Abstract
Background
Migraine is a complex, multifaceted, and disabling headache disease that is often complicated by gastrointestinal (GI) conditions, such as gastroparesis, functional dyspepsia, and cyclic vomiting syndrome (CVS). Functional dyspepsia and CVS are part of a spectrum of disorders newly classified as disorders of gut–brain interaction (DGBI). Gastroparesis and functional dyspepsia are both associated with delayed gastric emptying, while nausea and vomiting are prominent in CVS, which are also symptoms that commonly occur with migraine attacks. Furthermore, these gastric disorders are comorbidities frequently reported by patients with migraine. While very few studies assessing GI disorders in patients with migraine have been performed, they do demonstrate a physiological link between these conditions.
Objective
To summarize the available studies supporting a link between GI comorbidities and migraine, including historical and current scientific evidence, as well as provide evidence that symptoms of GI disorders are also observed outside of migraine attacks during the interictal period. Additionally, the importance of route of administration and formulation of migraine therapies for patients with GI symptoms will be discussed.
Methods
A literature search of PubMed for articles relating to the relationship between the gut and the brain with no restriction on the publication year was performed. Studies providing scientific support for associations of gastroparesis, functional dyspepsia, and CVS with migraine and the impact these associations may have on migraine treatment were the primary focus. This is a narrative review of identified studies.
Results
Although the association between migraine and GI disorders has received very little attention in the literature, the existing evidence suggests that they may share a common etiology. In particular, the relationship between migraine, gastric motility, and vomiting has important clinical implications in the treatment of migraine, as delayed gastric emptying and vomiting may affect oral dosing compliance, and thus, the absorption and efficacy of oral migraine treatments.
Conclusions
There is evidence of a link between migraine and GI comorbidities, including those under the DGBI classification. Many patients do not find adequate relief with oral migraine therapies, which further necessitates increased recognition of GI disorders in patients with migraine by the headache community.
Keywords: cyclic vomiting syndrome, disorders of gut–brain interaction, functional dyspepsia, gastric motility, gastroparesis, migraine
Abbreviations
- ANS
autonomic nervous system
- AUC
area under the curve
- CGRP
calcitonin gene‐related peptide
- CI
confidence interval
- CNS
central nervous system
- CVS
cyclic vomiting syndrome
- CUNV
chronic unexplained nausea and vomiting
- DGBI
disorders of gut–brain interaction
- EPS
epigastric pain syndrome
- GCSI
Gastroparesis Cardinal Symptom Index
- GI
gastrointestinal
- IBS
irritable bowel syndrome
- NTS
nucleus tractus solitarius
- OR
odds ratio
- PDS
postprandial distress syndrome
- SD
standard deviation
- SEM
standard error of the mean
- TGV
trigeminovascular
- TNFα
tumor necrosis factor‐alpha
INTRODUCTION
Migraine is defined by the International Classification of Headache Disorders (3rd edition) as a common, disabling primary headache disorder. 1 According to the Global Burden of Disease Study in 2016, migraine was the sixth most prevalent disorder, afflicted ~1 billion individuals, and was more predominant in women (18.9%) compared to men (9.8%) globally. 2 Migraine is defined as a recurrent headache disorder with moderate or severe headache attacks that can last for 4–72 h and are accompanied by nausea and/or photophobia and phonophobia. 1 In particular, the nausea that accompanies migraine strongly contributes to the burden and disability associated with migraine. 3 The American Migraine Prevalence and Prevention study conducted in 2009 revealed that patients with migraine who experienced high‐frequency nausea had significantly higher odds of occupational disability or taking medical leave, and increased headache pain severity and impact. 3 In addition to nausea, other gastrointestinal (GI) symptoms may be present with migraine and include vomiting, diarrhea, reflux, and constipation. 4 , 5 A large survey in the United States completed by 29,727 participants reported that 73% and 29% of patients with migraine experienced nausea and vomiting, respectively. 5 In a large analysis of women with migraine, nausea and vomiting were reported by 61.6% of those with migraine with aura and 66.0% without aura. 6 Furthermore, a variety of GI conditions have been associated with migraine, such as inflammatory bowel disease, celiac disease, irritable bowel syndrome (IBS), Helicobacter pylori infection, cyclic vomiting syndrome (CVS), functional dyspepsia, and gastroparesis. 7 , 8 , 9 , 10 , 11 , 12 , 13 The Rome Foundation recently introduced the term, disorders of gut–brain interaction (DGBI), which is defined as “a group of disorders classified by GI symptoms related to any combination of motility disturbances, visceral hypersensitivity, altered mucosal and immune function, gut microbiota, and/or central nervous system (CNS) processing.” 14 The Rome Foundation is an independent not‐for‐profit organization that provides support for science‐based activities to assist in DGBI diagnosis and treatment. 15 Functional dyspepsia and CVS are included under the DGBI classification, thus, suggesting that an interaction between the brain and gut exists in these common disorders. 14 There is significant overlap between idiopathic gastroparesis and functional dyspepsia such that both are associated with delayed gastric emptying in the absence of a mechanical obstruction, as well as impaired fundic accommodation and gastric hypersensitivity in functional dyspepsia, 16 , 17 while episodic nausea, vomiting, and abdominal pain are present in CVS. 18 In our recently completed Phase 3 STOP 301 study, 19 38.4% of our patients (N = 354) had comorbid GI disorders at study entry. Gastroesophageal reflux disease was the most prevalent (20.3%), followed by IBS (5.6%), constipation (4.0%), and dyspepsia (3.1%). There is evidence of an association between migraine and GI disorders in the literature. In this paper, we will review the historical and current state of scientific evidence that exists for a relationship between migraine and GI comorbidities, and how their association may impact migraine treatment.
METHODS
For this narrative review, a literature search of PubMed with no restriction on the publication year for articles relating to the relationship between the gut and the brain was performed. Search terms included gut‐brain connection, gut‐brain axis, gastric disorders AND migraine OR headache, gastroparesis AND migraine OR headache, functional dyspepsia AND migraine OR headache, and CVS AND migraine OR headache. Studies of primary focus were manually identified, which included those that investigated the association of migraine with three gastric disorders (gastroparesis, functional dyspepsia, and CVS) as well as studies reporting an impact of GI symptoms on migraine therapies. Since the objective of this narrative review was to provide historic and current scientific evidence linking gastric disorders with migraine and their effect on migraine management, inclusion criteria included all study types (i.e., case reports, randomized‐controlled trials, reviews, etc.). Manual reads of the reference section of articles were also completed. All authors reviewed the identified studies reported in this review.
PATHOPHYSIOLOGICAL SIMILARITIES BETWEEN GI COMORBIDITIES AND MIGRAINE
The pathophysiology of both migraine and GI comorbidities, including DGBI, is complex and neither has been fully elucidated 20 ; however, scientific evidence does show an overlap in pathophysiology between these complex disorders. Investigating this overlap can help shed light on a common pathophysiological abnormality or biological mechanism. Currently, there is evidence to implicate a shared pathophysiology between migraine and GI disorders, which is believed to occur via an interaction of numerous factors including inflammatory mediators, gut microbiota, neuropeptides, and the serotonin pathway. 21 Particularly, the autonomic nervous system (ANS) is thought to play an important role in the association between migraine and GI dysfunction due to the similarities in their symptom profiles, which both include nausea, vomiting, dyspepsia, and gastroparesis. 20 , 22 Further, ANS abnormalities have been described in migraine 23 , 24 , 25 and various upper GI disorders, such as diabetic and idiopathic gastroparesis, 26 , 27 CVS, 28 , 29 and functional dyspepsia 30 , 31 (Figure 1). Under normal conditions, digestion is regulated by a bidirectional interaction between the gut and brain through an interplay of the enteric nervous system, the interstitial cells of Cajal, fibroblast‐like cells, gastric smooth muscle, the CNS, and the ANS. 32 , 33 Afferent sensory information from the GI tract is transmitted by the vagus nerve to the nucleus tractus solitarius (NTS) of the brainstem, which then leads to efferent sympathetic or parasympathetic innervation of the GI tract to modulate GI function by either decreasing or increasing GI secretion and motility, respectively. 27 , 34 Under pathological conditions, delayed gastric emptying (i.e., gastroparesis) may occur either due to a loss or injury to the interstitial cells of Cajal, which may result from macrophage‐driven immune dysregulation and oxidative stress, and/or in some individuals, can involve the loss of enteric nerves and fibrosis in muscle layers. 33
FIGURE 1.
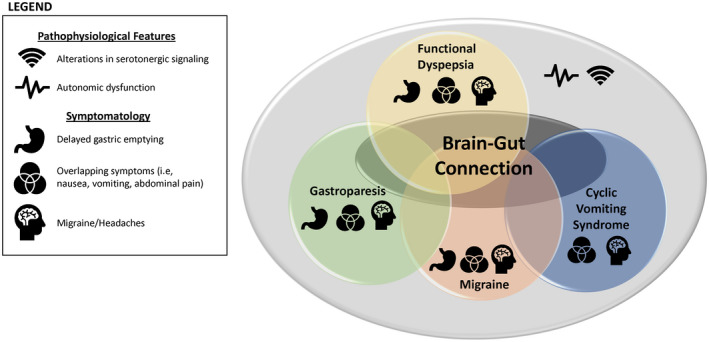
A brain–gut connection between migraine and gastric disorders. There is evidence in the literature supporting a brain‐gut connection. 21 This review further corroborates this association by providing evidence of shared pathophysiological features, such as alterations in serotonergic signaling 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 and autonomic dysfunction, 20 , 22 , 23 , 24 , 26 , 27 , 28 , 30 , 31 and overlapping symptomatology between migraine, 1 , 53 and GI disorders including gastroparesis, functional dyspepsia, and cyclic vomiting syndrome, often presenting as comorbidities in either condition 7 , 10 , 11 , 54 , 55 , 56 , 57 , 58
Nausea, vomiting, and delayed gastric emptying are part of the migraine symptom profile. 1 , 4 , 5 , 7 , 59 The NTS is involved in regulating vomiting, and in migraine it is suggested that the activation of the trigeminovascular (TGV) system during a migraine attack leads to neuronal activation in the NTS, which may cause nausea and vomiting. 60 , 61 The pathophysiology of migraine, especially the nausea and vomiting and alterations in gastric emptying, involves serotonergic signaling. Serotonin, both a vasodilator and vasoconstrictor, acts as a modulator of nociceptive pain, and decreased activity of 5‐HT1B/1D receptors has been thought to activate the TGV system involved in the initiation of a migraine attack. 35 , 36 , 37 The gut contains ~95% of the body's total serotonin, and numerous studies utilizing serotonergic pharmacologic agents have reported that serotonin may play a role in regulating gastric emptying and symptoms associated with GI dysfunction. 38 , 39 , 40 , 41 Serotonin acting on 5‐HT1P receptors initiates peristaltic and secretory reflexes, while stimulation of 5‐HT4 receptors enhances the release of neurotransmitters in reflex pathways. 5‐HT3 receptors activate extrinsic sensory nerves, regulating information transmitted from the gut to the brain, and blocking their activity can delay intestinal motility, while activating 5‐HT3 receptors on visceral afferent fibers can result in emesis. 40 , 62 Furthermore, a decrease in serotonergic signaling in mucosa occurs during inflammation. 40 , 63 Studies assessing the effects of 5‐HT1A agonists have shown enhanced gastric accommodation and improvement in postprandial symptoms in patients with functional dyspepsia as well as improvements in common gastroparetic symptoms such as nausea and vomiting in patients with gastroparesis. 42 , 43 In addition to the serotonergic receptors mentioned above, other receptor subtypes that are expressed in the GI tract have also been implicated in the pathogenesis of migraine, which include adrenergic and dopaminergic receptor subtypes, and are summarized in Table 1.
TABLE 1.
Selective receptors implicated in migraine pathophysiology are expressed in the GI tract
| Major receptors | Expression in the GI tract | Evidence supporting implication in migraine pathogenesis |
|---|---|---|
| Serotonergic 40 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 | 5‐HT1A, 5‐HT1P | 5‐HT1A, 5‐HT1B, 5‐HT1D, 5‐HT1F |
| 5‐HT2A, 5‐HT2B | 5‐HT2A, 5‐HT2B | |
| 5‐HT3 | ||
| 5‐HT4 | ||
| 5‐HT7 | ||
| Adrenergic 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 | α2 | α2 |
| β1 | β1 | |
| β2 | β2 | |
| β3 | ||
| Dopaminergic 80 , 106 | D1‐5 | D2‐5 |
| CGRP 107 , 108 , 109 | CLR/RAMP1 | CLR/RAMP1 |
This table includes a list of key receptors located in the GI tract that are also implicated in the pathogenesis of migraine in both animal and human studies.
Abbreviations: 5‐HT, 5‐hydroxytryptamine; CGRP, calcitonin gene‐related peptide; CLR, calcitonin‐like receptor; GI, gastrointestinal; RAMP1, receptor activity modifying protein 1.
The neuropeptide calcitonin gene‐related peptide (CGRP) has been implicated in migraine pathophysiology based on biological mechanisms as well as clinical trials demonstrating efficacy in migraine patients treated with pharmacologic agents that target CGRP and its receptors. 110 , 111 , 112 , 113 , 114 αCGRP and βCGRP are isoforms of CGRP that are predominantly expressed in sensory and enteric neurons, respectively, and have been shown to innervate a variety of areas within the digestive system. 114 , 115 , 116 Both the release of CGRP from parasympathetic perivascular and trigeminal fibers in migraine and the alterations in intestinal microbiota and gut permeability in response to stress factors can lead to the release of proinflammatory mediators. These, in turn, can affect nociceptive responses in the trigeminal pathway and lead to migraine development. 22 , 117 , 118 , 119 , 120 The release of proinflammatory molecules can also result in increased susceptibility to inflammatory disorders via the activation of the hypothalamic–pituitary–adrenal axis, such as those affecting the GI system. 21 , 117 , 118 In particular, high levels of CGRP and low pain thresholds for gastric distention have been reported in patients with functional dyspepsia who are positive for Helicobacter pylori, highlighting a potential role for CGRP in pain mechanisms in functional dyspepsia pathophysiology. 121 Additionally, CGRP has been shown to inhibit gastric acid secretion and may suppress food intake, and alterations in gut microbiota may affect signaling of CGRP. 122 , 123 A summary of the biologic mechanisms that may contribute to the relationship between migraine and gastric disorders can be found in Figure 2.
FIGURE 2.
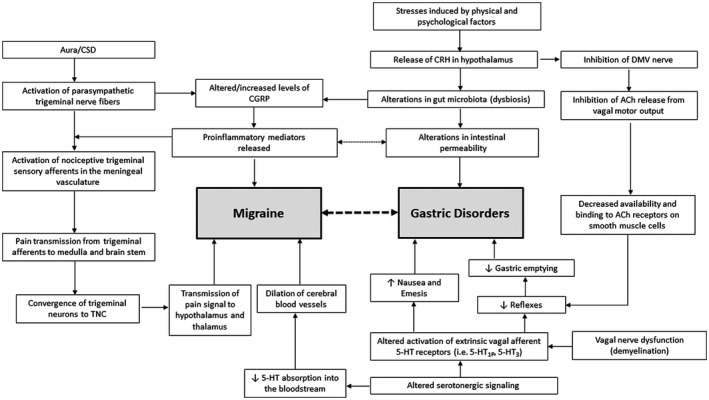
Proposed mechanisms explaining the relationship between migraine and gastric disorders. 21 , 131 A bidirectional relationship exists between migraine and gastric disorders, which is influenced by autonomic dysfunction; specifically, altered sympathetic and parasympathetic activity and changes in the gut microbiota profile, which are mediated by various cytokines, hormones, and neurotransmitters. 21 In migraine, cortical spreading depression (CSD) activates pain pathways that originate from the parasympathetic trigeminal nerve fibers and results in the release of calcitonin gene‐related peptide (CGRP) and proinflammatory mediators, which are implicated in both migraine and gastrointestinal (GI) disorder pathophysiology. 22 , 124 In gastric disorders, it is suggested that stress induced by physical and psychological factors causes the release of corticotrophin‐releasing hormone (CRH), which leads to alterations in gut microbiota and intestinal permeability, the release of proinflammatory mediators, and the inhibition of acetylcholine release, resulting in GI dysfunction. Another major factor contributing to this relationship is an alteration in serotonergic signaling, which can activate the TGV system involved in the initiation of a migraine attack and lead to the development of symptoms of gastric disorders including nausea, emesis, and delayed gastric emptying by altering GI reflex pathways or activating GI serotonin receptors. 21 , 62 Abbreviations: 5‐HT, 5‐hydroxytryptamine; ACh, acetylcholine; DMV, dorsal motor nucleus of the vagus; TGV, trigeminovascular; TNC, trigeminal nucleus caudalis
A pathophysiological relationship between migraine and gastric disorders is further supported by overlap in some pharmacological agents used in the treatment of both disorders. Domperidone, a dopamine receptor antagonist with gastrokinetic and antiemetic features, is used for the treatment of gastroparesis and was shown to prevent a majority of migraine attacks with early administration (i.e., at the time of early warning symptoms) at the higher doses. 132 , 133 , 134 Used to treat gastroparesis and nausea, metoclopramide is a dopamine receptor antagonist that was reported to be effective as an intravenous acute treatment of migraine at multiple doses. 134 , 135 , 136 CVS and migraine also overlap with regard to medications used to prevent or treat both conditions, which include tricyclic antidepressants and antiepileptic drugs as prophylaxis, and triptans and antiemetics as abortive therapies. 18 Similarly, agents used to treat migraine may also be efficacious in treating some GI disorders. One study reported the effectiveness of propranolol, a beta‐blocker used for migraine prevention, in increasing colonic motility in 10 patients with IBS. 137 , 138 Tricyclic antidepressants are used for migraine prevention and as first‐line therapy for pain‐predominant DGBIs such as functional dyspepsia, IBS, and CVS. 138 , 139 , 140 Noninvasive vagal nerve stimulation, which stimulates myelinated sensory afferent vagal fibers, is approved by the Food and Drug Administration as abortive therapy in patients with migraine and cluster headache. 141 , 142 Two separate open‐label pilot studies using the same device in patients with gastroparesis demonstrated improvement in gastroparesis symptoms and acceleration of gastric emptying. 142 , 143 While there are indications that the pathophysiologies of migraine and gut disorders are linked in many individuals, there is still work to be done to elucidate the mechanisms involved. Early research into the pathogenesis and links between these two disorders is sparse. A recent in vivo study utilizing several monogenic mouse models with mutations of genes linked to migraine phenotypes reported no presence of gastroparesis or delayed small intestinal motility, suggesting an absence of heritable characteristics between migraine and gastroparesis. 144 One recent in vivo mouse model that explicitly evaluated the role of the gut microbiome in the development of migraine‐like pain demonstrated that antibiotic treatment prolonged nitroglycerin‐induced acute migraine‐like pain, but this pain prolongation was completely blocked by genetic deletion of tumor necrosis factor‐alpha (TNFα) or injection of a TNFα receptor antagonist. These results suggest that gut microbiota dysbiosis contributes to migraine‐like pain by upregulating TNFα levels in the trigeminal nociceptive system, supporting a proposed link between these disorders. 145 We look forward for future studies elucidating this potential pathophysiologic link between migraine and gut disorders.
GI COMORBIDITIES AND MIGRAINE: GASTROPARESIS, FUNCTIONAL DYSPEPSIA, AND CYCLIC VOMITING SYNDROME
Gastroparesis and migraine
A diagnosis of gastroparesis is confirmed with a 4‐h gastric emptying evaluation by scintigraphy and exclusion of other etiologies. 146 , 147 To assess symptom severity, the Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Upper Gastrointestinal Symptoms, can be used. It comprises three subscales (nausea and vomiting, postprandial fullness and early satiety, and bloating) that the patient scores based on the past 2 weeks. 148 The Gastroparesis Cardinal Symptom Index − Daily Diary, a more accurate and comprehensive version of the GCSI that allows patients to record symptoms daily, is also available. 149 The natural history of gastroparesis is largely unknown, and the prevalence is difficult to estimate; however, the prevalence of diagnosed gastroparesis in the US population has been estimated at 24.2 per 100,000 persons. 150 , 151 According to population‐based studies, individuals are predominantly female and the most common symptoms, either persistent or episodic, are nausea and vomiting, but can also include abdominal pain, bloating, weight loss, postprandial fullness, and early satiety. 44 , 45 , 150 Importantly, abdominal pain is a common but underrecognized symptom that contributes to a decrease in quality of life. 46 , 47 In individuals with idiopathic gastroparesis and abdominal pain, those with severe abdominal pain were more likely to have overlapping migraine than those with milder symptoms. 54 Major etiologies of gastroparesis are diabetic, post‐surgical, and idiopathic, and in general, an idiopathic etiology is the most common. 45 , 150 , 152 A National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) study that characterized 243 patients with idiopathic gastroparesis reported that delayed gastric emptying was mild (≤20% gastric retention) in 45%, moderate (>20%–35% retention) in 27%, and severe (>35% retention) in 28% of patients. 44 Historical evidence supporting an association between migraine and delayed gastric emptying emerged from early reports of alterations in gastric motility in patients experiencing a migraine attack. 153 , 154 Later, experimental studies by Volans reported a delay in effervescent aspirin absorption during a migraine attack, but not during the headache‐free period, suggesting that delayed gastric emptying occurs during spontaneous migraine attacks. 59 A comprehensive summary of key studies assessing gastric emptying in patients with migraine is provided in Table 2. Boyle et al. 48 examined delay in gastric emptying utilizing the epigastric impedance method in 46 patients with migraine and 64 individuals without migraine. There was no difference in the mean time to half gastric emptying in those without migraine (mean ± standard deviation [SD]: 9 ± 5 min) compared to those with migraine outside an attack (mean ± SD: 10.1 ± 5.3 min). However, in 14 patients who experienced severe or moderate migraine attacks that were sometimes associated with pain, nausea, and photophobia, the mean time to half gastric emptying ranged from 6 to >60 min. 48 The hypothesis that gastric emptying was delayed only during a migraine attack was questioned when studies decades later demonstrated delay in gastric emptying during visually induced migraine and during the headache‐free, interictal period. 7 , 49 Aurora et al. evaluated gastric emptying using gastric scintigraphy in 10 patients with migraine during the ictal and interictal period and 10 age‐ and sex‐matched individuals without migraine. In patients with migraine, the time to half emptying after a visually induced migraine attack was delayed ictally (78%) and interictally (80%), and the mean time to half emptying during the interictal period was significantly longer in patients with migraine (mean ± SD: 188.8 ± 100.6 min) compared to patients without migraine (mean ± SD: 111.8 ± 38.6 min; p = 0.0168). 7 In a subsequent case report, Aurora et al. replicated these findings when assessing gastric emptying by gastric scintigraphy in a single patient with spontaneous migraine (124 min), a single patient with induced migraine (182 min), and a single patient with migraine during the interictal period (243 min) compared to normative values (112 min). 49 These results suggest that individuals with migraine may experience a delay in gastric motility both during and outside of a migraine attack, challenging previous theories that delays occur only during an attack. 48 , 49 , 59 However, when Yalcin et al. assessed liquid phase gastric emptying in seven patients with migraine during the interictal period, seven patients with migraine during the ictal period, and seven individuals without migraine, findings contradictory to those of Aurora and colleagues were reported. The mean time to half gastric emptying was similar between patients with migraine outside a migraine attack (mean ± SD: 26.29 ± 9.45 min) and those without migraine (mean ± SD: 26.14 ± 5.61 min), compared to patients with migraine during an attack (mean ± SD: 48.00 ± 18.72 min). 50 The difference in methodology used to evaluate gastric emptying during the liquid phase in this study may explain the observation of delayed gastric emptying only in patients with migraine during an attack and not interictally. More recent and extensive evidence is provided in a retrospective study that evaluated gastroparesis‐like symptoms in patients from the NIDDK Gastroparesis Clinical Research Consortium. A total of 711 patients were studied, including 516 patients with gastroparesis and 195 patients with chronic unexplained nausea and vomiting (CUNV). 36.6% of patients with gastroparesis reported having migraine attacks (Table 3). Patients with migraine headaches also had a more severe GCSI (odds ratio [OR] 1.24, 95% confidence interval [CI] 1.05–1.45, p = 0.009), increased trait anxiety (OR 1.16, 95% CI = 1.03–1.32, p = 0.02), and were less likely to be diabetic (OR = 0.67, 95% CI = 0.48–0.94, p = 0.02) compared to those without migraine headaches. 55 These data suggest that the comorbid association of migraine and GI disorders is indeed common and worthy of more detailed investigation.
TABLE 2.
Experimental studies assessing gastric emptying in patients with migraine
| Study | N | Subject group | T 1/2 (min) a | Detection method |
|---|---|---|---|---|
| Boyle 1990 48 | 46 | Patients with migraine—outside of attack | 10.1 ± 5.3 | Epigastric impedance |
| 14 | Patients with migraine—during attack | 6–<60 | ||
| 64 | Individuals without migraine—controls | 9 ± 5 | ||
| Aurora 2006 7 | 10 | Patients with migraine—interictal | 188.8 ± 100.6 | Gastric scintigraphy |
| 9 | Patients with migraine—ictal | 149.9 ± 69.4 | ||
| 10 | Individuals without migraine—controls | 111.8 ± 38.6 | ||
| Aurora 2007 49 | 1 | Patients with migraine—interictal | 243 | Gastric scintigraphy |
| 1 | Patients with migraine—spontaneous migraine | 124 | ||
| 1 | Patients with migraine—induced migraine | 182 | ||
| N/A | Control—normative value | 112 | ||
| Yu 2012 52 | 27 | Patients with migraine without GI symptoms interictally | 100.82 ± 23.94 | Gastric scintigraphy |
| 32 | Functional dyspepsia patients | 125.51 ± 52.55 | ||
| 12 | Healthy individuals—controls | 95.23 ± 23.29 | ||
| Yalcin 2012 50 | 7 | Patients with migraine—interictal | 26.29 ± 9.447 | Liquid phase gastric scintigraphy |
| 7 | Patients with migraine—ictal | 48.0 ± 18.717 | ||
| 7 | Individuals without migraine—controls | 26.14 ± 95.610 |
This table includes a comprehensive list of experimental studies assessing gastric emptying in patients with migraine utilizing different methodologies and patient populations.
Abbreviations: GI, gastrointestinal; N/A, not available.
T 1/2 is the time to half gastric emptying; values presented as mean ± standard deviation for all studies with the exception of the Aurora 2007 study.
TABLE 3.
Prevalence of comorbidities in patients with gastroparesis or CUNV
| Gastroparesis a (N = 516) | CUNV b (N = 195) | Total (N = 711) | p value c | Population estimates (US) | |
|---|---|---|---|---|---|
| Severe abdominal pain d | 237 (45.9%) | 66 (33.9%) | 303 (42.6%) | 0.004 | |
| Migraine headache | 189 (36.6%) | 69 (35.4%) | 258 (36.3%) | 0.79 | 15.3% |
| Endometriosis e | 71 (16.6%) | 20 (12.5%) | 91 (15.5%) | 0.25 | 6.1% |
| Fibromyalgia | 67 (13.0%) | 24 (12.3%) | 91 (12.8%) | 0.90 | 1.75% |
| Chronic fatigue syndrome | 44 (8.5%) | 11 (5.6%) | 55 (7.7%) | 0.27 | 0.52%–1.04% |
| Interstitial cystitis | 18 (3.5%) | 7 (3.6%) | 25 (3.5%) | 1.00 | 1.9%–6.53% f |
This table presents the prevalence of the most frequent comorbidities associated with patients with gastroparesis and CUNV from the National Institute of Diabetes and Digestive and Kidney Diseases Gastroparesis Clinical Research Consortium study. 55 , 155 , 156 , 157 , 158 , 159 , 160
Abbreviation: US, United States.
Gastroparesis based on delayed gastric emptying scintigraphy >60% retention at 2 hours or >10% retention at 4 hours
CUNV is defined as chronic unexplained nausea and vomiting, based on non‐delayed gastric emptying scintigraphy
p‐values derived from Fisher's exact tests
Severe abdominal pain based on a score of 4 (severe) or 5 (very severe) on the patient assessment of upper gastrointestinal symptom severity (PAGI‐SYM) questionnaire (scale of 0–5)
89 males excluded from endometriosis counts
Range includes calculated prevalence estimates based on definitions spanning a range of sensitivity and specificity for both men and women.
Functional dyspepsia and migraine
According to the Rome IV criteria, functional dyspepsia is subdivided into postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS). Functional dyspepsia is defined by bothersome early satiety or postprandial fullness ≥3 days per week (PDS) or bothersome epigastric pain or epigastric burning ≥1 day per week in the past 3 months (EPS) with a ≥6‐month history, respectively. 161 Additional symptoms may also include bloating, belching, and nausea. 14 A delay in gastric emptying of solids and liquids is also present in 23% and 35% of patients with functional dyspepsia, respectively. 51 Further, there is an overlap in symptomatology between gastroparesis and functional dyspepsia. According to the Gastroparesis Registry of 106 patients with gastroparesis, the majority of patients also met Rome criteria for the PDS subtype of functional dyspepsia. 46 While the etiology of functional dyspepsia remains to be determined, the prevalence varies between geographic location and diagnostic criteria, ranging from ~5% to 40% globally. 161 , 162 Di Stefano et al. observed that migraine is a common comorbidity in patients with functional dyspepsia and postprandial symptoms. In a study of 60 patients with functional dyspepsia, 38 with PDS and 22 with EPS, 68% experienced migraine without aura. Of those with EPS, 54% experienced migraine without aura, which was not correlated with ingesting a meal, while 76% of patients with PDS experienced migraine, where 89% of migraine attacks were correlated with meal ingestion. Interestingly, patients with PDS and moderate to severe migraine experienced significantly greater fullness (2.2 ± 0.7 vs. 1.9 ± 0.7; p = 0.02) and early satiety (2.0 ± 0.8 vs. 1.7 ± 0.8; p = 0.01) compared to PDS patients with mild or no migraine, while patients with EPS and severe migraine experienced less severe epigastric burning (0.7 ± 0.7 vs. 1.6 ± 0.8; p = 0.008) and bloating (1.3 ± 1.0 vs. 2.3 ± 0.7; p = 0.01) compared to EPS patients with mild or no migraine. However, the mean time to gastric emptying in patients with PDS and EPS was similar irrespective of migraine severity. 10 Pucci et al. observed meal‐induced hypersensitivity of the stomach in patients with functional dyspepsia and migraine. Visceral sensitivity was evaluated by assessing sensitivity thresholds to mechanical distention by utilizing an instrument called the barostat following a liquid meal. The discomfort threshold following a liquid meal was significantly lower in seven patients with functional dyspepsia and migraine without aura (mean ± SD: 7 ± 4 mm Hg; p < 0.01) compared to seven patients with functional dyspepsia without migraine (mean ± SD: 11 ± 4 mm Hg) and seven healthy volunteers (mean ± SD: 11 ± 6 mm Hg). 163 In a study by Yu et al. 52 gastric emptying was assessed by gastric scintigraphy in 27 patients with migraine without GI symptoms during the interictal period, 32 patients with functional dyspepsia, and 12 healthy volunteers. The mean gastric half emptying time in patients with functional dyspepsia (mean ± SD: 125.51 ± 52.55 min) was longer than in patients with migraine (mean ± SD: 100.82 ± 23.94 min; p = 0.035) and healthy volunteers (mean ± SD: 95.25 ± 23.29 min; p = 0.021). Further, in patients with migraine, the gastric half emptying time was similar in patients who did experience vomiting (mean ± SD: 98.1 ± 22.8 min) or nausea (mean ± SD: 103.8 ± 22.1 min) compared to those who did not experience vomiting (mean ± SD: 103.5 ± 24.7 min) or nausea (mean ± SD: 95.0 ± 29.9 min), suggesting that gastric half emptying time was not associated with vomiting or nausea experienced during the interictal period (Table 1). 52 It is important to note that the grading criteria of gastric emptying used in this study has been questioned, since the proportions of gastric emptying in the functional dyspepsia patients and healthy volunteers do not align with previous studies. Further, it had been suggested that the exclusion of patients with migraine who experience GI symptoms during the interictal period does not allow for an accurate assessment of an association between migraine and functional dyspepsia. 164
CVS and migraine
According to the Rome IV criteria, CVS is defined by stereotypical episodes of acute‐onset vomiting lasting <1 week, ≥3 discrete episodes in the prior year, and 2 episodes in the past 6 months, occurring ≥1 week apart; and absence of vomiting between episodes, although milder symptoms can be present. Symptoms are also required to have been present for the past 3 months with onset at least 6 months prior. 53 Although CVS is typically regarded as a pediatric condition, it is also present in adults, 11 , 18 and although few data have been published, the prevalence of CVS in adults has been reported to range from 0.7% to 2% in the United States, Canada, and the United Kingdom. 53 Depending on the stage of CVS, common symptoms may include nausea, sweating, epigastric pain, fatigue, feeling hot or cold, GI disturbances, and/or violent attacks of vomiting or retching. Some individuals may experience little to no symptoms during the inter‐episodic phase. 11 , 18 The etiology of CVS is unclear and it may arise from stress triggers, and less frequently dietary triggers, but an association with migraine is suggested based on similarities in triggers and symptomatology, which include antecedent auras, associated headaches, phonophobia, and photophobia. 18 , 28 Migraine is also a frequent comorbidity present in individuals with CVS, including children. 56 , 57 In a retrospective study by Fleisher et al. of 40 adult patients with CVS, 70% experienced migraine headaches during or between CVS episodes. 11 In a large, retrospective study by Bhandari et al. in adult patients with CVS (N = 20,952) and age‐matched patients without CVS (N = 44,262), migraine was a comorbidity observed more frequently in CVS patients (9%) compared to patients without CVS (3%; p < 0.001). 165 Additionally, Kumar et al. observed that 43% of adult patients with CVS experienced migraine in a retrospective analysis. 58 Further, a whole‐brain analysis of patients with CVS and episodic migraine by Ellingsen et al. revealed that patients with CVS and episodic migraine showed reduced sensorimotor connectivity in the mid/posterior insula, an area important for nausea and viscerosensory processing, compared to healthy controls, highlighting a shared pathophysiology between these conditions. 166
THE IMPACT OF GI COMORBIDITIES ON MIGRAINE TREATMENT
Nausea is an extremely disabling symptom of migraine and may impact when in an evolving attack a patient uses an oral treatment. In a large survey of 500 patients with migraine, nausea and vomiting were reported to affect a patient's willingness to take an oral medication in 30.5% and 42.2% of patients with migraine, respectively. 167 , 168 Gastric motility dysfunction and vomiting may also affect the rate and efficiency of absorption of drugs with high intestinal permeability. 167 Early studies assessing the pharmacokinetics of migraine treatment demonstrated that the absorption rates of oral formulations of headache medications were impaired in patients with migraine compared to the same patients with migraine during the headache‐free period or to patients without migraine. Volans 59 assessed effervescent aspirin absorption by measuring plasma salicylate levels at 30 and 60 min after ingestion of aspirin in 42 migraine patients. Compared to patients without migraine at 30 min (mean: 7.88 ± 0.40 mg/100 mL), patients with migraine demonstrated a statistically significant impairment in the mean rate of aspirin absorption during an acute attack (mean: 4.77 ± 0.43 mg/100 mL) but not during the headache‐free period (mean: 7.04 ± 0.64 mg/100 mL). At 30 min, 19 out of 42 migraine patients experienced plasma salicylate levels below the 2.5% lower confidence limit for individuals without migraine (i.e., 4.42 mg/100 mL), and delayed absorption was present more frequently with increasing headache and GI symptom severity. 59 Tokola and Neuvonen assessed the absorption of paracetamol in 9 female outpatients with migraine and determined that the mean peak serum concentration of paracetamol was significantly lower during the migraine phase (mean ± standard error of the mean [SEM]: 109 ± 14 μmol/L) than it was during the migraine‐free period (mean ± SEM: 144 ± 16 μmol/L; p < 0.05). During a migraine attack, the area under the curve (AUC)0‐2 h was decreased by 17%, the AUC0‐4 h by 14%, and the AUC0‐6 h by 12% (p < 0.05) compared to the headache‐free period. There was also a significant correlation between nausea at the beginning of the study and the decrease in the AUCs during a migraine attack at 0–3, 0–4, and 0–6 h (p < 0.05–<0.01). 169 Tokola and Neuvonen 170 also assessed the absorption of tolfenamic acid, a nonsteroidal anti‐inflammatory drug, in 7 patients with migraine and reported that mean AUC0‐2 h was significantly lower during the migraine phase (mean ± SEM: 2.00 ± 0.66 mg*h/L) compared to the headache‐free period (mean ± SEM: 4.07 ± 0.61 mg*h/L; p < 0.05), while the t max was significantly higher during the migraine phase (mean ± SEM: 2.86 ± 0.36 h) compared to the headache‐free period (mean ± SEM: 1.69 ± 0.20 h; p < 0.05). Pre‐treatment with metoclopramide, a p‐aminobenzamide derivative that increases gastric motility and emptying, during migraine attacks increased the serum concentration of tolfenamic acid at 1.5 h. There was also no association between impairment in tolfenamic acid absorption during a migraine attack and attack duration or severity. 170
There is also evidence that oral administration of some triptans results in varying plasma concentrations among patients with migraine during and outside of an attack. Thomsen et al. demonstrated that zolmitriptan was less rapidly absorbed during a migraine attack (median C max0‐4: 7.9 ng/mL; n = 20), which included those patients who vomited, compared to the migraine‐free period (median C max0‐4: 12.6 ng/mL; n = 18), which is consistent with a delay in gastric emptying. 171 Further, the efficacy of triptans in treating migraine is increased when combined with medications that enhance GI motility. Schulman and Dermott 172 reported the efficacy of combining orally administered sumatriptan with either metoclopramide or placebo to treat moderate to severe migraine in 16 patients with migraine with or without aura. A headache response, defined as moderate or severe to mild or no pain at 2 h, was observed in 7/16 (44%) of patients receiving sumatriptan with metoclopramide compared to 5/16 (31%) with placebo. Three additional patients who received sumatriptan and metoclopramide reported meaningful migraine relief, as defined by the patient. Therefore, meaningful relief was achieved in 10/16 (63%) headaches treated with sumatriptan and metoclopramide compared to 5/16 (31%) headaches treated with sumatriptan and placebo, suggesting that adding metoclopramide to sumatriptan may benefit those patients who do not receive adequate relief with triptans alone. 172 Krymchantowski et al. 173 assessed whether combining rizatriptan with trimebutine (β‐[dimethylamino]‐β‐ethylfenethylalcohol‐3,4,5‐trimetoxibenzoate), an opioid derivative that can activate receptors in the GI tract, would improve pain freedom compared to rizatriptan alone. Rizatriptan with trimebutine resulted in complete resolution of 30 out of 64 migraine attacks (46.8%) 1‐h post‐dose, compared with only 8 out of 64 attacks (12.5%) in patients treated with rizatriptan alone (p < 0.01). Complete resolution of migraine attacks was observed at even higher rates at 2 h (73.4% vs. 31.2%; p < 0.001) and 4 h (79.7% vs. 31.2%; p < 0.001) post‐dose, respectively. 173 Interestingly, there are several reports of delayed gastric motility associated with the use of sumatriptan in healthy volunteers. Sakamoto et al. demonstrated that the time to gastric half emptying, as measured by a continuous 13C breath test (BreathID system; Exalenz Bioscience Ltd.) in healthy individuals following ingestion of a liquid meal was a median of 131.84 (range: 103.13–168.70) minutes in the group receiving oral sumatriptan compared to 120.27 (range: 89.61–138.25) minutes in the control group (p = 0.0166). 174 Houghton et al. reported that in healthy individuals administered intravenous sumatriptan, the time to half gastric emptying, as measured by gamma scintillation, was delayed following a liquid meal (mean ± SD: 96 ± 40 min) compared to those who received placebo (mean ± SD: 76 ± 33 min). 175 Further, acute migraine therapies that are CGRP receptor antagonists or CGRP monoclonal antibodies have also been shown to cause constipation in some patients, suggesting that these agents are unable to provide patients relief from GI symptoms; however, data on the effect these molecules have on gastric emptying are not yet available. 176 Recognition of GI and DGBI comorbidities in patients with migraine is important for patients who experience GI symptoms and do not have relief from migraine symptoms using an oral abortive treatment. Non‐oral routes of administration should be considered in these patients as they may positively impact them. 167 , 177 Importantly, efficacious, non‐oral abortive therapies may also reduce the need for patients to seek chronic migraine medications that can be expensive or oral daily preventives that are suboptimal with regard to efficacy and tolerability.
CONCLUSION
The association between GI comorbidities, including those under the DGBI classification, and migraine may be underrecognized. Very few studies assessing DGBI, specifically gastroparesis, functional dyspepsia, and CVS, in patients with migraine have been performed, but the similarities and frequent comorbid presentation suggest a strong physiological link connecting these disorders that remains to be elucidated. It is evident that there is significant overlap with migraine and GI symptoms, which has been shown to affect the timing of drug administration by a patient due to nausea and fear of vomiting as well as the absorption of oral migraine medications. Attention to GI disorders and DGBI as comorbidities of migraine may be particularly important if patients with symptoms of nausea, vomiting, and/or abdominal pain do not experience relief from migraine symptoms using an oral migraine medication. Specifically, if a patient has cycled through several different orally administered migraine therapies without adequate relief, an assessment of GI comorbidities should be warranted. Non‐oral routes of administration and formulation of migraine therapies should be considered early in migraine management.
CONFLICT OF INTEREST
Sheena K. Aurora, Sutapa Ray, and Stephen B. Shrewsbury are full‐time employees of Impel NeuroPharma and are stockholders in Impel NeuroPharma. Nada Hindiyeh serves on advisory boards for Amgen, Eli Lilly, Lundbeck, and Zosano Pharma. Linda Nguyen serves on an advisory board for Gemelli and consults for Pendulum, Neurogastrx, Ironwood, Eli Lilly, and Alnylam.
AUTHOR CONTRIBUTIONS
Conception and design: Sheena K. Aurora, Stephen B. Shrewsbury, Sutapa Ray, Nada Hindiyeh, Linda Nguyen. Acquisition of data: Sheena K. Aurora, Stephen B. Shrewsbury, Sutapa Ray, Nada Hindiyeh, Linda Nguyen. Analysis and interpretation of data: Sheena K. Aurora, Stephen B. Shrewsbury, Sutapa Ray, Nada Hindiyeh, Linda Nguyen. Drafting the manuscript: Sheena K. Aurora, Stephen B. Shrewsbury, Sutapa Ray, Nada Hindiyeh, Linda Nguyen. Revising it for intellectual content: Sheena K. Aurora, Stephen B. Shrewsbury, Sutapa Ray, Nada Hindiyeh, Linda Nguyen. Final approval of the completed manuscript: Sheena K. Aurora, Stephen B. Shrewsbury, Sutapa Ray, Nada Hindiyeh, Linda Nguyen.
ACKNOWLEDGMENTS
Writing and editorial assistance was provided by IMPRINT Science, New York, NY, and was supported by Impel NeuroPharma. The authors are fully responsible for the content, editorial decisions, and opinions expressed in the current article. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number 3U01DK073983‐11S1.
REFERENCES
- 1. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2016 Headache Collaborators . Global, regional, and national burden of migraine and tension‐type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lipton RB, Buse DC, Saiers J, Fanning KM, Serrano D, Reed ML. Frequency and burden of headache‐related nausea: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2013;53:93‐103. [DOI] [PubMed] [Google Scholar]
- 4. Aamodt AH, Stovner LJ, Hagen K, Zwart JA. Comorbidity of headache and gastrointestinal complaints. The Head‐HUNT Study. Cephalalgia. 2008;28:144‐151. [DOI] [PubMed] [Google Scholar]
- 5. Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646‐657. [DOI] [PubMed] [Google Scholar]
- 6. Schürks M, Buring JE, Kurth T. Migraine, migraine features, and cardiovascular disease. Headache. 2010;50:1031‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aurora SK, Kori SH, Barrodale P, McDonald SA, Haseley D. Gastric stasis in migraine: more than just a paroxysmal abnormality during a migraine attack. Headache. 2006;46:57‐63. [DOI] [PubMed] [Google Scholar]
- 8. Moisset X, Bommelaer G, Boube M, et al. Migraine prevalence in inflammatory bowel disease patients: a tertiary‐care centre cross‐sectional study. Eur J Pain. 2017;21:1550‐1560. [DOI] [PubMed] [Google Scholar]
- 9. Su J, Zhou XY, Zhang GX. Association between Helicobacter pylori infection and migraine: a meta‐analysis. World J Gastroenterol. 2014;20:14965‐14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Stefano M, Pucci E, Miceli E, et al. Prevalence and pathophysiology of post‐prandial migraine in patients with functional dyspepsia. Cephalalgia. 2019;39:1560‐1568. [DOI] [PubMed] [Google Scholar]
- 11. Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic vomiting syndrome in 41 adults: the illness, the patients, and problems of management. BMC Med. 2005;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serratrice J, Disdier P, de Roux C, Christides C, Weiller PJ. Migraine and coeliac disease. Headache. 1998;38:627‐628. [DOI] [PubMed] [Google Scholar]
- 13. Georgescu D, Reisz D, Gurban CV, et al. Migraine in young females with irritable bowel syndrome: still a challenge. Neuropsychiatr Dis Treat. 2018;14:21‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil. 2017;23:151‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Rome Foundation . About the Rome Foundation. 2020. https://theromefoundation.org/about/. Accessed July 1, 2020. [Google Scholar]
- 16. Masaoka T, Tack J. Gastroparesis: current concepts and management. Gut Liv. 2009;3:166‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med. 2015;373:1853‐1863. [DOI] [PubMed] [Google Scholar]
- 18. Yu ES, Priyadharsini SSY, Venkatesan T. Migraine, cyclic vomiting syndrome, and other gastrointestinal disorders. Curr Treat Options Gastroenterol. 2018;16:511‐527. [DOI] [PubMed] [Google Scholar]
- 19. Shrewsbury SB, Hoekman J, Jeleva M. Patient acceptability of a novel upper nasal delivery system for dihydroergotamine mesylate using the Precision Olfactory Delivery (POD®) device—results from the open‐label STOP 301 trial. Presented at the American Headache Society Virtual Annual Scientific Meeting, June 2020. [Google Scholar]
- 20. Cámara‐Lemarroy CR, Rodriguez‐Gutierrez R, Monreal‐Robles R, Marfil‐Rivera A. Gastrointestinal disorders associated with migraine: a comprehensive review. World J Gastroenterol. 2016;22:8149‐8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arzani M, Jahromi SR, Ghorbani Z, et al. Gut‐brain axis and migraine headache: a comprehensive review. J Headache Pain. 2020;21:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hindiyeh N, Aurora SK. What the gut can teach us about migraine. Curr Pain Headache Rep. 2015;19:33. [DOI] [PubMed] [Google Scholar]
- 23. Shechter A, Stewart WF, Silberstein SD, Lipton RB. Migraine and autonomic nervous system function: a population‐based, case‐control study. Neurology. 2002;58:422‐427. [DOI] [PubMed] [Google Scholar]
- 24. Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41:629‐637. [DOI] [PubMed] [Google Scholar]
- 25. Dalla Volta G, Anzola GP, DiMonda V. The disappearance of the ”cold patch” in recovered migraine patients: thermographic findings. Headache. 1991;31:305‐309. [DOI] [PubMed] [Google Scholar]
- 26. Campbell IW, Heading RC, Tothill P, Buist TA, Ewing DJ, Clarke BF. Gastric emptying in diabetic autonomic neuropathy. Gut. 1977;18:462‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen L, Wilson LA, Miriel L, et al. Autonomic function in gastroparesis and chronic unexplained nausea and vomiting: relationship with etiology, gastric emptying, and symptom severity. Neurogastroenterol Motil. 2020;32:e13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Venkatesan T, Prieto T, Barboi A, et al. Autonomic nerve function in adults with cyclic vomiting syndrome: a prospective study. Neurogastroenterol Motil. 2010;22:1303‐1307.e1339. [DOI] [PubMed] [Google Scholar]
- 29. Hejazi RA, Lavenbarg TH, Pasnoor M, et al. Autonomic nerve function in adult patients with cyclic vomiting syndrome. Neurogastroenterol Motil. 2011;23:439‐443. [DOI] [PubMed] [Google Scholar]
- 30. Lorena SL, Figueiredo MJ, Almeida JR, Mesquita MA. Autonomic function in patients with functional dyspepsia assessed by 24‐hour heart rate variability. Dig Dis Sci. 2002;47:27‐31. [DOI] [PubMed] [Google Scholar]
- 31. Tominaga K, Fujikawa Y, Tsumoto C, et al. Disorder of autonomic nervous system and its vulnerability to external stimulation in functional dyspepsia. J Clin Biochem Nutr. 2016;58:161‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286‐294. [DOI] [PubMed] [Google Scholar]
- 33. Grover M, Farrugia G, Stanghellini V. Gastroparesis: a turning point in understanding and treatment. Gut. 2019;68:2238‐2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Browning KN. Role of central vagal 5‐HT3 receptors in gastrointestinal physiology and pathophysiology. Front Neurosci. 2015;9:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aggarwal M, Puri V, Puri S. Serotonin and CGRP in migraine. Ann Neurosci. 2012;19:88‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kilinc E, Guerrero‐Toro C, Zakharov A, et al. Serotonergic mechanisms of trigeminal meningeal nociception: implications for migraine pain. Neuropharmacology. 2017;116:160‐173. [DOI] [PubMed] [Google Scholar]
- 37. Tepper SJ, Rapoport AM, Sheftell FD. Mechanisms of action of the 5‐HT1B/1D receptor agonists. Arch Neurol. 2002;59:1084‐1088. [DOI] [PubMed] [Google Scholar]
- 38. Coleman NS, Marciani L, Blackshaw E, et al. Effect of a novel 5‐HT3 receptor agonist MKC‐733 on upper gastrointestinal motility in humans. Aliment Pharmacol Ther. 2003;18:1039‐1048. [DOI] [PubMed] [Google Scholar]
- 39. Degen L, Matzinger D, Merz M, et al. Tegaserod, a 5‐HT4 receptor partial agonist, accelerates gastric emptying and gastrointestinal transit in healthy male subjects. Aliment Pharmacol Ther. 2001;15:1745‐1751. [DOI] [PubMed] [Google Scholar]
- 40. Gershon MD. Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20:3‐14. [DOI] [PubMed] [Google Scholar]
- 41. Talley NJ, Van Zanten SV, Saez LR, et al. A dose‐ranging, placebo‐controlled, randomized trial of alosetron in patients with functional dyspepsia. Aliment Pharmacol Ther. 2001;15:525‐537. [DOI] [PubMed] [Google Scholar]
- 42. Tack J, Janssen P, Masaoka T, Farré R, Van Oudenhove L. Efficacy of buspirone, a fundus‐relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2012;10:1239‐1245. [DOI] [PubMed] [Google Scholar]
- 43. Malamood M, Roberts A, Kataria R, Parkman HP, Schey R. Mirtazapine for symptom control in refractory gastroparesis. Drug Des Devel Ther. 2017;11:1035‐1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long‐term follow‐up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398‐2404. [DOI] [PubMed] [Google Scholar]
- 46. Nguyen LA, Snape WJ, Jr . Clinical presentation and pathophysiology of gastroparesis. Gastroenterol Clin North Am. 2015;44:21‐30. [DOI] [PubMed] [Google Scholar]
- 47. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427‐438.e300‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boyle R, Behan PO, Sutton JA. A correlation between severity of migraine and delayed gastric emptying measured by an epigastric impedance method. Br J Clin Pharmacol. 1990;30:405‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aurora S, Kori S, Barrodale P, Nelsen A, McDonald S. Gastric stasis occurs in spontaneous, visually induced, and interictal migraine. Headache. 2007;47:1443‐1446. [DOI] [PubMed] [Google Scholar]
- 50. Yalcin H, Okuyucu EE, Ucar E, Duman T, Yilmazer S. Changes in liquid emptying in migraine patients: diagnosed with liquid phase gastric emptying scintigraphy. Intern Med J. 2012;42:455‐459. [DOI] [PubMed] [Google Scholar]
- 51. Sarnelli G, Caenepeel P, Geypens B, Janssens J, Tack J. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98:783‐788. [DOI] [PubMed] [Google Scholar]
- 52. Yu YH, Jo Y, Jung JY, Kim BK, Seok JW. Gastric emptying in migraine: a comparison with functional dyspepsia. J Neurogastroenterol Motil. 2012;18:412‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aziz I, Palsson OS, Whitehead WE, Sperber AD, Simrén M, Törnblom H. Epidemiology, clinical characteristics, and associations for Rome IV functional nausea and vomiting disorders in adults. Clin Gastroenterol Hepatol. 2019;17:878‐886. [DOI] [PubMed] [Google Scholar]
- 54. Parkman HP, Wilson LA, Hasler WL, et al. Abdominal pain in patients with gastroparesis: associations with gastroparesis symptoms, etiology of gastroparesis, gastric emptying, somatization, and quality of life. Dig Dis Sci. 2019;64:2242‐2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nguyen L, Donithan M, Tonascia J, Van Natta M. 2nd Federation of neurogastroenterology and motility meeting, 25–28 August 2016, Hyatt Regency San Francisco, San Francisco, California, USA. Neurogastroenterol Motil. 2016;28(suppl. 1):5‐108. [DOI] [PubMed] [Google Scholar]
- 56. Gelfand AA. Episodic syndromes of childhood associated with migraine. Curr Opin Neurol. 2018;31:281‐285. [DOI] [PubMed] [Google Scholar]
- 57. Irwin S, Barmherzig R, Gelfand A. Recurrent gastrointestinal disturbance: abdominal migraine and cyclic vomiting syndrome. Curr Neurol Neurosci Rep. 2017;17:21. [DOI] [PubMed] [Google Scholar]
- 58. Kumar N, Bashar Q, Reddy N, et al. Cyclic vomiting syndrome (CVS): is there a difference based on onset of symptoms—pediatric versus adult? BMC Gastroenterol. 2012;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Volans GN. Migraine and drug absorption. Clin Pharmacokinet. 1978;3:313‐318. [DOI] [PubMed] [Google Scholar]
- 60. Hoskin KL, Lambert GA, Donaldson C, Zagami AS. The 5‐hydroxytryptamine1B/1D/1F receptor agonists eletriptan and naratriptan inhibit trigeminovascular input to the nucleus tractus solitarius in the cat. Brain Res. 2004;998:91‐99. [DOI] [PubMed] [Google Scholar]
- 61. Miller AD, Ruggiero DA. Emetic reflex arc revealed by expression of the immediate‐early gene c‐fos in the cat. J Neurosci. 1994;14:871‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kohler DR, Goldspiel BR. Ondansetron: a serotonin receptor (5‐HT3) antagonist for antineoplastic chemotherapy‐induced nausea and vomiting. DICP. 1991;25:367‐380. [DOI] [PubMed] [Google Scholar]
- 63. Endo T, Minami M, Hirafuji M, et al. Neurochemistry and neuropharmacology of emesis—the role of serotonin. Toxicology. 2000;153:189‐201. [DOI] [PubMed] [Google Scholar]
- 64. Branchek TA, Mawe GM, Gershon MD. Characterization and localization of a peripheral neural 5‐hydroxytryptamine receptor subtype (5‐HT1P) with a selective agonist, 3H‐5‐hydroxyindalpine. J Neurosci. 1988;8:2582‐2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fiorica‐Howells E, Maroteaux L, Gershon MD. Serotonin and the 5‐HT(2B) receptor in the development of enteric neurons. J Neurosci. 2000;20:294‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kirchgessner AL, Liu MT, Raymond JR, Gershon MD. Identification of cells that express 5‐hydroxytryptamine1A receptors in the nervous systems of the bowel and pancreas. J Comp Neurol. 1996;364:439‐455. [DOI] [PubMed] [Google Scholar]
- 67. Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pan H, Galligan JJ. 5‐HT1A and 5‐HT4 receptors mediate inhibition and facilitation of fast synaptic transmission in enteric neurons. Am J Physiol. 1994;266:G230‐G238. [DOI] [PubMed] [Google Scholar]
- 69. Sidhu M, Cooke HJ. Role for 5‐HT and ACh in submucosal reflexes mediating colonic secretion. Am J Physiol. 1995;269:G346‐G351. [DOI] [PubMed] [Google Scholar]
- 70. Tonini M, Vicini R, Cervio E, et al. 5‐HT7 receptors modulate peristalsis and accommodation in the guinea pig ileum. Gastroenterology. 2005;129:1557‐1566. [DOI] [PubMed] [Google Scholar]
- 71. Cassidy EM, Tomkins E, Dinan T, Hardiman O, O'Keane V. Central 5‐HT receptor hypersensitivity in migraine without aura. Cephalalgia. 2003;23:29‐34. [DOI] [PubMed] [Google Scholar]
- 72. De Vries P, Heiligers JP, Villalón CM, Saxena PR. Blockade of porcine carotid vascular response to sumatriptan by GR 127935, a selective 5‐HT1D receptor antagonist. Br J Pharmacol. 1996;118:85‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Goadsby PJ, Classey JD. Evidence for serotonin (5‐HT)1B, 5‐HT1D and 5‐HT1F receptor inhibitory effects on trigeminal neurons with craniovascular input. Neuroscience. 2003;122:491‐498. [DOI] [PubMed] [Google Scholar]
- 74. Goadsby PJ, Hoskin KL. Serotonin inhibits trigeminal nucleus activity evoked by craniovascular stimulation through a 5HT1B/1D receptor: a central action in migraine? Ann Neurol. 1998;43:711‐718. [DOI] [PubMed] [Google Scholar]
- 75. Hamel E. The biology of serotonin receptors: focus on migraine pathophysiology and treatment. Can J Neurol Sci. 1999;26(suppl. 3):S2‐S6. [DOI] [PubMed] [Google Scholar]
- 76. Schmuck K, Ullmer C, Kalkman HO, Probst A, Lubbert H. Activation of meningeal 5‐HT2B receptors: an early step in the generation of migraine headache? Eur J Neurosci. 1996;8:959‐967. [DOI] [PubMed] [Google Scholar]
- 77. Srikiatkhachorn A, Suwattanasophon C, Ruangpattanatawee U, Phansuwan‐Pujito P. 2002 Wolff Award. 5‐HT2A receptor activation and nitric oxide synthesis: a possible mechanism determining migraine attacks. Headache. 2002;42:566‐574. [DOI] [PubMed] [Google Scholar]
- 78. Villalón CM, Sánchez‐López A, Centurión D. Operational characteristics of the 5‐HT1‐like receptors mediating external carotid vasoconstriction in vagosympathectomized dogs. Close resemblance to the 5‐HT1D receptor subtype. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:550‐556. [DOI] [PubMed] [Google Scholar]
- 79. Ferrari MD, Färkkilä M, Reuter U, et al. Acute treatment of migraine with the selective 5‐HT1F receptor agonist lasmiditan—a randomised proof‐of‐concept trial. Cephalalgia. 2010;30:1170‐1178. [DOI] [PubMed] [Google Scholar]
- 80. Cook RO, Shrewsbury SB, Ramadan NM. Reduced adverse event profile of orally inhaled DHE (MAP0004) vs IV DHE: potential mechanism. Headache. 2009;49:1423‐1434. [DOI] [PubMed] [Google Scholar]
- 81. Krobert KA, Bach T, Syversveen T, Kvingedal AM, Levy FO. The cloned human 5‐HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:620‐632. [DOI] [PubMed] [Google Scholar]
- 82. De Ponti F. Pharmacology of serotonin: what a clinician should know. Gut. 2004;53:1520‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Glatzle J, Sternini C, Robin C, et al. Expression of 5‐HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002;123:217‐226. [DOI] [PubMed] [Google Scholar]
- 84. Fiorica‐Howells E, Hen R, Gingrich J, Li Z, Gershon MD. 5‐HT2A receptors: location and functional analysis in intestines of wild‐type and 5‐HT2A knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G877‐G893. [DOI] [PubMed] [Google Scholar]
- 85. Segelcke D, Messlinger K. Putative role of 5‐HT2B receptors in migraine pathophysiology. Cephalalgia. 2016;37:365‐371. [DOI] [PubMed] [Google Scholar]
- 86. Nasser Y, Ho W, Sharkey KA. Distribution of adrenergic receptors in the enteric nervous system of the guinea pig, mouse, and rat. J Comp Neurol. 2006;495:529‐553. [DOI] [PubMed] [Google Scholar]
- 87. Valet P, Senard JM, Devedjian JC, et al. Characterization and distribution of alpha 2‐adrenergic receptors in the human intestinal mucosa. J Clin Invest. 1993;91:2049‐2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. De Ponti F, Giaroni C, Cosentino M, Lecchini S, Frigo G. Adrenergic mechanisms in the control of gastrointestinal motility: from basic science to clinical applications. Pharmacol Ther. 1996;69:59‐78. [DOI] [PubMed] [Google Scholar]
- 89. Andersson KE, Vinge E. β‐Adrenoceptor blockers and calcium antagonists in the prophylaxis and treatment of migraine. Drugs. 1990;39:355‐373. [DOI] [PubMed] [Google Scholar]
- 90. van de Ven LL, Franke CL, Koehler PJ. Prophylactic treatment of migraine with bisoprolol: a placebo‐controlled study. Cephalalgia. 1997;17:596‐599. [DOI] [PubMed] [Google Scholar]
- 91. Worz R, Reinhardt‐Benmalek B, Grotemeyer K‐H, Foh M. Bisoprolol and metoprolol in the prophylactic treatment of migraine with and without aura—a randomized double‐blind cross‐over multicenter study. Cephalalgia. 1991;11:152‐153. [Google Scholar]
- 92. Roberts SJ, Papaioannou M, Evans BA, Summers RJ. Characterization of beta‐adrenoceptor mediated smooth muscle relaxation and the detection of mRNA for beta1‐, beta2‐ and beta3‐adrenoceptors in rat ileum. Br J Pharmacol. 1999;127:949‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Anthony A, Schepelmann S, Guillaume JL, et al. Localization of the β(beta)3‐adrenoceptor in the human gastrointestinal tract: an immunohistochemical study. Aliment Pharmacol Ther. 1998;12:519‐525. [DOI] [PubMed] [Google Scholar]
- 94. Masterson CG, Durham PL. DHE repression of ATP‐mediated sensitization of trigeminal ganglion neurons. Headache. 2010;50:1424‐1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Baglole CJ, Davison JS, Meddings JB. Epithelial distribution of neural receptors in the guinea pig small intestine. Can J Physiol Pharmacol. 2005;83:389‐395. [DOI] [PubMed] [Google Scholar]
- 96. Li ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild‐type and knock‐out mice. J Neurosci. 2006;26:2798‐2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vaughan CJ, Aherne AM, Lane E, Power O, Carey RM, O'Connell DP. Identification and regional distribution of the dopamine D(1A) receptor in the gastrointestinal tract. Am J Physiol Regul Integr Comp Physiol. 2000;279:R599‐R609. [DOI] [PubMed] [Google Scholar]
- 98. Li Y, Zhang Y, Zhang XL, et al. Dopamine promotes colonic mucus secretion through dopamine D(5) receptor in rats. Am J Physiol Cell Physiol. 2019;316:C393‐C403. [DOI] [PubMed] [Google Scholar]
- 99. Barbanti P, Bronzetti E, Ricci A, et al. Increased density of dopamine D5 receptor in peripheral blood lymphocytes of migraineurs: a marker for migraine? Neurosci Lett. 1996;207:73‐76. [DOI] [PubMed] [Google Scholar]
- 100. Charbit AR, Akerman S, Goadsby PJ. Comparison of the effects of central and peripheral dopamine receptor activation on evoked firing in the trigeminocervical complex. J Pharmacol Exp Ther. 2009;331:752‐763. [DOI] [PubMed] [Google Scholar]
- 101. Charbit AR, Akerman S, Goadsby PJ. Dopamine: what's new in migraine? Curr Opin Neurol. 2010;23:275‐281. [DOI] [PubMed] [Google Scholar]
- 102. de Sousa SC, Karwautz A, Wöber C, et al. A dopamine D4 receptor exon 3 VNTR allele protecting against migraine without aura. Ann Neurol. 2007;61:574‐578. [DOI] [PubMed] [Google Scholar]
- 103. DaSilva AF, Nascimento TD, Jassar H, et al. Dopamine D2/D3 imbalance during migraine attack and allodynia in vivo. Neurology. 2017;88:1634‐1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Barbanti P, Fabbrini G, Ricci A, et al. Migraine patients show an increased density of dopamine D3 and D4 receptors on lymphocytes. Cephalalgia. 2000;20:15‐19. [DOI] [PubMed] [Google Scholar]
- 105. Peroutka SJ, Wilhoit T, Jones K. Clinical susceptibility to migraine with aura is modified by dopamine D2 receptor (DRD2) NcoI alleles. Neurology. 1997;49:201‐206. [DOI] [PubMed] [Google Scholar]
- 106. Marmura MJ. Use of dopamine antagonists in treatment of migraine. Curr Treat Options Neurol. 2012;14:27‐35. [DOI] [PubMed] [Google Scholar]
- 107. Cottrell GS, Alemi F, Kirkland JG, Grady EF, Corvera CU, Bhargava A. Localization of calcitonin receptor‐like receptor (CLR) and receptor activity‐modifying protein 1 (RAMP1) in human gastrointestinal tract. Peptides. 2012;35:202‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Moore EL, Salvatore CA. Targeting a family B GPCR/RAMP receptor complex: CGRP receptor antagonists and migraine. Br J Pharmacol. 2012;166:66‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hay DL, Walker CS. CGRP and its receptors. Headache. 2017;57:625‐636. [DOI] [PubMed] [Google Scholar]
- 110. Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381:2230‐2241. [DOI] [PubMed] [Google Scholar]
- 111. Dodick DW. CGRP ligand and receptor monoclonal antibodies for migraine prevention: evidence review and clinical implications. Cephalalgia. 2019;39:445‐458. [DOI] [PubMed] [Google Scholar]
- 112. Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene‐related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381:142‐149. [DOI] [PubMed] [Google Scholar]
- 113. Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6:573‐582. [DOI] [PubMed] [Google Scholar]
- 114. Edvinsson L, Ho TW. CGRP receptor antagonism and migraine. Neurotherapeutics. 2010;7:164‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mulderry PK, Ghatei MA, Spokes RA, et al. Differential expression of alpha‐CGRP and beta‐CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience. 1988;25:195‐205. [DOI] [PubMed] [Google Scholar]
- 116. Sternini C, Anderson K. Calcitonin gene‐related peptide‐containing neurons supplying the rat digestive system: differential distribution and expression pattern. Somatosens Mot Res. 1992;9:45‐59. [DOI] [PubMed] [Google Scholar]
- 117. Zinni M, Zuena AR, Marconi V, et al. Maternal exposure to low levels of corticosterone during lactation protects adult rat progeny against TNBS‐induced colitis: a study on GR‐mediated anti‐inflammatory effect and prokineticin system. PLoS One. 2017;12:e0173484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. de Punder K, Pruimboom L. Stress induces endotoxemia and low‐grade inflammation by increasing barrier permeability. Front Immunol. 2015;6:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183‐187. [DOI] [PubMed] [Google Scholar]
- 120. Edvinsson L. Sensory nerves in man and their role in primary headaches. Cephalalgia. 2001;21:761‐764. [DOI] [PubMed] [Google Scholar]
- 121. Mönnikes H, van der Voort IR, Wollenberg B, et al. Gastric perception thresholds are low and sensory neuropeptide levels high in Helicobacter pylori‐positive functional dyspepsia. Digestion. 2005;71:111‐123. [DOI] [PubMed] [Google Scholar]
- 122. Fischer JA, Born W. Novel peptides from the calcitonin gene: expression, receptors and biological function. Peptides. 1985;6(suppl. 3):265‐271. [DOI] [PubMed] [Google Scholar]
- 123. Holzer P, Farzi A. Neuropeptides and the microbiota‐gut‐brain axis. Adv Exp Med Biol. 2014;817:195‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Aurora SK, Papapetropoulos S, Kori SH, Kedar A, Abell TL. Gastric stasis in migraineurs: etiology, characteristics, and clinical and therapeutic implications. Cephalalgia. 2013;33:408‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Burgos‐Vega C, Moy J, Dussor G. Meningeal afferent signaling and the pathophysiology of migraine. Prog Mol Biol Transl Sci. 2015;131:537‐564. [DOI] [PubMed] [Google Scholar]
- 126. Xiao Y, Richter JA, Hurley JH. Release of glutamate and CGRP from trigeminal ganglion neurons: role of calcium channels and 5‐HT1 receptor signaling. Mol Pain. 2008;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. van Lelyveld N, Ter Linde J, Schipper M, Samsom M. Serotonergic signalling in the stomach and duodenum of patients with gastroparesis. Neurogastroenterol Motil. 2008;20:448‐455. [DOI] [PubMed] [Google Scholar]
- 128. Evans RW, Whyte C. Cyclic vomiting syndrome and abdominal migraine in adults and children. Headache. 2013;53:984‐993. [DOI] [PubMed] [Google Scholar]
- 129. Taché Y, Martinez V, Million M, Rivier J. Corticotropin‐releasing factor and the brain‐gut motor response to stress. Can J Gastroenterol. 1999;13(suppl. A):18a‐25a. [DOI] [PubMed] [Google Scholar]
- 130. Reddymasu SC, Bonino J, McCallum RW. Gastroparesis secondary to a demyelinating disease: a case series. BMC Gastroenterol. 2007;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Raybould HE, Glatzle J, Robin C, et al. Expression of 5‐HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2003;284:G367‐G372. [DOI] [PubMed] [Google Scholar]
- 132. Waelkens J. Dopamine blockade with domperidone: bridge between prophylactic and abortive treatment of migraine? A dose‐finding study. Cephalalgia. 1984;4:85‐90. [DOI] [PubMed] [Google Scholar]
- 133. Brogden RN, Carmine AA, Heel RC, Speight TM, Domperidone AGS. A review of its pharmacological activity, pharmacokinetics and therapeutic efficacy in the symptomatic treatment of chronic dyspepsia and as an antiemetic. Drugs. 1982;24:360‐400. [DOI] [PubMed] [Google Scholar]
- 134. Acosta A, Camilleri M. Prokinetics in gastroparesis. Gastroenterol Clin North Am. 2015;44:97‐111. [DOI] [PubMed] [Google Scholar]
- 135. Friedman BW, Mulvey L, Esses D, et al. Metoclopramide for acute migraine: a dose‐finding randomized clinical trial. Ann Emerg Med. 2011;57:475‐482.e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Shakhatreh M, Jehangir A, Malik Z, Parkman HP. Metoclopramide for the treatment of diabetic gastroparesis. Expert Rev Gastroenterol Hepatol. 2019;13:711‐721. [DOI] [PubMed] [Google Scholar]
- 137. Abrahamsson H, Dotevall G. Effects of propranolol on colonic pressure in patients with irritable bowel syndrome. Scand J Gastroenterol. 1981;16:1021‐1024. [DOI] [PubMed] [Google Scholar]
- 138. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1‐18. [DOI] [PubMed] [Google Scholar]
- 139. Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for functional gastrointestinal disorders (disorders of gut‐brain interaction): a Rome Foundation working team report. Gastroenterology. 2018;154:1140‐1171.e1141. [DOI] [PubMed] [Google Scholar]
- 140. Silberstein SD. Preventive migraine treatment. Continuum (Minneap Minn). 2015;21:973‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Mwamburi M, Liebler EJ, Staats PS. Patient experience with non‐invasive vagus nerve stimulator: gammaCore patient registry. Am J Manag Care. 2020;26:S15‐S19. [DOI] [PubMed] [Google Scholar]
- 142. Paulon E, Nastou D, Jaboli F, Marin J, Liebler E, Epstein O. Proof of concept: short‐term non‐invasive cervical vagus nerve stimulation in patients with drug‐refractory gastroparesis. Frontline Gastroenterol. 2017;8:325‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gottfried‐Blackmore A, Adler EP, Fernandez‐Becker N, Clarke J, Habtezion A, Nguyen L. Open‐label pilot study: non‐invasive vagal nerve stimulation improves symptoms and gastric emptying in patients with idiopathic gastroparesis. Neurogastroenterol Motil. 2020;32:e13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sprouse Blum AS, Lavoie B, Haag M, et al. No gastrointestinal dysmotility in transgenic mouse models of migraine. Headache. 2020;60:396‐404. [DOI] [PubMed] [Google Scholar]
- 145. Tang Y, Liu S, Shu H, Yanagisawa L, Tao F. Gut microbiota dysbiosis enhances migraine‐like pain via TNFα upregulation. Mol Neurobiol. 2020;57:461‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Burlen J, Runnels M, Mehta M, et al. Efficacy of gastric electrical stimulation for gastroparesis: US/European comparison. Gastroenterol Res. 2018;11:349‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592‐1622. [DOI] [PubMed] [Google Scholar]
- 148. Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient‐assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141‐150. [DOI] [PubMed] [Google Scholar]
- 149. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670‐680. [DOI] [PubMed] [Google Scholar]
- 150. Jung HK, Choung RS, Locke GR, 3rd , et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rey E, Choung RS, Schleck CD, Zinsmeister AR, Talley NJ, Locke GR, 3rd . Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ibele AR, Gould JC, eds. Gastroparesis: A Comprehensive Approach to Evaluation and Management. Cham, Switzerland: Springer Nature Switzerland AG; 2020. [Google Scholar]
- 153. Kaufman J, Levine I. Acute gastric dilatation of stomach during attack of migraine. Radiology. 1936;27:301‐302. [Google Scholar]
- 154. Carstairs LS. Headache and gastric emptying time. Proc R Soc Med. 1958;51:790‐793; discussion 791‐793. [PMC free article] [PubMed] [Google Scholar]
- 155. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58:496‐505. [DOI] [PubMed] [Google Scholar]
- 156. Fuldeore MJ, Soliman AM. Prevalence and symptomatic burden of diagnosed endometriosis in the United States: national estimates from a cross‐sectional survey of 59,411 women. Gynecol Obstet Invest. 2017;82:453‐461. [DOI] [PubMed] [Google Scholar]
- 157. Valdez AR, Hancock EE, Adebayo S, et al. Estimating prevalence, demographics, and costs of ME/CFS using large scale medical claims data and machine learning. Front Pediatr. 2019;6:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F. The prevalence and characteristics of fibromyalgia in the 2012 national health interview survey. PLoS One. 2015;10:e0138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. J Urol. 2013;189:141‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Talley NJ, Walker MM, Holtmann G. Functional dyspepsia. Curr Opin Gastroenterol. 2016;32:467‐473. [DOI] [PubMed] [Google Scholar]
- 162. Mahadeva S, Ford AC. Clinical and epidemiological differences in functional dyspepsia between the East and the West. Neurogastroenterol Motil. 2016;28:167‐174. [DOI] [PubMed] [Google Scholar]
- 163. Pucci E, Di Stefano M, Miceli E, Corazza GR, Sandrini G, Nappi G. Patients with headache and functional dyspepsia present meal‐induced hypersensitivity of the stomach. J Headache Pain. 2005;6:223‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lee KJ. Gastric emptying in patients with functional dyspepsia and patients with migraine. J Neurogastroenterol Motil. 2013;19:116‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bhandari S, Venkatesan T. Clinical characteristics, comorbidities and hospital outcomes in hospitalizations with cyclic vomiting syndrome: a nationwide analysis. Dig Dis Sci. 2017;62:2035‐2044. [DOI] [PubMed] [Google Scholar]
- 166. Ellingsen DM, Garcia RG, Lee J, et al. Cyclic vomiting syndrome is characterized by altered functional brain connectivity of the insular cortex: a cross‐comparison with migraine and healthy adults. Neurogastroenterol Motil. 2017;29:e13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Rapoport AM, Freitag F, Pearlman SH. Innovative delivery systems for migraine: the clinical utility of a transdermal patch for the acute treatment of migraine. CNS Drugs. 2010;24:929‐940. [DOI] [PubMed] [Google Scholar]
- 168. Silberstein SD. Migraine symptoms: results of a survey of self‐reported migraineurs. Headache. 1995;35:387‐396. [DOI] [PubMed] [Google Scholar]
- 169. Tokola RA, Neuvonen PJ. Effect of migraine attacks on paracetamol absorption. Br J Clin Pharmacol. 1984;18:867‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Tokola RA, Neuvonen PJ. Effects of migraine attack and metoclopramide on the absorption of tolfenamic acid. Br J Clin Pharmacol. 1984;17:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Thomsen LL, Dixon R, Lassen LH, et al. 311C90 (zolmitriptan), a novel centrally and peripheral acting oral 5‐hydroxytryptamine‐1D agonist: a comparison of its absorption during a migraine attack and in a migraine‐free period. Cephalalgia. 1996;16:270‐275. [DOI] [PubMed] [Google Scholar]
- 172. Schulman EA, Dermott KF. Sumatriptan plus metoclopramide in triptan‐nonresponsive migraineurs. Headache. 2003;43:729‐733. [DOI] [PubMed] [Google Scholar]
- 173. Krymchantowski AV, Filho PF, Bigal ME. Rizatriptan vs. rizatriptan plus trimebutine for the acute treatment of migraine: a double‐blind, randomized, cross‐over, placebo‐controlled study. Cephalalgia. 2006;26:871‐874. [DOI] [PubMed] [Google Scholar]
- 174. Sakamoto Y, Sekino Y, Yamada E, et al. Effect of sumatriptan on gastric emptying: a crossover study using the BreathID system. World J Gastroenterol. 2012;18:3415‐3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Houghton LA, Fowler P, Keene ON, Read NW. Effect of sumatriptan, a new selective 5HT1‐like agonist, on liquid gastric emptying in man. Aliment Pharmacol Ther. 1992;6:685‐691. [DOI] [PubMed] [Google Scholar]
- 176. Yuan H, Spare NM, Silberstein SD. Targeting CGRP for the prevention of migraine and cluster headache: a narrative review. Headache. 2019;59(suppl. 2):20‐32. [DOI] [PubMed] [Google Scholar]
- 177. Rapoport AM. How to Choose a Preventive Medication for Migraine; 2018. https://americanheadachesociety.org/wp‐content/uploads/2018/05/Alan_Rapoport_‐_Migraine_Prevention_Medications.pdf. Accessed July 2, 2020. [Google Scholar]


