Abstract
Aims
The present study aimed to evaluate the cost‐effectiveness of the 5‐day remdesivir regimen compared with standard of care among severe COVID‐19 patients in China, the evidence on which is essential to inform the necessity of securing access to remdesivir.
Methods
A dynamic transmission model that extended the susceptible–exposed–infected–recovered framework by incorporating asymptomatic, presymptomatic and waiting‐to‐be‐diagnosed patients was constructed to conduct the cost‐effectiveness analysis from the healthcare system perspective. To estimate epidemic parameters, the model was first calibrated to the observed epidemic curve in Wuhan from 23 January to 19 March 2020. Following the calibration, the infected compartment was replaced by 3 severity‐defined health states to reflect differential costs and quality of life associated with disease gravity. Costs and quality‐adjusted life year (QALY) outcomes of 9 million simulated people were accrued across time to evaluate the incremental cost‐effectiveness ratio of remdesivir. As robustness checks, an alternative modelling technique using decision tree, additional epidemic scenarios representing different epidemic intensities, and 1‐way parameter variations were also analysed.
Results
Remdesivir treatment cost CN¥97.93 million more than standard of care. Also, the net QALY gain from 5‐day remdesivir treatment was 6947 QALYs. As such, the incremental cost‐effectiveness ratio was CN¥14 098/QALY, substantially lower than the gross domestic product per capita threshold. The peak daily number of severe cases was 19% lower in the remdesivir treatment strategy. Overall, results were robust in alternative scenarios and sensitivity analyses.
Conclusion
Given the cost‐effectiveness profile, access to remdesivir for severe COVID‐19 patients in China should be considered.
Keywords: cost‐effectiveness, COVID‐19, hospitalized, remdesivir
What is already known about this subject
Remdesivir may have benefit for clinical improvement of COVID‐19 patients compared with standard of care.
What this study adds
The 5‐day remdesivir treatment is cost‐effective compared with standard of care for the treatment of severe COVID‐19 patients in China if priced the same as the international market.
Ensuring access to remdesivir for severe COVID‐19 patients may benefit the healthcare system in China.
1. INTRODUCTION
Over 30 million COVID‐19 cases have been confirmed worldwide as of 29 October 2020, accounting for >1 million deaths. 1 With the cases re‐surging in numerous parts of the world and its societal impacts still mounting differentially across countries, 2 identifying and delivering economic and effective pharmacological therapies for the patients remains an important task on top of current nonpharmacological interventions that are commonplace globally. 3
Recent meta‐analyses of randomized clinical trials (RCTs) provided evidence that remdesivir, an antiviral small molecule drug, might improve clinical manifestation among moderate to severe COVID‐19 patients. 4 An additional meta‐analysis suggested that remdesivir may reduce 14‐day mortality. 5 Remdesivir was also the only medication approved for hospitalized COVID‐19 patients by the US Food and Drug Administration as of 23 October 2020. However, direct evidence on mortality benefit of remdesivir is still at large. In the meantime, the hefty price tags of remdesivir create uncertainty in its value profiles. The acquisition costs of the 5‐day regimen of remdesivir set by the manufacturer vary across markets, totalling US$3120 for commercial payers in the USA and US$2340 for Medicare and public payers in other high‐income countries. 6 The list prices are within the cost‐effective range in the USA according to an analysis by the Institute for Clinical and Economic Review that assumed direct mortality benefit from remdesivir. 7
Outside of the USA, the cost‐effectiveness profiles of remdesivir have not been documented to our knowledge. As such, we aimed to conduct an economic evaluation of 5‐day remdesivir treatment plus standard of care (SoC) compared with SoC alone in the Chinese setting under different epidemic scenarios. Information on the cost‐effectiveness of remdesivir constitutes the evidence base for related regulatory, financial and clinical decision‐making, including the necessity of securing access and the choice of clinical pathways, thereby providing implications for policymaking by healthcare providers and reimbursement agencies.
2. METHODS
2.1. Setting
The study evaluated the incremental costs‐effectiveness ratio of the 5‐day remdesivir regimen from the healthcare system perspective in China. To that end, the study assessed the cost‐effectiveness of using remdesivir to treat severe hospitalized COVID‐19 patients compared with SoC. The analysis pertains to the policy question of whether it is cost‐effective to treat severe COVID‐19 patients with remdesivir in China, which is further related to whether the therapy should be accessible by severe patients. To extensively examine the cost‐effectiveness of remdesivir, several epidemic scenarios of adopting remdesivir were used, among which the reported epidemic intensity in Wuhan, China during January–March 2020 that represented the gravest local situation in China to date was considered the benchmark scenario.
2.2. Model
A dynamic compartment transmission model was constructed to simulate the public health, clinical, and economic outcomes under alternative courses corresponding to different interventions. The model was constructed with 2 processes. In the first process, the conventional susceptible–exposed–infected–recovered (SEIR) framework was extended to accommodate asymptomatic and presymptomatic infectivity associated with COVID‐19. 8 , 9 , 10 , 11 Specifically, a model with a susceptible–exposed–asymptomatic–presymptomatic–awaiting diagnosis–infected–recovered (SEAPWIR) structure similar to that used in a previously published study was created. 12 The structure of the model is displayed in Figure 1A. The model can be also described using the ordinary differential equations (ODEs) in Table S1. However, time progresses continuously in ODEs, which is not straightforward for the accrual of costs and health outcomes. Hence, a discrete‐time version of the SEAPWIR compartment model was constructed. It was assumed that cases in the W and I classes might die of COVID‐19 since both classes contained severe cases.
FIGURE 1.
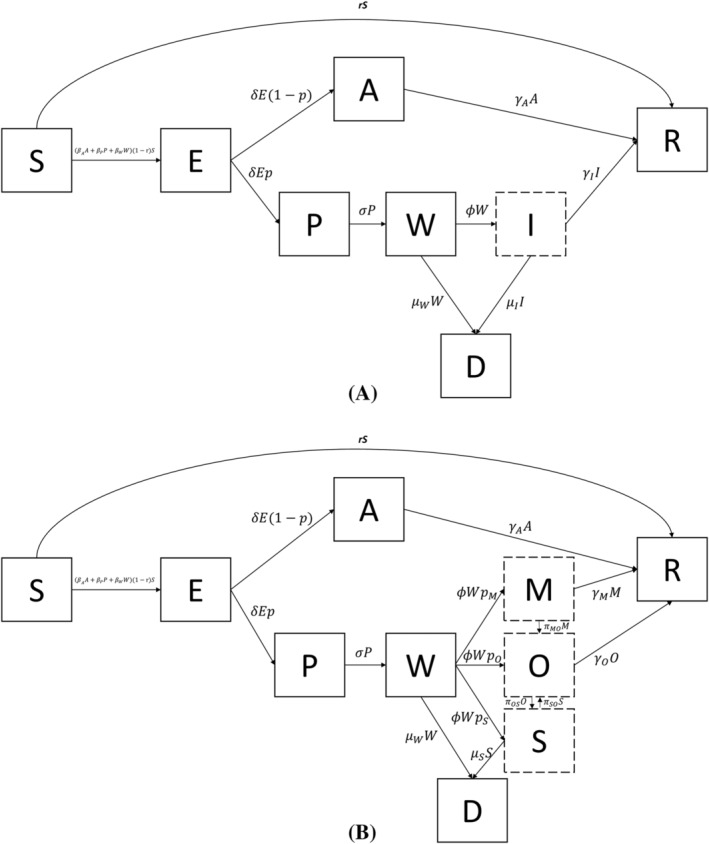
The structures of the SEAPWIR and the SEAPWIR–Markov model. A: The structure of the SEAPWIR model; B: the structure of the SEAPWIR–Markov model in which I was replaced by M, O, and S. S, susceptible; E: exposed; A: asymptomatic; P: presymptomatic; W: waiting‐to‐be‐diagnosed; I: infected; D: dead; R: recovered; M: mild; O: moderate; S: severe
With this specification, β A , β P and β W represented the transmission rate of the A class, the P class, and the W class, respectively. These 3 classes were the infective compartments of the model. Individuals in the I class were assumed to be institutionalized and quarantined, thereby becoming incapable of transmitting the virus. Therefore, the force of infection was β A A + β P P + β W I. When homogenous mixing across classes was assumed, the SEIR model and its extensions could not reflect any flattening trends of epidemic curves far earlier than when herd immunity could be reached, which was the situation in Wuhan where the number of cases started to level off at around 50 000. For example, Hou et al. showed that the majority of people in Wuhan would be infected when the daily contact rate was toggled from 18 to 6 as long as a well‐mixed SEIR model was used, even though the peak time was shifted out. 13 To incorporate the flattening trend without population immunity saturation, the original structure was modified to allow physical distancing barriers. Specifically, a proportion r of the S class individuals moved to the R class directly in each daily time step to reflect reduced risk by physical distancing barriers whereas the rest remained susceptible. Among the remaining susceptible individuals, an individual might become infected and move into the E class for a latency period, after which the individual would either become asymptomatic but infective (A) with a probability of 1 − p or become presymptomatic and infective (P) with a probability of p. Those in the P class then started to have symptom onset but took some time to be diagnosed (W) as was the case in Wuhan in the first quarter of 2020. Once diagnosed, the symptomatic individuals were treated and quarantined (I). Those in the A and I classes could recover from the disease. Also, those in W and I classes might die of the disease. An inventory of the parameters of the compartment model is provided in Table 1.
Once the SEAPWIR model was constructed in the first process, the second process was to modify the structure such that the model could also account for the differential severity‐related clinical manifestations. To that end, the I class was replaced by 3 health states, namely mild (M), moderate (O) and severe (S). 14 , 15 This mimics the common practice of Markov state‐transition modelling in health economics with each of the state represent the average profile of patients of the corresponding severity. As was compliant with the standard “Markovian assumption”, the subsequent transition probabilities of patients in each of the state depended on their current state but not disease history. 16 , 17 The M state housed the individuals that only had upper respiratory symptoms whereas the O state enveloped those who had nonsevere lower respiratory symptoms. The S state represented the weighted average profile of severe and critical patients by pooling the 2 severity categories regarding clinical, quality of life and resource utilization consequences. The modified structure of the model, which is referred to as the SEAPWIR–Markov model hereafter, is displayed in Figure 1B. The ODEs corresponding to the Markov submodel are listed in Table S1. To be in line with the SEAPWIR model, it was assumed that only cases in the W class and the S state could die of COVID‐19. Also, cases could transit from mild to moderate and from moderate to severe. They could also transit back from severe to moderate. However, it was assumed that the moderate cases recover directly without having to go through the mild state while the severe cases did have to transit to moderate before recovery.
2.3. Epidemic parameters
To estimate the epidemic parameters of the model in Figure 1A, it was necessary to set some of the parameters as fixed and calibrate the values of the rest of the parameters by fitting the simulated data to the observed data. 18 The parameters were set as fixed if available from the literature. Specifically, the fixed parameters included δ, p, σ, ϕ, μ W , μ I and γ I , leaving β A , β P , β W and γ A to be calibrated. A study that followed the viral shedding courses of 94 COVID‐19 patients estimated the incubation period to be 2.5 days. 19 Hence, δ was set at 1/2.5. A study on the Diamond Princess cruise ship, a closed system that allowed ascertainable follow‐up of patients by symptoms, estimated that about 17.9% of the cases were asymptomatic throughout the infection episode, which was used as the input value for p. 20 It has also been estimated that infectiousness started 2.5 days prior to symptom onset. 19 Therefore, σ was also set as 1/2.5. Once symptomatic, it took 3.3 days on average to be diagnosed during the Wuhan epidemic, 21 giving rise to an estimate of 1/3.3 for ϕ. Confirmed cases in Wuhan had a mean institutionalized period of 17 days, the inverse of which was used as γ I . The overall mortality rate of the confirmed cases, μ I , was 0.0015/person‐day. 22 Without precise information, it was assumed that those in the W class had the same severity profiles as the I class such that the morality rate of the W class, μ W , was equal to μ I . To conduct the calibration, the sum squared residuals (SSR) of the simulated and observed numbers of cumulative confirmed cases over 24 January–19 March 2020 was minimized by setting 23 January 2020 as the initial state. Numbers of individuals in classes S, A, L and I at the initial state were mandatory for the model calibration. The initial number of people in S class was assumed to be the same as the population in Wuhan. 23 These numbers were imputed using the proportions of the corresponding classes in relation to the number of confirmed cases. The values are also listed in Table 1.
There were several spikes in the raw epidemic curve in Wuhan that were unrelated to the true number of cases, causing a fuzzy observed curve. 28 , 29 Hence, we first estimated a 3‐parameter logistic growth curve using the observed data on the cumulative confirmed cases from 24 January–19 March 2020 to smooth the epidemic curve. The calibration was implemented using Oracle Crystal Ball 11. Then the proposed SEAPWIR model was fitted to the smoothed curve.
In the second process in which the I class was replaced by the Markov structure, not only the fixed and calibrated epidemic parameters but also the transition probabilities between the disease severity states as well as their neighbouring states were required. According to studies in China, 4.5% of the patients had nonpneumonia whereas 81% had no or mild pneumonia. Therefore, p M and p O were set at 0.045 and 0.765, respectively. 22 , 24 Following these, p S was calculated to be 0.190. These percentages were also used to derive μ S from μ I , the former of which was therefore 0.0079/person‐day. To the extent that studies on the disease progression of COVID‐19 patients typically pool mild and moderate patients together, π MO and π OS were assigned the identical value in the present analysis. To obtain these values, the percentage of mild and moderate patients that had any progression over 28 days from a study in China (28.5%) was used. 25 Specifically, the value of 0.012 for π MO and π OS was estimated by solving (1 − π MO )28 = 0.715. Similarly, π SO was obtained by solving simple ODEs such that the cumulative proportion of severe patients who had clinical improvement matched that reported for the placebo arm of a clinical trial of remdesivir among severe patients over 28 days. 26 The recovery time of the mild cases was estimated using the duration of viral shedding among mild cases from a Japanese study subtracted by the days spent in the E, P and W compartments, the reverse of which was γ M , 27 whereas the value of γ O took that of γ I .
2.4. Treatment regimen and effectiveness
The interventions of interest were a 5‐day remdesivir treatment strategy and SoC. According to clinical trials and meta‐analyses published in the literature, the 5‐day remdesivir treatment, although shorter in treatment and lower in costs, was noninferior to and probably more efficacious than its 10‐day counterpart. 28 , 29 , 30 The intervention strategy was formally defined as allowing all individuals who entered the severe state to receive the 5‐day remdesivir regimen and SoC while only providing SoC to mild and moderate patients. The comparator strategy was providing SoC to patients of any severity. Studies on the therapeutic profiles of COVID‐19 patients in China indicated that SoC typically encompassed lopinavir/ritonavir, ribavirin, arbidol, chloroquine phosphate, hydroxychloroquine and glucocorticoids, most of which had no effectiveness evidence from RCTs. 31 , 32
The effect of treatment was represented by the odds ratio (OR) of clinical improvement. Clinical studies typically engage ordinal scales to measure clinical improvement. In the present study, the effect of remdesivir on clinical improvement was applied to transition probabilities from relatively grave conditions to healthier states. A meta‐analysis estimated that the 5‐day remdesivir treatment was associated with an OR of 1.81 for clinical improvement. 30
2.5. Costs and utility inputs
The outcomes to be compared across interventions were costs and quality‐adjusted life years (QALYs). The types of costs that were accounted for in the present analysis included reverse transcription–polymerase chain reaction (RT‐PCR) test fee for diagnosis and discharge of all infected and symptomatic persons, 1‐time outpatient costs of mild patients, bed costs of mild patients during quarantine, hospitalization costs of moderate patients, hospitalization costs of severe patients, SoC medication costs of moderate patients, SoC medication costs of severe patients, and remdesivir acquisition costs, all of which were in 2020 CN¥.
The costs of each RT‐PCR test for COVID‐19 in China was based on the official charge in Hubei province. 33 The mild patients were assumed to incur no further direct treatment costs beyond the 1‐time visit costs. Therefore, they only accrued daily bed costs (not necessarily hospital beds) in designated quarantine facilities over the clinical course. Data on bed costs were based on a cost analysis of COVID‐19 patients in China, 34 whereas the 1‐time visit was assumed to cost CN¥500. Of note, the 1‐time visit costs did not impact the incremental costs across interventions because every patient had to incur it once upfront regardless of intervention strategies. The same study that estimated the bed costs also reported the inpatient medical costs and medication costs of moderate, severe, and critical patients, which were applied to the corresponding states in the present analysis. To reconcile the reported data and the set‐up of our model, the data on the severe and critical patients were pooled by taking the weighted average values to match the S state in the present analysis. Based on World Health Organization (WHO) estimates, 15–20% of severe patients would become critical. 35 Accordingly, the medium value of 17.5% was used as the proportion of critical patients in the S state. To be consistent with the clinical trials, patients in the remdesivir‐treated arm incurred both SoC medication costs and remdesivir acquisition costs, the latter of which was assumed equivalent to the pricing in high‐income countries other than the USA. 6 The input values of the cost parameters are provided in Table 2.
TABLE 2.
Treatment effectiveness, healthcare utilization and health state utility value (HSUV) inputs
| Input value | Included in OWSAs and PSA | Standard deviation in PSA | Distribution | Source | |
|---|---|---|---|---|---|
| Treatment effectiveness | 1.81 | Yes | 0.123 (log scale) | logNormal | 30 |
| Percentage of critical patients among severe patients | 17.5% | No | NA | NA | 35 |
| Costs of RT‐PCR/test | Cn¥160 | Yes | 10% of mean | Gamma | 33 |
| One‐time visit costs | Cn¥500 | No | NA | NA | Assumption |
| Daily bed costs | Cn¥11 | No | NA | NA | 34 |
| SoC medication costs of moderate patients/d | Cn¥898 | Yes | 10% of mean | Gamma | 34 |
| SoC medication costs of severe patients/d | Cn¥2375 | Yes | 10% of mean | Gamma | 34 |
| SoC medical costs of moderate patients/d | Cn¥582 | Yes | 10% of mean | Gamma | 34 |
| SoC medical costs of severe patients/d | Cn¥1538 | Yes | 10% of mean | Gamma | 34 |
| Acquisition costs of 5‐day remdesivir regimen | CN¥16 600 | Yes | 10% of mean | Gamma | 6 |
| Utility weight of the W class | 0.626 | No | NA | NA | Estimated |
| Utility weight of the mild patients | 0.995 | No | NA | NA | 36 |
| Utility weight of the moderate patients | 0.614 | Yes | 10% of mean | Beta | 36 |
| Utility weight of the severe patients | 0.588 | Yes | 10% of mean | Beta | 36 |
| QALY loss/Covid‐19 death | 11.27 | No | NA | NA | 22 , 37 , 38 |
Abbreviation: OWSA, 1‐way sensitivity analysis; PSA, probabilistic sensitivity analysis; NA, not applicable; RT‐PCR, reverse transcription–polymerase chain reaction; SoC, standard of care; QALY, quality‐adjusted life year.
Health state utility values (HSUVs) were mandatory inputs to weight survival time for the calculation of QALYs. To date, studies on the HSUVs of COVID‐19 patients were absent. As such, utility values associated with different respiratory disease severity states from the 2010 WHO global burden of disease and studies on the utility values of severe acute respiratory infection inpatients and influenza outpatients in China were used. 36 The disutility value of mild patients was 0.005 based on the estimates for acute infectious disease patients by WHO global burden of disease, 36 whereas the utility scores of moderate and severe cases based on the studies on Chinese influenza and severe acute respiratory infection patients were 0.614 and 0.588, respectively. 39 , 40 The utility weight of the W class was estimated as the weighted average of the mild, moderate and severe patients. The numbers are also presented in Table 2. Of note, simply accruing outcomes over the 55‐day time horizon would lead to substantial underestimation of the benefit of the superior strategy since a sizeable fraction of the benefit as measured by QALYs were attributable to reduced mortality. To comprehensively capture the clinical benefit, it was necessary to enclose both avoided QALY loss due to fewer deaths and increased quality‐adjusted life days (QALDs) due to more days spent in healthier states. To that end, the QALY loss associated with each COVID‐19 death was attached to deceased individuals in the model. Using the age distribution of the deceased patients in China, we estimated that the mean age of the fatal COVID‐19 cases was 69 years. 22 In 2019, the remaining life expectancy of 69‐year‐old individuals in China was 17 years. 37 In addition, the annual discount rate of 5% was used per the China Guideline for Pharmacoeconomic Evaluation. 38 Each death was assigned a loss of 11.27 QALYs. Given the short period of simulation, outcomes incurred within the analytic period were not discounted. Both outcomes were averaged across the population to obtain the incremental cost‐effectiveness ratio (ICER) which was defined as incremental costs/QALY. A willingness‐to‐pay threshold of once the gross domestic product per QALY was used, which was CN¥70 892 (US$10276) in 2019. 41 , 42
2.6. Exploratory and sensitivity analyses
The healthcare systems and resources in numerous parts of the world were drained during the first wave of the pandemic, highlighting the importance of healthcare capacity planning. As an exploratory outcome, the study looked at the peak numbers of severe cases under both strategies, the difference of which was considered the number of hospital beds that could be saved by the clinically superior strategy. Whereas the present modelling analysis did consider the quarantine and costs of mild and moderate patients in temporary shelter hospitals, 43 the peak numbers of hospital beds as exploratory outcomes only pertained to severe patients since the hospitalization requirement of the severe patients was clinically necessary regardless of public health strategies and, therefore, represented 1 of the most critical challenges in healthcare resourcing.
In addition, the cost‐effectiveness profiles were re‐examined under alternative scenarios in which r was changed to 20% and 5 times of its original values. Intuitively interpreted, such change represented variations in the stringency of social distancing policies. By varying the stringency of physical distancing in the model, these scenarios aimed to mimic the 2 extremes of epidemic situations representing the most severely hit countries and the other cities in mainland China, respectively.
In 1‐way sensitivity analyses (OWSAs), the epidemic and state‐transition probability parameters, the costs parameters other than the daily bed costs for the mild patients and the 1‐time visit costs, the HSUVs of the moderate and severe patients, and the discount rate were varied to examine the robustness of the results. Tables 1 and 2 contain indicators for whether the parameters were included in OWSAs. The parameters used in OWSAs were also included in a probabilistic sensitivity analysis. Normal distributions were used for transmission coefficients whereas beta distributions were used for the rest of the epidemic parameters. More, all state‐transition related parameters were assumed to follow beta distributions. HSUVs and the percentage of severe patients that were critical also followed beta distributions. Even more, γ distributions were used for costs inputs and a log‐normal distribution was used for the OR of treatment effect. Unless directly available from the literature, the standard errors of the parameter inputs (standard deviations of the parameter sampling distributions) were assumed to be 10% of the mean values. The distribution assumptions and specifications of the parameters are also listed in Tables 1 and 2.
TABLE 1.
Epidemic parameters of the SEAPWIR dynamic transmission model and the Markov sub‐model
| Symbol/parameter | Definition | Input value | Calibrated value | Source | Distribution type | Included in OWSAs and PSA | Standard deviation in PSA |
|---|---|---|---|---|---|---|---|
| β A | The transmission coefficient of the A class | NA | 7.30 × 10−7 | Calibrated | Normal | 7.30 × 10−7 | 10% of mean |
| β P | The transmission coefficient of the L class | NA | 1.00 × 10−9 | Calibrated | Normal | 1.00 × 10−9 | 10% of mean |
| β W | The transmission coefficient of the I class | NA | 5.20 × 10−7 | Calibrated | Normal | 5.20 × 10−7 | 10% of mean |
| r | Fraction moving into social distancing | NA | 0.160 | Calibrated | Beta | 0.160 | 10% of mean |
| δ | Conversion rate of the latent class to asymptomatic and presymptomatic classes | 0.400/d | NA | 19 | Beta | 0.400 | 10% of mean |
| p | The probability of not developing symptoms throughout the course | 0.179 | NA | 22 | NA | NA | NA |
| σ | Rate of transition from presymptomatic to symptomatic | 0.400/d | NA | 24 , 44 , 45 | Beta | 0.400 | 10% of mean |
| ϕ | Rate of diagnosis of the symptomatic | 0.303/d | 21 | Beta | 0.303 | 10% of mean | |
| μ I | The case‐fatality rate of the W class | 0.00150/d | NA | 22 | NA | NA | NA |
| μ W | The case‐fatality rate of the I class | 0.00150/d | NA | Assumed the same as μ I | Beta | 0.00150 | 10% of mean |
| γ A | The recovery rate of the A class | NA | 0.940/d | Calibrated | Beta | 0.940 | 10% of mean |
| γ I | The recovery rate of the I class | 0.0588/d | NA | 46 | NA | NA | NA |
| St = 0 | Initial number in the S class | 9 000 000 | NA | 23 | NA | NA | NA |
| At = 0 | Initial number in A class | 6 | NA | Imputed | NA | NA | NA |
| Lt = 0 | Initial number in L class | 252 | NA | Imputed | NA | NA | NA |
| It = 0 | Initial number in I class | 280 | NA | Imputed | NA | NA | NA |
| p M | Percentage of mild patients | 0.0450 | NA | 22 , 24 | Beta | 10% of mean | |
| p O | Percentage of moderate patients | 0.765 | NA | 22 , 24 | Beta | 10% of mean | |
| p S | Percentage of severe patients | 0.190 | NA | 22 , 24 | Beta | 10% of mean | |
| π MO | Transition probability from mild to moderate | NA | 0.0192/d | Assumed the same as π OS | Beta | 10% of mean | |
| π OS | Transition probability from moderate to severe | NA | 0.0192/d | Estimated | Beta | 10% of mean | |
| π SO | Transition probability from severe to moderate | NA | 0.0288/d | Estimated | Beta | 10% of mean | |
| γ M | Recovery rate of mild patients | 0.0935 | NA | 27 | Beta | 10% of mean | |
| γ O | Recovery rate of moderate patients | 0.0588 | NA | Assumed the same asγ I | Beta | 10% of mean | |
| μ S | Case‐fatality of severe patients | 0.0079 | NA | Estimated | Beta | 10% of mean |
OWSA, 1‐way sensitivity analysis; PSA, probabilistic sensitivity analysis; NA, not applicable.
To the extent that only the consequences to the treated patients instead of the population were of interest to specific decisionmakers in the Chinese healthcare system, a decision tree model was additionally constructed to re‐analyse the research question by only modelling the severe patients. In the decision tree model, the severe patients were further split into severe and critical patients who had differential costs, HSUVs, recovery time and time to death over a 29‐day follow‐up period. The follow‐up period was chosen to be consistent with the ACCT‐1 trial along with the effectiveness data on time to recovery and time to death. 47 The parameters of the decision tree model are presented in Table S2. The structure of the decision tree model is depicted in Figure S1.
The analyses were programmed using Excel 2019 spreadsheet and VBA except the decision tree model, which was conducted using TreeAge Pro 2019.
3. RESULTS
The calibrated parameters of the SEAPWIR epidemic model are presented in Table 1. The observed epidemic curve, the smoothed curve, and the fitted curve using the SEAPWIR model are depicted in Figure 2. Visually examined, the fitted curve closely resembled the smoothed curve.
FIGURE 2.
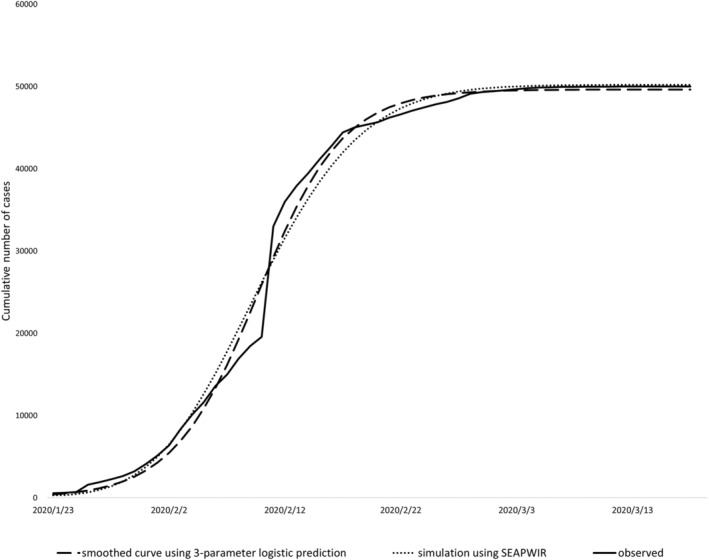
The observed epidemic curve, the smoothed curve of the observed epidemic, and the simulated epidemic curve using the calibrated SEAPWIR model
The base‐case cost‐effectiveness results are presented in Table 3. The 5‐day remdesivir strategy and the SoC strategy cost about CN¥2353.58 million and CN¥2255.65 million over the 55‐day period, respectively, totalling CN¥97.93 million incremental costs. Also, the QALDs associated with the 2 strategies were 512 677 159 and 512 651 191. Accordingly, the incremental QALDs were 25 968. More, the QALY loss due to COVID‐19 deaths corresponding to the 2 strategies were 27 150 and 34 026, amounting to 6875 QALY loss avoided by the 5‐day remdesivir strategy. Taken together, the net gain from 5 days of remdesivir treatment was 6947 QALYs. As such, the ICER was CN¥14 098/QALY, substantially lower than the WTP threshold. The maximum daily numbers of severe cases were 8163 and 10 082, respectively, representing 1919 fewer or 19% lower hospital bed requirement to accommodate all severe cases. The numbers of severe cases across time are plotted in Figure S2.
TABLE 3.
Base‐case results of the cost‐effectiveness analysis
| SoC | Remdesivir | Incremental | |
|---|---|---|---|
| Total costs (CN¥) | 2 255 649 459 | 2 353 580 729 | 97 931 270 |
| QALDs during the analytic period (QALY equivalent) | 512 651 191 (1 403 562) | 512 677 159 (1 403 634) | 25 968 (71) |
| QALY loss due to death | 34 026 | 27 150 | 6875 |
| QALY | NA | NA | 6947 |
| ICER (CN¥/QALY) | NA | NA | 14 098 |
SoC, standard of care; QALD, quality‐adjusted life day; QALY, quality‐adjusted life year; ICER, incremental cost‐effectiveness ratio.
When r was decreased to 20% of the base‐case value, over 0.83 million people would be infected over the 55‐day period, accounting for almost 10% of the simulated population. In this scenario, remdesivir was still cost‐effective with an ICER of CN¥25 499/QALY. When r was increased to 5× its original value, remdesivir remained cost‐effective with an ICER of CN¥8817/QALY.
Figure S3 depicts the results of the OWSA. Based on the results, the 5‐day remdesivir regimen remained cost‐effective in all but 1 scenario. Specifically, it was no longer cost‐effective when the RR of treatment effect dropped by 30% to 1.27, which drove the ICER slightly above the threshold. Figure S4 illustrates the cost‐effectiveness acceptability curve based on the results of the probabilistic sensitivity analysis. The 5‐day remdesivir regimen had a 98% probability of being cost‐effective.
The base‐case and OWSA results of the decision tree model are displayed in Figure S5. The ICER in the base case was CN¥24 673/QALY and remained below the WTP threshold in all scenarios.
4. DISCUSSION
In the present study, we investigated the cost‐effectiveness of the 5‐day remdesivir regimen compared with SoC for the treatment of severe COVID‐19 patients in China. Healthcare systems around the world, many of which were financially disadvantaged even before the pandemic, face double threats of overwhelming patient visits and budget shocks due to pandemic‐related economic downturns. 48 , 49 , 50 Hence, it is important to identify cost‐effective therapies for the patients. Our results suggest that 5‐day remdesivir treatment is cost‐effective for severe COVID‐19 patients vs. SoC if remdesivir is priced coherently with international markets outside of the USA. The results were robust to changes in epidemic intensities, modelling methods and most of the parameters. However, the results were relatively sensitive to changes in effectiveness estimates, which were reflected by the OWSAs of both the dynamic transmission model and the static decision tree model. In addition to cost‐effectiveness, the analysis also demonstrated that the remdesivir strategy lowered the apex of required hospitalization capacity.
Our findings are favourable to the potential use of remdesivir among severe COVID‐19 patients in China from the healthcare system perspective and provide the evidence base for potential decision‐making of remdesivir among COVID‐19 patients. As such, ensuring reasonable access to remdesivir in the undesirable scenario that severe cases resurface again may benefit the resilience of the public health system in China, which faces the challenge of balancing resource allocation between the pandemic and several additional population health priorities. 51 , 52 , 53 Although not of focus in the analysis, OWSAs results suggested that reducing the waiting time from symptom onset to diagnosis and treatment favoured the cost‐effectiveness profile of remdesivir. Reducing the waiting time would lead to earlier pharmacological intervention in effect. Therefore, timely initiation of remdesivir treatment may be preferred to delayed treatment. Of note, evidence from the present analysis only endorses the use of remdesivir among severe COVID‐19 patients. In addition to expediting recovery of patients, freeing up hospital resources with remdesivir treatment may have indirect benefit for other patients who need hospital beds, thereby serving to reinforce the capacity of healthcare facilities and keeping the clinical responses manageable. Without such effect, it is not impossible that stuffed hospitals may see an exacerbation of clinical performance.
Whereas it is tempting to only engage static models for the present research topic given that pharmacological interventions are not meant to impact infection, the transmission model did offer additional insights that would have otherwise not been available in static models. First, it allowed us to estimate the cost‐effectiveness of remdesivir for the healthcare system by averaging the costs and QALY outcomes of all COVID patients over the population. A static model such as the decision tree model we used would starts withy not the population but a hypothetical cohort of the severe hospitalized COVID patients and would only compute the outcomes of the treated patients, which means the epidemic scenarios would not have been accounted for in the results. In this regard, a transmission model is important for the understanding of the economic profile of remdesivir in the context of healthcare system. In addition, we estimated the maximum daily hospitalizations that reflected the required hospitalization capacity of the healthcare system to prepare for the pandemic. In the present analysis, we used the population size of Wuhan as the benchmark. By assuming time‐invariant incidence, a static model in which a hypothetical cohort of an arbitrary patient size would not have captured such impacts as well since the growth of the epidemics was not reflected.
The analysis has several limitations that call for discretion. First, RCTs failed to provide evidence that remdesivir significantly reduced the mortality of COVID‐19 patients of any severity, yet the set‐up of our models partially transferred its clinical benefit into reduced mortality, which was an inevitable consequence per the logic of modelling. Second, only the strategy of treating severe patients with remdesivir was considered, leaving the evidence on the economic profiles of remdesivir among moderate patients at large. Third, SoC may vary across countries, yet the present analysis did not distinguish SoC in China from that in other countries when using the effectiveness data. Fourth, data on several parameters including the HSUVs of COVID‐19 patients were still absent in the literature such that their values might not be reliable. Although sensitivity analyses were conducted to examine the robustness of the results, the ranges of the variation did not necessarily cover all plausible domains.
5. CONCLUSION
The 5‐day remdesivir treatment is cost‐effective for the treatment of severe hospitalized COVID‐19 patients under the observed epidemic intensities in China compared with SoC from the healthcare system perspective if the former is priced at CN¥16 600 (US$2340) for the full treatment course. The results were robust in alternative epidemic scenarios. Therefore, ensuring access to remdesivir in China may benefit the healthcare system when confronted with potential re‐surges.
COMPETING INTERESTS
All authors claim no conflict of interest related to the submitted work.
CONTRIBUTORS
Y.J.: study concept, study design, programming, data analysis, and manuscript drafting; D. Cai: data curation and manuscript revision; D. Chen: data curation and manuscript revision; S.J.: study design and manuscript revision; L.S.: study design and manuscript revision; J.W.: study concept and manuscript revision.
Supporting information
TABLE S1 Ordinary differential equations of the SEAPWIR and Markov structures.
TABLE S2 Parameters in the decision tree analysis
FIGURE S1 Structure of the decision tree model.
FIGURE S2 The numbers of severe cases across time among the simulated population with the 2 treatment strategies. SoC, standard of care.
FIGURE S3 The results of the 1‐way sensitivity analyses of the dynamic transmission model. RR, relative risk; RT‐PCR, reverse transcription–polymerase chain reaction.
FIGURE S4 The cost‐effectiveness acceptability curve using the results of the probabilistic sensitivity analysis of the dynamic transmission model (currency: CN¥). The 5‐day remdesivir regimen had a 95% probability of being cost‐effective.
FIGURE S5 Base‐case and 1‐way sensitivity analysis results of the decision tree model. EV: expected value of ICER in the base case; HSUV, health state utility value; ICER, incremental cost‐effectiveness ratio; QALY, quality‐adjusted life year; SoC, standard of care; WTP, willingness to pay.
ACKNOWLEDGEMENTS
Lei Si is funded by a National Health and Medical Research Council Early Career Fellowship of Australia (Grant number GNT1139826). The authors did not receive any grant from funding agencies in the public, commercial, or not‐for‐profit sectors for the submitted work.
Jiang Y, Cai D, Chen D, Jiang S, Si L, Wu J. Economic evaluation of remdesivir for the treatment of severe COVID‐19 patients in China under different scenarios. Br J Clin Pharmacol. 2021;87(11):4386-4396. 10.1111/bcp.14860
Principal investigator: This work was not an interventional study and did not have a principal investigator.
DATA AVAILABILITY STATEMENT
The data analysed during the study are presented in the article and its supplementary materials. Program code used in the submitted work is available from the corresponding author upon reasonable requests.
REFERENCES
- 1. World Health Organization . WHO Coronavirus Disease (COVID‐19) Dashboard. 2021. [cited 2021 Feb 22]; Available from: https://covid19.who.int/
- 2. Grima S, Kizilkaya M, Rupeika‐Apoga R, Romānova I, Dalli Gonzi R, Jakovljevic M. A Country Pandemic Risk Exposure Measurement Model. Risk Manag Healthc Policy. 2020;13:2067‐2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reshetnikov V, Mitrokhin O, Shepetovskaya N, Belova E, Jakovljevic M. Organizational measures aiming to combat COVID‐19 in the Russian Federation: the first experience. Expert Rev Pharmacoecon Outcomes Res. 2020;20(6):571‐576. [DOI] [PubMed] [Google Scholar]
- 4. Siemieniuk RA et al. Drug treatments for covid‐19: living systematic review and network meta‐analysis. BMJ. 2020;370:m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kow CS, Aldeyab M, Hasan SS. Effect of remdesivir on mortality in patients with COVID‐19: A meta‐analysis of randomized control trials. J Med Virol. 2021;93(4):1860‐1861. [DOI] [PubMed] [Google Scholar]
- 6. Wise J. Remdesivir: US purchase of world stocks sparks new “hunger games,” warn observers. BMJ. 2020;370:m2661. [DOI] [PubMed] [Google Scholar]
- 7. Hill A. ICER releases pricing models for potential COVID‐19 treatments. PharmacoEcon Outc News. 2020;853:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. del Rio C, Malani PN. COVID‐19—New Insights on a Rapidly Changing Epidemic. JAMA. 2020;323(14):1339–1340. [DOI] [PubMed] [Google Scholar]
- 9. Chan JF‐W, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. The Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID‐19. 2020. Published online February;21:2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Institute of Infectious Diseases . Field Briefing: Diamond Princess COVID‐19 Cases. 2020. [cited 2020 Feb 20]; Available from: https://www.niid.go.jp/niid/en/2019-ncov-e/9407-covid-dp-fe-01.html
- 12. Jiang Y, Cai D, Chen D, Jiang S. The cost‐effectiveness of conducting three versus two reverse transcription‐polymerase chain reaction tests for diagnosing and discharging people with COVID‐19: evidence from the epidemic in Wuhan China . BMJ Glob Health. 2020;5(7):e002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hou C et al. The effectiveness of the quarantine of Wuhan city against the Corona Virus Disease 2019 (COVID‐19): well‐mixed SEIR model analysis. J Med Virol. 2020;92(7):841–848. [DOI] [PubMed] [Google Scholar]
- 14. European Centre for Disease Prevention and Control . Guidance for discharge and ending isolation in the context of widespread community transmission of COVID‐19 – first update. 2020. [cited 2020 Apr 15]; Available from: https://www.ecdc.europa.eu/en/publications-data/covid-19-guidance-discharge-and-ending-isolation
- 15. World Health Organization . Criteria for releasing COVID‐19 patients from isolation. 2020. [cited 2020 Jun 20]; Available from: https://www.who.int/publications/i/item/criteria-for-releasing-covid-19-patients-from-isolation
- 16. Neumann PJ et al. Cost‐effectiveness in health and medicine. Oxford University Press; 2016. [Google Scholar]
- 17. Caro JJ, Möller J, Getsios D. Discrete event simulation: the preferred technique for health economic evaluations? Value Health. 2010;13(8):1056‐1060. [DOI] [PubMed] [Google Scholar]
- 18. Tan X, Yuan L, Zhou J, Zheng Y, Yang F. Modeling the initial transmission dynamics of influenza A H1N1 in Guangdong Province China . Int J Infect Dis. 2013;17(7):e479‐e484. [DOI] [PubMed] [Google Scholar]
- 19. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID‐19. Nat Med. 2020;26(5):672‐675. [DOI] [PubMed] [Google Scholar]
- 20. Mizumoto K, Katsushi K, Alexander Z, Gerardo C. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID‐19) cases on board the Diamond Princess cruise ship, Yokohama, Japan. Euro Surveill. 2020;25(10). 10.2807/1560-7917.es.2020.25.10.2000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Linton NM, Tetsuro K, Yichi Y, et al. Incubation Period and Other Epidemiological Characteristics of 2019 Novel Coronavirus Infections with Right Truncation: A Statistical Analysis of Publicly Available Case Data. J Clin Med. 2020;9(2):538. 10.3390/jcm9020538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . Vital Surveillances: The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID‐19) — China, 2020. China CDC Weekly. 2020;2(8):113‐122. [PMC free article] [PubMed] [Google Scholar]
- 23. Collman, A. 5 million people left Wuhan before China quarantined the city to contain the coronavirus outbreak. 2020. [cited 2020 Feb 28]; Available from: https://www.businessinsider.com/5-million-left-wuhan-before-coronavirus-quarantine-2020-1
- 24. Yang Y et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. MedRxiv. 2020;2020. 02.10.20021675 [Google Scholar]
- 25. Cen Y, Chen X, Shen Y, et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019—a multi‐centre observational study. Clin Microbiol Infect. 2020;26(9):1242‐1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Y, Zhang D, du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395(10236):1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miyamae Y, Hayashi T, Yonezawa H, et al. Duration of viral shedding in asymptomatic or mild cases of novel coronavirus disease 2019 (COVID‐19) from a cruise ship: A single‐hospital experience in Tokyo Japan . Int J Infect Dis. 2020;97:293‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid‐19. N Engl J Med. 2020.383(19):1827‐1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid‐19 ‐ Preliminary Report. N Engl J Med. 2020;383(19):1813‐1826. [DOI] [PubMed] [Google Scholar]
- 30. Jiang Y et al. Effectiveness of remdesivir for the treatment of hospitalized COVID‐19 persons: A network meta‐analysis. J Med Virol. 2021.93 1171‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan L, Jiang S, Yang X, Wang Z, Yang C. COVID‐19 Drug Treatment in China. Current Pharmac Rep. 2020;6(4):146‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choudhry N, Zhao X, Xu D, et al. Chinese Therapeutic Strategy for Fighting COVID‐19 and Potential Small‐Molecule Inhibitors against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2). J Med Chem. 2020;63(22):13205‐13227. [DOI] [PubMed] [Google Scholar]
- 33. Ma, X. What is the cost of polymerase chain reaction test of COVID‐19? Substantial heterogenity across different regions. (in Chinese). 2020. [cited 2020 Apr 15]; Available from: https://www.yicai.com/news/100592680.html
- 34. Li XZ, Jin F, Zhang JG, et al. Treatment of coronavirus disease 2019 in Shandong, China: a cost and affordability analysis. Infect Dis Poverty. 2020;9(1). 10.1186/s40249-020-00689-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization . Covid‐19 transmission questions. 2020. [cited 2020 Jun 24]; Available from: https://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/update-21-epi-win-covid-19-transmission-q-a.pdf?sfvrsn=796a4b2b_2
- 36. Institute for Health Metrics and Evaluation . Global Burden of Disease Study 2010 (GBD 2010) Disability Weights. 2020. [cited 2020 Jun 29]; Available from: http://ghdx.healthdata.org/record/ihme-data/gbd-2010-disability-weights
- 37. World Health Organization . Life tables by country China. 2020. [cited 2020 Dec 26]; Available from: http://apps.who.int/gho/data/?theme=main%26vid=60340
- 38. Liu G et al. China guidelines for pharmacoeconomic evaluations 2019. Beijing, China: Chinese Pharmaceutical Association; 2019. [Google Scholar]
- 39. Yang J, Jit M, Zheng Y, et al. The impact of influenza on the health related quality of life in China: an EQ‐5D survey. BMC Infect Dis. 2017;17(1):686. 10.1186/s12879-017-2801-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou L et al. Measuring Quality of Life of Hospitalized Patients with Severe Acute Respiratory Infection by EQ‐ 5D Instrument: an Exploratory Study. Prac Prev Med. 2012;19:644‐647. [Google Scholar]
- 41. Robinson LA, Hammitt JK, Chang AY, Resch S. Understanding and improving the one and three times GDP per capita cost‐effectiveness thresholds. Health Policy Plan. 2016;32(1):141‐145. [DOI] [PubMed] [Google Scholar]
- 42. China National Bureau of Statistics . China's economy expands 6.1% in 2019, in line with official target. 2020. [cited 2020 Jul 28]; Available from: http://english.www.gov.cn/news/videos/202001/18/content_WS5e22a44ec6d0db64b784cc17.html
- 43. Chen S, Zhang Z, Yang J, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. The Lancet. 2020;395(10232):1305‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang X, Rayner S, Luo M‐H. Does SARS‐CoV‐2 Has a Longer Incubation Period than SARS and MERS? J Med Virol. 2020;92(5):476‐478. 10.1002/jmv.25708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med. 2020;382(13):1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pan, F. , et al., Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID‐19) Pneumonia. 2020. Published online on Feb 13, 2020(0): p. 200370.
- 47. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid‐19 — Final Report. 383, 19; 2020:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krstic K, Westerman R, Chattu VK, Ekkert NV, Jakovljevic M. Corona‐Triggered Global Macroeconomic Crisis of the Early 2020s. Int J Environ Res Public Health. 2020;17(24):9404. 10.3390/ijerph17249404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jakovljevic M, Sugahara T, Timofeyev Y, Rancic N. Predictors of (in)efficiencies of Healthcare Expenditure Among the Leading Asian Economies ‐ Comparison of OECD and Non‐OECD Nations. Risk Mana Health Po. 2020;13:2261‐2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jakovljevic M, Matter‐Walstra K, Sugahara T, et al. Cost‐effectiveness and resource allocation (CERA) 18 years of evolution: maturity of adulthood and promise beyond tomorrow. Cost Effecti Reso Alloc. 2020;18(1):15. 10.1186/s12962-020-00210-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jakovljevic M, Timofeyev Y, Ranabhat CL, et al. Real GDP growth rates and healthcare spending ‐ comparison between the G7 and the EM7 countries. Gl He. 2020;16(1):64. 10.1186/s12992-020-00590-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jakovljevic M, Potapchik E, Popovich L et al. Evolving Health Expenditure Landscape of the BRICS Nations and Projections to 2025. Health Econ. 2017;26(7):844‐852. [DOI] [PubMed] [Google Scholar]
- 53. Jakovljevic M, Jakab M, Gerdtham U, et al. Comparative financing analysis and political economy of noncommunicable diseases. J Med Econ. 2019;22(8):722‐727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Ordinary differential equations of the SEAPWIR and Markov structures.
TABLE S2 Parameters in the decision tree analysis
FIGURE S1 Structure of the decision tree model.
FIGURE S2 The numbers of severe cases across time among the simulated population with the 2 treatment strategies. SoC, standard of care.
FIGURE S3 The results of the 1‐way sensitivity analyses of the dynamic transmission model. RR, relative risk; RT‐PCR, reverse transcription–polymerase chain reaction.
FIGURE S4 The cost‐effectiveness acceptability curve using the results of the probabilistic sensitivity analysis of the dynamic transmission model (currency: CN¥). The 5‐day remdesivir regimen had a 95% probability of being cost‐effective.
FIGURE S5 Base‐case and 1‐way sensitivity analysis results of the decision tree model. EV: expected value of ICER in the base case; HSUV, health state utility value; ICER, incremental cost‐effectiveness ratio; QALY, quality‐adjusted life year; SoC, standard of care; WTP, willingness to pay.
Data Availability Statement
The data analysed during the study are presented in the article and its supplementary materials. Program code used in the submitted work is available from the corresponding author upon reasonable requests.


