Abstract
Venetoclax (Ven) combined with a hypomethylating agent (HMA) has now emerged as an effective treatment regimen for acute myeloid leukemia, in both de novo and relapsed/refractory setting. The current multicenter study retrospectively examined Ven + HMA treatment outcome among 32 patients (median age 69 years; 59% males) with blast‐phase myeloproliferative neoplasm (MPN‐BP). Pre‐leukemic phenotype included essential thrombocythemia (ET)/post‐ET myelofibrosis (34%), polycythemia vera (PV)/post‐PV myelofibrosis (38%) and primary myelofibrosis (28%). Twenty‐nine study patients were fully annotated cytogenetically and molecularly (NGS): 69% harbored complex karyotype and/or mutations, including TP53 (41%), IDH1/2 (21%), ASXL1 (21%), N/KRAS (14%), SRSF2 (10%), EZH2 (10%) and U2AF1 (7%). All patients received Ven combined with either azacitidine (n = 12) or decitabine (n = 20); either up front (n = 23) or after failing another induction therapy (n = 9). Complete remission with (CR) or without (CRi) count recovery was achieved in 14 (44%) patients and was more likely to occur in the absence of pre‐leukemic PV/post‐PV myelofibrosis phenotype (p < .01), complex karyotype (p < .01) or K/NRAS (p = .03) mutations; seven of eight patients (88%) without vs four of 21 (19%) with complex karyotype or K/NRAS mutation achieved CR/CRi (p < .01); all 11 informative patients with pre‐leukemic PV/post‐PV myelofibrosis phenotype displayed complex karyotype (p < .01). In contrast, neither TP53 (p = .45) nor IDH1/2 (p = .63) mutations affected response. Compared to historical controls treated with HMA alone (n = 26), the CR/CRi rate (44% vs 4%) and median survival (8 vs 5.5 months) were more favorable with Ven + HMA, but without significant difference in overall survival. Importantly, six patients with CR/CRi subsequently received allogeneic hematopoietic stem cell transplant (AHSCT). Note, Ven + HMA produces robust CR/CRi rates in MPN‐BP, especially in the absence of RAS mutations and complex karyotype, thus enabling AHSCT, in some patients.
1. INTRODUCTION
Blast phase (BP) transformation is the most feared complication in patients with myeloproliferative neoplasms (MPN) and its 20‐year estimated incidence was approximately 9% for primary myelofibrosis (PMF), 4% for polycythemia vera (PV) and 3% for essential thrombocythemia (ET). 1 A 2018‐published report of 410 patients with MPN‐BP included 248 patients from the Mayo Clinic in whom treatment details were available for retrospective review; most of these patients received supportive care, because of their older age (median age 67 years) and presence of comorbidities. Whereas, 69 (28%) and 26 (10%) patients received acute myeloid leukemia (AML)‐like induction chemotherapy or a hypomethylating agent (HMA), respectively; the corresponding complete remission (CR)/CR with incomplete count recovery (CRi) rates were 59% and 4%. 2 Although overall median survival was only 3.6 months, the 3‐year survival rate was significantly higher (32%) among 24 patients who received allogeneic hematopoietic stem cell transplant (AHSCT), compared to those who either achieved CR/CRi but were not transplanted (19%; n = 24) or the rest of the study population without documented CR/CRi or AHSCT (1%; n = 200). The study also confirmed similar survival between CR and CRi. 2
The above‐outlined observations suggested the apparently crucial role of AHSCT in securing long‐term survival in MPN‐BP as well as the favorable short‐term survival impact of chemotherapy‐induced CR/CRi. Our recently published experience in AML patients above age 70 years was similar in confirming the survival advantage for intensive chemotherapy, for otherwise fit patients, and the value of HMA‐based therapy for prolonging short‐term survival in patients not considered fit to receive AML‐like induction chemotherapy. 3 The majority of patients with MPN‐BP are older than age 65 years and often suffer from comorbidities that make them ineligible for intensive chemotherapy. 2 Accordingly, HMA‐based treatment regimens are gaining favor in treating such patients. 4 , 5 , 6 In order to improve upon the therapeutic benefit from HMA, recent studies in both de novo and relapsed/refractory AML patients unfit for intensive therapy have demonstrated the added value of venetoclax (Ven) in terms of both attaining higher response rates and longer short‐term survival, leading to its FDA approval for use in elderly or unfit AML patients. 7 , 8 , 9 , 10 , 11 , 12 , 13 Similarly, preliminary reports involving small numbers of patients with MPN‐BP have also suggested therapeutic activity for Ven + HMA. 14 , 15 Herein, we describe a multicenter experience in the use of Ven + HMA in 32 consecutive cases with MPN‐BP, including assessment of CR/CRi rates, cytogenetic and molecular predictors of response and comparison of survival impact with historical controls receiving HMA alone or intensive chemotherapy.
2. METHODS
The current multicenter study includes a total of 32 consecutive patients with MPN‐BP treated with Ven + HMA combination therapy at the Mayo Clinic (Rochester MN, Arizona, Florida), USA (n = 27), University of Florence, Florence, Italy (n = 3) and University of Montreal, Montreal, Canada (n = 2). Study patients were retrospectively recruited after institutional review board approval. Diagnosis of MPN‐BP required the presence of ≥20% blasts in either the peripheral blood or bone marrow; 16 patients with isolated extramedullary accumulation of blasts (myeloid sarcoma) were excluded. Pertinent phenotypic details of chronic phase MPN including type of MPN, driver mutation profile, disease course and treatment details were abstracted. Similarly, laboratory findings at the time of diagnosis of BP‐MPN were reviewed, along with results of cytogenetic and molecular studies performed by conventional karyotype/fluorescence in situ hybridization, and next‐generation sequencing (NGS), respectively. Disease risk and response were assessed according to the 2017 European Leukemia Net (ELN) criteria. 17 All patients received Ven in a three‐day ramp‐up during cycle one with tumor lysis prophylaxis. The Ven dose was adjusted based on drug interactions particularly with azole antifungal prophylaxis. Azacitidine 75 mg/m2 days 1–7 or decitabine 20 mg/m2 days 1–5 were administered as part of the combination therapy. Bone marrow biopsy was obtained after either cycle one or two based on treating physician discretion. Treatment cycle delays/interruptions and dose reductions were also determined by the treating physician. Follow up for each patient was updated in March 2021. Patient characteristics are summarized by frequency (percentage) for categorical variables and median (range) values for continuous variables. Determinants of treatment response were assessed by chi‐square or Fisher's exact test for nominal data and Wilcoxon rank‐sum test for continuous variables. Overall survival was evaluated by the Kaplan–Meier method with differences between groups compared by log‐rank test. Analyses were performed using JMP Pro 14.0.0 software package, SAS Institute, Cary, NC and significance was defined as p value <.05.
3. RESULTS
3.1. Patient characteristics
A total of 32 patients with MPN‐BP (median age 69 years, range 47–81; 59% males) received Ven + HMA of which 23 and nine patients were treated in the first line and relapsed/refractory setting, respectively. Patients with relapsed/refractory disease had received either one (n = 6) or two (n = 3) prior therapies which included “7cytarabine + 3idarubicin” (n = 4), “5cytarabine + 2idarubicin” (n = 1), liposomal daunorubicin/cytarabine (n = 3), cladribine (n = 1). Second line therapies comprised of FLAG‐IDA, gemtuzumab ozogamicin, and enasidenib in one patient each. Moreover, prior HMA therapy for a median of two cycles (range; 2–5 cycles) were administered in a total of six patients; three each in the upfront and relapsed/refractory group respectively. Only one patient in our cohort underwent prior AHSCT. MPN‐BP evolved from ET/post‐ET myelofibrosis (post‐ET MF) in 11 (34%), PV/post‐PV MF in 12 (38%) and PMF in nine (28%) patients. Driver mutation profile included JAK2 in 81% of the patients and CALR in 13%; other mutations included TP53 in 12 patients (41%), TET2 in eight (28%), ASXL1 in six (21%), IDH1/2 in six (21%), N/KRAS in four (14%), RUNX1 in four (14%), SRSF2 in six (10%), EZH2 in six (10%) and U2AF1 in two (7%). Cytogenetic abnormalities were present in 79% of patients and classified as either intermediate (24%) or poor risk (76%); among the latter, 69% were classified as complex. Table 1 lists patient characteristics at time of leukemic transformation, treatment details, response rates, and overall outcome. Table 2 provides a comparison of patients treated in the front‐line vs relapsed/refractory setting and illustrates similarities within the two groups with the exception of age; patients treated upfront were older with median age 70 years vs 60 years for those with relapsed/refractory disease (p = .02).
TABLE 1.
Clinical characteristics at time of leukemic transformation for 32 patients with blast phase myeloproliferative neoplasm (BP‐MPN) treated with hypomethylating agent (HMA) and venetoclax stratified by achievement of complete response (CR) or CR with incomplete count recovery (CRi)
| Variables | All patients | Patients in CR/CRi | Patients not in CR/CRi | p value |
|---|---|---|---|---|
| N = 32 | N = 14 | N = 18 | ||
| Age in years, median (range) | 69 (47–81) | 69 (53–81) | 68.5 (47–81) | .84 |
| Male, n (%) | 19 (59) | 9 (47) | 10 (53) | .62 |
| MPN type, n (%) | ||||
| ‐ ET/ Post‐ET MF | 11 (34) | 8 (73) | 3 (27) | |
| ‐ PV/Post‐PV MF | 12 (38) | 1 (8) | 11 (92) | <.01 |
| ‐ PMF | 9 (28) | 5 (56) | 4 (44) | |
| Driver mutation, n (%) | n = 31 | n = 13 | n = 18 | |
| ‐JAK2 | 25 (81) | 9 (36) | 16 (64) | .14 |
| ‐CALR | 4 (13) | 2 (50) | 2 (50) | |
| ‐ Triple negative | 2 (6) | 2 (100) | 0 (0) | |
| Mutations on NGS, n (%) | n = 29 | n = 12 | n = 17 | |
| ‐TP53 | 12 (41) | 4 (33) | 8 (67) | .45 |
| ‐TET2 | 8 (28) | 4 (50) | 4 (50) | .56 |
| ‐ASXL1 | 6 (21) | 2 (33) | 4 (67) | .65 |
| ‐IDH1/2 | 6 (21) | 3 (50) | 3 (50) | .63 |
| ‐RUNX1 | 4 (14) | 1 (25) | 3 (75) | .46 |
| ‐N/KRAS | 4 (14) | 0 (0) | 4 (100) | .03 |
| ‐SRSF2 | 3 (10) | 1 (33) | 2 (67) | .76 |
| ‐EZH2 | 3 (10) | 1 (33) | 2 (67) | .76 |
| ‐U2AF1 | 2 (7) | 1 (50) | 1 (50) | .80 |
| Splenomegaly, n (%) | 13 (41) | 4 (31) | 9 (69) | .22 |
| Treatment for MPN | ||||
| ‐Hydroxyurea | 25 (78) | 9 (36) | 16 (64) | .09 |
| ‐Anagrelide | 4 (13) | 0 (0) | 4 (100) | .02 |
| ‐Ruxolitinib | 6 (19) | 1 (17) | 5 (83) | .12 |
| ‐ Other a | 2 (6) | 1 (100) | 1 (100) | .85 |
| Time to AML in months, median (range) | 128 (3–468) | 87 (3–468) | 146 (4–404) | .55 |
| Hemoglobin, g/dL, median (range) | 8.3 (4.6–15.9) | 8.8 (4.6–15.9) | 7.7 (5.4–10.4) | .09 |
| Leukocyte count x 109/L, median (range) | 4.8 (0.5–60.6) | 3.2 (0.5–40) | 6.3 (0.9–60.6) | .51 |
| Platelet count x 109/L, median (range) | 103 (15–920) | 94 (15–321) | 146 (15–920) | .14 |
| Circulating blasts % b , median (range) | 22 (0–78) | 24 (0–58) | 22 (6–78) | .92 |
| Bone marrow blasts % b , median (range) | 31 (5–90) | 35 (9–89) | 30 (5–90) | .53 |
| Karyotype available, n (%) | n = 29 (91%) | n = 11 | n = 18 | |
| ‐Normal karyotype | 6 (21) | 4 (67) | 2 (33) | .11 |
| ‐Complex karyotype | 20 (69) | 4 (20) | 16 (80) | <.01 |
| ‐Monosomal karyotype | 17 (59) | 4 (24) | 13 (76) | .05 |
| European LeukemiaNet (ELN) cytogenetic risk stratification, n (%) | n = 29 | n = 11 | n = 18 | .06 |
| ‐Favorable | 0 (0) | 0 (0) | 0 (0) | |
| ‐Intermediate | 7 (24) | 5 (71) | 2 (29) | |
| ‐Adverse | 22 (76) | 7 (32) | 15 (68) | |
| Blast phase status at the start of Venetoclax + HMA therapy, n (%) | ||||
| ‐Untreated | 23 (72) | 11 (48) | 12 (52) | .45 |
| ‐ Relapsed/Refractory | 9 (28) | 3 (33) | 6 (67) | |
| Prior HMA therapy, n (%) | 6 (19) | 2 (33) | 4 (67) | .56 |
| HMA used n (%) | ||||
| ‐Azacitidine | 12 (38) | 6 (50) | 6 (50) | .58 |
| ‐Decitabine | 20 (62) | 8 (40) | 12 (60) | |
| Dose of venetoclax (median, range) | 200 (70–400) | 200 (70–400) | 300 (100–400) | .66 |
| Number of cycles (median, range) | 3 (1–7) | 3.5 (1–7) | 3 (1–6) | .19 |
| Relapse after response (n, %) | 4 (13) | 4 (29) | n/a | n/a |
| Allogeneic transplant following response, n (%) | 6 (14) | 6 (43) | n/a | n/a |
| Follow up from BP‐MPN diagnosis, months (median, range) | 7 (2–24) | 7.5 (2–24) | 7 (2–24) | .61 |
| Follow up from start of Venetoclax +HMA, months (median, range) | 5.5 (1–24) | 6.5 (1–24) | 4.5 (1–19) | .33 |
| Median overall survival in months, median, (range) | 8 (1–24) | 9 (1–24) | 7 (1–24) | .18 |
Bold faced values are statistically significant, p < .05.
Abbreviations: ET, essential thrombocythemia, n/a, not applicable; PMF, primary myelofibrosis; PV, polycythemia vera.
Other includes decitabine, lenalidomide and prednisone.
blast percentage was ≥20% either in the peripheral blood or bone marrow.
TABLE 2.
Clinical characteristics at time of leukemic transformation followed by response status and treatment complications for 32 patients with blast phase myeloproliferative neoplasm (BP‐MPN) treated with hypomethylating agent (HMA) and venetoclax
| Variables | All patients | Untreated | Relapsed/refractory | p value |
|---|---|---|---|---|
| N = 32 | N = 23 | N = 9 | ||
| Age in years, median (range) | 69 (47–81) | 70 (53–81) | 60 (47–81) | .02 |
| Male, n (%) | 19 (59) | 14 (74) | 5 (26) | .78 |
| MPN type, n (%) | ||||
| ‐ ET/ Post‐ET MF | 11 (34) | 9 (82) | 2 (18) | |
| ‐ PV/Post‐PV MF | 12 (38) | 8 (67) | 4 (33) | .65 |
| ‐ PMF | 9 (28) | 6 (67) | 3 (33) | |
| Driver mutation, n (%) | n = 31 | n = 22 | n = 9 | |
| ‐ JAK2 | 25 (81) | 17 (68) | 8 (32) | |
| ‐ CALR | 4 (13) | 3 (75) | 1 (25) | .47 |
| ‐ Triple negative | 2 (6) | 2 (100) | 0 (0) | |
| Mutations on NGS | n = 29 | n = 21 | n = 8 | |
| ‐TP53 | 12 (41) | 9 (75) | 3 (25) | .79 |
| ‐TET2 | 8 (28) | 6 (75) | 2 (25) | .85 |
| ‐ASXL1 | 6 (21) | 5 (83) | 1 (17) | .48 |
| ‐IDH1/2 | 6 (21) | 4 (67) | 2 (33) | .73 |
| ‐RUNX1 | 4 (14) | 3 (75) | 1 (25) | .90 |
| ‐N/KRAS | 4 (14) | 2 (50) | 2 (50) | .30 |
| ‐SRSF2 | 3 (10) | 2 (67) | 1 (33) | .82 |
| ‐EZH2 | 3 (10) | 3 (100) | 0 (0) | .15 |
| ‐U2AF1 | 2 (7) | 1 (50) | 1 (50) | .46 |
| Splenomegaly, n (%) | 13 (41) | 8 (62) | 5 (38) | .28 |
| Treatment for MPN | ||||
| ‐Hydroxyurea | 25 (78) | 18 (72) | 7 (28) | .98 |
| ‐Anagrelide | 4 (13) | 3 (75) | 1 (25) | .88 |
| ‐Ruxolitinib | 6 (19) | 5 (83) | 1 (17) | .47 |
| ‐ Other a | 2 (6) | 1 (50) | 1 (50) | .49 |
| Time to AML in months, median (range) | 128 (3–468) | 144 (3–468) | 71 (4–238) | .19 |
| Hemoglobin, g/dL, median (range) | 8.3 (4.6–15.9) | 8.2 (4.6–15.9) | 8.8 (6.5–9.9) | .86 |
| Leukocyte count x 109/L, median (range) | 4.8 (0.5–60.6) | 4.3 (0.99–40) | 12.2 (0.5–60.6) | .15 |
| Platelet count x 109/L, median (range) | 103 (15–920) | 103 (15–466) | 176 (15–920) | .16 |
| Circulating blasts % b , median (range) | 22 (0–78) | 22 (1–78) | 6 (0–58) | .79 |
| Bone marrow blasts % b , median (range) | 31 (5–90) | 32 (5–90) | 30 (22–70) | .79 |
| Chromosomal abnormalities, n (%) | n = 29 (91%) | n = 21 | n = 8 | |
| ‐Normal karyotype | 6 (21) | 4 (67) | 2 (33) | .73 |
| ‐Complex karyotype | 20 (69) | 14 (70) | 6 (30) | .66 |
| ‐Monosomal karyotype | 17 (59) | 13 (76) | 4 (24) | .56 |
| European LeukemiaNet (ELN) cytogenetic risk stratification, n (%) | n = 29 | n = 21 | n = 8 | .95 |
| ‐Favorable | 0 (0) | 0 (0) | 0 (0) | |
| ‐Intermediate | 7 (24) | 5 (71) | 2 (29) | |
| ‐Adverse | 22 (76) | 16 (73) | 6 (27) | |
| HMA used, n (%) | ||||
| ‐Azacitidine | 12 (38) | 9 (75) | 3 (25) | .76 |
| ‐Decitabine | 20 (62) | 14 (70) | 6 (30) | |
| Dose of venetoclax (median, range) | 200 (70–400) | 400 (70–400) | 200 (70–400) | .17 |
| Number of cycles (median, range) | 3 (1–7) | 3 (1–7) | 3 (1–6) | .25 |
| Treatment complications, n (%) | ||||
| ‐Neutropenic fever/sepsis | 10 (31) | 8 (80) | 2 (20) | .48 |
| ‐Pancytopenia | 17 (53) | 13 (76) | 4 (26) | .54 |
| ‐Intracranial hemorrhage | 1 (3) | 1 (100) | 0 (0) | .41 |
| ‐Hepatic dysfunction | 3 (9) | 2 (67) | 1 (33) | .84 |
| ‐Gastrointestinal toxicity | 3 (9) | 1 (33) | 2 (67) | .14 |
| Cycle delays, n (%) | 11 (34) | 9 (82) | 2 (18) | .35 |
| Venetoclax dose adjustment, n (%) | 14 (44) | 9 (64) | 5 (36) | .40 |
| HMA dose adjustment, n (%) | 7 (22) | 5 (71) | 2 (29) | .98 |
| Response status | ||||
| ‐CR/CRi | 14 (44) | 11 (72) | 3 (28) | |
| ‐PR | 4 (13) | 3 (75) | 1 (25) | .61 |
| ‐MLFS | 2 (6) | 2 (100) | 0 (0) | |
| ‐Persistent disease | 12 (38) | 7 (58) | 5 (42) | |
| ‐Residual MPN features on bone marrow biopsy | 6 (19) | 5 (83) | 1 (27) | .47 |
| Relapse after response n, (%) |
n = 14 4 (13) |
n = 11 4 (100) |
n = 3 0 (0) |
.13 |
| Allogeneic transplant following response, n (%) | 6 (19) | 5 (83) | 1 (27) | .47 |
| Follow up from BP‐MPN diagnosis, months (median, range) | 7 (2–24) | 7 (2–24) | 9 (4–24) | .14 |
| Follow up from start of Venetoclax +HMA, months (median, range) | 5.5 (1–24) | 5 (1–24) | 6 (2–19) | .86 |
| Median overall survival in months, median, (range) | 8 (1–24) | 8 (1–24) | 8 (4–24) | .68 |
Bold faced values are statistically significant, p < .05.
Abbreviations: CR, complete remission; CRi, CR with incomplete count recovery; ET, essential thrombocythemia; MLFS, morphological leukemia free state; PMF, primary myelofibrosis; PR, partial remission, PV, polycythemia vera.
Other includes decitabine, lenalidomide and prednisone.
blast percentage was ≥20% either in the peripheral blood or bone marrow.
3.2. Treatment details and response
Twenty (62%) patients received decitabine and the remainder azacitidine with median final Ven dose of 200 mg (range, 70–400 mg) administered for a median of three cycles (range, 1–7 cycles). Eleven (34%) patients experienced cycle delays/interruptions; moreover, Ven and HMA dose reductions were instituted in 14 (44%) and seven (22%) of patients, respectively. Pancytopenia related to therapy was a frequent occurrence noted in about half of treated patients (n = 17) and was complicated by neutropenic fever or sepsis in 10 patients (31%) and one death related to intracranial hemorrhage. The incidence of pancytopenia was similar among patients with pre‐leukemic ET/post ET‐MF (29%), PV/post PV‐MF (29%) or PMF (42%) phenotype (p = .21). Furthermore, three patients each had hepatic function abnormalities and gastrointestinal toxicity in the form of anorexia, fatigue, and diarrhea associated with therapy.
Response was evaluable in all 32 patients; CR/CRi was documented in 14 (44%) patients and included 10 (31%) patients with CR and four (13%) with CRi. The remainder of the responses included morphological leukemia free state (MLFS) in two (6%) patients and partial response (PR) in four (13%). Residual morphological features of MPN were noted in a total of six patients which included four with CR/CRi. Among the 14 patients who achieved CR/CRi, median time to response was 1.5 months (range; 1–6 months) with median duration of response of 3.5 months (range, 0.5–10 months). Importantly, the achievement of CR/CRi enabled six (43%) of 14 responding patients to undergo AHSCT. Relapses occurred in a total of four of 14 (29%) responding patients, one of which occurred following AHSCT.
3.3. Predictors of response
We observed no difference in CR/CRi rates between patients who received Ven + HMA upfront (48%) or after failing another induction chemotherapy (33%; p = .45). Note, CR/CRi was more likely to occur in the absence of pre‐leukemic PV phenotype (p < .01), complex karyotype (p < .01) or K/NRAS (p = .03) mutations; seven of eight patients (88%) without vs four of 21 (19%) with complex karyotype or K/NRAS mutation achieved CR/CRi (p < .01). The latter two genetic markers were independently predictive of poor response whereas all 11 informative patients with pre‐leukemic PV phenotype displayed complex karyotype (p < .01). Upon evaluation of the impact of prior therapies on Ven + HMA treatment response; we observed that none of four patients previously exposed to anagrelide therapy (p = .02) and only one of six (17%) exposed to ruxolitinib therapy (p = .12) achieved CR/CRi. Additional trends worth highlighting included lower responses observed with JAK2 mutation (p = .14), lower hemoglobin level (p = .09), higher platelet count (p = .14) and exposure to hydroxyurea (p = .09). In contrast, age (p = .84), leukocyte count (p = .51), circulating or bone marrow blast % (p = .92, .53), dose of venetoclax (p = .66), prior HMA exposure (p = .56) or type of HMA (p = .58) did not appear to influence response. Figure 1 illustrates the correlation between mutations and likelihood of attaining CR/CRi from Ven + HMA; the only significant correlation was with N/KRAS mutation (p = .03), predicting lower CR/CRi rate, as already mentioned above; none of the other mutations tested affected response; CR/CRi rates were, in the presence or absence of TP53 (33% vs 47%, p = .45), IDH1/2 (50% vs 50% p = .63), ASXL1 (33% vs 43% p = .65), SRSF2 (33% vs 42% p = .76), EZH2 (33% vs 42% p = .76) and U2AF1 (50% vs 41% p = .80).
FIGURE 1.
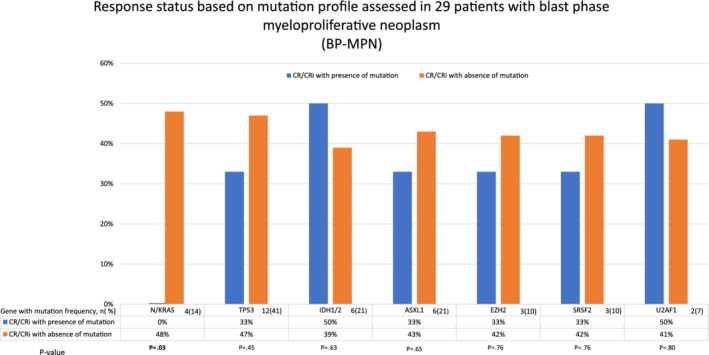
Response status based on mutation profile assessed in 29 patients with blast phase myeloproliferative neoplasm (BP‐MPN) [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Survival
After a median follow up of 5.5 months (range; 1–24 months), from the start of treatment with Ven + HMA, 23 (72%) patients have died with causes of death known in 20 patients: progressive disease (n = 10), infection (n = 9), and bleeding (n = 1). Among six patients bridged to AHSCT, four have since died, mainly from transplant related complications (n = 3) and one from disease relapse. Overall median survival was 8 months (range; 1–24 months) and longer in patients achieving CR/CRi (9 months) vs those who did not achieve CR/CRi (7 months; p = .18).
Similarly, patients bridged to AHSCT demonstrated a trend towards prolonged survival (9 vs 7 months with or without transplant respectively; p = .17). Predictors of shortened survival included presence of complex karyotype (7 vs 10 months; p = .05) and N/KRAS mutations (3 vs 7 months; p = .06). On the other hand, presence of TP53 (p = .22), ASXL1 (p = .19), EZH2 (p = .23), IDH1/2 (p = .65), U2AF1 (p = .17) mutations did not appear to impact survival. The small number of evaluable patients in each category precluded further multivariable analyses.
Outcome in the 32 patients treated with Ven + HMA was compared to historical controls from Mayo Clinic database of patients with MPN‐BP treated with either HMA alone (n = 26) or intensive chemotherapy (n = 69). Both CR/CRi rates (44% vs 4%) and median survival (8 vs 5.5 months) appeared more favorable with Ven + HMA vs HMA alone, but there was not significant difference in overall survival between the two HMA‐based treatment regimens (p = .3; Figure 2); however, longer‐term survival favored intensive chemotherapy over both HMA alone and Ven + HMA (2‐year survival rates were 20%, 9% and 9%, respectively; p = .13; Figure 2).
FIGURE 2.
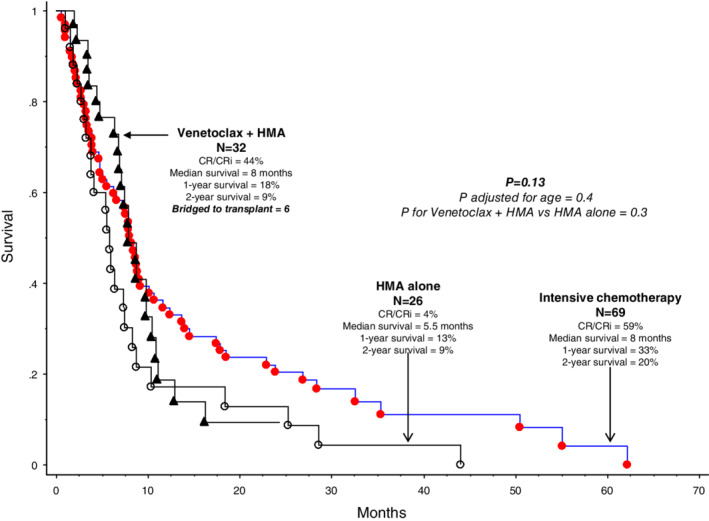
Retrospective comparison of survival data in 32 patients with blast phase myeloproliferative neoplasm treated with venetoclax + hypomethylating agent (HMA) vs 26 patients treated with HMA alone vs 69 patients treated with intensive chemotherapy [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
The dire prognosis associated with MPN‐BP has been underlined by a number of previously published studies where estimates of median survival had ranged from 1.5 to 2.5 months in patients treated with supportive care only, from 2.5 to 10 months in those receiving HMA or other less intensive chemotherapy, and from 3.9 to 9.4 months in patients receiving AML‐like induction chemotherapy. 18 The only treatment modality that has thus far shown to secure long‐term survival in MPN‐BP, albeit in a small percentage of patients, is AHSCT, with 3‐year reported survival rates ranging from 16% to 33%. 18 Consistently, in a recent retrospective study of 39 MPN‐BP patients reported by the Japan Society for Hematopoietic Cell Transplantation, including various donor sources (unrelated 15 cases; umbilical cord blood 16), reported a 2‐year survival rate of 29%, despite the fact that 82% of the patients were not in remission at the time of transplantation. 19 In another study from the European Group for Blood and Marrow Transplantation registry, 46 patients with MPN‐BP received AHSCT for acute leukemia evolving from myelofibrosis and achieved a 3‐year progression‐free and overall survival rates of 26% and 33%, respectively; 20 in the particular study, survival outcome was significantly better in the presence of CR/CRi at time of transplantation. In our own experience at the Mayo Clinic and the AIRC‐Gruppo Italiano Malattie Mieloproliferative (AGIMM), AHSCT for MPN‐BP resulted in 3‐year survival rates of over 30% in both cohorts. 2 Furthermore, among the 24 transplanted patients in the Mayo cohort, outcome was similar whether or not CR/CRi was documented at time of transplant (p = .6). 18 Accordingly, our current stance in the management of MPN‐BP in patients who are considered to be fit includes AML‐like induction chemotherapy followed by AHSCT, after adequate clearance of peripheral blood and bone marrow blasts.
In contrast to the case with AHSCT, currently available drugs, investigational or otherwise, have yet to show a significant impact on long‐term survival in patients with MPN‐BP, without the assistance of consolidation therapy with AHSCT. Both ruxolitinib and Ven have recently been investigated for their additional value to HMA therapy in MPN‐BP. In a recently published report of 25 patients with either MPN‐BP or accelerated phase MPN, ruxolitinib was administered at 25 mg twice daily for the induction cycle followed by 10 mg twice daily for subsequent cycles, in combination with decitabine 20 mg/m2 for 5 consecutive days in a 28‐day cycle. 21 The study included 15 patients with MPN‐BP, of whom none achieved CR and only 8% achieved CRi; it should be noted that the particular study applied response criteria that are different from those used in the current study. 22 Unlike the case with ruxolitinib+HMA, preliminary reports of combination chemotherapy with Ven + HMA, in MPN‐BP, have been more promising. 14 , 15 In a small study of nine patients with MPN‐BP, three (33%) were reported to achieve CR/CRi, including two who were subsequently transitioned to AHSCT; overall median survival was 4.5 months and the only three patients remaining alive at the time of publication have had AHSCT. 14 We have previously published our preliminary experience in 14 patients with MPN‐BP, including two who presented with myeloid sarcoma; 15 one of the latter two patients experienced partial resolution of the extramedullary tumor, documented by imaging studies, after treatment with Ven + HMA; the remaining 12 patients with bone marrow involvement are included in the current report.
The CR/CRi rate seen in our patients with Ven + HMA treated MPN‐BP (44%) are similar to those we previously reported in AML, using the same combination therapy, in both front‐line (n = 44; 50%) and relapsed/refractory setting (n = 42; 36%). 9 As was the case in the current study, the CR/CRi rate of 50% in previously untreated AML was superior to the 23% observed in a matched AML cohort treated with HMA alone (p < .01) and response rates were not affected by TP53 or IDH1/2 mutations; 9 furthermore, median survival was longer in the presence of CR/CRi (15 vs 3 months; p < .01). In the current study, we identified RAS mutations and complex karyotype as independent predictors of poor response to Ven + HMA while no associations with IDH1/2 mutations were apparent; incidentally, the association between poor response and preleukemic PV phenotype was explained by the latter clustering with complex karyotype. Interestingly, we have recently identified RAS/CBL mutations as predictors of poor treatment response to ruxolitinib, in patients with myelofibrosis. 23 In a previous study of relapsed/refractory AML in older patients, IDH2 and NPM1 mutations were associated with higher response rates and durable remissions while an association with IDH1 was less certain. 24 The frequency of TP53 mutations in our study was higher than previously reported, including our prior work where the incidence was 16%; incidentally, in the latter study, the only mutation that affected survival was RUNX1. 25 A higher TP53 mutation frequency (27%) was reported in an earlier study and almost all in JAK2 mutated cases. 26 Therefore, one possible explanation might be the fact that our patient population was enriched for JAK2 mutated cases (81%). Regardless, TP53 mutations in the current study did not affect either response rate (p = .45) or overall survival (p = .22). The underlying mechanisms of resistance or sensitivity to Ven‐based combination chemotherapy are currently under investigation and the role of specific mutations, in this regard, requires further clarification. 24 In the meantime, more recent studies suggest the value of Ven‐based combination chemotherapy might extend to more intensive settings that might be applicable to patients with MPN‐BP. 10 , 27 , 28 Taken together, the observations from the current study are encouraging in terms of the potential value of Ven + HMA therapy in MPN‐BP, as a bridge to AHSCT, with room for improvement.
FINANCIAL DISCLOSURES
A.M.V. participated in advisory board of AbbVie.
Gangat N, Guglielmelli P, Szuber N, et al. Venetoclax with azacitidine or decitabine in blast‐phase myeloproliferative neoplasm: A multicenter series of 32 consecutive cases. Am J Hematol. 2021;96:781–789. 10.1002/ajh.26186
DATA AVAILABILITY STATEMENT
Data available upon request.
REFERENCES
- 1. Szuber N, Mudireddy M, Nicolosi M, et al. 3023 Mayo Clinic patients with myeloproliferative neoplasms: risk‐stratified comparison of survival and outcomes data among disease subgroups. Mayo Clin Proc. 2019;94:599‐610. [DOI] [PubMed] [Google Scholar]
- 2. Tefferi A, Mudireddy M, Mannelli F, et al. Blast phase myeloproliferative neoplasm: Mayo‐AGIMM study of 410 patients from two separate cohorts. Leukemia. 2018;32:1200‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Begna KH, Gangat N, Al‐Kali A, et al. Acute myeloid leukemia after age 70 years: a retrospective comparison of survival following treatment with intensive versus HMA +/− venetoclax chemotherapy. Am J Hematol. 2021;96:E108‐E111. [DOI] [PubMed] [Google Scholar]
- 4. Badar T, Kantarjian HM, Ravandi F, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high‐risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk Res. 2015;39:950‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in accelerated and blast‐phase myeloproliferative neoplasms. Blood Adv. 2018;2:3572‐3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bose P, Verstovsek S, Cortes JE, et al. A phase 1/2 study of ruxolitinib and decitabine in patients with post‐myeloproliferative neoplasm acute myeloid leukemia. Leukemia. 2020;34:2489‐2492. [DOI] [PubMed] [Google Scholar]
- 7. Mei M, Aldoss I, Marcucci G, Pullarkat V. Hypomethylating agents in combination with venetoclax for acute myeloid leukemia: update on clinical trial data and practical considerations for use. Am J Hematol. 2019;94:358‐362. [DOI] [PubMed] [Google Scholar]
- 8. Gangat N, Tefferi A. Venetoclax‐based chemotherapy in acute and chronic myeloid neoplasms: literature survey and practice points. Blood Cancer J. 2020;10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morsia E, McCullough K, Joshi M, et al. Venetoclax and hypomethylating agents in acute myeloid leukemia: Mayo Clinic series on 86 patients. Am J Hematol. 2020;95:1511‐1521. [DOI] [PubMed] [Google Scholar]
- 10. Pollyea DA, Pratz K, Letai A, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow‐up from a phase 1b study. Am J Hematol. 2021;96:208‐217. [DOI] [PubMed] [Google Scholar]
- 11. DiNardo CD, Maiti A, Rausch CR, et al. 10‐day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single‐Centre, phase 2 trial. Lancet Haematol. 2020;7:e724‐e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617‐629. [DOI] [PubMed] [Google Scholar]
- 13. DiNardo CD, Rausch CR, Benton C, et al. Clinical experience with the BCL2‐inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93:401‐407. [DOI] [PubMed] [Google Scholar]
- 14. Tremblay D, Feld J, Dougherty M, et al. Venetoclax and hypomethylating agent combination therapy in acute myeloid leukemia secondary to a myeloproliferative neoplasm. Leuk Res. 2020;98:106456. [DOI] [PubMed] [Google Scholar]
- 15. Gangat N, Morsia E, Foran JM, Palmer JM, Elliott MA, Tefferi A. Venetoclax plus hypomethylating agent in blast‐phase myeloproliferative neoplasm: preliminary experience with 12 patients. Br J Haematol. 2020;191:e120‐e124. [DOI] [PubMed] [Google Scholar]
- 16. Arber DA. The 2016 WHO classification of acute myeloid leukemia: what the practicing clinician needs to know. Semin Hematol. 2019;56:90‐95. [DOI] [PubMed] [Google Scholar]
- 17. Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yogarajah M, Tefferi A. Leukemic transformation in myeloproliferative neoplasms: a literature review on risk, characteristics, and outcome. Mayo Clin Proc. 2017;92:1118‐1128. [DOI] [PubMed] [Google Scholar]
- 19. Takagi S, Masuoka K, Uchida N, et al. Allogeneic hematopoietic cell transplantation for leukemic transformation preceded by Philadelphia chromosome‐negative myeloproliferative neoplasms: a Nationwide survey by the adult acute myeloid leukemia working Group of the Japan Society for hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:2208‐2213. [DOI] [PubMed] [Google Scholar]
- 20. Alchalby H, Zabelina T, Stubig T, et al. Allogeneic stem cell transplantation for myelofibrosis with leukemic transformation: a study from the myeloproliferative neoplasm subcommittee of the CMWP of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2014;20:279‐281. [DOI] [PubMed] [Google Scholar]
- 21. Mascarenhas JO, Rampal RK, Kosiorek HE, et al. Phase 2 study of ruxolitinib and decitabine in patients with myeloproliferative neoplasm in accelerated and blast phase. Blood Adv. 2020;4:5246‐5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642‐4649. [DOI] [PubMed] [Google Scholar]
- 23. Coltro G, Rotunno G, Mannelli L, et al. RAS/CBL mutations predict resistance to JAK inhibitors in myelofibrosis and are associated with poor prognostic features. Blood Adv. 2020;4:3677‐3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135:791‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lasho TL, Mudireddy M, Finke CM, et al. Targeted next‐generation sequencing in blast phase myeloproliferative neoplasms. Blood Adv. 2018;2:370‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rampal R, Ahn J, Abdel‐Wahab O, et al. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc Natl Acad Sci U S A. 2014;111:E5401‐E5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Venugopal S, Maiti A, DiNardo CD, et al. Decitabine and venetoclax for IDH1/2‐mutated acute myeloid leukemia. Am J Hematol. 2021;96:E154‐E157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maiti A, Qiao W, Sasaki K, et al. Venetoclax with decitabine vs intensive chemotherapy in acute myeloid leukemia: a propensity score matched analysis stratified by risk of treatment‐related mortality. Am J Hematol. 2021;96:282‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available upon request.


