Dear Editor,
The coronavirus disease 2019 (COVID‐19) has been associated to a wide clinical spectrum of skin manifestations, including chilblain‐like, urticarial, vesicular, maculopapular, livedoid and vasculitic lesions, among others. 1 , 2 However, the exact pathophysiology for the appearance of skin lesions is still unknown. Several hypotheses have been suggested, including viral hypersensitivity reactions, overexpression of type I interferons, COVID‐19 induced coagulopathy, thrombotic microangiopathy and direct viral damage. 3 , 4 , 5 , 6 Potentially, some skin manifestations could also appear after vaccination with mRNA vaccines encoding the spike (S) protein of SARS‐CoV‐2. A delayed hypersensitivity reaction at the injection site of Moderna (mRNA‐1273) 7 and Pfizer‐BioNTech, Puurs, Belgium (BNT162b2) 8 vaccines has been recently described in the mass media as ‘COVID‐arm’. The mRNA‐1273 vaccine clinical trial reported delayed injection‐site reactions (onset after day 8) in 0.8% participants after the first dose and in 0.2% after the second dose. 7 The BNT162b2 clinical trial does not differentiate between immediate and delayed injection‐site reactions, with an overall incidence of 5–7% after the first and second dose. 8 In addition, delayed inflammatory reactions to dermal fillers have also been described. 9
We designed a retrospective study to characterize the skin manifestations of the BNT162b2 mRNA COVID‐19 vaccine in a tertiary referral hospital of Spain. A registry of vaccine‐related side effects was created by the Occupational Health Department, including delayed injection‐site reactions (Table 1). This vaccination campaign was conducted from January 11 to February 12 2021. Physical examination and duration of the skin manifestations were either directly evaluated or indirectly evaluated through clinical pictures. A skin biopsy was also performed in two cases.
Table 1.
Characteristics and demographic data of the subjects with delayed injection‐site reaction obtained from the registry of the BNT162b2 mRNA COVID‐19 vaccine
| Characteristics | |
|---|---|
| Number of subjects | 103 |
| Age, mean, years (range) | 40.4 (20‐64) |
|
Sex, male (%) Sex, female (%) |
12 (11.7%) 91 (88.3%) |
|
After 1st dose After 2nd dose |
49 (47.6%) 54 (52.4%) |
| Itch (%) | 70 (68.0%) |
|
Duration <8h (%) Duration 8‐24h (%) Duration 24‐72h (%) Duration >72h (%) |
23 (22.3%) 28 (27.1%) 38 (36.9%) 14 (13.6%) |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
From 4775 subjects that underwent BNT162b2 mRNA vaccination, a total of 864 overall side effects were registered (18.1%). The mean age was 43.2 years (range 19–72), and 721 (83.4%) patients were female. A delayed injection‐site reaction (Fig. 1a) was present in 103 subjects (2.1%), either after the first dose (49/103; 47.6%) or after the second dose (54/103, 52.4%). 16/49 subjects (32.7%) had recurrent lesions with the second dose. It lasted for less than 8 h in 23 patients (22.3%), between 8 and 24 h in 27 patients (26.2%), between 48 and 72 h in 38 patients (36.9%) and more than 72 h in 14 patients (13.6%). Itch was reported in 70 patients (68.0%). Five patients (4.9%) also presented disseminated lesions. None of these patients developed anaphylactic symptoms. In addition, two cases (2/4775; 0.04%) of vaccine‐related urticaria were registered, lasting less than a week and responding to oral antihistamines. Histologic examination of a delayed injection‐site reaction (Fig. 1b) showed a superficial and deep perivascular lymphocytic infiltrate, with dilated vessels and intraluminal neutrophils. Immunohistochemistry for the SARS‐CoV‐2 spike 1A9 protein (GeneTex, Irvine, CA, USA) was negative.
Figure 1.
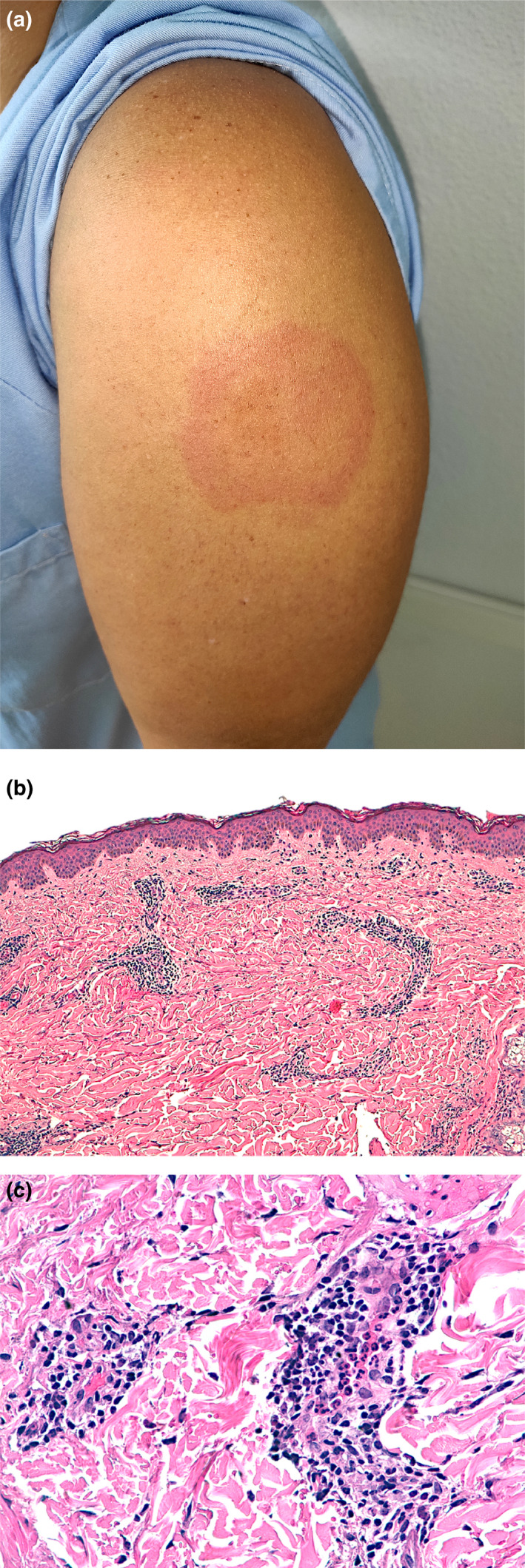
(a) A 34‐year‐old female healthcare worker showing a slightly indurated erythematous targetoid patch at the injection‐site reaction of 6 × 4.5 cm diameter, 8 days after the first dose of the BNT162b2 vaccine. (b) Histologic examination shows a superficial and deep perivascular lymphocytic infiltrate (H‐E ×100). (c) Close‐up examination shows dilated vessels, repleted with intraluminal neutrophils. An increased number of eosinophils or mast cells was not present (H‐E ×400).
Currently, there are scarce reports of skin side effects related to COVID‐19 vaccines. Recently, a case series of delayed large local reactions to the mRNA‐1273 vaccine has been published, including 12 cases. 10 The median onset was on day 8 (range 4–11) after the first dose and resolved in a median of 6 days (range 2–11). Half of the patients had similar recurrent reactions after the second dose.
This delayed injection‐site reaction shows similar features to COVID‐19 exanthems. 3 Whether it corresponds to a hypersensitivity reaction to the spike protein or to different components of the vaccine is still unknown. We also found two cases of urticaria triggered by the vaccine, in a similar fashion to the actual SARS‐CoV‐2 infection. 6 The main limitation of the study is the self‐reported and retrospective nature of the registry, so skin manifestations are probably under‐ascertained. No severe cutaneous reactions were present in the study, suggesting that the BNT162b2 mRNA vaccine has a good safety profile regarding skin side effects.
Funding source
This article has no funding source.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgements
The patients in this manuscript have given written informed consent to publication of their case details. Written consent was obtained from the patients included in this study. All human studies are approved by an Institutional Review Board.
References
- 1. Galván Casas C, Català A, Carretero Hernández G et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183: 71–77. 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marzano AV, Genovese G, Moltrasio C et al. The clinical spectrum of COVID‐19‐associated cutaneous manifestations: an Italian multicentre study of 200 adult patients. J Am Acad Dermatol 2021; 84: 1356–1363. 10.1016/j.jaad.2021.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garrido Ruiz MC, Santos‐Briz Á, Santos‐Briz Á et al. Spectrum of clinicopathologic findings in COVID‐19‐induced skin lesions: demonstration of direct viral infection of the endothelial cells. Am J Surg Pathol 2021; 45: 293–303. [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Cao W, Xiao M et al. Clinical and coagulation characteristics in 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi Zhonghua Xueyexue Zazhi 2020; 41: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kolivras A, Dehavay F, Delplace D et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathologic findings. JAAD Case Rep 2020; 6: 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kubanov AA, Deryabin DG. Skin manifestations in COVID‐19 provide a clue for disease’s pathophysiology understanding. J Eur Acad Dermatol Venereol JEADV 2021; 35: e3–e4. 10.1111/jdv.16902 [DOI] [PubMed] [Google Scholar]
- 7. Baden LR, El Sahly HM, Essink B et al. Efficacy and Safety of the mRNA‐1273 SARS‐CoV‐2 Vaccine. N Engl J Med 2021; 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Munavalli GG, Guthridge R, Knutsen‐Larson S et al. COVID‐19/SARS‐CoV‐2 virus spike protein‐related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res 2021; 9: 1–15. 10.1007/s00403-021-02190-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blumenthal KG, Freeman EE, Saff RR et al. Delayed large local reactions to mRNA‐1273 vaccine against SARS‐CoV‐2. N Engl J Med 2021; 384: 1273–1277. 10.1056/NEJMc2102131 [DOI] [PMC free article] [PubMed] [Google Scholar]


