Summary
Background
Gastroparesis is defined by delayed gastric emptying with associated symptoms in the absence of mechanical obstruction.
Aim
To evaluate pharmacokinetics and pharmacodynamics of felcisetrag, a highly selective 5‐HT4 receptor agonist, on total gut transit in patients with documented delayed gastric emptying of solids.
Methods
Single‐centre, placebo‐controlled study of 36 participants receiving placebo, 0.1mg, 0.3mg or 1.0mg of felcisetrag I.V. infusion, daily, for 3 days. At baseline, each participant completed a 4h, 99mTc‐egg meal (300 kcal, 30% fat) gastric emptying test. Following infusion (Day 2), gastric, small bowel and colonic transit of solids were measured over 48h (same meal plus 111In‐charcoal delivered in methacrylate‐coated capsule). Samples were collected for pharmacokinetics. The primary endpoint was gastric emptying T1/2. Statistical analysis used baseline parameters as covariates (ANCOVA).
Results
Patients (22 idiopathic, 14 diabetic gastroparesis) were randomised to felcisetrag (0.1 mg, n = 10; 0.3 mg, n = 9; 1.0 mg, n = 7) or placebo (n = 10). Compared to placebo, felcisetrag significantly accelerated gastric emptying T 1/2, colonic filling at 6h, and 10% small bowel transit time (overall P < 0.01; all three doses individually Bonferroni corrected P < 0.05) for all three measurements. Ascending colon emptying (T 1/2) was significantly accelerated (all doses), and colonic transit at 48 hours was accelerated with 0.1 mg and 0.3 mg felcisetrag compared to placebo. Pharmacokinetic results were dose proportional. Felcisetrag was well tolerated with no clinically significant findings from clinical laboratory, vital signs or ECG.
Conclusion
I.V. felcisetrag significantly accelerated gastric, small bowel and colonic transit in patients with gastroparesis, and should be further evaluated for short‐term treatment of gastric and intestinal motility disorders.
ClinicalTrials.gov #NCT03281577
Examples of gastric content at 120 minutes after meal ingestion in the 4 treatment groups.
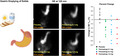
1. INTRODUCTION
Gastroparesis is a chronic gastrointestinal disorder affecting stomach motility. 1 It is defined by delayed gastric emptying with associated symptoms in the absence of mechanical obstruction. 1 , 2 Symptoms of gastroparesis include nausea, vomiting, early satiety, postprandial fullness, upper abdominal pain and bloating, which are known as the cardinal symptoms. 2 , 3 Among 1287 adults presenting with upper gastrointestinal symptoms, Park et al. demonstrated that there were about a quarter each with abnormal gastric emptying, or abnormal gastric accommodation, or a combination of the two motor dysfunctions; 4 a subset of 108 patients with diabetes showed similar proportions of abnormal gastric functions. 5
Prokinetics are frequently used treatments for a variety of chronic, slow motility‐related disorders (e.g. constipation, constipation‐predominant irritable bowel syndrome, gastroparesis). Patients with gastroparesis may experience acute exacerbations resulting in vomiting that may necessitate hospitalization; 6 such patients may benefit from availability of an effective parenterally administered prokinetic agent. Current prokinetic agents such as dopamine D2 antagonists, metoclopramide or domperidone, and macrolide antibiotics may be limited by adverse events, including neurological adverse events such as akathisia and tardive dyskinesia with metoclopramide; risks of prolongation of corrected QT (QTc) interval and sudden cardiac death with domperidone; and tachyphylaxis with erythromycin. 7 There is an unmet need for effective prokinetic agents, particularly for acute exacerbations that preclude oral administration of such agents.
Another class of drugs used in the treatment of motility disorders is the 5‐hydroxytryptamine‐4 (5‐HT4) receptor agonists. 8 , 9 5‐HT4 agonists vary in chemical structure and selectivity for the 5‐HT4 receptors in the gastrointestinal tract. 9 , 10 Highly selective and potent 5HT4 agonists are less likely to cause cardiac conduction abnormalities, for which cisapride, a non‐specific 5HT4 agonist, was withdrawal from the market for all indications. 11 Prucalopride, a highly specific 5HT4 agonist, significantly enhanced gastric emptying, small bowel transit and colonic transit in patients with chronic constipation. 12 5‐HT4a, 4b and 4c receptors are abundantly, but differentially, expressed throughout the gut. Felcisetrag has about 10‐ to 100‐fold greater affinity for human 5‐HT4 receptor splice variants (5‐HT4a, 4b and 4c) compared to prucalopride (P. Wade, personal communication, 13 October 2020).
Treatment of gastroparesis can be challenging due to limited availability of approved or efficacious therapeutic options despite the use of dietary changes, vitamin and nutrition supplementation, anti‐emetics and prokinetic agents. 2 Although not approved for treatment of gastroparesis, recent data from a small cross‐over study suggest that prucalopride significantly enhances gastric emptying and decreases symptoms in patients with idiopathic gastroparesis. 13
Felcisetrag is a highly selective and potent 5‐HT4 receptor agonist with prokinetic activity throughout the gastrointestinal tract in experimental models. 11 , 14 , 15 After intravenous administration in healthy volunteers and in treatment of enteral feeding intolerance in critically ill patients, felcisetrag demonstrated prokinetic activity when administered over a short term. 16 As an intravenously administered drug, felcisetrag may be useful in treating acute gastrointestinal motility disorders such as post‐operative gastrointestinal dysfunction, acute exacerbations of gastroparesis (which may result in hospitalization) 6 , 17 and enteral feeding intolerance in critically ill patients.
The aim of this study was to evaluate the pharmacokinetics and pharmacodynamics effects of felcisetrag on gastric, small intestinal and colonic transit in patients with diabetes reporting symptoms of gastroparesis, or in patients with idiopathic gastroparesis; both groups having previously documented delay in gastric emptying of solids. Patients with diabetes and constipation may require prokinetic treatment, as has been demonstrated using the acetylcholinesterase inhibitor, pyridostigmine. 18 It is interesting to note that, in that study, about 37% (11/30) of the patients also had delayed gastric emptying (T1/2 >150 minutes) measured by the same scintigraphic method used in the current study. Therefore, demonstration of the prokinetic effects of felcisetrag through the different regions of the gastrointestinal tract would be useful to assess its potential for slow transit disorders in the entire gut.
2. MATERIALS AND METHODS
2.1. Study design and ethical approval
This was a phase IIa dose‐ranging, randomised, parallel‐group, double‐blind, placebo‐controlled study conducted at a single medical centre. The study was approved by Mayo Clinic Institutional Review Board (IRB #17‐006648). Participants were randomised (based on computer‐generated schema) to felcisetrag 0.1, 0.3 or 1.0 mg, or placebo, given as a 60‐minute i.v. infusion once a day for 3 days. The random allocation sequence was retained in the Mayo Clinic Research Pharmacy, and all study personnel and participants were blinded to treatment allocation. Interventions were assigned according to randomisation by study pharmacist. Both interventions appeared identical.
2.2. Patients
Patients with diabetes reporting symptoms of gastroparesis with previously documented delayed gastric emptying, or patients with a diagnosis of idiopathic gastroparesis were eligible for this study. Males or non‐pregnant, non‐breastfeeding females, between 18 and 65 years of age, with a body mass index (BMI) ≥16 and ≤40 kg/m2 were included. Patients were enrolled by study coordinator (KA) and fellows (VC, JB, PV, XJW). Patients presented to the Clinical Research Trials Unit (CRTU) at Mayo Clinic in Rochester, MN for screening history, physical examination and baseline evaluation. During treatment, patient's recorded symptoms of gastroparesis 19 and bowel function based on a daily stool diary. 20 Exclusion criteria were poorly controlled diabetes (HbA1c >12%), medications that alter gastrointestinal motility [opioids, antiemetics, prokinetics, stimulant laxatives, anticholinergics and glucagon‐like peptide‐1 (GLP‐1) analogues or agonists], other structural gastrointestinal diseases, abnormal baseline labs, underlying liver disease, QTcF ≥460 msec, significant cardiac, pulmonary, renal, haematological, neurological, psychiatric disease and substance use such as cannabis.
2.3. Patient and public involvement
Patients were first invited to participate in the research process by electronic or phone invitation or by public advertisement on the Mayo Clinic campus in Rochester, MN. The research question(s) and outcome measures were developed based on previously validated measurements of the transit of radiolabeled solids, as well as the use of validated instruments to appraise symptoms and bowel function as well as evidence that a significant change in the primary endpoint had been shown to be associated with clinical benefit. 21 The public was not involved in the design of this study; participants consented to be included, but they did not provide any feedback regarding the conduct of the study, or the burden of the intervention, or time required to participate in the research. When the study is published in peer‐reviewed literature, the study results will be disseminated to participants and to relevant wider patient communities.
2.4. Study protocol
The study was registered at ClinicalTrials.gov #NCT03281577. An overview of the study design is shown in Figure 1. The study consisted of three periods: screening, baseline and treatment, and a follow‐up phone call 10 to 14 days later; further details of the protocol are included in the Supplemental Materials.
FIGURE 1.
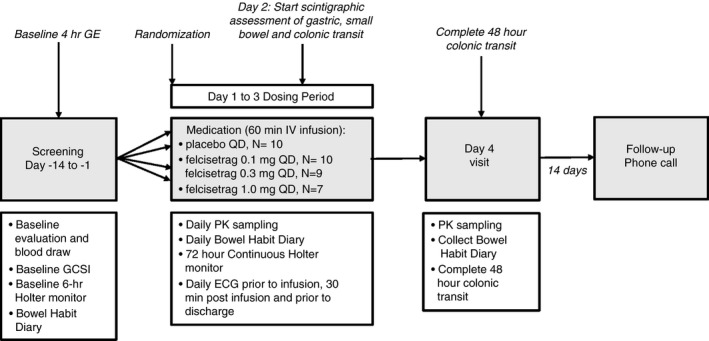
Study design
2.5. Gastric emptying of solids, small bowel and colonic transit by scintigraphy
Gastric emptying of solids was measured at baseline by scintigraphy. A radiolabelled meal [99mTc‐labelled technetium sulphur egg meal (296 kcal, 32% fat)] was ingested after a 12‐hour fast. Subsequently, abdominal images were obtained for 2 minutes in duration with anterior and posterior gamma camera at 0, 1, 2, 3 and 4 hours.
On Day 2, gastric emptying, and small bowel and colonic transit were assessed as in prior studies 20 , 21 , 22 , 23 , 24 , 25 , 26 (detailed in Supplemental Materials). Data analysis including gastric emptying, colonic filling at 6 hours, small bowel transit time, overall colonic transit time and ascending colon emptying T1/2 are included in the Supplemental Materials.
2.6. Pharmacokinetics measurements
Pharmacokinetics measurements were done on collected plasma samples obtained in the opposite arm from where the infusion was delivered. This was done at previously set intervals relative to the i.v. infusion on Days 1, 2, 3 and 4.
2.7. Endpoints
The primary endpoint was gastric emptying T 1/2. The secondary endpoints were small bowel transit (colonic filling at 6 hours and SBTT10%), colonic transit (ascending colon emptying T 1/2 and colonic geometric centre at 24 and 48 hours) and felcisetrag pharmacokinetics (T max, C max, AUC and clearance half‐time), safety and tolerability.
2.8. Pharmacokinetics
Plasma samples were analysed for felcisetrag using validated liquid chromatography with tandem mass spectrometry methods. The lower limit of quantification for felcisetrag is 5.00 pg/mL
2.9. Statistical analysis
2.9.1. Pharmacokinetics
Pharmacokinetic parameters were determined from the concentration‐time data for all evaluable subjects using non‐compartmental analysis. Actual sampling times, rather than scheduled sampling times, were used in all pharmacokinetic computations involving sampling times for plasma pharmacokinetic parameters. The following pharmacokinetic parameters were calculated: AUCτ: Days 1 to 3; C max: Days 1 to 3; and C trough: Days 2, 3 and 4.
The plasma concentrations of felcisetrag were summarized by treatment group over each scheduled sampling time point using descriptive statistics. In addition, the mean plasma concentrations of felcisetrag vs time were generated by treatment group.
2.9.2. Pharmacodynamics
The statistical analysis by intention‐to‐treat population used baseline parameters (where available) as covariates (ANCOVA). ANCOVA was used to assess the effect of treatment on the T1/2 of gastric emptying of solids, including the following as covariates: gastroparesis type (diabetic or idiopathic), age, gender, BMI and the baseline measurement of gastric emptying T1/2. Multiplicity adjusted and unadjusted 95% two‐sided confidence intervals were presented. Normality was tested using the Shapiro‐Wilk W test with criteria <0.01.
The effects of treatment on the secondary endpoints were also assessed using an ANCOVA model. The covariates considered for inclusion in the analyses were gastroparesis type (diabetic or idiopathic), age, gender, BMI and the baseline measurement of the respective endpoint. Dunnett’s test, multiplicity adjusted and unadjusted 95% two‐sided confidence intervals, and Kruskal‐Wallis test were used as outlined for primary endpoint.
2.9.3. Pharmacokinetics‐pharmacodynamics relationship
Felcisetrag gastric half‐emptying time models were estimated using the NLME Library (version 3.1‐140) in R (version 3.6.1). The best model was selected by Akaike’s Information Criterion (AIC). Colonic geometric centre models were estimated using the BRMS Library (version 2.10.0) in R (version 3.6.1). The best model was selected by widely accepted information criterion (WAIC). Final models were evaluated by visual predictive check.
2.9.4. Safety
Safety analyses included adverse events, clinical laboratory evaluations, vital sign results and 12‐lead ECG results. All summaries of safety data were based on patients in the safety set.
2.9.5. Patient reported outcomes
Information on the global score using the Gastroparesis Cardinal Symptom Index (GCSI), as well as bowel movement frequency per day and average stool consistency on the Bristol Stool Form Scale were tabulated and analysed.
2.9.6. Sample size considerations
Table S1 summarises data for the primary and secondary PD response measures and uses the (relative) per cent coefficient of variation (% CV) to estimate the effect size detectable with 80% power, based on a two‐sample z‐test (i.e. assuming the variation values were known) at a two‐sided alpha level of 0.05. The effect size is the difference in group means as a percentage of the overall mean for each response and assumes 12 subjects per group. The ANCOVA provided 80% power to detect similar (pairwise) differences using a pooled estimate of variation across all three groups and potentially even smaller effect sizes by adjusting for important covariates. An effect size of at least 30% for the primary endpoint is considered to be clinically important.
2.9.7. Pre‐planned interim analysis
An interim analysis was conducted on 18 September 2018, after the first 18 patients had completed all assessments for the purpose of assessing the variance in responses and for re‐calculation of the effect sizes demonstrable with the observed variance and, therefore, a re‐assessment of number of participants in the study. The interim analysis was conducted independently of the entire clinical trial team, and the only communication received pertained to the number to be studied in each treatment arm; this was communicated to the Mayo Clinic Research Pharmacy. Table S1 shows the a priori power statement based on the variance used to plan the study with 12 patients per treatment arm, and the final power statement as a result of the interim analysis. Based on the results of the pre‐planned interim analysis, one of the felcisetrag doses stopped enrolling. The specific dose that stopped enrolling was not specified in order to maintain the blind of the study team.
2.10. Role of the funding source
This was a single‐centre study at Mayo Clinic in Rochester, MN. The trial was sponsored by Takeda Pharmaceuticals International Co., Cambridge, MA, USA. Felcisetrag was supplied by Takeda. The investigative team at one site in the United States was responsible for recruitment, enrolment and follow‐up of patients.
All authors had access to the study data and reviewed and approved the final manuscript.
3. RESULTS
3.1. Patient characteristics
Thirty‐six Caucasian patients (30 women, mean age 44.2 ± 12.5 years, and BMI 26.1 ± 5.2 kg/m2) entered and completed the study between January 2018 and July 2019. Twenty‐two patients had idiopathic gastroparesis and the other 14 had diabetic gastroparesis. Gastric half‐emptying time for solids was 186.3 ± 58.2 minutes at baseline. Table 1 summarizes the patient demographics.
TABLE 1.
Characteristics, changes in gastric emptying, small bowel and colonic transit as well as bowel functions in patients with diabetic or idiopathic gastroparesis based on treatment group (placebo vs increasing doses of felcisetrag)
| Data show mean ± SD unless otherwise specified | Placebo (n = 10) | Felcisetrag 0.1 mg (n = 10) | Felcisetrag 0.3 mg (n = 9) |
Felcisetrag 1.0 mg (n=7) |
||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (years) | 46.2 ± 15.7 | 46.8 ± 12.5 | 42.3 ± 11.8 | 40.3 ± 8.75 | ||
| Female sex n | 8 | 10 | 7 | 5 | ||
| Gastroparesis n (idiopathic/diabetic) | 7/3 | 7/3 | 3/6 | 5/2 | ||
| BMI (kg/m2) | 24.8 ± 4.9 | 28.4 ± 5.5 | 27.7 ± 5.4 | 22.6 ± 3.1 | ||
| Baseline GCSI score (scale 0‐5) | 2.6±1.0 | 2.5±.72 | 3.0±.66 | 3.2±.61 | ||
| Gastric emptying T 1/2 (min) compared to baseline after two doses of felcisetrag or placebo | ||||||
| Baseline | 206.3 ± 70.8 | 183.3 ± 43.9 | 178.4 ± 57.2 | 172.0 ± 61.9 | ||
| Post‐two doses of assigned Rx | 198.1 ± 35.0 | 142.1 ±20.2 | 128.7 ± 44.2, | 121.8 ± 28.8 | ||
| Percent Δ from baseline (%) | ||||||
| Least‐squares mean | −1.0 | −26.8 | −28.6 | −42.8 | ||
| Difference from placebo | −25.8 | −27.5 | −41.8 | |||
| 95% confidence interval | (−38.9, −12.7) | (−42.1, −13.0) | (−56.4, −27.1) | |||
| P value | <0.001 | <0.001 | <0.001 | |||
| Colonic and small bowel transit parameters | ||||||
| Colonic filling at 6 hours (%) | 31.3 ± 25.38 | 55.6 ± 31.31* | 86.4 ± 19.76*** | 75.3 ± 31.70** | ||
| Small bowel transit time 10%, min | 225.6 ± 78.3 | 145.7 ± 60.6* | 106.3 ± 48.9* | 135.3 ± 64.0* | ||
| T 1/2 of ascending colon emptying (h) | 19.1 ± 13.2 | 8.2 ± 8.28* | 5.0 ±3.98** | 7.6 ± 5.03* | ||
| Colonic geometric centre 24 h | 1.97 ± 1.30 | 3.79 ± 1.14** | 3.47 ± 1.33 | 2.98 ± 1.14 | ||
| Colonic geometric centre 48 h | 3.32 ± 1.29 | 4.41 ± 1.12* | 4.55 ± 0.90* | 3.79 ± 1.04 | ||
| P values * P < 0.05, **P < 0.01, ***P < 0.001 | ||||||
| Bowel functions [number and consistency; median (minimum, maximum)] | ||||||
| # BM/day (Baseline) | 1.33 (1.0, 7.5) | 1.0 (0.0, 4.0) | 1.5 (0.0, 4.0) | 1.5 (0.0, 3.7) | ||
| # BM/day (average 3 days’ Rx) | 0.7 (0.0, 6.3) | 2.0 (0.3, 5.0) | 2.0 (0.3, 3.3) | 2.0 (1.0, 2.7) | ||
| Baseline stool consistency scale | 4.9 (3.0, 7.0) | 1.0 (1.0, 6.5) | 2.9 (1.0, 7.0) | 1.4 (1.0, 5.9) | ||
| Stool consistency (average 3 days’ Rx) | 4.0 (2.0, 6.7) | 5.1 (1.0, 6.8) | 5.6 (1.0, 7.0) | 5.7 (4.9, 7.0) | ||
Δ, change; BM, bowel movements; BMI, body mass index; GCSI, Gastroparesis Cardinal Symptom Index; SD, standard deviation; T 1/2, half‐time; stool consistency based on Bristol Stool Form Scale; Rx, treatment.
3.2. Conduct of the study
Twenty‐six patients were randomised using a computer‐generated list to receive felcisetrag intravenously (n = 10 to 0.1 mg, n = 9 to 0.3 mg and n = 7 to 1.0 mg doses) from Days 1 to 3. Ten patients were randomised to receive placebo intravenously from Days 1 to 3. Baseline characteristics according to the treatment allocation group are summarised in Table 1. One patient dropped out of the study due to difficulty with i.v. access. Another patient was withdrawn from the study due to a serious adverse event (SAE) (abdominal pain and lipase elevation without evidence of pancreatitis). This patient had type 1 diabetes mellitus with a history of chronic abdominal pain as well as fluctuations in serum lipase prior to participation in this trial. The abdominal pain was not consistent with typical clinical features of pancreatitis (e.g. radiation to the back) and there was no evidence of pancreatitis on abdominal imaging in association with the pain experienced during the study. We have follow‐up information up to 1 year after the increase in serum lipase which occurred during the trial. He has undergone endoscopic ultrasound which did not reveal any structural abnormalities in the pancreas, and he has had further episodes of pain similar to that observed prior to, as well as during the trial. Therefore, it was deemed that this SAE was unrelated to felcisetrag. All other patients participated in the full study protocol as planned. Figure 2 shows the CONSORT flow chart.
FIGURE 2.
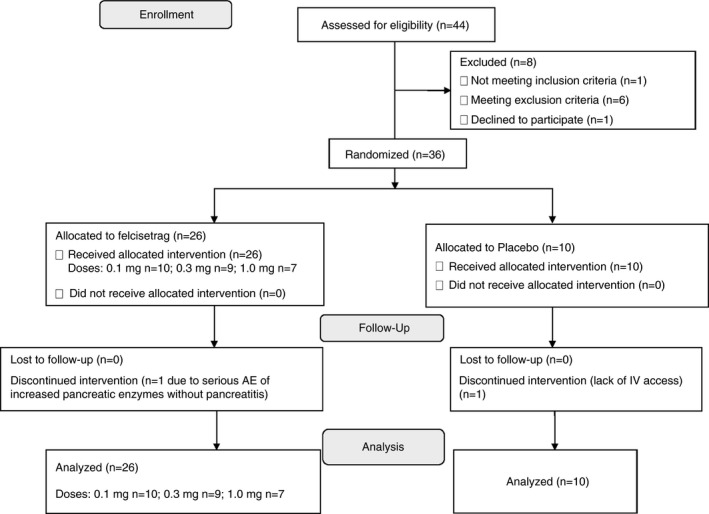
CONSORT flow diagram
3.3. Gastric emptying of solids (GE T 1/2) and small intestinal transit
At baseline, GE T 1/2 was similar across the four groups and was delayed in all groups (Table 1), consistent with a diagnosis of gastroparesis. After two i.v. doses of felcisetrag, GE T 1/2 was accelerated with all three doses of felcisetrag compared to placebo (Table 1). The absolute change (Table S2) and percent change in GE T1/2 were significantly more rapid than baseline across all doses compared to placebo (P < 0.001 for all dose groups compared to placebo (Table 1; Figures 3, 4, 5).
FIGURE 3.
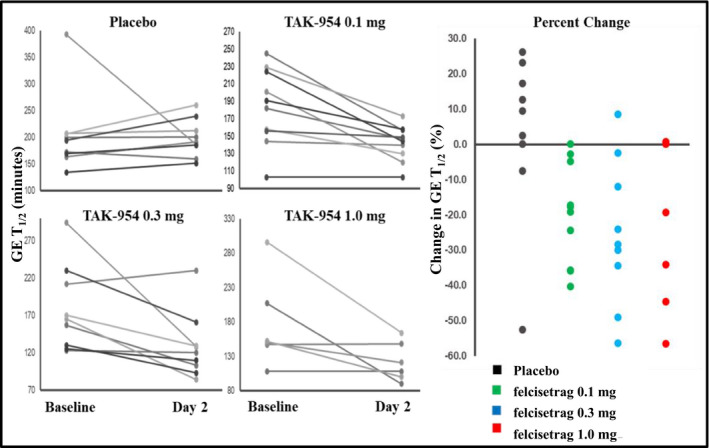
Effects of felcisetrag (0.1 mg, 0.3 mg, 1.0 mg) and placebo on GE T1/2 at baseline and Day 2, and percent change in GE T1/2 from baseline GE T1/2 = gastric half‐emptying time
FIGURE 4.
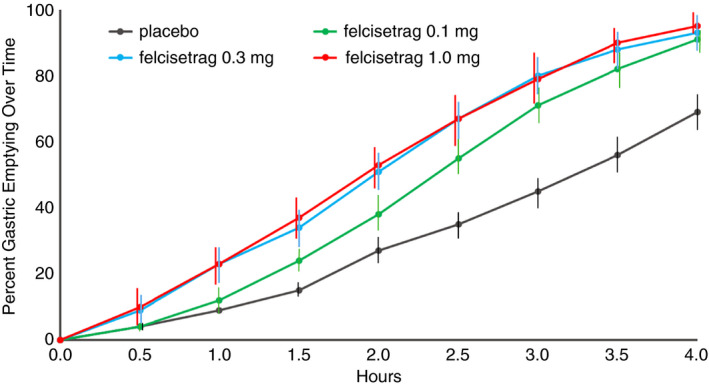
Gastric emptying of solids (% emptied) over time in patients with diabetic or idiopathic gastroparesis based on treatment group on Day 2 of placebo or increasing doses of felcisetrag; data show mean at each time point
FIGURE 5.
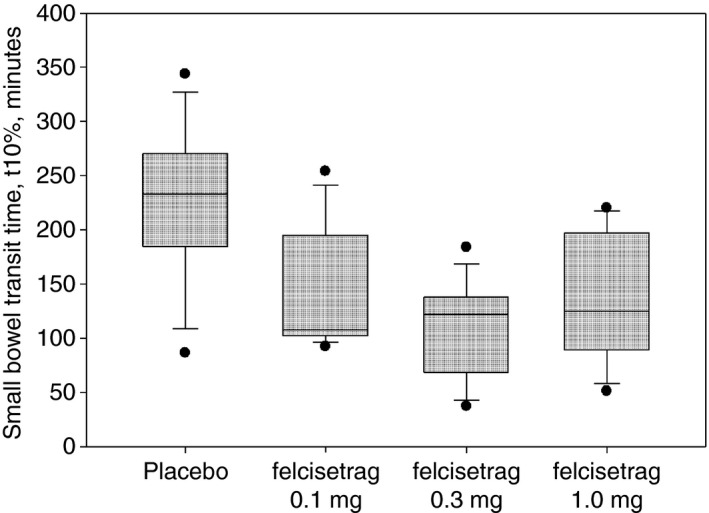
Small bowel transit time 10% in patients with diabetic or idiopathic gastroparesis based on treatment group on Day 2 of placebo or increasing doses of felcisetrag; data show median, IQR, time point
Furthermore, the effect on gastric emptying of solids was first noted after 30 minutes in the felcisetrag 0.3 mg and 1.0 mg groups, and after 60 minutes in the felcisetrag 0.1 mg group compared to placebo (Figure 4). The number of patients with more than 10% change (corresponding to an average ~20 minutes) from baseline in GE T 1/2 was also higher in all three treatment arms compared to placebo [placebo: 1/9, felcisetrag 0.1 mg: 7/10, felcisetrag 0.3 mg: 7/9, felcisetrag 1.0 mg: 4/6 (Figure 3)].
Table S2 shows the comparison between each dose of felcisetrag and placebo for the entire patient cohort as well as the groups with idiopathic or diabetic gastroparesis. While there appears to be greater efficacy, given the significant differences from placebo in the idiopathic gastroparesis group, we note the small sample size may compromise the ability to document acceleration of gastric emptying in the patients with diabetes and gastroparesis. Colonic filling at 6 hours was significantly increased overall and with all doses of felcisetrag compared to placebo (Table 1; P < 0.05).
Small bowel transit time 10% (SBTT10%) was also decreased overall and with all doses compared to placebo (Table 1; Figure 5; P < 0.05).
3.4. Colonic transit
The ascending colon emptying T1/2 was significantly accelerated with all three doses of felcisetrag compared to placebo (Table 1; P < 0.05).
Table 1 shows the numerical differences in colonic transit at 48 hours (GC, geometric center; GC48) in all four groups, with placebo having the lowest GC at all time points (Figure 6). We noted an effect on colonic transit starting at 30 minutes after i.v. infusion of felcisetrag in all three dose groups (Figure 4). Colonic transit at 24 hours (GC24) and GC48 was numerically greater than placebo at all three doses (Table 1). The difference in colonic transit was statistically significant for the felcisetrag 0.1 mg dose at 24 hours, and for the felcisetrag 0.1 mg and 0.3 mg dosing groups at 48 hours compared to placebo (Table 1). Changes in colonic transit were associated with increase in number of bowel movements and looser stool consistency (see Supplemental Material and Table 1).
FIGURE 6.
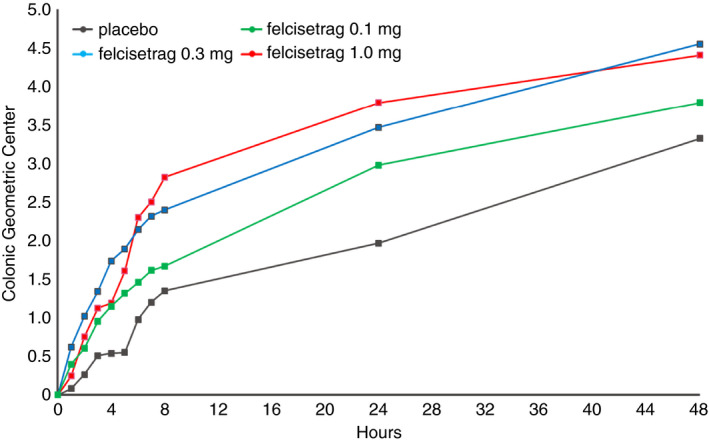
Progression of colonic geometric centre over time in patients with diabetic or idiopathic gastroparesis based on treatment group after 48 hours of placebo or increasing doses of felcisetrag; data show mean at each time point for the different treatment groups
3.5. Adverse events
Felcisetrag was well tolerated. There were no patient deaths. One patient withdrew due to poor i.v. access and one was withdrawn due to a serious adverse event (mild elevated pancreatic enzymes without evidence of pancreatitis; not drug related). Adverse events were mostly mild or moderate and frequency was generally comparable between felcisetrag doses and placebo (Tables S3 and S4).
A few patients had >30 milliseconds change in QTcF from baseline; however, none of those receiving felcisetrag had QTcF >500 milliseconds. There were no significant ECG changes in all treatment groups. One asymptomatic participant had a recorded QTcF >500 milliseconds, and this patient was eventually discovered to have been in the placebo group. The most common adverse events were diarrhoea and nausea, possibly secondary to enhanced pharmacologic effect in individual patients.
No clinically significant findings were identified from clinical laboratory results, vital signs or ECG.
3.6. Pharmacokinetics
The maximum plasma concentration (C max) of felcisetrag and the area under the curve (AUC τ) were similar from Day 1 to Day 3 at each dose level as seen in Figure 5. Both C max and AUCτ were approximately dose proportional at 0.1 mg, 0.3 mg, and 1 mg dose levels (Figure S1).
3.7. Pharmacokinetics and pharmacodynamics relationship
The felcisetrag/gastric half‐emptying model (Figure S2) was a mixed‐effects E max model with baseline effect. Felcisetrag enhanced gastric emptying with an Emax trend, suggesting that maximal receptor agonism was achieved over the dose range of 0.1–1.0 mg. The estimated concentration at the end of the infusion (C eoi) causing a half‐maximal decrease in gastric half‐emptying time (EC50) was 1262 pg/mL, similar to C max 1637 pg/mL observed for 0.1 mg dose. The 90% maximal effect (EC90) was 11 400 pg/mL, which was similar to the Cmax (16 029 pg/mL) observed for the 1.0 mg dose.
For colonic geometric centre modelling (Figure S3), the felcisetrag dose rather than exposure was selected for the model based on model parsimony and the fact that exposure metrics did not improve the model. The felcisetrag/colonic geometric centre model was a zero‐inflated beta‐regression model with dose‐ and time‐responsive effects on zero inflation, a time effect on the beta distribution shape and scale, and an E max effect of dose on the beta distribution scale. Felcisetrag dose responsively decreased the time in small bowel transit (decreasing zero inflation metric by a logit function of dose) and increased colonic transit velocity as measured by colonic geometric centre (by an E max function of dose where ED50 was <0.1 mg). All studied doses were at near maximal effect for colonic transit.
4. DISCUSSION
This dose‐ranging, phase 2 trial of the effects of felcisetrag in patients with diabetic or idiopathic gastroparesis has demonstrated that this 5‐HT4 receptor agonist is efficacious in accelerating GE T 1/2 across all doses compared to placebo. After two doses of felcisetrag, there was a significant improvement in GE T 1/2 and a significantly higher per cent change from baseline across all three doses compared to placebo. The exposure response modelling of gastric emptying confirms this dose relationship, as, even at the lowest dose of felcisetrag 0.1 mg, the C max at the end of the infusion approximated the EC50%, resulting in a significant effect on gastric emptying. In addition, the mean absolute change from baseline GE T1/2 at all three doses ranged from 41.2 to 54.8 minutes, and all were more than 20.4 minutes. According to a recent meta‐analysis, an acceleration in GE T 1/2 >20.4 minutes is associated with a clinically meaningful improvement in upper gastrointestinal symptoms. 21 The efficacy of felcisetrag on symptoms, for example in patients with acute exacerbations of gastroparesis, will require further studies focused on clinical patient response endpoints.
In addition, felcisetrag accelerated colonic filling at 6 hours (CF6) (a surrogate of small bowel and orocecal transit) and colonic transit at 24 and 48 hours, which suggests a role of felcisetrag on both upper and lower gastrointestinal transit. The acceleration in colonic transit, as measured by the geometric centre and ascending colon emptying T 1/2, was noted at the lowest dose of felcisetrag (0.1 mg), but there still was an effect at the 0.3 mg and 1.0 mg groups compared to placebo at 24 and 48 hours. There also was a change in the average number of bowel movements from baseline at 3 days, after three doses of felcisetrag, which is consistent with the accelerated colonic transit reported. Indeed, the modelling analysis suggests that there was no dose/exposure response and that all doses tested in the current study were active.
These findings are consistent with the pharmacokinetics properties of 5‐HT4 agonists and suggest a potential role for felcisetrag in improving regional and whole gut transit. The conduct of this study in patients with gastroparesis demonstrates the potential of felcisetrag in the context of a gastrointestinal motility disorders characterised by slow transit and, in the future, this may include conditions such as post‐operative ileus, acute exacerbation of gastroparesis or disorders of enteral feeding in the intensive care unit setting following appropriate clinical evaluation and regulatory approval.
Furthermore, felcisetrag is a highly selective 5‐HT4 receptor agonist. As such it would be expected to have a preferable safety profile. In this study, felcisetrag was well tolerated as a short‐term administration in patients with idiopathic or diabetic gastroparesis. Most of the adverse events reported were gastrointestinal symptoms such as nausea and diarrhoea, which are typically seen in patients using prokinetics like 5‐HT4 agonists. Most importantly, in this small study, there were no clinically significant cardiac effects of felcisetrag. This is an important point, since in developing a 5‐HT4 receptor agonist, the risk of cardiovascular adverse events is of serious concern. In the current study, while a few patients had >30 milliseconds change in QTcF from baseline, none of those receiving felcisetrag had a QTcF >500 milliseconds, and there were no significant ECG changes in all treatment groups.
The study did include a pre‐planned interim analysis which was performed for assessment of the variance in responses to the study medication, given that this was the first study conducted in humans evaluating gastrointestinal and colonic transit. Such variances in the measurements obtained on medication could not be predicted for the pre‐study power calculations. Thus, the a priori sample size calculation was based on the variance in the transit endpoints, based on a large sample size of healthy volunteers. After the first 18 patients had been studied, a statistical team, independent of the clinical research team at Mayo Clinic, conducted a further analysis of the observed variance in order to inform the research pharmacy on the number of participants that should be randomised to each of the four treatment groups. As a result of this interim analysis, enrolment into one of the felcisetrag doses was stopped. The specific dose that stopped enrolling was not specified in order to maintain the blinding of the entire clinical study team.
This study has limitations that impact its generalisability. First, the treatment period was short (3 days), but this is a phase 2 trial with a main goal to show acute pharmacodynamics effects of felcisetrag and safety in the gastroparesis cohort. Second, the study was not designed to assess the effects of the medication on symptoms, and the 3‐day treatment phase is clearly too short for patients to show improvement in symptoms of chronic gastroparesis with a prokinetic agent, rather than an agent directed to specific symptoms such as anti‐nausea or anti‐emetic agents. On the other hand, we know from the literature that an improvement of the magnitude observed in this study was >20.4 minutes in GE T1/2 that correlated well with a clinically meaningful improvement in upper gastrointestinal symptoms. The clinical relevance of the short‐lived acceleration of gastric emptying has been previously shown in critically ill patients requiring enteral feeding, where felcisetrag was beneficial in its effects on normalising gastric emptying compared to metoclopramide. 16 A third minor limitation is that the results of the current study are only applicable to patients with idiopathic or diabetic gastroparesis. These are, however, the predominant forms of gastroparesis encountered in clinical practice, and gastroparesis serves as a surrogate of impaired gastrointestinal motility and, therefore, is a useful model to assess clinical pharmacology of felcisetrag for such disorders. A fourth limitation is that the medication is administered i.v. which can limit its future utility to the inpatient settings or to patients with i.v. access. Future formulations of felcisetrag should focus on a more accessible route of administration such as the oral route.
In conclusion, felcisetrag, an i.v. administered, highly selective 5‐HT4 receptor agonist, significantly accelerated gastric emptying, orocecal as well as small bowel transit, and colonic transit compared to placebo in patients with gastroparesis with previously confirmed delayed gastric emptying. The pharmacokinetic/pharmacodynamic modelling supports the findings of this study and indicates that all doses investigated were sufficient to enhance gastric, small bowel and colonic transit. Felcisetrag was well tolerated, and its efficacy and safety should be further assessed in phase 2B and 3 trials. These results support further evaluation of felcisetrag for the treatment of gastric and intestinal motility disorders.
AUTHORSHIP
Guarantor of the article: Michael Camilleri is the guarantor of the article; he takes responsibility for the integrity of the work as a whole, from inception to published article.
Authors’ contributions: Victor Chedid: Fellow investigator who looked after patients in the clinical research unit; design of study. Justin Brandler: Fellow investigator who looked after patients in the clinical research unit. Kayla Arndt: Coordinator of all research studies and recruitment of patients. Priya Vijayvargiya: Fellow investigator who looked after patients in the clinical research unit. Xiao Jing Wang: Fellow investigator who looked after patients in the clinical research unit. Duane Burton: Lab manager and analysis of all gastrointestinal and colonic transit data. W. Scott Harmsen: Statistician; coordinator of randomization with Mayo Research Pharmacy. Jenifer Siegelman: assisting on protocol development and interpretation of scintigraphy results. Chunlin Chen: pharmacokinetic analysis and pharmacokinetic/pharmacodynamic modelling. Yinzhong Chen: responsible for developing statistical analysis plan and statistical analysis. Cristina Almansa: assisting on study design, protocol development, and interpretation of results. George Dukes: consultation on study design, protocol development, and interpretation of results. Michael Camilleri: Principal Investigator, design of study.
All authors approved the final version of the article, including the authorship list.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Declaration of personal interests: Michael Camilleri is funded for single‐centre research studies by Allergan and Takeda. He serves as an advisor to Allergan, Takeda, Ironwood with compensation paid to his employer, Mayo Clinic. The other authors have no conflicts of interest. Drs. Cristina Almansa, Jenifer Siegelman, Chunlin Chen, Yinzhong Chen and George Dukes were all employees of Takeda Pharmaceuticals at the time of this research. Dr. Almansa is currently an employee of Ironwood Pharmaceuticals, Dr. Chunlin Chen is currently an employee of Bayer, and Dr. Yinzhong Chen is currently an employee of Radius Health.
Chedid V, Brandler J, Arndt K, et al. Randomised study: effects of the 5‐HT4 receptor agonist felcisetrag vs placebo on gut transit in patients with gastroparesis. Aliment Pharmacol Ther. 2021;53:1010–1020. 10.1111/apt.16304
The Handling Editor for this article was Professor Alexander Ford, and it was accepted for publication after full peer‐review.
Funding information
This study was funded by research grant TAK‐954‐2003 from Takeda Pharmaceuticals International Co., Cambridge, MA, USA. Dr. Camilleri’s studies on gastroparesis are funded by NIH R01‐DK122280 grant.
DATA AVAILABILITY STATEMENT
All data are reported in the paper.
REFERENCES
- 1. Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nature Reviews Disease Primers. 2018;4:41. [DOI] [PubMed] [Google Scholar]
- 2. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670–680. [DOI] [PubMed] [Google Scholar]
- 4. Park S‐Y, Acosta A, Camilleri M, et al. Gastric motor dysfunction in patients with functional gastroduodenal symptoms. Am J Gastroenterol. 2017;112:1689–1699. [DOI] [PubMed] [Google Scholar]
- 5. Chedid V, Brandler J, Vijayvargiya P, et al. Characterization of upper gastrointestinal symptoms, gastric motor functions, and associations in patients with diabetes at a referral center. Am J Gastroenterol. 2019;114:143–154. [DOI] [PubMed] [Google Scholar]
- 6. Shahsavari D, Zhao H, Ehrlich AC, et al. Factors associated with hospital admissions and readmissions in patients with gastroparesis using the Nationwide Readmission Database. J Clin Gastroenterol. 2020;54:801–805. [DOI] [PubMed] [Google Scholar]
- 7. Thielemans L, Depoortere I, Perret J, et al. Desensitization of the human motilin receptor by motilides. J Pharmacol Exp Ther. 2005;313:1397–1405. [DOI] [PubMed] [Google Scholar]
- 8. Manabe N, Wong BS, Camilleri M. New‐generation 5‐HT4 receptor agonists: potential for treatment of gastrointestinal motility disorders. Expert Opin Investig Drugs. 2010;19:765–775. [DOI] [PubMed] [Google Scholar]
- 9. De Maeyer JH, Lefebvre RA, Schuurkes JA. 5‐HT4 receptor agonists: similar but not the same. Neurogastroenterol Motil. 2008;20:99–112. [DOI] [PubMed] [Google Scholar]
- 10. Shin A, Camilleri M, Kolar G, et al. Systematic review with meta‐analysis: highly selective 5‐HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment Pharmacol Ther. 2014;39:239–253. [DOI] [PubMed] [Google Scholar]
- 11. Beattie DT, Higgins DL, Ero MP, et al. An in vitro investigation of the cardiovascular effects of the 5‐HT(4) receptor selective agonists, velusetrag and TD‐8954. Vascul Pharmacol. 2013;58:150–156. [DOI] [PubMed] [Google Scholar]
- 12. Bouras EP, Camilleri M, Burton DD, et al. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–360. [DOI] [PubMed] [Google Scholar]
- 13. Carbone F, Van den Houte K, Clevers E, et al. Prucalopride in gastroparesis: a randomized placebo‐controlled crossover study. Am J Gastroenterol. 2019;114:1265–1274. [DOI] [PubMed] [Google Scholar]
- 14. Beattie DT, Armstrong SR, Vickery RG, et al. The pharmacology of TD‐8954, a potent and selective 5‐HT(4) receptor agonist with gastrointestinal prokinetic properties. Front Pharmacol. 2011;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McKinnell RM, Armstrong SR, Beattie DT, et al. Discovery of TD‐8954, a clinical stage 5‐HT(4) receptor agonist with gastrointestinal prokinetic properties. Bioorg Med Chem Lett. 2013;23:4210–4215. [DOI] [PubMed] [Google Scholar]
- 16. Chapman MJ, Jones KL, Almansa C, et al. Blinded, double‐dummy, parallel‐group, phase 2a randomized clinical trial to evaluate the efficacy and safety of a highly selective 5‐hydroxytryptamine type 4 receptor agonist in critically ill patients with enteral feeding intolerance. J Parenter Enteral Nutr 2020. Jan 28. 10.1002/jpen.1732. Online ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang YR, Fisher RS, Parkman HP. Gastroparesis‐related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol. 2008;103:313–322. [DOI] [PubMed] [Google Scholar]
- 18. Bharucha AE, Low P, Camilleri M, et al. A randomised controlled study of the effect of cholinesterase inhibition on colon function in patients with diabetes mellitus and constipation. Gut. 2013;62:708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index‐Daily Diary (GCSI‐DD). Neurogastroenterol Motil. 2012;24:456–463. [DOI] [PubMed] [Google Scholar]
- 20. Zinsmeister AR, Burton D, Camilleri M. Pharmacodynamic and clinical endpoints for functional colonic disorders: statistical considerations. Dig Dis Sci. 2013;58:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vijayvargiya P, Camilleri M, Chedid V, et al. Effects of promotility agents on gastric emptying and symptoms: a systematic review and meta‐analysis. Gastroenterology. 2019;156:1650–1660. [DOI] [PubMed] [Google Scholar]
- 22. Camilleri M, Colemont LJ, Phillips SF, et al. Human gastric emptying and colonic filling of solids characterized by a new method. Am J Physiol. 1989;257:G284–G290. [DOI] [PubMed] [Google Scholar]
- 23. Greydanus MP, Camilleri M, Colemont LJ, et al. Ileocolonic transfer of solid chyme in small intestinal neuropathies and myopathies. Gastroenterology. 1990;99:158–164. [DOI] [PubMed] [Google Scholar]
- 24. Ohe MRVD, Camilleri M, Kvols LK, et al. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993;329:1073–1078. [DOI] [PubMed] [Google Scholar]
- 25. von der Ohe MR, Camilleri M, Thomforde GM, et al. Differential regional effects of octreotide on human gastrointestinal motor function. Gut. 1995;36:743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saslow SB, Scolapio JS, Camilleri M, et al. Medium‐term effects of a new 5HT3 antagonist, alosetron, in patients with carcinoid diarrhoea. Gut. 1998;42:628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All data are reported in the paper.


