Abstract
Neuroimaging techniques that can sensitivity characterize healthy brain aging and detect subtle neuropatholo-gies have enormous potential to assist in the early detection of neurodegenerative conditions such as Alzheimer’s disease. Magnetic resonance elastography (MRE) has recently emerged as a reliable, high-resolution, and especially sensitive technique that can noninvasively characterize tissue biomechanical properties (i.e., viscoelasticity) in vivo in the living human brain. Brain tissue viscoelasticity provides a unique biophysical signature of neuroanatomy that are representative of the composition and organization of the complex tissue microstructure. In this article, we detail how progress in brain MRE technology has provided unique insights into healthy brain aging, neurodegeneration, and structure-function relationships. We further discuss additional promising technical innovations that will enhance the specificity and sensitivity for brain MRE to reveal considerably more about brain aging as well as its potentially valuable role as an imaging biomarker of neurodegeneration. MRE sensitivity may be particularly useful for assessing the efficacy of rehabilitation strategies, assisting in differentiating between dementia subtypes, and in understanding the causal mechanisms of disease which may lead to eventual pharmacotherapeutic development.
Keywords: Aging, Brain, Neuroimaging, Mechanical properties, Biomarkers, Stiffness
1. Introduction
With increasing age, the human brain is subjected to structural, chemical, molecular, and electro-physiological changes that impact its ability to function. Physical signs of brain aging can be identified through conventional neuroimaging, where it is frequently reported that the volume of the brain declines with age at a rate of around 5% per decade after age 40 (Peters, 2006), most likely due to the degeneration and death of neurons and oligodendrocytes (Anderton, 2002). Tissue volume loss is accompanied by increases in ventricular volume and other cerebrospinal fluid spaces, as well as the appearance of white matter lesions, alterations to brain vasculature, and depletion of neurotransmitters. While brain aging is inevitable to some extent, it is also a complex and heterogeneous process with no uniform signature. The interplay between both genetic and environmental factors are likely to determine the degree and rate of brain aging, which ultimately reflects in the high inter-individual variability in neurocognitive abilities observed in the elderly. As a result, new techniques are needed to enhance the study of the mechanisms behind healthy aging to better understand individual differences in the aging process, and to assist ongoing efforts to combat age-associated neurodegeneration and eventual disease and disorder.
Quantitative neuroimaging contrasts provide an attractive option to more precisely measure age-related changes to brain health and microstructure. Compared to morphometric measures, which capture tissue geometry that is presumably influenced by microstructure, quantitative contrasts offer measures more directly related to microscale characteristics (Paus, 2018). Recently, magnetic resonance elastography (MRE) (Muthupillai et al., 1995) has been applied to study brain health through imaging tissue biomechanics (Hiscox et al., 2016; Murphy et al., 2019). MRE probes tissue viscoelasticity non-invasively and in vivo, which is sensitive to brain tissue architecture and reflects the composition and organization of the underlying microstructure (Guo et al., 2019; Sack et al., 2013). Since the first brain MRE publication in 2005, the field has experienced considerable growth with now almost 150 dedicated publications (determined via PubMed search June 2020 using PubMed with key words “brain” and “magnetic resonance elastography”; Fig. 1). These papers comprise both methodological enhancements and clinical applications, with studies of healthy aging and age-related neurological disease providing the most robust body of literature in the field. Several different research groups across the world have now reported that the brain becomes softer with increasing age, which is likely to reflect a loss of neurons and their synaptic connections. Through continuous developments and more advanced methodologies, brain MRE has transitioned to a reliable, specific, and especially sensitive neuroimaging modality (Mariappan et al., 2010, Manduca et al., 2021). As a result, the field has begun to delve further into understanding more precise localized aging effects, which ultimately has implications for improving understanding of age-related cognitive decline and neurodegeneration.
Fig. 1.
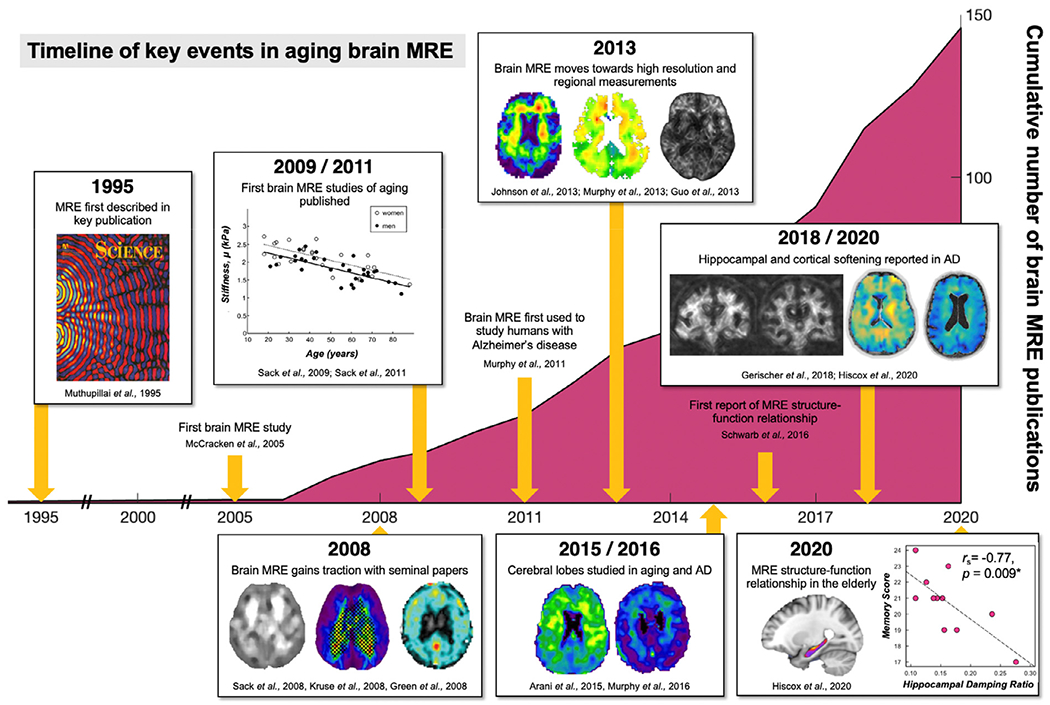
Summary of the key events in brain aging MRE and the growth of brain MRE studies since the first publication in 2005. Manuscript production in absolute numbers per year. Search was performed in June 2020 using PubMed with key words “brain” and “magnetic resonance elastography” (Green et al., 2008, Curtis L Johnson et al., 2013, Kruse et al., 2008, McCracken et al., 2005).
1.1. Magnetic resonance elastography of the brain
Regardless of the organ of interest, there are three main components that constitute an MRE examination. First, shear waves of sufficient amplitude must be delivered into the tissue by gentle vibration using an external mechanical actuator; second, the resulting tissue displacements need to be encoded into the MRI signal using customized phase-contrast pulse sequences; and third, displacement images must be inverted using a mathematical reconstruction to recover and quantify the underlying mechanical properties of the tissue. Mechanical actuators for brain MRE need to gently vibrate the entire head to induce shear tissue deformation, typically at 50 or 60 Hz; brain MRE pulse sequences should capture full vector wave fields at high spatial resolution across the entire brain; and inversion algorithms should account for significant material heterogeneity due to the varying types of cerebral tissue. An overview of a typical brain MRE procedure is illustrated in Fig. 2. The vibrations used for brain MRE have an extremely small, micron-level amplitude (Ehman et al., 2008) and consequently are safe and well tolerated, even in the elderly (Hiscox et al., 2018).
Fig. 2.
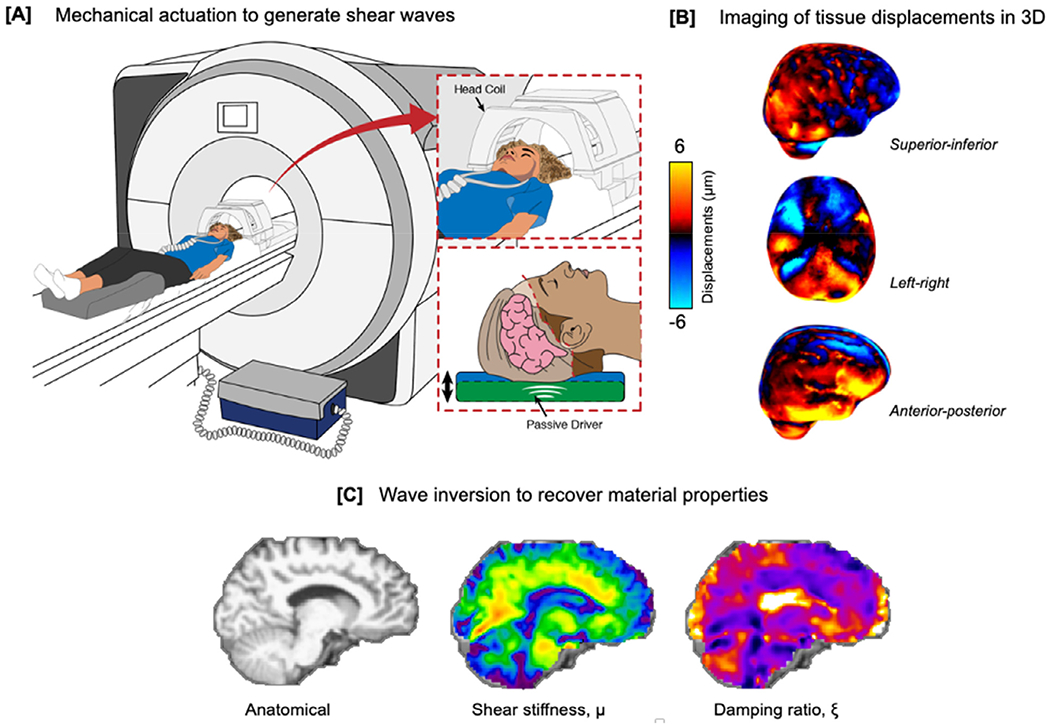
Overview of a typical brain magnetic resonance elastography investigation. (a) An external pneumatic mechanical actuator is used to gently vibrate the head and generate steady-state shear wave fields in brain tissue; (b) specialized phase-contrast MRI sequences image the resulting displacements through synchronization with applied vibration; and (c) an inversion algorithm is used to recover mechanical properties from the imaged wave field: μ, shear stiffness (kPa), and ξ, damping ratio (dimensionless).
Brain tissue exhibits viscoelastic behavior and the most common output measures from MRE express both the elastic and viscous components through the complex shear modulus . Here, the storage modulus (G’ reflects the elastic properties that describe the ability of a material to return to its original shape after deformation forces have been removed. In contrast, the loss modulus (G”) is associated with the viscous properties of tissue that cause attenuation of the waves as they travel through a material and relate to the absorption of mechanical energy. All MRE studies report parameters related to these terms, a full summary of which has been thoroughly described by Manduca et al., 2021. Regardless of which of these material parameters are reported across studies, all calculations rely on a series of mathematical assumptions within the inverse solution, with the brain typically modelled as a heterogenous, isotropic, and incompressible material.
In single frequency brain MRE studies, it has become increasingly common to report the composite parameter of shear stiffness, , which is the resistance of a viscoelastic material to an applied harmonic forcing, and is equivalent to the density × wave speed squared (Manduca et al., 2001) or shear wave speed reported by other groups (Herthum et al., 2021). In this case, μ can be regarded as a wave-field parameter in which measurements describe a purely elastic object that exhibits the observed wavelength at the driving frequency. To capture the full viscoelastic behavior, reporting is then typically coupled with a dimensionless parameter such as the damping ratio, which describes the relative viscous-to-elastic behavior of tissue, with higher values indicative of greater relative viscosity (Manduca et al., 2021). While the earliest brain MRE studies did not regularly report parameters related to the loss modulus, it has now become more common due to increased confidence in measurement accuracy and reliability (Johnson et al., 2016). Note that while μ and ξ are often reported for single frequency MRE studies, multifrequency MRE experiments instead seek to fit general dispersion behavior of brain tissue. In these cases, parameters such as the magnitude of the complex modulus and its phase angle, are often reported instead (Barnhill et al., 2019; Gerischer et al., 2017; Manduca et al., 2021).
The measured mechanical properties obtained from MRE reflect microstructural characteristics of neural tissue (Sack et al., 2013). Just as the constitutive elements of man-made structures determine their rigidity and flexibility, so too does the microenvironment determine the macroscopic mechanical response of the brain. Evidence from animal models combined with ex vivo histology of tissue slices has suggested that stiffness may reflect the composition of individual tissue components. For example, increased stiffness has been observed due to the generation of new neurons from a dopamine defect (Klein et al., 2014), to the density of intermediate filaments induced from reactive gliosis (Lu et al., 2011), and to increased myelin content (Weickenmeier et al., 2016, 2017), while decreased brain stiffness has been associated with neuronal loss in a mouse model of stroke (Freimann et al., 2013) and demyelination provoked by a cuprizone diet (Schregel et al., 2012). On the other hand, the relative viscous-to-elastic behavior described by the damping ratio or phase angle is thought to generally reflect the geometric organization of tissue components and their network complexity (Guo et al., 2012; I Sack et al., 2013). In a recent developmental mouse model, viscosity was associated with actin crosslinking and axonal organization (Guo et al., 2019). As such, the unique sensitivity and contrast afforded by MRE offers considerable potential for providing new insights into age-related neurological changes through a quantitative representation of the microstructural constituents of tissue.
As effects of aging on brain tissue integrity is both subtle and largely localized to specific neuroanatomical structures, researchers have begun to employ high-resolution MRE methods to improve mapping of brain biomechanics. This has been made possible through continued technological advances to imaging and inversion schemes. For example, the development of spiral MRE pulse sequences (Johnson et al., 2013, 2014), finite-element based nonlinear inversion (McGarry et al., 2012; Van Houten et al., 1999), multi-frequency MRE (Guo et al., 2013; Papazoglou et al., 2012; Barnhill et al., 2018), and inversion with machine learning and artificial neural networks (Murphy et al., 2018, 2020), have each contributed to improving the validity, reliability, and sensitivity of brain MRE. While beyond the remit of this review, we encourage interested readers to refer to the citations provided for aspects of MRE methods that would be relevant to studying the aging brain. As a result of these contributions, brain MRE has moved away from reporting average tissue stiffness of the brain parenchyma, to reliable measures that enable the study of individual neuroanatomical regions, including white matter tracts (Anderson et al., 2016; Johnson et al., 2013; Romano et al., 2012), subcortical gray matter structures (Hetzer et al., 2018; Johnson et al., 2016), hippocampal subfields (Daugherty et al., 2020; Delgorio et al., 2020), and the cerebral cortex (Hiscox et al., 2020a; McIlvain et al., 2020). In the following sections we examine how high-resolution MRE has allowed researchers to obtain sensitive and reliable mechanical measures to better understand age and disease. Example high-resolution MRE maps of a healthy young, and older adult are illustrated in Fig. 3.
Fig. 3.
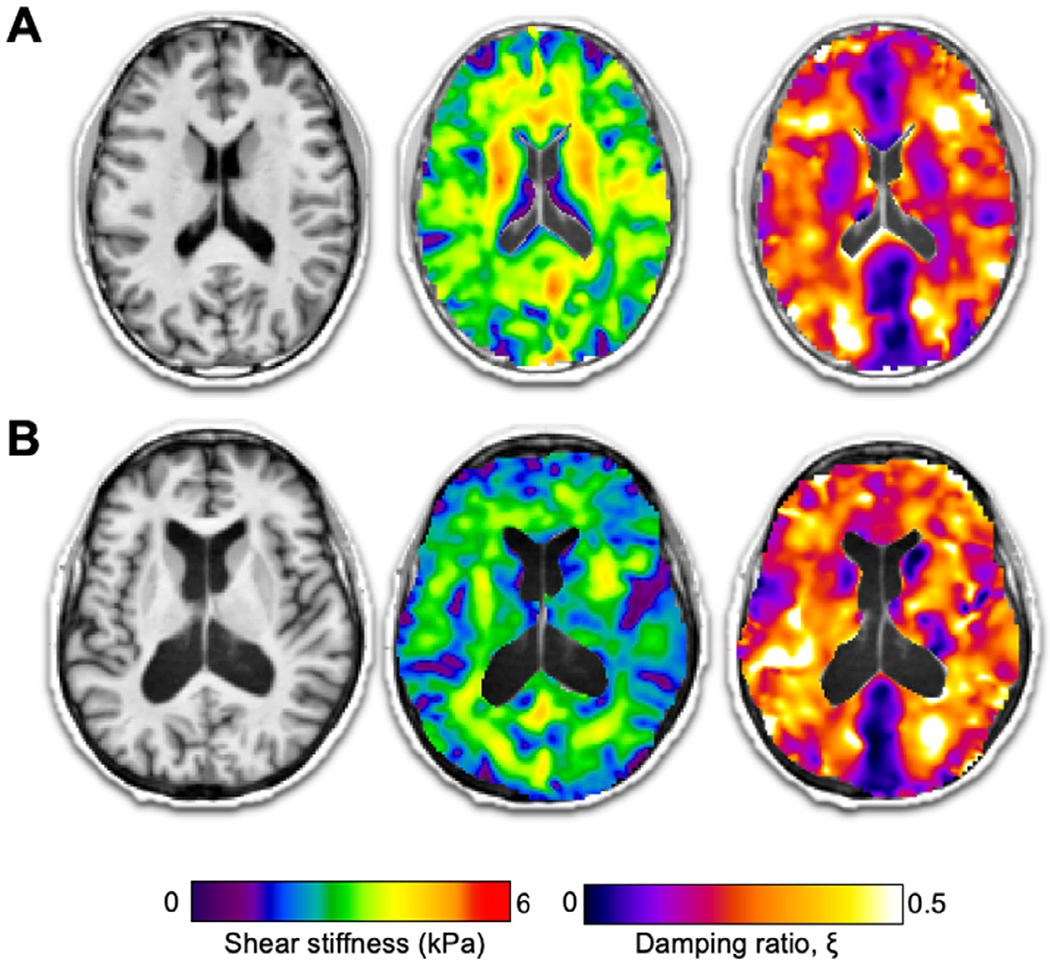
Structural T1−, weighted MRI images, and high-resolution MRE shear stiffness, μ, and damping ratio, ξ, maps for a (A) 23-year-old male, and (B) 65-year-old female. Widespread softer tissue and increased damping behavior is visibly notable in the older adult. Note that MRE inversion techniques do not model fluids and therefore are not valid in CSF spaces, including the lateral ventricles.
1.2. Viscoelasticity of the aging brain
Several studies have utilized MRE to study healthy brain aging; information regarding the MRE protocols used as well as the main results are provided in Table 1. In one of the earliest brain MRE studies, Sack, et al., investigated healthy brain aging in adults aged between 18 and 88 years and reported a significant linear decline in whole-brain averaged stiffness (Sack et al., 2009). The annual 0.8% reduction in stiffness was broadly speculated to be attributable to the degeneration of neurons and oligodendrocytes. Shortly afterwards, the same group reported that the decline in whole-brain stiffness was three times greater than changes in brain volume measurements (Sack et al., 2011). These were important discoveries as, first, it suggested that MRE may be more sensitive at detecting aging effects beyond those obtained from structural MRI, and second, that MRE measures were independent of geometry effects and in fact reflected a tissue-intrinsic structure alteration.
Table 1.
Summary of Brain MRE studies that have investigated healthy aging.
| Author | N | Frequency (Hz) | MRE resolution | Inversion algorithm | Age-range | Annual change in cerebral stiffness | Maximum regional change per year | Minimum regional change per year |
|---|---|---|---|---|---|---|---|---|
| Sack et al., 2009 | 55 | 25, 37.5, 50, 67.5 | 2.3 × 2.3 × 6 mm3 | Direct inversion + multi-frequency fit | 18 – 88 years | −0.025 kPa | Inner ROI: −0.036 kPa | Posterior: −0.013 kPa |
| Arani et al., 2015 | 45 | 60 | 3.0 × 3.0 × 3.0 mm3 | Direct inversion | 56 – 89 years | −0.011 kPa | Temporal lobe: −0.014 kPa | Cerebellum: −0.001 kPa |
| Hiscox et al., 2018 | 12 young vs 12 old | 50 | 1.6 × 1.6 × 1.6 mm3 | Nonlinear inversion | 19 – 30 versus 66 - 73 years | −0.006 kPa | Caudate: −0.016 kPa | Hippocampus: −0.005 kPa |
| Takamura et al., 2020 | 50 | 60 | 3.0 × 3.0 × 3.0 mm3 | Direct inversion | 20 - 69 years | −0.008 kPa | Sensory-motor ROI: −0.014 kPa | Cerebellum: −0.002 kPa |
| Lv et al., 2020 | 46 | 40, 60, 80, 90 | 1.8 × 1.8 × 3 mm3 | Direct inversion + multi-frequency fit | 26 - 76 years | −0.012 kPa | Caudate: −0.012 kPa | Hippocampus −0.001 kPa |
| Delgorio et al., 2020 | 54 | 50 | 1.25 × 1.25 × 1.25 mm3 | Nonlinear inversion | 23 – 81 years | N/A | Entorhinal cortex: −0.014 kPa | Subiculum: −0.011 kPa |
μ2 was used as the multifrequency viscoelastic shear stiffness outcome from Lv et al., 2020.
With improved MRE imaging methods to capture whole-brain, 3D MRE displacement data coupled with more stable inversion protocols, the ability to accurately quantify brain stiffness moved from non-specific global measures to examining lobar regions (Murphy et al., 2013). As a result, Arani, et al., studied the effect of healthy aging on regional brain stiffness measurements in 45 older participants aged between 56 and 89 years (Arani et al., 2015). Complementing the previous studies mentioned, a significant negative linear correlation was observed between age and global cerebrum stiffness, in addition to a linear age-related decline in stiffness within the individual frontal, occipital, parietal, and temporal lobes. Interestingly, no relationship between brain stiffness and age was observed in the cerebellum or sensory-motor regions. Similar results were reported by Takamura, et al., who found softening to all four lobes in 50 participants across a younger age range (20–60 years) (Takamura et al., 2020), but also not in the cerebellum. This study also reported how stiffness of all lobes differed between participants in their twenties compared with participants in their sixties, whereas volumetric differences between the two groups were minimal. These studies demonstrated the specificity for MRE to detect age-related effects within only certain brain partitions and which were also greater than age-related reductions in volume.
Using higher resolution MRE methods at a 1.6 mm isotropic resolution, Hiscox, et al., investigated aging effects on the mechanical properties of a range of subcortical gray matter structures, which are expected to support cognitive processes that may decline in old age (Hiscox et al., 2018). A sample of 12 healthy older adults (age range: 66–73 years) were found to exhibit reduced brain stiffness in the amygdala, caudate, pallidum, putamen, and thalamus compared to a group of 12 healthy young adults (age range: 19–30 years). Moreover, differences in MRE properties were independent of concomitant volume differences, which is suggestive of the additive value of MRE over traditional volumetric measures. The hippocampus did not exhibit a statistically significant difference between groups, although it did show a trend in the same direction for being softer in the older adult group; the absence of statistical significance was attributed to low statistical power given by the small sample size.
As the spatial resolution of brain MRE continued to evolve, Delgorio, et al., recently demonstrated the feasibility of performing MRE of the hippocampal subfields through the development of a specifically tailored high-resolution MRE protocol that combined 1.25 mm isotropic displacement data with NLI parameters tuned for high sensitivity and reliability (Delgorio et al., 2020). Viscoelasticity was quantified for four subregions including cornu ammonis areas 1 and 2 combined (CA1-CA2), dentate gyrus and cornu ammonis area 3 combined (DG-CA3), entorhinal cortex, and subiculum. In the cross-sectional study involving 54 participants (age range: 23–81 years), older age was associated with softer subfields with the magnitude of aging effects dependent on region. Entorhinal cortex showed the greatest annual change (0.014 kPa), whereas the subiculum exhibited the lowest (0.011 kPa). These results complement existing structural MRI and diffusion data in which differential age-related trajectories in subfield volumes have been observed (Daugherty et al., 2016; Mueller et al., 2007; Raz et al., 2005). There is evidence, however, that age-related variability in the volume of the entorhinal cortex and subiculum are minimal (Daugherty et al., 2016), suggesting that brain stiffness may be more sensitive marker to the early stages of age-related neurodegeneration.
Aging is also a common target for the application of new brain MRE methods to demonstrate the ability to recover “biologically-relevant” effects, such as with higher resolution inversion approaches (Barnhill et al., 2018; Murphy et al., 2018). One area of development that has received attention recently is in recovery of anisotropic mechanical properties in the brain with MRE. White matter tracts consist of highly-aligned, myelinated axonal fiber bundles, which cause anisotropic mechanical properties, and as such, stiffness of white matter is both regionally and directionally dependent (Anderson et al., 2016; Prange and Margulies, 2002; Smith et al., 2020). However, typical MRE methods assume mechanical isotropy to simplify analysis that has so far limited the ability to reliably assess the stiffness of white matter tracts and how it is affected by aging. A recent study attempted to study aging effects on white matter anisotropy in the brain, but the results were generally equivocal, likely due to methods with insufficient sensitivity or reliability (Kalra et al., 2019). Future work adopting advanced anisotropic MRE methods, including transverse isotropic (McGarry et al., 2020; Schmidt et al., 2018) or orthotropic models (Romano et al., 2014, 2012), may identify age-related softening of white matter tracts and changes in anisotropy. Previous studies using diffusion MRI have shown that aging causes a decrease in anisotropy, as well as anincrease in radial diffusivity (Davis et al., 2009), which has been linked with demyelination (Song et al., 2002). As brain stiffness is sensitive to myelination (Weickenmeier et al., 2016, 2017), studies of white matter tracts using appropriate anisotropic MRE methods may reveal similar effects.
In summary, the consensus reached from several separate MRE research groups is that the brain softens with advancing age, with the cerebrum experiencing an approximate reduction in stiffness by 0.008 kPa per year. The magnitude of softening is also region specific, suggesting that some brain regions are more vulnerable and/or resilient to age-related microstructural changes. It should be noted that the degree of reported softeningalso varies between studies, as shown in Table 1. This may be attributed to a number of reasons, such as the age differences in the sample population or, most likely, to the variety of MRE methods used including 2D or 3D wave acquisitions, actuation frequency, imaging spatial resolution, or the assumptions required in inversion algorithms. Another potentially confounding variable may be how studies have differed in their approach to handling voxels that contain CSF. Some studies have subtracted CSF-containing voxels for ROIs prior to inversion (Hiscox et al., 2020a; Hiscox et al., 2020b) or have used adaptive postprocessing techniques in conjunction with mask erosion to remove atrophy bias (Hughes et al., 2015; Murphy et al., 2013;Huston et al., 2015), while others have presumably ignored the issue entirely. The exact contribution of these approaches to data analysis, however, are not conclusively known. Despite the small deviations in the actual rates of change, the fundamental finding of brain tissue softening with increasing age is remarkably consistent and persists despite a host of differences in study protocols. We also note that while these annual changes are still small, they are well within published test-retest repeatability estimates for brain MRE (Barnhill et al., 2018; Johnson et al., 2016; Murphy et al., 2013). While longitudinal studies of brain aging with MRE do not yet exist, it is likely that current MRE methods would be unable to robustly detect a change in brain stiffness from year-to-year in a single individual. Studies on groups or populations will therefore be needed to observe the expected viscoelastic aging effects.
While the majority of brain MRE studies mentioned have reported age-related effects on stiffness showing tissue softening, the effects of aging on viscous behavior are less understood. Early studies found a general preservation of the relative viscous properties of the brain (Sack et al., 2011, 2008). With higher resolution and an optimized inversion protocol, Delgorio, et al., report that the individual hippocampal subfields show an age-related linear increase in the damping ratio, reflecting greater viscous-to-elastic properties in older age (Delgorio et al., 2020). These results are consistent with a previous study on the whole hippocampus which reported 21% higher damping ratio in older compared to younger adults (Hiscox et al., 2018). Additionally, the magnitude of the age relationship varied according to subfield damping ratio, indicating differential patterns of brain aging. For example, results suggest that the subiculum may undergo accelerated aging compared to the entorhinal cortex, which may open up new opportunities to study the successive emergence of neurodegeneration within the hippocampus. There has been speculation that higher damping ratio in older age may be reflective of more disorganized microstructural interactions from an increased proportion of mobile tissue components caused by common features of hippocampal aging, such as increased oxidative stress, altered protein processing, and dysregulated metabolism (Fan et al., 2017). These results are consistent with both a mouse model that had reported the viscous properties of the hippocampus increase with age (Munder et al., 2018), as well as human studies that have linked higher damping ratio to poorer cognitive performance (Hiscox et al., 2020b; Johnson et al., 2018; Schwarb et al., 2016, 2017), which are discussed later in this article.
1.3. MRE studies of dementias
Increasing age is the single greatest risk factor for the development of Alzheimer’s disease (AD), which is the most common cause of dementia. The study of healthy brain aging is therefore essential for improving understanding of the underlying neurological mechanisms that may eventually lead to mild cognitive impairment (MCI) and AD development. While atrophy is a common consequence of aging, the accumulation of toxic proteins is required to meet the pathological criteria for AD; namely, the appearance of neurofibrillary tangles in the hippocampus, and the deposition of ß-amyloid and neuritic infiltrate in association cortices (Halliday, 2017; Blennow and Zetterberg, 2018). How these processes may impact tissue mechanics is of interest for disease detection with MRE offering a novel contrast for observing subtle microstructural changes.
MRE has been used to study AD more than any other neurological condition. In the first proof-of-concept MRE study of AD in humans, Murphy, et al. reported global brain stiffness was 7% lower in biomarker-confirmed patients when compared with age-matched healthy control participants (Murphy et al., 2011). The same study also showed that ß-amyloid deposition alone was insufficient to cause a change in observed stiffness, as brain stiffness did not differ between cognitively healthy participants who were amyloid positive or amyloid negative, as measured through PET imaging. As such, it was reported that MRE may be most sensitive to downstream neurodegenerative effects particularly in patients who exhibited clinical symptoms and were in the later stages of the AD continuum. Despite reports that ß-amyloid plaques are far stiffer than surrounding brain tissue (Knowles and Buehler, 2011; Mattana et al., 2017), it is important to note that ß-amyloid plaques are distinct, discontinuous, and only form a relatively small volume fraction of the entire tissue; therefore, the effective stiffness of such a material will be dominated by changes in the softer matrix. A regional investigation of brain stiffness subsequently followed and illustrated the specificity of MRE for detecting more substantial softening to the frontal, parietal, and temporal lobes in AD patients (Fig. 4A) (Murphy et al., 2016), in accordance with what is known regarding the spatial localization of AD pathology. Brain stiffness was further shown to correlate with disease severity as assessed by established AD biomarkers including amyloid PET imaging and hippocampal volume (Murphy et al., 2016).
Fig. 4.
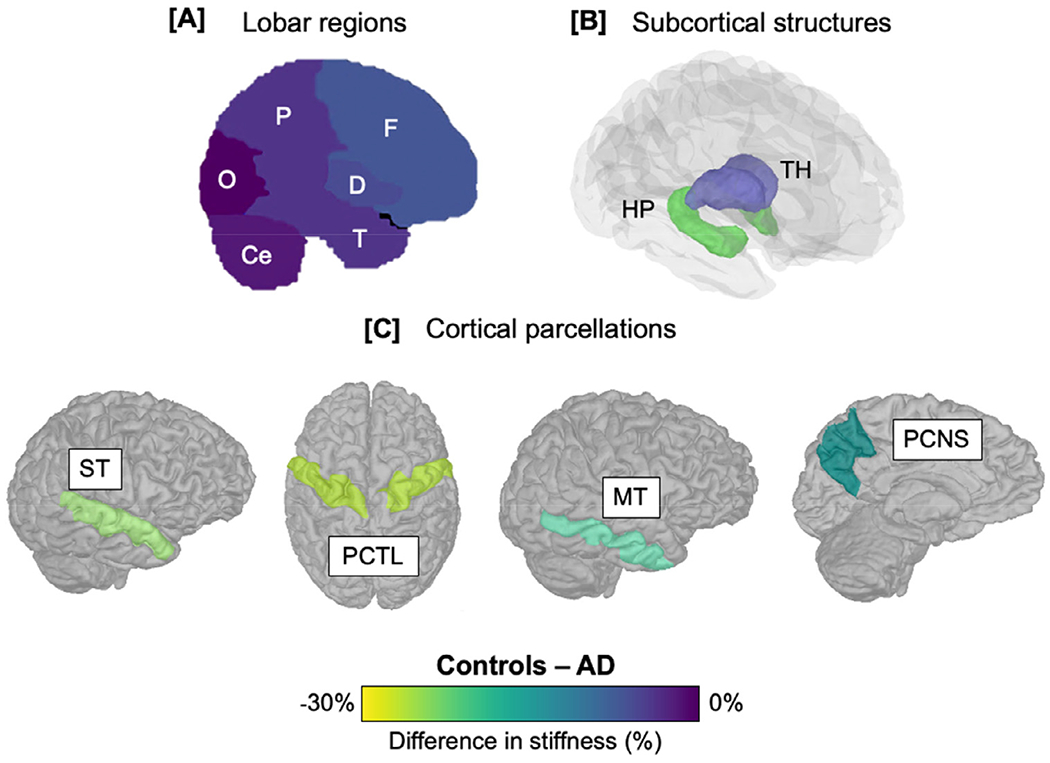
Summary of main findings that have used MRE to investigate brain stiffness in Alzheimer’s disease (AD). Color bar indicates percentage difference in AD patients compared to healthy controls (HC) in each of the respective studies. (A) Frontal (F), parietal (P), temporal (T) lobes, and a composite measure of deep gray/white matter (D) show AD-related softening, whereas the occipital lobe (O) remains unaffected; (B) analysis of subcortical structures indicate that the hippocampus (HP) is softer in AD, whereas the stiffness of the thalamus (TH) is relatively preserved; (C) cortical gray matter structures such as the superior temporal (ST), precentral (PCTL), precuneus (PCNS), and middle temporal (MT) cortex, show the greatest differences between AD and HC. These studies demonstrate the importance of obtaining high-resolution images for the investigation of ROIs with expected pathophysiology for greater sensitivity. Study A = Murphy et al., 2016; study B = Gerischer et al., 2018; study C = Hiscox et al., 2020a).
The hippocampus is an integral structure for memory encoding and consolidation and hippocampal neuronal loss and atrophy is incorporated into the pathological criteria for AD (Jack et al., 2011). As a result, there was anticipation that MRE would provide additional information regarding hippocampal health in AD patients. To ensure sufficient resolution for imaging its smaller structure, Gerischer, et al., from a different research group, adopted a multifrequency MRE protocol. Stiffness of the hippocampus was 22% lower in AD patients compared to controls, yet no differences were observed in the thalamus (Fig. 4B). These results illustrated both the sensitivity and specificity of MRE to detect differences in a region of interest known to be vulnerable to the disease. The diagnostic accuracy of AD was also improved with the incorporation of hippocampal stiffness with two other MRI-based hippocampal parameters. Compared with mean diffusivity from diffusion imaging and hippocampal volume alone, the addition of hippocampal stiffness yielded significantly higher classifier scores and thus greater discriminative value between healthy controls and AD patients (Gerischer et al., 2017).
More recently, a voxel-wise analysis using higher-resolution MRE was performed by Hiscox, et al. to investigate the viscoelasticity of the cerebral cortex in AD (Hiscox et al., 2020a). Brain tissue softening in AD was localized to specific areas within the frontal, parietal, and temporal cortices, supporting the previous study by Murphy, et al. that had investigated larger lobar regions (Murphy et al., 2016). More precisely, the middle temporal and superior temporal gyri, precuneus, operculum, and precentral gyri were softer in AD, which were independent from differences in cortical volumes (Fig. 4C). These results are consistent with existing studies which have shown how these regions are particularly affected early in the course of AD (Agosta et al., 2012; Jin et al., 2012; Migliaccio et al., 2015; Zhang et al., 2015), and likely account for some of the clinical symptoms of dementia. Interestingly, the spatial patterns of lower stiffness and smaller volumes in AD were not identical, suggesting that MRE may provide an indication of changes in tissue integrity not spatially localized to volume loss (Hiscox et al., 2020a). However, as with any cross-sectional study, the inference of the temporal progression cannot be determined.
The sensitivity of MRE to AD pathophysiology has been explored in animal studies, which have shown that stiffness is altered in both APP-PS162 and APP2363 mouse models of the disease (Munder et al., 2018; Murphy et al., 2012). At this stage there is limited data to confirm the neurobiological correlates of reduced stiffness in AD, but it has been hypothesized that it may reflect degradation of the extracellular matrix (due to amyloid deposition), loss of normal cytoskeletal architecture (due to tau hyper-phosphorylation), or altered synaptic connectivity (Murphy et al., 2019). Factors such as a decrease in the neuron-to-glial cell ratio may also contribute to the overall softening observed in AD (Hall et al., 2020). Nevertheless, the suggestion of a disease-related loss of microstructural integrity measured with MRE is consistent with well-established findings reported across numerous neuroimaging modalities and postmortem analyses.
The MRE studies of AD have shown changes in viscoelasticity within regions that correspond to the expected pathophysiology of AD, and they reflect how improvements in the resolution of MRE mechanical property maps have driven investigations that target affected neuroanatomical regions. A similar example is an MRE investigation into frontotemporal dementia (FTD), which found reduced stiffness only within the frontal and temporal lobes, as its name suggests (Huston et al., 2015). The reduction in stiffness in FTD was 9% for each lobe when compared to healthy controls, whereas no differences in stiffness were observed for any other lobar region. ElSheikh, et al. reviewed different signatures of softening in lobar regions in other dementias, including AD and FTD, and found distinct patterns of softening that differ between dementia subtypes (ElSheikh et al., 2017). These results indicate that MRE may be a useful non-invasive alternative to assist in the difficulties currently faced in the differential diagnosis of these conditions.
Collectively, these studies illustrate that MRE detects a physical transformation of the diseased brain through viscoelasticity that goes beyond what is observed in healthy aging. Offering a novel contrast sensitive to brain tissue health, brain MRE may be a valuable biomarker for characterizing healthy aging and at detecting deviations from a normal aging trajectory. Reliable biomarkers are urgently sought to detect the pathophysiology of AD during the pre-symptomatic stage to improve understanding of disease etiology and assist in providing metrics for the testing of candidate therapies. At the current time, however, longitudinal MRE studies in aging and disease have not been published. As such, we recommend that brain MRE could be a valuable addition to large-scale epidemiological studies such as the UK Biobank (Miller et al., 2016; Sudlow et al., 2015) and Lothian Birth Cohort (LBC) (Deary et al., 2011; Taylor et al., 2018) as well as in disease-modifying clinical trials of dementia such as the PREVENT-Dementia project (Habib et al., 2017; Mak et al., 2017; McKeever et al., 2020; McKiernan et al., 2020).
1.4. Viscoelastic structure-function relationships
An exciting new direction in brain MRE has been the study of how tissue biomechanics may relate to individual differences in cognitive function, which has implications for studying age-related cognitive decline and the links between brain health and cognitive impairment. Based on the relationship between tissue mechanical properties and microstructural organization, it was hypothesized that viscoelasticity may relate to brain function, which would allow these measures to be more fully interpretable with regard to brain health. Several studies have now examined so-called viscoelastic structure-function relationships that have found an association between MRE measures and outcomes on behavioural tasks. Although these studies to date have largely been conducted on healthy, young adults, they nevertheless highlight the potential for MRE to characterize memory-related impairments and other cognitive deficits related to early brain alterations in older age.
The first investigation that reported a significant viscoelastic structure-function relationship was performed by Schwarb, et al., who measured the viscoelastic properties of the hippocampus in twenty healthy young adult males aged 18–33 years (Schwarb et al., 2016). Each participant underwent a high-resolution MRE scan and performed a computerized spatial reconstruction task – a particularly sensitive measure of relational memory – to obtain individual scores of memory performance (Monti et al., 2015; Watson et al., 2013). Results indicated a strong, significant relationship between hippocampal damping ratio and memory performance (i.e., task reconstruction accuracy) (r = −0.72, p < 0.001), such that higher memory scores were associated with lower damping ratio, or more elastic material behavior (Fig. 5A). Furthermore, volumetric measures of the hippocampus were not associated with relational memory scores, suggesting that damping ratio may be an explicit measure of tissue integrity particularly sensitive to cognitive outcomes.
Fig. 5.
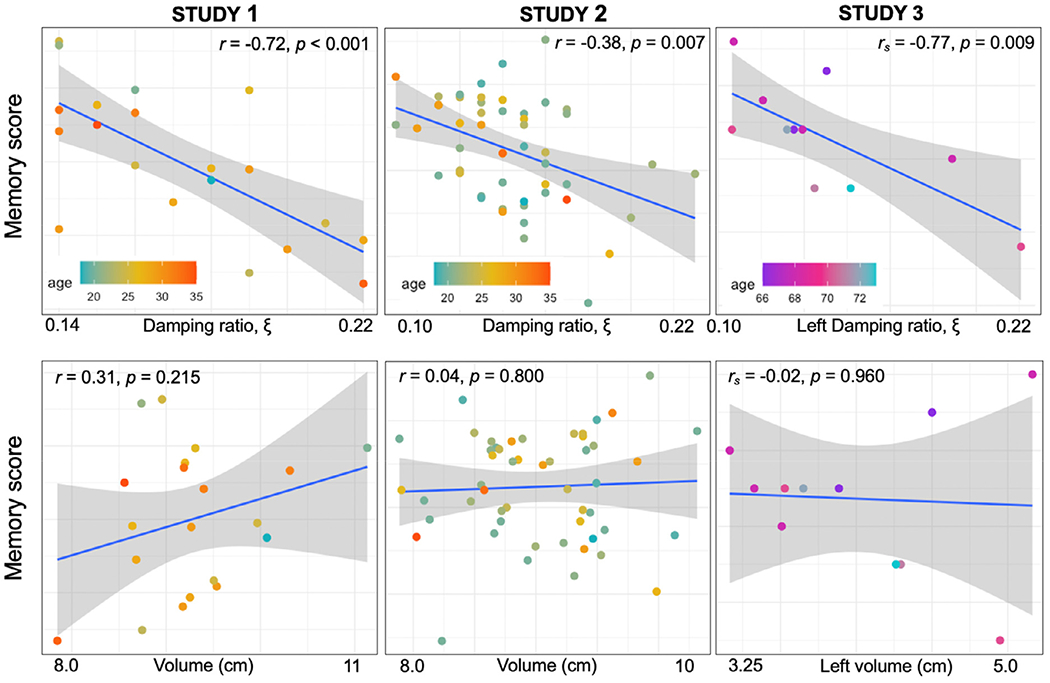
Results from three studies that have investigated the structure-function relationship between hippocampal viscoelasticity and memory score. In all studies, lower damping ratio was associated with better scores of memory performance, whereas hippocampal volume showed no relationship with performance in these tasks. Memory scores are from a spatial reconstruction task in studies 1 and 2 and from a verbal paired associates task in study 3. The blue line shows the regression line and the gray shaded region indicates the 95% confidence intervals. Study 1 = Schwarb et al., 2016; study 2 = Schwarb et al., 2017; study 3 = Hiscox et al., 2020b.
These compelling, yet preliminary, results were subsequently followed up in a larger sample of fifty-one participants, including both young men and women. Importantly, results remained consistent to those originally reported with lower damping ratio indicative of greater memory performance (Schwarb et al., 2017) (Fig. 5B) Neither hippocampal volume nor stiffness was associated with relational memory scores, thereby reinforcing the original findings that the relative elastic-to-viscous properties of the hippocampus are most strongly associated with relational memory function. Utilizing the same sample of participants, a recent report demonstrated that the damping ratio of the CA3-DG subregion of the hippocampus was able to predict relational memory accuracy, which in fact replicated most of the variance in performance that was explained by the entire hippocampus (Daugherty et al., 2020). Importantly, the CA3-DG region has been shown to support the information binding necessary to support relational memory and ensure successful performance in the administered task (Daugherty et al., 2017; Johnston et al., 2016; Rolls, 2016; Yassa and Stark, 2011). As such, there remains the opportunity to map and dissociate relationships between individual differences in specific domains of memory function with hippocampal subfield-specific viscoelasticity(Carey et al., 2019; Carr et al., 2017; Zammit et al., 2017).
There has also been preliminary work on the relationship between hippocampal viscoelasticity and memory in a healthy older adult population (Hiscox et al., 2020b) which revealed the same trend as observed with younger adults (Fig. 5C): higher damping ratio scores are associated with poorer memory performance, as assessed with a verbal paired associates (VPA) subtest from the Wechsler Memory Scale – Revised (WMS-R) (Wechsler and Stone, 1987). More specifically, the association was localized to the left hippocampus, which may be attributed to the role of the left hippocampus in the storage of verbal material, thus supporting the concept of functional hemispheric lateralization (Papanicolaou et al., 2002; Trenerry et al., 1993). No significant association was observed between VPA score and the right hippocampus damping ratio, or any stiffness or volumetric measure. A single dissociation, however, is not sufficient to demonstrate specificity for mapping cognitive function, and future studies should therefore identify more precisely cognitive functions supported by lateral hippocampi.
While these results show considerable promise for further exploring the hippocampal-memory relationship, extending the viscoelastic structure-function relationship to other brain regions and cognitive domains was essential to demonstrate that MRE was generalizable and relevant to wider brain mapping approaches. As a result, Johnson, et al., sought to examine and compare relationships of performance in relational memory (associated with the hippocampus) and fluid intelligence (associated with the orbitofrontal cortex) with the viscoelasticity of the associated structures (Johnson et al., 2018). In a sample of fifty-three healthy, young adults, a significant relationship between orbitofrontal cortex damping ratio and fluid intelligence performance (measured using a figure series task) was observed in addition to the hippocampal-memory relationship (measured using a spatial reconstruction task). Most importantly, however, was that a significant double dissociation between the orbitofrontal-fluid intelligence relationship and the hippocampal-relational memory relationship was also identified. These results provided clear evidence that the viscoelastic properties of each brain structure reflected performance in the expected cognitive domain, but not the other, strongly supporting the specificity of regional MRE measures. A similar finding has recently been reported by Schwarb, et al., who applied a context-dependent relational memory task and MRE to report correlations between hippocampal viscoelasticity and memory and between the viscoelasticity of the ventromedial prefrontal cortex and rule learning (Schwarb et al., 2019).
As is the case in most brain MRE applications and findings, the precise biological mechanism that results in lower damping ratio and greater cognitive function is not known. Previous hypotheses have suggested that lower damping ratio in the hippocampus may reflect elements of neurogenesis (Munder et al., 2018; Schwarb et al., 2017), which persists throughout adulthood (Kumar et al., 2019), resulting in an opportunity to influence hippocampal microstructure through lifestyle modifications (van Praag et al., 2005;van Praag et al., 1999). Yet as similar viscoelastic structure-function relationships are observed in other key brain areas, additional candidate mechanisms need to be considered. For example, greater connectivity within neuronal networks could plausibly reduce tissue viscosity, or conversely, disorganized microstructural interactions in the extracellular matrix may lead to increased viscosity. Other biological factors such as the tissue microvasculature (Jugé et al., 2015), and actin crosslinking and axonal organization (Guo et al., 2019) have been coupled to tissue viscosity and could account for part of the observed relationships. Important to note is how an increase in damping ratio may not be representative of a single structural or biological process. At first, the higher damping ratio may seem to represent an elevated imaginary loss modulus, yet as thoroughly discussed in this review, aging also causes a loss in stiffness and thus the real storage modulus, which could also affect the damping ratio. Whether the viscoelastic structure function relationships reported here can be explained by a reduction in the storage modulus (with the loss modulus staying relatively constant), or an actual increase to the loss modulus, is likely to infer separate structural changes. Targeted studies in animal models should therefore seek to disentangle the contributions from both the storage and loss modulus to more definitively understand the underlying neurobiology related to performance in functional tasks.
Regardless, the emergence of the viscoelastic structure-function relationship is only in its infancy, and there remain unlimited opportunities to explore key questions within cognitive, emotion, and social neuroscience, and age-related alterations in these fundamental processes. In what is usually interpreted as a compensatory mechanism, the elderly brain relies on increased network integration and a more distributed set of cortical regions than younger adults to maintain successful levels of performance during demanding tasks (Cabeza, 2002; Crowell et al., 2020). As a result, future MRE studies could study the mechanical integrity of more complex cortical networks that support both specialized and distributed information processing (Smith Bassett and Bullmore, 2006) using powerful quantitative techniques such as graph theoretical analysis (Bassett and Bullmore, 2009). This concept is supported by a recent demonstration of the sensitivity for MRE to map risk-taking behaviors in adolescents to a maturational imbalance between the reward and controls systems as revealed through viscoelasticity (McIlvain et al., 2020).
Finally, an exciting new development within the field is functional MRE (fMRE), an analogue to fMRI, in which researchers have begun to explore whether mechanical properties of localized regions respond accutely to stimulation or cognitive processes (Lan et al., 2020; Patz et al., 2019). In essence, these studies have unearthed a new functional contrast mechanism that shows how changes in brain stiffness respond to stimuli which can be observed on a time scale of 100 milliseconds. Importantly, the changes in stiffness appear to be 60 times faster than the fluctuations in blood oxygen levels (BOLD) typically captured by fMRI, allowing an assessment of neuronal activity at high speed (Patz et al., 2019). Work by a different research group also showed an agreement between fMRE and fMRI activation maps using a visual task, with changes in tissue stiffness within the visual cortex significantly greater than the BOLD signal change on a single-subject level (Lan et al., 2020). Future work will need to explore the relationship between baseline brain viscoelasticity and the magnitude of changes reported to stimuli to discover any possible interaction between the two. For instance, is the change in stiffness observed in fMRE impacted by baseline brain mechanics? Does the softer, aged brain exhibit more or less mechanical activation? While much work on fMRE remains in the future, fMRE provides an exciting extension to MRE and offers strong support for the relationship between tissue mechanics and functional processes.
1.5. Future directions
Efforts are ongoing within brain MRE development to fully exploit the inherently high sensitivity of MRE to changes in brain tissue health which may ultimately enhance our understanding of the physiological processes’ underling brain aging. While MRE displacement fields can now be captured at a comparable spatial resolution to a standard structural anatomical scan, further innovative work is needed to capture MRE displacement fields nearer the histological resolution. This will be required to investigate smaller and more complex structures such as the specific layers of the neocortex, with each layer likely to possess differing mechanical characteristics due to the relative distribution of neurons and glial cells (Lu et al., 2006). Future work will also need to reduce the current methodological constraints in inversion strategies. For example, while existing inversion algorithms are being optimised for improved sensitivity and/or reliability (Delgorio et al., 2020), more advanced mechanical models such as anisotropy and poroelasticity (McGarry et al., 2015, 2019; Solamen et al., 2019; Solamen 2020) or artifical intelligence approaches to inversion such as those proposed by Murphy et al., 2020) should be applied in aging populations (Murphy et al., 2018; Matthew C 2020). With an even more detailed and accurate characterization of brain mechanics, the multiple nuclei of the hypothalamus could also be a region of interest. Animal models have revealed that accelerated aging may be caused by a decline in hypothalamic neural stem cells, suggesting that the hypothalamus may in fact control the rate of aging (Zhang et al., 2017). Interestingly, replenishment of these cells was shown to slow and even reverse signs of aging, opening up opportunities to develop strategies for delaying age-related disease and extending the human lifespan. Whether MRE can provide a neuroimaging correlate of such processes will certainly be of interest.
As is the case with all brain MRE investigations, the biological interpretation of viscoelasticity and the mechanisms underlying viscoelastic changes in aging warrant serious investigation. Understanding how changes in tissue microstructure impacts the macroscopic mechanical response will be critical for the interpretation of the MRE signal to explain brain structure, function, and pathology. While some valuable work has been performed in this area, it has largely been limited to cross-sectional or observational studies. To establish causal links between viscoelasticity and microstructural characteristics, a recent developmental model combined MRE with histological and mass spectrometry proteome analysis during an extended period of mouse brain adolescence (Guo et al., 2019). Results revealed brain stiffness increased alongside the progressive accumulation of microtubular structures, myelination, cytoskeleton linkage, and cell-matrix attachment, whereas the phase angle decreased alongside downregulated actin crosslinking and axonal organization (Guo et al., 2019). It would be naïve to suggest that the observed decrease in stiffness and increase in viscosity in older age would simply mirror these effects, as the underlying presence of common age-related structural changes, including ß-amyloid burden, white matter microbleeds, enlarged perivascular spaces, or simply neuronal loss, will likely interact and have an impact on brain viscoelasticity. Therefore, a similar longitudinal model in brain aging would be highly desirable to obtain a better understanding of the neurobiological correlates of viscoelasticity directly related to senescence.
Evidence from animal models (Guo et al., 2019) and the recent application of MRE in the study of children and adolescents (Johnson and Telzer, 2018; McIlvain et al., 2018; E.F. 2020; Yeung et al., 2019) converge to a general observation that brain tissue stiffens during development at a time of rapid and significant myelination and synaptic pruning. The natural next questions are at what age or stage do viscoelastic properties reach a plateau, how long do these properties remain at their optimal levels, and what then causes a loss of brain stiffness and concomitant loss in tissue integrity? As biomechanical cues guide proliferation, differentiation, and maturation of neurons (Hall et al.,2020), which are essential processes in tissue regeneration (Mousavi and Hamdy Doweidar, 2015), does the decline in stiffness accelerate with age? Different brain structures are likely to follow different trajectories through development and aging, with high inter-individual variability in accordance with similar patterns observed for volumetry. Longitudinal studies will not only be able to provide answers to some of these questions but would also allow for a more direct and precise account of age-related changes of mechanical properties on an individual basis. This would also permit analyses of the temporal progression of changes in viscoelasticity and how they may relate to volume loss. Current conjecture is that the microstructural changes observed from MRE would predate those of volume loss, similar to the effects reported for diffusion imaging (Weston et al., 2015); however, this remains an open area of investigation.
A new trend in neuroscience is to establish biomarkers of the neu-roanatomical aging process in order to provide risk-assessments and predictions of cognitive dysfunction and age-associated brain diseases at single subject level (Franke and Gaser, 2019). For example, a popular area of research has combined structural and functional MRI with advanced machine learning methods to characterize individual brain health, or so-called “brain-predicted age” (Franke et al., 2010), (Cole et al., 2019). The brain age-delta (i.e. Δ: the brain’s estimated age minus the individual’s chronological age) has been shown as a heritable metric for monitoring cognitively healthy aging, as well as for the early identification of individuals with high-risk of age-associated disorders and mortality (Cole et al., 2018). In particular, baseline brain-predicted age scores have been shown to be significantly more accurate for predicting conversion to AD than conversion predictors based on chronological age, hippocampal volumes, cognitive scores, and CSF biomarkers (Gaser et al., 2013). Still, these models are not perfect and applying new features of brain aging obtained from MRE could improve model accuracy to make more precise judgments about individual risk for AD development. Other potential implementations include determining reference curves for healthy brain aging and discovering both protective and harmful environmental influences on brain health through comparison with the expected aging trajectory.
Finally, brain MRE may also be a clinically useful technique for identifying appropriate rehabilitation strategies and for enhancing our understanding of disease mechanisms to potentially accelerate the discovery of novel drug targets. For example, identification of a deviation from the expected healthy trajectory in structures such as the hippocampus may present opportunities for individuals to adopt relevant techniques to improve or maintain function by strengthening neural pathways through exercise, diet, cognitive training, or pharmaceutical intervention. This concept is supported by findings that have linked differences in blood lipid profiles (Sanjana et al., 2020) and body mass index (Hetzer et al., 2020) with viscoelasticity measured through MRE. Hippocampal viscoelasticity has also been identified as playing a mediating role between greater aerobic fitness, as measured with VO2,max, and greater memory function (Schwarb et al., 2017). Greater levels of physical activity are well documented to improve cognitive processes and memory (Di Liegro et al., 2019), and these results suggest that viscoelasticity may provide a noninvasive imaging marker to examine the mechanisms behind this relationship. For example, if MRE measures were explicitly representative of excessive synapse loss, this could accelerate the discovery and efficient deployment of novel pharmaceutical agents that promote dendrite and synapse regeneration (Agostinone et al., 2018). Additional support for using MRE to study the rehabilitative effects on brain health is provided by a preliminary investigation into the benefits of an exercise intervention in patients with multiple sclerosis (Sandroff et al., 2017). As a result of the exercise program, patients with MS exhibited significantly improved memory scores which corroborated with changes in hippocampal stiffness and damping ratio. Inevitably, large scale intervention studies are needed to more fully investigate this phenomenon.
Concluding remarks
Brain MRE has developed into a powerful neuroimaging technique for examining the integrity and complexity of the tissue microstructure through the analysis of its physical architecture. In this review, we have provided an overiew of studies that have utilized MRE to study healthy brain aging and age-related neurological disease, demonstrating how technological advances have substantially evolved knowledge regarding the biomechanics of the brain in health and disease. With further technical innovation including faster, higher-resolution MRE sequences, more advanced mechanical models, and the integration of artifical intelligence approaches to inversion, MRE has the potential to reveal vastly more about the relationship between brain biomechanics, health, structure and function.. We hope that this review highlights the growing utility of brain MRE to study brain aging to motivate its adoption into established longitudinal imaging protocols and within clinical trials to fully exploit the diagnostic potential for the early detection and differentiation of neurodegenerative diseases.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01-AG058853 and R01-EB027577).
Footnotes
Data and code availability statement
Not applicable
Declaration of Competing Interest
None.
References
- Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M, 2012. Resting state fMRI in Alzheimer’s disease: beyond the default mode network. Neurobiol. Aging 33 (8), 1564–1578. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Agostinone J, Alarcon-Martinez L, Gamlin C, Yu W-Q, Wong ROL, Di Polo A, 2018. Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain 141 (7), 1963–1980. doi: 10.1093/brain/awy142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AT, Van Houten EEW, McGarry MDJ, Paulsen KD, Holtrop JL, Sutton BP, Georgiadis JG, Johnson CL, 2016. Observation of direction-dependent mechanical properties in the human brain with multi-excitation MR elastography. J. Mech. Behav. Biomed. Mater doi: 10.1016/j.jmbbm.2016.03.005. http://www.sciencedirect.com/science/article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton BH, 2002. Ageing of the brain. Mech. Ageing Dev 123 (7), 811–817. doi: 10.1016/S0047-6374(01)00426-2. [DOI] [PubMed] [Google Scholar]
- Arani A, Murphy MC, Glaser KJ, Manduca A, Lake DS, Kruse SA, Jack CR, Ehman RL, Huston J, 2015. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage 111, 59–64. doi: 10.1016/j.neuroimage.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhill E, Nikolova M, Ariyurek C, Dittmann F, Braun J, Sack I, 2019. Fast robust dejitter and interslice discontinuity removal in MRI phase acquisitions: application to magnetic resonance elastography. IEEE Trans. Med. Imaging 38 (7), 1578–1587. doi: 10.1109/TMI.2019.2893369. [DOI] [PubMed] [Google Scholar]
- Barnhill Eric, Davies PJ, Ariyurek C, Fehlner A, Braun J, Sack I, 2018. Heterogeneous Multifrequency Direct Inversion (HMDI) for magnetic resonance elastography with application to a clinical brain exam. Med. Image Anal 46, 180–188. doi: 10.1016/j.media.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Bassett Danielle S, Bullmore ET, 2009. Human brain networks in health and disease. Curr. Opin. Neurol 22 (4), 340–347. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett Danielle Smith, Bullmore E, 2006. Small-world brain networks. Neuroscientist 12 (6), 512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Blennow K, Zetterberg H, 2018. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J. Intern. Med 284 (6), 643–663. doi: 10.1111/joim.12816. [DOI] [PubMed] [Google Scholar]
- Cabeza R, 2002. Hemispheric asymmetry reduction in older adults: The HAROLD model. In: Psychology and Aging, 17. American Psychological Association, pp. 85–100. doi: 10.1037/0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Carey D, Nolan H, Kenny RA, Meaney J, 2019. Dissociable age and memory relationships with hippocampal subfield volumes in vivo:data from the Irish Longitudinal Study on Ageing (TILDA). Sci. Rep 9 (1), 10981. doi: 10.1038/s41598-019-46481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr VA, Bernstein JD, Favila SE, Rutt BK, Kerchner GA, Wagner AD, 2017. Individual differences in associative memory among older adults explained by hippocampal subfield structure and function. Proc. Natl. Acad. Sci 114 (45), 12075. doi: 10.1073/pnas.1713308114,LP –12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Ritchie SJ, Bastin ME, Valdés Hernández MC, Muñoz Maniega S, Royle N, Corley J, Pattie A, Harris SE, Zhang Q, Wray NR, Redmond P, Marioni RE, Starr JM, Cox SR, Wardlaw JM, Sharp DJ, Deary IJ, 2018. Brain age predicts mortality. Mol. Psychiatry 23 (5), 1385–1392. doi: 10.1038/mp.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole James H, Marioni RE, Harris SE, Deary IJ, 2019. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Mol. Psychiatry 24 (2), 266–281. doi: 10.1038/s41380-018-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell CA, Davis SW, Beynel L, Deng L, Lakhlani D, Hilbig SA, Palmer H, Brito A, Peterchev AV, Luber B, Lisanby SH, Appelbaum LG, Cabeza R, 2020. Older adults benefit from more widespread brain network integration during working memory. Neuroimage, 116959 doi: 10.1016/j.neuroimage.2020.116959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Bender AR, Raz N, Ofen N, 2016. Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus 26 (2), 220–228. doi: 10.1002/hipo.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Flinn R, Ofen N, 2017. Hippocampal CA3-dentate gyrus volume uniquely linked to improvement in associative memory from childhood to adulthood. Neuroimage 153, 75–85. doi: 10.1016/j.neuroimage.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Schwarb HD, McGarry MDJ, Johnson CL, Cohen NJ, 2020. Magnetic resonance elastography of human hippocampal subfields: CA3-dentate gyrus viscoelasticity predicts relational memory accuracy. J. Cogn. Neurosci 1–10. doi: 10.1162/jocn_a_01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R, 2009. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage 46 (2), 530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Gow AJ, Pattie A, Starr JM, 2011. Cohort profile: the Lothian birth cohorts of 1921 and 1936. Int. J. Epidemiol 41 (6), 1576–1584. doi: 10.1093/ije/dyr197. [DOI] [PubMed] [Google Scholar]
- Delgorio P, Hiscox LV, Daugherty AM, Sanjana F, Pohlig RT, Ellison JM, Martens CR, Schwarb H, McGarry MDJ, Johnson CL, 2020. Effect of aging on the viscoelastic properties of hippocampal subfields assessed with a reliable, high-resolution MR elastography protocol. Cereb. Cortex [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Liegro CM, Schiera G, Proia P, Di Liegro I, 2019. Physical activity and brain health. Genes (Basel) 10 (9), 720. doi: 10.3390/genes10090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehman EC, Rossman PJ, Kruse SA, Sahakian AV, Glaser KJ, 2008. Vibration safety limits for magnetic resonance elastography. Phys. Med. Biol doi: 10.1088/0031-9155/53/4/007. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSheikh M, Arani A, Perry A, Boeve BF, Meyer FB, Savica R, Ehman RL, Huston J, 2017. MR elastography demonstrates unique regional brain stiffness patterns in dementias. Am. J. Roentgenol 209, 403–408. doi: 10.2214/AJR.16.17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Wheatley EG, Villeda SA, 2017. Mechanisms of hippocampal aging and the potential for rejuvenation. Annu. Rev. Neurosci 40 (1), 251–272. doi: 10.1146/an-nurev-neuro-072116-031357. [DOI] [PubMed] [Google Scholar]
- Franke K, Gaser C, 2019. Ten years of BrainAGE as a neuroimaging biomarker of brain aging: what insights have we gained? Front Neurol 10, 789. https://www.frontiersin.org/article/10.3389/fneur.2019.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke K, Ziegler G, Klöppel S, Gaser C, 2010. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage 50 (3), 883–892. doi: 10.1016/j.neuroimage.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Freimann FB, Muller S, Streitberger K, Guo J, Rot S, Ghori A, Vajkoczy P, Reiter R, Sack I, Braun J, 2013. MR elastography in a murine stroke model reveals correlation of macroscopic viscoelastic properties of the brain with neuronal density. NMR Biomed 26, 1534–1539. doi: 10.1002/nbm.2987. [DOI] [PubMed] [Google Scholar]
- Gaser C, Franke K, Klöppel S, Koutsouleris N, Sauer H, Initiative ADN, 2013. BrainAGE in mild cognitive impaired patients: predicting the conversion to Alzheimer’s disease. PLoS ONE 8 (6), e67346. doi: 10.1371/journal.pone.0067346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerischer LM, Fehlner A, Köbe T, Prehn K, Antonenko D, Grittner U, Braun J, Sack I, Flöel A, 2017. Combining viscoelasticity, diffusivity and volume of the hippocampus for the diagnosis of Alzheimer’s disease based on magnetic resonance imaging. Neuroimage Clin. 18, 485–493. doi: 10.1016/j.nicl.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MA, Bilston LE, Sinkus R, 2008. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 21, 755 764. doi: 10.1002/nbm.1254. [DOI] [PubMed] [Google Scholar]
- Guo J, Bertalan G, Meierhofer D, Klein C, Schreyer S, Steiner B, Wang S, Vieira da Silva R, Infante-Duarte C, Koch S, Boehm-Sturm P, Braun J, Sack I, 2019. Brain maturation is associated with increasing tissue stiffness and decreasing tissue fluidity. Acta Biomater. 99, 433–442. doi: 10.1016/j.actbio.2019.08.036. [DOI] [PubMed] [Google Scholar]
- Guo J, Hirsch S, Fehlner A, Papazoglou S, Scheel M, Braun J, Sack I, 2013. Towards an elastographic atlas of brain anatomy. PLoS ONE 8 (8), e71807. doi: 10.1371/journal.pone.0071807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Posnansky O, Hirsch S, Scheel M, Taupitz M, Braun J, Sack I, 2012. Fractal network dimension and viscoelastic powerlaw behavior: II. An experimental study of structure-mimicking phantoms by magnetic resonance elastography. Phys. Med. Biol 57 (12), 4041–4053. doi: 10.1088/0031-9155/57/12/4041. [DOI] [PubMed] [Google Scholar]
- Habib M, Mak E, Gabel S, Su L, Williams G, Waldman A, Wells K, Ritchie K, Ritchie C, O’Brien JT, 2017. Functional neuroimaging findings in healthy middle-aged adults at risk of Alzheimer’s disease. Ageing Res. Rev 36, 88–104. doi: 10.1016/j.arr.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Hall CM, Moeendarbary E, Sheridan GK, 2020. Mechanobiology of the brain in ageing and Alzheimer’s disease. Eur. J. Neurosci doi: 10.1111/ejn.14766, n/a (n/a), ejn.14766. [DOI] [PubMed] [Google Scholar]
- Halliday G, 2017. Pathology and hippocampal atrophy in Alzheimer’s disease. Lancet Neurol. 16 (11), 862–864. doi: 10.1016/S1474-4422(17)30343-5. [DOI] [PubMed] [Google Scholar]
- Herthum H, Dempsey SCH, Samani A, Schrank F, Shahryari M, Warmuth C, Tzschätzsch H, Braun J, Sack I, 2021. Superviscous properties of the in vivo brain at large scales. Acta Biomater. 121, 393–404. doi: 10.1016/j.actbio.2020.12.027. [DOI] [PubMed] [Google Scholar]
- Hetzer S, Birr P, Fehlner A, Hirsch S, Dittmann F, Barnhill E, Braun J, Sack I, 2018. Perfusion alters stiffness of deep gray matter. J. Cereb. Blood Flow Metab 38 (1), 116–125. doi: 10.1177/0271678X17691530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer S, Hirsch S, Braun J, Sack I, Weygandt M, 2020. Viscoelasticity of striatal brain areas reflects variations in body mass index of lean to overweight male adults. Brain Imaging Behav. 14 (6), 2477–2487. doi: 10.1007/s11682-019-00200-w. [DOI] [PubMed] [Google Scholar]
- Hiscox LV, Johnson CL, Barnhill E, McGarry MDJ, Huston III J, van Beek EJR, Starr JM, Roberts N, 2016. Magnetic resonance elastography (MRE) of the human brain: technique, findings and clinical applications. Phys. Med. Biol 61, 401–437. doi: 10.1088/0031-9155/61/24/R401. [DOI] [PubMed] [Google Scholar]
- Hiscox LV, Johnson CL, McGarry MDJ, Marshall H, Ritchie CW, van Beek EJR, Roberts N, Starr JM, 2020a. Mechanical property alterations across the cerebral cortex due to Alzheimer’s disease. Brain Commun. 2. doi: 10.1093/braincomms/fcz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox LV, Johnson CL, McGarry MDJ, Perrins M, Littlejohn A, van Beek EJR, Roberts N, Starr JM, 2018. High-resolution magnetic resonance elastography reveals differences in subcortical gray matter viscoelasticity between young and healthy older adults. Neurobiol. Aging 65, 158–167. doi: 10.1016/j.neurobiolaging.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox LV, Johnson CL, McGarry MDJ, Schwarb H, van Beek EJR, Roberts N, Starr JM, 2020b. Hippocampal viscoelasticity and episodic memory performance in healthy older adults examined with magnetic resonance elastography. Brain Imaging Behav. 14 (1), 175–185. doi: 10.1007/s11682-018-9988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JD, Fattahi N, Van Gompel J, Arani A, Lanzino G, Link M, Meyer F, Ehman R, Huston J 3rd, 2015. Higher resolution magnetic resonance elastography for the evaluation of intratumoral heterogeneity in meningiomas. J. Neurol. Surg. B. doi: 10.1055/s-0035-1546604. http://www.ncbi.nlm.nih.gov/pubmed/26197204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston J, Murphy MC, Boeve BF, Fattahi N, Arani A, Glaser KJ, Manduca A, Jones DT, Ehman RL, 2015. Magnetic resonance elastography of frontotemporal dementia. J. Magn. Reson. Imaging 43, 474–478. doi: 10.1002/jmri.24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Barkhof F, Bernstein MA, 2011. Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criterion for Alzheimer’s disease. Alzheimers Dement 7 (4), 474–485. e4. doi: 10.1016/j.jalz.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Pelak VS, Curran T, Nandy RR, Cordes D, 2012. A preliminary study of functional abnormalities in aMCI subjects during different episodic memory tasks. Magn. Reson. Imaging 30 (4), 459–470. doi: 10.1016/j.mri.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Schwarb H, Horecka KM, McGarry MDJ, Hillman CH, Kramer AF, Cohen NJ, Barbey AK, 2018. Double dissociation of structure-function relationships in memory and fluid intelligence observed with magnetic resonance elastography. Neuroimage 171, 99–106. doi: 10.1016/j.neuroimage.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, McGarry MDJ, Gharibans AA, Weaver JB, Paulsen KD, Wang H, Olivero WC, Sutton BP, Georgiadis JG, 2013a. Local mechanical properties of white matter structures in the human brain. Neuroimage 79, 145–152. doi: 10.1016/j.neuroimage.2013.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Schwarb H, McGarry MDJ, Anderson AT, Huesmann GR, Sutton BP, Cohen NJ, 2016. Viscoelasticity of subcortical gray matter structures. Hum. Brain Mapp 37, 4221–4233. doi: 10.1002/hbm.23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Telzer EH, 2018. Magnetic resonance elastography for examining developmental changes in the mechanical properties of the brain. Dev. Cogn. Neurosci 33, 81–176. doi: 10.1016/j.dcn.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson Curtis L, Holtrop JL, McGarry MDJ, Weaver JB, Paulsen KD, Georgiadis JG, Sutton BP, 2014. 3D multislab, multishot acquisition for fast, whole-brain MR elastography with high signal-to-noise efficiency. Magn. Reson. Med 71 (2), 477–485. doi: 10.1002/mrm.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson Curtis L, McGarry MDJ, Van Houten EEW, Weaver JB, Paulsen KD, Sutton BP, Georgiadis JG, 2013b. Magnetic resonance elastography of the brain using multishot spiral readouts with self-navigated motion correction. Magn. Reson. Med 70 (2), 404–412. doi: 10.1002/mrm.24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston ST, Shtrahman M, Parylak S, Gonçalves JT, Gage FH, 2016. Paradox of pattern separation and adult neurogenesis: a dual role for new neurons balancing memory resolution and robustness. Neurobiol. Learn. Mem 129, 60–68. doi: 10.1016/j.nlm.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugé L, Petiet A, Lambert SA, Nicole P, Chatelin S, Vilgrain V, Van Beers BE, Bilston LE, Sinkus R, 2015. Microvasculature alters the dispersion properties of shear waves – a multi-frequency MR elastography study. NMR Biomed. 28 (12), 1763–1771. doi: 10.1002/nbm.3438. [DOI] [PubMed] [Google Scholar]
- Kalra P, Raterman B, Mo X, Kolipaka A, 2019. Magnetic resonance elastography of brain: comparison between anisotropic and isotropic stiffness and its correlation to age. Magn. Reson. Med. 82 (2), 671–679. doi: 10.1002/mrm.27757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Hain EG, Braun J, Riek K, Mueller S, Steiner B, Sack I, 2014. Enhanced adult neurogenesis increases brain stiffness: in vivo magnetic resonance elastography in a mouse model of dopamine depletion. PLoS ONE 9 (3), e92582. doi: 10.1371/jour-nal.pone.0092582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles TPJ, Buehler MJ, 2011. Nanomechanics of functional and pathological amyloid materials. Nat. Nanotechnol. 6 (8), 469–479. doi: 10.1038/nnano.2011.102. [DOI] [PubMed] [Google Scholar]
- Kruse SA, Rose GH, Glaser KJ, Manduca A, Felmlee JP, Jack CR Jr, Ehman RL, 2008. Magnetic resonance elastography of the brain. Neuroimage 39 (1), 231–237. doi: 10.1016/j.neuroimage.2007.08.030, Jan 1Epub 2007 Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Pareek V, Faiq MA, Ghosh SK, Kumari C, 2019. Adult neurogenesis in humans: a review of basic concepts, history, current research, and clinical implications. Innov. Clin. Neurosci 16 (5–6), 30–37. https://pubmed.ncbi.nlm.nih.gov/31440399. [PMC free article] [PubMed] [Google Scholar]
- Lan PS, Glaser KJ, Ehman RL, Glover GH, 2020. Imaging brain function with simultaneous BOLD and viscoelasticity contrast: fMRI/fMRE. Neuroimage 211, 116592. doi: 10.1016/j.neuroimage.2020.116592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-B, Franze K, Seifert G, Steinhauser C, Kirchhoff F, Wolburg H, Guck J, Janmey P, Wei E-Q, Kas J, Reichenbach A, 2006. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc. Natl. Acad. Sci 103 (47), 17759–17764. doi: 10.1073/pnas.0606150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Yun-Bi, Iandiev I, Hollborn M, Körber N, Ulbricht E, Hirrlinger PG, Pannicke T, Wei E-Q, Bringmann A, Wolburg H, Wilhelmsson U, Pekny M, Wiedemann P, Reichenbach A, Käs JA, 2011. Reactive glial cells: increased stiffness correlates with increased intermediate filament expression. FASEB J. 25 (2), 624–631. doi: 10.1096/fj.10-163790. [DOI] [PubMed] [Google Scholar]
- Mak E, Gabel S, Mirette H, Su L, Williams GB, Waldman A, Wells K, Ritchie K, Ritchie C, O’Brien J, 2017. Structural neuroimaging in preclinical dementia: from microstructural deficits and grey matter atrophy to macroscale connectomic changes. Ageing Res. Rev 35, 250–264. doi: 10.1016/j.arr.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Manduca A, Oliphant T, Dresner M, Mahowald J, Kruse S, Amromin E, Felmlee J, Greenleaf J, Ehman R, 2001. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med. Image Anal 5, 237–254. doi: 10.1016/S1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- Manduca Armando, Bayly PJ, Ehman RL, Kolipaka A, Royston TJ, Sack I, Sinkus R, Van Beers BE, 2021. MR elastography: principles, guidelines, and terminology. Magn. Reson. Med 85 (5), 2377–2390. doi: 10.1002/mrm.28627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan YK, Glaser KJ, Ehman RL, 2010. Magnetic resonance elastography: a review. Clin. Anat doi: 10.1002/ca.21006. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattana S, Caponi S, Tamagnini F, Fioretto D, Palombo F, 2017. Viscoelasticity of amyloid plaques in transgenic mouse brain studied by Brillouin microspectroscopy and correlative Raman analysis. J. Innov. Opt. Health Sci 10 (06), 1742001. doi: 10.1142/S1793545817420019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken PJ, Manduca A, Felmlee J, Ehman RL, 2005. Mechanical transient-based magnetic resonance elastography. Magn. Reson. Med 53 (3), 628–639. doi: 10.1002/mrm.20388, Mar. [DOI] [PubMed] [Google Scholar]
- McGarry MDJ, Johnson CL, Sutton BP, Georgiadis JG, Van Houten EEW, Pattison AJ, Weaver JB, Paulsen KD, 2015. Suitability of poroelastic and viscoelastic mechanical models for high and low frequency MR elastography. Med. Phys 42 (2), 947–957. doi: 10.1118/1.4905048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry MD, Van Houten EE, Johnson CL, Georgiadis JG, Sutton BP, Weaver JB, Paulsen KD, 2012. Multiresolution MR elastography using nonlinear inversion. Med. Phys 39 (10), 6388–6396. doi: 10.1118/1.4754649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry Matthew, Van Houten E, Solamen L, Gordon-Wylie S, Weaver J, Paulsen K, 2019. Uniqueness of poroelastic and viscoelastic nonlinear inversion MR elastography at low frequencies. Phys. Med. Biol 64 (7), 75006. doi: 10.1088/1361-6560/ab0a7d. [DOI] [PubMed] [Google Scholar]
- McGarry MDJ, Van Houten E, Guertler C, Okamoto R, Smith D, Sowinski D, Johnson C, Bayly P, Weaver J, Paulsen K, 2020. A heterogenous, time harmonic, nearly incompressible transverse isotropic finite element brain simulation platform for MR elastography. Phys. Med. Biol doi: 10.1088/1361-6560/ab9a84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvain G, Clements RG, Magoon EM, Spielberg JM, Telzer EH, Johnson CL, 2020. Viscoelasticity of reward and control systems in adolescent risk taking. Neuroimage 215, 116850. doi: 10.1016/j.neuroimage.2020.116850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvain G, Schwarb H, Cohen NJ, Telzer EH, Johnson CL, 2018. Mechanical properties of the in vivo adolescent human brain. Dev. Cogn. Neurosci 34, 27–33. doi: 10.1016/j.dcn.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeever A, Paris AF, Cullen J, Hayes L, Ritchie CW, Ritchie K, Waldman AD, Wells K, Busza A, Carriere I, O’Brien JT, Su L, 2020. Hippocampal subfield volumes in middle-aged adults at risk of dementia. J. Alzheimer’s Dis 75, 1211–1218. doi: 10.3233/JAD-200238. [DOI] [PubMed] [Google Scholar]
- McKiernan EF, Mak E, Dounavi M-E, Wells K, Ritchie C, Williams G, Su L, O’Brien J, 2020. Regional hyperperfusion in cognitively normal <em>APOE ε4</em> allele carriers in mid-life: analysis of ASL pilot data from the PREVENT-Dementia cohort. J. Neurol. Neurosurg. Amp; Psychiatry doi: 10.1136/jnnp-2020-322924, jnnp-2020-322924. [DOI] [PubMed] [Google Scholar]
- Migliaccio R, Agosta F, Possin KL, Canu E, Filippi M, Rabinovici GD, Rosen HJ, Miller BL, Gorno-Tempini ML, 2015. Mapping the progression of atrophy in early- and late-onset Alzheimer’s disease. J. Alzheimer’s Dis 46, 351–364. doi: 10.3233/JAD-142292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KL, Alfaro-Almagro F, Bangerter NK, Thomas DL, Yacoub E, Xu J, Bartsch AJ, Jbabdi S, Sotiropoulos SN, Andersson JLR, Griffanti L, Douaud G, Okell TW, Weale P, Dragonu I, Garratt S, Hudson S, Collins R, Jenkinson M, …, Smith SM, 2016. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci 19 (11), 1523–1536. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM, Cooke GE, Watson PD, Voss MW, Kramer AF, Cohen NJ, 2015. Relating hippocampus to relational memory processing across domains and delays. J. Cogn. Neurosci doi: 10.1162/jocn_a_00717. https://www.ncbi.nlm.nih.gov/pmc/articles/PM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SJ, Hamdy Doweidar M, 2015. Role of mechanical cues in cell differentiation and proliferation: a 3D numerical model. PLoS ONE 10 (5), e0124529. doi: 10.1371/journal.pone.0124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Stables L, Du AT, Schuff N, Truran D, Cashdollar N, Weiner MW, 2007. Measurement of hippocampal subfields and age-related changes with high resolution MRI at 4T. Neurobiol. Aging 28 (5), 719–726. doi: 10.1016/j.neurobiolaging.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder T, Pfeffer A, Schreyer S, Guo J, Braun J, Sack I, Steiner B, Klein C, 2018. MR elastography detection of early viscoelastic response of the murine hippocampus to amyloid β accumulation and neuronal cell loss due to Alzheimer’s disease. J. Magn. Reson. Imaging 47 (1), 105–114. doi: 10.1002/jmri.25741. [DOI] [PubMed] [Google Scholar]
- Murphy MC, Cogswell PM, Trzasko JD, Manduca A, Senjem ML, Meyer FB, Ehman RL, Huston J 3rd, 2020. Identification of Normal Pressure Hydrocephalus by Disease-Specific Patterns of Brain Stiffness and Damping Ratio. Invest. Radiol 55 (4), 200–208. doi: 10.1097/RLI.0000000000000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MC, Huston J, Jack CR, Glaser KJ, Senjem ML, Chen J, Manduca A, Felmlee JP, Ehman RL, 2013. Measuring the characteristic topography of brain stiffness with magnetic resonance elastography. PLoS ONE 8, e81668. doi: 10.1371/journal.pone.0081668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Huston J 3rd, Ehman R, 2019. MR elastography of the brain and its application in neurological diseases. Neuroimage 176–183. doi: 10.1016/j.neuroimage.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MC, Curran GL, Glaser KJ, Rossman PJ, Huston J, Poduslo JF, Jack CR, Felmlee JP, Ehman RL, 2012. Magnetic resonance elastography of the brain in a mouse model of Alzheimer’s disease: initial results. Magn. Reson. Imaging 30 (4), 535–539. doi: 10.1016/j.mri.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MC, Huston J 3rd, Jack JCR, Glaser KJ, Manduca A, Felmlee JP, Ehman RL, 2011. Decreased brain stiffness in Alzheimer’s disease determined by magnetic resonance elastography. J. Magn. Reson. Imaging 34, 494–498. doi: 10.1002/jmri.22707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MC, Jones DT, Jack CR, Glaser KJ, Senjem ML, Manduca A, Felmlee JP, Carter RE, Ehman RL, Huston J, 2016. Regional brain stiffness changes across the Alzheimer’s disease spectrum. Neuroimage: Clin. 10, 283–290. doi: 10.1016/j.nicl.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy Matthew C, Manduca A, Trzasko JD, Glaser KJ, Huston J III, Ehman RL, 2018. Artificial neural networks for stiffness estimation in magnetic resonance elastography. Magn. Reson. Med 80 (1), 351–360. doi: 10.1002/mrm.27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthupillai R, Lomas D, Rossman P, Greenleaf J, Manduca A, Ehman R, 1995. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 269, 1854–1857. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Katz JS, Wright AA, 2002. The hippocampus and memory of verbal and pictorial material. Learn. Mem 9 (3), 99–104. doi: 10.1101/lm.44302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazoglou S, Hirsch S, Braun J, Sack I, 2012. Multifrequency inversion in magnetic resonance elastography. Phys. Med. Biol 57 (8), 2329–2346. doi: 10.1088/0031-9155/57/8/2329. [DOI] [PubMed] [Google Scholar]
- Patz S, Fovargue D, Schregel K, Nazari N, Palotai M, Barbone PE, Fabry B, Hammers A, Holm S, Kozerke S, Nordsletten D, Sinkus R, 2019. Imaging localized neuronal activity at fast time scales through biomechanics. Sci. Adv 5 (4). doi: 10.1126/sciadv.aav3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, 2018. Imaging microstructure in the living human brain: a viewpoint. Neuroimage 182, 3–7. doi: 10.1016/j.neuroimage.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Peters R, 2006. Ageing and the brain. Postgrad. Med. J 964. doi: 10.1136/pgmj.2005.036665, https://www.ncbi.nlm.nih.gov/pmc/articles/PM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange MT, Margulies SS, 2002. Regional, directional, and age-dependent properties of the brain undergoing large deformation. J. Biomech. Eng 124 (2), 244–252. doi: 10.1115/1.1449907. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD, 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15 (11), 1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rolls ET, 2016. Pattern separation, completion, and categorisation in the hippocampus and neocortex. Neurobiol. Learn. Mem 129, 4–28. doi: 10.1016/j.nlm.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Romano A, Guo J, Prokscha T, Meyer T, Hirsch S, Braun J, Sack I, & Scheel M (2014). In vivo waveguide elastography: effects of neurodegeneration in patients with amyotrophic lateral sclerosis. Magn. Reson. Med, , \href{ 10.1002/mrm.25067}{1755–17. [DOI] [PubMed] [Google Scholar]
- Romano Anthony, Scheel M, Hirsch S, Braun J, Sack I, 2012. In vivo waveguide elastography of white matter tracts in the human brain. Magn. Reson. Med 68 (5), 1410–1422. doi: 10.1002/mrm.24141. [DOI] [PubMed] [Google Scholar]
- Sack I, Streitberger K-J, Krefting D, Paul F, Braun J, 2011. The influence of physiological aging and atrophy on brain viscoelastic properties in humans. PLoS ONE 6, e23451. doi: 10.1371/journal.pone.0023451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack I, Johrens K, Wurfel J, Braun J, 2013. Structure-sensitive elastography: on the viscoelastic powerlaw behavior of in vivo human tissue in health and disease. Soft Matter 24. doi: 10.1039/C3SM50552A.23494476 [DOI] [Google Scholar]
- Sack Ingolf, Beierbach B, Hamhaber U, Klatt D, Braun J, 2008. Non-invasive measurement of brain viscoelasticity using magnetic resonance elastography. NMR Biomed. 21 (3), 265–271. doi: 10.1002/nbm.1189. [DOI] [PubMed] [Google Scholar]
- Sack Ingolf, Beierbach B, Wuerfel J, Klatt D, Hamhaber U, Papazoglou S, Martus P, Braun J, 2009. The impact of aging and gender on brain viscoelasticity. Neuroimage 46 (3), 652–657. doi: 10.1016/j.neuroimage.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Sandroff BM, Johnson CL, Motl RW, 2017. Exercise training effects on memory and hippocampal viscoelasticity in multiple sclerosis: a novel application of magnetic resonance elastography. Neuroradiology 59, 61–67. doi: 10.1007/s00234-016-1767-x. [DOI] [PubMed] [Google Scholar]
- Sanjana F, Delgorio PL, Hiscox LV, DeConne TM, Hobson JC, Cohen ML, Johnson CL, Martens CR, 2020. Blood lipid markers are associated with hippocampal viscoelastic properties and memory in humans. J. Cereb. Blood Flow Metab doi: 10.1177/0271678X20968032, 0271678X20968032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JL, Tweten DJ, Badachhape AA, Reiter AJ, Okamoto RJ, Garbow JR, Bayly PV, 2018. Measurement of anisotropic mechanical properties in porcine brain white matter ex vivo using magnetic resonance elastography. J. Mech. Behav. Biomed. Mater 79, 30–37. doi: 10.1016/j.jmbbm.2017.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schregel K, Wuerfel nee Tysiak E, Garteiser P, Gemeinhardt I, Prozorovski T, Aktas O, Merz H, Petersen D, Wuerfel J, Sinkus R, 2012. Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance elastography. Proc. Natl. Acad. Sci 109 (17), 6650–6655. doi: 10.1073/pnas.1200151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarb H, Johnson CL, Daugherty AM, Hillman CH, Kramer AF, Cohen NJ, Barbey AK, 2017. Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. Neuroimage 153, 179–188. doi: 10.1016/j.neuroimage.2017.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarb H, Johnson CL, Dulas MR, McGarry MDJ, Holtrop JL, Watson PD, Wang JX, Voss JL, Sutton BP, Cohen NJ, 2019. Structural and functional MRI evidence for distinct medial temporal and prefrontal roles in context-dependent relational memory. J. Cogn. Neurosci 31, 1857–1872. doi: 10.1162/jocn_a_01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarb H, Johnson CL, McGarry MDJ, Cohen NJ, 2016. Medial temporal lobe viscoelasticity and relational memory performance. Neuroimage 132, 534–541. doi: 10.1016/j.neuroimage.2016.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Guertler CA, Okamoto RJ, Romano AJ, Bayly PV, Johnson CL, 2020. Multi-excitation MR elastography of the brain: wave propagation in anisotropic white matter. J. Biomech. Eng doi: 10.1115/1.4046199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solamen LM, Gordon-Wylie SW, McGarry MD, Weaver JB, Paulsen KD, 2019. Phantom evaluations of low frequency MR elastography. Phys. Med. Biol 64 (6), 65010. doi: 10.1088/1361-6560/ab0290. [DOI] [PubMed] [Google Scholar]
- Solamen LM, McGarry MDJ, Fried J, Weaver JB, Lollis SS, Paulsen KD, 2020. Poroelastic mechanical properties of the brain tissue of normal pressure hydrocephalus patients during lumbar drain treatment using intrinsic actuation MR elastography. Acad. Radiol doi: 10.1016/j.acra.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH, 2002. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17 (3), 1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R, 2015. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12 (3), e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura T, Motosugi U, Sasaki Y, Kakegawa T, Sato K, Glaser KJ, Ehman RL, Onishi H, 2020. Influence of age on global and regional brain stiffness in young and middle-aged adults. J. Magn. Reson. Imaging 51 (3), 727–733. doi: 10.1002/jmri.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Pattie A, Deary IJ, 2018. Cohort profile update: the Lothian birth cohorts of 1921 and 1936. Int. J. Epidemiol. 47 (4). doi: 10.1093/ije/dyy022, 1042–1042r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenerry MR, Jack CR, Ivnik RJ, Sharbrough FW, Cascino GD, Hirschorn KA, Marsh WR, Kelly PJ, Meyer FB, 1993. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology 43 (9), 1800. doi: 10.1212/WNL.43.9.1800,LP −1800. [DOI] [PubMed] [Google Scholar]
- Van Houten E, Paulsen K, Miga M, Kennedy F, Weaver J, 1999. An overlapping subzone technique for MRbased elastic property reconstruction. Magn. Reson. Med 42, 779–786. doi:. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH, 1999. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci 96 (23), 13427. doi: 10.1073/pnas.96.23.13427, LP −13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH, 2005. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci 25 (38), 8680. doi: 10.1523/JNEU-ROSCI.1731-05.2005, LP −8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PD, Voss JL, Warren DE, Tranel D, Cohen NJ, 2013. Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus 23 (7), 570–580. doi: 10.1002/hipo.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, Stone CP, 1987. Wechsler Memory Scale-Revised. Psychological Corporation [Google Scholar]
- Weickenmeier J, de Rooij R, Budday S, Steinmann P, Ovaert TC, Kuhl E, 2016. Brain stiffness increases with myelin content. Acta Biomater. 42, 265–272. doi: 10.1016/j.actbio.2016.07.040. [DOI] [PubMed] [Google Scholar]
- Weickenmeier Johannes, de Rooij R, Budday S, Ovaert TC, Kuhl E, 2017. The mechanical importance of myelination in the central nervous system. J. Mech. Behav. Biomed. Mater doi: 10.1016/j.jmbbm.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Weston PSJ, Simpson IJA, Ryan NS, Ourselin S, Fox NC, 2015. Diffusion i maging changes in grey matter in Alzheimer’s disease: a potential marker of early neurodegeneration. Alzheimer’s Res. Ther. 7 (1), 47. doi: 10.1186/s13195-015-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL, 2011. Pattern separation in the hippocampus. Trends Neurosci. 34 (10), 515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung J, Jugé L, Hatt A, Bilston LE, 2019. Paediatric brain tissue properties measured with magnetic resonance elastography. Biomech. Model. Mechanobiol 18 (5), 1497–1505. doi: 10.1007/s10237-019-01157-x. [DOI] [PubMed] [Google Scholar]
- Zammit AR, Ezzati A, Zimmerman ME, Lipton RB, Lipton ML, Katz MJ, 2017. Roles of hippocampal subfields in verbal and visual episodic memory. Behav. Brain Res 317, 157–162. doi: 10.1016/j.bbr.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Yalin, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D, 2017. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 548 (7665), 52–57. doi: 10.1038/nature23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Yudong, Dong Z, Phillips P, Wang S, Ji G, Yang J, Yuan T-F, 2015. Detection of subjects and brain regions related to Alzheimer’s disease using 3D MRI scans based on eigenbrain and machine learning. Front. Comput. Neurosci 9, 66. https://www.frontiersin.org/article/10.3389/fncom.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]


