Abstract
As public distribution of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is underway, prevention of coronavirus disease 2019 (COVID‐19) relies on minimizing spread. In this study, chlorhexidine gluconate was investigated as a topical antimicrobial agent against SARS‐CoV‐2. This was a randomized, prospective cohort study using chlorhexidine as an oral rinse and posterior oropharyngeal spray in hospitalized COVID‐19 patients. The primary outcome was presence or absence of laboratory‐confirmed SARS‐CoV‐2 in the oral and oropharyngeal cavities after 4 days of chlorhexidine use and standard of care (study group) or standard of care only (control group). SARS‐CoV‐2 was eliminated from the oropharynx in 62.1% of patients who used chlorhexidine as an oral rinse, versus 5.5% of the control group patients. Among patients who used a combination of oral rinse and oropharyngeal spray, 86.0% eliminated oropharyngeal SARS‐CoV‐2, versus 6.3% of control patients. Chlorhexidine is a simple and safe addition to current COVID‐19 prevention guidelines and may play a significant role in reducing disease spread.
Keywords: antiviral agents, coronavirus, COVID‐19, disinfectants, dissemination, epidemiology, SARS‐CoV‐2, shedding
Highlights
Chlorhexidine is a simple and safe addition to the current COVID‐19 prevention guidelines.
When used with vaccination, proper social distancing, mask wearing, and hand hygiene, chlorhexidine may contribute to a more robust disease prevention regimen.
1. BACKGROUND
Since the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in December 2019, there have been over 113 million confirmed cases of coronavirus disease 2019 (COVID‐19) worldwide resulting in over 2.5 million deaths. 1 , 2 In the absence of effective systemic antimicrobial agents, prophylaxis is crucial for disease control. The gold standard of epidemic disease prophylaxis is a combination of vaccine immunization, use of prophylactic antimicrobial agents, and isolation from the causal microorganism. As public distribution of a SARS‐CoV‐2 vaccine is underway, prophylactic recommendations currently focus on isolation from the virus via social distancing, mask wearing, hand washing, and disease tracing. 3 There is emerging evidence that topical antimicrobial agents may also be useful in preventing COVID‐19 disease spread. 4 , 5 In this study, the authors investigated the use of chlorhexidine gluconate as an oropharyngeal antimicrobial agent against SARS‐CoV‐2 infection.
2. MATERIALS AND METHODS
This was a prospective cohort study performed at four community hospitals in Los Angeles, CA (East Los Angeles Doctors Hospital, Community Hospital of Huntington Park, Alhambra Hospital Medical Center, and Garfield Medical Center). The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committees of the Pipeline Health and AHMC Healthcare hospital systems. Informed consent was obtained from all subjects involved in the study. COVID‐19 patients who were admitted to the COVID‐19 wards at the above hospitals between May 20, 2020, and December 15, 2020 were identified for the study. All COVID‐19 diagnoses were confirmed via detection of SARS‐CoV‐2 in nasopharyngeal swab specimens by real‐time reverse transcriptase‐polymerase chain reaction (rRT‐PCR) by WestPac Labs with the use of the cobas 6800 SARS‐CoV‐2 test (Roche Molecular Systems). The study population included patients who were able to follow instructions to use chlorhexidine as an oral rinse. Patients with nasogastric or endotracheal tubes placed were excluded from the study. Patients who were symptomatic for over a week before admission were also excluded.
From this population, patients were randomly assigned to the study and control groups. Both groups received the standard of care in their treatment plans. The study group was given chlorhexidine to use as an oropharyngeal rinse while the control group was not. For each administration of chlorhexidine, a unit dose cup containing 0.5 ounces (15 ml) of commercially available chlorhexidine gluconate (0.12%) was provided to each patient. Patients were then observed to self‐administer the solution as a thorough oral rinse for 30 s twice a day. After 4 days, the oropharynx was swabbed and tested for the presence of SARS‐CoV‐2 by rRT‐PCR.
Since an oral rinse alone would not reach the posterior oropharynx effectively, a chlorhexidine spray was added to the oral rinse regimen in the second study group. After the patient used chlorhexidine as an oral rinse as stated above, a provider used a spray applicator to deliver three sprays (a total of approximately 1.5 ml) of the chlorhexidine solution to the posterior oropharynx. To open the posterior pharynx, the patient was instructed to vocalize “ah” for 5 s while the solution was sprayed. This process was performed twice a day for 4 days. After 4 days of chlorhexidine administration, the oropharynx was swabbed and tested for presence of SARS‐CoV‐2 by rRT‐PCR. A paired t‐test was used to compare findings, with a significance level of .05.
Fourteen nurses and one physician in the hospitals where this study was conducted followed the above chlorhexidine oral rinse with oropharyngeal spray regimen twice a day in addition to social distancing, mask wearing, and hand washing to prevent contracting COVID‐19. The healthcare workers were followed for the duration of the study, and adherence to the chlorhexidine regimen and COVID‐19 status was self‐reported. The rate of SARS‐CoV‐2 infection among this group was compared to the rate of infection among all healthcare workers in their respective hospitals.
3. RESULTS
684 patients with positive SARS‐CoV‐2 infection were identified (Figure 1). The mean onset of symptoms was 5.6 days before admission (standard deviation, 2.3; range, 1–16). 390 patients were excluded for symptom onset of greater than 6 days before admission, placement of nasogastric or endotracheal tubes, or inability to follow instructions to use chlorhexidine. 58% of the study population was male, 42% was female, and the median age was 62 years (range, 23–89). 294 patients were included in analysis, with 159 patients in the study group receiving chlorhexidine and 135 patients in the control group. All patients received the standard of care for COVID‐19, which included remdesivir, anticoagulation, steroids, and oxygen therapy. There was no significant difference in response to treatment between the two groups.
Figure 1.
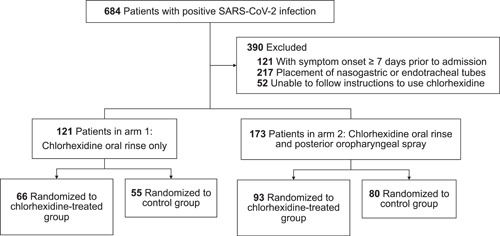
Participant flow in a randomized control trial of chlorhexidine in hospitalized COVID‐19 patients
3.1. Oropharyngeal specimens after oral rinse only
A total of 121 patients were included in the analysis of using chlorhexidine as an oral rinse in COVID‐19 patients (Table 1). Out of the 66 patients in the chlorhexidine‐treated group, 41 (62.1%) were found to be negative and 25 (37.9%) were found to be positive for SARS‐CoV‐2 in the oropharynx. On the same testing in the control group of 55 patients, 3 (5.5%) tested negative and 52 (94.6%) tested positive. The differences in the results were statistically significant between the two groups (p < .01).
Table 1.
Detection of SARS‐CoV‐2 by rRT‐PCR in the oropharynx of COVID‐19 patients after a 4‐day course of chlorhexidine oropharyngeal rinse
| Positive | Negative | Total | |
|---|---|---|---|
| Chlorhexidine‐treated group | 25 (37.9%) | 41 (62.1%) | 66 |
| Control group | 52 (94.5%) | 3 (5.5%) | 55 |
Note: Patients in the study group used a chlorhexidine oral rinse twice a day for 4 days. Patients in the control group received no chlorhexidine. Oropharyngeal specimens tested for presence of SARS‐CoV‐2 by rRT‐PCR on the fourth day. P‐value of chlorhexidine‐treated versus control groups was <.01.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. Oropharyngeal specimens after oral rinse with oropharyngeal spray
Oropharyngeal specimens from 173 patients were included in the evaluation of using a chlorhexidine oral rinse with additional posterior oropharyngeal chlorhexidine spray in COVID‐19 patients (Table 2). 80 out of 93 (86.0%) of the chlorhexidine‐treated patients were negative for presence of SARS‐CoV‐2, while 5 out of 80 (6.2%) of the control patients were found to be negative for the virus. The differences in the results were statistically significant between the two groups (p < .01).
Table 2.
Detection of SARS‐CoV‐2 by rRT‐PCR in the oropharynx of COVID‐19 patients after a 4‐day course of chlorhexidine oropharyngeal rinse and posterior oropharyngeal spray
| Positive | Negative | Total | |
|---|---|---|---|
| Chlorhexidine‐treated group | 13 (14.0%) | 80 (86.0%) | 93 |
| Control group | 75 (93.8%) | 5 (6.2%) | 80 |
Note: Patients in the study group used a chlorhexidine oral rinse and posterior oropharyngeal spray twice a day for 4 days. Patients in the control group received no chlorhexidine. Oropharyngeal specimens tested for presence of SARS‐CoV‐2 by rRT‐PCR on the fourth day. P‐value of chlorhexidine‐treated versus control groups was <.01.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.3. Use of chlorhexidine in healthcare workers
A group of 15 healthcare workers (14 nurses and 1 physician) used chlorhexidine as an oral rinse and oropharyngeal spray twice a day as described above for the duration of the study. None were observed to develop SARS‐CoV‐2 infection during the course of this study. In contrast, the rate of COVID‐19 among all healthcare workers in their respective hospitals during the same time period was near 50%.
3.4. Adverse effects
No adverse effects of using chlorhexidine as outlined were observed.
4. DISCUSSION
Chlorhexidine is an antimicrobial agent that is commonly used in the preoperative preparation of skin to prevent postoperative infections, dental plaque prevention, and the prevention of ventilator‐associated pneumonia. 6 , 7 , 8 Due to its cationic nature, chlorhexidine has been shown to be effective in killing enveloped viruses. 9 As SARS‐CoV‐2 is an enveloped virus, chlorhexidine has been shown to be effective against SARS‐CoV‐2. 10
The data from this study show a significant elimination of SARS‐CoV‐2 with chlorhexidine application in the oral cavity and pharynx. In an effort to deliver chlorhexidine to the posterior oropharynx more effectively, a spray applicator was used to apply chlorhexidine to the posterior oropharynx directly. In this study population, the addition of the posterior oropharyngeal chlorhexidine spray was associated with the elimination of SARS‐CoV‐2 from the oropharynx in 86.0% of patients, compared with 62.1% in patients who used chlorhexidine as an oral rinse alone. This result suggests that applying chlorhexidine to the posterior oropharynx greatly improved clearance of SARS‐CoV‐2 from the oropharynx.
Chlorhexidine was noted to be very effective in preventing SARS‐CoV‐2 infection in a small group of healthcare workers when compared with the general hospital healthcare worker population in this study. The observation is encouraging, but the formal analysis was not performed on this group. Further study with a larger cohort is needed to investigate the use of chlorhexidine in preventing COVID‐19 in healthcare workers.
The authors propose that the use of chlorhexidine as an oropharyngeal rinse may serve two purposes: (1) to prevent viral spread from COVID‐19 patients to others and (2) to prevent SARS‐CoV‐2 infection in the case of exposure to the virus. For prevention of viral spread from SARS‐CoV‐2‐infected individuals to others, the authors recommend using chlorhexidine gluconate 0.12% mouthwash twice a day as follows: (1) spray 1 ml to the nares, (2) rinse the throat thoroughly with 15 ml for at least 30 s, and (3) use a spray applicator to spray the posterior throat three times (1.5 ml). This process may be continued until the virus is naturally cleared from the body, which takes approximately 2–3 weeks. 11 For postexposure prophylaxis, the authors recommend the above chlorhexidine regimen for 2–4 days. The authors must emphasize that chlorhexidine cannot be used to treat COVID‐19, and strongly caution against ingesting chlorhexidine in any attempt to eradicate the disease.
4.1. Limitations
This study has several limitations. First, the method of chlorhexidine application performed in this study does not reach the nasopharynx, which is a route of respiratory droplet transmission. 12 Chlorhexidine could theoretically be applied to the anterior nares, and further study is needed to explore this topic. Second, the study was not blinded, which potentially led to biases in patient care and data reporting. Third, the study was conducted before the SARS‐CoV‐2 variants were identified in the United States. 13 Chlorhexidine should theoretically be effective against SARS‐CoV‐2 variants given its effect against enveloped viruses broadly, but variant strains were not investigated in this study.
5. CONCLUSIONS
Chlorhexidine used as an oral rinse and posterior oropharyngeal spray is a simple and safe addition to the current COVID‐19 prevention guidelines and may have significant effects on controlling the spread of the disease. When used with vaccination, proper social distancing, mask wearing, and hand washing, chlorhexidine may contribute to a more robust disease prevention regimen.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Huang YH, Huang JT. Use of chlorhexidine to eradicate oropharyngeal SARS‐CoV‐2 in COVID‐19 patients. J Med Virol. 2021;93:4370‐4373. 10.1002/jmv.26954
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author on reasonable request.
REFERENCES
- 1. World Health Organization . Weekly Epidemiological Update – 2 March 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update---2-march-2021. Accessed March 8, 2021.
- 2. Khokhar M, Roy D, Purohit P, Goyal M, Setia P. Viricidal treatments for prevention of coronavirus infection. Pathog Glob Health. 2020;114(7):349‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Coronavirus Disease 2019 (COVID‐19): How to Protect Yourself and Others. Atlanta, GA: US Department of Health and Human Services, CDC; 2020.
- 4. Carrouel F, Gonçalves LS, Conte MP, et al. Antiviral activity of reagents in mouth rinses against SARS‐CoV‐2. J Dent Res. 2020;100(2):124‐132. 10.1177/0022034520967933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meyers C, Robison R, Milici J, et al. Lowering the transmission and spread of human coronavirus. J Med Virol. 2021;93(3):1605‐1612. [DOI] [PubMed] [Google Scholar]
- 6. Anderson DJ, Podgorny K, Berríos‐Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones CG. Chlorhexidine: is it still the gold standard? Periodontol. 1997;2000:55‐62. [DOI] [PubMed] [Google Scholar]
- 8. Houston S, Hougland P, Anderson JJ, LaRocco M, Kennedy V, Gentry LO. Effectiveness of 0.12% chlorhexidine gluconate oral rinse in reducing prevalence of nosocomial pneumonia in patients undergoing heart surgery. Am J Crit Care. 2002;11(6):567‐570. [PubMed] [Google Scholar]
- 9. Denton GW. Chlorhexidine. In: Block SS, ed. Disinfection, Sterilization and Preservation. 4th ed. Philadelphia: Lea and Febiger; 1991:274‐289. [Google Scholar]
- 10. Yoon JG, Yoon J, Song JY, et al. Clinical significance of a high SARS‐CoV‐2 viral load in the saliva. J Korean Med Sci. 2020;35(20):e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID‐19. Lancet Infect Dis. 2020;20(6):656‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dhand R, Li J. Coughs and sneezes: their role in transmission of respiratory viral infections, including SARS‐CoV‐2. Am J Respir Crit Care Med. 2020;202(5):651‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Firestone MJ, Lorentz AJ, Wang X, et al. First identified cases of SARS‐CoV‐2 variant B.1.1.7 in Minnesota – December 2020‐January 2021. Morb Mortal Wkly Rep. 2021;70(8):278‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.


