically, many women became pregnant after recovery from COVID‐19 or vaccination. In summary, immune responses against natural SARS‐CoV‐2 infection or vaccination do not impair fertility. 18
ADE of infection
ADE is a rare phenomenon observed in several animal models and humans. After infection or vaccination, antibodies produced by the host against various pathogenic viruses interact with the pathogen but aggravate the infection when the host is subsequently infected with the same pathogen. This phenomenon is mediated by the production of antigen‐specific antibodies that do not neutralize the pathogenic viruses and are incorporated into host cells via Fc receptors. Among various mammalian coronaviruses, feline infectious peritonitis virus (FIPV) causes severe infectious peritonitis in cats after a second infection by ADE. 19 Fortunately, no ADE has been reported in human coronaviruses, but during the development phase of the SARS vaccine, a theoretical possibility of ADE at the T cell level has been reported in rhesus monkeys. 20 As we cannot completely discount the possibility of ADE, careful observation of a second infection with COVID‐19 among naturally infected or vaccinated people is indispensable. 21
Vaccination policies of other countries
According to the American College of Obstetricians and Gynecologists (ACOG) and the American Advisory Committee on Immunization Practices (ACIP), data on the safety of the COVID‐19 vaccine administered during pregnancy are still under investigation, but the ACIP did not exclude pregnant subjects from vaccination.
As pregnant and lactating women were excluded from Pfizer's and Moderna's COVID‐19 vaccine clinical trials, there are still no solid safety data available to know if the vaccine is safe for pregnant or lactating people. However, according to Moderna, rats vaccinated with the Moderna COVID‐19 vaccine before or during pregnancy have not shown any safety concerns. In addition, as of November 14, 2020, Pfizer reported that 23 pregnant individuals, including 12 in the vaccine group, were inadvertently enrolled in the clinical trial. These pregnancies are now progressing without problems. Moderna reported 13 pregnancies in the clinical trial, including six in the vaccine group and seven in the placebo group. All of these pregnancies are so far uneventful for both mothers and fetuses. 22 , 23 The Joint Committee on Immunization (JCVI) also advises that there is no risk of vaccination to lactating women. Furthermore, considering the vaccine's mechanism of action and safety profile, the COVID‐19 mRNA vaccine is not considered to increase the risk of infertility. 24
In the UK, the JCVI advocated that the evidence is insufficient to recommend routine use of the COVID‐19 vaccine during pregnancy. However, vaccination may be considered during pregnancy for conditions in which COVID‐19 infection may cause serious illness (e.g., after organ transplantation, immunosuppressive drug use, dialysis therapy, and other immunosuppressive conditions).
The British Society of Obstetricians and Gynaecologists (BSOG) states that vaccination during pregnancy should be considered for women who are front‐line health or social care workers or who have an underlying condition that puts them at very high risk of serious complications if infected with COVID‐19. It also states that women who are trying to become pregnant do not need to avoid pregnancy after vaccination. 25
Possible induction of autoimmune disorders
Compared to conventional methods, mRNA vaccines are expected to be more effective in preventing infection. However, the disadvantage of mRNA vaccines is that the frequency of side effects cannot be predicted since they have not yet been commercialized. Several researchers are concerned that injected RNA may act as a neoantigen or modify autoantigens to induce autoimmune diseases. 26 However, from experimental and clinical observations, the frequency of allergies and autoimmune diseases is expected to be quite rare since the administered mRNA will eventually be degraded. Both RNA vaccines have been administered to over 30 million people in the USA, and there have been no reports of autoimmune diseases among them.
Organogenesis and vaccination
Currently, over 30 types of vaccines are approved in Japan. Of these, six (BCG, mumps, rubella, varicella‐zoster virus [VZV], yellow fever, and rotavirus) are live vaccines, all of which are contraindicated in pregnant women. Among them, only the anti‐rubella vaccine is potentially teratogenic. However, vaccine strains possess low virulence, and there have been no reports of fetal malformation after vaccination during pregnancy. Commercially available anti‐SARS‐CoV‐2 vaccines are mRNA vaccines or adenovirus vector vaccines, and the possibility of replication in the placenta or uptake by the fetus is not probable. However, we cannot completely rule out the integration of exogenous RNA into the host genome by endogenous reverse transcriptase originating from endogenous retroviruses in the placenta, and we recommend that vaccination be avoided during the period of organogenesis. It is not necessary to hesitate to vaccinate people who wish to become pregnant or for vaccinated women to avoid pregnancy after receiving the mRNA COVID‐19 vaccine.
Vaccination for spouses, parents, and older children
In both Japan and foreign countries, husbands whose wives are pregnant are important targets for vaccination to prevent intrafamilial transmission. If the pregnant woman has older children, transmission from the children to the mother can occur, but vaccination for children is not yet approved. If a woman is living together with the elderly or sees them frequently, they should be vaccinated. This is also recommended considering that elderly individuals are more likely to become seriously ill if they contract COVID‐19.
Indication for COVID‐19 vaccination in Japan
The Ministry of Health, Labour, and Welfare (MHLW) of Japan officially licensed Pfizer's vaccine on February 14. 27 To achieve herd immunity, the government recommends that citizens aged 16 and over be vaccinated with their consent but excludes pregnant women from mandatory efforts. However, this policy does not mean that pregnant women should refrain from being vaccinated but rather that they should not be denied access to vaccination if the situation warrants it. In other words, it is taking the same position as in other countries that healthcare workers and pregnant women at risk, including those with obesity, diabetes, and so on, should be actively vaccinated. We truly appreciate that the government officials are considering our recommendations (Table 1).
Table 1.
Priority for COVID‐19 vaccination by the Ministry of Health, Labour, and Welfare (February 14, 2021)
|
Concluding Remarks
Generalized vaccination is the most essential way to control the current COVID‐19 pandemic. Although we lack enough clinical information of long‐term side effects, we can expect protective effects of vaccines to terminate this pandemic because many countries decreased numbers of new cases, severely ill patients as well as mortalities. Considering that more than two million people worldwide have lost their lives to COVID‐19, it is essential to protect as many people as possible by vaccination. It is also essential to enlighten uninformed people including pregnant and nursing mothers who are dominated by a baseless aversion to vaccines by correct official information by government and academies.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
This manuscript was partially supported by the Ministry of Health, Labour and Welfare of Japan (grant number 20CA2033).
References
- 1. Gerberding JL, Haynes BF. Vaccine innovations ‐ past and future. N Engl J Med. 2021;384(5):393–6. [DOI] [PubMed] [Google Scholar]
- 2. Miyasaka M. COVID‐19 and immunity: quo vadis?. Int immunology. 2021. 10.1093/intimm/dxab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chagla Z. The BNT162b2 (BioNTech/Pfizer) vaccine had 95% efficacy against COVID‐19 ≥7 days after the 2nd dose. Ann Intern Med. 2021;174(2):JC15. [DOI] [PubMed] [Google Scholar]
- 4. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poland GA, Ovsyannikova IG, Kennedy RB. SARS‐CoV‐2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayakawa S, Komine‐Aizawa S, Mor GG. Covid‐19 pandemic and pregnancy. J Obstet Gynaecol Res. 2020;46(10):1958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Volz E, Hill V, McCrone JT, Price A, Jorgensen D, O'Toole Á, et al. Evaluating the effects of SARS‐CoV‐2 spike mutation D614G on transmissibility and pathogenicity. Cell. 2021;184(1):64–75.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. JSIDOG and JSOG Recommendation (in Japanese). [Cited 28 Feb 2021.] Available from URL: (http://www.jsog.or.jp/news/pdf/20210127_COVID19.pdf).
- 11. CDC . Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer‐BioNTech COVID‐19 Vaccine — United States, December 14–23, 2020. [Cited 28 Feb 2021.] Available from URL: (https://www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm).
- 12. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines ‐ a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AV, Dorrell L. Viral vectors as vaccine platforms: from immunogenicity to impact. Curr Opin Immunol. 2016;41:47–54. [DOI] [PubMed] [Google Scholar]
- 14. Grigoryan L, Pulendran B. The immunology of SARS‐CoV‐2 infections and vaccines. Semin Immunol. 2020;50:101422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kadire SR, Wachter RM, Lurie N. Delayed second dose versus standard regimen for Covid‐19 vaccination. N Engl J Med. 2021;384:e28. [DOI] [PubMed] [Google Scholar]
- 16. CDC . Interim Clinical Considerations for Use of mRNA COVID‐19 Vaccines Currently Authorized in the United States. [Cited 28 Feb 2021.] Available from URL: (https://www.cdc.gov/vaccines/covid‐19/info‐by‐product/clinical‐considerations.html).
- 17. Roberts RM, Ezashi T, Schulz LC, Sugimoto J, Schust DJ, Khan T, et al. Syncytins expressed in human placental trophoblast. Placenta. 2021. 10.1016/j.placenta.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu‐Culligan A, Iwasaki A. The False Rumors About Vaccines That Are Scaring Women. New York Times Opinion. [Cited 26 Jan 2021.] https://www.nytimes.com/2021/01/26/opinion/covid‐vaccine‐rumors.html?fbclid=IwAR1E04zmdaotQKr2Ncm.
- 19. Takano T, Yamada S, Doki T, Hohdatsu T. Pathogenesis of oral type I feline infectious peritonitis virus (FIPV) infection: antibody‐dependent enhancement infection of cats with type I FIPV via the oral route. J Vet Med Sci. 2019;81(6):911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou J, Wang W, Zhong Q, Hou W, Yang Z, Xiao SY, et al. Immunogenicity, safety, and protective efficacy of an inactivated SARS‐associated coronavirus vaccine in rhesus monkeys. Vaccine. 2005;23(24):3202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody‐dependent enhancement and SARS‐CoV‐2 vaccines and therapies. Nat Microbiol. 2020;5(10):1185–91. [DOI] [PubMed] [Google Scholar]
- 22. FDA Briefing Document . Moderna COVID‐19 Vaccine. [Cited 28 Feb.] Abailable from URL: (https://www.fda.gov/media/144434/download).
- 23. Rasmussen SA, Kelley CF, Horton JP, Jamieson DJ. Coronavirus disease 2019 (COVID‐19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American College of Obstetricians and Gynecologists . Vaccinating Pregnant and Lactating Patients Against COVID‐19. [Cited 28 Feb 2021.] Available from URL: (https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19).
- 25. Royal College of Obstetricians and Gynaecologists . Updated advice on COVID‐19 vaccination in pregnancy and women who are breastfeeding. [Cited 28 Feb.] Available from URL: (https://www.rcog.org.uk/en/news/updated‐advice‐on‐covid‐19‐vaccination‐in‐pregnancy‐and‐women‐who‐are‐breastfeeding/).
- 26. Vojdani A, Kharrazian D. Potential antigenic cross‐reactivity between SARS‐CoV‐2 and human tissue with a possible link to an increase in autoimmune diseases. Clinical immunology (Orlando, Fla). 2020;217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The Ministry of Health, Labor and Welfare of Japan . Announcement of SARS‐CoV‐2 Vaccines (in Japanese). [Cited 28 Feb 2021.] Available from URL: (https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00218.html


