Abstract
Background
The coronavirus disease‐2019 (COVID‐19) outbreak has presented unique dermatologic challenges due to respiratory protective equipment (RPE)–related skin conditions.
Objective
To objectively evaluate the effects of RPE including medical masks and respirators on the skin barrier by measuring various physiological properties of the skin.
Methods
A cross‐sectional study was designed. Twenty healthy healthcare workers were included in this study. Skin parameters including skin hydration, transepidermal water loss (TEWL), erythema, sebum secretion, pH, and skin temperature were measured in the RPE‐covered and RPE‐uncovered areas of the face 4 and 8 hours after wearing RPE and 14 hours after not wearing RPE.
Results
Skin hydration, TEWL, erythema, pH, and skin temperature increased in the RPE‐covered areas after wearing RPE for 4 and 8 hours. By contrast, in the RPE‐uncovered areas, skin hydration decreased and TEWL, erythema, and pH showed minimal changes over time. Based on the repeated‐measure analysis, the changes in skin physiological properties over time were significantly different between RPE‐covered and RPE‐uncovered areas.
Conclusion
We observed that skin physiological characteristics change with the prolonged use of RPE such as medical masks and respirators. These changes may lead to various adverse skin reactions after long‐term use.
Keywords: adverse skin reaction, COVID‐19, healthcare workers, medical mask, respirators, respiratory protective equipment, skin biophysical properties
The coronavirus disease‐2019 (COVID‐19) outbreak has presented unique dermatologic challenges related to respiratory protective equipment–related skin conditions.
Skin hydration, transepidermal water loss, erythema, pH, and skin temperature increased after wearing the respiratory protective equipment for a prolonged time, which may lead to various adverse skin reactions.
Our results would help physicians understand the mechanism behind skin reactions and allow for comprehensive preventive measures that would be important in the present situation wherein COVID‐19 continues to persist.
Abbreviations
- COVID‐19
coronavirus disease‐2019
- HCW
healthcare worker
- IR
infrared
- KCA
KF94 respirator–covered area
- KF94
Korean Filter 94 respirator
- KUA
K94 respirator–uncovered area
- MCA
medical mask–covered area
- MUA
medical mask–uncovered area
- PPE
personal protective equipment
- RM‐ANOVA
Repeated measures analysis of variance
- RPE
respiratory protective equipment
- SC
stratum corneum
- TEWL
transepidermal water loss
1. INTRODUCTION
The coronavirus disease‐2019 (COVID‐19) pandemic is one of the most devastating viral outbreaks in modern history. Although the world has suffered from other deadly pandemics in the past, the impact of the COVID‐19 pandemic is unprecedented. The extremely wide and rapid spread of this pandemic, which was enabled by globalization, has forced us to recognize the importance of foundational measures of disease control, including “physical distancing,” “physical isolation,” and universal infection control precautions including handwashing and the use of personal protective equipment (PPE). Because the virus is mainly spread via respiratory droplets, respiratory protective equipment (RPE) such as medical masks (surgical masks) or respirators (filtering facepiece) is arguably the most important piece of PPE. 1 , 2
However, prolonged daily use of RPE itself can lead to physical and psychological disturbances especially among healthcare workers (HCWs). 3 In fact, there has been an increasing number of reports on RPE‐related skin conditions among HCWs fighting against COVID‐19, with the prevalence estimated up to 74%. 4 , 5 , 6 , 7 , 8 , 9 A recent study even reported that 21% of HCWs suffered from work absenteeism due to various RPE‐related facial dermatoses. 10 However, there is insufficient objective data regarding the effect of RPE on the skin barrier. Therefore, this study aimed to objectively evaluate the effects of RPE such as wearing medical masks or respirators on the skin barrier by measuring various physiological properties of the skin.
2. MATERIALS AND METHODS
2.1. Study design and participants
Twenty healthy HCWs with no history of skin diseases or skin changes at the test sites were included in the study. Exclusion criteria were (a) the use of topical or systemic corticosteroids, retinoids, or other medications that can alter the skin condition for 1 month before inclusion and during the study; and (b) nonadherence to the study protocol.
The study protocol was approved by the Institutional Review Board (approval number: P2007‐1336, P&K Skin ResearchCente), and the study conformed to the guidelines of the Declaration of Helsinki.
2.2. Wearing RPE and checking area
After being evaluated for eligibility, participants were randomly assigned to wear either a Korean Filter 94 respirator (KF94 respirator; 3M Corporation, St. Paul, MN, USA), which is equivalent to the European FFP2 respirator, or a medical mask (surgical mask; Kimberly‐Clark, Roswell, GA, USA). Participants were given the same face wash and moisturizer (Laviderm; HP&C Ltd, Seoul, Korea) to use for at least one week before the start of the study. Participants were prohibited from using all other skincare products other than the provided face wash and moisturizer and receiving any skincare procedures that could alter their skin condition until the end of the study.
For each participant, the facial skin was divided into an RPE‐covered area and an RPE‐uncovered area, and two points were designated for measurement in each region. The points of measurement were defined such that the same point could be selected at each measurement time (Figure 1). The two values obtained from the two points in each RPE‐covered and uncovered area were averaged to obtain a single value for each region.
FIGURE 1.
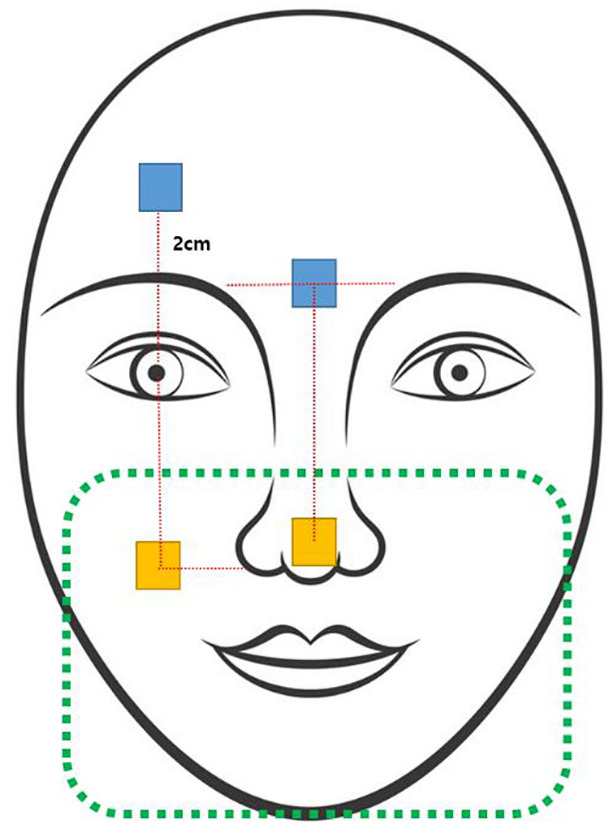
Points of measurement of skin properties on the face. Yellow box: measurement points on the respiratory protective equipment–covered area; blue box: measurement points on the respiratory protective equipment–uncovered area
Measurements were performed four times in total. At baseline, participants gently washed their faces with water and were acclimatized to an indoor environment without RPE for 30 minutes. Baseline measurements (V0) were performed at approximately 8 am on the first day. After wearing the RPE for 4 hours, the second measurements (V1) were taken again at around 12 pm. After wearing the RPE for another 4 hours, the third measurements (V2) were taken at around 4 pm. After three measurements on the first day, participants were instructed to return to their homes and not wear the RPE until the next morning. The fourth measurements (V3) were performed the next morning at around 8 am (approximately 14 hours after the last use of RPE).
Between the measurement periods, all participants were allowed to continue their usual routines, but only in the outpatient setting. Furthermore, all participants were guided to adhere to the study protocol that is wearing either a KF94 respirator or a medical mask without additional protective equipment such as face shields or other facial coverings.
2.3. Measurement of skin parameters and skin temperature
Corneometer CM825, Tewameter TM300, Mexameter MX18, Sebumeter SM815, and Skin‐pH‐Meter PH905 (Courage & Khazaka GmbH, Cologne, Germany) were used to assess skin hydration, transepidermal water loss (TEWL), erythema, sebum secretion, and pH. All measurements were taken in accordance with the manufacturer's guidelines. During the measurement, the room temperature was maintained at a constant temperature of 20 to 22°C and a relative humidity range of 40% to 60%.
The skin temperature of the RPE‐covered area was evaluated using infrared (IR) thermography. IR thermography is a noninvasive method that detects IR energy emitted from an object and converts it to temperature to display an image of temperature distribution. At each measurement time, the facial temperature of the perioral region of each participant was recorded using a 14‐bit digital IR camera (FLIR SC660 QWIP; FLIR Systems, Danderyd, Sweden).
2.4. Statistical analysis
Data were analysed using the SPSS package (SPSS for Windows, version 24.0; SPSS, Inc, Chicago, IL). Repeated measures analysis of variance (RM‐ANOVA) was used to analyse the change in skin physiological properties and temperature due to RPE use by time and group. Initial data were analysed using the Mauchly test of sphericity, with the Greenhouse–Geisser adjustment to correct for the lack of sphericity. The Bonferroni method was used to control the type I error rate for post hoc procedures. Consolidated data were analysed using independent sample t tests. A P value <.05 was considered statistically significant.
3. RESULTS
The baseline demographic characteristics of the participants are summarized in Table 1. There was no significant difference in sex, age, or average duration of RPE use per day between the KF94 respirator group and the medical mask group (Table 1).
TABLE 1.
Baseline characteristics of the participants in each group
| Demographic parameters | Respirator (KF94 respirator) (n = 10) | Medical mask (n = 10) | P value a |
|---|---|---|---|
| Sex, n (%) | >.99 | ||
| Male | 1 (10.0) | 1 (10.0) | |
| Female | 9 (90.0) | 9 (90.0) | |
| Age (years) | |||
| Mean | 37.5 | 34.7 | .614 |
| SD | 10.83 | 13.43 | |
| Median | 39.5 | 29.5 | |
| Average duration of RPE use per day, n (%) | .795 | ||
| <4 hours | 1 (10.0) | 3 (30.0) | |
| 4–8 hours | 3 (30.0) | 0 (0) | |
| >8 hours | 6 (60.0) | 7 (70.0) |
Calculated using the Student t test.
Abbreviations: RPE, respiratory protective equipment; SD, standard deviation.
3.1. Skin hydration
At the baseline, the KF94 respirator–covered area (KCA) and medical mask–covered area (MCA) had significantly lower skin hydration values than the KF94 respirator–uncovered area (KUA) and medical mask–uncovered area (MUA). After wearing the RPE for 4 and 8 hours, skin hydration in the KCA and MCA remained significantly lower than that in the KUA and MUA (Figure 2A; P < .001). However, skin hydration in the KCA and MCA increased while skin hydration in the KUA and MUA remained relatively constant over time with RPE use. After 14 hours without wearing RPE (V3), the skin hydration returned to values similar to those at baseline in the RPE‐covered areas regardless of the type of mask. Based on the RM‐ANOVA, the changes in skin hydration over time were statistically significantly different between KCA and KUA as well as MCA and MUA (Figure 2A; P < .001).
3.2. Transepidermal water loss
At the baseline, TEWL was greater in the KCA and MCA than in the KUA and MUA. Furthermore, TEWL in the KCA and MCA increased while TEWL in the KUA and MUA remained relatively constant over time with RPE use. Thus, after 8 hours of RPE use, TEWL in the KCA was statistically significantly greater than that in the KUA (Figure 2B; P < .05). TEWL in the MCA was also statistically significantly greater than that in the MUA after 4 and 8 hours of RPE use (Figure 2B; P < .05). After 14 hours without RPE (V3), TEWL returned to values similar to those at baseline in the RPE‐covered area. Based on the RM‐ANOVA, the changes in TEWL over time were statistically significantly different between KCA and KUA as well as MCA and MUA (Figure 2B; P < .001).
FIGURE 2.
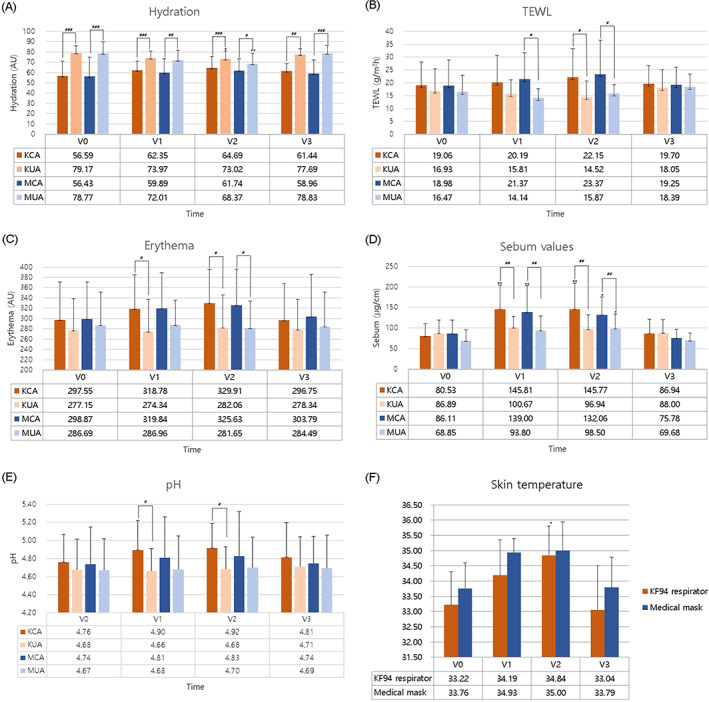
Skin properties on the face, including (A) hydration, (B) transepidermal water loss (TEWL), (C) erythema, (D) sebum values, (E) pH, and (F) skin temperature. P‐values <.05 are significant. *P < .05, **P < .01, compared with baseline. #P < .05, ##P < .01, ###P < .001, compared with the uncovered area. V0: Baseline; V1: 4 hours after wearing the respiratory protective equipment (RPE); V2: 8 hours after wearing the RPE; V3: 14 hours after taking off the RPE. Abbreviations: KCA, KF94 respirator‐covered area; KUA, KF94‐uncovered area; MCA, medical mask–covered area; MUA, medical mask–uncovered area
3.3. Skin erythema
At the baseline, the KCA and MCA had higher skin erythema levels than the KUA and MUA. Furthermore, skin erythema in the KCA and MCA increased while skin erythema in the KUA and MUA remained relatively constant over time with RPE use. Thus, after 4 and 8 hours of RPE use, skin erythema in the KCA was statistically significantly greater than that in the KUA (Figure 2C; P < .05). Skin erythema in the MCA was also statistically significantly greater than that in the MUA after 8 hours of RPE use (Figure 2C; P < .05). After 14 hours without RPE (V3), skin erythema returned to values similar to those at baseline in the RPE‐covered area. However, based on the RM‐ANOVA, the changes in skin erythema over time were not statistically significantly different between KCA and KUA as well as MCA and MUA.
3.4. Sebum secretion
At the baseline, sebum secretion levels were similar in both RPE‐covered and RPE‐uncovered areas. Furthermore, sebum secretion increased over time in both areas, but the changes were greater in the RPE‐covered area. Thus, after 4 and 8 hours of RPE use, sebum secretion levels were statistically significantly greater in the RPE‐covered areas than in the RPE‐uncovered areas (Figure 2D; P < .01). After 14 hours without RPE (V3), sebum secretion levels returned to near baseline levels in the RPE‐covered area. Based on the RM‐ANOVA, the changes in sebum secretion over time were statistically significantly different between the KCA and KUA (Figure 2D; P < .001), although not significantly different between the MCA and MUA.
3.5. pH
At the baseline, the KCA and MCA had higher pH levels than the KUA and MUA. Furthermore, pH levels in the KCA and MCA increased, whereas minimal changes were observed in the KUA and MUA over time with RPE use. Thus, after 4 and 8 hours of RPE use, skin pH level in the KCA was statistically significantly greater than that in the KUA (Figure 2E; P < .05). After 14 hours without RPE (V3), skin pH returned to values near baseline in the RPE‐covered area. However, based on the RM‐ANOVA, changes in the skin pH over time were not statistically significantly different between KCA and KUA as well as MCA and MUA.
3.6. Facial skin temperature
IR thermography images demonstrated a temperature increase at mask–skin contact sites after 4 and 8 hours of RPE use in both types of RPE. After 8 hours of RPE use, the skin temperature in the KCA and MCA increased by 1.62 and 1.24°C, respectively. After 14 hours without RPE (V3), the skin temperature returned to values similar to those at baseline in the RPE‐covered areas (Figure 2F). Representative IR thermography images are shown in Figure 3.
FIGURE 3.
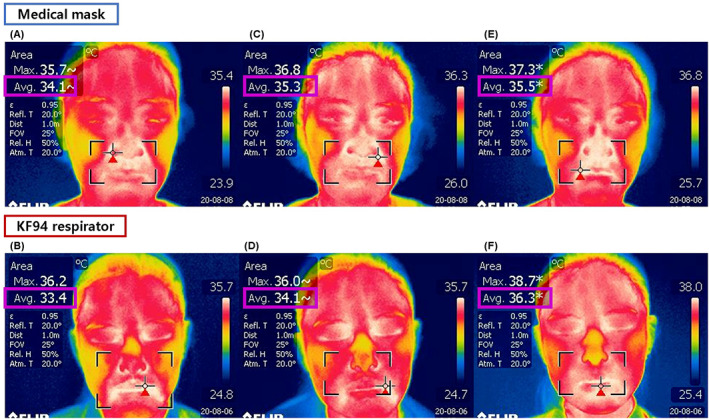
Infrared photographs were taken at (A, B) baseline, (C, D) after 4 hours of wearing the respiratory protective equipment, and (E, F) after 8 hours of wearing the respiratory protective equipment
4. DISCUSSION
To elucidate the pathophysiological mechanism underlying adverse skin reactions to RPE, we aimed to investigate the changes in skin properties after RPE use by evaluating various noninvasive, in vivo measurements of skin properties. In a previous study by Hua et al, 11 the authors evaluated short‐term skin reactions (up to 4 hours after donning) to RPE. Here, we aimed to evaluate skin reactions after wearing RPE for a longer time (4 and 8 hours after donning) as well as skin condition after stopping RPE use (14 hours without RPE overnight). The experiment mimicked the “ideal” daily schedule of HCWs while considering minimal RPE use time, considering the fact that most HCWs often work overtime and wear RPE for at least eight hours or longer.
First, skin hydration values reflect the water content of the stratum corneum (SC), and TEWL represents the diffusion of condensed water through the SC. 12 , 13 At baseline, the skin hydration values were significantly lower and TEWL values were higher in the RPE‐covered area than in the RPE‐uncovered area. This may be due to anatomical differences; it is well known that areas around the nasolabial fold area and middle cheek have poor hydration compared with the temple or forehead. 14 , 15 However, after wearing RPE for 4 and 8 hours, hydration and TEWL of the RPE‐covered area increased over time. This maybe because, in the RPE‐covered area, continuous expiration and occlusion increase local humidity, skin temperature, and sweating. 16 Roberge et al 17 has previously shown that relative humidity in the dead space inside an N95 mask increased over time, reaching levels as high as 93% after just 60 minutes of use. Clinically, this microclimate with increased temperature and humidity would make the facial skin condition similar to diapered skin with local disruption of the skin barrier. 18 It is also known that a higher content of water in the SC can facilitate dermal absorption of chemicals. 16 , 19 Thus, RPE‐covered skin can become more susceptible to various allergens or chemical irritants, which can increase the risk of contact dermatitis.
Second, similar to the changes in skin hydration and TEWL, skin erythema also increased over time in the RPE‐covered area. Furthermore, an increase in skin temperature on the RPE‐covered area was confirmed by IR thermography. Erythema may be due to the direct pressure effect of RPE or maybe a result of cutaneous blood vessel dilatation, which is a normal physiological response to increased temperature. However, skin erythema and high temperature may also indicate inflammation and increased skin permeability. 20
Third, sebum levels increased both in the RPE‐covered area and the RPE‐uncovered area. This can be explained by the circadian changes in sebum secretion. 21 However, the increase in sebum values was greater in the RPE‐covered area compared with the RPE‐uncovered area. Cunliffe et al 22 have reported a significant relationship between skin temperature and sebum excretion rate, where sebum secretion was increased by 10% as the local temperature increased by 1°C. In another study, Cunliffe et al 23 also found that sebum excretion rate rose significantly following occlusion with surgical tape, confirming the view that an obstruction to the outflow of sebum with keratin hydration increases sebum secretion rate. Therefore, the increase in skin temperature and occlusion would have led to a greater increase in sebum secretion in the RPE‐covered area compared with the uncovered area. Clinically, excessive sebum secretion may lead to enlarged pores, acne, seborrheic dermatitis, or “Maskne,” which is a variant of acne mechanica that occurs in the O‐zone due to the use of PPE. 24 , 25
Last but not least, skin pH also continuously increased in the RPE‐covered area over time. The acidic milieu of the skin is important for epidermal permeability barrier homeostasis, restoration of the disrupted barrier, and nonspecific antimicrobial defence of the skin. 26 , 27 , 28 , 29 The RPE‐covered area is constantly exposed to the oral fluid that carries both dangerous and innocuous viral and bacterial agents. 30 , 31 Therefore, on the RPE‐covered skin with high surface pH and thus with compromised antimicrobial defense and healing ability, these bacteria can fuel skin irritation and infections, leading to various RPE‐related adverse skin reactions. 5
Overall, we observed that skin hydration, TEWL, erythema, sebum secretion, pH, and skin temperature increased over time on the RPE‐covered skin. These results are consistent with the results reported by Hua et al 11 on the short‐term effect of RPE on skin. Based on these findings, Hua et al 11 concluded that skin reactions to the RPE are characterized by a compromised skin barrier function. However, it is unclear whether the increase in TEWL and pH of the RPE‐covered area reflects the disrupted barrier function. It is more likely that a transient increase in TEWL and pH observed in thus study occurred due to the temporary increase in sweating, humidity, and temperature. The fact that these values returned to baseline after not wearing RPE overnight supports this view. Regardless of this fact, we observed a continuous increase in skin hydration, TEWL, sebum secretion, and pH over time in the RPE‐covered skin, unlike the normal, RPE‐uncovered skin. This repetitive and sustained overhydration and elevation of surface TEWL, pH, and sebum secretion can eventually lead to local disruption of the skin barrier function, which may contribute to the development of various RPE‐related dermatoses. Lastly, the changes in skin hydration, erythema, sebum secretion, and pH were greater in KF94 respirators than in medical masks, although the differences were not statistically significant. However, because our study was limited by a small sample size and short study period, further studies are needed to clarify this finding.
As mentioned above, the limitations of our study include the small sample size and a relatively short study period. Further studies with larger sample sizes and longer study periods are needed to fully elucidate the long‐term or cumulative effects of RPE‐related skin changes. Furthermore, noninvasive in vivo measurements of skin biophysical properties are inevitably affected by the instrument‐ and environment‐related variables as well as individual‐originating factors. 32 Hence, efforts to minimize these variables are essential when performing these studies. In our study, we performed measurements in temperature‐ and relative humidity–controlled rooms according to the manufacturer's guidelines. Furthermore, to maintain the same skin condition at the baseline, we guided the participants to wash their faces and acclimatize in the measurement rooms for 30 minutes, as recommended by EEMCO guidance for the in vivo assessment of biochemical properties of the human skin. 12 In addition, the RPE‐uncovered area measurements were included as relevant controls for each participant, which served as its own control for each individual.
Therefore, while it is important to strictly adhere to PPE guidelines in this pandemic, measures should be implemented to protect the skin barrier, thereby preventing the paradoxical situation in which protective measures become a risk factor for various dermatoses. While adverse skin reactions may not be considered severe conditions, they are often known to reduce effective workforce due to work absenteeism, and can cause additional medical expenses. To this end, several recommendations regarding the use of RPE have been suggested by experts around the world. 7 , 33 , 34 Based on the previous recommendations and our study results, we have summarized the prevention strategies for RPE‐related skin reactions in Table 2.
TABLE 2.
Recommendation and prevention strategies for RPE‐related skin reactions
| How to wear masks |
|
| Skin care during mask use |
|
| Managing heat and sweating |
|
Abbreviations: RPE, respiratory protective equipment.
5. CONCLUSION
The COVID‐19 pandemic has forced the global population to adopt new ways of living, including the daily and compulsory use of masks. Wearing masks is crucial for preventing airborne diseases and cannot be easily substituted. However, as shown in various reports, wearing RPE for extended periods, as has occurred in the era of COVID‐19, can have potentially serious consequences. Therefore, dermatologists must identify the mechanisms responsible for adverse skin conditions due to RPE use in order to devise proper preventive measures.
AUTHOR CONTRIBUTIONS
Hye Sung Han: Conceptualization; data curation; formal analysis; investigation; methodology; resources; writing‐original draft; writing‐review & editing. Sun Hye Shin: Data curation; investigation; resources; validation; visualization; writing‐review & editing. Jae Wan Park: Data curation; formal analysis; methodology; project administration; resources; validation; writing‐review & editing. Kapsok Li: Data curation; formal analysis; methodology; software; supervision; validation; visualization; writing‐review & editing. Beom joon Kim: Conceptualization; funding acquisition; investigation; project administration; supervision; validation; writing‐review & editing. Kwang Ho Yoo: Conceptualization; data curation; formal analysis; funding acquisition; project administration; software; supervision; validation; visualization; writing‐review & editing.
CONFLICTS OF INTEREST
The authors have no conflict of interest to declare.
IRB APPROVAL STATUS
The study protocol was approved by the Institutional Review Board (approval number: P2007‐1336).
ACKNOWLEDGEMENTS
The patients in this study provided written informed consent regarding the publication of their clinical information and photos. This research was supported by the Chung‐Ang University Research Grants in 2020.
Han HS, Shin SH, Park JW, Li K, Kim BJ, Yoo KH. Changes in skin characteristics after using respiratory protective equipment (medical masks and respirators) in the COVID‐19 pandemic among healthcare workers. Contact Dermatitis. 2021;85:225–232. 10.1111/cod.13855
Funding information Chung‐Ang University Research Grants in 2020
Contributor Information
Beom Joon Kim, Email: beomjoon74@gmail.com.
Kwang Ho Yoo, Email: psyfan9077@naver.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Tang JW, Liebner TJ, Craven BA, Settles GS. A Schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J R Soc Interface. 2009;6(Suppl 6):S727‐S736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leung CC, Lam TH, Cheng KK. Mass masking in the COVID‐19 epidemic: people need guidance. Lancet. 2020;395(10228):945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID‐19 pandemic: a systematic review and meta‐analysis. Brain Behav Immun. 2020;88:901‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gheisari M, Araghi F, Moravvej H, Tabary M, Dadkhahfar S. Skin reactions to non‐glove personal protective equipment: an emerging issue in the COVID‐19 pandemic. J Eur Acad Dermatol Venereol. 2020;34(7):e297‐e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease‐2019. J Am Acad Dermatol. 2020;82(5):1215‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Foo CC, Goon AT, Leow YH, Goh CL. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome–a descriptive study in Singapore. Contact Dermatitis. 2006;55(5):291‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desai SR, Kovarik C, Brod B, et al. COVID‐19 and personal protective equipment: treatment and prevention of skin conditions related to the occupational use of personal protective equipment. J Am Acad Dermatol. 2020;83(2):675‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin P, Zhu S, Huang Y, et al. Adverse skin reactions among healthcare workers during the coronavirus disease 2019 outbreak: a survey in Wuhan and its surrounding regions. Br J Dermatol. 2020;183(1):190‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang Q, Song S, Zhou J, et al. The prevalence, characteristics, and prevention status of skin injury caused by personal protective equipment among medical staff in fighting COVID‐19: a multicenter, cross‐sectional study. Adv Wound Care (New Rochelle). 2020;9(7):357‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34(8):e378‐e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hua W, Zuo Y, Wan R, et al. Short‐term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83(2):115‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rogiers V, Group E . EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol Appl Skin Physiol. 2001;14(2):117‐128. [DOI] [PubMed] [Google Scholar]
- 13. Imhof RE, De Jesus ME, Xiao P, Ciortea LI, Berg EP. Closed‐chamber transepidermal water loss measurement: microclimate, calibration and performance. Int J Cosmet Sci. 2009;31(2):97‐118. [DOI] [PubMed] [Google Scholar]
- 14. Voegeli R, Gierschendorf J, Summers B, Rawlings AV. Facial skin mapping: from single point bio‐instrumental evaluation to continuous visualization of skin hydration, barrier function, skin surface pH, and sebum in different ethnic skin types. Int J Cosmet Sci. 2019;41(5):411‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zlotogorski A. Distribution of skin surface pH on the forehead and cheek of adults. Arch Dermatol Res. 1987;279(6):398‐401. [DOI] [PubMed] [Google Scholar]
- 16. Kezic S, Nielsen JB. Absorption of chemicals through compromised skin. Int Arch Occup Environ Health. 2009;82(6):677‐688. [DOI] [PubMed] [Google Scholar]
- 17. Roberge RJ, Kim JH, Coca A. Protective facemask impact on human thermoregulation: an overview. Ann Occup Hyg. 2012;56(1):102‐112. [DOI] [PubMed] [Google Scholar]
- 18. Kleesz P, Darlenski R, Fluhr JW. Full‐body skin mapping for six biophysical parameters: baseline values at 16 anatomical sites in 125 human subjects. Skin Pharmacol Physiol. 2012;25(1):25‐33. [DOI] [PubMed] [Google Scholar]
- 19. Warner RR, Stone KJ, Boissy YL. Hydration disrupts human stratum corneum ultrastructure. J Invest Dermatol. 2003;120(2):275‐284. [DOI] [PubMed] [Google Scholar]
- 20. Luo J, Hu H. Thermally activated TRPV3 channels. Curr Top Membr. 2014;74:325‐364. [DOI] [PubMed] [Google Scholar]
- 21. Le Fur I, Reinberg A, Lopez S, Morizot F, Mechkouri M, Tschachler E. Analysis of circadian and ultradian rhythms of skin surface properties of face and forearm of healthy women. J Invest Dermatol. 2001;117(3):718‐724. [DOI] [PubMed] [Google Scholar]
- 22. Cunliffe WJ, Burton JL, Shuster S. The effect of local temperature variations on the sebum excretion rate. Br J Dermatol. 1970;83(6):650‐654. [DOI] [PubMed] [Google Scholar]
- 23. Cunliffe WJ, Perera WD, Tan SG, Williams M, Williams S. Pilo‐sebaceous duct physiology. 2. The effect of keratin hydration on sebum excretion rate. Br J Dermatol. 1976;94(4):431‐434. [DOI] [PubMed] [Google Scholar]
- 24. Shuo L, Ting Y, KeLun W, Rui Z, Rui Z, Hang W. Efficacy and possible mechanisms of botulinum toxin treatment of oily skin. J Cosmet Dermatol. 2019;18(2):451‐457. [DOI] [PubMed] [Google Scholar]
- 25. Teo WL. Diagnostic and management considerations for “maskne” in the era of COVID‐19. J Am Acad Dermatol. 2021;84(2):520‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121(2):345‐353. [DOI] [PubMed] [Google Scholar]
- 27. Schmid‐Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19(6):296‐302. [DOI] [PubMed] [Google Scholar]
- 28. Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001;117(1):44‐51. [DOI] [PubMed] [Google Scholar]
- 29. Fluhr JW, Behne MJ, Brown BE, et al. Stratum corneum acidification in neonatal skin: secretory phospholipase A2 and the sodium/hydrogen antiporter‐1 acidify neonatal rat stratum corneum. J Invest Dermatol. 2004;122(2):320‐329. [DOI] [PubMed] [Google Scholar]
- 30. Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang Q, Li H, Shen S, et al. Study of the micro‐climate and bacterial distribution in the deadspace of N95 filtering face respirators. Sci Rep. 2018;8(1):17382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darlenski R, Sassning S, Tsankov N, Fluhr JW. Non‐invasive in vivo methods for investigation of the skin barrier physical properties. Eur J Pharm Biopharm. 2009;72(2):295‐303. [DOI] [PubMed] [Google Scholar]
- 33. Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health‐care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33(4):e13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Balato A, Ayala F, Bruze M, et al. European task force on contact dermatitis statement on coronavirus disease‐19 (COVID‐19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34(8):e353‐e354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


