To the Editor:
Newly diagnosed multiple myeloma (NDMM) patients who achieve and sustain minimal residual disease (MRD) negative complete response/stringent complete response (CR/sCR) demonstrate clinical benefit with prolonged progression‐free survival and overall survival. 1 In this phase I/II clinical trial, we investigated twice weekly high doses of carfilzomib (45 and 56 mg/m2) in combination with lenalidomide and dexamethasone. We also explored the prevailing doctrine of fixed number of cycles of induction therapy by integrating MRD testing into the clinic to guide the total number of cycles delivered during induction therapy. To our knowledge, this is the first trial reporting results that fully incorporate MRD testing into a clinical decision‐making algorithm for NDMM.
We conducted a single center phase I/II clinical trial investigating high doses of twice‐weekly carfilzomib in combination with lenalidomide and dexamethasone NDMM patients. The study (NCT 02937571) was approved by Memorial Sloan Kettering Institutional Review Board. Phase I consisted of a standard 3 + 3 schema design based on dose‐limiting toxicities occurring in cycle one. Phase II used a Simon's optimal two‐stage design at the MTD dose to determine proportion of patients achieving CR/sCR MRD negativity within 12 cycles (CR/sCR MRD‐negative unpromising rate 20% and promising rate 45%). Treatment consisted of 28‐day cycles with carfilzomib 20/45 mg/m2 or 20/56 mg/m2 on days 1, 2, 8, 9 15 and 16; lenalidomide 25 mg on days 1–21′ and dexamethasone 40 mg weekly (cycles 1–4) then 20 mg weekly (after cycle four). Patients achieving CR/sCR (defined by IMWG 2016 criteria) underwent MRD testing. Patients achieving MRD‐negative status received two additional cycles from conversion time and ceased further protocol therapy, while patients with less than an MRD‐negative response continued therapy until treatment completion (max 12 cycles), disease progression, or unacceptable toxicity. Patients deemed eligible for autologous hematopoietic cell transplantation (AHCT) underwent stem cell collection after six cycles, and then continued with therapy. Decision about AHCT and maintenance was deferred until after completion of protocol therapy. Multiparametric flow cytometry MRD assessments were performed on the first pull of each aspirate in a single 10‐color tube with a limit of detection of at least 6x10−6 (ie, three cells in 1 million) with at least 3 million cell acquisitions. 2
Between October 2016 and June 2018, 29 patients with NDMM gave consent and enrolled in the study. Data cutoff was May 14, 2020. Baseline on the 29 patients in demographics are outlined (online Appendix S1, Figures [Link], [Link], Tables S1 and S2). Nine patients were treated in the phase I portion of the trial (3 ‐ KRd‐45 and 6 ‐ KRd‐56). In the phase I portion, no DLTs occurred and the MTD of 56 mg/m2 of twice‐weekly carfilzomib was further investigated. An additional 20 patients [eight patients stage one + 11 patients stage two + one patient withdrew consent] were enrolled and treated with KRd‐56 in phase II. One patient withdrew consent after one cycle of therapy was evaluable for toxicity, and 28 patients were evaluable for toxicity and response. The median follow‐up for the study was 36.7 months (95% CI: 27.6–39.8 months). In phase 1, no DLTs occurred in KRd‐45 or KRd‐56 dose level cohorts within first cycle. The most common grade 3/4 hematologic treatment related adverse events (TRAE) was lymphopenia 7(24%) and the most common grade 3/4 non‐hematologic TRAE was electrolyte abnormalities 10 (34%) (Figure 1A). Thirteen serious adverse events occurred: seven (24%) infections, one (3%) atrial fibrillation, one (3%) non‐ST elevation myocardial infarction (NSTEMI), one (3%) thromboembolic, one (3%) decreased ejection fraction, one (3%) gastrointestinal perforation, and one (3%) severe constipation. All grade cardiac TRAE was 17% with serious grade 3/4 at the following: atrial fibrillation two (6%), NSTEMI myocardial infarction one (3%), and decreased ejection fraction one (3%).
FIGURE 1.
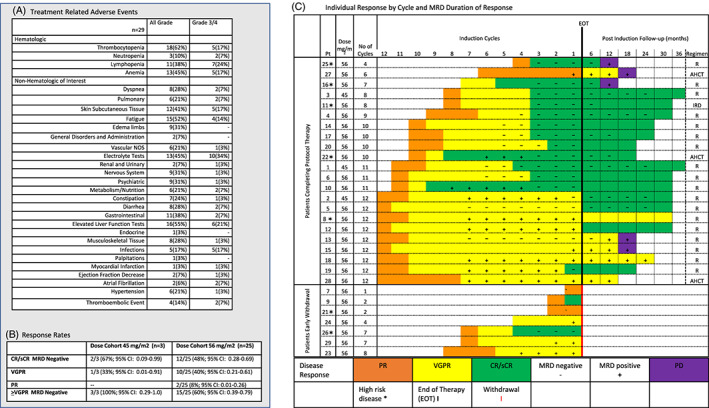
(A) Treatment Related Adverse Events of Interest. Complete list online Appendix S1, Figures [Link], [Link], Tables S1 and S2. (B) Overall Response Rates by Dosing Cohort. (C) Individual Response by Cycle and Follow‐up MRD Duration of Response R‐ Lenalidomide, AHCT – Autologous hematopoietic cell transplant, IRD – ixaxomib, lenalidomide, dexamethasone. Twenty two patients completed cycle trial therapy and remain in follow‐up. For the 22 patients completing the study treatment, 18 patients went onto lenalidomide maintenance (7 VGPR and 11 CR/sCR MRD negative), three patients received AHCT immediately (2 VGPR +1 PR), and one patient received ixazomib‐based therapy. A total of five patients progressed during follow‐up period and went onto other second line therapies not shown (1 AHCT, 3 daratumumab‐based therapy, and 1 ixazomib‐based therapy). Seven patients came off study early. One patient (PT 7) was evaluable for toxicity but unevaluable for response due withdrawal of consent after one cycle of therapy. Six patients came off of study early but evaluable for response: two patients withdrew from trial due to personal choice (PT 23 and 24), two patients came off due to rash or allergy (PT 29 and 21), one patient came off study due to non‐ST elevation myocardial infarction (PT 9), and one patient came off due to persistent neutropenia after seven cycles (PT 26)
Best responses at MTD dose level KRd‐56 were CR/sCR MRD‐negative 12/25 (48%; 95% CI: 0.28–0.69), CR/sCR MRD unconfirmed 1/25 (4%; 95% CI: 0–0.2), VGPR 10/25 (40%; 95% CI: 0.21–0.61), PR 2/25 (8%; 95% CI: 0.01–0.26), ORR 25/25 (100%; 95% CI: 0.86–1.0), and ≥VGPR serum response with MRD‐negative bone marrow 15/25 (60%; 95% CI: 0.39–0.79) (Figure 1B). The median number of cycles for patients to achieve CR/sCR MRD‐negative status were eight (range, 2–12), and median number of cycles delivered to the CR/sCR MRD‐negative group were 10 (range, 2–12). Median PFS for both KRd‐56 and combined cohorts were not reached while 36‐month PFS rates were 73% (95% CI: 55%‐96%) for KRd‐56 and 77% (95% CI: 60%‐97%) for combined. Median OS was not reached for KRd‐56 cohort or combined cohorts. Additionally, with high‐risk disease being defined as R‐ISS III or unfavorable cytogenetics, 36‐month PFS was 84% (95% CI: 66%‐100%) standard‐risk vs 50% (95% CI: 22%‐100%) high‐risk in KRD‐56 cohort (P = .07) and 87% (95% CI: 72%‐100%) standard‐risk vs 50% (95% CI: 22%‐100%) high‐risk in combined cohorts (P = .03). In comparing outcomes between CR/sCR MRD‐negative patients and all others, 24‐month PFS in a one‐year landmark analysis was 89% (95% CI: 0.71–1) vs 64% (95% CI: 0.38–1) for KRd‐56 cohort (P = .3). For individual dosing cohorts and both combined dosing cohorts, the median duration of MRD‐negative response sustained in the bone marrow was not reached. At the post‐induction 12‐month MRD time point, 11/13 (85%) CR/sCR MRD‐negative patients remain MRD‐negative sustained or clinically sustained in a CR/sCR serological response, while 2/13 (15%) CR/sCR MRD‐negative patients converted to MRD positivity before meeting criteria for progressive disease (PD) (Figure 1C). Among three patients that were VGPR MRD‐negative at end of induction, two patients seroconverted to CR/sCR and remain MRD‐negative at the 12‐month follow‐up mark (patient two and five), while one patient turned MRD‐positive at 12‐month time‐point before having PD (patient 13). For the six patients who were MRD‐positive at the conclusion of induction therapy, two remain MRD‐positive at the 12‐month MRD time‐point, two patients seroconverted to CR/sCR from VGPR (MRD uncertain), and two patients sustained their MRD‐positive response at the 12‐month MRD time‐point before PD.
In this phase I/II study, we found KRd‐56 to be tolerable and effective in NDMM patients. The overall toxicity profile of KRd‐45 and KRd‐56 was comparable to KRd‐36 regimens 3 with an all‐grade cardiac TRAE of 17% and grade 3/4 of 3%‐6%, similar to previously described all‐grade at 18.1% and grade 3/4 at 8.2%. 4 The KRd‐56 regimen yielded a CR/sCR MRD‐negative rate of 48% and a ≥VGPR MRD‐negative rate of 60%, meeting primary endpoint and similar to KRd‐36 regimens with and without AHCT.3, 5 As an exploratory approach, our study took an alternative strategy to the existing framework of a fixed number of cycles followed by upfront AHCT by delivering personalized tailored number of cycles based on MRD response and delaying the timing of AHCT. The MRD status directed induction is supported by the observation in the IFM 2009 study that NDMM patients achieving MRD‐negative disease status (10−6) receiving RVd combination therapy followed by maintenance irrespective of upfront or delayed AHCT showed no difference in OS 6 . Ongoing studies, such as the FORTE trial (NCT02203643), are currently investigating outcomes between four cycles of KRD‐AHCT‐4 cycles of KRD and12 KRD cycles, but treatment assignments in this study are independent of MRD responses. We found that the median number of cycles delivered to patients achieving CR/sCR MRD‐negative status was eight cycles (range, 2–12), higher than the usual four cycle limit threshold followed by immediate AHCT and notably ranging in a wide number of cycles needed to achieve MRD negativity. This highlights the individualized nature of disease response. Our study capped maximum number of cycles at 12. While it is unclear if additional therapy would have further deepened response rates, we found that three patients converted from VGPR to CR/sCR during maintenance. Accordingly, most patients went onto maintenance therapy and inferior PFS was not observed. The PFS outcomes were similar between MRD‐negative and MRD‐positive response groups, possibly due to short follow‐up or lack of consistency in post‐trial therapies. For high‐risk disease, 36‐month PFS favored standard‐risk disease patients. Admittedly, it is unclear whether obtaining a simple MRD‐negative response after induction therapy is an optimal strategy for decision making in the high‐risk disease setting due to rapidly evolving kinetics. Perhaps MRD surveillance is particularly relevant in high‐risk disease patients and these patients should be considered for intensification regardless of MRD status. Although numbers are limited, this is highlighted in our study by five out of seven high‐risk cytogenetic patients achieving CR/sCR MRD negativity during induction but durability only lasted in two patients (one‐AHCT and one‐ixazomib‐lenalidomide therapy post‐induction) and the other two high‐risk disease CR/sCR MRD‐negative patients clinically progressed after lenalidomide maintenance (one dropped out). Despite these observations, an MRD response‐adapted approach demonstrated 36‐month PFS rates at 73% (95% CI: 55%‐96%) for KRd‐56 and 77% (95% CI: 60%‐97%) for combined dosing cohorts, similar to published studies of 50%‐80% PFS at 36 months.3, 5 At current follow‐up, median PFS was not reached using an MRD response adapted approach, and approximately 85% remained CR/sCR and/or MRD‐negative sustained at the post‐12 month MRD follow‐up time‐point. By way of this unique proof‐in‐concept trial design, we demonstrated the safety and efficacy of KRd‐56 as an induction regimen for NDMM, yielding high rates of MRD negativity while examining the role of MRD testing into clinical management. Future work needs to be done to validate MRD response adapted approaches and sustainability in clinical practice.
CONFLICT OF INTEREST
N.K. – Research Funding – Amgen; Consulting – Medimmune/Astra Zeneca S.M. – Honoraria ‐ PleXus Communications, Physician Education Resource; Research Funding – Juno Therapeutics (Bristol‐Myers Squibb Company), Allogene Therapeutics, Janssen Oncology, Takeda A.L. – Consulting/Honoraria ‐ BMS, Janssen, Amgen, Boehringer, Genmab; Research Funding – BMS, Janssen H.H. – Consultancy – Novartis, Research Funding – Celgene/BMS, Takeda E.S. ‐ Patents/Royalties for CAR T cells to treat multiple myeloma, BMS; Consulting: BMS, Fate Therapeutics, Precision Biosciences; N.L. – Employed by Janssen; M.H. – Research Funding – Daiichi Sankyo, GSK, Amgen, Consultancy – Intellisphere D.C. – none M.S. –Consulting: Angiocrine Bioscience, Inc., Omeros Corporation, McKinsey & Company, Kite – A Gilead Company; Speaking engagement: i3Health (CME); G.S. – Research Funding – Amgen, Janssen; O.L. – MorphoSys; H.L. – Honoraria – Takeda, Sanofi, Pfizer; Advisory board – Takeda, Sanofi, Pfizer, Janssen, Celgene, Caelum, Abbvie, Karyopharm; Research Funding – Takeda, Janssen; Consultancy – Caelum, Karyopharm; M.A. – Honorarium: Invivoscribe, Inc.; C.H.: Honorarium, Invivoscribe; M.R. – none; A.D. – Consultancy – Roche, Physicians Education Resource, Seattle Genetics, Takeda, EUSA Pharma, Abbvie, Research Funding – Corvus, NC S.G. – Research Funding – Jazz, Kite, Actinuum, CSL Behring, Pfizer, Quintiles, Janssen, Amgen, Sanofi, Celgene/BMS, Adienne; O.L. – Consultancy – Adaptive, Amgen, Celgene/BMS, Janssen, Glenmark, Cellectis, Juno, Pfizer; Honoraria – Amgen, Celgene/BMS, Janssen, Glenmark, Cellectis, Seattle Genetics, Pfizer; Research Funding – Amgen, Celgene/BMS, Glenmark, Seattle Genetics, Karyopharm, Pfizer, Binding Site; Independent Data Monitoring Committee – Janssen, Takeda; D.M., E.T., B.D., M.S., K.W., K.J., D.V., A.S., T.P., A.D., S.D., C.T., U.S., S.L. report no disclosures.
Supporting information
Appendix S1. Supporting Information.
Figure S1 Median Time to CR/sCR MRD Negative response.
Figure S2 Landmark Analysis at 1 year ‐ PFS Comparison by MRD Negative Disease Status vs. Positive Response Disease Status.
Figure S3 Duration of Response.
Table S1 Demographics
Table S2 Adverse Events ‐ Detailed
Funding information This work is supported through the following Amgen; Medication Supply – Amgen, Bristol Meyers Squibb (Formerly Celgene); Grants –P30 CA 008748, K08 CA241400, Multiple Myeloma Research Foundation (MMRF) translational research grant GC242190, Sawiris Family Foundation and Parker Institute for Cancer Immunotherapy; Awards – Kroll Award 2019
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Munshi NC, Avet‐Loiseau H, Anderson KC, et al. A large meta‐analysis establishes the role of MRD negativity in long‐term survival outcomes in patients with multiple myeloma. Blood Adv. 2020;4(23):5988‐5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roshal M, Flores‐Montero JA, Gao Q, et al. MRD detection in multiple myeloma: comparison between MSKCC 10‐color single‐tube and EuroFlow 8‐color 2‐tube methods. Blood Adv. 2017;1(12):728‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Landgren O, Sonneveld P, Jakubowiak A, et al. Carfilzomib with immunomodulatory drugs for the treatment of newly diagnosed multiple myeloma. Leukemia. 2019;33(9):2127‐2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waxman AJ, Clasen S, Hwang WT, et al. Carfilzomib‐associated cardiovascular adverse events: a systematic review and meta‐analysis. JAMA Oncol. 2018;4(3):e174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jasielec J, Kubicki T, Raje N, et al. Carfilzomib, lenalidomide, and dexamethasone plus transplant in newly diagnosed multiple myeloma. Blood. 2020;136:2513‐2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Attal M, Lauwers‐Cances V, Hulin C, et al. Lenalidomide, Bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311‐1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Figure S1 Median Time to CR/sCR MRD Negative response.
Figure S2 Landmark Analysis at 1 year ‐ PFS Comparison by MRD Negative Disease Status vs. Positive Response Disease Status.
Figure S3 Duration of Response.
Table S1 Demographics
Table S2 Adverse Events ‐ Detailed
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


