Abstract
Background and Aims
Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) associated acute liver injury (ALI) has been linked to poor outcomes in adults. Here we compare characteristics in children with elevated ALT (E‐ALT) in two distinct manifestations of the infection, multisystem inflammatory syndrome‐children (MIS‐C) and coronavirus disease 2019 (COVID‐19).
Methods
This is a retrospective study of patients ≤21 years of age with positive for SARS‐CoV‐2 PCR. E‐ALT was defined as alanine aminotransferase (ALT) > 40 U/L. Bivariate analysis and multivariable logistic regression were obtained to describe differences in children with and without E‐ALT in COVID‐19 and MIS‐C.
Results
E‐ALT was detected in 36% of the 291 patients; 31% with COVID‐19, and 51% with MIS‐C. E‐ALT in COVID‐19 was associated with obesity (P < .001), immunocompromised status (P = .04), and chronic liver disease (P = .01). In the regression models, E‐ALT in COVID‐19 was associated with higher c‐reactive protein (OR 1.08, P = .01) after adjusting for common independent predictors. Children with E‐ALT and MIS‐C were more often boys (P = .001), Hispanic (P = .04), or Black (P < .001). In MIS‐C, male gender (OR 5.3, P = .02) and Black race (OR 4.4, P = .04) were associated with increased odds of E‐ALT. Children with E‐ALT in both cohorts had significantly higher multiorgan dysfunction, longer hospitalization, and ICU stay. Children with MIS‐C had 2.3‐fold increased risk of E‐ALT compared to COVID‐19. No association was found between E‐ALT and mortality.
Conclusion
E‐ALT with SARS‐CoV‐2 presents as elevated transaminases without hepatic synthetic dysfunction. Patients with either manifestation of SARS‐CoV‐2 infection and E‐ALT experienced more severe disease.
Keywords: acute liver failure, acute liver injury and MISC, COVID‐19 ALI in children, elevated ALT, liver involvement in SARS‐CoV2
Abbreviations
- μg/mL
microgram per milliliter
- ALI
acute liver injury
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CDC
centers for disease control and prevention
- CI
confidence interval
- CK
creatine kinase
- CLD
chronic liver disease
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- DILI
drug‐induced liver injury
- E‐ALT
elevated alanine aminotransferase
- g/dL
gram per deciliter
- ICD‐10
international classification of diseases 10th revision
- ICU
intensive care unit
- IL‐6
interleukin 6
- INR
international normalized ratio
- IQR
interquartile range
- IU/L
international units per liter
- k/μL
kilo per microliter
- LDH
lactate dehydrogenase
- mg/dL
milligram per deciliter
- mg/L
milligram per liter
- MIS‐C
multisystem inflammatory disease in children
- mm3
cubic millimeter
- NAFLD
non‐alcoholic fatty liver disease
- ng/mL
nanogram per milliliter
- NYC
New York City
- OR
odds ratio
- pg/mL
picogram per milliliter
- pro‐BNP
pro b‐type natriuretic peptide
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
- SD
standard deviation
- U/L
units per liter
- ULN
upper limit of normal
Lay summary.
Children with either COVID‐19 or MIS‐C who developed concomitant elevated alanine aminotransferase were at risk of a more severe disease course including longer hospitalization and ICU stay.
Obese children immunocompromised and children with chronic liver disease with COVID‐19 were more frequently to develop hepatitis.
Children with MIS‐C had overall higher chances of getting hepatitis compared to children with COVID‐19
1. INTRODUCTION
The clinical manifestations of severe acute respiratory syndrome (SARS)‐associated coronavirus 2 (SARS‐CoV‐2) infection are typically mild in children. 1 , 2 , 3 , 4 , 5 , 6 , 7 However, severe symptoms and even deaths from Coronavirus Disease 2019 (COVID‐19) can occur. 8 , 9 A separate pediatric‐specific clinical presentation arises in the form of an entity termed Multisystem Inflammatory Syndrome (MIS‐C), characterized by a persistent acute febrile illness progressing to multiorgan dysfunction. 10 , 11
The prevalence of acute liver injury (ALI) in adult patients with COVID‐19 ranges from 15%‐78%, with most studies reporting 20%‐30% among hospitalized patients. 12 , 13 , 14 ALI, defined as an elevation in aminotransferases and typically presenting as hepatocellular histological injury with mild acute cholestasis, has been documented in adults and children with SARS‐CoV‐2 infection. Most adult patients experience transient mild to moderate elevation of liver enzymes. 12 , 13 , 14 , 15 , 16 Severe ALI (alanine aminotransferase [ALT] values 5x above the upper limit of normal [ULN]) was reported to correlate with disease severity and poor outcomes in adults, including death. 13 , 17
In contrast, less is known about the association between elevated ALT (E‐ALT) and disease severity in children with SARS‐CoV‐2. In this study, we looked at elevations in ALT (E‐ALT), as a surrogate marker for liver injury, similar to what has been done in adult studies. 13 We previously published a focused description of the association of hepatitis with disease severity in a cohort of 44 patients with MIS‐C included within this report. 18 We now further expand the scope of understanding of liver involvement in two clinical presentations of SARS‐CoV‐2. We provide critical data on its associating factors, epidemiology, and their contribution to the consequences of elevated ALT in children with COVID‐19 versus MIS‐C.
2. METHODS
In this retrospective study, we included patients ≤21 years old evaluated between March 14, 2020 and June 30, 2020, in the inpatient or outpatient clinical setting of two large children's hospitals in New York City (Morgan Stanley Children's Hospital of New York‐Presbyterian and Children's Hospital at Montefiore). The Institutional Review Board approved this study at both centers. All research was conducted following the Declaration of Helsinki guidelines of good practice.
Patients were identified by the international classification of diseases 10th revision (ICD‐10) code for a positive COVID‐19 test (U07.1) and/or by an institutional COVID‐19 or MIS‐C database. Only participants with confirmed SARS‐CoV‐2 infection with detection of the virus via nasal swab‐derived real‐time reverse polymerase chain reaction were included in the COVID‐19 cohort. MIS‐C was defined by the modified criteria of the Centers for Disease Control and Prevention (CDC) (see supplement). 19 We excluded children without liver tests.
Clinical, demographic, laboratory, and anthropometric data were collected. We have defined E‐ALT as a peak elevation of ALT >40 U/L, as these values were reported to fall above the 97th percentile for all ages and both sexes in a large cohort of healthy children. 20 Measures of hepatic synthetic dysfunction, such as international normalized ratio (INR) and bilirubin, were not included in this definition, given the multifactorial reasons for abnormal values in this clinical setting. 13 Each patient's medical records were manually reviewed for the presence of comorbidities.
E‐ALT was further categorized as mild to moderate (ALT > 40 ≤ 200 U/L) and severe (ALT > 200 U/L). Due to the small number of patients with severely E‐ALT and unchanged statistical significance when combined with mild to moderate elevations in ALT, all analyses were subsequently dichotomized as E‐ALT present (ALT > 40 U/L) or absent (ALT ≤ 40 U/L) within each cohort. We also evaluated AST, bilirubin, albumin, and INR values. Obesity was defined as body mass index (BMI) above 95th percentile and/or BMI ≥30 kg/m2 as applicable. An immunocompromised state was defined by the presence of malignancy requiring chemotherapy or radiation, recipients of bone marrow or solid organ transplant, or among patients receiving other immunosuppressive therapy or biologics including those with inflammatory bowel disease.
Student's t‐test or Mann–Whitney U‐test was performed for continuous variables. Chi‐square or Fisher's exact test was used for categorical variables to identify differences between the presence or absence of E‐ALT within each cohort (COVID‐19 and MIS‐C) and to compare the differences between them. Variables were presented as frequency (percentage), mean with standard deviation (SD), or median with interquartile range (IQR), as appropriate.
Multivariable logistic regression was used to examine the potential risk factors for E‐ALT by cohort (COVID‐19 and MIS‐C) and for the combined data set. Clinically significant variables and/or those with P < .25 in bivariate analysis were selected. Due to the small sample size in each cohort, the association between the potential risk factors and E‐ALT was tested sequentially, while controlling for age, gender, and race in each model, as well as for other predictors. Backward variable selection was used to compare E‐ALT in MIS‐C to E‐ALT in COVID‐19 until all variables were significant, while controlling for age, gender, and race. P‐values <0.05 were considered statistically significant. All analyses were performed using SAS 9.4.
3. RESULTS
A total of 291 patients were included in the study: 220 (76%) and 71 (24%) were diagnosed with COVID‐19 and MIS‐C respectively (Figure 1). Elevated ALT was detected in 36% (n = 105) of all children, 31% (n = 69) of children with COVID‐19, and 51% (n = 36) of children with MIS‐C. Severe injury was noted in 8% and 4% of children with COVID‐19 (Figure 1; Table S1) and MIS‐C respectively. One child with MIS‐C developed acute liver failure and recovered.
FIGURE 1.
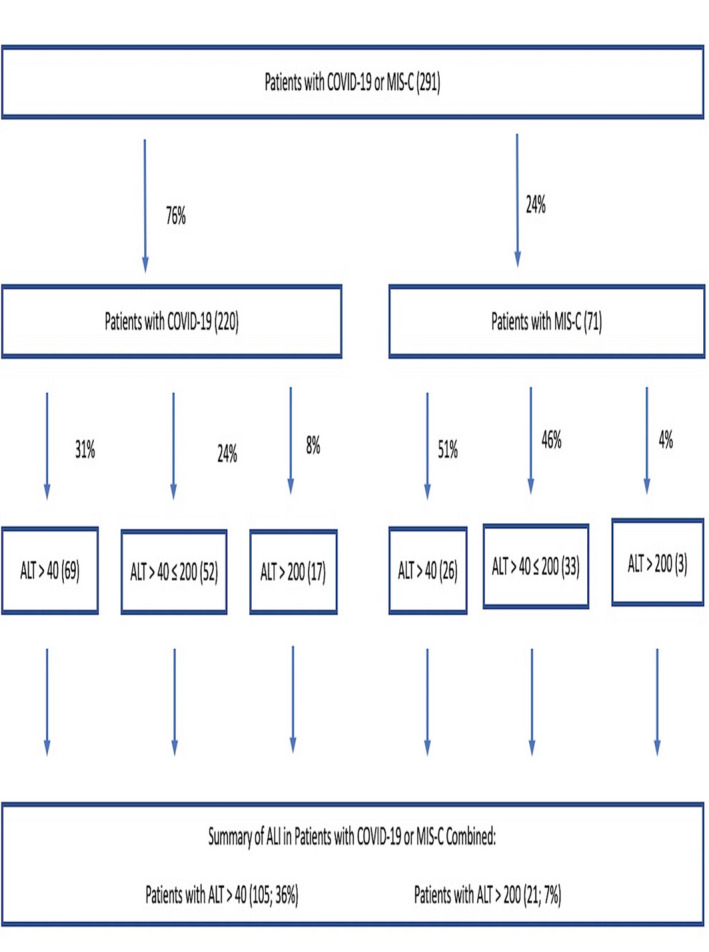
Flow‐chart of study cohort demonstrating the differences of ALI in the COVID‐19 and MIS‐C cohorts; A total of 291 patients were included in the study: 220 (76%) and 71 (24%) were diagnosed with COVID‐19 and MIS‐C, respectively. ALI was detected in 31% (n = 69) of children with COVID‐19 and 51% (n = 36) of children with MIS‐C. Severe injury defined as ALT > 200 U/L was noted in 8% of children with COVID‐19 and 4% of children with MIS‐C, respectively. Abbreviations: ALI, acute liver injury; ALT, alanine aminotransferase; COVID‐19, Coronavirus disease 2019; MIS‐C, system inflammatory syndrome in children
3.1. Liver involvement in COVID‐19
Children with E‐ALT were significantly older, with a median age of 16 vs 11 years (P =.001). No differences were observed in race, gender, or ethnicity. E‐ALT was associated with obesity (P <.001), immunocompromised status (P =.04), presence of any malignancy (P =.01), and chronic liver disease (CLD) (P =.01). Underlying CLD was present in 13% of children with E‐ALT. The etiologies of CLD in patients in whom prior medical history could be discerned were non‐alcoholic fatty liver disease (NAFLD) (six patients), biliary atresia (1), liver transplant recipient (1), and glycogenic hepatopathy (1) (Tables 1, 2; Tables S1 and S2 ).
TABLE 1.
Patient characteristics in children with COVID‐19 and MIS‐C with and without Elevated ALT
| COVID‐19 | MIS‐C | COVID‐19 vs MIS‐C a | |||||
|---|---|---|---|---|---|---|---|
| ALT ≤ 40 U/L (n = 151) | ALT > 40 U/L (n = 69) | P‐value | ALT ≤ 40 U/L (n = 35) | ALT > 40 U/L (n = 36) | P‐value | P‐value | |
| Demographics | |||||||
| Age (years), median (IQR) | 11 (2‐17) | 16 (8‐20) | 0.001 | 6 (2‐10) | 9.5 (6.0‐13.5) | 0.01 | 0.01 |
| Male sex, n (%) | 87 (58) | 49 (71) | 0.06 | 11 (31) | 25 (69) | 0.001 | 0.87 |
| Hispanic ethnicity, n (%) | 50 (43) | 19 (31) | 0.13 | 14 (48) | 16 (53) | 0.69 | 0.04 |
| Black race, n (%) | 26 (26) | 7 (14) | 0.08 | 6 (24) | 13 (59) | 0.01 | <0.001 |
| Comorbidities | |||||||
| Asthma, n (%) | 25 (17) | 17 (25) | 0.16 | 2 (6) | 6 (17) | 0.26 | 0.35 |
| Chronic liver disease, n (%) | 4 (3) | 9 (13) | 0.01 | 0 | 0 | ‐ | 0.03 |
| Congenital heart disease, n (%) | 10 (7) | 2 (3) | 0.35 | 0 | 0 | ‐ | 0.54 |
| Diabetes mellitus, n (%) | 11 (7) | 7 (10) | 0.48 | 0 | 1 (2.8) | 1.00 | 0.26 |
| Immunosuppression, n (%) | 9 (6) | 11 (16) | 0.02 | 0 | 0 | ‐ | 0.01 |
| Malignancy, n (%) | 5 (3) | 10 (15) | 0.01 | 0 | 0 | ‐ | 0.01 |
| Obesity, n (%) | 28 (23) | 34 (54) | <0.001 | 6 (19) | 11 (31) | 0.26 | 0.02 |
| Clinical course characteristics | |||||||
| ICU admission, n (%) | 28 (19) | 24 (35) | 0.01 | 15 (43) | 23 (64) | 0.08 | 0.01 |
| ICU length of stay (days) b | 2.1 (±6.8) | 5.3 (±12.1) | 0.01 | 1.8 (±2.6) | 3.6 (±3.9) | 0.04 | 0.08 |
| Hospital length of stay (days) b | 6.1 (± 12.1) | 15.8 (± 27.1) | <0.001 | 5 (3‐7) | 8 (5‐10) | 0.001 | 0.48 |
| Multiorgan dysfunction, n (%) | 6 (4) | 12 (17) | 0.001 | 5 (14) | 11 (31) | 0.10 | 0.12 |
| Respiratory failure, n (%) | 18 (12) | 21 (30) | 0.001 | 1 (3) | 11 (31) | 0.01 | 0.99 |
| Cardiac dysfunction, n (%) | 13 (9) | 12 (17) | 0.06 | 16 (46) | 20 (56) | 0.41 | <0.001 |
| Kidney dysfunction, n (%) | 12 (8) | 5 (7) | 0.85 | 5 (14) | 10 (28) | 0.16 | 0.01 |
| Death, n (%) | 4 (3) | 3 (4) | 0.68 | 0 | 0 | ‐ | 0.55 |
| Therapeutic agent use | |||||||
| Remdesivir, n (%) | 7 (5) | 7 (10) | 0.23 | 0 | 3 (8) | 0.24 | 1.0 |
| Systemic steroids, n (%) | 17 (11) | 23 (33) | <0.001 | 26 (74) | 29 (81) | 0.53 | <0.001 |
| Antibiotics, n (%) | 44 (51) | 31 (76) | 0.01 | 8 (80) | 15 (88) | 0.61 | 0.48 |
Abbreviations: ICU, intensive care unit; IQR, interquartile ranges; SD, standard deviation.
For additional variables, please see supplemental tables.
compares children with elevated ALT values defined as ALT >40 U/L in COVID‐19 vs MIS‐C.
Values reported as mean (±SD).
TABLE 2.
Laboratory characteristics in children with COVID‐19 and MIS‐C with and without elevated ALT
| COVID‐19 | MIS‐C | COVID‐19 vs MIS‐C a | |||||
|---|---|---|---|---|---|---|---|
| ALT ≤ 40 U/L (n = 151) | ALT > 40 U/L (n = 69) | P‐value | ALT ≤ 40 U/L (n = 35) | ALT > 40 U/L (n = 36) | P‐value | P‐value | |
| Liver tests | |||||||
| Median (IQR) | |||||||
| ALT (U/L), peak | 19 (15‐25) | 98 (58‐197) | <0.001 | 23 (15‐31) | 77 (58‐117) | <0.001 | 0.16 |
| AST (U/L), peak | 31 (21‐43) | 78 (53‐137) | <0.001 | 30 (26‐34) | 88 (62‐124) | <0.001 | 0.84 |
| Total bilirubin (mg/dL), peak | 0.4 (0.2‐0.8) | 0.6 (0.4‐1.0) | 0.001 | 0.4 (0.3‐0.6) | 0.8 (0.6‐1.8) | <0.001 | 0.02 |
| Albumin (g/dL), nadir | 4.3 (3.7‐4.8) | 3.5 (2.9‐4.2) | <0.001 | 3.3 (2.5‐4.0) | 2.7 (2.3‐3.2) | 0.02 | <0.001 |
| Coagulation tests | |||||||
| Median (IQR) | |||||||
| INR, peak n = 127 | 1.2 (1.1‐1.3) | 1.2 (1.1‐1.3) | 0.51 | 1.2 (1.1‐1.4) | 1.3 (1.2‐1.4) | 0.23 | 0.01 |
| D Dimer (ug/mL), peak | 1.2 (0.5‐3.2) | 2.2 (0.9‐6.3) | 0.05 | 3.0 (1.5‐4.5) | 4.8 (3.2‐15.3) | 0.01 | 0.01 |
| Complete blood count | |||||||
| Mean (±SD) | |||||||
| White blood cell count (103/mm3), peak | 11.0 (±6.5) | 14.3 (±14.5) | 0.02 | 16.4 (±9.7) | 21.1 (±13.8) | 0.11 | 0.02 |
| Acute lymphocytic count (cells/ mm3), nadir | 2,295 (±1,641) | 1,380 (±1,150) | <0.01 | 2,005 (±1967) | 1,382 (±1234) | 0.33 | 0.99 |
| Platelet count (103/mm3), peak | 250 (±129) | 210 (±120) | 0.03 | 235 (±136) | 134 (±96) | 0.001 | 0.01 |
| Inflammatory markers | |||||||
| Median (IQR) | |||||||
| CRP (mg/dL), peak | 1.4 (0.5‐5.7) | 13.6 (3.6‐23.4) | <0.001 | 14.0 (2.9‐25.2) | 19.0 (6.0‐30.0) | 0.07 | 0.06 |
| Ferritin (ng/mL), peak | 144 (71‐382) | 1208 (368 −2629) | <0.001 | 344 (171‐531) | 759 (508‐1641) | <0.001 | 0.69 |
| IL 6 (pg/mL), peak | 22.9 (7.3‐78.7) | 61.4 (9.0‐315.0) | 0.30 | 213.1 (31.0‐315.0) | 234.6 (86.6‐315.0) | 0.51 | 0.04 |
| LDH (U/L), peak | 287 (217‐386) | 464 (353‐738) | <0.001 | 315 (288‐409) | 370 (300‐482) | 0.07 | 0.19 |
| Other tests | |||||||
| Median (IQR) | |||||||
| CK (IU/L), peak | 138 (77‐226) | 223 (100‐662) | 0.12 | 120 (67‐168) | 280 (78‐983) | 0.18 | 0.93 |
Clinically relevant and significant values depicted.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CRP, c‐reactive protein; g/dL, gram per deciliter; ICU, intensive care unit; IL 6, interleukin 6; INR, international normalized ratio; IU/L, international units per liter; k/uL, kilo per microliter; LDH, lactate dehydrogenase; mg/dl, milligram per deciliter; mg/L, milligrams per liter; mm3, cubic millimeter; ng/mL, nanogram per milliliter; pg/ml, picograms per milliliter; U/L, unit per liter; ug/mL, microgram per milliliter.
For additional variables, please see supplemental tables.
compares children with elevated ALT defined as ALT > 40 U/L in COVID‐19 vs MIS‐C.
3.2. Liver involvement in MIS‐C
Children with E‐ALT were also significantly older, with a median age of 9.5 vs 6 years (P = .01). E‐ALT was associated with Black race (P = .01) and male gender (P = .001). Most children had no prior medical history and no differences in BMI were noted (Tables 1, 2; Tables S3).
3.3. Liver involvement in MIS‐C compared to COVID‐19
Liver involvement in both cohorts was characterized by statistically significant elevation of median ALT above 2x ULN with mild cholestasis. MIS‐C hepatitis was associated with decreased albumin (median 2.7, P < .001) and thrombocytopenia (mean 134, P = .01). Children with E‐ALT and MIS‐C were more frequently Hispanic (P = .04) and of Black race (P < .001). Cardiac dysfunction (P < .001), kidney injury (P = .01), elevated troponin (P = .03), higher pro‐B natriuretic peptide (P < .001), higher median D‐dimer levels (P = .01), and higher peak interleukin‐6 (P = .04) were more frequently recorded in the MIS‐C cohort with E‐ALT (Tables 1, 2; Tables S4).
Children with E‐ALT in both cohorts had a more severe disease course with a greater prevalence of multiorgan dysfunction (P = .001 in both cohorts), respiratory failure (P = .001 and 0.01 in COVID‐19 and MIS‐C, respectively), longer hospitalization (P < .001 and .001), and longer ICU stay (P = .01 and 0.04). No significant association was found between E‐ALT and mortality in COVID‐19 (see Table S6 for details), and no deaths were recorded in the MIS‐C cohort. In multivariable analysis, E‐ALT in the COVID‐19 cohort was associated with an increase in c‐reactive protein (CRP) level (OR 1.08 with 95% confidence interval [CI] [1.03‐1.15]) after adjustment for age, gender, race, obesity, CLD, and ICU admission (Table 3). In the MIS‐C cohort, male gender and Black race were associated with more than 5‐ and 4‐fold increased E‐ALT odds, respectively, when adjusted for age and CRP (Table 3). The association with gender was consistent in all multivariable regression models; Black race was also associated with E‐ALT when adjusted for obesity but did not reach statistical significance in other models (Table S5).
TABLE 3.
Predictors of ALT elevations comparing Children with COVID‐19 and MIS‐C
| COVID‐19 | MIS‐C | |||||
|---|---|---|---|---|---|---|
| OR | 95% Confidence interval | P‐value | OR | 95% Confidence interval | P‐value | |
| Age | 1.02 | 0.94‐1.11 | 0.64 | 1.14 | 0.97‐1.34 | 0.11 |
| Male sex | 2.34 | 0.73‐7.46 | 0.15 | 5.29 | 1.25‐22.43 | 0.02 |
| Black race | 0.28 | 0.06‐1.30 | 0.10 | 4.43 | 1.01‐18.81 | 0.04 |
| Obesity | 1.67 | 0.55‐5.07 | 0.37 | ‐ | ‐ | ‐ |
| Chronic liver disease | 2.39 | 0.32‐17.95 | 0.40 | ‐ | ‐ | ‐ |
| ICU admission | 2.63 | 0.66‐10.44 | 0.17 | ‐ | ‐ | ‐ |
| Peak CRP | 1.08 | 1.03‐1.15 | 0.01 | 1.02 | 0.96‐1.08 | 0.63 |
Multivariable logistic regression comparing children without and with elevated ALT. E‐ALT defined as ALT > 40 U/L. Obesity defined as body mass index ≥95th percentile in patients <18 years‐old or ≥30 kg/m2 in patients 19‐21 years‐old, COVID‐19 cohort: ALT ≤ 40 U/L (n = 151) and ALT > 40 U/L (n = 69)
MIS‐C cohort: ALT ≤ 40 U/L (n = 35) and ALT > 40 U/L (n = 36).
Abbreviations: ALT, alanine aminotransferases; CI, Confidence interval; COVID‐19, coronavirus disease‐2019; CRP, c‐reactive protein; MIS‐C, multisystem inflammatory syndrome in children; OR, odds ratio.
Children with MIS‐C had 2.3× increased odds of elevated ALT levels than children with COVID‐19 after adjusting for age and race (Table 4).
TABLE 4.
Predictors of elevated ALT among children comparing COVID‐19 vs MIS‐C
| OR | 95% Confidence interval | P value | |
|---|---|---|---|
| Age | 1.05 | 0.99‐1.11 | 0.06 |
| Male sex | 2.33 | 1.22‐4.47 | 0.01 |
| Black race | 0.84 | 0.42‐1.69 | 0.62 |
| Presence of MIS‐C | 2.30 | 1.10‐4.87 | 0.03 |
Multivariable logistic regression comparing ONLY children with elevated ALT defined as ALT >40 U/L in COVID‐19 (n = 69) versus MIS‐C (n = 36).
Abbreviations: ALT, alanine aminotransferase; COVID‐19, coronavirus disease‐2019, OR: odds ratio; MIS‐C, multisystem inflammatory syndrome in children.
4. DISCUSSION
In our study, 36% of children developed E‐ALT, which was also associated with mild cholestasis. Irrespective of their SARS‐CoV‐2 manifestations, those with E‐ALT experienced more severe clinical courses. The association of E‐ALT coupled with severe COVID‐19 has been documented in the adult literature but only scarcely in pediatric literature. 13 , 17 , 18 , 21 The E‐ALT mechanism in SARS‐CoV‐2 is not fully understood, but under investigation are an effect of viral lesions in hepatic/cholangiocyte cells, inflammatory damage, hypoxic/shock‐related circulatory compromise, endothelial dysfunction, microthrombi formation, and drug toxicity. 22 , 23 , 24 The direct viral cytopathic effect in the pathophysiology of E‐ALT via the cell receptors for angiotensin‐converting enzyme II and transmembrane protease serine 2, invoked in the transmission of SARS‐CoV‐2, is supported by the demonstration of SARS‐CoV‐2 in the liver parenchyma in patients with higher liver enzyme elevation. 25
As previously reported, children with E‐ALT in the COVID‐19 cohort more frequently carried an underlying medical condition such as immunocompromised state (including malignancy), or CLD, significantly contrasting with those with E‐ALT in MIS‐C. 3 , 4 , 26 In our study, although children with MIS‐C had >2× higher odds of any degree of elevation in ALT levels compared to children with COVID‐19 (51% vs 31%), severe elevation, defined as ALT >200 U/L was observed at a higher frequency in children with COVID‐19 (8% vs 4%) (Figure 1). A possible explanation of why a higher percentage of children with COVID‐19 (as opposed to MIS‐C) had a severe elevation (ALT > 200 U/L) could be due to the significantly greater number of comorbidities that the COVID‐19 population possessed that can contribute to hepatitis, including obesity. It mirrors prior observations that MIS‐C affects previously healthy children, 27 but SARS‐Cov2 can induce a severe cytokine storm with a hyperinflammatory state with either disease entity. The pathophysiology of organ damage is unknown, although differences in cytokines and autoantibodies profiles have been identified. 28
Obesity was present in both cohorts, albeit significantly more prevalent in children with E‐ALT in COVID‐19. Among them, only six children were previously diagnosed with NAFLD. We cannot exclude the possibility of a higher prevalence of NAFLD within this cohort due to our retrospective design limitations. It has been proposed that patients with NAFLD may be susceptible to a worsening viral oxidative stress when infected with a hepatotoxic virus. 29
It is worth noting that the three patients who died of COVID‐19 and developed E‐ALT and the single patient in the MIS‐C cohort who developed acute liver failure were obese (Table S6). Adult literature also highlights the association between clinical course severity and obesity in COVID‐19. It further implies that increased morbidity may be due to obesity‐related impact on cytokine dysfunction or impaired immune response in leptin resistance, raising concerns that obesity influences disease behavior. 30 , 31 , 32 This observation is particularly relevant given the increasing prevalence of obesity in our children and is an essential practical point for the bedside physician. 33
Racial disparities in SARS‐CoV‐2 infection, including a higher disease prevalence and mortality among adult Blacks and Hispanics, have been noted, but these observations are based on limited data. 34 Population‐based data from MIS‐C in NYC also concluded a disproportionate MIS‐C burden among Black and Hispanic children. 35 It is unclear whether this finding represents an actual phenomenon or skewness due to missing race/ethnicity data for most confirmed, non‐hospitalized, and nonfatal cases. 35 We did not identify any racial differences predisposing children to E‐ALT in COVID‐19. Black and Hispanic children with MIS‐C were more frequently affected by E‐ALT compared to those with COVID‐19. Furthermore, children of Black race may be disproportionately impaired by E‐ALT in MIS‐C.
We cannot exclude that some children with COVID‐19 or MIS‐C had additional liver insults including ischemia, congestion, and/or drug‐induced liver injury (DILI). No differences in antibiotics or Remdesivir use between children with and without E‐ALT in both cohorts were observed, making DILI less probable.
There are multiple strengths to our study. We have analyzed one of the largest North American cohort of pediatric patients with E‐ALT in MIS‐C, and COVID‐19 admitted to two tertiary care centers in a former epicenter of the pandemic. Our centers follow similar protocols for diagnosis, treatment, and management and serve diverse patient populations, allowing us to generalize the laboratory values and scrutinize patients' disease courses across both centers.
Our study cohort is significantly smaller than those of adult studies, limiting our ability to model all predictors of E‐ALT used in multivariate analyses of adult populations. Additionally, we were unable to evaluate the full impact of CLD in the full etiology of elevated ALT levels due to lack of preadmission laboratory tests and imaging. Our study, similarly, to others, is burdened by missing race/ethnicity data due to retrospective design.
In conclusion, liver involvement associated with SARS‐CoV‐2 infection typically leads to a temporary moderate elevation of liver tests without significant hepatic synthetic function impairment. Patients with SARS‐CoV‐2 infection and E‐ALT are at risk of a more severe disease course including longer hospitalization and ICU stays. Children require careful management and monitoring throughout their hospitalization and thereafter to establish resolution of elevated ALT values. Further studies need to provide mechanistic insights into the pathophysiology of underlying liver injury in both conditions.
CONFLICTS OF INTEREST
The authors declare no conflict of interest related to this study.
Supporting information
Table S1‐S7
Perez A, Cantor A, Rudolph B, et al. Liver involvement in children with SARS‐COV‐2 infection: Two distinct clinical phenotypes caused by the same virus. Liver Int. 2021;41:2068–2075. 10.1111/liv.14887
Handling Editor: Luca Valenti
Equal contribution: Adriana Perez and Amanda Cantor.
Corresponding co‐authors. Equal contribution: Mercedes Martinez and Nadia Ovchinsky.
Funding informations
This work was supported in part by the National Center for Advancing Translational Sciences (NCATS), components of the National Institutes of Health (NIH), through CTSA grant numbers UL1TR001073 (AR/BR) and KL2TR001071 (BR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. This work is also supported by the Einstein‐Mount Sinai Diabetes Research Center NIH‐5P60DK20541 (BR). Drs Gross‐Margolis and Miller receive support via NIH T32DK083256 (JM), NIH RO1NS01554 (KGM), and DoDPR160365 (KGM).
Contributor Information
Nadia Ovchinsky, Email: Novchins@montefiore.org.
Mercedes Martinez, Email: mm2479@cumc.columbia.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Bialek S, Gierke R, Hughes M, McNamara LA, Pilishvili T, Skoff T. Coronavirus disease 2019 in children — United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID‐19) in a children's hospital in New York City, New York. JAMA Pediatr. 2020;174(10):e202430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prevention CfDaC . Coronavirus disease 2019 (COVID‐19). CDC COVID Data Tracker. 2020. [cited 2020 06/19/2020]. Available from: https://covid.cdc.gov/covid‐data‐tracker/#cases_casesper100klast7days
- 6. Rajapakse N, Dixit D. Human and novel coronavirus infections in children: a review. Paediatr Int Child Health. 2020;41:1‐20. [DOI] [PubMed] [Google Scholar]
- 7. Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis KG. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to coronavirus disease 2019: A single center experience of 44 cases. Gastroenterol. 2020;159(4):1571‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID‐19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174(9):868‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prevention CfDaC , National Center for Health Statistics . Weekly updates by select demographic and geographic characteristics. Provisional death counts for coronavirus disease 2019 (COVID‐19). 2020. [cited 2020 12/02/2020]. Available from: https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/#AgeAndSex
- 10. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS‐CoV‐2 pandemic. Circulation. 2020;142(5):429‐436. [DOI] [PubMed] [Google Scholar]
- 12. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Phipps MM, Barraza LH, LaSota ED, et al. Acute liver injury in COVID‐19: prevalence and association with clinical outcomes in a large U.S. cohort. Hepatol. 2020;72(3):807‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee IC, Huo TI, Huang YH. Gastrointestinal and liver manifestations in patients with COVID‐19. J Chin Med Assoc. 2020;83(6):521‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henry BM, Benoit SW, de Oliveira MHS, et al. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID‐19): a pooled analysis and review. Clin Biochem. 2020;81:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perez A, Kogan‐Liberman D, Sheflin‐Findling S, Raizner A, Ahuja KL, Ovchinsky N. Presentation of severe acute respiratory syndrome‐coronavirus 2 infection as cholestatic jaundice in two healthy adolescents. J Pediatr. 2020;226:278‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai Q, Huang D, Yu H, et al. COVID‐19: abnormal liver function tests. J Hepatol. 2020;73(3):566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cantor A, Miller J, Zachariah P, DaSilva B, Margolis K, Martinez M. Acute hepatitis is a prominent presentation of the multisystem inflammatory syndrome in children: a single‐center report. Hepatol. 2020;72(5):1522‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prevention CfDaC . Multisystem Inflammatory Syndrome (MIS‐C) 2020. 2020. [cited 2020 12/09/2020]. Available from: https://www.cdc.gov/mis‐c/hcp/
- 20. Bussler S, Vogel M, Pietzner D, et al. New pediatric percentiles of liver enzyme serum levels (alanine aminotransferase, aspartate aminotransferase, γ‐glutamyltransferase): effects of age, sex, body mass index, and pubertal stage. Hepatol. 2018;68(4):1319‐1330. [DOI] [PubMed] [Google Scholar]
- 21. Kaushik S, Aydin SI, Derespina KR, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS‐C): a multi‐institutional study from New York City. J Pediatr. 2020;224:24‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrison AG, Lin T, Wang P. Mechanisms of SARS‐CoV‐2 transmission and pathogenesis. Trends Immunol. 2020;41(12):1100‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen H, Zou T‐H, Xuan B, et al. Single cell transcriptome revealed SARS‐CoV‐2 entry genes enriched in colon tissues and associated with coronavirus infection and cytokine production. Signal Transduct Target Ther. 2020;5(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luglio M, Tannuri U, de Carvalho WB, et al. COVID‐19 and liver damage: narrative review and proposed clinical protocol for critically ill pediatric patients. Clinics (Sao Paulo). 2020;75:e2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lagana SM, Kudose S, Iuga AC, et al. Hepatic pathology in patients dying of COVID‐19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33(11):2147‐2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heinz N, Griesemer A, Kinney J, et al. A case of an infant with SARS‐CoV‐2 hepatitis early after liver transplantation. Pediatr Transplant. 2020;24(8):e13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Godfred‐Cato S, Bryant B, Leung J, et al. COVID‐19‐associated multisystem inflammatory syndrome in children ‐ United States, March‐July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1074‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID‐19. Cell. 2020;183(4):968‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Y, Fan C, Chen Y, et al. Effect of hepatic steatosis on the progression of chronic hepatitis B: a prospective cohort and in vitro study. Oncotarget. 2017;8(35):58601‐58610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hussain A, Mahawar K, Xia Z, Yang W, El‐Hasani S. Obesity and mortality of COVID‐19. Meta‐analysis. Obes Res Clin Pract. 2020;14(4):295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Maurya R, Bhattacharya P, Dey R, Nakhasi HL. Leptin functions in infectious diseases. Front Immunol. 2018;9:2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rojas‐Osornio SA, Cruz‐Hernández TR, Drago‐Serrano ME, Campos‐Rodríguez R. Immunity to influenza: impact of obesity. Obes Res Clin Pract. 2019;13(5):419‐429. [DOI] [PubMed] [Google Scholar]
- 33. Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141(3):1999‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Webb Hooper M, Nápoles AM, Pérez‐Stable EJ. COVID‐19 and racial/ethnic disparities. JAMA. 2020;323(24):2466‐2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee EH, Kepler KL, Geevarughese A, et al. Race/ethnicity among children with COVID‐19–associated multisystem inflammatory syndrome. JAMA Netw Open. 2020;3(11):e2030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S7
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


