Abstract
The description of protective humoral and T cell immune responses specific against SARS-CoV-2 has been reported among immunocompetent (IC) individuals developing COVID-19 infection. However, its characterization and determinants of poorer outcomes among the at-risk solid organ transplant (SOT) patient population have not been thoroughly investigated. Cytokine-producing T cell responses, such as IFN-γ, IL-2, IFN-γ/IL-2, IL-6, IL-21, and IL-5, against main immunogenic SARS-CoV-2 antigens and IgM/IgG serological immunity were tracked in SOT (n = 28) during acute infection and at two consecutive time points over the following 40 days of convalescence and were compared to matched IC (n = 16) patients admitted with similar moderate/severe COVID-19. We describe the development of a robust serological and functional T cell immune responses against SARS-CoV-2 among SOT patients, similar to IC patients during early convalescence. However, at the infection onset, SOT displayed lower IgG seroconversion rates (77% vs. 100%; p = .044), despite no differences on IgG titers, and a trend toward decreased SARS-CoV-2-reactive T cell frequencies, especially against the membrane protein (7 [0–34] vs. 113 [15–245], p = .011, 2 [0–9] vs. 45 [5–74], p = .009, and 0 [0–2] vs. 13 [1–24], p = .020, IFN-γ, IL-2, and IFN-γ/IL-2 spots, respectively). In summary, our data suggest that despite a certain initial delay, SOT population achieve comparable functional immune responses than the general population after moderate/severe COVID-19.
KEYWORDS: adaptive immunity, basic (laboratory) research / science, clinical research / practice, COVID-19 infection, heart transplantation / cardiology, infection and infectious agents, kidney transplantation / nephrology, liver transplantation / hepatology, solid organ transplantation, T cell biology
1. INTRODUCTION
A novel coronavirus, designated as SARS-CoV-2, emerged in Wuhan, China, at the end of 2019 and has spread all over the globe in a logarithmic manner. The increasing number of fatal outcomes related to the Coronavirus Disease-2019 (COVID-19) has put global health institutions on high alert.
While most people remain asymptomatic or develop only mild symptoms during COVID-19,1 , 2 some specific group of patients seem to be at significantly higher risk of fatal outcomes,3 and among them recipients of solid organ transplants (SOT) most likely because they receive chronic immunosuppressive therapy that predominantly targets T cell adaptive immunity.4 Importantly, SOT patients represent an important prevalent high-risk population in whom the biology of the adaptive immunity specific to SARS-CoV-2 during COVID-19 has not yet been thoroughly investigated.
First studies evaluating immunocompetent (IC) convalescent individuals have shown the induction of neutralizing antibodies after primary infection5, 6, 7, 8 which seem to be detectable essentially among patients with more severe forms of COVID-19.9 , 10 Conversely, robust anti-viral T cell responses have been described after SARS-CoV-2 infection, which seem to correlate with the magnitude of SARS-CoV-2-specific IgG and IgA titers during the initial phase of convalescence11 and with the severity of COVID-19 infection.12 Interestingly, SARS-CoV-2-reactive T cell immunity seems to last for a longer period of time, even among seronegative convalescent patients13 and can discriminate those patients with the poorest outcomes.14
In this study, we aimed at investigating the IgM and IgG serological antibody responses as well as the SARS-CoV-2-reactive T cell responses against main four different structural viral proteins, Spike (S), Nucleocapsid (N), Membrane (M), and Envelope (E), in SOT recipients as compared to matched hospitalized IC healthy individuals due to COVID-19, both at the time of the acute infection phase and over the convalescent clinical course after infection, in order to provide mechanistic insights that could explain the recent epidemiological observations of a higher risk of poorer outcomes in SOT as compared to IC-infected patients.
2. MATERIAL AND METHODS
2.1. Patients of the study and clinical definitions
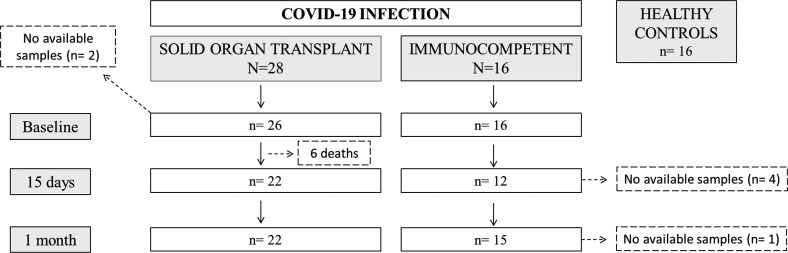
In this study, we evaluated 44 consecutive patients hospitalized between March 15 and April 18, 2020, at Bellvitge University Hospital (Barcelona, Spain) and Montpellier University Hospital (Montpellier, France) due to COVID-19 infection, and in whom peripheral blood mononuclear cells (PBMCs) and serum samples were available. All patients had been tested positive for SARS-CoV-2 infection by a RT-PCR analysis on nasopharyngeal swab samples. Among these 44 patients, 28 were SOT recipients and 16 IC patients, who were matched for age, gender, and severity of COVID-19 at study inclusion ( Figure 1; Table 1).
FIGURE 1.
Flowchart of the study
TABLE 1.
Demographic and clinical characteristics of patients infected by SARS-CoV-2
| SOT (N = 28) | IC (N = 16) | HC (n = 16) | P value | |
|---|---|---|---|---|
| Age (years, mean ± SD) | 59.4 ±13.6 | 59.4 ± 11.3 | 63.4 ± 10 | 0.531 |
| Sex (Female) (n, %) | 7 (25) | 7 (44) | 5 (31.3) | 0.437 |
| Comorbidities (n, %) | ||||
| Diabetes | 11 (39.3) | 1 (6.3) | N/A | 0.032 |
| Arterial hypertension | 19 (67.9) | 6 (37.5) | N/A | 0.051 |
| Obesity a | 6 (21.4) | 3 (18.8) | N/A | 0.868 |
| Pulmonary disease b | 2 (7.1) | 2 (12.5) | N/A | 0.614 |
| Heart disease c | 6 (21.4) | 2 (12.5) | N/A | 0.689 |
| Active neoplasm | 4 (14.3) | 1 (6.3) | N/A | 0.638 |
| ACEi/ARB use | 10 (35.7) | 2 (12.5) | N/A | 0.116 |
| Previous Influenza vaccine (yes) | 22 (78.6) | 7 (43.8) | 12 (75) | 0.082 |
| Clinical symptoms at onset (n, %) | ||||
| Cough | 18 (64.3) | 13 (81.3) | N/A | 0.314 |
| Dyspnea | 10 (35.7) | 7 (43.8) | N/A | 0.749 |
| Diarrhea | 14 (50) | 6 (37.5) | N/A | 0.534 |
| Myalgias | 11 (39.3) | 7 (43.8) | N/A | 1.000 |
| Fever | 23 (82.1) | 16 (100) | N/A | 0.141 |
| Disease severity at enrollment (n, %) | ||||
| No oxygen therapy needed | 5 (17.9) | 1 (6.2) | N/A | 0.276 |
| Oxygen requirement (NO ARDS) | 8 (28.6) | 6 (37.5) | N/A | 0.738 |
| ARDS | 15 (53.6) | 9 (56.3) | N/A | 1.000 |
| Outcomes at the end of follow-up (n, %) | ||||
| Death | 6 (21.4) | 0 (0) | N/A | 0.072 |
| MV or Death | 9 (32.1) | 1 (6.2) | N/A | 0.05 |
| Sampling time points (days) | ||||
| Days from symptom onset to first time-point PBMC collection (median, IQR) | 15 (12–20) | 17 (10–18) | N/A | 0.794 |
| Days from symptom onset to second time-point PBMC collection (median, IQR) | 31 (25–40) | 32 (26–37) | N/A | 0.711 |
| Days from symptom onset to third time-point PBMC collection (median, IQR) | 48 (42–53) | 50 (44–54) | N/A | 0.225 |
| Days from positive PCR to first time-point collection (median, IQR) | 7 (5–12) | 6 (4–10) | N/A | 0.15 |
| Days from positive PCR to second time-point collection (median, IQR) | 23 (20–28) | 24 (20–26) | N/A | 0.762 |
| Days from positive PCR to third time-point collection (median, IQR) | 40 (36–44) | 41 (38–44) | N/A | 0.556 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARDS, acute respiratory distress syndrome; HC, healthy controls; IC, immunocompetent; MV, mechanical ventilation (invasive or non-invasive); PBMC, peripheral blood mononuclear cells; PCR, polymerase chain reaction; SOT, solid organ transplant.
Obesity: body mass index >30.
Pulmonary disease: chronic obstructive pulmonary disease, asthma, bronchiectasis, or sleep apnea-hypopnea syndrome.
Heart disease: congestive heart failure, coronary artery disease, atrial fibrillation, or valvular heart disease.
A total of 113 serially collected peripheral blood samples at three different time points of the disease were analyzed in this study—during the acute phase of infection (T1; median 16, IQR 12–19 days after symptom onset) and at two convalescence periods (T2; median 32, IQR 25–37 days, and T3; 49 days, IQR 43–53), which represented a median of 7 days, IQR 4–11 and 23 days, IQR 20–27 and 40 days, and IQR 37–44, after first positive PCR, respectively.
Additionally, PBMC samples from 16 non-immunosuppressed patients on the waiting list for kidney transplantation that were obtained 2 years before the COVID-19 outbreak (November 2018) and were stored in our biobank facilities were used as healthy controls (HC).
All clinical, demographic, and immunological patient characteristics as well as the main outcomes, such as mortality, or the need of invasive/non-invasive mechanical ventilation (MV) were recorded. COVID-19 disease severity was defined according to the level of oxygen support during hospitalization according to the World Health Organization interim guidance to define Acute Respiratory Distress Syndrome (bilateral opacities not explained by volume overload with an oxygen saturation/fraction of inspired oxygen ratio <315).15
The study was approved by the Ethical Review Boards (PR115/20) at each center and patients were recruited in the study after providing a signed informed consent.
2.2. Collection and management of serum and PBMC samples
Detailed description is depicted in Data S1.
2.3. Assessment of SARS-CoV-2-specific antibodies
IgM and IgG antibodies against SARS-CoV-2 were detected by a chemiluminescence technique, using the MaglumiTM 2019 nCov-IgM and the MaglumiTM 2019 nCov-IgG tests (Snibe Diagnostic) on a Maglumi 2000® analyzer (Snibe Diagnostic), according to the manufacturer’s instructions. Detailed information is provided in Data S1.
2.4. Assessment of cytokine-producing SARS-CoV-2-reactive T cell responses
SARS-CoV-2-reactive T cell responses were evaluated using a multicolor FluoroSpot Immune assay kit (AID® Gmbh). Distinct cytokine-producing T cell frequencies were assessed: effector (IFN-γ), proliferative (IL-2) and central (IFN-γ/IL-2) memory Th1 responses, IL-5 and IL-21 Th2 responses, and IL-6 pro-inflammatory T cell responses. The main four structural SARS-CoV-2 proteins, Spike Glycoprotein (S), Membrane Protein (M), Nucleoprotein (N), and Envelope Small Membrane Protein (E) (JPT®), were used for stimulation in the multicolor FluoroSpot Immune assay individually. Overlapping peptide pools covering the whole Influenza virus antigen length (AID® Gmbh) were also tested. In each test, complete medium alone and Pokeweed (PWM) mitogen were used as negative and positive controls, respectively. Any antigen-specific ELISPOT test with less than 5 spots/2 × 105 PBMC was considered as negative when assessed in a qualitative manner. Precise information is provided in Data S1.
2.5. Statistics
Continuous variables were expressed as mean ±SD or median and IQR and categorical variables as number of total (n) and percentage (%). A comparison between groups was performed using Pearson’s χ2 test for categorical data. Continuous measurements were compared among groups using Kruskal-Wallis and Mann-Whitney U test for non-normally distributed data, while ANOVA and t tests were used when data were normally distributed. p-values <.05 were considered statistically significant. SARS-CoV-2-reactive cellular and humoral responses were centered and scaled and heatmap was built by means of the pheatmap R package 16 using Euclidean distance and complete method as agglomeration method. R package version 1.0.12 was used https://CRAN.R-project.org/package=pheatmap. All other analyses were performed using SPSS version 26 software, and graphs were generated using GraphPad Prism version 8.0 software (GraphPad Software).
3. RESULTS
3.1. Patients of the study
Forty-four hospitalized patients with COVID-19 disease confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) were included: 28 SOT recipients and 16 IC patients. Eighteen (64.3%) kidney, five (17.9%) heart, and five (17.9%) liver transplants composed the SOT group, with a median time after transplantation of 9 ± 7 years (IQR 3–14) and were receiving a calcineurin inhibitor (CNI)-based immunosuppressant scheme (67.9%). Also, 16 individuals in whom PBMC samples were retrieved and stored at our biobank facilities in 2018 were included in the study (Figure 1).
Main clinical, demographic, and immunological characteristics are depicted in Table 1. As shown, SOT and IC patients of the study were matched for age, sex, and main comorbidities, but IC patients were less diabetic. The degree of COVID-19 severity and time of assessment were not different between groups. After a follow-up of 40 days (37–44), six (13.6%) patients passed away, they were all SOT (three liver, two kidney, and one heart transplant recipient). The composite outcome depicted as requirement of MV or death did also occur more frequently among SOT (9 [32.1%] SOT vs. 1 [6.2%] IC; p = .05). First time-point blood samples were retrieved prior to this composite outcome.
We further evaluated 16 healthy control (HC) individuals in whom PBMC samples had been retrieved in 2018, before the SARS-CoV-2 pandemic, and were also matched for age and gender with the other two study groups. As expected, previous influenza vaccination rate was lower among the IC group (43.8%) as compared to SOT (78.6%) and HC (75%) groups (p = .082).
3.2. Circulating lymphocytes and functional adaptive immunity during acute and convalescent COVID-19 infection
Our first analysis showed that while both SOT and IC patients displayed abnormally low total lymphocyte counts, this lymphopenia was more pronounced for SOT recipients (866 ± 427 vs. 1531 ± 490 in IC; p < .001). Total lymphocyte counts in HC were 1564 ±427 and were significantly higher than SOT at T1 (p < .001) (Figure S2).
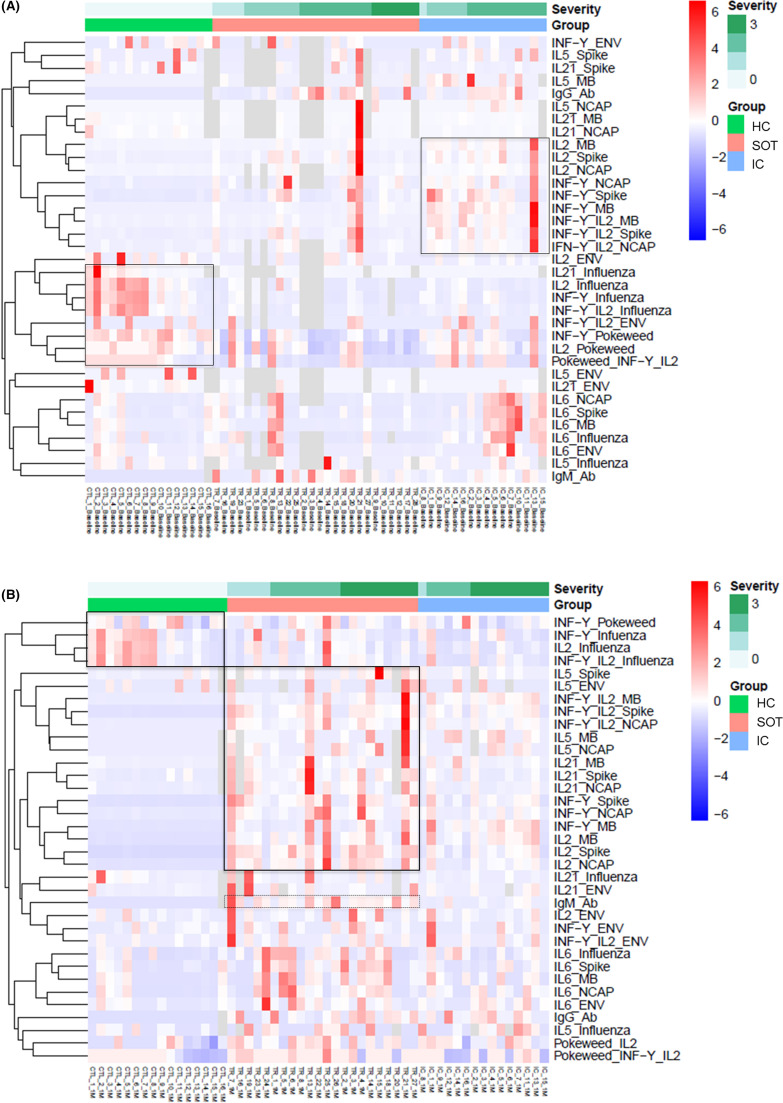
As shown in Figure 2A and Figure S3A, during acute infection (T1), SARS-CoV-2-reactive T cell responses against four main viral antigens were more predominantly detected among IC patients than within SOT and especially among those with higher severity index. Notably, no SARS-CoV-2-reactive responses were observed among HC. IgG and IgM serological immunity against SARS-CoV-2 was detected within both SOT and IC. At the last convalescent period (T3) (Figure 2B and Figure S3B), SARS-CoV-2-reactive T cell immune responses were now detectable within the SOT group while they had faded in IC patients. Likewise, more predominant IgM responses were observed among SOT than IC, whereas IgG-specific antibodies were similarly detected.
FIGURE 2.
Heatmaps generated by hierarchical clustering of SARS-CoV-2-specific and non-specific immune responses for SOT, IC patients, and HC, according to the COVID-19 disease severity (0 = no oxygen need; 1 = oxygen need; 2 = acute respiratory distress syndrome, 3 = death). Immune responses used for clustering were differentially expressed (fold change >2, false discovery rate p < .05). Gray fields indicate missing values. (A) Heatmap performed at first time point during acute COVID-19 infection (7; 4–11 days after the diagnosis) among 26 SOT, 16 IC, and 16 HC. (B). Heatmap performed during the early convalescent period (40; 37–44 days after the diagnosis) of COVID-19 disease in 22 SOT, 15 IC, and 16 HC [Color figure can be viewed at wileyonlinelibrary.com]
Conversely, non-SARS-CoV-2-reactive T cell immune responses against influenza and a polyclonal stimuli (PWM) were significantly weaker within both SOT and IC as compared to HC at baseline, which persisted during the convalescence period.
3.3. SARS-CoV-2-reactive T cell immunity during acute and early convalescent COVID-19 infection
No correlation was observed between absolute lymphocyte counts and SARS-CoV-2-reactive T cell frequencies for each antigen-specific cytokine-producing T cell (IFN-γ, IL-2, IFN-γ/IL-2, IL-6, IL-21, and IL-5) at any time point of the study (Table S1).
3.4. SARS-CoV-2-reactive T cell function during acute COVID-19 infection
A strong correlation was observed between all four SARS-CoV-2 antigen responses (Table S2), showing a wide and different range of T cell frequencies.
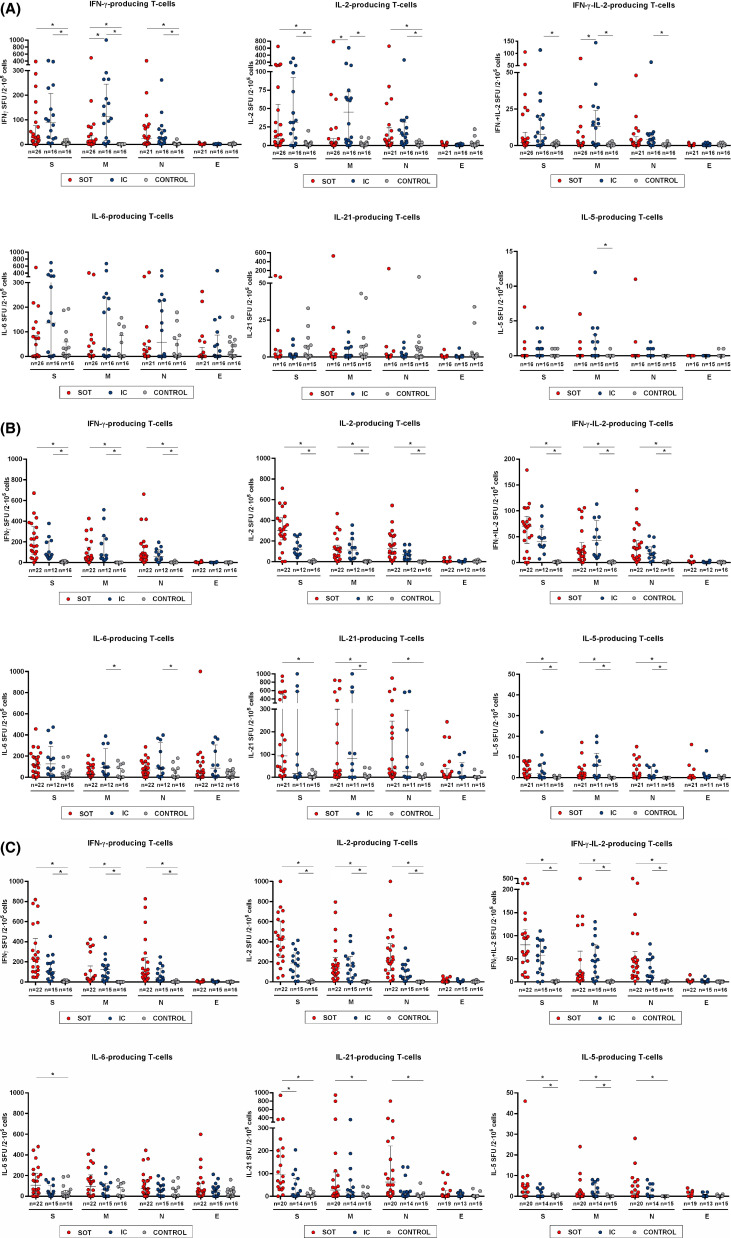
As illustrated in Figure 3A and described in Table S3, as compared to IC individuals, SOT displayed numerically lower IFN-γ, IL-2, and IFN-γ/IL-2-producing T cell frequencies, although being statistically significant only for antigen M (7 [0–34] vs. 113 [15–245], p = .011; 2 [0–9] vs. 45 [5–74], p = .009, and 0 [0–2] vs. 13 [1–24], p = .020, for IFN-γ, IL-2, and IFN-γ/IL-2 spots in SOT and IC, respectively). A certain detectable IL-6 stimulation was widely detected in all evaluated patients, including HC thus suggesting a general non-antigen-specific immune response. Notably, IL-21 and IL-5-producing T cells against SARS-CoV-2 were barely detectable in both SOT and IC patients at this time point. As also illustrated, the highest frequencies were observed for T cells only producing IFN-γ, whereas the lowest for those polyfunctional IFN-γ/IL-2-producing T cells.
FIGURE 3.
Cytokine profile of T cell responses against main structural SARS-CoV-2 proteins Spike (S), Membrane (M), Nucleoprotein (N), and Envelope (E). Frequencies of IFN-γ, IL-2, IFN-γ/IL-2, IL-6, IL-5, and IL-21-producing T cells were assessed among the three study group samples at different time points. *p < .05, calculated with Kruskal-Wallis test. (A) T1 = 16; 12–19 days. (B) T2 = 32; 25–37 days. (C) T3 = 49; 43–53 days after symptom onset [Color figure can be viewed at wileyonlinelibrary.com]
While IC patients showed similarly high T cell immune responses against both antigens S and M, the highest immune response among SOT was only against antigen S. Of note, T cell responses against antigen E were barely detectable in all infected patients (Figure S4A).
As illustrated in Figure S5A, a higher proportion of SARS-CoV-2 T cell non-responders was observed among SOT as compared to IC, and especially those IFN-γ/IL-2-producing T cells.
3.5. Progression of SARS-CoV-2-reactive T cell immunity during COVID-19 convalescence
We next sequentially monitored these patients at two consecutive time points during convalescence periods: at T2; 32 (IQR 25–37) and T3; 49 (IQR 43–53) days after symptom onset, which represents a median of 11 (IQR 3–16) and 27 (IQR 22–30) days after discharge, respectively. Similar to T1, a strong correlation of T cell responses was observed between the different SARS-CoV-2 antigens at both time points (Tables S4–S5).
Unlike during acute infection, there were in general no longer differences between SOT and IC regarding the distinct SARS-CoV-2-reactive T cell responses (Figure 3B; Tables S6–S7). However, at T3, while no statistically significant differences were noted between groups, numerically higher SARS-CoV-2-reactive T cell responses in SOT as compared to IC patients were observed, and particularly against antigen S for IL-2 and IL-21 (425 [242–606] vs. 181 [58–289], p = .07 and 107 [36–212] vs. 10 [2–83], p = .025, respectively) (Figure 3C). Similarly, as during the acute infection phase, while the strongest T cell responses among IC were driven against SARS-CoV-2 antigens S and M, the predominant T cell response among SOT was against antigen S but not to antigen M (Figure S4B,C). Also, almost no detectable T cell responses were observed against SARS-CoV-2 antigen E. As also illustrated in Figures S5B,C, now at T2 and T3, the great majority of both SOT and IC patients showed detectable SARS-CoV-2-reactive T cell frequencies.
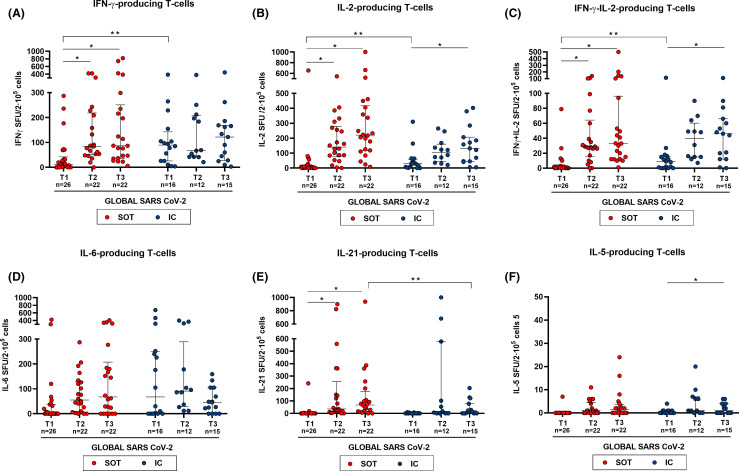
To examine the kinetics of SARS-CoV-2-reactive T cell responses over time in the two groups, we assessed the global SARS-CoV-2-reactive T cell immune responses by means of the median T cell frequencies against the three main immunogenic antigens (S, M, and N) in each patient and at each time point. As shown in Figure 4, both SOT and IC developed a rapid increase of global SARS-CoV-2-reactive T cell responses until T3. Notably, these functional changes were more evident among SOT as compared to IC patients, which fundamentally occurred between T1 and T2. As previously described at the single antigen level, SOT displayed weaker global SARS-CoV-2-reactive T cell frequencies at baseline than IC patients (11 [1–42] vs. 90 [26–143] spots, p = .003 and; 6 [0–15] vs. 30 [4–60] spots, p = .049; 1 [0–2] vs. 9 [0–16], p = .050; for IFN-γ, IL-2, and IFN-γ/IL-2, respectively).
FIGURE 4.
Global T cell responses specific to SARS-CoV-2 at different time points (median T cell frequencies against the three SARS-CoV-2 immunogenic antigens: S, M, and N). At T1, N = 42 (SOT = 26, IC = 16); T2, N = 34 (SOT = 22, IC = 12), and T3, N = 37 (SOT = 22, IC = 15). Median and IQR are shown. Intragroup paired analysis; *p < .05 evaluated with Friedman’s test. Significant intergroup differences (IC vs. SOT) are also shown; **p < .05 (analyzed by Mann-Whitney U test) [Color figure can be viewed at wileyonlinelibrary.com]
3.6. SARS-CoV-2-specific serological immunity in SOT and IC with severe COVID-19
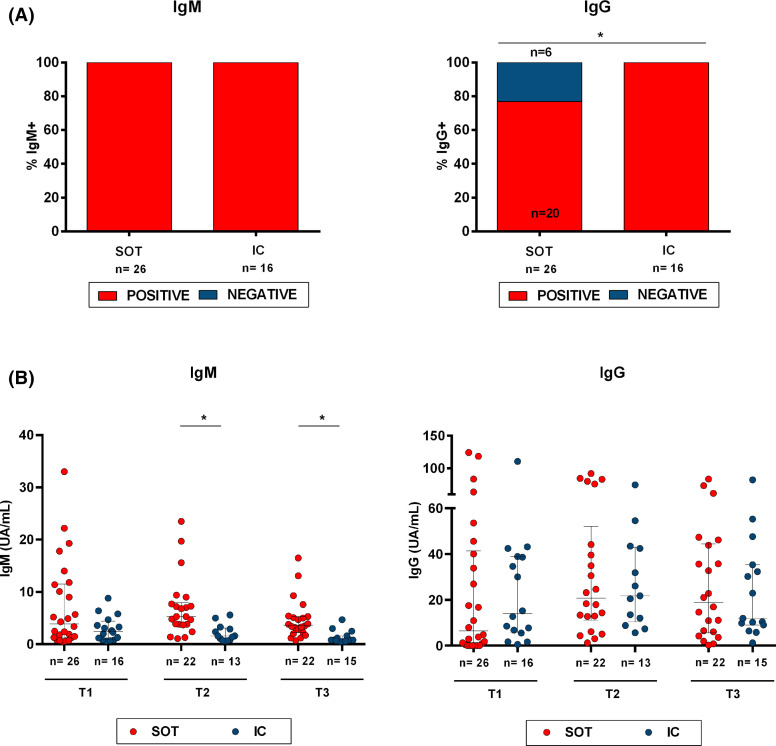
All infected patients showed detectable SARS-CoV-2-specific IgM titers at baseline ( Figure 5A) and remained detectable in the following two time points. Conversely, while all 16 IC patients showed detectable virus-specific IgG titers already at T1, 6/26 (23%) SOT did not (p = .044). All SOT seroconverted at T2 and remained positive until T3. Nevertheless, while no differences were observed regarding quantitative IgG titers between the two groups at any time point, IgM titers, albeit detectable, seemed to be cleared from the circulation much faster among IC than in SOT over time (Figure 5B). Indeed, at T2 and T3, IC showed significantly lower IgM titers than SOT patients (1.6 [0.75–3.1] vs. 5.3 [3.7–7.7] UA/ml, p = .001 at T2 and 0.8 [0.6–1.6] vs. 3.5 [1.9–5.3] UA/ml; p < .001 at T3).
FIGURE 5.
IgM and IgG antibody responses to SARS-CoV-2. (A) Percentage at T1 of SOT and IC patients with detectable SARS-CoV-2-specific IgM and IgG class-switching. *p < .05 (Chi-square test). (5B) IgM and IgG titers for every time point and study group (SOT and IC). *p < .05 (Mann-Whitney test analysis) [Color figure can be viewed at wileyonlinelibrary.com]
Of note, patients without IgG class-switch seroconversion displayed lower SARS-CoV-2-reactive IL-2-producing T cell frequencies against antigens S and M than patients with IgG serology (6 [1–9] vs. 28 [4–98], p = .073 and 1 [0–5] vs. 7 [2–63], p = .067 for IL-2-producing T cells against antigens S and M, respectively).
3.7. T cell immunity against influenza and polyclonal stimulation during COVID-19
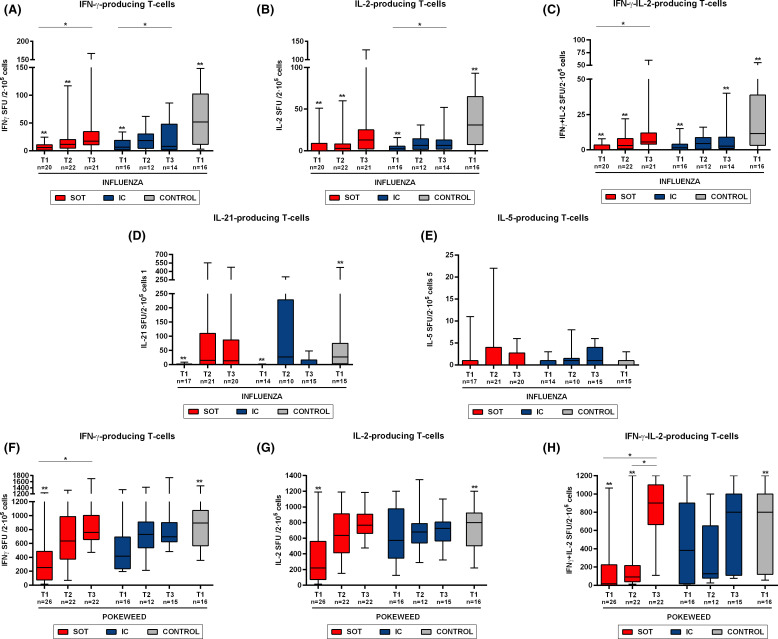
To investigate the degree of general immune impairment in patients developing moderate/severe COVID-19 infection, we assessed non-SARS-CoV-2-reactive T cell responses to influenza peptides and to a strong polyclonal T cell stimulation with PWM. To note, a correlation was found between these antigens, mainly for IFN-γ-producing T cells at the two first time points of evaluation, T1 (r = .403, p = .015) and T2 (r = .403 p = .015). No differences were observed between SOT and IC patients regarding both influenza and PWM T cell responses at any time point. Remarkably, both SOT and IC individuals displayed significantly lower IFN-γ, IL-2, IFN-γ/IL-2, and IL-21 T cell responses against both stimuli as compared to HC, which lasted in some cases until T3 ( Figure 6), despite significant vaccination rates.
FIGURE 6.
T cell responses against non-specific SARS-CoV-2 antigens (influenza and PWM) at the different time points of study. Percentile 5–95 represented by whiskers; median and IQR inside the boxes. Intragroup paired analysis; *p < .05 evaluated with Friedman’s test. Significant differences with healthy controls are shown by **p < .05 (analyzed by Mann-Whitney U test). No differences were found between IC and SOT [Color figure can be viewed at wileyonlinelibrary.com]
3.8. Baseline SARS-CoV-2-reactive T cell immunity and clinical outcomes among SOT
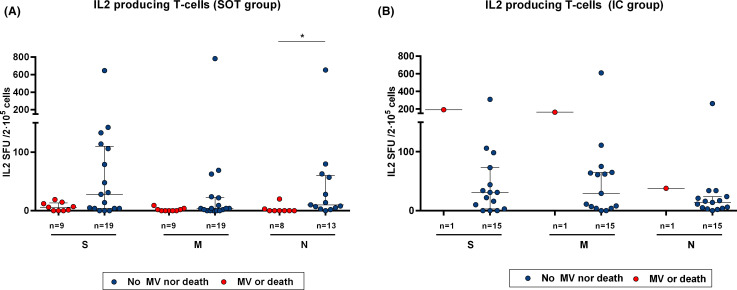
In our study, 10 (22.7%) patients required MV or died during the follow-up, being nine SOT. As depicted in Table S8, we did not find any differences regarding main clinical or demographic within the whole study population. Likewise, no differences were observed when analyzing SARS-CoV-2-reactive T cell responses and outcomes (data not shown). However, and since almost no fatal events occurred within the IC group in our study, we then focused on the SOT group. Also, no clinical nor demographical variables discriminated a poorer clinical evolution. Nevertheless, while no differences were observed regarding most SARS-CoV-2-reactive T cell responses, SOT with the poorest outcomes displayed lower IL-2-producing T cell frequencies against main three immunogenic SARS-CoV-2 antigens as compared to those with better clinical results (0 [0–3] vs. 10 [4–60] p = .003; 6 [0–13] vs. 28 [4–110] p = .085; and 0 [0–3] vs. 4 [0–22] p = .075 for antigens N, S, and M, respectively) ( Figure 7A). Intriguingly, the only patient of the IC group who required MV showed robust IL-2-producing T cell frequencies against the three viral antigens (Figure 7B). Furthermore, the proportion of IgG seroconversion was numerically lower among those with worse outcomes (80% vs. 62.5%, p = .245).
FIGURE 7.
Baseline SARS-CoV-2-specific IL-2-producing T cell frequencies and clinical outcomes in SOT and IC patients with severe COVID-19 infection. IL-2-producing frequencies between patients with a poor outcome (VM or death) and those with a favorable clinical evolution. (A) SOT patients **p < .05 (analyzed by Mann-Whitney U test). (B) IC patients. Only one IC patient required mechanical ventilation [Color figure can be viewed at wileyonlinelibrary.com]
In terms of immunosuppression, while mycophenolate was broadly withdrawn in our cohort (Table S9), no differences were found between patients with or without CNI-based immunosuppressive regimens at T1. Also, no differences were observed at the successive time points for those patients who had the CNI withdrawn during the infection phase (data not shown).
4. DISCUSSION
In this study, we investigated the magnitude and kinetics of adaptive immunity, both serological and specific T cell responses to main four immunogenic SARS-CoV-2 antigens among chronically immunocompromised SOT recipients and compared them to matched IC individuals developing the same moderate/severe COVID-19 infection. Here, we show that SOT patients achieve a similarly robust serological and functional T cell immune response comparable to that of IC patients during early COVID-19 convalescence. Nonetheless, a certain delay achieving such strong immune responses was observed among SOT, depicted by lower IgG seroconversion rates and cytokine-producing T cell frequencies, especially against the membrane antigen, as compared to IC patients during the acute infection onset. Moreover, we also describe that among SOT, those patients developing the worst clinical outcomes displayed more deprived SARS-CoV-2-reactive IL-2-producing T cell immune responses as compared to patients with better clinical results.
A widely reported viral-related effect is the severe peripheral lymphopenia observed during COVID-19 infection.17, 18, 19 Indeed, it was particularly severe among SOT as compared to IC patients, a finding that would seem to be most likely favored in this group of patients by the chronic immunosuppressive therapy these patients follow. However, we did not observe any correlation between total lymphocyte counts and the different SARS-CoV-2-reactive T cell responses, thus illustrating the importance of not only measuring total cell numbers but also their antigen-specific function.
So far, a number of studies have shown the contribution of T cell immunity specific to SARS-CoV-2 in COVID-19 patients.20 However, most of them have exclusively focused in patients without previous underlying immune condition such as SOT, and have not assessed the magnitude and relevance of different peripheral T cell immune subsets against the distinct viral antigens both during the acute infection phase as well as during the convalescence period.11 , 13 , 21 Herein, we first show that an important proportion of patients, both SOT and IC, display a wide range of SARS-CoV-2-reactive T cell responses, already in a very early phase of the disease. Globally, and as previously reported, main functional T cell responses were observed against three viral antigens: Spike (S), Membrane (M), and Nucleocapsid (N),11 , 22, 23, 24 but not against Envelope (E).
Different studies have described the significantly higher risk of fatal outcomes among SOT developing COVID-19 infection as compared to healthy population.4 , 25, 26, 27 While the main hypothesis for these poorer outcomes is sustained on their T cell immunocompromised status, no evaluation of their anti-viral immune response, both at the time of acute infection and during convalescence, has been reported yet. In our study, the lower IFN-γ, IL-2, and IFN-γ/IL-2-producing T cell frequencies against SARS-CoV-2, especially against antigen M, along with the higher proportion of patients with no detectable SARS-CoV-2-reactive T cell responses and the lower IgG seroconversion rates at the infection onset in SOT as compared to IC patients, suggest a certain delay of SOT to achieve a similarly robust initial adaptive immune response than IC patients, most likely due to their chronic immunosuppressive therapy. Nonetheless, a rapid increase of such adaptive T cell immunity, similar to that of IC, is achieved by SOT during early COVID-19 convalescence.
Interestingly, a progressive emergence of both IL-5- and IL-21-producing T cells was detected during the convalescent period in both groups. Although we did not phenotypically characterize these immune cells due to the lack of viable cell samples, these data suggest the fact that for an optimal B-cell activation, cognate T cell help, most likely through antigen-specific follicular helper T cells, is needed.21
As similarly described in a recent published report,28 we did not find any specific clinical, demographic, or immunological factors influencing worse clinical outcomes within the whole study group. Nonetheless, among the SOT group, significantly lower IL-2-producing T cell frequencies were observed in patients with the poorest clinical evolution. Conversely, the sole IC patient also needing MV support exhibited significantly more robust IL-2-specific T cell responses than SOT with the same severe outcome, a finding in line with a recent report 14 suggesting that patients with advanced age and higher comorbidity index showed higher IL-2 but decreasing portions of IFN-γ-secreting cells, in particular against antigen N. This different biological observation between SOT and IC may most likely rely in the chronic immunosuppressive effect of transplant immunotherapies, which abrogate IL-2 production on T cells.29
Importantly, SARS-CoV-2-reactive T cell responses and antibody titers progressively increased over time, during the convalescent period. Interestingly, this enhancement was more pronounced among SOT, who reached similar or even higher functional T cell and serological immune responses than IC patients. Interestingly, longer SARS-CoV-2 viral shedding has been reported among immunosuppressed patients,30 , 31 which might account to some extent for a longer persistence of antigen stimulation ultimately leading to higher SARS-CoV-2-reactive T cell frequencies among SOT at later time points. This is of importance, since these data show that SOT patients may develop an optimal and sustained adaptive immune response, despite receiving chronic immunosuppressive therapy. Thus, vaccination against SARS-CoV-2 should be highly encouraged also among this prevalent high-risk population.32
In line with previous works,33 , 34 non-specific T cell immune assessment did also reveal a severe global immune impairment of moderate/severe COVID-19, which was similarly depressed both in SOT and IC patients. Indeed, influenza and PWM-derived T cell responses were significantly abrogated at the acute phase of the infection, displaying a progressive restoration over time. In fact, influenza-specific memory T cell responses did not reach the same frequencies as those observed among healthy controls at the end of the follow-up, thus highlighting that recovery of adaptive immunity in some individuals was not fully achieved yet. These results underscore the difference between inflammation and adaptive immunity, which may raise concern about the hypothesis of potential therapeutic effects of some immunosuppressive agents, such as cyclosporine, aiming at reducing systemic inflammatory state in these patients.35 , 36
Finally, we did not find SARS-CoV-2-reactive T cell responses against any of the four viral antigens in any HC thus, no evidence for T cell immune cross-reactivity was observed in out cohort, at least in vitro. Despite the presence of IL-6-producing T cell responses against SARS-CoV-2 in HC suggesting unspecific T cell stimulation, the assessment of SARS-CoV-2-reactive IL-6-producing T cell frequencies over time showed a similar pattern than that also observed in other T cell compartments.
There are some limitations in this study such as the small sample size evaluated, which was directly influenced by the difficulty in obtaining biological samples during acute COVID-19 infection. While our FluoroSpot assay allowed us to investigate in a functional manner the frequencies of different cytokine-producing T cells reactive to distinct SARS-CoV-2 antigens at single cell level, we could not describe the predominant T cell subset compartment, either CD4+ or CD8+ T cells, responsible of these SARS-CoV-2-reactive T cells. Although previous reports have shown a predominant role of SARS-CoV-2-reactive CD4+ T cells, CD8+ T cells do also account for a robust anti-viral T cell immunity.11
In summary, this study describes that despite the strong general immune impairment occurring in patients with severe acute COVID-19 infection, SARS-CoV-2 elicits robust adaptive immune responses also in SOT recipients, both at the cellular and humoral level, although with a certain functional immune delay as compared to IC individuals. Notably, the robust immune response against the virus during convalescence strongly supports the need of active immunization with the up-coming vaccines also in SOT patients.
ACKNOWLEDGMENTS
We thank CERCA Program / Generalitat de Catalunya for their institutional support. We want to particularly acknowledge the patients and the Biobank HUB-ICO-IDIBELL (PT17/0015/0024) integrated in the Spanish National Biobanks Network for their collaboration. The authors acknowledge Ms Gema Cerezo and Ms Iris Alvarez Teubel for careful management of all biological samples, and all kidney transplant unit staff for their support and care of the patients, especially in the context of this pandemic.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
AUTHOR CONTRIBUTIONS
A.F.: Designed the study, collected the data, performed experiments, analyzed the data, drafted the article, and revised the article critically. L.D.: Designed the study, collected the data, performed experiments, analyzed the data, drafted the article, and revised the article critically. I.T.: Performed experiments. N.S.: Collected the data and revised the article critically. V.P.: Collected the data and revised the article critically. J.C.: Revised the article critically. L.L.L.: Revised the article critically. M.M.: Collected the data and revised the article critically. J.J.L.: Performed statistical analyses, J.A.S.: Performed statistical analyses. E.R: Revised the article critically. A.C.: Revised the article critically. A.T.: Performed research and revised the article critically. R.U.: Collected the data and revised the article critically. E.C.: Designed the study, performed research, collected the data, analyzed the data and revised the article critically. E.M.: Revised the article critically. N.M.: Revised the article critically. A.M.: Revised the article critically. A.O.: Revised the article critically. J.M.C.: Revised the article critically. M.L.Q.: Revised the article critically. O.T.: Revised the article critically. O.B.: Conceived and designed the study, analyzed the data, drafted the article, and revised the article critically.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information This work was supported by the Instituto de Salud Carlos III (ISCIII) (grant number COV20/00324).
Footnotes
Alexandre Favà and Laura Donadeu equally contributed as first authors to this work.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Supplementary Material
REFERENCES
- 1.Guan W-J, Ni Z-Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He XI, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020. 10.1038/s41591-020-0869-5 [DOI] [PubMed]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA - J Am Med Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotez PJ, Corry DB, Strych U, Bottazzi ME. COVID-19 vaccines: neutralizing antibodies and the alum advantage. Nat Rev Immunol. 2020;20(7):399–400. doi: 10.1038/s41577-020-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seydoux E, Homad LJ, MacCamy AJ, et al. Analysis of a SARS-CoV-2-infected individual reveals development of potent neutralizing antibodies with limited somatic mutation. Immunity. 2020;53(1) doi: 10.1016/j.immuni.2020.06.001. 98-105.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer AS, Jones FK, Nodoushani A, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5(52):1–13. doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Pan Z, Yue S, et al. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Sig Transduct Target Ther. 2020;5(1):180. doi: 10.1038/s41392-020-00301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7) doi: 10.1016/j.cell.2020.05.015. 1489-1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F, Gan R, Zhen Z, et al. Adaptive immune responses to SARS-CoV-2 infection in severe versus mild individuals. Sig Transduct Target Ther. 2020;5(1):156. doi: 10.1038/s41392-020-00263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekine T, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183(1) doi: 10.1016/j.cell.2020.08.017. 158-168.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sattler A, Angermair S, Stockmann H, et al. SARS-CoV-2 specific T-cell responses and correlations with COVID-19 patient predisposition. J Clin Invest. 2020;130(12):6477–6489. doi: 10.1172/JCI140965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical management of COVID-19. WHO Information Web Site. https://www.who.int/publications/i/item/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Updated May 27, 2020. Accessed November 3, 2020.
- 16.Kolde R. Pheatmap: Pretty Heatmaps [Software]. 2019.
- 17.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzoni A, Salvati L, Maggi L, et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest. 2020;130(9):4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 21.Ni L, Ye F, Cheng M-L, et al. Report detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals ll report detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52(6) doi: 10.1016/j.immuni.2020.04.023. 971-977.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poland GA, Ovsyannikova IG, Kennedy RB. Review SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;6736(20) doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babel N, Anft M, Blazquez-Navarro A, et al. Immune monitoring facilitates the clinical decision in multifocal COVID-19 of a pancreas-kidney transplant patient. Am J Transplant. 2020;20(11):3210–3215. doi: 10.1111/ajt.16252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Candon S, et al. T cell and antibody responses to SARS-CoV-2: experience from a French transplantation and hemodialysis center during the COVID-19 pandemic. Am J Transplant. 2020;00:1–10. doi: 10.1111/ajt.16348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favà A, Cucchiari D, Montero N, et al. Clinical characteristics and risk factors for severe COVID-19 in hospitalized kidney transplant recipients: a multicentric cohort study. Am J Transplant. 2020;20(11) doi: 10.1111/ajt.16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97(6):1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thieme JC, Anft M, Paniskaki K, et al. Robust T cell response toward spike, membrane, and nucleocapsid SARS-CoV-2 proteins is nor associated with recovery in critical COVID-19 patients. Cell Reports Medicine. 2020;1(6):100092. doi: 10.1016/j.xcrm.2020.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 30.Caillard S, Benotmane I, Gautier Vargas G, Perrin P, Fafi-Kremer S. SARS-CoV-2 viral dynamics in immunocompromised patients. Am J Transplant. 2020;ajt.16353. [DOI] [PMC free article] [PubMed]
- 31.Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383(26):2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peiris M, Leung GM. What can we expect from first-generation COVID-19 vaccines? Lancet. 2020. [DOI] [PMC free article] [PubMed]
- 33.Remy KE, Mazer M, Striker DA, et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. 2020;5(17) doi: 10.1172/jci.insight.140329. e140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilk AJ, Rustagi A, Zhao NQ, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26(7):1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willicombe M, Thomas D, McAdoo S. COVID-19 and calcineurin inhibitors: should they get left out in the storm? J Am Soc Nephrol. 2020;31(6):1145–1146. doi: 10.1681/ASN.2020030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoot TS, Kerckhoffs APM, Hilbrands LB, van Marum RJ. Immunosuppressive drugs and COVID-19: a review. Front Pharmacol. 2020;11:1333. doi: 10.3389/fphar.2020.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.