Key Points
Question
Are neuronal autoantibodies associated with impaired cognitive function in patients with lung cancer?
Findings
In this cross-sectional study of 167 consecutive patients with lung cancer, 37% of patients had neuronal autoantibodies; 45% of patients with small cell lung cancer and 34% of patients with non–small cell lung cancer were autoantibody positive. Patients with neuronal autoantibodies had increased odds of cognitive impairment compared with patients without autoantibodies; 17% of patients had autoantibodies against currently unknown neuronal antigens, which were also associated with higher odds of cognitive impairment.
Meaning
The study’s findings suggested that neuronal autoantibodies might represent a pathogenic factor in cancer-related cognitive impairment among patients with lung cancer; autoantibodies against currently unknown epitopes have potentially relevant consequences for cognitive function, which warrants further characterization.
Abstract
Importance
Paraneoplastic neurological syndromes are associated with neuronal autoantibodies, and some of these autoantibodies are associated with neuropsychological symptoms. The most common underlying tumor is lung cancer. The association of neuronal autoantibodies with cognitive deficits has not been systematically investigated in patients with small cell lung cancer (SCLC) and non–small cell lung cancer (NSCLC).
Objective
To assess the frequency of neuronal autoantibodies in patients with lung cancer and analyze their association with cognitive function.
Design, Setting, and Participants
This prospective, cross-sectional study included 167 patients with lung cancer (both SCLC and NSCLC) recruited at a single lung cancer center in Berlin, Germany, between June 2015 and April 2016. Detailed neuropsychological testing was performed in a carefully selected subgroup of 97 patients (from which patients with potential confounding factors were excluded). Investigators were blinded to patients’ autoantibody status and cognitive test results. Data were analyzed from May 2016 to December 2019.
Main Outcomes and Measures
Prevalence of neuronal autoantibodies and their association with cognitive impairment. The evaluation of autoantibodies as potential risk factors for cognitive impairment was performed using bayesian logistic regression models.
Results
Among 167 patients with lung cancer (median age, 66.0 years [interquartile range, 59.0-72.0 years]; 105 men [62.9%]), 127 had NSCLC, and 40 had SCLC. Brain-directed autoantibodies were detected in 61 of 167 patients (36.5%); 33 patients (19.8%) had known autoantibodies and 28 patients (16.8%) had autoantibodies against currently unknown antigens that were detected through immunohistochemical analysis. Cognitive impairment was found in 65 of 97 patients (67.0%). Among patients with SCLC, the odds of cognitive impairment for those with any autoantibodies was 11-fold higher (odds ratio [OR], 11.0; 95% credible interval [CrI], 1.2-103.6) than that of autoantibody-negative patients, and the increased odds were independent of age, sex, and neurological deficit. Among patients with NSCLC, those with immunoglobin A autoantibodies targeting the N-methyl-d-aspartate receptor had a relevantly increased odds of verbal memory deficits (OR, 182.8; 95% CrI, 3.1-10 852.4). Autoantibodies against currently unknown antigens were also associated with increased odds of cognitive impairment (OR, 2.8; 95% CrI, 0.6-12.1).
Conclusions and Relevance
In this prospective, cross-sectional study, more than one-third of patients with lung cancer had neuronal autoantibodies that were found to be associated with cognitive impairment. These autoantibodies might represent a potentially treatable mechanism of immune-mediated cognitive impairment among patients with lung cancer.
This cross-sectional study assesses the frequency of neuronal autoantibodies in patients with small cell and non–small cell lung cancer and examines the association of these autoantibodies with cognitive function.
Introduction
Paraneoplastic neurological syndromes (PNSs) are neurological disorders occurring in the context of an underlying tumor.1 These disorders are immune mediated and associated with cancer-induced immune responses, including neuronal autoantibodies..2 Small cell lung cancer (SCLC) is the most frequent cancer associated with PNS, but non–small cell lung cancer (NSCLC) is also commonly found in patients with PNS.3 In a prospective study of 264 patients with SCLC, almost 10% developed a PNS, and many patients, even those without a diagnosis of PNS, had 1 or more neuronal autoantibody.4
A high prevalence of neuronal autoantibodies was observed in a 2017 retrospective study of 323 patients with different types of cancer, including SCLC and NSCLC.5 These serum autoantibodies mainly targeted neuronal cell surface proteins and were associated with mild cognitive impairment.5 This finding was replicated in a prospective blinded study indicating that neuronal autoantibodies were associated with deficits in all cognitive domains among patients with melanoma.6 Some of these autoantibodies (immunoglobin A [IgA] and immunoglobin M [IgM] autoantibodies targeting the N-methyl-d-aspartate receptor [NMDAR]) had previously been associated with slow cognitive impairment and were also detected in a subgroup of patients with dementia.7,8
Cognitive deficits in patients with lung cancer (both SCLC and NSCLC) are frequent, clinically relevant, and occur independent of tumor treatment.9 However, the association of neuronal autoantibodies with cognitive deficits has not been systematically investigated in patients with SCLC and NSCLC.
This cross-sectional study examined the prevalence of neuronal autoantibodies in patients with SCLC and NSCLC, independent of tumor stage or treatment. This analysis included previously characterized anti-intracellular (AIC) autoantibodies and neuronal surface autoantibodies as well as brain-directed autoantibodies with currently unidentified neuronal antigens. In addition, detailed cognitive testing was performed among a carefully selected subgroup after exclusion of patients with potential confounders (eg, brain metastases and the receipt of brain radiotherapy), comparing autoantibody-positive with autoantibody-negative patients with SCLC and NSCLC. We hypothesized that patients with neuronal autoantibodies are more likely to have cognitive impairment compared with patients without neuronal autoantibodies.
Methods
This cross-sectional study was approved by the research ethics board of Charité–Universitätsmedizin in Berlin, Germany. All participants provided written informed consent. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies.
Patients
A total of 167 consecutive patients with lung cancer were recruited at a large lung cancer center (Evangelische Lungenklinik Berlin) in Berlin, Germany, between June 2015 and April 2016. For assessment of autoantibody prevalence, all patients with a histologically confirmed diagnosis of lung cancer, regardless of lung cancer subtype, cancer stage, or treatment, were considered for the study. Clinical data on tumor type, tumor stage, treatment, medical history, and medication receipt were obtained from patients’ medical records (Table 1).
Table 1. Participant Characteristics.
| Characteristic | No. (%) | ||
|---|---|---|---|
| All patients (N = 167) | Patients with SCLC (n = 40) | Patients with NSCLC (n = 127)a | |
| Age, median (IQR) | 66.0 (59.0-72.0) | 63.5 (59.5-69.3) | 66.0 (59.0-72.0) |
| Sex | |||
| Male | 105 (62.9) | 25 (62.5) | 80 (63.0) |
| Female | 62 (37.1) | 15 (37.5) | 47 (37.0) |
| Clinical cancer stageb | |||
| I | 17 (10.2) | 0 | 17 (13.4) |
| II | 12 (7.2) | 1 (2.5) | 11 (8.7) |
| III | 42 (25.2) | 15 (37.5) | 27 (21.3) |
| IV | 93 (55.7) | 23 (57.5) | 70 (55.1) |
| Unknown | 3 (1.8) | 1 (2.5) | 2 (1.6) |
| Metastases | |||
| No | 72 (43.1) | 16 (40.0) | 56 (44.1) |
| Yes | 93 (55.7) | 23 (57.5) | 70 (55.1) |
| Unknown | 2 (1.2) | 1 (2.5) | 1 (0.8) |
| Site of metastases | |||
| Lung, pleura, and/or pericardium (M1a) | 70 (41.9) | 16 (40.0) | 54 (42.5) |
| Visceral | 38 (22.8) | 13 (32.5) | 25 (19.7) |
| Bones | 21 (12.6) | 6 (15.0) | 15 (11.8) |
| Distant lymph nodes | 20 (12.0) | 8 (20.0) | 12 (9.4) |
| Soft tissue | 7 (4.2) | 2 (5.0) | 5 (3.9) |
| Brain | 13 (7.8) | 3 (7.5) | 10 (7.9) |
| Treatment | |||
| Chemotherapyc | 91 (54.5) | 27 (67.5) | 64 (50.4) |
| Radiotherapyd | 47 (28.1) | 14 (35.0) | 33 (26.0) |
| Surgery of primary tumore | 38 (22.8) | 1 (2.5) | 37 (29.1) |
| Surgery of metastases | 5 (3.0) | 0 | 5 (3.9) |
| Targeted therapyf | 8 (4.8) | 0 | 8 (6.3) |
| Immunotherapy (IVIG) | 3 (1.8) | 2 (5.0) | 1 (0.8) |
| Time since initial diagnosis, median (IQR), mo | 2.0 (1.0-5.5) | 1.0 (1.0-5.0) | 2.0 (1.0-6.5) |
| Other current or previous cancer | 38 (22.8) | 8 (20.0) | 30 (23.6) |
| Lung cancerg | 10 (6.0) | 1 (2.5) | 9 (7.1) |
| Other cancer | 28 (16.8) | 7 (17.5) | 21 (16.5) |
| Family history of cancer | 55/101 (54.5) | 16/28 (57.1) | 39/73 (53.4) |
| Medical history | |||
| Smokingh | 148 (88.6) | 37 (92.5) | 111 (87.4) |
| Pack-years, median (IQR) | 40.0 (30.0-50.0) | 40.0 (30.0-50.0) | 40.0 (30.0-50.0) |
| Arterial hypertension | 110 (65.9) | 23 (57.5) | 87 (68.5) |
| Pulmonary disease | 90 (53.9) | 17 (42.5) | 73 (57.5) |
| Cardiovascular disease | 84 (50.3) | 15 (37.5) | 69 (54.3) |
| Gastrointestinal disease | 51 (30.5) | 9 (22.5) | 42 (33.1) |
| Neurological disease | 43 (25.7) | 15 (37.5) | 28 (22.0) |
| Diabetes | 42 (25.1) | 9 (22.5) | 33 (26.0) |
| Urological or nephrological disease | 41 (24.6) | 8 (20.0) | 33 (26.0) |
| Thyroid disease | 39 (23.4) | 11 (27.5) | 28 (22.0) |
| Psychiatric disease | 16 (9.6) | 6 (15.0) | 10 (7.9) |
| Autoimmune disease | 9 (5.4) | 2 (5.0) | 7 (5.5) |
| Rheumatic disease | 6 (3.6) | 2 (5.0) | 4 (3.1) |
| Paraneoplastic disease | 10 (6.0) | 8 (20.0) | 2 (1.6) |
| Autoantibody-associated PNS | 6 (3.6) | 6 (15.0) | 0 |
| SIADH | 2 (1.2) | 2 (5.0) | 0 |
| Hypercalcemia | 2 (1.2) | 0 | 2 (1.6) |
Abbreviations: IQR, interquartile range; IVIG, intravenous immunoglobin; NSCLC, non–small cell lung cancer; PNS, paraneoplastic neurological syndrome; SCLC, small cell lung cancer; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
Among 127 patients with NSCLC, 62 (48.8%) had adenocarcinoma, 43 (33.9%) had squamous cell carcinoma, and 22 (17.3%) had other cancers, including 2 patients (1.6%) with large cell carcinoma, 1 patient (0.8%) with large cell neuroendocrine carcinoma, 2 patients (1.6%) with typical carcinoid tumors, 1 patient (0.8%) with atypical carcinoid tumors, and 16 patients (12.6%) with NSCLC not otherwise specified.
Based on 2010 cancer staging criteria from the Union for International Cancer Control.
A total of 58 patients (34.7%) were currently receiving chemotherapy.
A total of 47 patients (28.1%) had local radiotherapy (primary tumor), and 10 patients (6.0%) had brain radiotherapy.
A total of 31 patients (18.6%) had a resection margin of R0, 3 patients (1.8%) had a resection margin of R1, 4 patients (2.4%) had an unknown resection margin.
A total of 3 patients (1.8%) received bevacizumab (vascular endothelial growth factor antibody) therapy, 2 patients (1.2%) received nintedanib (tyrosine kinase inhibitor) therapy, and 3 patients (1.8%) received other therapy.
A total of 6 patients (3.6%) had a history of lung cancer (cured), and 4 patients (2.4%) had synchronous second carcinoma.
Data on smoking were missing for 8 patients.
Neuronal Autoantibody Analysis
Serum samples were analyzed using well-established commercial assays, including cell-based assays (CBAs) and frozen brain tissue sections using immunohistochemical analysis (IHC) (BIOCHIP mosaics; EUROIMMUN AG) (eMethods in the Supplement). Autoantibody testing was performed by investigators (including K.R.) who were blinded to neuropsychological test results.
Neuropsychological Tests
To assess the association between autoantibodies and cognitive function, a carefully selected subgroup of 97 patients (from which patients with potential confounding factors were excluded) received detailed neuropsychological testing and neurological examinations. Patients were excluded if they were older than 80 years, had brain metastases or a history of severe neurological or psychiatric disorders (eg, stroke, epilepsy, or dementia), received brain radiotherapy, had surgery less than 5 days before study enrollment, and were not fluent in German (eMethods in the Supplement).
Cognitive function was assessed by investigators (M.-M.W., T.S., and K.F.) who were blinded to patients’ autoantibody status using a battery of standardized neuropsychological tests for verbal memory (Verbal Learning and Memory Test10), visuospatial memory (Rey-Osterrieth Complex Figure test11), working memory (digit span forward and backward), attention (Test of Attentional Performance12), and executive function (Test of Attentional Performance, Stroop Color and Word Test,13 and Regensburger Word Fluency Test14) (eMethods in the Supplement). Patient-reported outcome measures for fatigue, depression, quality of life, and subjective cognitive performance are listed in the eMethods in the Supplement.
Cognitive impairment as a binary outcome was defined using criteria from the International Cognition and Cancer Task Force (ICCTF),15 in which cognitive impairment was defined as a test score of 2 SDs or more below the test-specific reference cohort on at least 1 test of a cognitive domain. The respective reference cohort for each test corresponded to a healthy German normative control group provided for each cognitive test. In addition, to assess specific cognitive domains according to ICCTF criteria, a cognitive domain deficit was defined as a test score of 2 SDs or more below the test-specific reference cohort on at least 1 domain subtest. To assess cognitive performance as a continuous measure across different tests and domains, neuropsychological test scores were t standardized using the mean and SD of autoantibody-negative patients (defined as patients with CBA-negative and IHC-negative results) as the reference group. Composite domain scores were computed for single cognitive domains using the mean of the derived t values of all domain subtests. An overall composite cognitive score was computed using the mean score of composite domain scores. Investigators (M.-M.W. and F.B.) who performed and analyzed the cognitive tests were blinded to autoantibody test results. All patients included in neuropsychological testing received a complete neurological examination.
Statistical Analysis
We calculated absolute and relative frequencies for categorical variables and medians and interquartile ranges (IQRs) for ordinal and continuous variables for the whole cohort as well as patients with lung cancer subtypes. The prevalence of autoantibodies, cognitive impairment, and domain deficits were reported along with Clopper-Pearson 95% CIs.16 Risk factors for cognitive impairment overall and in specific domains were investigated using bayesian logistic regression models with a weakly informative prior distribution (Cauchy distribution with a center of 0 and a scale of 2.5) to account for problems associated with small samples and adjust for confounding. The algorithm incorporated the prior distribution into standard logistic regression computation.17 Estimated odds ratios (ORs) and 95% credible intervals (CrIs) were then derived from 1 000 000 simulated draws from the approximate posterior distribution using the mean along with 2.5 and 97.5 percentiles. Cognitive performance was subsequently investigated in more detail using the raw and t-standardized neuropsychological scores to derive means and SEs, considering them normally distributed. Given the small number of patients in some subgroups, all results should be interpreted with caution, and the focus should be on effect estimates rather than statistical significance, especially because no correction for multiplicity was applied.
Statistical analysis was performed using IBM SPSS Statistics, version 23 (IBM SPSS); data handling and plotting packages in R software18,19; and bayesglm and sim functions in the R package arm for bayesian logistic regression models.20 Data were analyzed from May 2016 to December 2019.
Results
Among 167 patients with lung cancer (median age, 66.0 years [IQR, 59.0-72.0 years]; 105 men [62.9%]), 127 patients had NSCLC, and 40 patients had SCLC (Table 1). Most patients had stage IV cancer (70 of 127 patients [55.1%] with NSCLC vs 23 of 40 patients [57.5%] with SCLC) and metastases (M1a) to the lung, pleura, and/or pericardium (54 of 127 patients (42.5%) with NSCLC vs 16 of 40 patients [40.0%] with SCLC).
Frequency of Neuronal Autoantibodies
The presence of any neuronal autoantibody (defined as positive results on CBA or IHC analysis) was detected in 61 of 167 patients (36.5%; 95% CI, 29.2%-44.3%) with lung cancer, with a higher prevalence of autoantibodies found in 18 of 40 patients (45.0%; 95% CI, 29.3%-61.5%) with SCLC compared with 43 of 127 patients (33.9%; 95% CI, 25.7%-42.8%) with NSCLC (eFigure 1 in the Supplement). Autoantibodies against known antigens (ie, CBA-positive results) were detected in 33 of 167 patients (19.8%) (Table 2). Autoantibodies against intracellular antigens with the corresponding typical staining pattern detected on IHC analysis were only found in 8 of 40 patients (20.0%; 95% CI, 9.1%-35.6%) with SCLC. Detected AIC autoantibodies included autoantibodies against Hu, Ri, Ma2/Ta, Homer-3, Zic4, and SOX1 (Table 2). In contrast, autoantibodies against neuronal surface antigens had a similar frequency among patients with SCLC (4 of 40 patients [10.0%]) and NSCLC (14 of 127 patients [11.0%]). The most common neuronal surface autoantibodies were IgA and IgM autoantibodies targeting NMDAR (8 patients [4.8%] and 7 patients [4.2%], respectively). Other targets included myelin oligodendrocyte glycoprotein (MOG) (2 patients), pre–glycine receptor subunit α-1b (pre-GLRA1b) (2 patients), and leucine-rich glioma-inactivated 1 (LGI1) (1 patient).
Table 2. Autoantibody Prevalence in Patients With SCLC vs NSCLC.
| Autoantibody status | No. (%)a | ||
|---|---|---|---|
| All patients (N = 167) | Patients with SCLC (n = 40)a | Patients with NSCLC (n = 127) | |
| Autoantibody-positive | 33 (19.8) | 14 (35.0) | 19 (15.0) |
| 1 Autoantibody only | 27 (16.2) | 10 (25.0) | 17 (13.4) |
| Combination of 2 antibodiesb | 6 (3.6) | 4 (10.0) | 2 (1.6) |
| Localization of antigen | |||
| Surface antigen(s) only | 16 (9.6) | 2 (5.0) | 14 (11.0) |
| Intracellular antigen(s) only | 15 (9.0) | 10 (25.0) | 5 (3.9) |
| Both surface and intracellular antigens | 2 (1.2) | 2 (5.0) | 0 |
| Surface antigens | 18 (10.8) | 4 (10.0) | 14 (11.0) |
| NMDAR | 14 (8.4) | 4 (10.0) | 10 (7.9) |
| NMDAR IgMb | 8 (4.8) | 2 (5.0) | 6 (4.7) |
| NMDAR IgAb | 7 (4.2) | 2 (5.0) | 5 (3.9) |
| MOG | 2 (1.2) | 0 | 2 (1.6) |
| Pre-GLRA1b | 2 (1.2) | 0 | 2 (1.6) |
| LGI1 | 1 (0.6) | 0 | 1 (0.8) |
| Intracellular antigens | 17 (10.2) | 12 (30.0) | 5 (3.9) |
| Homer-3b | 5 (3.0) | 2 (5.0) | 3 (2.4) |
| Zic4b | 5 (3.0) | 5 (12.5) | 0 |
| Hub | 4 (2.4) | 4 (10.0) | 0 |
| Ri | 1 (0.6) | 1 (2.5) | 0 |
| Ma2 (Ta) | 1 (0.6) | 0 | 1 (0.8) |
| CARP VIII | 1 (0.6) | 0 | 1 (0.8) |
| SOX1c | 2 (1.2) | 2 (5.0) | 1 (0.8) |
Of the 40 patients with SCLC, 3 patients had combined carcinoma (SCLC and NSCLC). Numbers for these patients do not total 100% because of the combination of 2 autoantibodies.
Among those with SCLC, 2 patients had Hu and Zic4 autoantibodies, 1 patient had NMDAR IgM and Ri autoantibodies, and 1 patient had NMDAR IgM and Homer-3 autoantibodies. Among those with NSCLC, 1 patient had NMDAR IgM and IgA autoantibodies, and 1 patient had NMDAR IgM and LGI1 autoantibodies.
SOX1 autoantibodies were tested with EUROLINE immunoblots (EUROIMMUN AG)
Serum autoantibodies detected by brain tissue IHC analysis without corresponding autoantibody identification on CBA analysis (ie, currently unidentified autoantibodies) were found in 28 of 167 patients (16.8%; 95% CI, 11.4%-23.3%) with lung cancer, with a higher frequency among those with NSCLC (24 of 127 patients [18.9%]) vs those with SCLC (4 of 40 patients [10.0%]). Binding of serum autoantibodies to hippocampus tissue on IHC analysis was more frequent in those with SCLC (11 of 40 patients [27.5%]) vs those with NSCLC (10 of 127 patients [7.9%]) (eTable 1 in the Supplement). The demographic characteristics of different autoantibody groups are provided in eTable 2 in the Supplement.
The prevalence of any autoantibody was higher in male patients (41.0%; 95% CI, 31.5%-51.0%) compared with female patients (29.0%; 95% CI, 18.2%-41.9%) (eFigure 2 in the Supplement). This difference in prevalence was specifically found in neuronal surface autoantibodies (13.3% in male patients vs 6.5% in female patients).
Cognitive Impairment
A carefully selected subgroup of 97 patients received comprehensive standardized neuropsychological assessment after exclusion of patients with brain metastases and other potentially confounding severe neurological or psychiatric disorders (eMethods in the Supplement). The clinical details of patients in this subgroup were similar to those of the entire cohort (eTable 3 and eTable 4 in the Supplement).
Overall, cognitive impairment was found in 65 of 97 patients (67.0%) with lung cancer based on the ICCTF criteria used (ie, test score of 2 SDs or more below the test-specific reference cohort on ≥1 cognitive test). The most common cognitive domain deficits (ie, test score of 2 SDs or more below the test-specific reference cohort on ≥1 domain subtest) were found in executive function (44 of 96 patients [45.8%]) and attention (42 of 97 patients [43.3%]). The prevalence of cognitive impairment was similar between patients with SCLC (61.5%; 95% CI, 40.6%-79.8%) and NSCLC (69.0%; 95% CI, 56.9%-79.5%) and between male (69.6%; 95% CI, 55.9%-81.2%) and female (63.4%; 95% CI, 46.9%-77.9%) patients (eFigure 3 in the Supplement). The prevalence of cognitive impairment was also similar between different treatment groups (chemotherapy, radiotherapy, surgery, opioid analgesic therapy, and targeted therapy) and tumor stages (eTable 9 in the Supplement). Moreover, depression and mental health scale scores were similar between patients with and without cognitive impairment (eTable 5 and eFigure 4 in the Supplement).
Neuronal Autoantibodies and Cognitive Impairment
Patients With SCLC
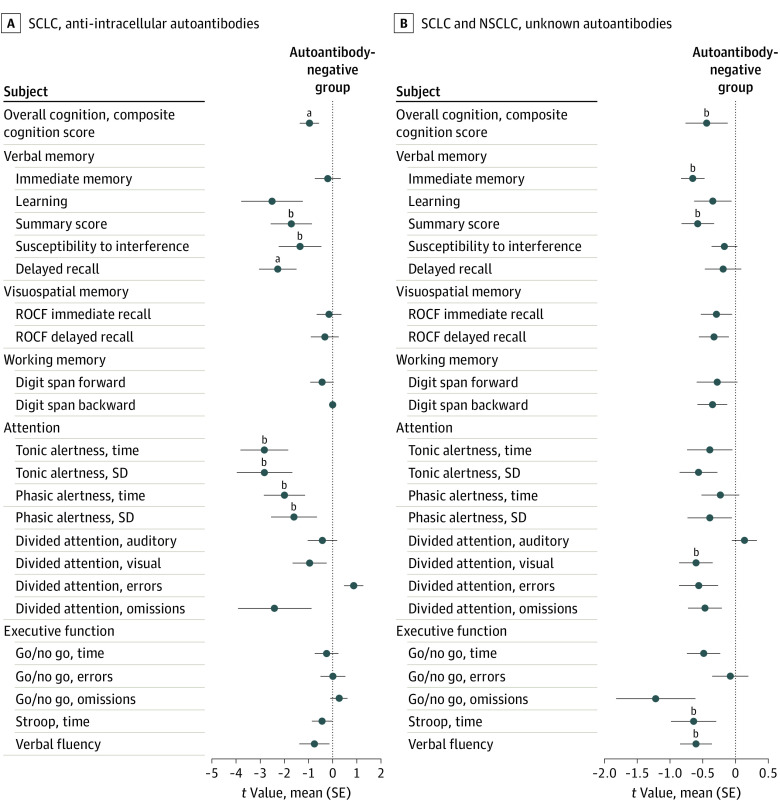
Among patients with SCLC, the odds of cognitive impairment among those with any neuronal autoantibodies were 11-fold higher than in autoantibody-negative patients (OR, 11.0; 95% CrI, 1.2-103.6) (Figure 1A), and the higher odds were independent of age, sex, and neurological deficits. Most patients with SCLC had AIC autoantibodies (12 of 40 patients [30.0%]). The presence of these autoantibodies was associated with substantially higher odds of cognitive impairment (OR, 8.3; 95% CrI, 0.7- 92.5), verbal memory deficits (OR, 44.0; 95% CrI, 1.4-1345.4), and attention deficits (OR, 36.8; 95% CrI, 2.9-474.1) (Figure 1B and C; eFigure 5 in the Supplement). Patients with SCLC who were AIC positive had worse overall cognitive performance compared with autoantibody-negative patients (mean [SE] t value for cognitive composite score, −0.97 [0.31] vs 0, respectively) (Figure 2A; eTable 6 in the Supplement), with impaired performance in the domains of verbal memory (verbal learning, delayed recall, and summary scores on the Verbal Learning and Memory Test) and attention (tonic and phasic alertness scores on the Test of Attentional Performance).
Figure 1. Cognitive Impairment and Neuronal Autoantibodies.
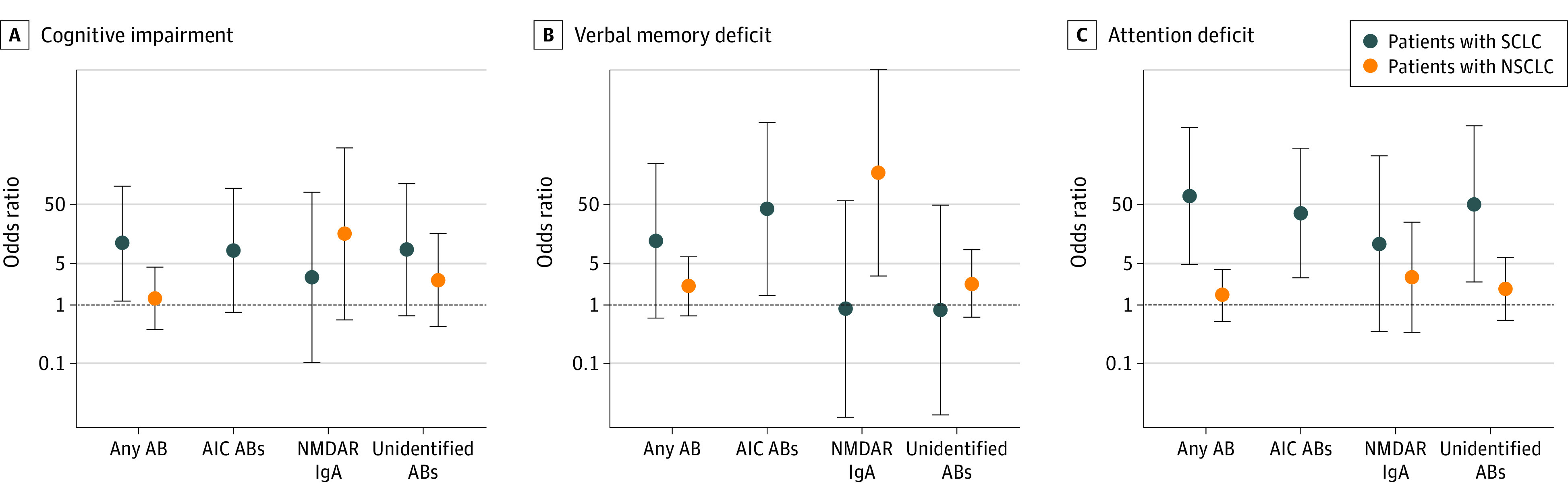
Odds ratio estimates with 95% credible intervals (CrIs) were based on bayesian logistic regression models. Derived estimates are shown for patients with the respective autoantibody compared with autoantibody-negative patients, in which each model was adjusted for sex, age, and neurological deficit. AB indicates autoantibody; AIC, anti-intracellular; IgA, immunoglobin A; NMDAR, N-methyl-d-aspartate receptor; NSCLC, non–small cell lung cancer; and SCLC, small cell lung cancer.
Figure 2. Standardized Cognitive Test Scores.
Autoantibody-negative patients are those with negative results for autoantibodies on tissue staining, and patients with currently unknown autoantibodies are those with positive results on tissue staining and no autoantibody detection on cell-based assays (recombinant cells). A, Comparison of cognitive performance across different cognitive tests among patients with small cell lung cancer (SCLC). Cognitive test scores were t standardized, and scores for 6 patients with SCLC and anti-intracellular autoantibodies were compared with scores from a reference group of 13 patients with no autoantibodies (as indicated by the vertical line at 0). The composite cognitive score was computed as the mean score of all cognitive tests. B, Comparison of cognitive performance across different tests among all patients with lung cancer (SCLC and non–small cell lung cancer). Cognitive test scores were t standardized, and scores for 17 patients with lung cancer and currently unknown autoantibodies were compared with scores from a reference group of 60 patients with no autoantibodies (as indicated by the vertical line at 0). The composite cognitive score was computed as the mean score of all cognitive tests. ROCF indicates Rey-Osterrieth Complex Figure test.
aP < .05.
bP < .01.
Patients With NSCLC
Among patients with NSCLC, most autoantibodies targeted the NMDAR. In those with NSCLC, the presence of IgA NMDAR autoantibodies was associated with substantially higher odds of a verbal memory deficit (OR, 182.8; 95% CrI, 3.1-10 852.4) (Figure 1B). Patients with NSCLC and IgA NMDAR autoantibodies had substantial deficits on verbal learning memory tasks compared with autoantibody-negative patients, especially with regard to delayed recall and recognition (eFigure 6 and eTable 7 in the Supplement). In a linear regression model, the presence of IgA NMDAR autoantibodies was associated with a decrease of 3.55 points (95% CI, −6.87 to −0.23 points) in the delayed recall score on the Verbal Learning and Memory Test, independent of age, sex, and neurological deficits. In contrast, among patients with IgM NMDAR autoantibodies, no increase in the odds of cognitive impairment (OR, 0.7; 95% CrI, 0.1-4.9) or verbal memory deficit (OR, 0.4; 95% CrI, 0.02-11.1) was found compared with autoantibody-negative patients.
Currently Unidentified Neuronal Autoantibodies
We next assessed all patients, independent of tumor subtype (SCLC or NSCLC), who had serum autoantibodies against brain tissue with currently unidentified target epitopes. These patients had higher odds of cognitive impairment (OR, 2.8; 95% CrI, 0.6-12.1), especially for an attention deficit (OR, 2.8; 95% CrI, 0.9-8.5), compared with patients with SCLC and NSCLC who did not have any brain-directed autoantibodies (Figure 1C). Patients with currently unidentified autoantibodies had a significantly reduced composite cognitive score compared with autoantibody-negative patients (mean [SE] t value, −0.44 [0.16] vs 0, respectively), with lower cognitive performance in verbal memory, attention, executive function, and verbal fluency (Figure 2B; eTable 8 in the Supplement).
Neurological Deficits Associated With Neuronal Autoantibodies
A total of 8 patients (4.8%) had a PNS; all of these patients had SCLC. Six patients (3.6%) already had a PNS diagnosis at the time of study inclusion. Diagnoses included Lambert-Eaton myasthenic syndrome (3 patients), neuronopathy (2 patients), and anti–Hu-associated limbic encephalitis (1 patient). Another 2 patients (1.2%) with SCLC received a diagnosis of anti–Hu-associated PNS (specifically, paraneoplastic cerebellar syndrome and paraneoplastic sensory neuronopathy) during study evaluation.
Patients with any neuronal autoantibodies were more likely to have neurological deficits (eg, polyneuropathy) detected during neurological examination compared with autoantibody-negative patients (28 of 37 patients [75.7%; 95% CI, 58.8%-88.2%] vs 33 of 59 patients [55.9%; 95% CI, 42.4%-68.8%], respectively) (eFigure 7 and eTable 10 in the Supplement). Patients with AIC autoantibodies had a high prevalence of neurological deficits (9 of 11 patients [81.8%]). All patients with AIC autoantibodies and cognitive impairment also had neurological deficits in contrast to autoantibody-negative patients with cognitive impairment (7 of 7 patients [100%] vs 21 of 31 patients [67.7%], respectively).
Discussion
This cross-sectional study assessed the prevalence of neuronal autoantibodies and their association with cognitive impairment in patients with SCLC and NSCLC. The findings indicated that 36.5% of patients with lung cancer had brain-directed autoantibodies (33.9% of those with NSCLC and 45.0% of those with SCLC). Overall, 19.8% of patients had autoantibodies against known neuronal antigens identified through well-established, commercially available CBAs, and another 16.8% of patients had currently unidentified brain-directed autoantibodies. Compared with autoantibody-negative patients, patients with brain-directed autoantibodies had deficits in a broad range of cognitive domains and a substantially increased rate of cognitive impairment.
Cancer-related cognitive impairment is an important complication among patients with cancer, and its clinical relevance has been increasingly recognized, which may also be owing to the increasing number of long-term cancer survivors.21 Among patients with lung cancer, cognitive function has been examined mainly in the context of prophylactic cranial radiotherapy, which has been associated with cognitive impairment in patients without subsequent brain metastases.22 Previous studies have found that cognitive impairment is present in patients with lung cancer before the receipt of any cancer treatment (eg, cranial radiotherapy or chemotherapy).9 Cancer-related cognitive impairment before or independent of cancer treatment has also been described in patients with a number of other cancer types, including breast and colorectal cancer.21,23 However, the pathogenesis of these treatment-independent cognitive changes remains unclear.24 The present study’s findings suggest that neuronal autoantibodies are associated with and might represent a potential pathogenic factor in cognitive impairment among patients with lung cancer.
The association of autoantibodies with cognitive impairment was particularly notable in patients with SCLC and was mainly found among patients with AIC autoantibodies and associated with deficits in verbal memory, visuospatial memory, and attention. Anti-intracellular autoantibodies have frequently been associated with SCLC and PNS, including cortical and limbic encephalitis (anti-Hu antibodies)25 or cerebellar syndromes (anti-Zic4 or anti–Homer-3 autoantibodies).26,27,28 The findings of the present study suggest that AIC autoantibodies may be associated with a broader clinical spectrum, including cognitive impairment, which has been previously described for other AIC autoantibodies, such as anti–glutamic acid decarboxylase (anti-GAD) and anti–rho GTPase activating protein 26 (anti-ARHGAP26).29,30
Among patients with NSCLC, the most common antibodies were directed against NMDAR (IgA/IgM isotype). Notably, NMDAR antibodies of the IgA isotype were associated with verbal memory deficits. Together, these findings add to the results of a recent prospective study of patients with melanoma,6 which found an association between neuronal autoantibodies and cancer-related cognitive impairment, as well as similar findings from a previous retrospective study of different types of cancer.5
The exact pathophysiological mechanisms underlying the observed association between neuronal autoantibodies and cognitive impairment warrant further investigation. This interaction could either be causal (eg, the autoantibodies directly interfere with neuronal function and cause cognitive impairment) or indicative of an underlying (not yet identified) pathogenic mechanism that is associated with both autoantibody seropositivity and cognitive impairment. However, previous studies reported direct molecular and electrophysiological consequences of IgA and IgM NMDAR autoantibodies (eg, a substantial decrease of neuronal NMDARs and reduction of NMDAR-mediated currents).7,31,32 Moreover, IgA and IgM NMDAR autoantibodies have previously been associated with slow cognitive impairment and have been identified in a subset of patients with dementia.7,8 In contrast, AIC autoantibodies do not appear to have direct pathogenic consequences; rather, these autoantibodies indicate a T-cell–mediated immune response targeting the central nervous system.33 Furthermore, it should be noted that patients with lung cancer in the present cohort did not have NMDAR encephalitis, a severe autoimmune encephalitis with a characteristic neuropsychiatric syndrome that is associated with IgG NMDAR antibodies.34 However, interestingly, the major factor associated with long-term morbidity in these typically young patients is persistent cognitive impairment.35,36
The prevalence of neuronal autoantibodies observed in this study is similar to that of a previous study of patients with SCLC4; however, a direct comparison is precluded by a different set of investigated autoantibodies. In addition to well-defined autoantibodies, we observed currently unidentified, brain-directed IgG autoantibodies in 16.8% of patients using immunofluorescence techniques in rodent and monkey brain tissue. Immunofluorescence in these patients indicated typical staining patterns, clearly suggesting the presence of autoantibodies against neuronal epitopes. However, the samples had negative results on CBA analysis, so the exact neuronal epitopes targeted by these autoantibodies remain to be identified. In this process, preliminary results could already identify neuronal epitopes that included hexokinase-1, ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 3, and vesicle-fusing ATPase as targets of some of these unidentified autoantibodies. Notably, this study’s data suggested that patients with these autoantibodies against currently unknown central nervous system epitopes were more likely to have cognitive impairment, suggesting a clinical relevance of these new autoantibodies. Therefore, further studies are needed to identify the target epitopes and better characterize associated clinical symptoms.
The definition of cognitive impairment in the present study followed ICCTF criteria, which were established to facilitate comparability of cognitive test results between different studies of cancer-related cognitive impairment. Using these criteria, we found that a high proportion of patients with lung cancer (67.0%) had cognitive impairment, with a similar prevalence between patients with SCLC and NSCLC. This overall proportion is higher compared with the prevalence reported in a previous study of patients with other cancers (eg, cognitive impairment in 37% of patients with melanoma, among whom the same cognitive test battery was administered).6 This prevalence is also higher than that of another previous study, which reported a cognitive impairment frequency of 30% among patients with both SCLC and NSCLC.9 Differences from the present study include a different set of neuropsychological tests, a smaller sample, and the fact that ICCTF criteria were not applied. Furthermore, our study included a larger overall number of tests and more tests for attention and executive function, which likely increased sensitivity.
Limitations
This study has limitations. These limitations include small samples and consecutive low power in subgroup analyses when evaluating patients with SCLC and NSCLC as well as different autoantibody types separately. To make the best use of the data, different strategies, such as bayesian logistic regression analysis, were used. Although this study did not find any association between cancer treatment (chemotherapy, radiotherapy, or targeted therapy) and cognitive impairment, it was not designed to address these questions, and the findings should, in this respect, be interpreted with caution.
Conclusions
In this cross-sectional study, a high prevalence of neuronal autoantibodies was observed among patients with lung cancer, and these neuronal autoantibodies were associated with clinically relevant cognitive impairment. These autoantibodies might represent a potentially treatable mechanism of immune-mediated cognitive impairment among patients with lung cancer.
eMethods 1. Patients
eMethods 2. Neuronal Autoantibody Analysis
eMethods 3. Neuropsychological Tests
eTable 1. Prevalence of Brain Tissue Staining in Patients With Lung Cancer: SCLC vs NSCLC
eTable 2. Demographic Characteristics of Patients by Antibody
eTable 3. Demographic Characteristics of Patients With Cognitive Testing Results
eTable 4. Demographic Characteristics of Tested Patients by Antibody
eTable 5. Demographic Characteristics of Patients by Cognitive Impairment
eTable 6. Cognitive Test Scores of Patients With SCLC: AIC Antibodies vs No Antibodies
eTable 7. Cognitive Test Scores of Patients With NSCLC: VLMT IgA NMDAR Antibodies vs No Antibodies
eTable 8. Cognitive Test Scores of All Patients With Lung Cancer: Unknown Antibodies vs No Antibodies
eTable 9. Model Adjusted for Opioid Therapy
eTable 10. Neuronal Autoantibodies and Neurological Deficits in Patients With Lung Cancer
eFigure 1. Antibody Prevalence
eFigure 2. Antibody Prevalence by Sex
eFigure 3. Cognitive Impairment by Sex
eFigure 4. Mental Health and Depression Scale Scores and Cognitive Impairment
eFigure 5. Cognitive Domain Deficits: Working Memory, Visuospatial Memory, and Executive Function
eFigure 6. Verbal Memory Performance in Patients With IgA NMDAR Antibodies
eFigure 7. Neurological Deficits
eReferences
References
- 1.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543-1554. doi: 10.1056/NEJMra023009 [DOI] [PubMed] [Google Scholar]
- 2.Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7(4):327-340. doi: 10.1016/S1474-4422(08)70060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giometto B, Grisold W, Vitaliani R, Graus F, Honnorat J, Bertolini G; PNS Euronetwork . Paraneoplastic neurologic syndrome in the PNS Euronetwork database: a European study from 20 centers. Arch Neurol. 2010;67(3):330-335. doi: 10.1001/archneurol.2009.341 [DOI] [PubMed] [Google Scholar]
- 4.Gozzard P, Woodhall M, Chapman C, et al. Paraneoplastic neurologic disorders in small cell lung carcinoma: a prospective study. Neurology. 2015;85(3):235-239. doi: 10.1212/WNL.0000000000001721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finke C, Bartels F, Lutt A, Pruss H, Harms L. High prevalence of neuronal surface autoantibodies associated with cognitive deficits in cancer patients. J Neurol. 2017;264(9):1968-1977. doi: 10.1007/s00415-017-8582-0 [DOI] [PubMed] [Google Scholar]
- 6.Bartels F, Strönisch T, Farmer K, Rentzsch K, Kiecker F, Finke C. Neuronal autoantibodies associated with cognitive impairment in melanoma patients. Ann Oncol. 2019;30(5):823-829. doi: 10.1093/annonc/mdz083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pruss H, Holtje M, Maier N, et al. IgA NMDA receptor antibodies are markers of synaptic immunity in slow cognitive impairment. Neurology. 2012;78(22):1743-1753. doi: 10.1212/WNL.0b013e318258300d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doss S, Wandinger KP, Hyman BT, et al. High prevalence of NMDA receptor IgA/IgM antibodies in different dementia types. Ann Clin Transl Neurol. 2014;1(10):822-832. doi: 10.1002/acn3.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simo M, Root JC, Vaquero L, et al. Cognitive and brain structural changes in a lung cancer population. J Thorac Oncol. 2015;10(1):38-45. doi: 10.1097/JTO.0000000000000345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmstaedter C, Lendt M, Lux S. Verbal Learning and Memory Test (VLMT). Hogrefe; 2001. [Google Scholar]
- 11.Stern RA, Javorsky DJ, Singer EA, et al. BQSS: the Boston Qualitative Scoring System for the Rey Osterrieth Complex Figure. Psychological Assessment Resources; 1999. [Google Scholar]
- 12.Zimmermann P, Fimm B. Test of Attentional Performance. Version 2. 3rd ed. Psytest; 2012. [Google Scholar]
- 13.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643-662. [Google Scholar]
- 14.Aschenbrenner S, Tucha O, Lange KW. Regensburg Word Fluency Test (RWT). Hogrefe; 2000. [Google Scholar]
- 15.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703-708. doi: 10.1016/S1470-2045(10)70294-1 [DOI] [PubMed] [Google Scholar]
- 16.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404-413. doi: 10.1093/biomet/26.4.404 [DOI] [Google Scholar]
- 17.Gelman A, Jakulin A, Pittau MG, Su YS. A weakly informative default prior distribution for logistic and other regression models. Ann Appl Stat. 2008;2(4):1360-1383. doi: 10.1214/08-AOAS191 [DOI] [Google Scholar]
- 18.R Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2020. Accessed March 2021. https://www.r-project.org/
- 19.Wickham H, Averick M, Bryan J, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4(43):1686-1691. doi: 10.21105/joss.01686 [DOI] [Google Scholar]
- 20.Gelman A, Su YS. Data analysis using regression and multilevel/hierarchical models. Version 1.11-2. R Foundation for Statistical Computing; 2020. Accessed March 2021. https://cran.r-project.org/package=arm
- 21.Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer–related cognitive impairment in adults. CA Cancer J Clin. 2015;65(2):123-138. doi: 10.3322/caac.21258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gondi V, Paulus R, Bruner DW, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys. 2013;86(4):656-664. doi: 10.1016/j.ijrobp.2013.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vardy JL, Dhillon HM, Pond GR, et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J Clin Oncol. 2015;33(34):4085-4092. doi: 10.1200/JCO.2015.63.0905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192-201. doi: 10.1038/nrc2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graus F, Keime-Guibert F, Rene R, et al. Anti–Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124(pt 6):1138-1148. doi: 10.1093/brain/124.6.1138 [DOI] [PubMed] [Google Scholar]
- 26.Bataller L, Wade DF, Graus F, Stacey HD, Rosenfeld MR, Dalmau J. Antibodies to Zic4 in paraneoplastic neurologic disorders and small-cell lung cancer. Neurology. 2004;62(5):778-782. doi: 10.1212/01.WNL.0000113749.77217.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoftberger R, Sabater L, Ortega A, Dalmau J, Graus F. Patient with Homer-3 antibodies and cerebellitis. JAMA Neurol. 2013;70(4):506-509. doi: 10.1001/jamaneurol.2013.1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuliani L, Sabater L, Saiz A, Baiges JJ, Giometto B, Graus F. Homer 3 autoimmunity in subacute idiopathic cerebellar ataxia. Neurology. 2007;68(3):239-240. doi: 10.1212/01.wnl.0000251308.79366.f9 [DOI] [PubMed] [Google Scholar]
- 29.Bartels F, Pruss H, Finke C. Anti-ARHGAP26 autoantibodies are associated with isolated cognitive impairment. Front Neurol. 2018;9:656. doi: 10.3389/fneur.2018.00656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takagi M, Ishigaki Y, Uno K, et al. Cognitive dysfunction associated with anti-glutamic acid decarboxylase autoimmunity: a case-control study. BMC Neurol. 2013;13:76. doi: 10.1186/1471-2377-13-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruss H, Finke C, Holtje M, et al. N-methyl-d-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012;72(6):902-911. doi: 10.1002/ana.23689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castillo-Gomez E, Oliveira B, Tapken D, et al. All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class. Mol Psychiatry. 2017;22(12):1776-1784. doi: 10.1038/mp.2016.125 [DOI] [PubMed] [Google Scholar]
- 33.Lancaster E, Dalmau J. Neuronal autoantigens—pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8(7):380-390. doi: 10.1038/nrneurol.2012.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalmau J, Armangue T, Planaguma J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057. doi: 10.1016/S1474-4422(19)30244-3 [DOI] [PubMed] [Google Scholar]
- 35.Finke C, Kopp UA, Pruss H, Dalmau J, Wandinger KP, Ploner CJ. Cognitive deficits following anti–NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2012;83(2):195-198. doi: 10.1136/jnnp-2011-300411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKeon GL, Robinson GA, Ryan AE, et al. Cognitive outcomes following anti–N-methyl-d-aspartate receptor encephalitis: a systematic review. J Clin Exp Neuropsychol. 2018;40(3):234-252. doi: 10.1080/13803395.2017.1329408 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Patients
eMethods 2. Neuronal Autoantibody Analysis
eMethods 3. Neuropsychological Tests
eTable 1. Prevalence of Brain Tissue Staining in Patients With Lung Cancer: SCLC vs NSCLC
eTable 2. Demographic Characteristics of Patients by Antibody
eTable 3. Demographic Characteristics of Patients With Cognitive Testing Results
eTable 4. Demographic Characteristics of Tested Patients by Antibody
eTable 5. Demographic Characteristics of Patients by Cognitive Impairment
eTable 6. Cognitive Test Scores of Patients With SCLC: AIC Antibodies vs No Antibodies
eTable 7. Cognitive Test Scores of Patients With NSCLC: VLMT IgA NMDAR Antibodies vs No Antibodies
eTable 8. Cognitive Test Scores of All Patients With Lung Cancer: Unknown Antibodies vs No Antibodies
eTable 9. Model Adjusted for Opioid Therapy
eTable 10. Neuronal Autoantibodies and Neurological Deficits in Patients With Lung Cancer
eFigure 1. Antibody Prevalence
eFigure 2. Antibody Prevalence by Sex
eFigure 3. Cognitive Impairment by Sex
eFigure 4. Mental Health and Depression Scale Scores and Cognitive Impairment
eFigure 5. Cognitive Domain Deficits: Working Memory, Visuospatial Memory, and Executive Function
eFigure 6. Verbal Memory Performance in Patients With IgA NMDAR Antibodies
eFigure 7. Neurological Deficits
eReferences