Abstract
Objective
The aim of this study was to investigate the association of obesity with in‐hospital coronavirus disease 2019 (COVID‐19) outcomes in different ethnic groups.
Methods
Patients admitted to hospital with COVID‐19 in the United Kingdom through the Clinical Characterisation Protocol UK (CCP‐UK) developed by the International Severe Acute Respiratory and emerging Infections Consortium (ISARIC) were included from February 6 to October 12, 2020. Ethnicity was classified as White, South Asian, Black, and other minority ethnic groups. Outcomes were admission to critical care, mechanical ventilation, and in‐hospital mortality, adjusted for age, sex, and chronic diseases.
Results
Of the participants included, 54,254 (age = 76 years; 45.0% women) were White, 3,728 (57 years; 41.1% women) were South Asian, 2,523 (58 years; 44.9% women) were Black, and 5,427 (61 years; 40.8% women) were other ethnicities. Obesity was associated with all outcomes in all ethnic groups, with associations strongest for black ethnicities. When stratified by ethnicity and obesity status, the odds ratios for admission to critical care, mechanical ventilation, and mortality in black ethnicities with obesity were 3.91 (3.13‐4.88), 5.03 (3.94‐6.63), and 1.93 (1.49‐2.51), respectively, compared with White ethnicities without obesity.
Conclusions
Obesity was associated with an elevated risk of in‐hospital COVID‐19 outcomes in all ethnic groups, with associations strongest in Black ethnicities.
Study Importance.
What is already known?
-
►
People of South Asian or Black ethnic origin have been shown to have a higher risk of infection, severe disease, and coronavirus disease 2019 (COVID‐19) mortality compared with those of White ethnicities.
-
►
Obesity is an established risk factor for COVID‐19 outcomes, but less is known about whether ethnicity acts to modify the strength of association observed with obesity or whether the risk remains consistent across ethnic groups.
What does this study add?
-
►
Compared with White individuals without obesity, all other combinations of obesity and ethnicity had a higher risk of admission to critical care, receiving mechanical ventilation, or mortality in those admitted to hospital with COVID‐19. However, the risk of all outcomes was greatest in those of Black ethnicity with obesity.
How might these results change the direction of research or the focus of clinical practice?
-
►
Black ethnic groups with obesity represent a particularly high‐risk group of patients, with implications for targeted public health and vaccination strategies and for identifying those most likely to suffer severe outcomes once admitted to hospital.
Introduction
Obesity and ethnicity are well‐described risk factors for coronavirus disease 2019 (COVID‐19) outcomes (1, 2, 3, 4, 5, 6, 7). People of South Asian or Black ethnic origin, in particular, have been shown to carry a higher risk of infection, severe disease, and COVID‐19 mortality compared with those of White ethnicities (1, 2, 3, 4, 5). In addition, individuals with obesity have around twice the risk of severe outcomes or mortality compared with normal‐weight individuals (6, 7). However, although obesity and ethnicity were shown to be independent of each other as risk factors for COVID‐19 outcomes, less is known about whether ethnicity acts to modify the strength of association observed with obesity or whether the risk remains consistent across different ethnic groups.
The hypothesis that ethnicity may modify associations between obesity and COVID‐19 outcomes is drawn from previous research, suggesting that the dose–repose relationship between levels of obesity and cardiometabolic health is steeper in minority ethnic communities compared with White populations: indeed, the higher the BMI, the greater the difference in health outcomes between minority ethnic groups and White Europeans (8, 9, 10, 11, 12). As cardiometabolic diseases are known risk factors for COVID‐19 outcomes (13, 14, 15, 16), it is possible that obesity may also act as a particularly important risk factor for severe COVID‐19 outcomes in minority ethnic communities. Early research supports this hypothesis, in which the risk of severe acute respiratory syndrome coronavirus 2 infection, severe disease, and COVID‐19 mortality in minority ethnic communities has been shown to be magnified in the presence of obesity (17, 18), although this has not been confirmed in all studies (19). However, evidence to date is preliminary and based on small cohorts with a limited number of outcomes, with minority ethnic groups analyzed as one category. As minority ethnic groups cover heterogeneous populations, it remains uncertain whether associations between obesity and COVID‐19 outcomes differ in all minority ethnic groups or how they apply to national in‐hospital settings.
Investigating whether obesity is a stronger risk factor for in‐patient outcomes in specific minority ethnic groups will help inform public health and vaccination strategies aimed at identifying and targeting patients at greatest risk along with informing in‐hospital clinical decision‐making. In this view, we investigated associations of obesity and ethnicity with in‐hospital critical care and mortality outcomes in patients admitted with COVID‐19 using data from the Clinical Characterisation Protocol UK (CCP‐UK), a preparedness protocol for severe emerging diseases developed by the International Severe Acute Respiratory and emerging Infections Consortium (ISARIC) cohort (20). Previous ISARIC CCP‐UK publications have helped characterize in‐hospital patients admitted with COVID‐19 (21) and have shown a greater risk of in‐hospital outcomes with both obesity and ethnicity (21) (Harrison et al. SSRN, doi:10.2139/ssrn.3618215, unpublished data) during early phases of the pandemic. Having previously established independent associations, this paper investigates their interaction.
We hypothesized that obesity will be a stronger risk factor for in‐hospital outcomes within some minority ethnic groups.
Methods
Cohort
The protocol, amendment history, case report form, information leaflets, consent forms, and details of the Independent Data and Material Access Committee for ISARIC CCP‐UK are available at https://isaric4c.net. The study was approved by the South Central ‐ Oxford C Research Ethics Committee in England (Reference 13/SC/0149) and by the Scotland Research Ethics Committee (Reference 20/SS/0028). For this study, we included participants with a coding of “Proven or high likelihood of infection with a pathogen of Public Health Interest,” reflecting that a preparedness protocol cannot assume a diagnostic test will be available for an emergent pathogen. From January 2020 onward, site training also emphasized the importance of only recruiting proven cases of COVID‐19. Participants were included in the analysis if information was available on hospital admittance date from the emergence of the COVID‐19 pandemic in the United Kingdom (UK) (February 6, 2020); a completed coding indicating that the hospital admission had been resolved through discharge or in‐hospital mortality; and ethnicity status. Data were available up until October 12, 2020.
Data were directly transcribed from routine health care into case report forms hosted on a Research Electronic Data Capture database (REDCap; https://projectredcap.org). Data collection was undertaken by research nurses, administrators, and medical students. Detailed demographic and clinical data were collected on admission, with follow‐up data on clinical care collected at day 3, 6, and 9. The outcome of hospital admission was coded on discharge or death.
Exposures
Obesity was coded as yes or no on assessment from the attending clinician. Clinical assessment was based on objective measurement of obesity, such as BMI (BMI ≥ 30 kg/m2) or abdominal girth, or on clinical judgment.
Ethnicity was transcribed from health care records. In order to be consistent with internationally applicable ethnicity definitions, ethnicity within ISARIC was classified as East Asian, South Asian, West Asian, Black, White, Latin American, aboriginal/First Nations, and other ethnic minority. For the purposes of this analysis and based on frequency, ethnicity was categorized as White, South Asian, Black, or other (East Asian, West Asian, Arab, Latin American, Aboriginal/First Nations, other).
Covariates
Age was measured to the nearest year based on the difference between date of birth and hospital admission date. Sex was coded as male or female. Chronic disease was based on clinician‐diagnosed status. In this study, we included diseases that have been consistently associated with COVID‐19 outcomes (13, 14, 15, 16): chronic cardiac disease (coronary artery disease, heart failure, congenital heart disease, cardiomyopathy, rheumatic heart disease), chronic kidney disease (diagnosed chronic kidney disease or estimated glomerular filtration rate <60 mL/min/1.73 m2), chronic pulmonary disease (chronic obstructive pulmonary disease [chronic bronchitis, emphysema], cystic fibrosis, bronchiectasis, interstitial lung disease, preexisting requirement for long‐term oxygen therapy), diabetes (type 1 or 2), and malignant neoplasm (current solid organ or hematological malignancy). In‐hospital treatment for COVID‐19 was coded at discharge or death. The two main treatment types of oral or intravenous corticosteroids (including dexamethasone) and antivirals were included in this analysis.
Outcome
The main outcomes were admission to a critical care facility (intensive care unit [level 3] or high‐dependency unit [level 2]), any deployed usage of a mechanical ventilation procedure (tracheal intubation or tracheostomy), and in‐hospital mortality.
Statistical analysis
We use logistic regression to quantify associations between obesity and outcomes stratified by ethnicity: odds ratios (ORs) were adjusted for age, sex, and the presence of comorbidities (diabetes, chronic heart disease, chronic kidney disease, chronic pulmonary disease, and cancer). Stratified analysis was undertaken to assess whether the pattern of association of obesity with assessed outcomes in each ethnic group was consistent across strata of age (<70 years, ≥70 years), sex and presence of any chronic disease (defined as diabetes, chronic heart disease, chronic kidney disease, chronic pulmonary disease, and cancer).
To further assess the pattern of associations for both obesity and ethnicity, we defined mutually exclusive groups of ethnicity and obesity and estimated ORs in each category compared with White individuals without obesity (reference group). Sensitivity analysis was undertaken to assess whether the association between mutually exclusive groups of ethnicity and obesity with mortality were independent of in‐hospital corticosteroid and antiviral treatments.
In order to account for missing obesity and covariate data, all analysis was conducted using multiple imputation through the Markov chain Monte Carlo imputation algorithm across five iterations. Associations with obesity within each ethnicity were also derived using a complete case data set as a sensitivity analysis.
All analyses were conducted in SPSS version 26 (IBM Corp., Armonk, New York). Data are reported with 95% CI unless reported otherwise; P < 0.05 was considered significant (e.g., in which the 95% CI does not cross the null).
Results
Of the 65,932 individuals included in this analysis, 54,254 (82.3%) were White, 3,728 (5.7%) South Asian, 2,523 (3.8%) Black, and 5,427 (8.8%) from other ethnic minorities: Table 1 shows the characteristics of the cohort. Compared with White individuals, all minority ethnic groups were younger. Black ethnicities had the highest frequency of coded obesity (13.0%), although there was no difference compared with White individuals when accounting for differences in age and sex (OR 1.00; 95% CI: 0.88‐1.13). Conversely, age and sex adjusted frequencies were lower in South Asian (0.84; 95% CI: 0.76‐0.94) and other minority ethnic groups (0.76; 95% CI: 0.69‐0.84) than in White individuals. When adjusted for age and sex, all minority ethnic groups had a higher risk of in‐hospital outcomes, with associations persisting after further adjustment for obesity and chronic disease (Table 2). For example, compared with White ethnicities, South Asian (1.27; 95% CI: 1.17‐1.38), Black (1.19; 95% CI: 1.08‐1.32), and other ethnicities (1.12; 95% CI: 1.04‐1.21) had a higher risk of mortality.
TABLE 1.
Cohort characteristics
| White (n = 54,254) | South Asian (n = 3,728) | Black (n = 2,523) | Other (n = 5,427) | |||||
|---|---|---|---|---|---|---|---|---|
| Categorical variables | Number | % | Number | % | Number | % | Number | % |
| Sex | ||||||||
| Men | 29,817 | 55.0 | 2,186 | 58.6 | 1,393 | 55.2 | 3,205 | 59.1 |
| Women | 24,340 | 44.9 | 1,536 | 41.2 | 1,128 | 44.7 | 2,213 | 40.8 |
| Missing | 97 | 0.2 | 6 | 0.2 | 2 | 0.1 | 9 | 0.2 |
| Chronic heart disease | ||||||||
| No | 33,016 | 60.9 | 2,630 | 70.5 | 1963 | 77.8 | 3,915 | 72.1 |
| Yes | 18,428 | 34.0 | 772 | 20.7 | 380 | 15.1 | 1,080 | 19.9 |
| Missing | 2,810 | 5.2 | 326 | 8.7 | 180 | 7.1 | 432 | 8.0 |
| Chronic pulmonary disease | ||||||||
| No | 40,846 | 75.3 | 3,123 | 83.8 | 2,187 | 86.7 | 4,508 | 83.1 |
| Yes | 10,403 | 19.2 | 240 | 6.4 | 145 | 5.7 | 482 | 8.9 |
| Missing | 3,005 | 5.5 | 365 | 9.8 | 191 | 7.6 | 437 | 8.1 |
| Diabetes | ||||||||
| No | 41,796 | 77.0 | 2,362 | 63.4 | 1,744 | 69.1 | 3,858 | 71.1 |
| Yes | 7,604 | 14.0 | 852 | 22.9 | 505 | 20.0 | 953 | 17.6 |
| Missing | 4,854 | 8.9 | 514 | 13.8 | 274 | 10.9 | 616 | 11.4 |
| Chronic kidney disease | ||||||||
| No | 41,688 | 76.8 | 2,874 | 77.1 | 1944 | 77.1 | 4,339 | 80.0 |
| Yes | 9,380 | 17.3 | 495 | 13.3 | 393 | 15.6 | 633 | 11.7 |
| Missing | 3,186 | 5.9 | 359 | 9.6 | 186 | 7.4 | 455 | 8.4 |
| Cancer | ||||||||
| No | 44,879 | 82.7 | 3,222 | 86.4 | 2,153 | 85.3 | 4,621 | 85.1 |
| Yes | 5,823 | 10.7 | 126 | 3.4 | 175 | 6.9 | 327 | 6.0 |
| Missing | 3,552 | 6.5 | 380 | 10.2 | 195 | 7.7 | 479 | 8.8 |
| Antiviral treatment | ||||||||
| No | 48,286 | 89.0 | 3,096 | 83.0 | 2,086 | 82.7 | 4,558 | 84.0 |
| Yes | 2,914 | 5.4 | 309 | 8.3 | 251 | 9.9 | 481 | 8.9 |
| Missing | 3,054 | 5.6 | 323 | 8.7 | 186 | 7.4 | 388 | 7.1 |
| Corticosteroid treatment | ||||||||
| No | 43,005 | 79.3 | 2,682 | 71.9 | 1995 | 79.1 | 4,135 | 76.2 |
| Yes | 8,108 | 14.9 | 715 | 19.2 | 340 | 13.5 | 883 | 16.3 |
| Missing | 3,141 | 5.8 | 331 | 8.9 | 2,335 | 92.5 | 409 | 7.5 |
| Obesity | ||||||||
| No | 40,399 | 74.5 | 2,597 | 69.7 | 1,815 | 71.9 | 4,019 | 74.1 |
| Yes | 5,363 | 9.9 | 404 | 10.8 | 328 | 13.0 | 543 | 10.0 |
| Missing | 8,492 | 15.7 | 727 | 19.5 | 380 | 15.1 | 865 | 15.9 |
| Mortality | ||||||||
| No | 37,236 | 68.6 | 2,845 | 76.3 | 1932 | 76.6 | 4,153 | 76.5 |
| Yes | 17,018 | 31.4 | 883 | 23.7 | 591 | 23.4 | 1,274 | 23.5 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mechanical ventilation | ||||||||
| No | 50,349 | 92.8 | 3,087 | 82.8 | 2,056 | 81.5 | 4,499 | 82.9 |
| Yes | 3,278 | 6.0 | 474 | 12.7 | 404 | 16.0 | 816 | 15.0 |
| Missing | 627 | 1.2 | 167 | 4.5 | 63 | 2.5 | 112 | 2.1 |
| Critical care | ||||||||
| No | 47,590 | 87.7 | 2,796 | 75.0 | 1,895 | 75.1 | 4,151 | 76.5 |
| Yes | 6,132 | 11.3 | 773 | 20.7 | 570 | 22.6 | 1,173 | 21.6 |
| Missing | 532 | 1.0 | 159 | 4.3 | 58 | 2.3 | 103 | 1.9 |
| Continuous variables | Median | Interquartile range | Median | Interquartile range | Median | Interquartile range | Median | Interquartile range |
| Age (y) | 76 | 63‐85 | 59 | 44‐73 | 59 | 47‐75 | 61 | 47‐76 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
TABLE 2.
The risk of critical care admission, mechanical ventilation, and in‐hospital mortality in minority ethnic patients compared with White patients
| In‐hospital outcome | White | South Asian | Black | Other |
|---|---|---|---|---|
| Model 1 | ||||
| Critical care admission | Reference | 1.40 (1.28‐1.53) | 1.66 (1.49‐1.83) | 1.53 (1.42‐1.65) |
| Mechanical ventilation | Reference | 1.57 (1.40‐1.76) | 2.17 (1.94‐2.44) | 1.99 (1.82‐2.17) |
| Mortality | Reference | 1.25 (1.15‐1.46) | 1.18 (1.06‐1.30) | 1.09 (1.02‐1.17) |
| Model 2 | ||||
| Critical care admission | Reference | 1.37 (1.26‐1.50) | 1.58 (1.43‐1.75) | 1.51 (1.40‐1.62) |
| Mechanical ventilation | Reference | 1.49 (1.33‐1.68) | 2.03 (1.80‐2.28) | 1.91 (1.75‐2.09) |
| Mortality | Reference | 1.27 (1.17‐1.38) | 1.19 (1.08‐1.32) | 1.12 (1.04‐1.21) |
Data are given as odds ratio (95% CI). Model 1 is adjusted for age and sex. Model 2 is adjusted for age, sex, obesity, diabetes, chronic heart disease, chronic kidney disease, chronic pulmonary disease, and cancer.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Obesity was consistently associated with admission to critical care, mechanical ventilation, and mortality in all ethnic groups (Table 3). However, for all outcomes, the association with obesity was strongest in Black ethnicities. For example, in White ethnicities, the OR for mortality in those with obesity compared with those without obesity was 1.23 (95% CI, 1.15‐1.32) whereas it was 1.98 (95% CI: 1.46‐2.68) for Black ethnicities. The association between obesity and in‐hospital mortality in South Asian (1.34; 95% CI: 1.03‐1.76) and other (1.22; 95% CI: 0.91‐1.62) minority ethnic groups was similar in magnitude to White ethnicities (Table 3). The pattern of association of obesity within each ethnic group was the same when the analysis was restricted to a complete case data set (Supporting Information Table S1). Associations within each ethnic group were also similar in men and women (Figure 1) but tended to be weaker in those with chronic disease or older (≥70 years) adults, particularly for mortality (Figure 1), in which no associations were seen in older adults for any ethnicity. The strongest association for obesity with mortality within assessed strata was observed in Black ethnicities without coexisting chronic disease (OR = 2.95; 95% CI: 1.84‐4.74) (Figure 1).
TABLE 3.
Risk of COVID‐19 outcomes in those with obesity compared with the reference of those without obesity when stratified by ethnicity
| In‐hospital outcome | White | South Asian | Black | Other |
|---|---|---|---|---|
| Model 1 | ||||
| Critical care admission | 2.07 (1.91‐2.24) | 1.63 (1.26‐2.11) | 2.42 (1.90‐3.09) | 1.94 (1.61‐2.34) |
| Mechanical ventilation | 2.11 (1.93‐2.32) | 1.66 (1.18‐2.34) | 2.44 (2.15‐3.31) | 1.91 (1.55‐2.34) |
| Mortality | 1.30 (1.22‐1.38) | 1.42 (1.09‐1.85) | 1.98 (1.46‐2.68) | 1.29 (0.97‐1.70) |
| Model 2 | ||||
| Critical care admission | 2.20 (2.03‐2.38) | 1.72 (1.32‐2.26) | 2.50 (1.95‐3.20) | 2.00 (1.66‐2.42) |
| Mechanical ventilation | 2.27 (2.06‐2.49) | 1.79 (1.27‐2.52) | 2.56 (1.95‐3.37) | 1.92 (1.56‐2.37) |
| Mortality | 1.23 (1.15‐1.32) | 1.34 (1.03‐1.76) | 1.98 (1.46‐2.68) | 1.22 (0.91‐1.62) |
Data are given as odds ratio (95% CI). Reference group is those without obesity within each ethnic strata. Model 1 is adjusted for age and sex. Model 2 is adjusted for age, sex, diabetes, chronic heart disease, chronic kidney disease, chronic pulmonary disease, and cancer.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 1.
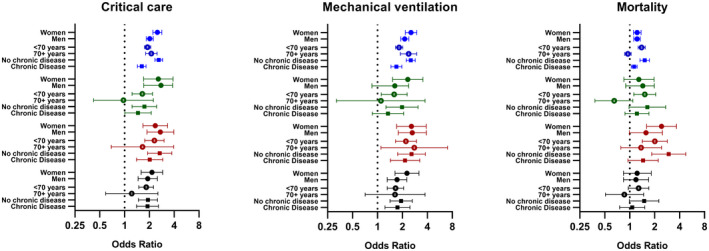
Associations of obesity (compared with those without obesity) with critical care, mechanical ventilation, and mortality for each ethnicity stratified by age, sex, and chronic disease. Error bars display 95% CI. Sex strata adjusted for age, diabetes, chronic heart disease, chronic kidney disease, chronic pulmonary disease, and cancer. Age strata adjusted for sex, diabetes, chronic heart disease, chronic kidney disease, chronic pulmonary disease, and cancer. Chronic disease strata adjusted for age and sex.
Figure 2 shows the association of mutually exclusive categories of obesity and ethnicity with outcomes. Compared with White ethnicities without obesity, all other combinations of obesity and ethnicity had a higher risk of admission to critical care or having mechanical ventilation, with obesity having a stronger association with risk than ethnicity. For example, the OR of admission to a critical care facility in Black ethnicities without obesity was 1.54 (95% CI: 1.38‐1.73) compared with White ethnicities without obesity, whereas the OR in Black ethnicities with obesity was 3.91 (95% CI: 3.13‐4.88). The same comparisons for mechanical ventilation were 1.99 (95% CI: 1.74‐2.27) and 5.03 (95% CI: 3.94‐6.63), respectively (Figure 2). South Asian and other minority ethnic groups without obesity had a similar risk to Black ethnicities without obesity, but the risk in those with obesity was less pronounced. For mortality in those without obesity, risk was marginally higher for Black (1.14; 95% CI: 1.02‐1.28), South Asian (1.27; 95% CI: 1.16‐1.40) and other (1.13; 95% CI: 1.05‐1.23) minority ethnic groups (Figure 2). When considering patients with obesity, the risk of mortality was elevated in all ethnic groups; those with Black ethnicity and obesity had the greatest risk (1.93; 95% CI: 1.49‐2.51) (Figure 2). The pattern and strength of association of ethnicity and obesity with mortality was not affected by further adjusting for in‐hospital corticosteroid and antiviral treatments (Supporting Information Figure S1).
Figure 2.
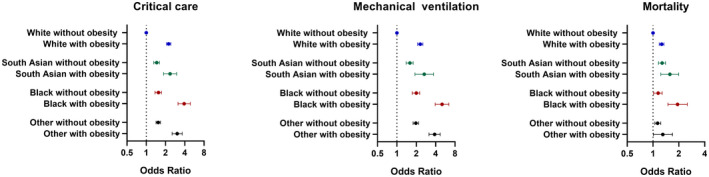
Risk of admittance to critical care, mechanical ventilation, and mortality across categories of obesity and ethnicity compared with White individuals without obesity. Error bars display 95% CI. Adjusted for age, sex, diabetes, chronic heart disease, chronic kidney disease, chronic pulmonary disease, and cancer.
Discussion
In this large national study of patients hospitalized with COVID‐19, obesity was associated with an increased risk of admission to critical care, receiving mechanical ventilation, and in‐hospital mortality in all ethnic groups, with associations strongest in those under 70 years of age or without other chronic diseases. However, the risk was consistently strongest in Black ethnicities with obesity when compared with all other ethnic and obesity groupings, with a four to five times greater risk of admission to critical care or receiving mechanical ventilation and around twice the risk of in‐hospital mortality compared with White ethnicities without obesity. The association of obesity with in‐hospital outcomes was stronger than that of ethnicity; in individuals without obesity, ethnicity was only marginally associated with in‐hospital mortality.
This study extends early preliminary findings from two different early analyses from the UK Biobank community cohort involving 1,087 positive cases and 189 deaths, in which obesity was a stronger predictor of positivity and COVID‐19 mortality in non‐White ethnicities (17, 18). The present study suggests that in patients admitted to hospital, a clinical coding of obesity is a stronger risk factor in Black ethnic groups specifically rather than for minority ethnic groups in general. Obesity in South Asian and Other ethnic minority groups carried a similar level of risk as it did for White ethnicities. There are several hypothesized mechanisms that may explain these findings. Obesity has been hypothesized to increase the risk of severe COVID‐19 through mechanisms linked to restricted pulmonary function and chronic inflammation (2, 22, 23, 24); adipose tissue has also been suggested as a viral reservoir (23). Patients from Black ethnicities may have greater inflammatory responses to infection (24, 25, 26), and excessive systemic innate inflammation due to obesity (“adipositis”) is associated with susceptibility to other infectious diseases (27). Because both clinical and genetic evidence strongly suggests that inflammatory processes drive mortality in COVID‐19 (28, 29), these mechanisms may place Black ethnicities with obesity at a higher risk of organ failure and death. It is also possible that sociodemographic factors, such as greater levels of deprivation and discrimination, may also act through a host of different mechanisms to lower resilience to infection or delay admission to hospital (24), which again could become more severe in the presence of obesity. However, further research is required to disentangle the mechanisms behind the observations reported in this paper.
Associations with obesity in each ethnic group were consistent across men and women but tended to be weaker in older (≥70 years) adults or those with coexisting chronic disease, particularly for the outcome of mortality, in which no association between obesity and mortality was seen in any ethnic group in older adults. Although there was a high level of uncertainty in older minority ethnic populations due to a younger average age and the resulting limited sample of older adults, the findings are consistent with another in‐hospital study in which associations of BMI with mortality or mechanical ventilation were largely attenuated in those over 70 years of age (30). Therefore, the findings from this study may only be generalizable to those under 70 years of age, with ethnic specific associations in older adults needing further investigation. Obesity may also be a particularly strong risk factor for severe COVID‐19 outcomes in otherwise healthy adults, where the highest odds of COVID‐19 mortality with obesity was seen in Black ethnicities without other coexisting chronic diseases.
Strengths of this analysis include the large multisite national sample with data captured from clinical records by trained individuals using standardized operating procedures, allowing the largest analysis of COVID‐19 outcomes with obesity and ethnicity to date. However, there are some important limitations. Obesity was defined through clinician assessment, with the prevalence an underestimate compared with levels that would have been expected based on population estimates (31). Thus, the coding of obesity possibly reflects more extreme phenotypes of obesity that are likely to prompt a clinical coding, and it is unknown whether this procedure was biased by ethnicity. However, the pattern of obesity prevalence in this sample is broadly consistent with national survey data for overweight and obesity prevalence (32), with rates higher in Black ethnic groups but lower in other minority ethnic groups compared with White ethnicities. It is also important to note that, in order to inform clinical care, analyzed risk factors need to reflect data that are readily available to treating clinical staff through clinical records. Therefore, the coding of obesity in this study may have real‐world utility as it corresponds to data collected within routine clinical care. Another potential limitation is that the coding of ethnicity was designed to be consistent with international definitions, rather than those designed for the UK populations. As such, it is acknowledged that the terms “Black,” “South Asian,” “White,” and “other” cover a wide range of different cultures and races. Consequently, our analyses may have masked important differences between further stratified ethnic groups. However, comparisons using these broad ethnic groupings are informative for understanding initial ethnic differences that can then be further and more granularly investigated. Finally, this analysis did not have access to potential sociodemographic confounders, such as deprivation, housing, or employment status. However, the purpose of this analysis was to highlight ethnic differences in the strength of obesity as a global risk factor for in‐hospital outcomes following admission with COVID‐19, rather than for supporting potential etiological conclusions around reducing levels of obesity per se.
In conclusion, although obesity was a consistent risk factor for adverse in‐hospital outcomes following admission with COVID‐19 within all ethnic groups, particularly for younger adults without coexisting chronic disease, the risk with obesity was greatest in Black ethnicities compared with other ethnic groups. Black ethnic groups with obesity therefore represent a particularly high‐risk group of patients with implications for targeted public health and vaccination strategies and for identifying those most likely to suffer severe outcomes once admitted to hospital.
Funding agencies
This work was supported by the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre (BRC), NIHR Applied Research Collaboration East Midlands (ARC EM), and a grant from the UK Research and Innovation ‐ Department of Health and Social Care (UKRI‐DHSC) COVID‐19 Rapid Response Rolling Call (MR/V020536/1 to TY); NIHR (award CO‐CIN‐01 to MGS); the Medical Research Council (MRC; grant MC_PC_19059 to MGS); and by the NIHR Health Protection Research Unit (HPRU) in Emerging and Zoonotic Infections at University of Liverpool. The funder/sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure
KK is supported by the NIHR ARC EM and TY by the NIHR BRC. KK is Director for the University of Leicester Centre for Black and Minority Ethnic Health, trustee of the South Asian Health Foundation, national NIHR ARC lead for Ethnicity and Diversity, and a member of the Independent Scientific Advisory Group for Emergencies (SAGE) and Chair of the SAGE subgroup on ethnicity and COVID‐19. MGS is a member of SAGE COVID‐19. MGS reports grants from DHSC NIHR UK, grants from MRC UK, grants from HPRU in Emerging and Zoonotic Infections, University of Liverpool, during the conduct of the study, and other support from Integrum Scientific LLC, Greensboro, North Carolina, outside the submitted work. The other authors declared no conflict of interest.
Supporting information
Supplementary Material
Acknowledgments
The ISARIC WHO CCP‐UK study was registered at https://www.isrctn.com/ISRCTN66726260 and designated an Urgent Public Health Research Study by NIHR.
The study protocol is available at https://isaric4c.net/protocols. This work uses data provided by patients and collected by the National Health Service (NHS) as part of their care and support campaign #DataSavesLives. We are grateful to the 2,648 frontline NHS clinical and research staff and volunteer medical students who collected the data in challenging circumstances and the generosity of the participants and their families for their individual contributions in these difficult times. We also acknowledge the support of Jeremy J. Farrar and Nahoko Shindo.
ISARIC Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators: consortium lead investigator: J. Kenneth Baillie; chief investigator: Malcolm G. Semple; co‐lead investigator: Peter JM Openshaw; ISARIC clinical coordinator: Gail Carson; co‐investigators: Beatrice Alex, Benjamin Bach, Wendy S. Barclay, Debby Bogaert, Meera Chand, Graham S. Cooke, Annemarie B. Docherty, Jake Dunning, Ana da Silva Filipe, Tom Fletcher, Christopher A. Green, Ewen M. Harrison, Julian A. Hiscox, Antonia Ying Wai Ho, Peter W. Horby, Samreen Ijaz, Saye Khoo, Paul Klenerman, Andrew Law, Wei Shen Lim, Alexander J. Mentzer, Laura Merson, Alison M. Meynert, Mahdad Noursadeghi, Shona C. Moore, Massimo Palmarini, William A. Paxton, Georgios Pollakis, Nicholas Price, Andrew Rambaut, David L. Robertson, Clark D. Russell, Vanessa Sancho‐Shimizu, Janet T. Scott, Louise Sigfrid, Tom Solomon, Shiranee Sriskandan, David Stuart, Charlotte Summers, Richard S. Tedder, Emma C. Thomson, Ryan S. Thwaites, Lance C. W. Turtle, Maria Zambon; project managers: Hayley Hardwick, Chloe Donohue, Jane Ewins, Wilna Oosthuyzen, Fiona Griffiths; data analysts: Lisa Norman, Riinu Pius, Tom M. Drake, Cameron J. Fairfield, Stephen Knight, Kenneth A. Mclean, Derek Murphy, Catherine A. Shaw; data and information system manager: Jo Dalton, Michelle Girvan, Egle Saviciute, Stephanie Roberts, Janet Harrison, Laura Marsh, Marie Connor, Sophie Halpin, Clare Jackson, Carrol Gamble; data integration and presentation: Gary Leeming, Andrew Law, Ross Hendry, James Scott‐Brown; material management: William Greenhalf, Victoria Shaw, Sarah McDonald; outbreak laboratory volunteers: Katie A. Ahmed, Jane A. Armstrong, Milton Ashworth, Innocent G. Asiimwe, Siddharth Bakshi, Samantha L. Barlow, Laura Booth, Benjamin Brennan, Katie Bullock, Benjamin W. A. Catterall, Jordan J. Clark, Emily A. Clarke, Sarah Cole, Louise Cooper, Helen Cox, Christopher Davis, Oslem Dincarslan, Chris Dunn, Philip Dyer, Angela Elliott, Anthony Evans, Lewis W. S. Fisher, Terry Foster, Isabel Garcia‐Dorival, Willliam Greenhalf, Philip Gunning, Catherine Hartley, Antonia Ho, Rebecca L. Jensen, Christopher B. Jones, Trevor R. Jones, Shadia Khandaker, Katharine King, Robyn T. Kiy, Chrysa Koukorava, Annette Lake, Suzannah Lant, Diane Latawiec, L. Lavelle‐Langham, Daniella Lefteri, Lauren Lett, Lucia A. Livoti, Maria Mancini, Sarah McDonald, Laurence McEvoy, John McLauchlan, Soeren Metelmann, Nahida S. Miah, Joanna Middleton, Joyce Mitchell, Shona C. Moore, Ellen G. Murphy, Rebekah Penrice‐Randal, Jack Pilgrim, Tessa Prince, Will Reynolds, P. Matthew Ridley, Debby Sales, Victoria E. Shaw, Rebecca K. Shears, Benjamin Small, Krishanthi S. Subramaniam, Agnieska Szemiel, Aislynn Taggart, Jolanta Tanianis‐Hughes, Jordan Thomas, Erwan Trochu, Libby van Tonder, Eve Wilcock, J. Eunice Zhang; local principal investigators: Kayode Adeniji, Daniel Agranoff, Ken Agwuh, Dhiraj Ail, Ana Alegria, Brian Angus, Abdul Ashish, Dougal Atkinson, Shahedal Bari, Gavin Barlow, Stella Barnass, Nicholas Barrett, Christopher Bassford, David Baxter, Michael Beadsworth, Jolanta Bernatoniene, John Berridge, Nicola Best, Pieter Bothma, David Brealey, Robin Brittain‐Long, Naomi Bulteel, Tom Burden, Andrew Burtenshaw, Vikki Caruth, David Chadwick, Duncan Chambler, Nigel Chee, Jenny Child, Srikanth Chukkambotla, Tom Clark, Paul Collini, Catherine Cosgrove, Jason Cupitt, Maria‐Teresa Cutino‐Moguel, Paul Dark, Chris Dawson, Samir Dervisevic, Phil Donnison, Sam Douthwaite, Ingrid DuRand, Ahilanadan Dushianthan, Tristan Dyer, Cariad Evans, Chi Eziefula, Chrisopher Fegan, Adam Finn, Duncan Fullerton, Sanjeev Garg, Sanjeev Garg, Atul Garg, Jo Godden, Arthur Goldsmith, Clive Graham, Elaine Hardy, Stuart Hartshorn, Daniel Harvey, Peter Havalda, Daniel B. Hawcutt, Maria Hobrok, Luke Hodgson, Anita Holme, Anil Hormis, Michael Jacobs, Susan Jain, Paul Jennings, Agilan Kaliappan, Vidya Kasipandian, Stephen Kegg, Michael Kelsey, Jason Kendall, Caroline Kerrison, Ian Kerslake, Oliver Koch, Gouri Koduri, George Koshy, Shondipon Laha, Susan Larkin, Tamas Leiner, Patrick Lillie, James Limb, Vanessa Linnett, Jeff Little, Michael MacMahon, Emily MacNaughton, Ravish Mankregod, Huw Masson, Elijah Matovu, Katherine McCullough, Ruth McEwen, Manjula Meda, Gary Mills, Jane Minton, Mariyam Mirfenderesky, Kavya Mohandas, Quen Mok, James Moon, Elinoor Moore, Patrick Morgan, Craig Morris, Katherine Mortimore, Samuel Moses, Mbiye Mpenge, Rohinton Mulla, Michael Murphy, Megan Nagel, Thapas Nagarajan, Mark Nelson, Igor Otahal, Mark Pais, Selva Panchatsharam, Hassan Paraiso, Brij Patel, Justin Pepperell, Mark Peters, Mandeep Phull, Stefania Pintus, Jagtur Singh Pooni, Frank Post, David Price, Rachel Prout, Nikolas Rae, Henrik Reschreiter, Tim Reynolds, Neil Richardson, Mark Roberts, Devender Roberts, Alistair Rose, Guy Rousseau, Brendan Ryan, Taranprit Saluja, Aarti Shah, Prad Shanmuga, Anil Sharma, Anna Shawcross, Jeremy Sizer, Richard Smith, Catherine Snelson, Nick Spittle, Nikki Staines, Tom Stambach, Richard Stewart, Pradeep Subudhi, Tamas Szakmany, Kate Tatham, Jo Thomas, Chris Thompson, Robert Thompson, Ascanio Tridente, Darell Tupper‐Carey, Mary Twagira, Andrew Ustianowski, Nick Vallotton, Lisa Vincent‐Smith, Shico Visuvanathan, Alan Vuylsteke, Sam Waddy, Rachel Wake, Andrew Walden, Ingeborg Welters, Tony Whitehouse, Paul Whittaker, Ashley Whittington, Meme Wijesinghe, Martin Williams, Lawrence Wilson, Sarah Wilson, Stephen Winchester, Martin Wiselka, Adam Wolverson, Daniel G. Wooton, Andrew Workman, Bryan Yates, Peter Young.
References
- 1. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature 2020;584:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Office for National Statistics (ONS) . Coronavirus (COVID‐19) related deaths by ethnic group, England and Wales: 2 March 2020 to 10 April 2020. Published May 7, 2020. Accessed July 28, 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/coronavirusrelateddeathsbyethnicgroupenglandandwales/2march2020to10april2020
- 3. Office for National Statistics (ONS) . Updating ethnic contrasts in deaths involving the coronavirus (COVID‐19), England and Wales: deaths occurring 2 March to 28 July 2020. Published October 16, 2020. Accessed October 26, 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/updatingethniccontrastsindeathsinvolvingthecoronaviruscovid19englandandwales/deathsoccurring2marchto28july2020
- 4. Pan D, Sze S, Minhas JS, et al. The impact of ethnicity on clinical outcomes in COVID‐19: a systematic review. EClinicalMedicine 2020;23:100404. doi: 10.1016/j.eclinm.2020.100404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID‐19: a systematic review and meta‐analysis. EClinicalMedicine 2020;29:100630. doi: 10.1016/j.eclinm.2020.100630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seidu S, Gillies C, Zaccardi F, et al. The impact of obesity on severe disease and mortality in people with SARS‐CoV‐2: a systematic review and meta‐analysis. Endocrinol Diabetes Metab 2020;4:e00176. doi: 10.1002/edm2.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Földi M, Farkas N, Kiss S, et al. Obesity is a risk factor for developing critical condition in COVID‐19 patients: a systematic review and meta‐analysis. Obes Rev 2020;21:e13095. doi: 10.1111/obr.13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Razak F, Anand S, Vuksan V, et al. Ethnic differences in the relationships between obesity and glucose‐metabolic abnormalities: a cross‐sectional population‐based study. Int J Obes 2005;29:656‐667. [DOI] [PubMed] [Google Scholar]
- 9. Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic‐specific BMI cutoff points for assessing diabetes risk. Diabetes Care 2011;34:1741‐1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ntuk UE, Gill JM, Mackay DF, Sattar N, Pell JP. Ethnic‐specific obesity cutoffs for diabetes risk: cross‐sectional study of 490,288 UK biobank participants. Diabetes Care 2014;37:2500‐2507. [DOI] [PubMed] [Google Scholar]
- 11. Tillin T, Sattar N, Godsland IF, Hughes AD, Chaturvedi N, Forouhi NG. Ethnicity‐specific obesity cut‐points in the development of type 2 diabetes–a prospective study including three ethnic groups in the United Kingdom. Diabet Med 2015;32:226‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gray LJ, Yates T, Davies MJ, et al. Defining obesity cut‐off points for migrant South Asians. PLoS One 2011;6:e26464. doi: 10.1371/journal.pone.0026464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh AK, Gillies CL, Singh R, et al. Prevalence of co‐morbidities and their association with mortality in patients with COVID‐19: a systematic review and meta‐analysis. Diabetes Obes Metab 2020;22:1915‐1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre‐existing comorbidities with COVID‐19 mortality: a systematic review and meta‐analysis. PLoS One 2020;15:e0238215. doi: 10.1371/journal.pone.0238215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahumud RA, Kamara JK, Renzaho AMN. The epidemiological burden and overall distribution of chronic comorbidities in coronavirus disease‐2019 among 202,005 infected patients: evidence from a systematic review and meta‐analysis. Infection 2020;48:813‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID‐19‐related mortality in England: a whole‐population study. Lancet Diabetes Endocrinol 2020;8:813‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sattar N, Ho FK, Gill JM, et al. BMI and future risk for COVID‐19 infection and death across sex, age and ethnicity: preliminary findings from UK biobank. Diabetes Metab Syndr 2020;14:1149‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Razieh C, Zaccardi F, Davies MJ, Khunti K, Yates T. Body mass index and risk of COVID‐19 across ethnic groups: analysis of UK Biobank. Diabetes Obes Metab 2020;22:1953‐1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID‐19: results from an integrated health care organization. Ann Intern Med 2020;173:773‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dunning JW, Merson L, Rohde GGU, et al. Open source clinical science for emerging infections. Lancet Infect Dis 2014;14:8‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369:m1985. doi:10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michalakis K, Ilias I. SARS‐CoV‐2 infection and obesity: common inflammatory and metabolic aspects. Diabetes Metab Syndr 2020;14:469‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kruglikov IL, Scherer PE. The role of adipocytes and adipocyte‐like cells in the severity of COVID‐19 infections. Obesity (Silver Spring) 2020;28:1187‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vepa A, Bae JP, Ahmed F, Pareek M, Khunti K. COVID‐19 and ethnicity: a novel pathophysiological role for inflammation. Diabetes Metab Syndr 2020;14:1043‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nédélec Y, Sanz J, Baharian G, et al. Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell 2016;167:657‐669.e21. [DOI] [PubMed] [Google Scholar]
- 26. Yeyeodu ST, Kidd LR, Kimbro KS. Protective innate immune variants in racial/ethnic disparities of breast and prostate cancer. Cancer Immunol Res 2019;7:1384‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clohisey S, Baillie JK. Host susceptibility to severe influenza A virus infection. Crit Care 2019;23:303. doi: 10.1186/s13054-019-2566-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19—final report. N Engl J Med 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pairo‐Castineira E, Clohisey S, Klaric L, et al. Genetic mechanisms of critical illness in Covid‐19. Nature 2020;591:92‐98. [DOI] [PubMed] [Google Scholar]
- 30. Hendren NS, de Lemos JA, Ayers C, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID‐19: results from the American Heart Association COVID‐19 Cardiovascular Disease Registry. Circulation 2020;143:134‐144. [DOI] [PubMed] [Google Scholar]
- 31. NHS . Health Survey for England 2017. Published December 4,; 2018. Accessed November 12, 2020. https://digital.nhs.uk/data‐and‐information/publications/statistical/health‐survey‐for‐england/2017 [Google Scholar]
- 32. GOV.UK . Overweight adults: by ethnicity over time. Published May 19, 2020. Accessed November 18, 2020. https://www.ethnicity‐facts‐figures.service.gov.uk/health/diet‐and‐exercise/overweight‐adults/latest#by‐ethnicity‐over‐time
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


