Summary
Glucocorticoid treatment increases venous thromboembolism (VTE) risk. Whether this is due to the medication or the underlying disease, or affects the risk of VTE recurrence, has been difficult to determine. The aim of our present study was to quantify the risk for first and recurrent VTE associated with oral glucocorticoids use, considering the underlying disease. A total of 2547 patients with VTE from the Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis (MEGA) study were linked to the Dutch Pharmaceutical Statistics register. The risk of first VTE during periods of exposure with oral glucocorticoids was estimated by the self‐controlled case series method and that of recurrent VTE was examined in a cohort design. The incidence rate ratio (IRR) of first VTE in the period of glucocorticoid treatment was 3·51 [95% confidence interval (CI) 2·55–4·80]. This IRR was 2·53 (95% CI 1·10–5·72) in the week before treatment started, 5·28 (95% CI 2·89–9·53) in the first 7 days of treatment, remained elevated afterwards and decreased to 1·55 (95% CI 0·85–3·12) after 6 months, as compared to unexposed periods. The hazard ratio for recurrence was 2·72 (95% CI 1·64–4·78) in treatment periods as compared with no treatment. The increased risk of VTE associated with oral glucocorticoid treatment is due to a combined effect of the treatment and the underlying disease, remaining high during the first months of prescription.
Keywords: thrombosis, glucocorticoids, coagulation, risk factors, inflammation
Introduction
Glucocorticoids are potent anti‐inflammatory drugs used for a variety of conditions, e.g. allergy, pulmonary, dermatological and rheumatic disorders and malignancies. 1 , 2 It is well‐known that long‐term use of glucocorticoids can cause severe adverse events, e.g. diabetes, osteoporosis, hypertension and arterial cardiovascular diseases. 3
The risk of venous thromboembolism (VTE) has also been reported to be two‐ to threefold higher with the use of glucocorticoids. 4 , 5 , 6 However, to what extent this is due to the medication or the underlying disease has been difficult to determine as several of the indications for which glucocorticoids are prescribed are considered provoking risk factors for VTE, 7 e.g. chronic diseases, 8 , 9 , 10 , 11 , 12 , 13 periods of disease exacerbation or flares, 11 , 14 , 15 comorbidities 16 and concomitant medications. 17 As the absolute risk of recurrence is much higher than that for first VTE, 18 it is worthwhile studying the effect of glucocorticoids prescription on recurrent VTE as well. As far as we know, such studies have not been performed to date.
We studied individuals who used oral glucocorticoids and their risk of first VTE employing the self‐controlled case‐series (SCCS) method, which eliminates between‐person confounding and time‐invariant confounding by design. 19 We also assessed the absolute risk of recurrent VTE with oral glucocorticoid therapy in a standard follow‐up design.
Patients and methods
Population description and clinical outcomes
This study enrolled patients from the population‐based case‐control study of the Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis (MEGA) study and from the MEGA follow‐up study. The MEGA study was approved by the Ethics Committee of the Leiden University Medical Center and written informed consent was obtained from all participants at the date of inclusion in the study.
Between February 1999 and August 2004, 4956 consecutive patients, aged 18–70 years, with a first acute VTE [deep vein thrombosis (DVT) or pulmonary embolism (PE)] were identified at six anticoagulation clinics in the Netherlands. 20 , 21 An extensive questionnaire on putative risk factors for VTE was completed and blood was sampled on the day of enrolment in the study. Information about the diagnosis of VTE was obtained from hospital discharge reports and general practitioners. The diagnosis of DVT was confirmed with Doppler ultrasonography and the diagnosis of PE was confirmed with a ventilation–perfusion lung scan, computed tomography of the chest or angiogram. Unprovoked VTE was defined as DVT or PE without surgery, trauma, plaster cast, pregnancy or immobilisation in the 3 months immediately before the event, prolonged travel in the 2 months immediately before the event, active malignancies in the 5 years prior to the event or hormone use (oral contraceptives or hormone‐replacement therapy) at the time of the event. Patients who had one or more of these risk factors at time of their thrombotic event were classified as having had a provoked VTE. 22
A total of 4731 patients agreed to participate in a follow‐up study (MEGA follow‐up study). These patients were followed from their first episode of acute VTE until 2007–2009 when the vital status of all MEGA follow‐up participants was acquired from the Dutch population registers, as described previously. 23 Questionnaires concerning recurrent VTE were sent by mail between June 2008 and July 2009. Questions were asked by telephone interview when questionnaires were not returned. During the same period information about recurrences was retrieved from the anticoagulation clinics, where patients were initially included for their first event and in case they moved house, also at the clinic nearest to their new address. The diagnosis of recurrence was made according to the information collected per patient. 23 We considered certain recurrences as outcome event only.
Exposure (use of oral glucocorticoids)
Information on oral glucocorticoid treatment was obtained by linking patients from the MEGA study to the Stichting Farmaceutische Kengetallen [SFK (Dutch Foundation for Pharmaceutical Statistics)] register (http://www.sfk.nl/english). 21 In the Netherlands, oral glucocorticoids are only available by prescription, and >95% of the community pharmacies are represented in this register. The SFK contains information about patient‐specific drugs dispensed: the generic name of a drug, the dose per pill, the Anatomical Therapeutic Chemical (ATC6) classification, the date of prescription, the total number of pills prescribed and the daily doses.
Linkage was based on a combination of age, sex, four‐digit postal code and vitamin K antagonist use within the first month after the initial VTE. Between 1999 and 2004, the inclusion period for the MEGA study, low‐molecular‐weight heparins (as well as direct oral anticoagulants) were not regularly prescribed for long‐term anticoagulation. 21 In total, 2547 (54%) patients in the MEGA study could be individually linked with the SFK. Linkage failure occurred mainly due to the failure in matching the four‐digit postal code of MEGA study participants with that of the SFK patients. Daily doses were missing in 2% of prescriptions and were imputed considering the average daily dose prescribed in the periods immediately previous and after the missing doses for each patient. A gap period between prescriptions lasting <4 weeks was considered as continuing treatment. A new prescription starting after >4 weeks from the end of the previous prescription was considered a new period of treatment.
Study design
We investigated the risk of first VTE with oral glucocorticoid treatment using the SCCS design, in which patients who experienced both the outcome (VTE) and exposure of interest are included in the analysis and there is no censoring by the outcome of interest. 24 The risk for the outcome is estimated comparing the within‐patients rate of events during periods of exposure and periods of non‐exposure. The SCCS method requires three key assumptions. 19 First, recurrent events must be independent. If this assumption is not met, the method can be applied by using the first event. In this study, we used the SCCS method to evaluate only first VTE. Second, the occurrence of an event must not alter the probability of subsequent exposure. Glucocorticoid use is a key therapy for several inflammatory and autoimmune diseases. The occurrence of VTE is not a contraindication (or indication) for glucocorticoid treatment. Therefore, there is no reason to assume that occurrence of a VTE alters the probability of glucocorticoid use. Even so, we included a pre‐exposure period to evaluate the risk of VTE shortly before the exposure. In case that the event alters the probability of exposure, the SCCS method can be applied if a pre‐exposure period is included. 25 Third, the occurrence of the event of interest must not censor or affect the observation period. In this study, VTE was not a reason for censoring. Using this approach, patients with a first VTE and at least one prescription of glucocorticoids during the inclusion period for the MEGA study (February 1999 to September 2004) were included in the analysis.
Next, in a longitudinal cohort study design, we investigated the association of recurrent VTE with glucocorticoid treatment. Patients with a first VTE who could be linked to the SFK register were included in this analysis. Duration of follow‐up was estimated as the time at risk from the date of the index (first) VTE to the end of follow‐up. The end of follow‐up was defined as the date of a recurrent event and in the absence of a recurrence, the date of completing the follow‐up questionnaire or the last date the patient was known to be recurrence free. Details of the end of follow‐up assessment were described previously. 23 The association between oral glucocorticoids and recurrent VTE was examined using two different approaches. First, we evaluated the recurrence rates of VTE in patients according to oral glucocorticoids use around the time of first VTE. Next, we investigated after the first event whether prescriptions of oral glucocorticoids during follow‐up affected the risk of recurrent VTE, as an approach to evaluate the safety of oral glucocorticoid treatment in patients with a prior VTE.
Statistical analysis
For the SCCS design, we defined periods of exposure and non‐exposure to oral glucocorticoids. The observation period used in the SCCS design for each patient was firstly divided into two periods: the period when patients were unexposed to glucocorticoids (unexposed period) and the total period of oral glucocorticoid treatment, defined as the date treatment started until the end date of the prescription.
Next, we separated a period immediately before the start of treatment to allow assessment of the effect of disease activity on the risk of VTE (shortly) before glucocorticoids were prescribed. Thereby, we could distinguish between disease effect (e.g. procoagulant state) and medication effects (e.g. pro‐coagulant response to glucocorticoids), as previously described. 26 , 27 In total, the exposure to oral glucocorticoids period was separated into five periods (Fig 1): (i) the 7‐day period immediately before a prescription was given, when we expected to observe the effect of the exacerbation of the disease on the risk of VTE, (ii) the first 7 days with glucocorticoid treatment, (iii) 8–30 days with oral glucocorticoid treatment, (iv) 31–180 days with oral glucocorticoid treatment and (v) >180 days with oral glucocorticoid treatment. Periods 2 and 3 represent short‐term use of oral glucocorticoid and periods 4 and 5 represent long‐term glucocorticoid use. 28 , 29 We considered non‐exposure periods if a prescription was not provided and was not given in the following 7 days.
Fig 1.
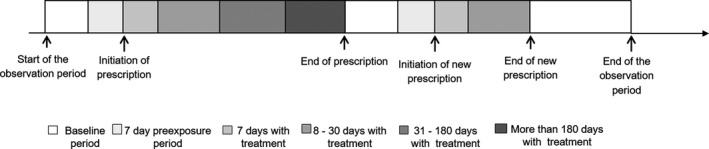
Study analysis. The observation period coincided with the inclusion period for the Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis (MEGA) study (between February 1999 and September 2004). Periods with treatment were defined according to the day of prescription. Six risk periods were defined: (i) baseline (unexposed) period (without oral glucocorticoids); (ii) 7‐day pre‐exposure period; (iii) first 7 days with oral glucocorticoids treatment; (iv) 8–30 days with oral glucocorticoids; (v) 31–180 days with oral glucocorticoids and (vi) >180 days with oral glucocorticoids (not drawn to scale). A new baseline period started the day after the end of prescription.
We also differentiated between agents according to their degree of anti‐inflammatory activity: hydrocortisone; prednisone, prednisolone and methylprednisolone; dexamethasone and betamethasone. 30 , 31
To standardise the glucocorticoid doses, we first converted the daily dose into equivalent prednisolone dose. 30 , 32 Next, we calculated the initial daily dose and 30‐day cumulative dose. The initial daily dose was a priori divided into four categories of clinical effect, 33 , 34 as ≤7·5 mg, 7·5–20 mg, 21–39 mg and ≥40 mg equivalent prednisolone dose/day. The 30‐day cumulative dose was categorised into three groups, as ≤300 mg, 300–2000 mg and >2000 mg equivalent prednisolone dose. Incidence rate ratios (IRRs) and 95% confidence intervals (95% CIs) for first VTE were estimated using conditional Poisson regression for the total period with glucocorticoid treatment and for the sub‐periods, as compared with the unexposed period. IRRs for first VTE were also estimated for types of oral glucocorticoids and initial and 30‐day cumulative prednisolone dose.
For the cohort study, we firstly estimated absolute risks of recurrence in patients with an otherwise provoked or unprovoked first VTE during a period of oral glucocorticoid treatment. Incidence rates and 95% CIs of recurrent VTE were estimated as the number of events over the total person time split for periods with and without glucocorticoid treatment. Periods with glucocorticoid treatment were defined as the total period of glucocorticoid use by a patient and then split into two periods; (i) first 180 days with treatment and (ii) >180 days with treatment. Hazard ratios (HRs) and 95% CIs for recurrent VTE were estimated using Cox regression with glucocorticoid treatment as a time‐dependent covariate and were adjusted for age and sex. All statistical analysis were performed with the use of STATA software (version 14·1; StataCorp., College Station, TX, USA).
Results
A total of 2547 patients with first VTE were linked to the SFK data register and were included in the study. Baseline demographic and clinical characteristics of these patients at the time of their first VTE are summarised in Table SI. The mean age was 51 years and 47% of the patients (n = 1197) were male. Patients who could be linked to the SFK registry had similar demographic and clinical characteristics as those who could not be linked to the SFK, as shown in Table SI.
Risk of first VTE with oral glucocorticoid treatment
A total of 363 patients with a first VTE received at least one outpatient prescription of oral glucocorticoids between February 1999 and September 2004 and were included in the SCCS analysis. Table I summarises the baseline demographic and clinical characteristics of these patients. The median [interquartile range (IQR)] number of prescriptions was 2 [1–3] per patient and the median (IQR) duration of oral glucocorticoid treatment was 102 (36–238) days per prescription. A total of 85 patients (23%) had their first VTE event during a period of glucocorticoid use.
Table I.
Demographic and clinical characteristics of patients included in the SCCS analysis. A total of 363 patients with a first VTE from the MEGA Study (the Netherlands, 1999–2004) received at least one outpatient prescription of oral glucocorticoids and were included in the SCCS analysis.
| Variable | Patients with VTE and a prescription of oral glucocorticoids (n = 363) |
|---|---|
| Age, years, mean (SD) | 54·2 (11·3) |
| N (%) | |
| Male | 168 (46) |
| DVT only | 182 (50) |
| PE ± DVT | 181 (50) |
| Provoked VTE | 241 (66) |
| Unprovoked VTE | 103 (28) |
| Inflammatory disease* | 84 (23) |
| Rheumatoid arthritis | 22 (26) |
| Multiple sclerosis | 3 (4) |
| Emphysema | 13 (15) |
| Chronic bronchitis | 46 (55) |
| Malignancy in the previous 5 years | 94 (26) |
| Time since diagnosis | |
| 0 to 3 months | 36 (39) |
| >3 months to 1 year | 22 (24) |
| >1 to 3 years | 23 (25) |
| >3 to 5 years | 12 (13) |
| Main sites of malignancy | |
| Lung | 18 (19) |
| Myeloma | 8 (9) |
| Other haematological malignancies† | 16 (17) |
| Gastrointestinal | 11 (12) |
| Breast | 12 (13) |
| Other female malignancies‡ | 5 (5) |
| Male malignancies§, * | 7 (7) |
| Urinary | 3 (3) |
DVT, deep vein thrombosis; MEGA study, Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis Case‐Control Study; PE, pulmonary embolism; SCCS, self‐controlled case series; VTE, venous thromboembolism.
Provoked VTE was considered if: malignancy, trauma/surgery/ immobilisation, plaster cast, oestrogen use, pregnancy/puerperium, travel >4 h
The diseases listed in this table were self‐reported. Data on chronic disease or malignancy in the past 5 years were missing in 38 patients. Data on the date of malignancy diagnosis was missing in one patient.
Non‐Hodgkin lymphoma, Hodgkin lymphoma, leukaemia.
Cervix, endometrium, ovary.
Prostate or testis cancer. Gastrointestinal cancer included: colon, stomach, oesophagus, liver or pancreas cancer. Urinary cancer included bladder or kidney cancer.
Table II shows the IRR of first VTE for oral glucocorticoid treatment. The risk of a first VTE event was 3·51‐fold (95% CI 2·55–4·80) higher in the total period of oral glucocorticoid treatment as compared with unexposed periods. The IRR of a first VTE event was 2·53 (95% CI 1·10–5·72) in the week before treatment started, reflecting the risk for VTE related to the underlying condition, and increased to 5·28 (95% CI 2·89–9·53) after glucocorticoid treatment started (first 7 days with treatment). The IRR of first VTE remained elevated (IRR 3·67, 95% CI 2·64–5·23) until 6 months of treatment and was reduced to 1·55 (95% CI 0·85–3·12) during prolonged treatment (>6 months of oral glucocorticoids), as compared with unexposed periods (Table II).
Table II.
Results from the SCCS analysis for the use of oral glucocorticoid and risk of first VTE. A total of 363 patients with a first VTE from the MEGA Study (the Netherlands, 1999–2004) received at least one outpatient prescription of oral glucocorticoids and were included in the SCCS analysis.
| All VTE episodes | |
|---|---|
| Oral glucocorticoid | (363 cases) |
| IRR (95% CI) | |
| Any oral glucocorticoid | |
| No oral glucocorticoid | reference |
| Use of glucocorticoid | 3·51 (2·50; 4·88) |
| Type of glucocorticoid drug | |
| No oral glucocorticoid | reference |
| Prednisone Prednisolone or Triamcinolon | 3·15 (2·23; 4·55) |
| Dexamethasone or Betamethasone | 3·39 (1·80; 6·56) |
| Initial daily equivalent prednisolone dose* | |
| 0 mg | reference |
| ≤7·5 mg | 3·68 (2·07; 6·45) |
| 7·5–20 mg | 3·08 (1·89; 4·92) |
| 21–39 mg | 3·72 (1·91; 7·23) |
| ≥40 mg | 4·04 (2·13; 7·67) |
| 30‐day cumulative equivalent prednisolone dose at the time of VTE# | |
| 0 mg | Reference |
| ≤300 mg | 3·38 (2·28; 5·02) |
| 300–2000 mg | 3·51 (2·34; 5·33) |
| >2000 mg | 4·87 (1·72; 14·01) |
| Days since prescription start date | |
| No oral glucocorticoid | reference |
| 7 days before prescription | 2·53 (1·10; 5·72) |
| First 7 days with glucocorticoid treatment | 5·28 (2·89; 9·53) |
| 8–30 days with glucocorticoid treatment | 3·83 (2·32; 6·34) |
| 31–180 days with glucocorticoid treatment | 3·59 (2·41; 5·27) |
| >180 days with glucocorticoid treatment | 1·55 (0·85; 3·12) |
CI, confidence interval; IRR, incidence rate ratio; MEGA study, Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis Case‐Control Study; SCCS, self‐controlled case series; VTE, venous thromboembolism.
Initial daily doses were obtained from the first prescription of the treatment.
Cumulative dose was calculated by summing the consecutive daily doses in the last 30 days of the treatment.
The observed risk of VTE did not change with the type of the oral glucocorticoid prescribed. The IRR of a first VTE was 3·15 (95% CI 2·23–4·55) for prednisone, prednisolone or triamcinolone use and 3·39 (95% CI 1·80–6·56) in periods of dexamethasone or betamethasone use, as compared with periods without oral glucocorticoid treatment. For this analysis we did not consider treatments with cortisone, hydrocortisone or with combinations of several types of glucocorticoids separately because the number of prescriptions in these groups was small. A dose‐dependent relationship between oral glucocorticoids and VTE risk was not observed with increases either in 30‐day cumulative dose or with the initial dose (Table II).
The IRRs for DVT (3·89, 95% CI 2·45–6·03) and PE (3·11, 95% CI 2·01–4·86) were similar and the IRR for unprovoked VTE (2·44, 95% CI 1·33–4·72) was lower than the IRR for provoked VTE (4·16, 95% CI 2·90–6·02). To rule out the effect of malignancy on the risk of provoked VTE, we performed a sensitivity analysis removing 94 patients who had VTE and malignancy. This analysis did not substantially change the results (Table III).
Table III.
Results from the SCCS analysis for the use of oral glucocorticoid and risk of different types of VTE during the glucocorticoid treatment period. A total of 363 patients with a first VTE from the MEGA Study (the Netherlands, 1999–2004) received at least one outpatient prescription of oral glucocorticoids and were included in the SCCS analysis.
| Type of VTE (n = 363) | IRR (95% CI) |
|---|---|
| DVT only (n = 182) | 3·89 (2·45; 6·03) |
| PE ± DVT (n = 181) | 3·11 (2·01; 4·86) |
| Unprovoked (n = 103) | 2·44 (1·33; 4·72) |
| Provoked (n = 241) | 4·16 (2·90; 6·02) |
| Provoked without malignancy (n = 147) | 5·03 (3·08; 8·23) |
CI, confidence interval; DVT, deep vein thrombosis; IRR, incidence rate ratio; MEGA study, Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis Case‐Control Study; PE, pulmonary embolism; SCCS, self‐controlled case series; VTE, venous thromboembolism.
Provoked VTE was considered if: malignancy, trauma/surgery/ immobilisation, plaster cast, oestrogen use, pregnancy/puerperium, travel >4 h; Unprovoked VTE was considered if no risk factor was present at the time of the event. We considered VTE provoked by malignancy if there was a diagnosis of neoplasia in the 5 years prior to the first VTE event. Data on malignancy was missing in four patients.
Risk of recurrent VTE with oral glucocorticoid treatment
All 2547 patients with a first VTE event who were linked to the SFK data register were included in these analyses. During a median (IQR) follow‐up of 2071 (632–2658) days, 2123 patients did not receive prescriptions of oral glucocorticoids and 424 patients received a total of 857 oral glucocorticoids prescriptions. The median (IQR) number of prescriptions was 1 (1–2) per patient and the median (IQR) duration of oral glucocorticoid treatment was 128 (46–304) days per prescription. The mean (SD) age of those who received oral glucocorticoid prescriptions was 53 (12) years, 47% were male and 19% had had a malignancy in the previous 5 years. Among those who did not receive oral glucocorticoid prescriptions, the mean (SD) age was 49 (12) years, 46% were male and 7% had had a malignancy in the previous 5 years.
A total of 367 patients had a recurrent VTE during the follow‐up period. Of them, 13 patients (8%) had a VTE recurrence during oral glucocorticoid use. As shown in Table IV, the absolute risk of recurrent VTE was 2·8%/year during periods without oral glucocorticoids (unexposed periods) and was 7·5%/year during periods of oral glucocorticoid treatment. The risk of recurrent VTE was 2·72‐fold greater (95% CI 1·64–4·78) during treatment periods as compared to baseline periods and did not vary substantially according to the duration of treatment.
Table IV.
Results from the cohort study for the risk of recurrent VTE related to periods of glucocorticoid use. All 2547 patients with a first VTE from the MEGA Study (the Netherlands, 1999–2004) who were linked to the Stichting Farmaceutische Kengetallen (SFK) data register were included in these analyses.
| Oral glucocorticoids | Observation years | Recurrent events | Incidence rate per 1000 person‐years (95% CI) | HR (95% CI) | HR (95% CI)* |
|---|---|---|---|---|---|
| Aggregated period of oral glucocorticoid treatment | |||||
| No oral glucocorticoid | 12435 | 354 | 28·47 (25·67; 31·55) | Reference | Reference |
| Oral glucocorticoid treatment | 172 | 13 | 75·38 (43·78; 129·82) | 2·45 (1·42; 4·36) | 2·72 (1·64; 4·78) |
| Periods with treatment | |||||
| No oral glucocorticoid | 12435 | 354 | 28·47 (25·67; 31·55) | Reference | Reference |
| ≤180 days with glucocorticoid treatment | 103 | 7 | 67·86 (32·28; 142·24) | 2·48 (1·19; 5·15) | 2·61 (1·23; 5·36) |
| >180 days with glucocorticoid treatment | 69 | 6 | 86·69 (39·04; 193·09) | 2·64 (1·23; 5·78) | 2·86 (1·26; 6·56) |
CI, confidence interval; HR, hazard ratio; IRR, incidence rate ratio; MEGA study, Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis Case‐Control Study; VTE, venous thromboembolism.
Adjusted for age and sex.
We also estimated the incidence rate of recurrent VTE separately for patients with provoked or unprovoked first VTE event during a period of glucocorticoid treatment. The results are shown in Table V. The lowest absolute risk of recurrent VTE was seen in patients with provoked first VTE who were not using glucocorticoids at the time of their event (2·2%/year). This was elevated in patients with an unprovoked first VTE (i.e. 4·5%/year) without glucocorticoids and in those who had their first VTE during a period of oral glucocorticoid treatment, either if their event was otherwise classified as provoked (4·9%/year) or unprovoked (7·8%/year). The age‐ and sex‐adjusted HRs for recurrent VTE were 1·6 (95% CI 1·2–2·0) in patients with unprovoked first VTE not associated with oral glucocorticoids, 2·1 (95% CI 1·2–3·8) in those with glucocorticoid‐related provoked first VTE and 2·3 (95% CI 1·2–7·0) in those with glucocorticoid‐related unprovoked VTE, as compared with patients with a provoked first event not associated with oral glucocorticoids (Table V). The results did not change substantially after excluding patients with a history of malignancy in the previous 5 years, as shown in Table VI.
Table V.
Results from the cohort study for the risk of recurrent VTE related to glucocorticoid use at the day of the first venous thromboembolism. All 2547 patients with a first VTE from the MEGA Study (the Netherlands, 1999–2004) who were linked to the Stichting Farmaceutische Kengetallen (SFK) data register were included in these analyses.
| Oral glucocorticoids | Observation years | Recurrent events | IRR per 1000 person‐years (95% CI) | HR (95% CI) | HR (95% CI)* |
|---|---|---|---|---|---|
| Provoked 1st VTE/no glucocorticoids | 8557 | 185 | 21·62 (18·67; 24·97) | Reference | Reference |
| Unprovoked 1st VTE/no glucocorticoids | 3447 | 156 | 45·26 (38·68; 52·94) | 2·09 (1·72; 2·56) | 1·62 (1·24; 2·0) |
| Provoked 1st VTE/glucocorticoids | 245 | 12 | 48·98 (27·82; 86·24) | 2·27 (1·26; 4·04) | 2·06 (1·22; 3·76) |
| Unprovoked 1st VTE/glucocorticoids | 64 | 5 | 78·41 (32·55; 188·32) | 3·37 (1·38; 8·34) | 2·29 (1·19; 7·04) |
1st VTE, first venous thromboembolism; CI, confidence interval; HR, hazard ratio; IRR, incidence rate ratio; MEGA study, Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis Case‐Control Study.
Adjusted for age and sex.
Table VI.
Results from the cohort study for the risk of recurrent VTE stratified by glucocorticoid use at the time of first venous thromboembolism, excluding patients with malignancy. All 2547 patients with a first VTE from the MEGA Study (the Netherlands, 1999–2004) who were linked to the Stichting Farmaceutische Kengetallen (SFK) data register were included in these analyses.
| Oral glucocorticoids | Observation years | Recurrent events | IRR per 1000 person‐years (95% CI) | HR (95% CI) | HR (95% CI)* |
|---|---|---|---|---|---|
| Provoked 1st VTE/no glucocorticoids | 7907 | 161 | 20·36 (17·39; 23·76) | Reference | Reference |
| Unprovoked 1st VTE/no glucocorticoids | 3447 | 156 | 45·26 (38·68; 52·94) | 2·22 (1·78; 2·77) | 1·64 (1·32; 2·13) |
| Provoked 1st VTE/glucocorticoids | 207 | 10 | 48·21 (25·89; 89·58) | 2·27 (1·24; 4·39) | 2·20 (1·21; 4·24) |
| Unprovoked 1st VTE/glucocorticoids | 64 | 5 | 78·43 (32·56; 188·32) | 3·58 (1·47; 8·84) | 2·44 (1·19; 7·34) |
1st VTE, first venous thromboembolism; CI, confidence interval; HR, hazard ratio; IRR, incidence rate ratio; MEGA study, Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis Case‐Control Study.
Adjusted for age and sex.
Discussion
In the present study, the risk of incident VTE was 3·5‐fold higher during periods of treatment with oral glucocorticoids than in periods without glucocorticoids, and were notably highest during the first weeks after prescription of glucocorticoids. These results are consistent with observational data from a large population‐based case‐control study 5 and from a recent SCCS study 6 that reported a roughly threefold increase in the risk of VTE. An important advantage of our present study is that the SCCS method by design adjusts for fixed confounders such as age, frailty and socioeconomic status, including both measured and unmeasured confounders. However, as with the other observational studies, 4 , 5 , 6 our present results are susceptible to time‐varying confounders, mainly represented by exacerbation of the underlying disease. To address this issue, we separated the total period of exposure to oral glucocorticoids into five periods representing the effect of the exacerbation of the disease on the risk of VTE and short‐ and long‐term glucocorticoid use. 28 , 29 Relative to the unexposed period, the risk of incident VTE increased by 2·5‐fold 1 week before the prescription of an oral glucocorticoid and by four to fivefold in the following weeks after the treatment started. These results demonstrate that there is an increased risk of VTE due to the underlying disease, particularly when the condition is severe enough to require a glucocorticoid treatment. However, after starting treatment this risk further increases and remains elevated during the entire treatment period, also when the disease activity is expected to be controlled. Therefore, our present findings suggest that oral glucocorticoid use adds to the disease‐associated risk of VTE. Pathophysiological studies support such an association from a causal point of view, as it has been shown previously in a randomised clinical trial that oral glucocorticoids induce a pro‐coagulant state in healthy individuals. 35
While observing a time‐dependent relationship, we did not find a clear dose–response association between oral glucocorticoids and VTE risk. We observed that VTE risk was increased >three‐times with doses as low as 7·5 mg (prednisone equivalent)/day. These findings are confirmatory to Waljee et al., 6 who have previously described a threefold increase in the risk of VTE with doses of <20 mg/day. Although the association between low‐dose glucocorticoids and VTE risk may appear counterintuitive, concerns about the side‐effects of low dosages exist 33 and several adverse events, including weight gain, eye cataract, acne and skin bruising, also occur in patients taking low doses of the drug. 36 Given that low‐dose glucocorticoids are often prescribed when the disease activity of the initial condition warranting its prescription is not severe, these results further suggest that oral glucocorticoids have a direct effect on the risk of VTE that seems independent of the underlying disease activity. Therefore, it is possible that VTE could be a Type B adverse event of oral glucocorticoids; i.e. an adverse event that is independent of dose and affects a previously susceptible population. 37
In our present study, oral glucocorticoids were associated not only with the risk of a first VTE event, but also with the risk of recurrent VTE. Having had a first VTE event while taking oral glucocorticoids doubled the risk of a recurrent event, regardless of the cause of the first event (provoked or unprovoked). Our present results also showed that the prognosis of glucocorticoid‐associated VTE was worse than that of unprovoked VTE, as the risk of recurrence was 1·6‐fold increased with unprovoked VTE and twofold increased with glucocorticoid‐associated VTE. Unprovoked VTE is a predictor of worse prognosis and may be an indication for continuous anticoagulation. 38 Therefore, a careful follow‐up of patients with glucocorticoid‐associated VTE may be necessary. We also observed that the risk of recurrent VTE was increased more than twice during a period of oral glucocorticoid use, regardless of the duration of the treatment. This finding highlights that patients with a prior VTE are under higher risk of recurrence in periods of glucocorticoid treatment indication. This finding further reinforces that careful follow‐up during these periods may be necessary.
Some limitations to our present study need to be considered in order to interpret the results. First, we had data on glucocorticoid use for only half of the patients of the MEGA study, as only half of the patients could be linked to the SFK registry. Linkage was based on age, sex, four‐digit postal code and anti‐coagulant use in the month after VTE, which did not lead to a unique match with a Dutch citizen for >54% of the patients. However, clinical characteristics of patients linked or not linked to the SFK registry were similar, suggesting no bias because of this limitation. Furthermore, the incidence rate of recurrent thrombosis among linked patients was similar to that of the entire MEGA study patients. The overall incidence rate of recurrent VTE amongst linked patients was 29·11 per 1000 person‐years (95% CI 26·25–32·21), while the reported incidence rate of recurrent VTE in the MEGA study was 26·40 per 1000 person‐years (95% CI 24·18–28·78). 39 Second, we could not detect the condition that motivated the prescription of oral glucocorticoids because these diagnoses were not available in the SFK registry. By using the SCCS method, we controlled for fixed confounding such as chronic disease and socio‐economic status. Time‐varying confounding was controlled for using information on the pre‐exposure period and initial and cumulative doses as surrogate measurements for disease severity. This approach seems justifiable as the condition must be sufficiently severe before a treatment with oral glucocorticoids is prescribed, particularly at high dose, and must be under control when the dose is reduced. 11 The choice of a 7‐day pre‐exposure period could be a limitation, as periods may vary according to the underlying disease. However, the 7‐day period was chosen because VTE events are mostly diagnosed within 7 days from the onset of symptoms 40 and we assumed this period to be representative of the underlying disease exacerbation peak as it shortly preceded the requirement for glucocorticoid treatment. Even though it is not possible with our present data to completely separate the effect of the drug from that of the underlying condition because these effects are coincident, it is worth noting that no observational study design would be capable of completely separating these effects, while SCCS with the use of different time periods is the design that comes closest to that purpose. Third, we only evaluated data on outpatient oral glucocorticoid treatment, so treatments during hospital stays were not available. Furthermore, treatments with non‐oral glucocorticoids (inhaled, injections, intestinal‐acting) were not evaluated because the systemic bioavailability of these drugs is heterogeneous, 41 , 42 particularly when compared with that of oral glucocorticoids. 43 Also, data on intravenous drugs were incomplete, as data on treatments during hospital stays were not available. Fifth, we only adjusted for age and sex in the Cox regression analysis because of small numbers and it is possible that other confounders played a role in the results on recurrent VTE.
In conclusion, patients receiving oral glucocorticoids have a more than threefold increase in the risk of a first VTE. Patients with prior VTE have a twofold increase in the risk of VTE recurrence during periods of oral glucocorticoid treatment. As oral glucocorticoids are commonly prescribed for a wide range of conditions, 44 awareness of the drug‐associated risk of VTE may improve treatment strategies to prevent this complication. Although there is not enough evidence to support changes in current indication for oral glucocorticoid treatment, patients with a higher risk of VTE, including those with a prior VTE event, should be treated and followed with caution. Given the risk of incident and recurrent VTE associated with oral glucocorticoids, future studies are warranted to examine whether prophylactic anticoagulation is beneficial for patients starting oral glucocorticoid treatment, in particular for those at high risk of VTE.
Author contributions
Fernanda A. Orsi performed the statistical analyses and drafted the manuscript; Willem M. Lijfering performed the statistical analyses, designed the study and revised the manuscript; Geert‐Jan Geersing and Frits R. Rosendaal revised the manuscript; Olaf M. Dekkers designed the study and revised the manuscript, Saskia le Cessie designed and supervised the statistical analysis and revised the manuscript, Suzanne C. Cannegieter was responsible for the study concept, designed the analysis and revised the manuscript.
Conflicts of interest
The authors declare no competing financial interests.
Supporting information
Table SI. Demographic and clinical characteristics of patients linked and not linked to the Stichting Farmaceutische Kengetallen (SFK) registry at time of first venous thromboembolism event.
Table SII. Baseline and demographic and clinical characteristics of patients (n = 2547) linked to the Stichting Farmaceutische Kengetallen (SFK) registry by exposure to oral glucocorticoids.
Acknowledgements
This study was supported by grants from Dutch‐Heart‐Foundation (NHS 98·113), Dutch‐Cancer‐Foundation (RUL 99/1992) and The Netherlands Organization for Scientific Research (912‐03‐033‐2003). Fernanda A. Orsi received financial support from São Paulo Research Foundation (FAPESP grant#2017/09506‐5). Role of the funding sources: the sponsors had no role in study design, data collection, data analysis, data interpretation, or the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
We express our gratitude to all individuals who participated in the MEGA study. The authors thank the Dutch Foundation for Pharmaceutical Statistics for making data available from the SFK registry.
References
- 1. Buttgereit F, Burmester GR, Lipworth BJ. Optimised glucocorticoid therapy: the sharpening of an old spear. Lancet. 2005;365:801–3. [DOI] [PubMed] [Google Scholar]
- 2. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23. [DOI] [PubMed] [Google Scholar]
- 3. Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huerta C, Johansson S, Wallander MA, Garcia Rodriguez LA. Risk factors and short‐term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167:935–43. [DOI] [PubMed] [Google Scholar]
- 5. Johannesdottir SA, Horvath‐Puho E, Dekkers OM, Cannegieter SC, Jorgensen JO, Ehrenstein V, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population‐based case‐control study. JAMA Intern Med. 2013;173:743–52. [DOI] [PubMed] [Google Scholar]
- 6. Waljee AK, Rogers MA, Lin P, Singal AG, Stein JD, Marks RM, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heit JA. Cardiovascular endocrinology: Risk of venous thromboembolism with glucocorticoids. Nat Rev Endocrinol. 2013;9:387–8. [DOI] [PubMed] [Google Scholar]
- 8. Avina‐Zubieta JA, Jansz M, Sayre EC, Choi HK. The risk of deep venous thrombosis and pulmonary embolism in primary Sjogren syndrome: a general population‐based study. J Rheumatol. 2017;44:1184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bertoletti L, Quenet S, Mismetti P, Hernandez L, Martin‐Villasclaras JJ, Tolosa C, et al. Clinical presentation and outcome of venous thromboembolism in COPD. Eur Respir J. 2012;39:862–8. [DOI] [PubMed] [Google Scholar]
- 10. Carruthers EC, Choi HK, Sayre EC, Avina‐Zubieta JA. Risk of deep venous thrombosis and pulmonary embolism in individuals with polymyositis and dermatomyositis: a general population‐based study. Ann Rheum Dis. 2016;75:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657–63. [DOI] [PubMed] [Google Scholar]
- 12. Peeters PJ, Bazelier MT, Uitdehaag BM, Leufkens HG, De Bruin ML, de Vries F. The risk of venous thromboembolism in patients with multiple sclerosis: the Clinical Practice Research Datalink. J Thromb Haemost. 2014;12:444–51. [DOI] [PubMed] [Google Scholar]
- 13. Zoller B, Pirouzifard M, Memon AA, Sundquist J, Sundquist K. Risk of pulmonary embolism and deep venous thrombosis in patients with asthma: a nationwide case‐control study from Sweden. Eur Respir J. 2017;49:1601014. [DOI] [PubMed] [Google Scholar]
- 14. Higgins PD, Skup M, Mulani PM, Lin J, Chao J. Increased risk of venous thromboembolic events with corticosteroid vs biologic therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13:316–21. [DOI] [PubMed] [Google Scholar]
- 15. Majoor CJ, Kamphuisen PW, Zwinderman AH, Ten Brinke A, Amelink M, Rijssenbeek‐Nouwens L, et al. Risk of deep vein thrombosis and pulmonary embolism in asthma. Eur Respir J. 2013;42:655–61. [DOI] [PubMed] [Google Scholar]
- 16. Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence‐based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–31. [DOI] [PubMed] [Google Scholar]
- 17. van der Goes MC, Jacobs JW, Boers M, Andrews T, Blom‐Bakkers MA, Buttgereit F, et al. Monitoring adverse events of low‐dose glucocorticoid therapy: EULAR recommendations for clinical trials and daily practice. Ann Rheum Dis. 2010;69:1913–9. [DOI] [PubMed] [Google Scholar]
- 18. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res 2016;118:1340–7. [DOI] [PubMed] [Google Scholar]
- 19. Whitaker HJ, Hocine MN, Farrington CP. The methodology of self‐controlled case series studies. Stat Methods Med Res. 2009;18:7–26. [DOI] [PubMed] [Google Scholar]
- 20. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. [DOI] [PubMed] [Google Scholar]
- 21. Timp JF, Cannegieter SC, Tichelaar V, Braekkan SK, Rosendaal FR, le Cessie S, et al. Antibiotic use as a marker of acute infection and risk of first and recurrent venous thrombosis. Br J Haematol. 2017;176:961–70. [DOI] [PubMed] [Google Scholar]
- 22. Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing GJ, Kyrle PA, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14:1480–3. [DOI] [PubMed] [Google Scholar]
- 23. Flinterman LE, van Hylckama VA, Cannegieter SC, Rosendaal FR. Long‐term survival in a large cohort of patients with venous thrombosis: incidence and predictors. PLoS Med. 2012;9:e1001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self‐controlled case series method. Stat Med. 2006;25:1768–97. [DOI] [PubMed] [Google Scholar]
- 25. Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. [DOI] [PubMed] [Google Scholar]
- 26. Man KK, Coghill D, Chan EW, Lau WCY, Hollis C, Liddle E, et al. Association of risk of suicide attempts with methylphenidate treatment. JAMA Psychiatry. 2017;74:1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong AY, Wong IC, Chui CS, Lee EH, Chang WC, Chen EY, et al. Association Between Acute Neuropsychiatric Events and Helicobacter pylori Therapy Containing Clarithromycin. JAMA Intern Med. 2016;176:828–34. [DOI] [PubMed] [Google Scholar]
- 28. Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long‐term side effects of glucocorticoids. Expert Opin Drug Safe. 2016;15:457–65. [DOI] [PubMed] [Google Scholar]
- 29. Strehl C, Bijlsma JW, de Wit M, Boers M, Caeyers N, Cutolo M, et al. Defining conditions where long‐term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann Rheum Dis. 2016;75:952–7. [DOI] [PubMed] [Google Scholar]
- 30. Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43:1216–27. [DOI] [PubMed] [Google Scholar]
- 31. Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary‐adrenal function. Am J Med. 1977;63:200–7. [DOI] [PubMed] [Google Scholar]
- 32. Jacobs JW, Bjjlsma JW. Glucocorticoid therapy. In: Firestein GS, Budd RC, Harris EDJ, McInnes IB, Ruddy S, Sergent JS, eds, Kelley’s Textbook of Rheumatology. Philadelphia, PA, USA: Sauders, 2008. p. 865–8. [Google Scholar]
- 33. Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, et al. Population‐based assessment of adverse events associated with long‐term glucocorticoid use. Arthritis Rheum. 2006;55:420–6. [DOI] [PubMed] [Google Scholar]
- 34. Santiago T, da Silva JA. Safety of low‐ to medium‐dose glucocorticoid treatment in rheumatoid arthritis: myths and reality over the years. Ann NY Acad Sci. 2014;1318:41–9. [DOI] [PubMed] [Google Scholar]
- 35. Majoor CJ, Sneeboer MM, de Kievit A, Meijers JC, van der Poll T, Lutter R, et al. The influence of corticosteroids on hemostasis in healthy subjects. J Thromb Haemost. 2016;14:716–23. [DOI] [PubMed] [Google Scholar]
- 36. Huscher D, Thiele K, Gromnica‐Ihle E, Hein G, Demary W, Dreher R, et al. Dose‐related patterns of glucocorticoid‐induced side effects. Ann Rheum Dis. 2009;68:1119–24. [DOI] [PubMed] [Google Scholar]
- 37. ASHP guidelines on adverse drug reaction monitoring and reporting. American Society of Hospital Pharmacy. Am J Health Syst Pharm. 1995;52:417–9. [DOI] [PubMed] [Google Scholar]
- 38. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 39. Timp JF, Braekkan SK, Lijfering WM, van Hylckama VA, Hansen JB, Rosendaal FR, et al. Prediction of recurrent venous thrombosis in all patients with a first venous thrombotic event: The Leiden Thrombosis Recurrence Risk Prediction model (L‐TRRiP). PLoS Medicine. 2019;16:e1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elliott CG, Goldhaber SZ, Jensen RL. Delays in diagnosis of deep vein thrombosis and pulmonary embolism. Chest. 2005;128:3372–6. [DOI] [PubMed] [Google Scholar]
- 41. Kapadia CR, Nebesio TD, Myers SE, Willi S, Miller BS, Allen DB, et al. Endocrine effects of inhaled corticosteroids in children. JAMA Pediatr. 2016;170:163–70. [DOI] [PubMed] [Google Scholar]
- 42. Wales D, Makker H, Kane J, McDowell P, O'Driscoll BR. Systemic bioavailability and potency of high‐dose inhaled corticosteroids: a comparison of four inhaler devices and three drugs in healthy adult volunteers. Chest. 1999;115:1278–84. [DOI] [PubMed] [Google Scholar]
- 43. Bonovas S, Nikolopoulos GK, Lytras T, Fiorino G, Peyrin‐Biroulet L, Danese S. Comparative safety of systemic and low‐bioavailability steroids in inflammatory bowel disease: Systematic review and network meta‐analysis. Br J Clin Pharmacol. 2018;84:239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laugesen K, Jorgensen JOL, Petersen I, Sorensen HT. Fifteen‐year nationwide trends in systemic glucocorticoid drug use in Denmark. Eur. J. Endocrinol. 2019;181:267–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Demographic and clinical characteristics of patients linked and not linked to the Stichting Farmaceutische Kengetallen (SFK) registry at time of first venous thromboembolism event.
Table SII. Baseline and demographic and clinical characteristics of patients (n = 2547) linked to the Stichting Farmaceutische Kengetallen (SFK) registry by exposure to oral glucocorticoids.


