Abstract
Objective
Autoantibodies, such as anti–citrullinated protein antibodies (ACPAs), have been described as inducing bone loss in rheumatoid arthritis (RA), which can also be reflected by bone mineral density (BMD). We therefore examined the association between osteoporosis and autoantibodies in two independent RA cohorts.
Methods
Dual x‐ray absorptiometry (DXA) of the lumbar spine and left hip was performed in 408 Dutch patients with early RA during 5 years of follow‐up and in 198 Swedish patients with early RA during 10 years of follow‐up. The longitudinal effect of ACPAs and other autoantibodies on several BMD measures was assessed using generalized estimating equations.
Results
In the Dutch cohort, significantly lower BMD at baseline was observed in ACPA‐positive patients compared to ACPA‐negative patients, with an estimated marginal mean BMD in the left hip of 0.92 g/cm2 (95% confidence interval [95% CI] 0.91–0.93) versus 0.95 g/cm2 (95% CI 0.93–0.97) (P = 0.01). In line with this, significantly lower Z scores at baseline were noted in the ACPA‐positive group compared to the ACPA‐negative group (estimated marginal mean Z score in the left hip of 0.18 [95% CI 0.08–0.29] versus 0.48 [95% CI 0.33–0.63]) (P < 0.01). However, despite clear differences at baseline, ACPA positivity was not associated with greater decrease in absolute BMD or Z scores over time. Furthermore, there was no association between BMD and higher levels of ACPAs or other autoantibodies (rheumatoid factor and anti–carbamylated protein antibodies). In the Swedish cohort, ACPA‐positive patients tended to have a higher prevalence of osteopenia at baseline (P = 0.04), but again, ACPA positivity was not associated with an increased prevalence of osteopenia or osteoporosis over time.
Conclusion
The presence of ACPAs is associated with significantly lower BMD at baseline, but not with greater BMD loss over time in treated RA patients. These results suggest that ACPAs alone do not appear to contribute to bone loss after disease onset when disease activity is well‐managed.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by polyarthritis and an increased risk of osteoporosis (1). It is known that patients with RA have twice the risk of sustaining osteoporosis‐related fractures compared to age‐matched controls, which is associated with high morbidity and mortality (2). Although some of the mechanisms leading to bone loss in RA have been clarified (such as the effect of cytokines), the precise relationship between the immunopathogenesis of RA (e.g., autoantibodies) and osteoporosis remains unclear.
One of the most important serological markers in RA is the presence of anti–citrullinated protein antibodies (ACPAs), which is a well‐known predictive marker of a more destructive disease course (3). ACPAs may affect systemic bone mineral density (BMD) loss, as seropositive patients (especially those with higher levels of ACPAs) have been described as having lower systemic BMD and a higher prevalence of osteoporosis (4, 5, 6).
There are two hypotheses for how ACPAs might affect BMD: 1) ACPAs represent a unique type of antibody able to directly induce bone loss or 2) ACPAs mediate bone loss only in the presence of concomitant inflammation. Regarding the first hypothesis, some data suggest that ACPAs can bind to and activate osteoclasts (7, 8), which leads to increased osteoclast‐mediated bone degradation and elevated serum levels of collagen degradation products such as RANKL (9). This process is believed to occur independently of inflammation status (6, 10), as bone remodeling starts even before the onset of clinical disease (11). In addition, altered bone metabolism has been observed in healthy subjects with ACPAs (12) and bone loss may develop in mice after injection of ACPAs (7), further supporting a possible direct pathogenic link between ACPAs and bone destruction in RA. However, chronic inflammation alone could also lead to bone degradation in RA via osteoclast activation mediated by proinflammatory cytokines (13, 14). ACPAs could therefore characterize a particular subset of RA with a more inflammatory profile that in turn could result in more bone loss. This hypothesis is supported by preliminary studies indicating that RA patients who have higher disease activity and higher levels of inflammation markers suffer from more bone loss (15). Lower BMD values in ACPA‐positive patients can also be attributed to more aggressive prednisone bridging in ACPA‐positive patients, which in itself is a risk factor for bone loss (16).
Longitudinal data, including detailed information about disease activity and treatment with disease‐modifying antirheumatic drugs (DMARDs) and glucocorticoids, are necessary to elucidate the exact association between ACPAs and bone loss in RA, which could provide insight into underlying biological mechanisms. We therefore performed an in‐depth investigation into the relation between autoantibodies and BMD by examining yearly dual x‐ray absorptiometry (DXA) scores in two independent cohorts of RA patients.
PATIENTS AND METHODS
Study design and patient selection
We used data from two large RA cohorts that were analyzed separately. The Dutch Induction therapy with Methotrexate and Prednisone in Rheumatoid Or Very Early arthritic Disease (IMPROVED) study is a multicenter, randomized controlled trial in which 610 patients with early untreated RA (symptom duration of <2 years) or undifferentiated arthritis received remission‐steered treatment between 2007 and 2010, with remission being defined as having a Disease Activity Score (DAS) of <1.6. For the Swedish cohort, 233 consecutively enrolled patients with early RA (symptom duration of <12 months), recruited between 1995 and 2005 in the area of the city of Malmö, were followed up according to a structured program. Detailed inclusion and exclusion criteria as well as the exact study protocols have been described previously (17, 18). For both studies, ethics approval was granted, and written informed consent was obtained from all patients.
At baseline, ACPA (anti‐CCP2) IgG and rheumatoid factor (RF) IgM were measured by standard clinical methods. In the Dutch cohort, antibodies directed against carbamylated proteins (anti‐CarP) were analyzed by a validated in‐house assay as described previously (19). RA was classified according to the 2010 American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) criteria for RA (20) in the Dutch cohort and the 1987 ACR criteria for RA (21) in the Swedish cohort. Data from RA patients ages 20 years and older with a known ACPA status were used for this study, resulting in 408 Dutch patients and 198 Swedish patients. Of the 408 Dutch patients with RA, a subgroup of 128 patients with a relatively high disease activity (mean DAS of >1.8 during the first two years after study inclusion) was selected for separate analyses to assess the association between ACPAs and BMD in the presence of increased levels of inflammation.
Measurements of BMD
BMD was assessed by DXA. In the Dutch cohort, DXA images were obtained of the left total hip, the first to fourth vertebrae of the lumbar spine (L1–L4), or the second to fourth vertebrae of the lumbar spine (L2–L4) every year for 5 years. For the Swedish cohort, DXA images of the left femoral neck and second to fourth vertebrae of the lumbar spine (L2–L4) were obtained at study inclusion and after 2, 5, and 10 years. Results for BMD were expressed as absolute values (in g/cm2), T scores (measured as standard deviations from the mean value in healthy young adults), or Z scores (measured as standard deviations from the mean value in an age‐, sex‐, and ethnicity‐matched control population [22]). Osteopenia was defined as a T score between −2.5 (a value of −2.5 not included) and −1.0 (a value of −1.0 included) at any location, and osteoporosis was defined as a T score of less than or equal to −2.5 at any location. Dutch centers used the Hologic densitometer system, whereas Swedish data derived from the Lunar densitometer system. For the Dutch cohort, lumbar scores were determined according to the Hologic Spine reference group, and femoral scores were determined according to the National Health and Nutrition Examination Survey femur reference population (23). BMD scores for the Swedish cohort were calculated using a cohort of healthy individuals (146 men and 178 women, ages 20–87 years) from the same area as the reference population (24).
Statistical analysis
First, univariate analyses were performed to determine which of the covariates should be included in the final models. Variables that were univariably associated with ACPA status and one of the outcome measures of interest (P ≤ 0.1) in at least one of the cohorts were included as covariates in the final models for both cohorts, namely: sex, age, body mass index (BMI), symptom duration, smoking status, and serum levels of 25‐hydroxyvitamin D. Furthermore, the following variables were added to the models based on literature and a priori hypotheses: prednisone usage, DAS scores (25), Health Assessment Questionnaire (HAQ) scores (26), and C‐reactive protein (CRP) levels.
The association between ACPAs and BMD over time was modeled using generalized estimating equations (GEE), which allow for missing data in the outcome and account for clinical and demographic factors that differ between the two groups. With repeated measurements of BMD scores as the dependent variable, we investigated whether ACPA status was associated with changes in BMD. The same was done for osteopenia or osteoporosis prevalence. An interaction term of ACPA status × time was added to determine whether yearly changes in the outcome variables were different between ACPA‐positive patients and ACPA‐negative patients. The final models were adjusted for the following baseline variables: age, sex, BMI, symptom duration, and smoking status. The final models were also adjusted for the following longitudinal time‐varying measurements: disease activity (as assessed by the DAS44), prednisone intake, the HAQ disability index, CRP levels, and serum levels of 25‐hydroxyvitamin D (levels of vitamin D only available for the Dutch cohort). Since there was no difference in the intake of antiosteoporotic medication (bisphosphonates, vitamin D, or calcium supplementation) between ACPA‐positive patients and ACPA‐negative patients, these variables were not included in the final analyses.
Due to missingness of data, multiple imputation by chained equations (MICE) with predictive mean matching on 5 nearest neighbors was used to create 20 imputed data sets. All data of variables considered relevant for BMD were included. For analyses conducted on these 20 imputed data sets, only results after imputation were reported, which did not differ from the results obtained before imputation. All statistical analyses of data from the Dutch cohort were performed using Stata version 14 software, and all analyses of data from the Swedish cohort were performed using IBM SPSS version 26. P values less than or equal to 0.05 were considered significant. The Holm‐Bonferroni method was used to correct the alpha level for multiple testing.
RESULTS
Patient characteristics
Baseline characteristics of all patients included in this study are displayed in Table 1. The only notable differences in demographic or clinical variables between ACPA‐positive and ACPA‐negative patients were DAS scores, HAQ scores, and BMI for the Dutch cohort and CRP levels for the Swedish cohort. Higher levels of disease activity measured in the Dutch ACPA‐negative group can be explained by the use of the 2010 ACR/EULAR criteria for RA, which indicate that in patients who are negative for ACPAs, a higher number of affected joints and higher levels of acute‐phase reactants are needed to meet the definition of RA. A higher BMI among Dutch ACPA‐negative patients is consistent with previous findings (27), as is the observed association between ACPAs and smoking (28) and between ACPAs and CRP (29) in the Swedish cohort.
Table 1.
Baseline characteristics of the rheumatoid arthritis patients in the Dutch and Swedish cohort*
|
Dutch cohort (n = 408) |
Swedish cohort (n = 198) |
|||||
|---|---|---|---|---|---|---|
|
ACPA‐positive (n = 268) |
ACPA‐negative (n = 140) |
P |
ACPA‐positive (n = 114) |
ACPA‐negative (n = 84) |
P | |
| Age, years | 52 ± 13 | 54 ± 14 | 0.27 | 61 ± 12 | 62 ± 16 | 0.78 |
| Female sex, no. (%) | 188 (70) | 92 (66) | 0.36 | 81 (71) | 61 (73) | 0.81 |
| BMI | 25.6 ± 4.3 | 26.6 ± 4.9 | 0.02 | 25.4 ± 4.1 | 24.9 ± 3.9 | 0.36 |
| Smoking status, no. (%) | ||||||
| Never | 151 (57) | 90 (65) | 0.09 | 25 (22) | 34 (42) | 0.01 |
| Ever | 116 (43) | 48 (35) | – | – | ||
| Former | – | – | 40 (36) | 25 (31) | ||
| Current | – | – | 47 (42) | 22 (27) | ||
| Symptom duration, median (IQR) weeks | 18 (9–36) | 14 (9–28) | 0.18 | 35 (26–44) | 31 (22–43) | 0.11 |
| CRP, median (IQR) mg/liter | 13 (6–29) | 11 (4–29) | 0.32 | 10 (<9–32) | <9 (<9–17) | 0.05 |
| DAS | 3.3 ± 0.9 | 3.6 ± 0.9 | <0.01 | 3.3 ± 1.2 | 3.2 ± 1.1 | 0.48 |
| HAQ | 1.1 ± 0.7 | 1.3 ± 0.7 | 0.02 | 0.8 ± 0.6 | 0.9 ± 0.7 | 0.29 |
| Calcium intake, mg/day | 822 ± 281 | 870 ± 327 | 0.13 | NA | NA | |
| Serum 25(OH)D, nmoles/liter | 61 ± 30 | 55 ± 27 | 0.06 | NA | NA | |
Except where indicated otherwise, values are the mean ± SD. P values were calculated using t‐tests, Mann‐Whitney U tests, or chi‐square tests for normally distributed, non‐normally distributed, and dichotomous variables, respectively. ACPA = anti–citrullinated protein antibody; BMI = body mass index; IQR = interquartile range; CRP = C‐reactive protein; DAS = Disease Activity Score; HAQ = Health Assessment Questionnaire; NA = not available; 25(OH)D = 25‐hydroxyvitamin D.
Patient characteristics and treatment at follow‐up visits are shown in Supplementary Table 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41623/abstract. The use of conventional synthetic DMARDs (csDMARDs), biological DMARDs (bDMARDs), and prednisone at later time points was generally lower among ACPA‐negative patients, as expected based on previous results of the IMPROVED study that showed a higher achievement of drug‐free remission in this subset of patients (30).
Lower BMD values at baseline in ACPA‐positive patients
In the Dutch cohort, a significantly lower absolute BMD at baseline was observed in ACPA‐positive patients compared to ACPA‐negative patients (Figures 1A and B). A similar result was observed for Z scores in this cohort (Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41623/abstract). For the Swedish cohort, ACPA‐positive patients also had slightly lower BMD values at baseline, but the difference was far less pronounced than in the Dutch cohort and did not reach statistical significance (Figures 1C and D). Notably, no conclusions can be drawn from statistical comparisons between the two cohorts, as the Dutch and Swedish data were analyzed in separate models.
Figure 1.
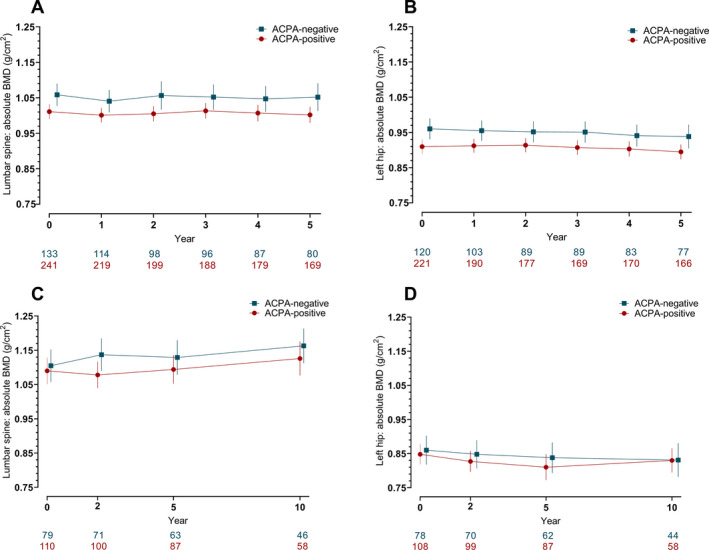
Raw data plots illustrating the yearly change in bone mineral density (BMD) measurements in two independent rheumatoid arthritis cohorts which were caterogized by anti–citrullinated protein antibody (ACPA) status. The Dutch cohort (A and B) and the Swedish cohort (C and D) of ACPA‐positive and ACPA‐negative patients received dual x‐ray absorptiometry (DXA) assessments at the lumbar spine and left hip at the indicated time points. Values below the graphs represent the number of patients with available DXA scans at each given time point in the ACPA‐positive and the ACPA‐negative group. Results are shown as the mean with error bars showing the 95% confidence intervals for both groups at the given time points.
The association between ACPA status and BMD measurements at baseline and over time was analyzed using GEE, the results of which are shown in Table 2. We found that ACPA positivity was significantly associated with lower absolute BMD values at baseline in the Dutch cohort, both at the lumbar spine (P = 0.03) and at the left hip (P = 0.01). Z scores at baseline were also significantly lower at both the left hip and lumbar spine in the ACPA‐positive group. Differences in BMD values or Z scores in the Swedish cohort did not reach statistical significance, although point estimates for the ACPA‐positive subset were slightly lower than for the ACPA‐negative subset at both measurement sites. When the final analyses for the Dutch and the Swedish cohort were adjusted for longitudinal intake of antiosteoporotic medication, the results did not change (Supplementary Table 2, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41623/abstract).
Table 2.
Generalized estimating equations (conducted on 20 imputed data sets) of the effect of ACPAs on baseline and longitudinal change in absolute BMD and Z scores and the association between ACPAs and the prevalence of osteopenia and osteoporosis over time*
| Dutch cohort | Swedish cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lumbar spine | Left hip (total hip) | Lumbar spine | Left hip (femoral neck) | |||||||||
| ACPA‐positive | ACPA‐negative | P | ACPA‐positive | ACPA‐negative | P | ACPA‐positive | ACPA‐negative | P | ACPA‐positive | ACPA‐negative | P | |
| Absolute BMD, g/cm2 | ||||||||||||
| Baseline EMM, (95% CI) | 1.01 (1.00, 1.03) | 1.05 (1.02, 1.08) | 0.03 | 0.92 (0.91, 0.93) | 0.95 (0.93, 0.97) | 0.01† | 1.10 (1.06, 1.14) | 1.13 (1.10, 1.17) | 0.12 | 0.85 (0.83, 0.88) | 0.90 (0.86, 0.94) | 0.22 |
| Yearly change, β (95% CI) | −0.002 (−0.004, 0.001) | 0.0004 (−0.004, 0.004) | 0.61 | −0.003 (−0.006, −0.001) | −0.004 (−0.008, 0.00002) | 0.89 | 0.003 (−0.002, 0.009) | 0.003 (−0.002, 0.009) | 0.95 | −0.003 (−0.007, 0.001) | −0.003 (−0.009, 0.002) | 0.92 |
| Z score | ||||||||||||
| Baseline EMM, (95% CI) | 0.32 (0.16, 0.47) | 0.62 (0.38, 0.86) | 0.04 | 0.18 (0.08, 0.29) | 0.48 (0.33, 0.63) | <0.01† | −0.15 (−0.36, 0.06) | 0.02 (−0.21, 0.26) | 0.13 | −0.22 (−0.45, 0.00) | −0.06 (−0.28, 0.17) | 0.12 |
| Yearly change, β (95% CI) | 0.038 (0.017, 0.058) | 0.060 (0.027, 0.094) | 0.43 | 0.008 (−0.007, 0.023) | 0.001 (−0.022, 0.025) | 0.37 | 0.035 (0.005, 0.064) | 0.033 (0.002, 0.064) | 0.93 | 0.004 (−0.026, 0.034) | 0.003 (−0.031, 0.037) | 0.98 |
| Prevalence of osteopenia, no. (%) | ||||||||||||
| Baseline | 94 (38.4) | 50 (37.6) | 0.48 | – | – | – | 37 (33.0) | 16 (20.2) | 0.04 | – | – | – |
| 5 years | 77 (43.8) | 31 (38.8) | 0.65‡ | – | – | – | 34 (38.6) | 25 (39.7) | – | – | – | – |
| 10 years | – | – | – | – | – | – | 26 (44.1) | 15 (32.6) | 0.56‡ | – | – | – |
| Prevalence of osteoporosis, no. (%) | ||||||||||||
| Baseline | 21 (8.6) | 9 (6.8) | 0.33 | – | – | – | 33 (29.5) | 26 (32.9) | 0.54 | – | – | – |
| 5 years | 14 (8.0) | 4 (5.0) | 0.66‡ | – | – | – | 28 (31.8) | 14 (22.2) | – | – | – | – |
| 10 years | – | – | – | – | – | – | 14 (23.7) | 12 (26.0) | 0.73‡ | – | – | – |
Models were adjusted for the following baseline variables: age, sex, body mass index, symptom duration, and smoking status. Models were also adjusted for the following longitudinal time‐varying measurements: Disease Activity Score, prednisone intake, Health Assessment Questionnaire score, C‐reactive protein levels, and serum 25‐hydroxyvitamin D levels (the latter only available for the Dutch cohort). Absolute bone mineral density (BMD) and Z score values are shown as point estimates with 95% confidence intervals (95% CIs) representing the estimated marginal means (EMMs) for baseline BMD and parameter estimates (β) for yearly change BMD. Osteopenia was defined as a T score between −2.5 (a value of −2.5 not included) and −1.0 (a value of −1.0 included) at any location, and osteoporosis was defined as a T score of less than or equal to −2.5 at any location. In the final generalized estimating equations model, osteopenia was defined as “at least osteopenia”, indicating a T score of less than or equal to −1.0, with a T score of more than −1.0 as reference. Osteoporosis was defined in a similar manner as osteopenia, with comparison groups using a T score of less than or equal to −2.5 versus a T score of more than −2.5 as reference. P values were calculated using Wald’s chi‐square test of model effects for anti–citrullinated protein antibodies (ACPAs) (e.g., baseline) and for the ACPA × time interaction (e.g., yearly change).
Difference between the groups remained significant after correction for multiple testing.
Yearly change P value with ACPA‐negative as reference group.
Given the possible negative influence of ACPAs on BMD, we expected the prevalence of osteopenia or osteoporosis to be higher among ACPA‐positive patients compared to ACPA‐negative patients. This was indeed the case in the Swedish cohort, wherein a significantly higher prevalence of osteopenia at baseline was found in the ACPA‐positive patients (P = 0.04) (Table 2). The prevalence of osteoporosis at baseline, though, did not differ between the two groups in the Swedish cohort. In the Dutch cohort, however, there was no association between ACPA positivity and a higher prevalence of osteopenia or osteoporosis at baseline.
In total, ACPA‐positive patients appeared to have slightly lower BMD values at baseline in both cohorts, although the BMD measurements in which this is reflected differed between the cohorts (absolute BMD value and Z score in the Dutch cohort versus osteopenia in the Swedish cohort). Although not all differences reached statistical significance after correction for multiple testing, ACPA‐positive patients overall had slightly lower BMD values at baseline in both cohorts.
No association between ACPA positivity and more loss of BMD over time
We hypothesized that ACPA‐positive patients would have a greater decline in BMD over time compared to ACPA‐negative patients. However, in contrast to the differences observed at baseline between the two groups, we found no association between ACPA status and yearly changes in BMD (Figure 1 and Table 2). ACPA positivity was not associated with a significantly greater decline in absolute BMD values during the follow‐up periods of 5 years (Dutch cohort) or 10 years (Swedish cohort) at either the left hip or the lumbar spine. Also, ACPA positivity was not associated with an increase in osteopenia or osteoporosis over time in either cohort. In line with this, changes in Z scores over time did not differ between the two groups at either the left hip or lumbar spine (Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41623/abstract).
No association between ACPA levels and BMD
To investigate whether higher levels of ACPAs are associated with greater BMD loss, we analyzed the association between ACPA IgG levels at inclusion and longitudinal BMD scores. We found that higher levels of ACPAs were not significantly associated with lower BMD values at baseline (Table 3). This was observed for absolute BMD values as well as for Z scores, at both lumbar and femoral sites. There was also no association between higher levels of ACPA IgG at baseline and more absolute BMD loss over time (Supplementary Figure 2, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41623/abstract).
Table 3.
Generalized estimating equations (conducted on non‐imputed data) of the association between ACPA IgG levels at inclusion and baseline and longitudinal change in absolute BMD and Z scores*
| Absolute BMD, g/cm2 | Z score | |
|---|---|---|
| Lumbar spine | ||
| Baseline | −0.002 (−0.017, 0.126) | 0.001 (−0.129, 0.132) |
| Yearly change | 0.001 (−0.001, 0.002) | 0.004 (−0.009, 0.017) |
| Left hip (total hip) | ||
| Baseline | 0.007 (−0.006, 0.019) | 0.074 (−0.016, 0.165) |
| Yearly change | 0.0003 (−0.002, 0.002) | −0.004 (−0.012, 0.004) |
Values are the β (95% confidence interval [95% CI]) for the association between anti–citrullinated protein antibody (ACPA) IgG levels at inclusion and absolute bone mineral density (BMD) and Z scores at baseline, and yearly change in absolute BMD and Z scores per 10‐fold (or log10) difference in ACPA IgG levels. Analyses were performed in 268 Dutch patients with rheumatoid arthritis who were positive for anti–citrullinated protein antibodies (ACPAs). Log10 transformation on ACPA IgG levels was applied in order to achieve normal distribution of levels. Models were adjusted for the following baseline variables: age, sex, body mass index, symptom duration, and smoking status. Models were also adjusted for the following longitudinal time‐varying measurements: Disease Activity Score, prednisone intake, Health Assessment Questionnaire score, C‐reactive protein levels, and serum 25‐hydroxyvitamin D levels.
Other autoantibodies not independently associated with BMD
In light of the associations that were observed between ACPA status and baseline BMD values, we extended our analyses in the Dutch cohort to other autoantibodies associated with RA (RF and anti‐CarP). Table 4 lists the differences in BMD measurements between seropositive and seronegative patients for the different autoantibodies. We found that RF‐positive patients had lower absolute BMD at baseline compared to RF‐negative patients (lumbar spine: P = 0.04). Similarly, the presence of anti‐CarP was associated with lower absolute BMD and Z scores at baseline (left hip: P = 0.04 and P = 0.04, respectively). Since both RF and anti‐CarP frequently occur simultaneously with ACPAs, the analyses were adjusted for ACPAs, after which both RF and anti‐CarP were found to no longer be associated with lower BMD scores at baseline at any given location. In contrast, the association between ACPAs and lower BMD values at baseline at the left hip remained significant after correction for the presence of RF and anti‐CarP. Consistent with previously described results for ACPAs, no association was found between RF positivity or anti‐CarP positivity and more decline in BMD over time. Finally, there was no baseline or longitudinal association between the quantitative number of autoantibodies present in a patient (ranging 0–3, among ACPAs, RF, and anti‐CarP) and (loss of) BMD either at baseline or over time.
Table 4.
Generalized estimating equations (conducted on 20 imputed data sets) of the effect of ACPA, RF, anti‐CarP and number of antibodies on baseline and longitudinal changes in BMD values and Z scores*
| Lumbar spine | Left hip (total hip) | |||||||
|---|---|---|---|---|---|---|---|---|
|
Absolute BMD, g/cm2 |
P |
Z score |
P |
Absolute BMD, g/cm2 |
P |
Z score |
P | |
| ACPAs | ||||||||
| Baseline, β (95% CI) | −0.04 (−0.07, −0.004) | 0.03 | −0.30 (−0.59, −0.01) | 0.04 | −0.03 (−0.06, −0.01) | 0.01† | −0.29 (−0.47, −0.11) | <0.01† |
| Yearly change, β (95% CI) | −0.001 (−0.01, 0.003) | 0.61 | −0.01 (−0.04, 0.02) | 0.44 | −0.0003 (−0.004, 0.004) | 0.89 | 0.01 (−0.01, 0.04) | 0.37 |
| ACPAs corrected for anti‐CarP and RF | ||||||||
| Baseline, β (95% CI) | −0.02 (−0.06, 0.01) | 0.18 | −0.20 (−0.51, 0.12) | 0.22 | −0.03 (−0.06, −0.003) | 0.03 | −0.28 (−0.48, −0.07) | 0.01† |
| Yearly change, β (95% CI) | −0.001 (−0.01, 0.003) | 0.54 | −0.01 (−0.05, 0.02) | 0.39 | −0.0005 (−0.004, 0.004) | 0.81 | 0.01 (−0.01, 0.03) | 0.42 |
| RF | ||||||||
| Baseline, β (95% CI) | −0.03 (−0.07, −0.001) | 0.04 | −0.27 (−0.57, 0.04) | 0.08 | −0.01 (−0.04, 0.01) | 0.27 | −0.13 (−0.32, 0.06) | 0.17 |
| Yearly change, β (95% CI) | −0.0004 (−0.005, 0.004) | 0.86 | −0.02 (−0.05, 0.01) | 0.27 | 0.0003 (−0.004, 0.004) | 0.88 | 0.001 (−0.02, 0.03) | 0.93 |
| RF corrected for ACPAs | ||||||||
| Baseline, β (95% CI) | −0.03 (−0.06, 0.11) | 0.18 | −0.18 (−0.50, 0.15) | 0.29 | −0.001 (−0.03, 0.03) | 0.97 | −0.01 (−0.21, 0.19) | 0.93 |
| Yearly change, β (95% CI) | 0.0001 (−0.004, 0.005) | 0.95 | −0.02 (−0.05, 0.02) | 0.41 | 0.001 (−0.004, 0.005) | 0.77 | −0.004 (−0.03, 0.02) | 0.78 |
| Anti‐CarP | ||||||||
| Baseline, β (95% CI) | −0.02 (−0.05, 0.01) | 0.20 | −0.18 (−0.46, 0.09) | 0.19 | −0.03 (−0.05, −0.001) | 0.04 | −0.19 (−0.37, −0.005) | 0.04 |
| Yearly change, β (95% CI) | −0.004 (−0.005, 0.004) | 0.85 | −0.001 (−0.04, 0.03) | 0.95 | 0.001 (−0.004, 0.005) | 0.80 | 0.002 (−0.02, 0.02) | 0.85 |
| Anti‐CarP corrected for ACPAs | ||||||||
| Baseline, β (95% CI) | −0.01 (−0.04, 0.02) | 0.59 | −0.08 (−0.37, 0.21) | 0.57 | −0.02 (−0.04, 0.011) | 0.24 | −0.09 (−0.29, 0.11) | 0.38 |
| Yearly change, β (95% CI) | −0.00003 (−0.005, 0.005) | 0.99 | 0.004 (−0.03, 0.04) | 0.83 | 0.001 (−0.004, 0.01) | 0.76 | −0.002 (−0.03, 0.02) | 0.84 |
| Number of antibodies corrected for ACPAs | ||||||||
| Baseline, β (95% CI) | −0.01 (−0.04, 0.01) | 0.24 | −0.11 (−0.31, 0.09) | 0.63 | −0.01 (−0.24, 0.01) | 0.48 | −0.06 (−0.19, 0.07) | 0.36 |
| Yearly change, β (95% CI) | −0.0004 (−0.002, 0.002) | 0.68 | −0.01 (−0.02, 0.01) | 0.37 | 0.0001 (−0.002, 0.002) | 0.95 | 0.003 (−0.01, 0.13) | 0.63 |
Data are shown for patients from the Dutch cohort. Models were adjusted for the following baseline variables: age, sex, body mass index, symptom duration, and smoking status. Models were also adjusted for the following longitudinal time‐varying measurements: Disease Activity Score, prednisone intake, Health Assessment Questionnaire score, C‐reactive protein levels, and serum 25‐hydroxyvitamin D levels. Point estimates and 95% confidence intervals (95% CIs) represent parameter estimates (β) for absolute BMD and Z scores at baseline and yearly change in absolute BMD and Z scores. P values were calculated using Wald’s chi‐square test of model effects for ACPAs, RF, and anti‐CarP (e.g., baseline) and for the antibody × time interaction (e.g., yearly change). Effect of number of antibodies (ranging 0–3, among ACPAs, rheumatoid factor [RF], and anti–carbamylated protein [anti‐CarP] antibodies) on baseline and longitudinal changes in bone mineral density (BMD) and Z scores was also assessed.
Difference remained significant after correction for multiple testing.
In summary, the association between autoantibody presence and lower BMD at baseline appears to be most clearly demonstrated for ACPAs, independent of the presence of other autoantibodies.
No association between ACPAs and BMD in patients with high levels of disease activity
Inflammation is hypothesized to play a role in BMD loss in RA (15). This raises the question of whether the lack of association observed between ACPAs and BMD loss over time could be due to the fact that there was very little disease activity, and thus inflammation, over time, especially in the Dutch patients who were treated with a treat‐to‐target approach with a DAS target of <1.6. Perhaps an association between ACPAs and BMD loss over time would have been apparent in the setting of higher levels of inflammation/disease activity. To investigate this, we attempted to identify a subgroup of patients with higher disease activity in the Dutch cohort. In light of the overall very low disease activity in this cohort, we defined this group with a higher disease activity as having a mean DAS of >1.8 during the first two years after study inclusion (not including the baseline visit). In this subgroup of 128 patients, no association was found between ACPAs and absolute BMD values at baseline in the lumbar spine or left hip (Figures 2A and B). In line with the results obtained from all patients included in the study (regardless of DAS), no association was found between ACPAs and more bone loss over time.
Figure 2.
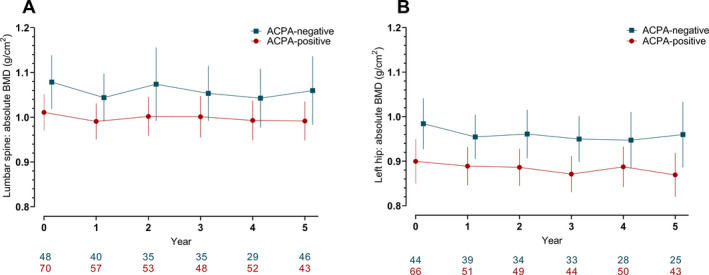
Raw data plots illustrating the yearly change in BMD measurements in 128 Dutch patients with rheumatoid arthritis who had high disease activity and who were categorized by ACPA status. High disease activity was classified as a patient having a mean Disease Activity Score of >1.8 during the first two years after study inclusion (baseline visit not included). BMD was measured at the lumbar spine (A) and left hip (B). Values below the graphs represent the number of patients with available DXA scans for each given time point in the ACPA‐positive and the ACPA‐negative group. Results are shown as the mean with error bars showing the 95% confidence intervals for both groups at the given time points. See Figure 1 for definitions.
DISCUSSION
To the best of our knowledge, our study is the first to investigate the important link between ACPAs and BMD in a longitudinal manner in untreated patients with early RA. In the present study, we found that ACPAs are associated with lower systemic BMD at disease onset in RA. This was particularly the case at femoral sites, where the observed values remained statistically significant after correction for multiple testing. However, in spite of differences in BMD between ACPA‐positive and ACPA‐negative patients at baseline, ACPA positivity is not associated with greater BMD loss over time in patients receiving standard clinical care or tight remission‐steered treatment. Finally, there is no association between BMD and other RA‐specific autoantibodies (such as RF and anti‐CarP), nor is there an association between BMD and the number of autoantibodies present in a patient.
Our results are consistent with previous findings showing lower BMD values among ACPA‐positive patients compared to ACPA‐negative patients at baseline. Moreover, this study is of important additive value, as it provides new insights into the course of BMD loss over time in patients with RA. Although no longitudinal differences were observed between the two groups, baseline differences were pronounced. Considering these results, it might be unlikely that the mere presence of ACPAs is sufficient to cause bone loss in RA, as ACPAs remain present after the start of treatment, yet ACPA‐positive patients do not exhibit more bone loss compared to ACPA‐negative patients. Our results therefore suggest alternative explanations than previous findings that have supported the theory that ACPAs induce bone loss independently of inflammation status by directly binding to osteoclasts, stimulating osteoclast differentiation and proliferation.
Instead, lower BMD in ACPA‐positive patients could possibly be an effect of inflammation. This hypothesis is supported by preliminary studies indicating that adequate suppression of disease activity, and thus inflammation, is key to prevent further bone loss and thereby stabilize BMD in patients with RA (13, 31). Furthermore, it has been suggested that suppression of inflammation effectively prevents bone loss in ACPA‐positive and ACPA‐negative patients in equal measure. Earlier studies have demonstrated that inhibition of interleukin‐8 interferes with osteoclastogenesis and thus prevents osteolysis (32, 33). Moreover, ACPAs are only associated with higher erosion scores in the clinically suspect arthralgia stage of RA when concomitant inflammation is present, indicating that inflammation functions as a key mediator in the link between ACPAs and erosion development (34). Since there is strong evidence that erosive disease and systemic BMD loss in RA have common pathways in their pathogenesis (35, 36), these results might also suggest an indirect association between ACPAs and bone loss via inflammation.
In the present study, we found a stronger association between BMD and ACPAs than between BMD and RF or anti‐CarP. This could be a reflection of the fact that due to for example their specific associations with certain genetic and environmental risk factors (37), ACPAs seem to represent a more discriminatory type of antibody compared to RF or Anti‐CarP that is able to define a particular subset of patients with RA. This specific subset of RA patients might also tend to experience more severe bone loss. In contrast to the findings of Orsolini et al (5), we found no level‐dependent effect of ACPAs on BMD at baseline.
Our study has several limitations. One limitation is that we do not know the natural course of BMD over time in the absence of therapeutic intervention. We cannot exclude the possibility that ACPAs might have been associated with BMD loss over time if patients had not been treated. However, this limitation is unavoidable in modern RA research, because all RA patients normally receive treatment. This limitation could also be seen as an advantage, as it afforded us the opportunity to assess the effect of autoantibody presence in the setting of optimal control of disease activity. Furthermore, treatment for osteoporosis, which was in part initiated based on the DXA results in the study, may have prevented further BMD loss during follow‐up. Although this could theoretically have affected our comparisons, we have no indication that medication for osteoporosis was preferentially prescribed to ACPA‐positive or ACPA‐negative patients. Another limitation is that we cannot exclude the possibility that DXA scans of the lumbar spine are sensitive to increasing degenerative and osteoarthritic changes associated with aging. This could explain why lumbar BMD measurement showed a very slight increase over time. Furthermore, differences regarding absolute BMD values and Z scores between ACPA‐positive and ACPA‐negative patients in the Dutch cohort were not exactly replicated in the Swedish cohort. This could be due to the fact that there were fewer Swedish patients, resulting in less power to detect differences. Finally, despite the clear statistically significant differences at baseline, absolute differences in mean BMD measures between ACPA‐positive and ACPA‐negative patients were minor, meaning the clinical relevance of these findings has yet to be established.
Our study also has several strengths, such as the use of two independent cohorts with large sample sizes. Because of the long follow‐up periods of 5 and 10 years, we were able to not only investigate the link between autoantibodies and BMD on a baseline level, but also to determine the impact of these autoantibodies on long‐term changes in BMD while accounting for various relevant covariates. By selecting patients diagnosed with early untreated arthritis, we were able to study the effect of autoantibodies on BMD without prior confounding by therapy.
In conclusion, we found that ACPA‐positive patients have a significantly lower BMD at baseline compared to ACPA‐negative patients. However, ACPA positivity is not associated with more bone loss over time in patients with early RA who are treated according to modern strategies. These results indicate that ACPAs alone do not seem to contribute to bone loss after the onset of clinical disease in the absence of severe inflammation.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Ms Amkreutz had full access to all the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Nilsson, Karlsson, Huizinga, Jacobsson, Allaart, Turesson, van der Woude.
Acquisition of data
Theander, Willim, Heimans, Nilsson, Karlsson, Åkesson, Jacobsson, Allaart.
Analysis and/or interpretation of data
Amkreutz, de Moel, Nilsson, Karlsson, Huizinga, Åkesson, Jacobsson, Allaart, Turesson, van der Woude.
Supporting information
Supplementary Material
Dr. Åkesson has received consulting fees, speaking fees, and/or honoraria from Amgen, UCB, Chugai, Astellas, and Renapharma (less than $10,000 each). Dr. Jacobsson has received consulting fees, speaking fees, and/or honoraria from Pfizer, AbbVie, Novartis, Eli Lilly, and Janssen (less than $10,000 each). No other disclosures relevant to this article were reported.
References
- 1. Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment [review]. Nat Rev Rheumatol 2012;8:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wysham KD, Shoback DM, Imboden JB Jr, Katz PP. Association of high anti–cyclic citrullinated peptide seropositivity and lean mass index with low bone mineral density in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2018;70:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van der Helm‐van Mil AH, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther 2005;7:R949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bugatti S, Bogliolo L, Vitolo B, Manzo A, Montecucco C, Caporali R. Anti‐citrullinated protein antibodies and high levels of rheumatoid factor are associated with systemic bone loss in patients with early untreated rheumatoid arthritis. Arthritis Res Ther 2016;18:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Orsolini G, Caimmi C, Viapiana O, Idolazzi L, Fracassi E, Gatti D, et al. Titer‐dependent effect of anti‐citrullinated protein antibodies on systemic bone mass in rheumatoid arthritis patients. Calcif Tissue Int 2017;101:17–23. [DOI] [PubMed] [Google Scholar]
- 6. Llorente I, Merino L, Ortiz AM, Escolano E, Gonzalez‐Ortega S, Garcia‐Vicuna R, et al. Anti‐citrullinated protein antibodies are associated with decreased bone mineral density: baseline data from a register of early arthritis patients. Rheumatol Int 2017;37:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest 2012;122:1791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catrina AI, Svensson CI, Malmstrom V, Schett G, Klareskog L. Mechanisms leading from systemic autoimmunity to joint‐specific disease in rheumatoid arthritis [review]. Nat Rev Rheumatol 2017;13:79–86. [DOI] [PubMed] [Google Scholar]
- 9. Hensvold AH, Joshua V, Li W, Larkin M, Qureshi F, Israelsson L, et al. Serum RANKL levels associate with anti‐citrullinated protein antibodies in early untreated rheumatoid arthritis and are modulated following methotrexate. Arthritis Res Ther 2015;17:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hafstrom I, Ajeganova S, Forslind K, Svensson B. Anti‐citrullinated protein antibodies are associated with osteopenia but not with pain at diagnosis of rheumatoid arthritis: data from the BARFOT cohort. Arthritis Res Ther 2019;21:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Schaardenburg D, Nielen MM, Lems WF, Twisk JW, Reesink HW, van de Stadt RJ, et al. Bone metabolism is altered in preclinical rheumatoid arthritis. Ann Rheum Dis 2011;70:1173–4. [DOI] [PubMed] [Google Scholar]
- 12. Kleyer A, Finzel S, Rech J, Manger B, Krieter M, Faustini F, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis 2014;73:854–60. [DOI] [PubMed] [Google Scholar]
- 13. Hoes JN, Bultink IE, Lems WF. Management of osteoporosis in rheumatoid arthritis patients. Expert Opin Pharmacother 2015;16:559–71. [DOI] [PubMed] [Google Scholar]
- 14. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- 15. Hauser B, Riches PL, Wilson JF, Horne AE, Ralston SH. Prevalence and clinical prediction of osteoporosis in a contemporary cohort of patients with rheumatoid arthritis. Rheumatology (Oxford) 2014;53:1759–66. [DOI] [PubMed] [Google Scholar]
- 16. Briot K, Roux C. Glucocorticoid‐induced osteoporosis. RMD Open 2015;1:e000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wevers‐de Boer K, Visser K, Heimans L, Ronday HK, Molenaar E, Groenendael JH, et al. Remission induction therapy with methotrexate and prednisone in patients with early rheumatoid and undifferentiated arthritis (the IMPROVED study). Ann Rheum Dis 2012;71:1472–7. [DOI] [PubMed] [Google Scholar]
- 18. Theander L, Willim M, Nilsson JA, Karlsson M, Akesson KE, Jacobsson LT, et al. Changes in bone mineral density over 10 years in patients with early rheumatoid arthritis. RMD Open 2020;6:e001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Moel EC, Derksen V, Stoeken G, Trouw LA, Bang H, Goekoop RJ, et al. Baseline autoantibody profile in rheumatoid arthritis is associated with early treatment response but not long‐term outcomes. Arthritis Res Ther 2018;20:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 21. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 22. Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J 2007;83:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. US Department of Health and Human Services . Plan and operation of the Third National Health and Nutrition Examination Survey, 1988‐94. Series 1: programs and collection procedures. July 1994. URL: https://www.cdc.gov/nchs/data/series/sr_01/sr01_032.pdf. [PubMed]
- 24. Karlsson MK, Gardsell P, Johnell O, Nilsson BE, Akesson K, Obrant KJ. Bone mineral normative data in Malmo, Sweden. Comparison with reference data and hip fracture incidence in other ethnic groups. Acta Orthop Scand 1993;64:168–72. [DOI] [PubMed] [Google Scholar]
- 25. Van der Heijde DM, van ’t Hof MA, van Riel PL, Theunisse LM, Lubberts EW, van Leeuwen MA, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis 1990;49:916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 27. Pedersen M, Jacobsen S, Klarlund M, Pedersen BV, Wiik A, Wohlfahrt J, et al. Environmental risk factors differ between rheumatoid arthritis with and without auto‐antibodies against cyclic citrullinated peptides. Arthritis Res Ther 2006;8:R133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van der Woude D, Catrina AI. HLA and anti‐citrullinated protein antibodies: building blocks in RA. Best Pract Res Clin Rheumatol 2015;29:692–705. [DOI] [PubMed] [Google Scholar]
- 29. Alemao E, Guo Z, Burns L, Frits M, Coblyn J, Weinblatt M, et al. Evaluation of the association between C‐reactive protein and anti–citrullinated protein antibody in rheumatoid arthritis: analysis of two clinical practice data sets [abstract]. Arthritis Rheumatol 2016;68 Suppl 10. URL: https://acrabstracts.org/abstract/evaluation-of-the-association-between-c-reactive-protein-and-anti-citrullinated-protein-antibody-in-rheumatoid-arthritis-analysis-of-two-clinical-practice-data-sets. [Google Scholar]
- 30. Heimans L, Akdemir G, Boer KV, Goekoop‐Ruiterman YP, Molenaar ET, van Groenendael JH, et al. Two‐year results of disease activity score (DAS)‐remission‐steered treatment strategies aiming at drug‐free remission in early arthritis patients (the IMPROVED‐study). Arthritis Res Ther 2016;18:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raterman HG, Lems WF. Pharmacological management of osteoporosis in rheumatoid arthritis patients: a review of the literature and practical guide. Drugs Aging 2019;36:1061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krishnamurthy A, Joshua V, Hensvold AH, Jin T, Sun M, Vivar N, et al. Identification of a novel chemokine‐dependent molecular mechanism underlying rheumatoid arthritis‐associated autoantibody‐mediated bone loss. Ann Rheum Dis 2016;75:721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kopesky P, Tiedemann K, Alkekhia D, Zechner C, Millard B, Schoeberl B, et al. Autocrine signaling is a key regulatory element during osteoclastogenesis. Biol Open 2014;3:767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ten Brinck RM, Toes RE, van der Helm‐van Mil AH. Inflammation functions as a key mediator in the link between ACPA and erosion development: an association study in Clinically Suspect Arthralgia. Arthritis Res Ther 2018;20:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guler‐Yuksel M, Bijsterbosch J, Goekoop‐Ruiterman YP, de Vries‐Bouwstra JK, Hulsmans HM, de Beus WM, et al. Changes in bone mineral density in patients with recent onset, active rheumatoid arthritis. Ann Rheum Dis 2008;67:823–8. [DOI] [PubMed] [Google Scholar]
- 36. Sambrook PN. The skeleton in rheumatoid arthritis: common mechanisms for bone erosion and osteoporosis? [editorial]. J Rheumatol 2000;27:2541–2. [PubMed] [Google Scholar]
- 37. Toes RE, van der Woude D. ACPA (anti‐citrullinated protein antibodies) and rheumatoid arthritis [editorial]. Acta Reumatol Port 2011;36:205–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


