Abstract
Aim
To assess the effects of oral semaglutide on postprandial glucose and lipid metabolism, and gastric emptying, in subjects with type 2 diabetes (T2D).
Materials and Methods
In this randomized, double‐blind, single‐centre, crossover trial, subjects with T2D received once‐daily oral semaglutide (escalated to 14 mg) followed by placebo, or vice versa, over two consecutive 12‐week periods. Glucose and lipid metabolism, and gastric emptying (paracetamol absorption) were assessed before and after two types of standardized meals (standard and/or fat‐rich) at the end of each treatment period. The primary endpoint was area under the glucose 0–5‐h curve (AUC0–5h) after the standard breakfast.
Results
Fifteen subjects were enrolled (mean age 58.2 years, HbA1c 6.9%, body weight 93.9 kg, diabetes duration 3.1 years; 13 [86.7%] males). Fasting concentrations of glucose were significantly lower, and C‐peptide significantly greater, with oral semaglutide versus placebo. Postprandial glucose (AUC0–5h) was significantly lower with oral semaglutide versus placebo (estimated treatment ratio, 0.71; 95% CI, 0.63, 0.81; p < .0001); glucose incremental AUC (iAUC0–5h/5h) and glucagon AUC0–5h were also significantly reduced, with similar results after the fat‐rich breakfast. Fasting concentrations of triglycerides, very low‐density lipoprotein (VLDL) and apolipoprotein B48 (ApoB48) were significantly lower with oral semaglutide versus placebo. AUC0–8h for triglycerides, VLDL and ApoB48, and triglycerides iAUC0–8h/8h, were significantly reduced after oral semaglutide versus placebo. During the first postprandial hour, gastric emptying was delayed (a 31% decrease in paracetamol AUC0–1h) with oral semaglutide versus placebo. One serious adverse event (acute myocardial infarction) occurred during oral semaglutide treatment.
Conclusion
Oral semaglutide significantly improved fasting and postprandial glucose and lipid metabolism, and delayed gastric emptying.
Keywords: dyslipidaemia, GLP‐1 analogue, glycaemic control, incretin therapy, pharmacodynamics, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes (T2D) is associated with a variety of metabolic defects, including insulin resistance and β‐cell failure, resulting in impaired metabolism of macronutrients. 1 Fasting hyperglycaemia, as well as postprandial hyperglycaemic excursions, contribute to the risk of complications associated with T2D, and reduction of both is likely to be necessary to optimally address this risk. 2 In addition, the dyslipidaemia that often accompanies T2D is a risk factor for cardiovascular disease, which is a leading cause of morbidity and mortality in people with T2D. 3 Achieving glycaemic control and managing cardiovascular risk factors are key aspects of the treatment of T2D. 3 , 4
Semaglutide is a glucagon‐like peptide‐1 (GLP‐1) analogue developed as subcutaneous (s.c.) and oral formulations for the treatment of T2D. Oral semaglutide has been co‐formulated with the absorption enhancer sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate (SNAC), and is the first GLP‐1 receptor agonist (GLP‐1RA) to be approved for oral administration. Both once‐daily oral and once‐weekly s.c. semaglutide improve glycaemic control and reduce body weight in subjects with T2D, with the s.c. formulation also shown to provide cardiovascular benefits. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14
In a previous study in subjects with obesity, s.c. semaglutide was reported to improve fasting and postprandial glucose, and lipid metabolism, and delay first‐hour gastric emptying, compared with placebo. 15 The aim of this trial was to determine the effect of oral semaglutide on postprandial glucose and lipid metabolism, and gastric emptying, compared with placebo, in subjects with T2D.
2. MATERIALS AND METHODS
2.1. Trial design
This was a phase 1, randomized, placebo‐controlled, double‐blind, two‐period crossover trial (NCT02773381) conducted at a single site in the UK (Covance Clinical Research Unit Ltd, Leeds, UK) from 2 June 2016 to 19 October 2018. The crossover design included two treatment periods, one with oral semaglutide and one with placebo tablets. Each treatment period lasted 12 weeks and 3 days, the last 4 days of which consisted of an in‐house meal test period (Figure 1). The two treatment periods were separated by 5–9 weeks, allowing for the wash‐out of semaglutide in plasma and of its effects.
FIGURE 1.
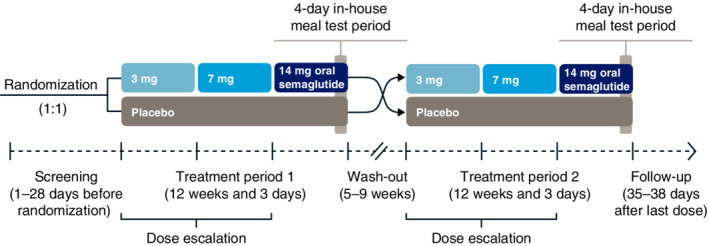
Trial design
The trial was conducted in compliance with the International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki.
2.2. Trial population
Eligible subjects were male or female, aged 18–75 years, diagnosed 90 days or more before screening with T2D (that was treated with diet and exercise and/or a stable dose of metformin for ≥30 days), with HbA1c 6.0%–9.0%, body mass index 20–38 kg/m2 and a stable body weight (<3 kg body weight change during 90 days before screening). Written informed consent was obtained from all subjects before any trial‐related activities. Full eligibility criteria are available in the supporting information.
2.3. Interventions
Subjects were randomized (see the supporting information for details) to a treatment sequence of either once‐daily oral semaglutide 14 mg followed by placebo, or vice versa. When subjects were receiving oral semaglutide, treatment was initiated at 3 mg (weeks 0–4), before escalating to 7 mg (weeks 4–8), and then 14 mg (weeks 8–12); subjects continued on 14 mg during the meal tests, resulting in a total of 31 days on this dose.
2.4. Assessments and endpoints
The pharmacodynamics (PD) of oral semaglutide were assessed during the 4‐day in‐house meal test period that occurred at the end of each treatment phase. During the meal tests, subjects continued to receive their randomized trial product once daily. On each day of the meal test period, subjects received a standardized breakfast (served 30 min after dosing of trial product, at approximately 8:30 AM), lunch, evening meal and evening snack box, and performed a 20‐min afternoon walk on a treadmill.
On day 1 of the meal test period, subjects were familiarized with the meals, physical activity and sleep schedule. The first meal test was performed on day 2 and assessed glucose metabolism after a standard breakfast. The standard breakfast had a total energy content of approximately 2.2 MJ (527 kcal) and a macronutrient composition of approximately 30 energy percentage (E%) fat, 15 E% protein and 55 E% carbohydrate. Blood sampling was performed before the meal (fasting) and over the 5 h postmeal (postprandial; Table S1) for measurement of glucose, glucagon, insulin and C‐peptide. The area under the concentration–time curve (AUC) from 0 to 5 h after the start of the meal (AUC0–5h) and the incremental AUC (iAUC0–5h/5h) were derived for each parameter from the concentration–time profiles. The AUC0–5h for glucose was the primary endpoint.
TABLE 1.
Baseline disease characteristics and demographics
| Parameter | Total (N = 15) |
|---|---|
| Sex, n (%) | |
| Male | 13 (86.7) |
| Female | 2 (13.3) |
| Race, n (%) | |
| White | 15 (100.0) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 15 (100.0) |
| Mean age, years (SD) | 58.2 (9.8) |
| Mean body weight, kg (SD) | 93.9 (14.9) |
| Mean BMI, kg/m2 (SD) | 30.8 (2.4) |
| Mean HbA1c, % (SD) | 6.9 (1.1) |
| Mean duration of diabetes, years (SD) | 3.1 (1.8) |
| Concomitant medications at screening, n (%) | |
| Anticholinergics: tropicamide | 12 (80.0) |
| Biguanides: metformin | 12 (80.0) |
| Statins: | 4 (26.7) |
| Atorvastatin | 3 (20.0) |
| Simvastatin | 1 (6.7) |
| Dihydropyridine derivatives: amlodipine | 3 (20.0) |
| ACE inhibitors: ramipril | 2 (13.3) |
| Angiotensin II antagonists: losartan | 1 (6.7) |
| Combinations and complexes of aluminium, calcium and magnesium compounds: calcium carbonate, calcium phosphate, kaolin, magnesium carbonate, magnesium hydroxide, magnesium oxide | 1 (6.7) |
| Multivitamins: vitamins not otherwise specified | 1 (6.7) |
| Unspecified herbal and traditional medicine: krill oil | 1 (6.7) |
| Vitamin A and D combination: cod liver oil | 1 (6.7) |
Abbreviations: ACE, angiotensin‐converting enzyme; BMI, body mass index; SD, standard deviation.
Gastric emptying was assessed on day 3 using the paracetamol absorption technique. 16 , 17 Subjects were given 1500 mg paracetamol dissolved in yoghurt at the start of the standard lunch. Blood samples were taken before, and for 5 h after, the meal (Table S1) to assess postprandial paracetamol absorption, and the paracetamol AUC 0–1 and 0–5 h after the start of the meal were calculated.
Glucose and lipid metabolism after a fat‐rich breakfast were assessed on day 4. The fat‐rich breakfast had a total energy content of approximately 3.5 MJ (844 kcal) and a macronutrient composition of approximately 65 E% fat, 15 E% protein and 20 E% carbohydrate. Blood sampling was performed before the meal, and over the 8 h postmeal (Table S1), for measurement of fasting and postprandial glucose, glucagon, insulin, C‐peptide, triglycerides, very low‐density lipoprotein (VLDL), free fatty acids (FFA) and apolipoprotein B48 (ApoB48). For each variable, the AUC0–8h and iAUC0–8h/8h were derived from the concentration–time profiles. In addition, fasting concentrations of low‐density lipoprotein (LDL), high‐density lipoprotein (HDL) and total cholesterol were measured before the fat‐rich breakfast. All of the lipid parameters were also assessed separately in statin users and non‐users.
Blood samples were also taken on the fourth, eighth and 12th week of each treatment period after administration of the last dose of oral semaglutide 3, 7 and 14 mg, to determine oral semaglutide pharmacokinetics (PK). Safety endpoints included the number of treatment‐emergent adverse events (AEs) and hypoglycaemic episodes (defined as either plasma glucose ≤3.9 mmol/L [70 mg/dL] or >3.9 mmol/L [70 mg/dL] in conjunction with hypoglycaemic symptoms) between the time of first dosing and the end of follow‐up, and changes in vital signs, electrocardiograms, clinical laboratory parameters and physical examinations, including eye examinations.
The effects of oral semaglutide on energy intake and appetite were also assessed in this trial and are reported by Gibbons et al. 18
Details of the analytical methods are provided in the supporting information.
2.5. Statistical methods
Analyses of PD and PK endpoints were based on the full analysis set (all randomized subjects who were exposed to at least one dose of trial product). The analyses of safety endpoints were based on the safety analysis set (all subjects who were exposed to at least one dose of trial product).
A sample size of 18 subjects was expected to give at least 90% power to detect a difference in the primary endpoint (AUC0–5h for glucose); further details are provided in the supporting information.
2.5.1. Pharmacodynamic endpoints
The AUC of the concentration–time curve was calculated using the linear trapezoidal method. Linear interpolation was used to estimate the concentration value at exactly 5 h after the standard breakfast (for glucose metabolism parameters), 8 h after the fat‐rich breakfast (for glucose and lipid metabolism parameters), and 1 and 5 h after the standard lunch (for paracetamol). The iAUC normalized by time span (5 h for the standard breakfast, 8 h for the fat‐rich breakfast) for glucose and lipid metabolism parameters was calculated as the AUC minus the product of baseline value measured immediately before the start of the meal multiplied by the time span. The iAUC could be negative.
The primary endpoint and secondary PD endpoints (fasting concentration, AUC and iAUC for each parameter) were analysed using an analysis of variance model, with the endpoint as a dependent parameter, and treatment (oral semaglutide or placebo), treatment period (two levels) and subject as fixed factors. The PD endpoints, except iAUC, were log‐transformed. The estimated differences in logarithmic values were back‐transformed and presented as ratios (oral semaglutide vs. placebo), with corresponding two‐sided 95% confidence intervals (CIs) and p‐values. For the iAUC, differences between oral semaglutide and placebo were estimated, together with corresponding two‐sided 95% CI and p‐values for the test of no difference. A ratio between iAUC means (oral semaglutide vs. placebo) was also provided, together with a 95% CI derived using Fieller's method. To explore the effects of concomitant statins, the statistical analyses of the lipid parameter endpoints were repeated separately for statin users and non‐users. As an ad hoc analysis, the effects of oral semaglutide on β‐cell function were further explored by calculation of the ratio for total AUC C‐peptide over total AUC glucose for the standard breakfast (0–5 h) and the fat‐rich breakfast (0–8 h), respectively. The log‐transformed ratio for the total AUC C‐peptide over total AUC glucose was analysed and presented in the same way as the primary endpoint.
2.5.2. Pharmacokinetic endpoints
The AUC0–24h and maximum concentration (Cmax) for oral semaglutide were analysed using a linear mixed model with the logarithmic transformed endpoint as a dependent parameter, treatment (3, 7 and 14 mg oral semaglutide) and treatment period as fixed factors, and subject as a random effect. From this model, the differences in logarithmic values between doses were back‐transformed to original scale and presented as ratios (14 vs. 7 mg, and 7 vs. 3 mg) with the corresponding two‐sided 95% CI.
3. RESULTS
3.1. Subject characteristics
In total, 53 subjects were screened, of whom 15 were enrolled and exposed to the trial product. Two enrolled subjects, both male, withdrew before the end of the trial; one because of an AE (acute myocardial infarction) and one who withdrew consent. Thirteen of the enrolled subjects were male, mean age was 58 years, mean HbA1c was 6.9% and the mean duration of T2D was 3 years (Table 1). Metformin, which was allowed as background medication, was the most common concomitant medication at screening, being used by 80% of subjects (Table 1). One subject on the placebo/oral semaglutide treatment sequence started concomitant atorvastatin while receiving oral semaglutide during the second treatment period.
3.2. Glucose metabolism
3.2.1. Glucose
Before the standard and fat‐rich breakfasts, fasting glucose was 22% and 23% lower, respectively, when subjects received oral semaglutide versus placebo (standard breakfast estimated treatment ratio [ETR], 0.78; 95% CI, 0.70, 0.87; p = .0004; fat‐rich breakfast ETR, 0.77; 95% CI, 0.73, 0.82; p < .0001; Figure 2 and Table S2). After the standard breakfast, the primary endpoint result of glucose AUC0–5h was 29% lower with oral semaglutide versus placebo, which was statistically significant (ETR, 0.71; 95% CI, 0.63, 0.81; p < .0001), and the iAUC0–5h/5h was also statistically significantly lower (estimated treatment difference [ETD], −1.25 mmol/L; 95% CI, −2.04, −0.45; p = .0053). After the fat‐rich breakfast, glucose AUC0–8h was 23% lower with oral semaglutide versus placebo (ETR, 0.77; 95% CI, 0.68, 0.87; p = .0007), with no significant difference in iAUC0–8h/8h.
FIGURE 2.
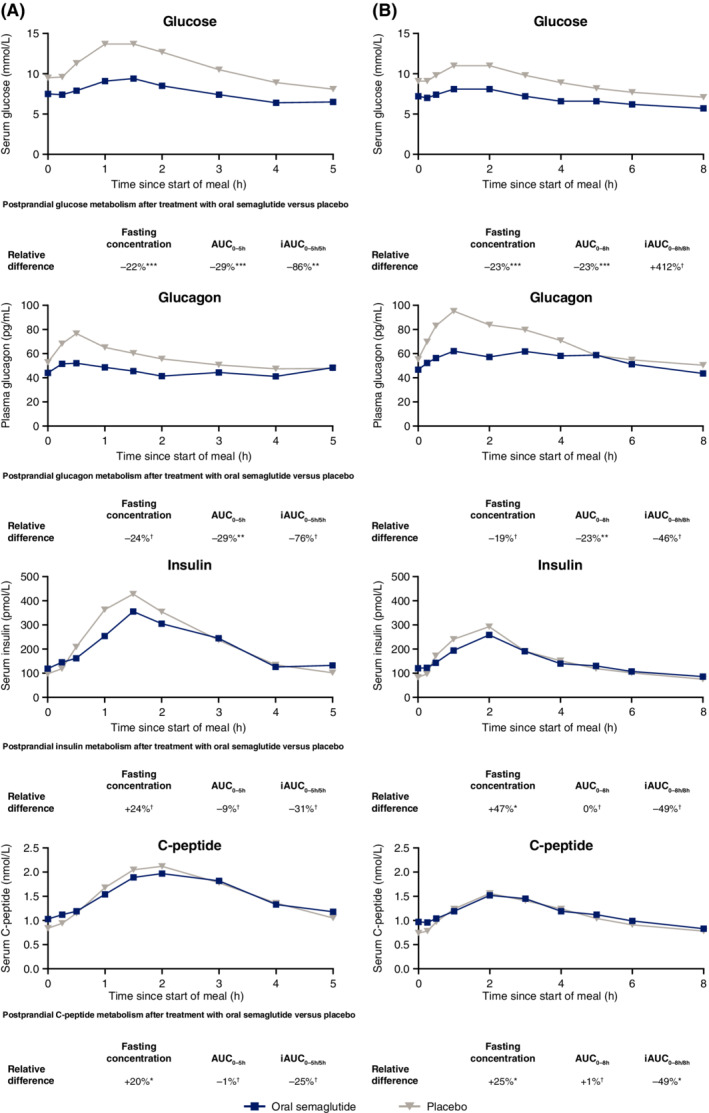
Mean postprandial glucose metabolism profiles after A, a standard breakfast; and B, a fat‐rich breakfast. Data are observed means. p‐values are for the ETR for fasting concentrations and AUC, and the ETD for iAUC, for oral semaglutide versus placebo. *p < .05; **p < .01; ***p < .001. †Difference was not statistically significant. AUC, area under the concentration–time curve; ETD, estimated treatment difference; ETR, estimated treatment ratio; iAUC, incremental area under the concentration–time curve
3.2.2. Glucagon
There was no significant difference in fasting glucagon before the standard or fat‐rich breakfasts when subjects received oral semaglutide versus placebo (Figure 2 and Table S2). After the standard breakfast, glucagon AUC0–5h was 29% lower with oral semaglutide versus placebo (ETR, 0.71; 95% CI, 0.59, 0.85; p = .0017); there was no significant difference in glucagon iAUC0–5h/5h. After the fat‐rich breakfast, glucagon AUC0–8h was 23% lower with oral semaglutide versus placebo (ETR, 0.77; 95% CI, 0.67, 0.89; p = .0027), with no significant difference in glucagon iAUC0–8h/8h.
3.2.3. Insulin and C‐peptide
Before the standard breakfast, there was no significant difference in fasting insulin, but fasting C‐peptide was 20% greater when subjects received oral semaglutide versus placebo (ETR, 1.20; 95% CI, 1.01, 1.42; p = .0389; Figure 2 and Table S2). Before the fat‐rich breakfast, fasting insulin was 47% greater (ETR, 1.47; 95% CI, 1.11, 1.96; p = .0132) and fasting C‐peptide 25% greater (ETR, 1.25; 95% CI, 1.05, 1.48; p = .0191) with oral semaglutide versus placebo.
After the standard breakfast, there were no significant differences between oral semaglutide and placebo in AUC0–5h or iAUC0–5h/5h for insulin or C‐peptide (Figure 2 and Table S2). After the fat‐rich breakfast, the iAUC0–8h/8h for C‐peptide was significantly lower with oral semaglutide versus placebo (ETD, −0.18 nmol/L; 95% CI, −0.32, −0.04; p = .0154); there were no significant differences in insulin AUC0–8h, insulin iAUC0–8h/8h or C‐peptide AUC0–8h.
The analysis of effects from oral semaglutide on β‐cell sensitivity to glucose showed significant increases from treatment with oral semaglutide in the AUC C‐peptide to AUC glucose ratios during the standard breakfast and the fat‐rich breakfast, respectively (ETR, 1.39; 95% CI, 1.07, 1.79; p = .0176; and ETR, 1.34; 95% CI, 1.07, 1.69; p = .0183) (Table S3).
3.3. Lipid metabolism
Before the fat‐rich breakfast, fasting concentrations were significantly lower for LDL (ETR, 0.88; 95% CI, 0.84, 0.94; p = .0005), total cholesterol (ETR, 0.88; 95% CI, 0.84, 0.91; p < .0001), triglycerides (ETR, 0.81; 95% CI, 0.72, 0.92; p = .0036), VLDL (ETR, 0.80; 95% CI, 0.67, 0.95; p = .0161) and ApoB48 (ETR, 0.75; 95% CI, 0.58, 0.98; p = .0350) when subjects received oral semaglutide versus placebo (Figure 3 and Table S4). There were no significant differences in fasting HDL or FFA.
FIGURE 3.
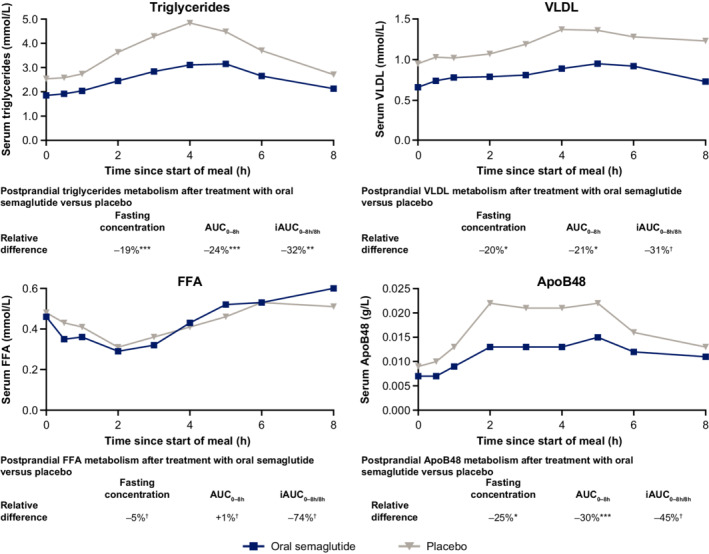
Mean postprandial lipid metabolism profiles after a fat‐rich breakfast. Data are observed means. p‐values are for the ETR for fasting concentrations and AUC, and the ETD for iAUC, for oral semaglutide versus placebo. *p < .05; **p < .01; ***p < .001. †Difference was not statistically significant. ApoB48, apolipoprotein B48; AUC, area under the concentration–time curve; ETD, estimated treatment difference; ETR, estimated treatment ratio; FFA, free fatty acids; iAUC, incremental area under the concentration–time curve; VLDL, very low‐density lipoprotein
After the fat‐rich breakfast, AUC0–8h values were significantly lower for triglycerides (ETR, 0.76; 95% CI, 0.64, 0.91; p = .0062), VLDL (ETR, 0.79; 95% CI, 0.68, 0.93; p = .0102) and ApoB48 (ETR, 0.70; 95% CI, 0.57, 0.85; p = .0025) with oral semaglutide versus placebo, with no significant difference for FFA (Figure 3 and Table S4). Oral semaglutide treatment resulted in a significantly lower iAUC0–8h/8h for triglycerides (ETD, −0.36 mmol/L; 95% CI, −0.68, −0.04; p = .0317), with no significant differences in iAUC0–8h/8h for VLDL, FFA or ApoB48.
Fasting and postprandial triglycerides, and postprandial ApoB48 were significantly lower with oral semaglutide than with placebo in statin users but not in non‐users, whereas fasting LDL was significantly lower in non‐users but not users (Table S5).
3.4. Gastric emptying
After the standard lunch, absorption of paracetamol was 31% lower in the first hour after the meal with oral semaglutide versus placebo (ETR, 0.69; 95% CI, 0.54, 0.87; p = .0050), but was not significantly different over 5 h (Figure 4 and Table S6).
FIGURE 4.
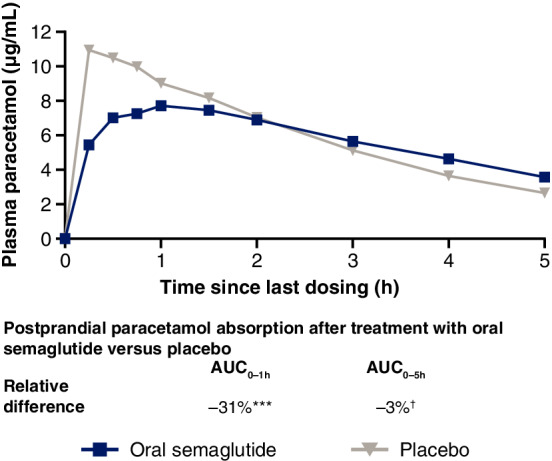
Mean paracetamol profile after a standard lunch. Data are observed means. p‐values are for the ETR for oral semaglutide versus placebo. ***p < .001. †Difference was not statistically significant. AUC, area under the concentration–time curve; ETR, estimated treatment ratio
3.5. Semaglutide pharmacokinetics
AUC0–24h and Cmax of semaglutide increased with increasing dose (Tables S7 and S8). The geometric mean terminal half‐life for oral semaglutide 14 mg was 154 h, and the median time to maximum concentration was 1 h for each of the three oral semaglutide doses (3, 7 and 14 mg).
3.6. Safety
Overall, 14 (93.3%) subjects reported a total of 144 AEs. There were 93 events reported in 14 (93.3%) subjects during oral semaglutide treatment, and 51 events in 13 (92.9%) subjects during placebo treatment (Table S9). The difference in the number of events was primarily due to gastrointestinal AEs with oral semaglutide. One serious AE (acute myocardial infarction) was reported during treatment with oral semaglutide and led to withdrawal. Apart from the one serious AE, which was severe, all the other events were mild or moderate in severity, and there were no deaths.
4. DISCUSSION
In this trial, we found that oral semaglutide 14 mg treatment resulted in clinically relevant and statistically significant improvements in fasting and postprandial glucose and lipid metabolism, and delayed gastric emptying during the first postprandial hour. In addition, we observed improvements in β‐cell sensitivity to glucose.
Improvements in glycaemic control have previously been demonstrated with oral semaglutide in people with T2D at different stages of disease in the PIONEER phase 3 clinical trial programme. 6 , 9 , 10 , 11 , 12 , 13 , 14 In the present trial in subjects with a comparatively short duration of disease who were well‐controlled on metformin, fasting C‐peptide and insulin concentrations were greater after once‐daily oral semaglutide treatment than after placebo, although the difference was not statistically significant for insulin before the standard breakfast. In the PIONEER 1 and 2 trials, oral semaglutide 14 mg treatment for up to 52 weeks generally resulted in improvements in these parameters compared with placebo and empagliflozin; 6 , 12 in PIONEER 2, these improvements occurred in patients with slightly more advanced disease, with a longer mean disease duration and higher mean baseline HbA1c than those in the present trial. The ad hoc analysis for β‐cell sensitivity to glucose points towards significant improvements in β‐cell sensitivity to glucose from treatment with oral semaglutide: the AUC C‐peptide over AUC glucose ratios during meal tests are on average 39% and 34% higher with oral semaglutide treatment compared with placebo in the standard breakfast and the fat‐rich meal, respectively. These effects of oral semaglutide on glucose metabolism are consistent with once‐weekly s.c. semaglutide in subjects with obesity, 15 as well as other GLP‐1RAs in T2D. 19 , 20 , 21
In terms of the effects on glucagon, the present trial identified a significantly lower glucagon AUC after oral semaglutide treatment compared with placebo after both the standard and fat‐rich breakfasts. The fasting concentrations and iAUC of glucagon after both meal tests were numerically lower with oral semaglutide than placebo, but the difference did not reach statistical significance, which is likely to be because of the small number of subjects in the trial. A trial of s.c. semaglutide in 37 subjects with T2D, with a longer mean diabetes duration and higher mean baseline HbA1c than in the present trial, demonstrated that semaglutide significantly reduces glucagon levels and improves β‐cell function. 22 It is likely that the same phenomena also occurred in the present study with oral semaglutide, since exposure–response analyses have shown that the route of administration does not affect treatment response. 23
The weight loss observed in this trial 18 may also have contributed to the observed reduction in postprandial glucose concentrations. Similar findings have been demonstrated with once‐weekly s.c. semaglutide and once‐daily liraglutide in subjects with obesity 15 , 24 or T2D. 25 This may be due to the increased insulin sensitivity that occurs following weight loss, 26 and a positive association between weight loss and reduced insulin resistance has been demonstrated for s.c. semaglutide. 27
This trial showed that fasting LDL and total cholesterol concentrations were lower with oral semaglutide versus placebo. However, no effect on HDL was observed. In addition, oral semaglutide treatment resulted in lower fasting and postprandial triglycerides than with placebo. There were generally similar effects on fasting lipid concentrations with oral semaglutide 14 mg compared with placebo and non‐GLP‐1RA active comparators reported in PIONEER trials. 6 , 7 , 11 , 12 , 13 The effect of oral semaglutide on triglycerides in this trial could be caused by the concomitant reduction of ApoB48 also observed after oral semaglutide treatment. The causal link could be the requirement of ApoB48 for the construction of chylomicrons, which are necessary for the absorption of triglycerides from the intestines. 28 Similar effects on triglycerides and ApoB48 were observed with s.c. semaglutide in subjects with obesity 15 and with other GLP‐1RAs in subjects with T2D. 25 , 29 , 30 Furthermore, liraglutide has been shown to reduce postprandial hyperlipidaemia by both increasing the catabolism of ApoB48 and reducing its production. 31 However, care should be taken when comparing findings from different trials because there may be differences in the fat meal tests used. To address this, an expert panel has recently recommended the use of a standardized fat tolerance test where postprandial triglycerides are measured 4 h after a high‐fat, sugary meal, such as 250 g of cream with 15 g of sugar (75 g of fat, 25 g of carbohydrates and 10 g of protein), for the evaluation of an abnormal (>2.5 mmol/L) response to fat indicative of increased cardiovascular risk. 32 By comparison, the fat‐rich meal in this trial contained approximately 60 g of fat.
Elevated total cholesterol, LDL and triglycerides, and reduced HDL, are associated with cardiovascular disease in people with T2D. 3 Improvements in these parameters were reported with oral semaglutide in the PIONEER 6 cardiovascular outcomes trial, 7 which met its primary objective of showing the cardiovascular safety of oral semaglutide. In addition, a cardiovascular outcomes trial with s.c. semalutide has shown cardiovascular risk reduction. 8 It has been suggested that the reduction of ApoB48 and subsequent decrease in postprandial dyslipidaemia could be one mechanism that may contribute to the beneficial cardiovascular effects observed with some GLP‐1RAs. 33 As lipid‐lowering agents, statins are recommended on an individual basis to people with T2D who are at risk of cardiovascular disease, 3 and so it was relevant to investigate whether the beneficial effects of oral semaglutide on lipid parameters were also present with concomitant statin use. The results from this analysis are presented in Table S5, but the number of subjects using statins in this trial was too low to allow reliable conclusions to be drawn.
GLP‐1RAs delay gastric emptying to varying degrees, which results in a prolonged absorption profile, as can be seen in Figure 4. This has been linked to the reduction in postprandial hyperglycaemia, and so may be one of the mechanisms by which these drugs improve glycaemic control. 34 We found that oral semaglutide delayed gastric emptying during the first hour compared with placebo, but total paracetamol absorption over 5 h after ingestion was unchanged; similar findings have been reported with s.c. semaglutide in subjects with obesity. 15 Delayed gastric emptying may contribute to the slight increases in exposure to metformin, furosemide and rosuvastatin that occur when administered with oral semaglutide. 35 , 36 It is also possible that by only causing a delay in initial gastric emptying, the overall clinical impact of oral semaglutide on drug–drug interactions may be limited.
It is noteworthy that an effect on gastric emptying, although only present in the first hour, could still be observed after continuous exposure to oral semaglutide for 12 weeks. However, it is unclear whether the effect on gastric emptying would persist with treatment for longer than 12 weeks, as in this trial oral semaglutide concentrations were only at steady‐state for approximately 1.5 weeks. Extrapolating from trials on gastric emptying with other GLP‐1RAs is difficult as there are few with treatment periods longer than the 12 weeks used in our trial. A trial in subjects with obesity noted that liraglutide significantly delayed gastric emptying compared with placebo after 16 weeks of treatment, but to a lesser extent compared with earlier in the trial, indicating the effect may diminish over time. 37
Semaglutide exposure following dosing of the two marketed doses of oral semaglutide (7 and 14 mg) was, as expected, 38 , 39 in line with previously published data. 40 Furthermore, there were no unexpected safety findings with oral semaglutide in this trial, and the safety profile was consistent with that observed in the PIONEER programme. 6 , 7 , 9 , 10 , 11 , 12 , 13 , 14
There are several limitations to this trial. First, the complexity of the trial design, which required multiple visits to the clinical trial facility as well as two 4‐day in‐house stays, affected recruitment and resulted in a small number of subjects being enrolled. As this was an exploratory trial, no correction for multiplicity was performed and so there is a risk of false positive results, particularly as many endpoints were tested. Second, gastric emptying was only measured using the paracetamol absorption test. While scintigraphy is the gold standard for measuring gastric emptying, it can provide inconsistent results because of the lack of standardized procedures. 41 By contrast, the paracetamol absorption method is able to provide a similar degree of accuracy to scintigraphy, is widely used and therefore comparable across trials, and is inexpensive. 42 , 43 Finally, the proportion of female subjects enrolled in the trial was low (13.3%), and all of the participants were White, which could have affected the generalizability of the results as the trial population may not have been representative of a general T2D population.
In conclusion, this trial showed that oral semaglutide significantly improves glucose and lipid metabolism and delays gastric emptying in subjects with T2D. These effects are expected to be clinically relevant, and are consistent with those shown with s.c. semaglutide and other GLP‐1RAs. The PK profile of oral semaglutide was as expected, and the safety profile was in line with the results of the global phase 3 PIONEER trials.
CONFLICT OF INTEREST
KD, STH, CB and TAB are employees, and KD, STH and TAB shareholders, of Novo Nordisk A/S, the sponsor of this trial. FA and AB are employees of Covance Clinical Research Unit Ltd, the site where the trial was conducted; they did not receive any direct funding, nor have financial affiliations with the trial sponsor.
AUTHOR CONTRIBUTIONS
AB, FA, STH and TAB designed the trial. KD, AB, FA and TAB were responsible for the conduct of the trial. KD, AB, FA, STH, TAB and CB were responsible for the collection, analysis and interpretation of data. All authors were involved in the writing, revision and final approval of the manuscript, and agree to be accountable for all aspects of the work.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14373.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGEMENTS
The authors would like to thank the subjects participating in this trial, the investigators, all trial site staff and all Novo Nordisk employees involved in the trial. The authors would also like to thank Jeppe Sturis PhD of Novo Nordisk for advice in relation to data interpretation, Christin Løth Hertz MD PhD of Novo Nordisk for reviewing the manuscript, and Sophie Walton MSc of Axis, a division of Spirit Medical Communications Group Limited, for assistance with medical writing and editorial support (funded by Novo Nordisk A/S). This study was funded by Novo Nordisk A/S, Søborg, Denmark.
Dahl K, Brooks A, Almazedi F, Hoff ST, Boschini C, Bækdal TA. Oral semaglutide improves postprandial glucose and lipid metabolism, and delays gastric emptying, in subjects with type 2 diabetes. Diabetes Obes Metab. 2021;23:1594–1603. 10.1111/dom.14373
Funding information This study was funded by Novo Nordisk A/S, Søborg, Denmark.
DATA AVAILABILITY STATEMENT
Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at novonordisk‐trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in data sets in a de‐identified/anonymised format.
REFERENCES
- 1. Defronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. [DOI] [PubMed] [Google Scholar]
- 2. Monnier L, Colette C. Postprandial and basal hyperglycaemia in type 2 diabetes: contributions to overall glucose exposure and diabetic complications. Diabetes Metab. 2015;41:6S9‐6S15. [DOI] [PubMed] [Google Scholar]
- 3. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;41:255‐323. [DOI] [PubMed] [Google Scholar]
- 4. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andreadis P, Karagiannis T, Malandris K, et al. Semaglutide for type 2 diabetes mellitus: a systematic review and meta‐analysis. Diabetes Obes Metab. 2018;20:2255‐2263. [DOI] [PubMed] [Google Scholar]
- 6. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42:1724‐1732. [DOI] [PubMed] [Google Scholar]
- 7. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841‐851. [DOI] [PubMed] [Google Scholar]
- 8. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 9. Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo‐controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515‐527. [DOI] [PubMed] [Google Scholar]
- 10. Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open‐label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:528‐539. [DOI] [PubMed] [Google Scholar]
- 11. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double‐blind, phase 3a trial. Lancet. 2019;394:39‐50. [DOI] [PubMed] [Google Scholar]
- 12. Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42:2272‐2281. [DOI] [PubMed] [Google Scholar]
- 13. Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321:1466‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42:2262‐2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hjerpsted JB, Flint A, Brooks A, Axelsen MB, Kvist T, Blundell J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first‐hour gastric emptying in subjects with obesity. Diabetes Obes Metab. 2018;20:610‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanaka M, Kuyama Y, Yamanaka M. Guide for judicious use of the paracetamol absorption technique in a study of gastric emptying rate of liquids. J Gastroenterol. 1998;33:785‐791. [DOI] [PubMed] [Google Scholar]
- 17. Willems M, Quartero AO, Numans ME. How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Dig Dis Sci. 2001;46:2256‐2262. [DOI] [PubMed] [Google Scholar]
- 18. Gibbons C, Blundell J, Hoff ST, Dahl K, Bauer R, Bækdal TA. Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes Metab. 2021;23:581‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flint A, Kapitza C, Hindsberger C, Zdravkovic M. The once‐daily human glucagon‐like peptide‐1 (GLP‐1) analog liraglutide improves postprandial glucose levels in type 2 diabetes patients. Adv Ther. 2011;28:213‐226. [DOI] [PubMed] [Google Scholar]
- 20. Linnebjerg H, Kothare PA, Skrivanek Z, et al. Exenatide: effect of injection time on postprandial glucose in patients with type 2 diabetes. Diabet Med. 2006;23:240‐245. [DOI] [PubMed] [Google Scholar]
- 21. Matthews JE, Stewart MW, De Boever EH, et al. Pharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide, a long‐acting glucagon‐like peptide‐1 mimetic, in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:4810‐4817. [DOI] [PubMed] [Google Scholar]
- 22. Kapitza C, Dahl K, Jacobsen JB, Axelsen MB, Flint A. Effects of semaglutide on beta cell function and glycaemic control in participants with type 2 diabetes: a randomised, double‐blind, placebo‐controlled trial. Diabetologia. 2017;60:1390‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Overgaard RV, Navarria A, Hertz C, Ingwersen S. Similar efficacy and gastrointestinal tolerability versus exposure for oral and subcutaneous semaglutide. Diabetologia. 2019;62:1‐600. Abstract 777.31384961 [Google Scholar]
- 24. van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once‐daily GLP‐1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non‐diabetic adults. Int J Obes (Lond). 2014;38:784‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hermansen K, Bækdal TA, Düring M, et al. Liraglutide suppresses postprandial triglyceride and apolipoprotein B48 elevations after a fat‐rich meal in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled, cross‐over trial. Diabetes Obes Metab. 2013;15:1040‐1048. [DOI] [PubMed] [Google Scholar]
- 26. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287‐302. [DOI] [PubMed] [Google Scholar]
- 27. Fonseca VA, Capehorn MS, Garg SK, et al. Reductions in insulin resistance are mediated primarily via weight loss in subjects with type 2 diabetes on semaglutide. J Clin Endocrinol Metab. 2020;104:4078‐4086. [DOI] [PubMed] [Google Scholar]
- 28. Hussain MM. Intestinal lipid absorption and lipoprotein formation. Curr Opin Lipidol. 2014;25:200‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Voukali M, Kastrinelli I, Stragalinou S, et al. Study of postprandial lipaemia in type 2 diabetes mellitus: exenatide versus liraglutide. J Diabetes Res. 2014;2014:304032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koska J, Sands M, Burciu C, et al. Exenatide protects against glucose‐ and lipid‐induced endothelial dysfunction: evidence for direct vasodilation effect of GLP‐1 receptor agonists in humans. Diabetes. 2015;64:2624‐2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vergès B, Duvillard L, Pais de Barros JP, et al. Liraglutide reduces postprandial hyperlipidemia by increasing ApoB48 (apolipoprotein B48) catabolism and by reducing ApoB48 production in patients with type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2018;38:2198‐2206. [DOI] [PubMed] [Google Scholar]
- 32. Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non‐fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498‐514. [DOI] [PubMed] [Google Scholar]
- 33. Vergès B, Charbonnel B. After the LEADER trial and SUSTAIN‐6, how do we explain the cardiovascular benefits of some GLP‐1 receptor agonists? Diabetes Metab. 2017;43(Suppl 1):2S3‐2S12. [DOI] [PubMed] [Google Scholar]
- 34. Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. 2013;36:1396‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bækdal T, Albayaty M, Manigandan E, Anderson TW, Skibsted S. A trial to investigate the effect of oral semaglutide on the pharmacokinetics of furosemide and rosuvastatin in healthy subjects. Diabetologia. 2018;61(Suppl 1):1‐620. Abstract 714. https://link.springer.com/content/pdf/10.1007/s00125-018-4693-0.pdf 30132038 [Google Scholar]
- 36. Bækdal T, Borregaard J, Hansen CW, Thomsen M, Anderson TW. Effect of oral semaglutide on the pharmacokinetics of lisinopril, warfarin, digoxin, and metformin in healthy subjects. Clin Pharmacokinet. 2019;58:1193‐1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Halawi H, Khemani D, Eckert D, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo‐controlled pilot trial. Lancet Gastroenterol Hepatol. 2017;2:890‐899. [DOI] [PubMed] [Google Scholar]
- 38. Novo Nordisk . RYBELSUS® semaglutide tablets prescribing information. 2020. https://www.novo-pi.com/rybelsus.pdf. Accessed 13 January 2021.
- 39. Novo Nordisk . Rybelsus summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product‐information/rybelsus‐epar‐product‐information_en.pdf. Accessed 13 January 2021.
- 40. Granhall C, Donsmark M, Blicher TM, et al. Safety and pharmacokinetics of single and multiple ascending doses of the novel oral human GLP‐1 analogue, oral semaglutide, in healthy subjects and subjects with type 2 diabetes. Clin Pharmacokinet. 2019;58:781‐791. [DOI] [PubMed] [Google Scholar]
- 41. Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753‐763. [DOI] [PubMed] [Google Scholar]
- 42. Näslund E, Bogefors J, Grybäck P, Jacobsson H, Hellström PM. Gastric emptying: comparison of scintigraphic, polyethylene glycol dilution, and paracetamol tracer assessment techniques. Scand J Gastroenterol. 2000;35:375‐379. [DOI] [PubMed] [Google Scholar]
- 43. Djerf P, Brundin M, Bajk M, Smedh U. Validation of the paracetamol absorption test for measuring gastric tube emptying in esophagectomized patients versus gold standard scintigraphy. Scand J Gastroenterol. 2015;50:1339‐1347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at novonordisk‐trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in data sets in a de‐identified/anonymised format.


