Abstract
Objectives
Effects of low energy, single‐pass helium plasma dermal resurfacing (PDR) treatment on brown spots, enlarged pores, and wrinkles—preliminary findings.
Methods
Twenty two subjects (64.6 ± 6.6 years) with Fitzpatrick Wrinkle and Elastosis Scale score (FWS) of ≤2 and seeking improvement of facial appearance were included in this subanalysis. All subjects received a single, one‐pass, full face, and low power helium PDR treatment. Standard digital images were collected using the VISIA‐CR (Canfield Scientific Inc.) at baseline and 3 months after treatment with images assessed for improvement in FWS and for improvements in brown spots, enlarged pores, and wrinkles by proprietary automated image processing algorithms.
Results
Nearly all subjects demonstrated ≥1‐point improvement in FWS and also reported improvement per modified Global Aesthetic Improvement Scale query. The numbers of brown spots and enlarged pores decreased by 45.1% and 28.3%, respectively. Stratification of brown spots data by presence or absence of post‐inflammatory hyperpigmentation revealed paradoxically conflicting data. The improvement detected in wrinkle area and mean wrinkle thickness was less pronounced with overall reductions of 13.4% and 4.8%, respectively. 37 Non‐serious adverse events (AEs) in 22 subjects were reported with most resolving within 14 days or less, and no serious AEs were observed.
Conclusions
While longer‐term follow‐up is needed, these early study results show that one single‐pass, low energy helium PDR treatment improves facial skin appearance both qualitatively and quantitatively. Studies evaluating higher energy levels and multiple treatment passes are ongoing.
Keywords: helium plasma, skin pore, Skin resurfacing, skin spot, wrinkle
1. INTRODUCTION
Skin aging is a natural process influenced by intrinsic and extrinsic factors 1 that can have a significant impact on self‐esteem and appearance of overall health. Improvement of facial skin appearance is among the most common reasons patients seek treatment from cosmetic dermatologists and aesthetic surgeons. While options for energy‐based skin rejuvenation are numerous those that lead to renewal of the upper skin layers through immediate (eg, CO2 laser 2 ; eg, erbium YAG laser 3 ; eg, helium PDR [2 or more passes with debridement of desiccated tissue after first pass] 4 ; eg, ablative fractional [laser] resurfacing 5 ) or delayed ablation (eg, nitrogen plasma skin regeneration 6 ; eg, helium PDR [single pass leaving desiccated tissue in place] 4 ; eg, non‐ablative fractional (laser) resurfacing 7 ) are considered to be the most effective.
Ablative skin resurfacing can be defined as the removal of upper skin layers that are then replaced through the natural skin healing process. 8 Ablative skin resurfacing is commonly employed in Fitzpatrick skin types I, II, and III for improvement of skin clarity and smoothing of skin texture; visible hallmarks of success include reduction of fine lines and wrinkles and reduction of dyschromia (including dark spots). 9 Although ablative skin resurfacing may also improve redness and pore size and visibility other approaches may be more reliable for these specific concerns (eg, treatment of telangiectasias with 532 or 1064 nm NdYAG laser; eg, improvement of pore visibility with Er‐YAG‐1470 nm NdYAG hybrid fractional laser).
Over the past several years, a helium plasma dermal resurfacing device (Renuvion; Apyx Medical) has emerged as a promising skin resurfacing option. 10
This device uses radiofrequency energy to energize the electrode in the handpiece and, when helium gas is passed over the energized electrode, helium plasma is generated which allows the conduction of the radiofrequency energy from the electrode to the targeted skin in the form of a precise helium plasma beam (Figure 1, helium plasma skin tissue interaction). 11
FIGURE 1.
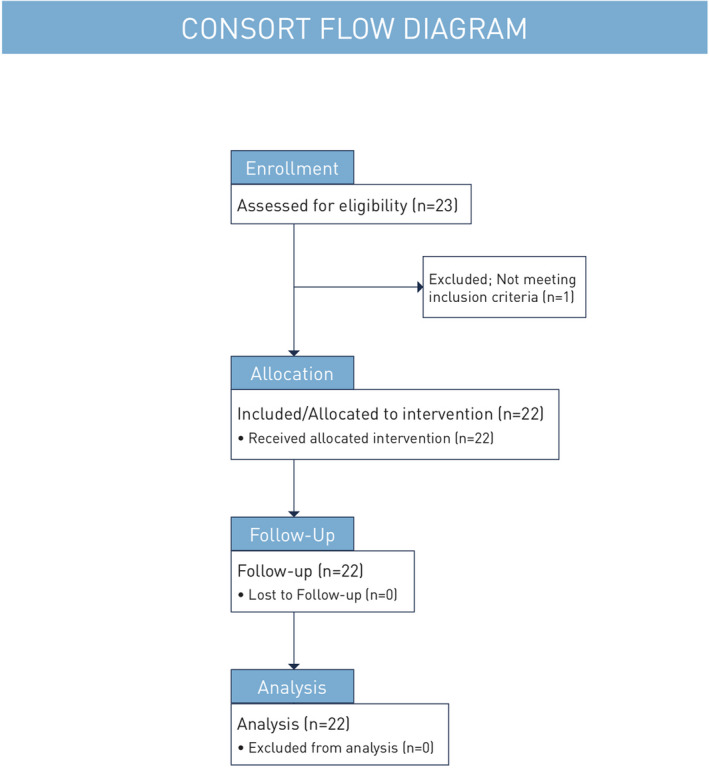
Consort flow diagram. Graphical depiction of subject enrollment, allocation, follow‐up, and analysis
It has recently been shown that one single‐pass, helium PDR treatment (20% power, 3 mm spot, continuous energy delivery with energy density approximately 40% lower than that of predicate nitrogen plasma device at 4.0 J, 6 mm spot, 2.5 Hz pulse speed) can improve the appearance of facial wrinkles with high subject and investigator satisfaction and relatively few unanticipated adverse events. 12 In this study, change in wrinkles was analyzed by independent dermatologists/plastic surgeons via scoring of standard digital images and in real life by the study investigator and subjects. To further characterize the effect of helium PDR treatment on facial skin, digital images collected before the procedure and at the 3‐month primary endpoint from one study site were analyzed with proprietary software algorithms for brown spots, enlarged pores, and wrinkles (see Methods). The results of this early quantitative analysis are presented herein.
2. METHODS
2.1. Study subjects
Study subjects aged at least 30 years old seeking improvement of facial appearance were included in this study. Subjects had a Fitzpatrick wrinkle and elastosis scale (FWS) 3 of at least 2, a Fitzpatrick skin scale score equal to or less than III and were willing to abstain from other facial cosmetic procedures throughout the study. Main exclusion criteria were pregnancy or lactation, active infection in treatment area, diabetes mellitus, skin barrier disruption (eg, abrasion or laceration), autoimmune disease, bleeding disorder, connective tissue disease, cancerous lesions on the face, susceptibility to keloid formation, recent use of isotretinoin or other medication that may interfere with skin healing, or facelift or facial injections within 1 year of the treatment.
This study was conducted in compliance with the declaration of Helsinki, was approved by a commercial Institutional Review Board (Western Institutional Review Board, Puyallup, WA), and informed consent was obtained from each study participant before any study procedures were performed.
2.2. Study design/treatment
This was a single‐arm prospective, multicenter study conducted in the United States of America between January and August 2018. This article presents a post hoc subanalysis that was conducted on images from subjects seen at one of the sites (Sarasota, Florida, USA; Figure 2, Consort Flow Diagram) (ClinicalTrials.gov Identifier: NCT03286283). Each subject received one treatment at baseline with the helium plasma device (Renuvion, Apyx Medical Corporation). After infiltration of tumescent anesthesia, the investigator treated facial skin with a single non‐overlapping pass, keeping a steady movement. While the study protocol required treatment of the perioral and periorbital zones treatment of the forehead, nose, and cheeks was optional. All subjects in the subgroup underwent full‐face treatment at 20% power with continuous energy delivery and helium flow of 4 L/min. Post‐treatment care included topical application of an occlusive layer of petroleum jelly and cool water and vinegar soaks. Skin care was transitioned to a gentle moisturizer upon re‐epithelialization. Follow‐up visits were performed 10 days, and 1‐, 3‐, and 6‐months after the procedure.
FIGURE 2.
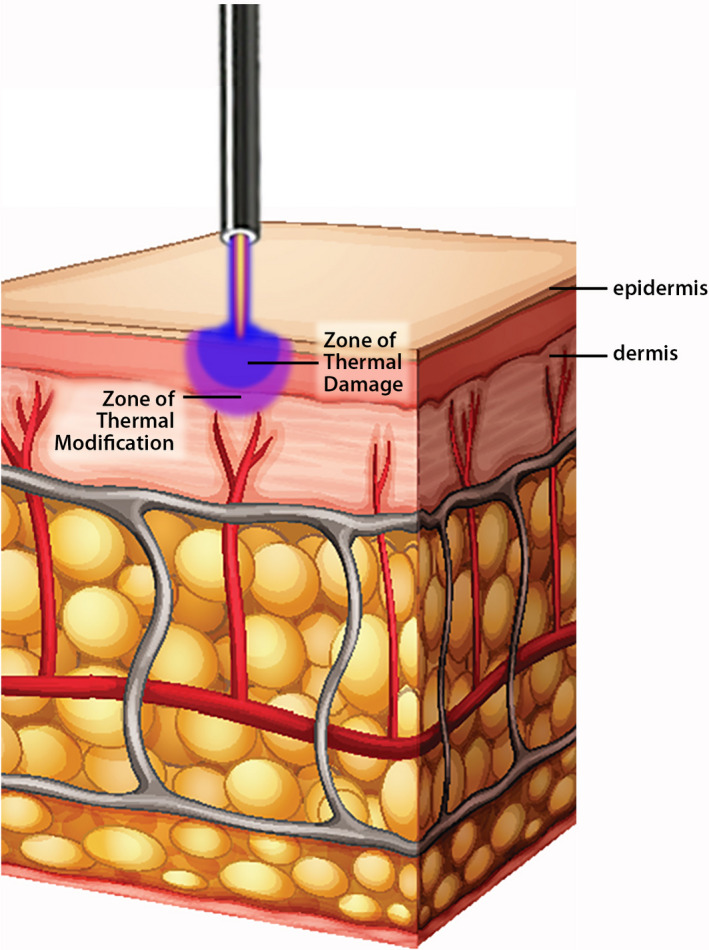
Helium plasma skin tissue interaction. Artistic rendering depicting helium plasma skin tissue interaction with helium plasma flow (beam) emanating from tip of handpiece above and impacting skin surface below with zones of irreversible thermal damage (blue zone, more superficial) and thermal modification (violet zone, deeper)
2.3. Image analysis
Standard images of the face were performed with the VISIA‐CR 2.3 (Canfield Scientific, Inc.) before and 3 months after treatment. Right and left oblique and frontal views were captured using a standard procedure. Areas of interest were adjusted manually for each subject, view, and visit by the image analysis technician. Any areas with facial hair or artifacts were excluded. Although VISIA‐CR images were obtained at the final 6‐month follow‐up visit, quantitative data for brown spots, enlarged pores, and wrinkles were not available for analysis.
Brown spots, enlarged pores, and wrinkles were analyzed by one or more proprietary automated image processing algorithms. The absorption of UV light by melanin was used to count the number of brown spots and to determine the total area covered by brown spots. Enlarged pore count and total surface area with enlarged pores were determined using an algorithm able to detect circular objects with a prespecified diameter range and minimum circularity threshold. Mean thickness of and total area covered by wrinkles were determined using oriented contrast‐based filters to detect curvilinear features.
2.4. Qualitative assessments
Changes in wrinkles based on FWS were analyzed by the investigator and three independent board‐certified dermatologists or plastic surgeon photographic reviewers (IPRs). FWS is a clinically validated assessment tool used to assess skin wrinkle severity and elastosis on a scale from 1 (mild) through 9 (severe). 8 The investigator evaluated FWSs during subject visits. IPRs used the digital images described above and were blinded to the treatment visit. A subject was considered a success if at least two out of three IPRs agreed that the subject reached at least one degree of wrinkle score reduction when comparing the score obtained at the 3‐month follow‐up with the baseline score. The modified Global Aesthetic Improvement Scale (mGAIS), a 7‐point scale from “Very Much Worse” to “Very Much Improved,” 13 was used by the subjects and the investigator to assess overall change compared to pre‐treatment.
2.5. Data analyses
When applicable, data obtained with both left and right oblique views were added to obtain a total count or area or averaged to obtain a mean thickness. Data are presented for both cheeks, both perioral and periorbital regions, and full forehead and nose. The overall score represents the full face. Data are presented as percentile changes relative to baseline (brown spots, enlarged pores, wrinkle thickness, and area) and numerical change relative to baseline (wrinkle thickness and area) except for the IPR assessed FWS that is presented as least square mean with 95% confidence interval of the mean. Statistics for Baseline Demographics (Table 1), Qualitative Assessment of Efficacy at 3 Months (Table 2), and Adverse Events (Table 3) were produced using statistical software (SAS version 9.3 or later, SAS Institute, Cary, NC) whereas KaleidaGraph version 4.5.3 (Synergy Software) was used for the descriptive statistics of brown spots, enlarged pores, and wrinkles. Paired t test was used to evaluate statistical significance for changes in brown spots and in enlarged pores whereas unpaired t test with unequal variance was used to assess significance for changes in brown spots between PIH and no PIH subgroups, and linear regression analysis was used to evaluate correlation between changes in wrinkle thickness and area.
TABLE 1.
Baseline demographics
| Characteristics | N = 22 |
|---|---|
| Age, mean ± SD (years) | 64.6 ± 6.6 |
| Sex, n (%) | |
| Female | 21 (95.5) |
| Male | 1 (4.5) |
| Race/Ethnicity a , n (%) | |
| Hispanic or Latino | 1 (4.5) |
| White | 21 (95.5) |
| Weight, mean ± SD (kg) | 70.1 ± 11.5 |
| Height, mean ± SD (cm) | 166.8 ± 6.1 |
| Fitzpatrick Phototyping Scale, (%) | |
| Type I | 1 (4.5) |
| Type II | 8 (36.4) |
| Type III | 13 (59.1) |
| FWS by IPR, LS Mean (95% CI) | 7.83 (7.05, 8.61) |
| FWS by Investigator, mean ± SD | 5.2 ± 1.1 |
Subgroup baseline demographics including age, sex, race/ethnicity, weight, height, Fitzpatrick skin phototype, Facial Wrinkle Score (FWS) by IPR (Independent Physician Reviewer) and by Investigator.
Abbreviations: CI, confidence interval; FWS, Fitzpatrick wrinkle and elastosis scale; IPR, independent photographic reviewers; LS, least square; SD, standard deviation.
Race/Ethnicity are not mutually exclusive categories.
TABLE 2.
Qualitative assessment of efficacy at 3 months
| Assessment |
3 months follow‐up N = 22 |
|---|---|
| ≥1‐point improvement in IPR‐FWS, n (%) | 21 (95.5%) |
| Change from baseline in IPR‐FWS, LS mean (95% CI) | −1.61 (−1.99, −1.23) |
| Change from baseline in Investigator‐FWS, mean ± SD | −2.6 ± 1.1 |
| Modified Global Aesthetic Improvement Scale, n (%) | Investigator | Subjects |
| Very much improved | 6 (27.3%) | 3 (13.6%) |
| Much improved | 14 (63.6%) | 7 (31.8%) |
| Improved | 2 (9.1%) | 10 (45.5%) |
| No change | 0 | 2 (9.1%) |
| Worse | 0 | 0 |
| Much worse | 0 | 0 |
| Very much worse | 0 | 0 |
Subgroup qualitative assessment of efficacy at 3 months including percent with >1 improvement in IPR‐FWS, change from baseline in IPR‐FWS and in Investigator‐FWS, Modified Global Aesthetic Improvement Scale results per Investigator and per Subjects.
Abbreviation: CI, confidence interval; FWS, Fitzpatrick wrinkle and elastosis scale; IPR, independent photographic reviewer; LS, least square; SD, standard deviation
TABLE 3.
Adverse events, single study site
| Anticipated | n | Percent |
|---|---|---|
| Hypersensitivity with one or more of: edema, erythema, induration, urticaria | 18 | 48.7 |
| Post‐inflammatory hyperpigmentation (temporary) | 2 | 5.4 |
| Acne | 1 | 2.7 |
| Pruritis | 3 | 8.1 |
| Pain | 1 | 2.7 |
| Subtotal | 25 | 67.6 |
| Non‐anticipated | n | Percent |
|---|---|---|
| Other treatment (device or procedure) related a | 5 | 13.5 |
| Non‐treatment related b | 5 | 13.5 |
| Hypertrophic scarring | 1 | 2.7 |
| Systemic effects | 1 | 2.7 |
| Subtotal | 12 | 32.4 |
Single study site anticipated and non‐anticipated adverse events by type with number and percent.
Other treatment‐related AEs included transient blurred vision and contact dermatitis.
Non–treatment‐related AEs included MRSA folliculitis, rash, diarrhea, and low‐grade temperature elevation.
3. RESULTS
A total of 22 participants were included in this subanalysis. Subjects' baseline characteristics are presented in Table 1. The mean age was 64.6 ± 6.6 years, and the vast majority of subjects were white females with Fitzpatrick skin type II or III.
The primary efficacy endpoint of ≥1‐point improvement in FWS at 3 months vs. baseline as assessed by IPRs was achieved in 21 of 22 subjects (95.5%) and self‐reported mGAIS scores showed improvement at 3 months in 91% of subjects (Table 2). Mean change in FWS from baseline to 3 months as assessed by IPRs was −1.61 indicating a decrease in wrinkle severity.
Overall, 37 adverse events (AEs) were reported by 20 subjects (90.9%), (Table 3). AEs reported in ≥1 subject included hypersensitivity to treatment (resulting in erythema, edema, induration, and/or urticaria) (n = 18, 48.7%), itching (n = 3, 8.1%), sensitivity to topical care (n = 3, 8.1%), and post‐inflammatory hyperpigmentation (n = 2, 5.4%). Two severe AEs were bronchitis and folliculitis associated with MRSA; however, neither were treatment related. The remaining AEs were mild to moderate in severity. For many subjects, AEs resolved within 7 to 14 days (n = 30, 81.1%) with 62.2% (n = 23) resolving within 7 days. Focal hypertrophic scarring of the lower chin in 1 subject responded well to serial triamcinolone injections (10 mg/ml).
A single treatment with the helium PDR device decreased the overall number of brown spots over the entire face by 45.1% at the 3‐month follow‐up visit with improvement ranging from −22.6% for the nose to over −55% for the periorbital and cheek regions; improvement was statistically significant (p < 0.05, paired t test) for all facial regions (Figure 3A). Decreases in facial area covered by brown spots were similar to decreases in number of brown spots for each facial area (data not shown). Of note, only two subjects reported moderate hyperpigmentation as an AE whereas the investigator identified 8 subjects with post‐inflammatory hyperpigmentation (PIH) of variable severity. Stratification of brown spots data by the presence or absence of PIH at 3 months showed a greater decrease in number of brown spots for all facial regions in the subgroup with PIH (n = 8) vs. that of the subgroup without PIH (n = 14) compared to baseline values but the differences were not statistically significant (p > 0.05, unpaired t test with unequal variance) (Figure 3B).
FIGURE 3.
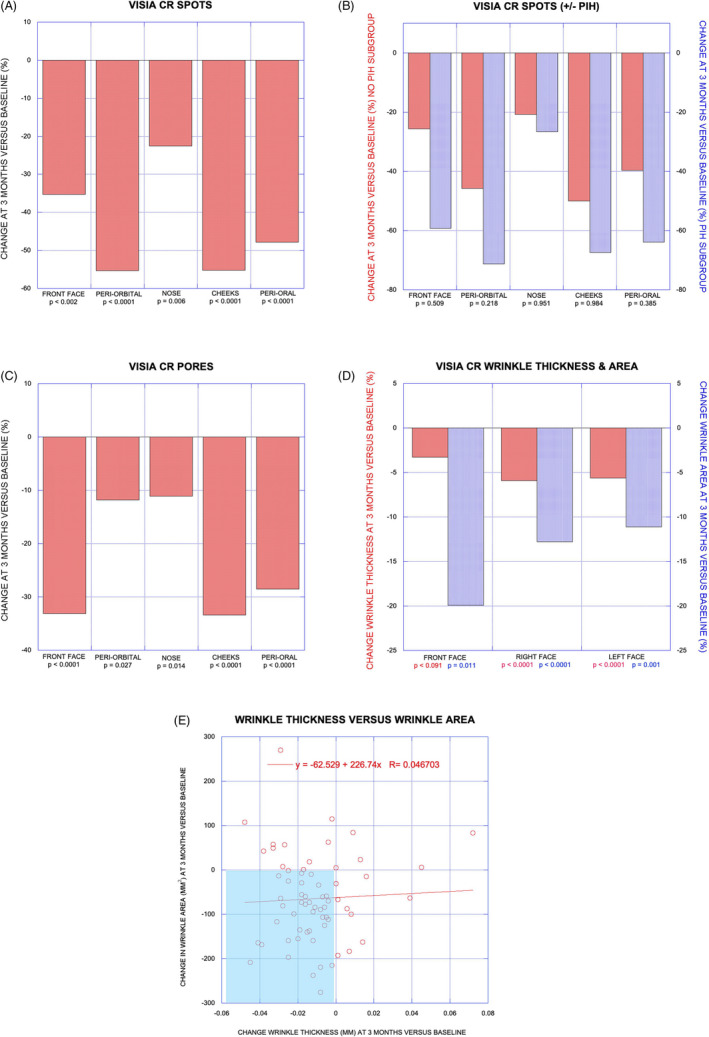
Subanalysis data in graphical format. VISIA CR spots (A) with change at 3 months vs. baseline (%) for individual facial zones and with p values indicating statistically significant improvement for all zones. VISIA CR spots with/without PIH (B) with change at 3 months vs. baseline (%) for No PIH and PIH subgroups and with p values eliminating statistically significant differences between groups. VISIA CR pores (C) with change at 3 months vs. baseline (%) for individual facial zones and with p values indicating statistically significant improvement for all zones. VISIA CR wrinkle thickness and area (D) showing greater improvement for wrinkle area and p values indicating statistically significant improvement in winkle area for each zone. Linear regression analysis for wrinkle thickness vs. wrinkle area (E) showing poor correlation
The total number of enlarged pores over the entire face decreased by 28.3% at the 3‐month follow‐up visit with improvement ranging from −11.1% for the nose to over −33% for the front face and cheek regions; improvement was statistically significant (p < 0.05, paired t test) for all facial regions (Figure 3C). Decreases in facial area covered by enlarged pores were similar to decreases in number of enlarged pores for each facial area (data not shown).
Improvement in mean wrinkle thickness was less pronounced than improvement in mean winkle area: overall reduction of wrinkle thickness was −4.8% vs. overall reduction in wrinkle area of −13.4%. Regional variance for improvement in wrinkle thickness and wrinkle area was greatest for the front face (including forehead) with −3.3% and −19.9%, respectively, vs. means of −5.8% and −12.0% for the sides of the face (Figure 3D). Change in wrinkle thickness vs. change in wrinkle area was plotted with 66 data points by combining data from the three facial regions in each of 22 subjects; although 40 of 66 data points fall within the quadrant representing both reduced wrinkle thickness and reduced wrinkle area linear regression analysis does not show good correlation between change in wrinkle thickness and change in wrinkle area (R 2 = 0.0022, Figure 3E).
Representative pre‐ and post‐treatment images including algorithm overlays for brown spots, enlarged pores, and wrinkles are presented in Figures 4, 5, and 6, respectively.
FIGURE 4.
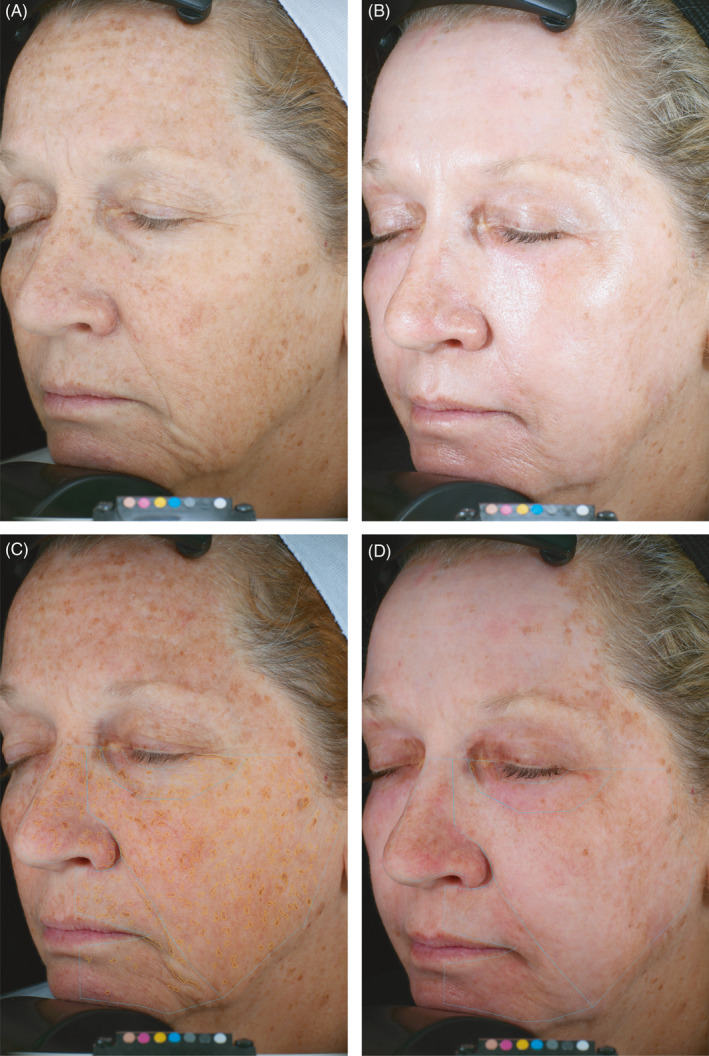
Quantitative evaluation of spots. 62‐year‐old female. Standard photographic (A, B) and cross‐polarized (C, D) left oblique images captured with the VISA‐CR 2.3 before (A, C) and 3 months after (B, D) a single treatment with helium PDR. Marked improvement in overall skin tone and texture evident in standard left oblique photographic images (B vs. A). Computer generated mask overlays for counted spots (164) and spot area (580 mm2) pre‐treatment (C) vs. counted spots (0) and spot area (0 mm2) 3 months after treatment (D)
FIGURE 5.
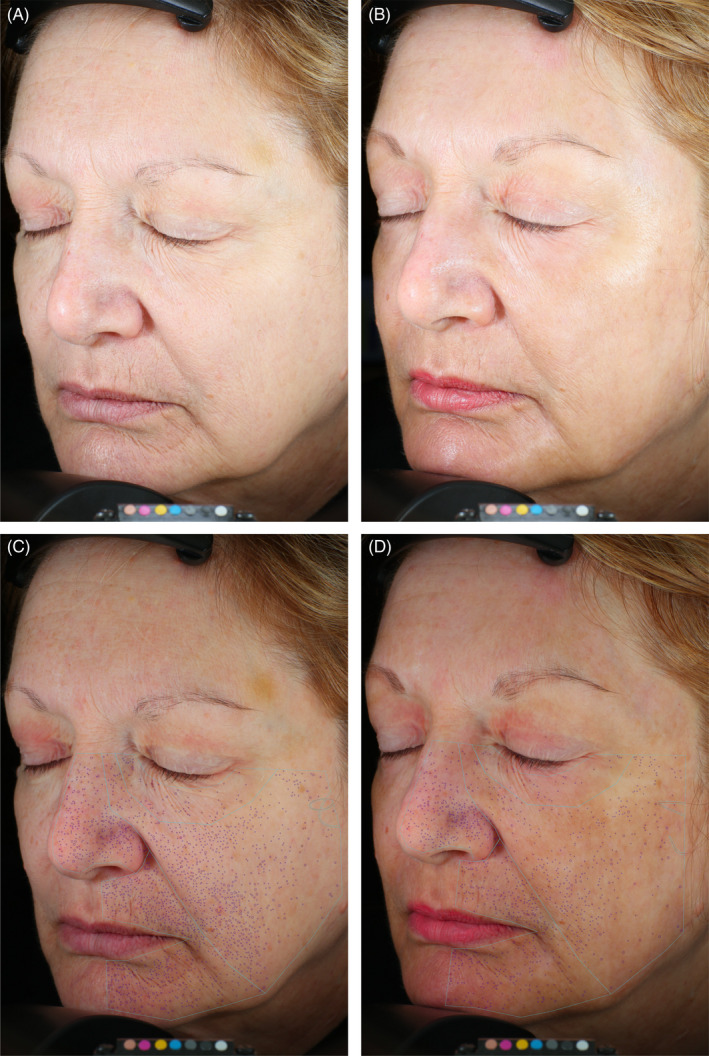
Quantitative evaluation of pores. 67‐year‐old female. Standard photographic (A, B) and cross‐polarized (C, D) left oblique images captured with the VISA‐CR 2.3 before (A, C) and 3 months after (B, D) a single treatment with helium PDR. Modest improvement in overall skin tone and texture evident but changes in pores more difficult to discern in standard left oblique photographic images (B vs. A). Computer generated mask overlays for counted pores (729) and pore area (115 mm2) pre‐treatment (C) vs. counted pores (372, −51%) and pore area (54 mm2, −53%) 3 months after treatment (D)
FIGURE 6.
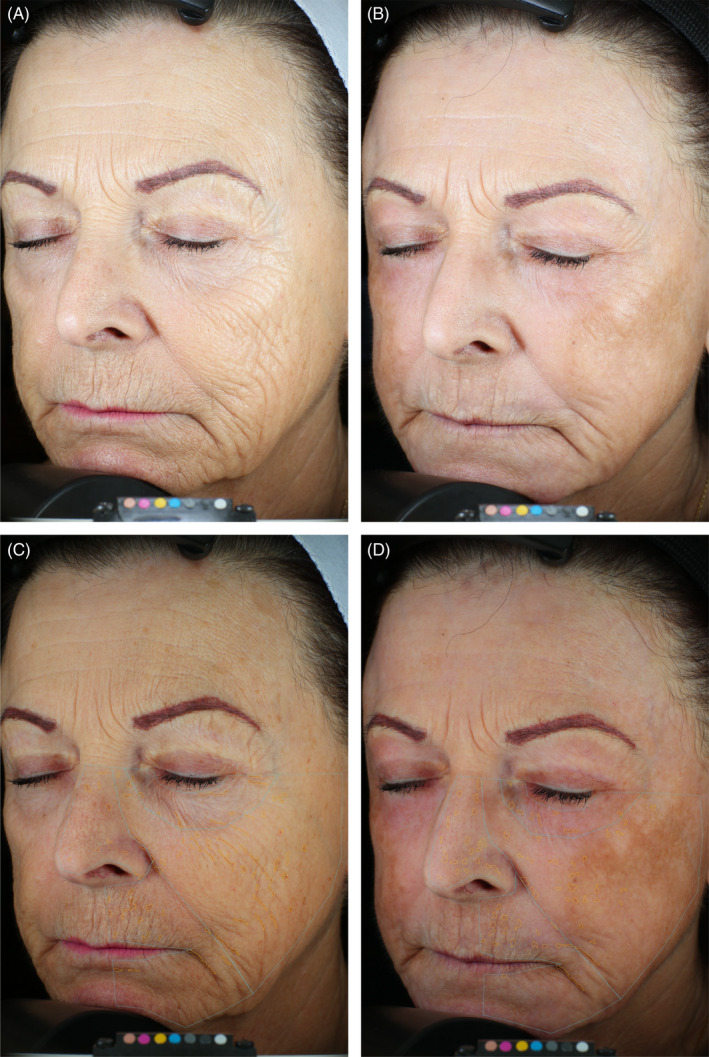
Representative improvement in wrinkles. 70‐year‐old female. Standard photographic (A, B) and cross‐polarized (C, D) left oblique images captured with the VISA‐CR 2.3 before (A, C) and 3 months after (B, D) a single treatment with helium PDR. Marked improvement in overall skin tone and texture with marked reduction in cheek and periorbital lines/wrinkles evident in standard left oblique photographic images (B vs. A). Computer generated mask overlays for wrinkle thickness (0.29 mm) and wrinkle area (703 mm2) pre‐treatment (C) vs. wrinkle thickness (0.25 mm, −14%) and wrinkle area (534 mm2, −24%) 3 months after treatment (D). Mild PIH evident over malar/zygomatic area of left cheek
4. DISCUSSION
This initial 3‐month post‐treatment quantitative subanalysis demonstrates that one, single‐pass helium PDR treatment can provide significant improvements in skin appearance by reducing brown spots, enlarged pores, and wrinkles, findings that are well‐aligned with the qualitative assessment of global improvement that showed that 21 of 22 subjects achieved the primary efficacy endpoint of ≥1‐point improvement in IPR‐FWS and that all 22 subjects had an improved appearance as assessed by the investigator using the mGAIS at 3 months post‐treatment. That these improvements can also positively impact self‐esteem and the appearance of overall health is supported by 91% self‐reported improvement in appearance per modified GAIS by this subgroup at 3 months post‐treatment.
Most AEs were anticipated (67.6%) and the majority of AEs resolved within 7 to 14 days (81%). Temporary facial and/or periorbital edema accounted for 21 of 37 reported AEs (56.8%). 1 patient developed focal hypertrophic scarring of the chin area that responded well to serial triamcinolone (10 mg/ml) injections. As with any energy‐based skin resurfacing treatment, it is imperative to carefully control energy delivery; for helium PDR treatment energy density may be controlled by adjustments of treatment speed (tip velocity), limiting power, implementing pulsing, or limiting treatment passes.
Stratification of this subgroup's VISIA CR analysis of number of brown spots by both facial region and presence or absence of post‐inflammatory hyperpigmentation (PIH, regional, or diffuse) at 3‐months suggested that the subgroup with PIH showed a greater decrease in number of brown spots for all facial regions vs. the no PIH subgroup compared to baseline values; however, the variance was not statistically significant (likely due to the small sample sizes resulting from stratification, Figure 3B). Nonetheless, the greater decreases that were observed in the PIH subgroup may result from differential effects of wrinkles and PIH on counted brown spots; with increased brown spots recorded pre‐treatment representing multiple false positives due to counting dark spots in the depth or shadows of deeper wrinkles and reduced ability of the VISA CR software algorithm to discriminate darker spots from increased background melanin pigmentation (false negative) or from fewer darker spots being present or both. Figure 7 highlights both of these phenomena with false positives from wrinkles present before treatment and potential false negatives related to PIH present at the 3‐month endpoint wherein zero brown spots were identified by the software algorithm. Of note, compliance with sun avoidance was a factor in development of PIH in some of the subjects and most (5 out of 8) subjects' PIH improved or resolved at the 6‐month endpoint without any specific intervention.
FIGURE 7.
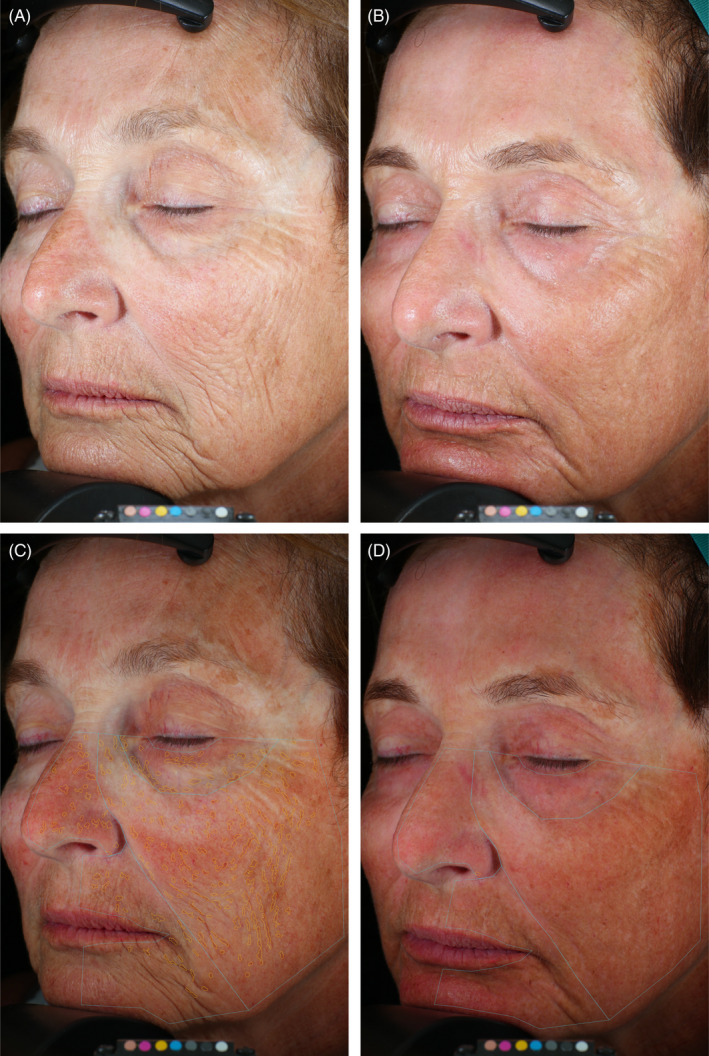
Wrinkles and post‐inflammatory hyperpigmentation and VISIA spots analysis. 67‐year‐old female. Standard photographic (A, B) and cross‐polarized (C, D) left oblique images captured with the VISA‐CR 2.3 before (A, C) and 3 months after (B, D) a single treatment with helium PDR. Variable skin tone outcome with areas of improvement (eg, forehead) and areas with mild PIH (eg, cheek) but marked improvement in overall skin texture with marked reduction in cheek and peri‐oral lines/wrinkles evident in standard photographic images (B vs. A). Computer generated mask overlays for counted spots (140, including false positives?) and spot area (463 mm2) pre‐treatment (C) vs. counted spots (0, including false negatives?) and spot area (0 mm2) 3 months after treatment (D)
Based on the results obtained with the qualitative tools, we could have expected the magnitude of the change in wrinkles (wrinkle area, wrinkle thickness) observed to be greater than what was obtained in this quantitative subanalysis. Qualitative analysis performed by independent photographic reviewers showed that almost all subjects had an improvement of at least one point in FWS (95.5% responders [21/22]) and significant changes from baseline (Table 2). Similarly, the global improvement using the mGAIS was at least “Improved” for the vast majority of the subjects (100%, investigator; 20 of 22 or 90.9%, subjects) (Table 2). The mildly diverging mGAIS assessments at 3 months after treatment may reflect availability of baseline photographs for comparison (investigator, mGAIS values higher) vs. subjects' impressions based on memory (mGAIS values lower).
Other studies have reported discrepancies in wrinkle analysis between VISIA and other tools. 14 , 15 , 16 , 17 , 18 Kwon et al 16 noted that the investigator global assessment (IGA) detected changes in wrinkles earlier than VISIA following treatment with a microneedle monopolar radiofrequency device. However, when a significant change was detected by VISIA it was more sensitive than the IGA in detecting differences between the two types of needles used. Similarly, in an efficacy assessment of a Deschampsia antarctica extract and high‐tolerance retinoid topical combination, a change in wrinkles was detected using Visioline 4 weeks after the treatment, but when the VISIA was used, a difference could only be detected 12 weeks after the treatment even if different VISIA devices and analyses were used 17 ; this may suggest that VISIA wrinkle analysis requires a greater minimum change from baseline (higher threshold) to detect a change in wrinkles.
Interestingly, in a recent comparison between VISIA and Image‐Pro Plus (Media Cybernetics Inc.), the sensitivity of the software was found to vary according to skin sites and VISIA was more sensitive for wrinkle assessment of the forehead. 18 In our quantitative wrinkle analysis, we found least improvement in wrinkle thickness but greatest improvement in wrinkle area in the front face (including forehead).
In contrast to variances observed between qualitative and quantitative VISIA CR assessment of wrinkles a recent study demonstrates high correlation between visual grading and pore size as assessed by VISIA CR. 19 While qualitative measures of pores were not incorporated into this study the quantitative reduction of enlarged pores by −28.3% herein is of greater magnitude than that reported with several other energy‐based treatments: for example, 17% improvement in pore score (percentage of skin surface with detectable pores per VISIA CR) with a series of six 1440‐nm laser treatments 20 ; eg, 4% reduction in average number of facial pores 2 months after a series of five broadband light treatments per VISIA CR. 21
Limitations of this subanalysis include the relatively short 3‐month post‐treatment endpoint, the inclusion of a limited number of subjects with substantial variation in results and therefore large standard deviations and the absence of a control group as is often the case in this kind of study.
5. CONCLUSION
While longer‐term follow‐up analysis is needed, these early findings indicate that helium PDR treatment at the modest energy level (20% power) and treatment approach (single pass) studied herein improves facial skin appearance qualitatively with improved FWS and mGAIS scores and quantitatively with significant reductions in dark spots, pore visibility, and wrinkle area. The ability of the VISIA software algorithm to detect changes in wrinkle thickness appears to lag behind qualitative findings.
ETHICAL APPROVAL
This study was conducted in compliance with the declaration of Helsinki, was approved by a commercial Institutional Review Board (Western Institutional Review Board, Puyallup, WA), and informed consent was obtained from each study participant before any study procedures were performed.
ACKNOWLEDGMENTS
This study was funded by Apyx Medical Corporation (Clearwater, FL, USA). The author is grateful to the four subjects who graciously provided permission to publish their photographs for the medical community. The author wants to thank Annie Levesque, MSc, for her assistance with the writing of the manuscript. Dr. Holcomb does not hold stock or stock options but is a paid consultant for Apyx Medical Corporation as well as a principal investigator in an ongoing multisite helium plasma dermal resurfacing study.
ClinicalTrials.gov Identifier: NCT03286283
[Correction added on April 14, 2021 after first online publication: a typo in the article title has been fixed.]
DATA AVAILABILITY STATEMENT
The data that supporting the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. McDaniel D, Farris P, Valacchi G. Atmospheric skin aging‐contributors and inhibitors. Cosmet Dermatol. 2018;17(2):124‐137. 10.1111/jocd.12518 [DOI] [PubMed] [Google Scholar]
- 2. Fitzpatrick RE, Rostan EE, Marchell N. Collagen tightening induced by carbon dioxide laser versus erbium: YAG laser. Lasers Surg Med. 2000;27(5):395‐403. [DOI] [PubMed] [Google Scholar]
- 3. Rostan EE, Fitzpatrick RE, Goldman MP. Laser resurfacing with a long pulse erbium: YAG laser compared to the 950 ms pulsed CO(2) laser. Lasers Surg Med. 2001;29(2):136‐141. 10.1002/lsm.1099 [DOI] [PubMed] [Google Scholar]
- 4. Holcomb JD, Duncan D, Lin M, et al. Helium plasma dermal resurfacing: consensus guidelines. Dermatological Rev. 2020;2:1‐11. 10.1002/der2.22 [DOI] [Google Scholar]
- 5. Hantash BM, Bedi VP, Kapadia B, et al. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg Med. 2007;39(2):96‐107. 10.1002/lsm.20468 [DOI] [PubMed] [Google Scholar]
- 6. Kilmer S, Semchyshyn N, Shah G, Fitzpatrick R. A pilot stdy on the use of a plasma skin regeneration device (Portrait PSR3) in full facial rejuvenation procedures. Lasers Med Sci. 2007;22(2):101‐109. 10.1007/s10103-006-0431-9 [DOI] [PubMed] [Google Scholar]
- 7. Narukar VA. Nonablative fractional laser resurfacing. Dermatol Clin. 2009;27(4):473‐478. 10.1016/j.det.2009.08.012 [DOI] [PubMed] [Google Scholar]
- 8. Fitzpatrick RE, Goldman MP, Satur NM, Tope WD. Pulsed carbon dioxide laser resurfacing of photo‐aged skin. Arch Dermatol. 1996;132(4):395‐402. [PubMed] [Google Scholar]
- 9. Holcomb JD. Versatility of the erbium YAG laser: from fractional skin rejuvenation to full‐field skin resurfacing. Facial Plast Surg Clin North Am. 2011;19(2):261‐273. 10.1016/j.fsc.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 10. Holcomb JD. Plasma energy skin rejuvenation. Facial Plast Surg Clin North Am. 2020;28(1):67‐74. 10.1016/j.fsc.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 11. Holcomb JD, Schucker A. Helium plasma skin regeneration: evaluation of skin tissue effects in a porcine model and comparison to nitrogen plasma skin regeneration. Lasers Surg Med. 2020;52(1):23‐32. 10.1002/lsm.23167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holcomb JD, Kelly M, Hamilton TK, DeLozier JB 3rd. A prospective study evaluating the use of helium plasma for dermal resurfacing. Lasers Surg Med. 2020;52(10):940‐951. 10.1002/lsm.23257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vandeputte J. Real‐world experience with volume augmentation using cohesive polydensified matrix hyaluronic acid gel: a retrospective single‐center analysis of 110 consecutive patients with medium‐ to long‐term follow‐up. J Clin Aesthet Dermatol. 2018;11(12):30‐39.PMID: 30666277. [PMC free article] [PubMed] [Google Scholar]
- 14. Linming F, Wei H, Angq L, et al. Comparison of two skin imaging analysis instruments: the VISIA® from Canfield vs the ANTERA 3D® from Miravex. Skin Res Technol. 2018;24(1):3‐8. 10.1111/srt.12381 [DOI] [PubMed] [Google Scholar]
- 15. Al‐Muriesh M, Zhang X, Wang Q, Huang C, An X. Efficacy of non‐invasive multisource radiofrequency treatment on periorbital rhytids using an imaging device. Lasers Surg Med. 2018;51(3):251‐255. 10.1002/lsm.23038 [DOI] [PubMed] [Google Scholar]
- 16. Kwon SH, Choi JY, Ahn GY, et al. The efficacy and safety of microneedle monopolar radiofrequency for the treatment of periorbital wrinkles. J Dermatolog Treat Sept. 2019;10:1‐5. 10.1080/09546634.2019.1662880 [DOI] [PubMed] [Google Scholar]
- 17. Perez DA, Truchuelo MT, Vitale M, Gonzalez‐Castro J. Efficacy of an antiaging treatment against environmental factors: Deschampsia anarctica extract and high‐tolerance retinoids combination. J Clin Aesthet Dermatol. 2019;12(7):E65‐E70.PMCID: PMC6715328. [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Shu X, Li Z, et al. Comparison of two kinds of skin imaging analysis software: VISIA® from Canfield and IPP® from Media Cybernetics. Skin Res Technol. 2018;24(3):379‐385. 10.1111/srt.12440 [DOI] [PubMed] [Google Scholar]
- 19. Dissanayake B, Kukizo M, Purwar A, et al. New image analysis tool for facial pore characterization and assessment. Skin Res Technol. 2019;25(5):631‐638. 10.1111/srt.12696 [DOI] [PubMed] [Google Scholar]
- 20. Saedi N, Petrell K, Arndt K, Dover J. Evaluating facial pores and skin texture after low‐energy nonablative fractional 1440‐nm laser treatments. J Am Acad Dermatol. 2013;68(1):113‐118. 10.1016/j.jaad.2012.08.041 [DOI] [PubMed] [Google Scholar]
- 21. Wenxin Y, Han Y, Wu X, et al. A split‐face randomized controlled trial of treatment with broadband light for enlarged facial pores. J Dermatolog Treat. 2020:Dec 4; 1‐5. 10.1080/09546634.2019.1698701 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supporting the findings of this study are available from the corresponding author upon reasonable request.


