Abstract
Aim
To examine the long‐term efficacy and safety of dapagliflozin, a sodium‐glucose co‐transporter‐2 (SGLT2) inhibitor used to treat type 1 diabetes, in the Japanese subpopulation of the DEPICT‐2 study.
Materials and Methods
Patients with type 1 diabetes were randomized to dapagliflozin 5 mg (n = 55), dapagliflozin 10 mg (n = 41) or placebo (n = 58) plus insulin for a 24‐week, double‐blind period followed by a 28‐week, single‐blind extension phase.
Results
From baseline to 24 weeks, dapagliflozin reduced HbA1c compared with placebo (mean change of −0.58% and −0.80% for 5 and 10 mg, respectively), and an HbA1c reduction was observed up to 52 weeks. Compared with placebo, dapagliflozin 5 and 10 mg increased the proportion of patients achieving HbA1c reductions of 0.5% or more without severe hypoglycaemia events and reduced glycaemic variability assessed via continuous glucose monitoring. Both dapagliflozin doses decreased body weight and total daily insulin dose at 24 weeks compared with placebo; these reductions were maintained up to 52 weeks. Diabetic ketoacidosis occurred in both dapagliflozin groups (one and two cases, respectively) but not with placebo.
Conclusions
Efficacy and safety results from the Japanese subpopulation of the DEPICT‐2 study were generally consistent with those from the overall population, indicating that long‐term dapagliflozin adjunct to insulin therapy improves glycaemic control without an increased risk of hypoglycaemia but with a risk of diabetic ketoacidosis in Japanese patients with type 1 diabetes.
Keywords: dapagliflozin, hypoglycaemia, Japanese subpopulation, long‐term efficacy, safety, type 1 diabetes
1. INTRODUCTION
Type 1 diabetes (T1D) is a chronic disorder with a tendency for childhood onset, although it can occur at any age, and accounts for 5%–10% of all diabetes cases worldwide. 1 In Japan, the population of patients with T1D is estimated at 82,000–110,000. 2 The current standard treatment for T1D primarily involves intensive insulin regimens that aim to lower HbA1c. 3 However, despite intensified insulin therapy, the majority of patients with T1D fail to achieve optimal glycaemic control, 4 and even among those who do achieve their HbA1c targets, hypoglycaemia and weight gain associated with intensive insulin use can lead to diminished quality of life. 5 , 6 , 7 According to 2018 statistics from the Japan Diabetes Clinical Data Management Study Group (JDDM), 8 mean HbA1c (a measure of long‐term blood glucose control) in patients with T1D was 7.76%, which is higher than the 7.03% observed in patients with type 2 diabetes (T2D) and exceeds the diabetes treatment target of 7.0%. 9 ,10 Although insulin therapy represents life‐saving treatment for patients with T1D, most patients experience treatment‐related hypoglycaemia that affects their daily lives, and severe hypoglycaemia is a life‐threatening risk.
Insulin therapy is also associated with a long‐term risk of weight gain. 9 ,10 JDDM statistics show that the mean body mass index (BMI) in Japanese patients has gradually increased from 22.21 kg/m2 in 2003 to 23.12 kg/m2 in 2018, 8 although the ideal BMI does not exceed 22 kg/m2 for both males and females in Japan and obesity is defined as a BMI of 25 kg/m2 or higher. 11 Furthermore, a BMI of 23 kg/m2 or higher has been shown to be a risk factor for insulin resistance in the Japanese population. 12 As weight gain is a risk factor for arteriosclerosis, 13 weight control may therefore represent an important long‐term prognostic factor. Furthermore, variability in blood glucose levels associated with insulin therapy can have an adverse effect on a patientʼs quality of life 14 and is an independent risk factor for multiple hypoglycaemic events. 15 Narrowing the variability of blood glucose is expected not only to prevent insulin‐induced hypoglycaemia and hyperglycaemia, but also to improve quality of life in patients with T1D. To ensure adequate glycaemic control, it is therefore important to evaluate the potential use of adjunct therapy to insulin in this patient population. In Japan, the only approved adjunct to insulin in patients with T1D is an α‐glucosidase inhibitor, which has limited efficacy and is associated with gastrointestinal symptoms such as flatus and diarrhoea, 16 underscoring the need for alternative non‐insulin treatment. Taken together, there is a high unmet medical need for a novel insulin adjunct therapy that can improve blood glucose levels without compounding the risks associated with insulin therapy (hypoglycaemia, weight gain and wide variability in blood glucose level) in Japanese patients with T1D.
Sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors, a class of antidiabetic medications, show significant potential in the treatment of hyperglycaemia. 16 Dapagliflozin is a highly selective SGLT2 inhibitor approved for the treatment of T2D, and has been shown to improve glycaemic control independent of insulin and to attenuate the weight gain associated with insulin therapy without increasing the risk of hypoglycaemia. 17 As part of the clinical development of dapagliflozin, two phase 3 studies were performed (DEPICT‐1 and DEPICT‐2), each with a 24‐week, double‐blind period 17 , 18 followed by a 28‐week extension period, for a total of 52 weeks. 19 , 20 The outcomes of these studies showed that dapagliflozin as an adjunct to insulin was associated with significant improvements in glycaemic control and weight reduction, without an increased risk of hypoglycaemia but with an increased risk of diabetic ketoacidosis (DKA) events compared with placebo. Furthermore, continuous glucose monitoring (CGM) data showed a reduction in the mean amplitude of glucose excursions.
The current analysis was performed to examine the efficacy and safety of dapagliflozin as an adjunct to insulin in Japanese patients enrolled in the DEPICT‐2 study. The study included a 28‐week extension, which allowed us to evaluate the long‐term (52‐week) efficacy and safety of dapagliflozin in Japanese patients with inadequately controlled T1D.
2. METHODS
2.1. Patients
The detailed inclusion and exclusion criteria for the DEPICT‐2 study have been published elsewhere. 19 Briefly, Japanese patients with T1D, aged 18–75 years, with a BMI of 18.5 kg/m2 or higher, an HbA1c of 7.5%–10.5%, and C‐peptide levels of less than 0.7 ng/mL, were eligible for participation. Patients were excluded if they had been treated with dapagliflozin or any other SGLT2 inhibitor at any time prior to enrolment or if they had been treated with insulin‐sensitizing agents (e.g. metformin or thiazolidinediones) in the 2 months prior to screening. The complete list of inclusion and exclusion criteria can be found in Table S1. In addition to study medication, patients were treated with insulin administered as multiple daily injections (MDI; three or more injections per day of basal and prandial insulin) or via continuous subcutaneous insulin infusion (CSII).
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice, and the study protocol. The study was approved by the institutional review boards and independent ethics committees at each participating centre. All patients provided written informed consent prior to participation. This study was registered on ClinicalTrials.gov (NCT02460978) and there were 29 participating sites in Japan.
2.2. Study design and treatments
This was a multicentre, randomized, double‐blind, parallel‐group, three‐arm, placebo‐controlled phase 3 study to evaluate the efficacy and safety of two doses of dapagliflozin (5 and 10 mg) as an adjunct to insulin in patients with T1D and inadequate glycaemic control. The study design comprised an 8‐week, lead‐in period, during which no study medication or placebo were administered; a 24‐week treatment period; and a 28‐week patient‐ and site‐blinded extension phase followed by a 4‐week follow‐up period (Figure S1). At the end of the lead‐in period, participants were randomly assigned 1:1:1 to dapagliflozin 5 mg, dapagliflozin 10 mg or placebo using a centralized blocked randomization schedule via an interactive voice response system. Randomization was stratified by current CGM use, method of insulin administration (MDI or CSII) and baseline HbA1c. Study medication or placebo was administered orally, once daily. Enrolled patients were prohibited from receiving antihyperglycaemic agents other than insulin and weight‐loss medication from screening to the end of the follow‐up period. A reduction in daily insulin dose by up to 20% for both basal and bolus insulin was recommended after the first dose of the study drug on day 1 to reduce the risk of hypoglycaemia, at the discretion of the investigator. Thereafter, attempts to uptitrate the daily insulin dose were recommended.
2.3. Assessments
HbA1c was measured centrally. Weight and height were measured at the study sites. CGM assessment was performed by patients using an electronic CGM sensor over a 2‐week period and data were reviewed by site. CGM was assessed at baseline and at weeks 12 and 24. Self‐monitored blood glucose values and ketones, measured in the blood as beta‐hydroxybutyrate, were measured using a device provided by the study sponsor. Safety and tolerability were evaluated by assessing adverse events (AEs), vital signs, physical examination findings, electrocardiogram and laboratory values, self‐measured glucose and ketone values, and DKA events. Patients were advised to measure their blood ketone levels following potential symptoms of DKA or during acute illness, and to contact the study centre if values above 0.6 mmol/L were noted.
2.4. Efficacy outcomes
The primary efficacy outcome was the adjusted mean change in HbA1c from baseline to week 24. Secondary efficacy outcomes included the % change from baseline to week 24 in body weight and total daily insulin dose, the change from baseline to week 24 in the mean value of 24‐h glucose readings obtained from CGM, the mean amplitude of glucose excursion of 24‐h glucose readings obtained from CGM, the % of 24‐h CGM values of 71–180 mg/dL, and the proportion of patients achieving an HbA1c reduction of 0.5% or higher from baseline to week 24 without severe hypoglycaemia. Exploratory endpoints were all efficacy analyses at/up to week 52.
2.5. Safety outcomes
The safety outcomes included AEs, as well as the frequency and severity of hypoglycaemia and DKA events. AEs were classified using the terms listed in the Medical Dictionary for Regulatory Activities (version 20.1). All potential DKA events reported by investigators during the study period were adjudicated by an independent blinded DKA adjudication committee. In addition, an external data monitoring committee periodically evaluated the incidence of hypoglycaemia and DKA events in addition to AEs, laboratory data and safety assessments.
2.6. Statistical methods
Efficacy analyses were carried out in the full analysis set, which consisted of all randomized patients who received at least one dose of study medication during the short‐term double‐blind period and who had a baseline assessment and any postbaseline assessment during the short‐term double‐blind period. Safety analyses were carried out in all patients who received at least one dose of study medication during the short‐term double‐blind period.
In the main analysis for DEPICT‐2, a total of 768 patients (256 per arm) was required to detect a statistical difference between each dapagliflozin treatment group and the placebo group at a two‐sided significance level of .0262, based on the Dunnett and Tamhane step‐up procedure, assuming that 5% of patients do not undergo a postbaseline assessment. 19 Of these 768 patients, 160 Japanese patients were planned for enrolment to carry out population‐specific analyses in a subpopulation comprising approximately 20% of the global study population.
The analyses for this subgroup were similar to those performed for the overall population in DEPICT‐2. 19 The primary analysis of the change in HbA1c from baseline to week 24 was based on a longitudinal repeated‐measures analysis using direct likelihood and an overall type 1 error rate of 5%. The model included the fixed categorical effects of treatment, week, randomization stratification factor (one term for each combination of all stratification factors), and treatment‐by‐week interaction, as well as the continuous fixed covariates of baseline measurement and baseline measurement‐by‐week interaction. For secondary outcomes, point estimates and two‐sided 95% confidence intervals (CIs) for the mean change within each treatment group and the difference in mean change between each dapagliflozin treatment group and placebo were calculated. The analyses in Japanese patients were prespecified in the study protocol. All statistical analyses were performed using SAS software.
3. RESULTS
3.1. Patients
Of 813 patients randomized and treated with study drug in DEPICT‐2, 154 Japanese patients were enrolled from 29 participating sites in Japan, and 55, 41 and 58 of these patients were randomized to the 5 mg dapagliflozin, 10 mg dapagliflozin and placebo groups, respectively. The disposition of the Japanese patients is shown in Table 1. Similar proportions of patients in each group completed the 24‐week (89.1%, 97.6% and 91.4%, respectively) and 52‐week (85.5%, 97.6% and 89.7%, respectively) treatment periods. The most common reason for treatment discontinuation in each treatment group at both 24 and 52 weeks was AEs. No patients had the status of lost to follow‐up by the end of the long‐term treatment period, and all patients were deemed to be treatment compliant. The key baseline characteristics and demographics of the Japanese patients are shown in Table 2 and were generally comparable between the three groups.
TABLE 1.
Patient disposition
| DAPA 5 mg (n = 55) | DAPA 10 mg (n = 41) | Placebo (n = 58) | |
|---|---|---|---|
| Completed short‐term treatment | 49 (89.1) | 40 (97.6) | 53 (91.4) |
| Did not complete short‐term treatment | 6 (10.9) | 1 (2.4) | 5 (8.6) |
| Reason for not completing short‐term treatment | |||
| Adverse event | 3 (5.5) | 1 (2.4) | 3 (5.2) |
| Patient request to discontinue study treatment | 1 (1.8) | 0 | 0 |
| Withdrawal of consent | 0 | 0 | 2 (3.4) |
| Other | 2 (3.6) | 0 | 0 |
| Completed short‐term treatment but did not enter long‐term treatment | 0 | 0 | 1 (1.7) |
| Entered long‐term treatment | 49 (89.1) | 40 (97.6) | 52 (89.7) |
| Completed long‐term treatment | 47 (85.5) | 40 (97.6) | 52 (89.7) |
| Reason for not completing long‐term treatment | |||
| Adverse event | 1 (1.8) | 0 | 0 |
| Patient requested to discontinue study treatment | 1 (1.8) | 0 | 0 |
Abbreviation: DAPA, dapagliflozin.
Data are shown as n (%).
TABLE 2.
Demographic and baseline characteristics
| DAPA 5 mg (n = 55) | DAPA 10 mg (n = 41) | Placebo (n = 58) | |
|---|---|---|---|
| Age (years, mean [SD]) | 46.2 (12.8) | 47.3 (9.4) | 47.0 (13.5) |
| Sex | |||
| Male | 29 (52.7) | 20 (48.8) | 24 (41.4) |
| Female | 26 (47.3) | 21 (51.2) | 34 (58.6) |
| Body weight (kg, mean [SD]) | 66.2 (11.9) | 67.8 (12.8) | 64.5 (13.1) |
| Body mass index (kg/m2, mean [SD]) | 24.0 (3.4) | 25.4 (3.5) | 24.8 (4.3) |
| Duration of T1D (years, mean [SD]) | 17.3 (11.3) | 16.0 (9.9) | 13.1 (9.5) |
| Total baseline insulin (IU, mean [SD]) | 50.6 (25.8) | 48.5 (24.1) | 46.4 (18.9) |
| Total basal insulin (IU, mean [SD]) | 20.0 (9.5) | 20.7 (10.3) | 19.1 (9.0) |
| Total bolus insulin (IU, mean [SD]) | 31.0 (18.0) | 29.0 (17.8) | 27.3 (11.4) |
| HbA1c (%, mean [SD]) | 8.4 (0.7) | 8.4 (0.6) | 8.5 (0.7) |
| HbA1c | |||
| <8% | 12 (21.8) | 9 (22.0) | 15 (25.9) |
| ≥8% to <9% | 32 (58.2) | 24 (58.5) | 27 (46.6) |
| ≥9% | 11 (20.0) | 8 (19.5) | 16 (27.6) |
| Fasting C‐peptide (ng/mL) | |||
| <lower limit of detection | 44 (80.0) | 31 (75.6) | 46 (79.3) |
| ≥0.05 | 11 (20.0) | 9 (22.0) | 12 (20.7) |
| Not reported | 0 | 1 (2.4) | 0 |
| Method of insulin administration | |||
| MDI | 47 (85.5) | 37 (90.2) | 54 (93.1) |
| CSII | 8 (14.5) | 4 (9.8) | 4 (6.9) |
| GFR (mL/min/1.73 m2) | |||
| <60 | 0 | 1 (2.4) | 0 |
| ≥60 and >90 | 21 (38.2) | 13 (31.7) | 24 (41.4) |
| ≥90 | 34 (61.8) | 27 (65.9) | 34 (58.6) |
Abbreviations: CSII, continuous subcutaneous insulin infusion; DAPA, dapagliflozin; GFR, glomerular filtration rate; MDI, multiple daily injection; SD, standard deviation; T1D, type 1 diabetes.
Data are shown as n (%) unless otherwise stated.
3.2. Efficacy
At week 24, a significant reduction in HbA1c was observed with both doses of dapagliflozin compared with placebo. The mean changes (95% CI) in HbA1c from baseline to week 24 were −0.58% (95% CI: −0.82%, −0.33%) and −0.80% (95% CI: −1.07%, −0.54%) for dapagliflozin 5 and 10 mg, respectively, versus placebo. At week 52, the mean changes were −0.22% (95% CI: −0.50%, 0.06%) for dapagliflozin 5 mg and −0.33% (95% CI: −0.63%, −0.04%) for dapagliflozin 10 mg (Figure 1A). For body weight, differences from baseline to week 24 were −3.75% (95% CI: −4.92%, −2.56%) in the dapagliflozin 5 mg group and −4.98% (95% CI: −6.22%, −3.72%) in the dapagliflozin 10 mg group compared with placebo, and from baseline to week 52 they were −4.66% (95% CI: −6.06%, −3.25%) in the dapagliflozin 5 mg group and −6.30% (95% CI: −7.75%, −4.82%) in the dapagliflozin 10 mg group (Figure 1B).
FIGURE 1.
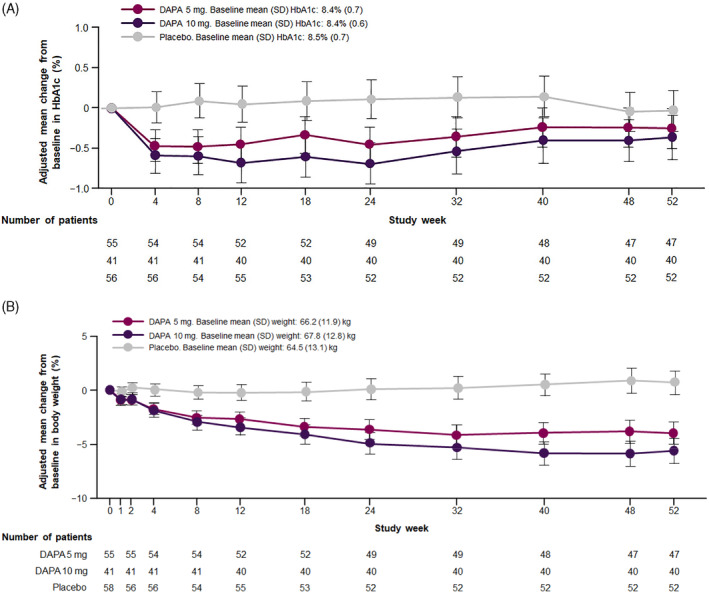
Adjusted mean change from baseline in (A) HbA1c and (B) body weight (kg). Error bars represent 95% confidence intervals. Abbreviations: DAPA, dapagliflozin; SD, standard deviation
The total daily insulin dose was reduced in the dapagliflozin 5 and 10 mg groups compared with placebo at week 24, and this effect was also seen at week 52 (Figure 2). The % reduction in insulin was similar with both basal and bolus administration at 24 weeks from baseline. For dapagliflozin 5 and 10 mg, the respective changes from baseline to week 24 were −9.53% (95% CI: −15.70%, −2.91%) and −16.63% (95% CI: −22.99%, −9.75%) with basal insulin, and −13.84% (95% CI: −19.32%, −8.00%) and −14.48% (95% CI: −20.54%, −7.95%) with bolus insulin. The proportion of patients who achieved a reduction in HbA1c of 0.5% or higher without severe hypoglycaemia events at week 24 was 45.5% and 61.0% in the dapagliflozin 5 and 10 mg groups, respectively, compared with 17.2% in the placebo group, and at week 52 was 34.5% and 41.5% in the dapagliflozin 5 and 10 mg groups, respectively, compared with 24.1% in the placebo group. The effects of dapagliflozin on other secondary outcomes are reported in the supporting information, including the change from baseline to week 24 in the mean value of 24‐h glucose readings obtained from CGM (Table S2), the mean amplitude of glucose excursion of 24‐h glucose readings obtained from CGM (Table S3), and the % of 24‐h CGM values of 71–180 mg/dL (Table S4). All three outcomes were improved with both dapagliflozin 5 and 10 mg compared with placebo.
FIGURE 2.
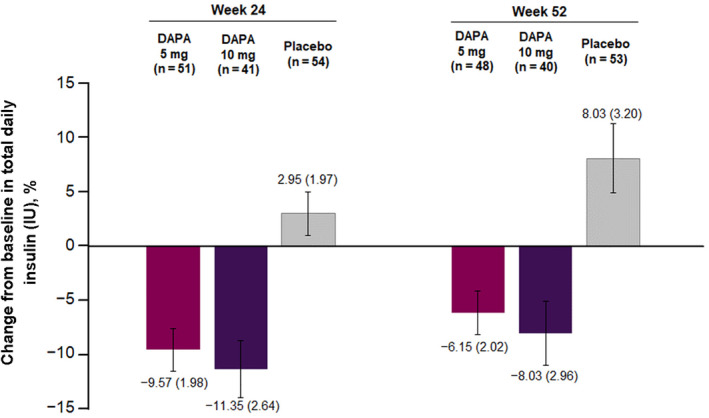
Percentage change from baseline in total daily insulin at weeks 24 and 52. Data are mean (standard error). Abbreviations: DAPA, dapagliflozin; IU, international unit. Total baseline insulin dose (IU, mean [SD]): DAPA 5 mg, 50.6 (25.8); DAPA 10 mg, 48.5 (24.1); placebo, 46.4 (18.9)
3.3. Safety
3.3.1. Adverse events
The incidence of overall AEs at week 52 was higher in the dapagliflozin treatment groups compared with placebo: 87.3%, 87.8% and 82.8% in the dapagliflozin 5 mg, dapagliflozin 10 mg and placebo groups, respectively (Table S5). Respective incidences were 21.8%, 14.6% and 8.6% for pollakiuria; 3.6%, 19.5% and 1.7% for thirst; 10.9%, 17.1% and 1.7% for weight decreased; 1.8%, 7.3% and 0% for rash; and 3.6%, 7.3% and 1.7% for constipation; and all incidences were higher in the dapagliflozin groups compared with placebo (Table S5). The majority of events were mild or moderate in intensity. The frequency of AEs leading to discontinuation was not higher in patients treated with dapagliflozin compared with placebo: 7.3% of patients in the dapagliflozin 5 mg group, 2.4% in the dapagliflozin 10 mg group and 5.2% in the placebo group. The incidence of serious AEs (SAEs) at week 52 was increased with dapagliflozin treatment compared with placebo (5.5% in the dapagliflozin 5 mg group, 9.8% in the dapagliflozin 10 mg group and 1.7% in the placebo group) (Table 3).
TABLE 3.
Adverse events
| Category a | DAPA 5 mg (n = 55) | DAPA 10 mg (n = 41) | Placebo (n = 58) |
|---|---|---|---|
| ≥1 AE | 48 (87.3) | 36 (87.8) | 48 (82.8) |
| ≥1 AE related to study drug | 23 (41.8) | 22 (53.7) | 12 (20.7) |
| ≥1 AE of hypoglycaemia | 46 (83.6) | 34 (82.9) | 50 (86.2) |
| AE leading to discontinuation | 4 (7.3) | 1 (2.4) | 3 (5.2) |
| Death | 0 | 0 | 0 |
| ≥1 SAE | 3 (5.5) | 4 (9.8) | 1 (1.7) |
| ≥1 SAE related to study drug | 3 (5.5) | 2 (4.9) | 0 |
| SAE leading to discontinuation | 3 (5.5) | 1 (2.4) | 0 |
| ≥1 SAE of hypoglycaemia | 1 (1.8) | 0 | 0 |
| SAE of hypoglycaemia leading to discontinuation | 1 (1.8) | 0 | 0 |
| ≥1 ketone‐related SAE | 1 (1.8) | 1 (2.4) | 0 |
| Ketone‐related SAE leading to discontinuation | 1 (1.8) | 0 | 0 |
Abbreviations: AE, adverse event; DAPA, dapagliflozin; SAE, serious adverse event.
Data are shown as n (%).
Only hypoglycaemia and diabetic ketoacidosis reported as an SAE are included in the AE, related AE, SAE, related SAE, and AE leading to discontinuation summary data.
3.3.2. Hypoglycaemia and DKA
Hypoglycaemic events of any type occurred in a similar proportion of patients in all three groups: 46 (83.6%) patients in the dapagliflozin 5 mg group, 34 (82.9%) in the dapagliflozin 10 mg group and 50 (86.2%) in the placebo group (Table 3). A severe hypoglycaemic event was reported for two (3.6%), one (2.4%) and zero (0%) patients in the dapagliflozin 5 mg, dapagliflozin 10 mg and placebo groups, respectively, which was lower than observed for the overall population (8.9%, 9.6% and 8.5%). DKA events were adjudicated as definite in one, two and zero patients in the dapagliflozin 5 mg, dapagliflozin 10 mg and placebo groups, respectively. Two of the three patients required hospitalization (one each in the dapagliflozin 5 mg and dapagliflozin 10 mg groups); both patients discontinued dapagliflozin treatment. Further details of the three cases of DKA are given in Table S6.
4. DISCUSSION
Patients with T1D face a number of challenges to achieve their treatment targets, including weight gain associated with intensive insulin treatment and a potential increase in cardiovascular risk. 13 SGLT2 inhibitors, including dapagliflozin, have a mechanism of action that is insulin‐independent 22 and they may be of benefit as adjunct therapies in the treatment of patients with T1D. However, caution should be exercised when treating patients with SGLT2 inhibitors as there appears to be potential for an increased risk of DKA associated with their use. 23
The current study showed that treatment with dapagliflozin improved HbA1c, reduced body weight and reduced total daily insulin dose at 24 weeks, and that these efficacy improvements were maintained for up to 52 weeks. In T2D, there are inherent demographic differences, for example in BMI, between Asian and Caucasian patients, 24 underscoring the importance of conducting the current study in the Japanese patient group, the only Asian population in DEPICT‐2. 18 , 20 These Japanese patients had similar baseline characteristics to those of the overall population in DEPICT‐2 18 , 20 ; the exceptions were mean BMI and proportion of CSII use, which were lower compared with the overall population, and mean age, which was slightly higher in Japanese patients. We recently completed a long‐term safety study of dapagliflozin as add‐on to insulin for 52 weeks in 151 Japanese patients with T1D. 25 A reduction in HbA1c was observed at week 24 following treatment with 5 or 10 mg dapagliflozin, and reduced HbA1c was observed up to week 52 for both doses with a similar safety profile to that observed in the current study. Taken together, the findings from the safety study 25 and the results of the current analysis support the overall favourable benefit‐risk profile of dapagliflozin for the treatment of Japanese patients with T1D.
The frequencies of AEs and SAEs were higher in the dapagliflozin groups compared with the placebo group, which was similar to the pattern observed in the overall population. The AE profile was consistent with that known for dapagliflozin, 26 although DKA events were more frequently observed in the T1D than in the T2D population. In the Japanese subpopulation of the study, DKA events were observed in three patients receiving dapagliflozin.
The results observed in the current study are consistent with those reported for the overall study population in DEPICT‐2, 21 with similar efficacy results observed with respect to the significant reduction in HbA1c, body weight and insulin dose at 24 weeks. The hypoglycaemic effect was less pronounced at week 52 in both the overall population and the Japanese population at the lower dose of dapagliflozin, which was potentially attributable to the unblinding of HbA1c during the 28‐week patient‐ and site‐blinded extension. No differences in dapagliflozin exposure are expected in Japanese patients with T1D compared with Western populations, indicating that no adjustment of dapagliflozin dose will be necessary when it is used as an adjunct to insulin in Japanese patients. 27 A recent phase 2 study of the SGLT2 inhibitor empagliflozin as an adjunct to insulin in Japanese patients with T1D showed that the pharmacodynamics, pharmacokinetics and safety profile of empagliflozin in this population was comparable with that of non‐Japanese patients 28 ; however, few other studies in Japanese patients have been published to date.
The SGLT2 inhibitor class has been associated in both T1D and T2D patients with an increase in the risk of DKA, including euglycaemic DKA, 23 which is generally much more prevalent in T1D than in T2D regardless of SGLT2 inhibitor use. In the current study, one out of three patients who developed DKA experienced euglycaemic DKA, which was attributed to insulin pump failure and periods of illness. Two of these three patients used CSII for insulin administration. All patients recovered, and one continued dapagliflozin treatment. Patients with T1D using SGLT2 inhibitors appear to be at a higher risk of DKA. 29 While DKA is frequently preventable with early detection and appropriate self‐monitoring, 30 particular attention must be paid to the risk of euglycaemic DKA (glucose levels of <14 mmol/L) in those receiving SGLT2 inhibitors. Hence, ketone measurement is recommended to detect potential DKA at an early stage and subsequently prevent progression to DKA when patients are particularly susceptible, such as during illness, when consuming alcohol, when dehydrated, following intense exercise, or after large reductions in insulin dose. 31 Of note, it is not recommended to reduce the insulin dose by more than 20% from baseline in patients receiving dapagliflozin. 32
This study had some limitations. First, comparatively few Japanese patients were enrolled. However, the target number of Japanese patients was prespecified in the protocol and satisfied regulatory requirements in Japan. Second, patients with a history of DKA or severe hypoglycaemia within 1 month prior to screening were excluded, which may limit generalizability to patients at a higher risk of DKA or severe hypoglycaemia. Finally, the close monitoring of DKA and hypoglycaemia during the study may have been more intense than commonly found in clinical practice.
In conclusion, the results of this phase 3 study show that in Japanese patients with inadequately controlled T1D, dapagliflozin 5 and 10 mg as an adjunct to insulin for up to 52 weeks is associated with improvements in glycaemic control and weight loss, without increasing the risk of hypoglycaemia. Furthermore, there were no differences in safety profiles between the dapagliflozin 5 and 10 mg groups. In the Japanese subpopulation, three events of DKA (including one case of euglycaemic DKA) were reported in the dapagliflozin groups, while no DKA was observed in the placebo group. The efficacy and safety results in the Japanese population were generally consistent with those from the overall population in the DEPICT‐2 study.
CONFLICT OF INTEREST
EA has received honoraria for lectures from AstraZeneca, MSD, Ono Pharmaceutical, Kowa Pharmaceutical, Sanofi, Mitsubishi Tanabe Pharma and Novo Nordisk Pharma; and has received scholarship grants from Astellas Pharma, Kowa Pharmaceutical, Sanofi, Daiichi Sankyo, Taisho Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Eli Lilly Japan, Novartis Pharma, Novo Nordisk Pharma and Pfizer. CM serves or has served on the advisory panel for Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd, Eli Lilly and Company, Novartis, Bristol‐Myers Squibb, AstraZeneca, Janssen Pharmaceuticals, Boehringer Ingelheim, Hanmi Pharmaceuticals, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Dianax and UCB; financial compensation for these activities has been received by KU Leuven. KU Leuven has received research support for CM from Medtronic, Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd, Eli Lilly and Company, Roche, Abbott, ActoBio Therapeutics and Novartis. CM also serves or has served on the speakersʼ bureau for Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd, Eli Lilly and Company, Boehringer Ingelheim, Astra Zeneca and Novartis; financial compensation for these activities has been received by KU Leuven. TS has received honoraria for lectures from Takeda Pharmaceutical and Sanofi; and has received research grants from Novo Nordisk Pharma, Sanofi, Takeda Pharmaceutical, AstraZeneca, Boehringer Ingelheim, Quintiles and Mitsubishi Tanabe Pharma. HM has no conflict of interest to disclose. HI has received research grants from AstraZeneca, Eli Lilly Japan, Novo Nordisk Pharma and Daiichi Sankyo. FT, NA, MA and NI are employees of AstraZeneca.
AUTHOR CONTRIBUTIONS
EA contributed to data analysis and interpretation, and manuscript review. CM contributed to the study design and conduct, interpretation of data, and manuscript review. TS contributed to data collection and manuscript review. HM contributed to data collection and manuscript review. HI contributed to data collection and manuscript review. FT contributed to the study design and conduct, data analysis and interpretation, and manuscript review. NA contributed to data analysis and interpretation, and manuscript review. MA contributed to data analysis and interpretation, and manuscript review. NI contributed to the study design and interpretation of data, and manuscript review. All the authors approved the final version of the manuscript prior to submission.
5.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14362.
Supporting information
Table S1 Study inclusion and exclusion criteria.
Table S2. Change in mean value of 24‐hour glucose readings at Week 24.
Table S3. Change in mean amplitude of glucose excursions at Week 24.
Table S4. Change in percentage of 24‐hour glucose readings in the range of >70 mg/dL to ≤180 mg/dL at Week 24.
Table S5. Most common adverse events (≥5%) at Week 52.
Table S6. Diabetic ketoacidosis events adjudicated by an independent adjudication committee.
Figure S1. Study design.
ACKNOWLEDGEMENTS
We thank Clare Cox, PhD, of Edanz Evidence Generation, for providing medical writing support, which was funded by AstraZeneca through EMC K.K. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Some results of this study were previously presented as a poster presentation at the 62nd Annual Meeting of the Japan Diabetes Society, Sendai, Miyagi Prefecture, Japan, 23–25 May 2019. This study was funded by AstraZeneca.
Araki E, Mathieu C, Shiraiwa T, et al. Long‐term (52‐week) efficacy and safety of dapagliflozin as an adjunct to insulin therapy in Japanese patients with type 1 diabetes: Subgroup analysis of the DEPICT‐2 study. Diabetes Obes Metab. 2021;23:1496–1504. 10.1111/dom.14362
Funding information This study was funded by AstraZeneca.
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data‐sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
REFERENCES
- 1. You W, Henneberg M. Type 1 diabetes prevalence increasing globally and regionally: the role of natural selection and life expectancy at birth. BMJ Open Diabetes Res Care. 2016;4:e000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ministry of Health, Labour and Welfare . Patient Survey 2014 and 2017. https://www.e-stat.go.jp/stat-search/files?page=1&toukei=00450022&tstat=000001031167. Accessed September 23, 2020.
- 3. Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014;2:CD009122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the US: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971‐978. [DOI] [PubMed] [Google Scholar]
- 5. Frier BM, Schernthaner G, Heller SR. Hypo‐glycemia and cardiovascular risks. Diabetes Care. 2011;34(suppl 2):S132‐S137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925‐1932. [DOI] [PubMed] [Google Scholar]
- 7. Harris S, Mamdani M, Galbo‐Jørgensen CB, Bøgelund M, Gundgaard J, Groleau D. The effect of hypoglycemia on health‐related quality of life: Canadian results from a multinational time trade‐off survey. Can J Diabetes. 2014;38:45‐52. [DOI] [PubMed] [Google Scholar]
- 8. Japan Diabetes Clinical Data Management Study Group . 2019. http://jddm.jp/data/index-2018.html. Accessed September 23, 2020.
- 9. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int. 2020;11:165‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Journal of Diabetes Investigation. 2020;11(4):1020‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tokunaga K, Matsuzawa Y, Kotani K. Ideal body weight estimated from body mass index with the lowest morbidity. Int J Obes. 1991;15:1‐5. [PubMed] [Google Scholar]
- 12. Okura T, Nakamura R, Fujioka Y, et al. Body mass index ≥23 is a risk factor for insulin resistance and diabetes in Japanese people: a brief report. PLoS One. 2018;13:e0201052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Purnell JQ, Zinman B, Brunzell JD, DCCT/EDIC Research Group . The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation. 2013;127:180‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vanstone M, Rewegan A, Brundisini F, Dejean D, Giacomini M. Patient perspectives on quality of life with uncontrolled type 1 diabetes mellitus: a systematic review and qualitative meta‐synthesis. Ont Health Technol Assess Ser. 2015;15:17. [PMC free article] [PubMed] [Google Scholar]
- 15. Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia. 2007;50:2553‐2561. [DOI] [PubMed] [Google Scholar]
- 16. Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig. 2018;9:657‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S, Dapagliflozin 006 Study Group . Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124‐136. [DOI] [PubMed] [Google Scholar]
- 18. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT‐1): 24‐week results from a multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:864‐876. [DOI] [PubMed] [Google Scholar]
- 19. Mathieu C, Dandona P, Gillard P, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT‐2 study): 24‐week results from a randomized controlled trial. Diabetes Care. 2018;41:1938‐1946. [DOI] [PubMed] [Google Scholar]
- 20. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT‐1 52‐week study. Diabetes Care. 2018;41:2552‐2559. [DOI] [PubMed] [Google Scholar]
- 21. Mathieu C, Rudofsky G, Phillip M, et al. Long‐term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT‐2 study): 52‐week results from a randomized controlled trial. Diabetes Obes Metab. 2020;22:1516‐1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wright EM, Loo DDF, Hirayama B. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733‐794. [DOI] [PubMed] [Google Scholar]
- 23. Meyer EJ, Gabb G, Jesudason D. SGLT2 inhibitor‐associated euglycemic diabetic ketoacidosis: a south Australian clinical case series and Australian spontaneous adverse event notifications. Diabetes Care. 2018;41:e47‐e49. [DOI] [PubMed] [Google Scholar]
- 24. Møller JB, Pedersen M, Tanaka H, et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care. 2014;3:796‐804. [DOI] [PubMed] [Google Scholar]
- 25. Araki E, Watada H, Uchigata Y, et al. Efficacy and safety of dapagliflozin in Japanese patients with inadequately controlled type 1 diabetes (DEPICT‐5): 52‐week results from a randomized, open‐label, phase III clinical trial. Diabetes Obes Metab. 2020;22:540‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TWA, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf. 2014;37:815‐829. [DOI] [PubMed] [Google Scholar]
- 27. Sokolov V, Yakovleva T, Ueda S, et al. Urinary glucose excretion after dapagliflozin treatment: an exposure‐response modelling comparison between Japanese and non‐Japanese patients diagnosed with type 1 diabetes mellitus. Diabetes Obes Metab. 2019;21:829‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shimada A, Hanafusa T, Yasui A, et al. Empagliflozin as adjunct to insulin in Japanese participants with type 1 diabetes: results of a 4‐week, double‐blind, randomized, placebo‐controlled phase 2 trial. Diabetes Obes Metab. 2018;20:2190‐2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA Adverse Event Reporting System. Diabetologia. 2017;60:1385‐1389. [DOI] [PubMed] [Google Scholar]
- 30. Goldenberg RM, Berard LD, Cheng AY, et al. SGLT2 inhibitor–associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis. Clin Ther. 2016;38:2654‐2664. [DOI] [PubMed] [Google Scholar]
- 31. Danne T, Gard S, Peters AL, et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium‐glucose cotransporter (SGLT) inhibitors. Diabetes Care. 2019;42:1147‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Henry RR, Dandona P, Pettus J, Mudaliar S, Xu J, Hansen L. Dapagliflozin in patients with type 1 diabetes: a post hoc analysis of the effect of insulin dose adjustments on 24‐hour continuously monitored mean glucose and fasting β‐hydroxybutyrate levels in a phase IIa pilot study. Diabetes Obes Metab. 2017;19:814‐821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Study inclusion and exclusion criteria.
Table S2. Change in mean value of 24‐hour glucose readings at Week 24.
Table S3. Change in mean amplitude of glucose excursions at Week 24.
Table S4. Change in percentage of 24‐hour glucose readings in the range of >70 mg/dL to ≤180 mg/dL at Week 24.
Table S5. Most common adverse events (≥5%) at Week 52.
Table S6. Diabetic ketoacidosis events adjudicated by an independent adjudication committee.
Figure S1. Study design.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data‐sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.


