Abstract
Objective.
To investigate the relationship between molecular subtype, intraperitoneal (IP) disease dissemination patterns, resectability, and overall survival (OS) in advanced high-grade serous ovarian cancer (HGSOC).
Methods.
Patients undergoing primary surgery for stage III-IV HGSOC at Mayo Clinic from 1994 to 2011 were categorized into three IP disease dissemination patterns: upper abdominal or miliary; lower abdominal; and pelvic. Residual disease was defined as 0 (RD0), 0.1–0.5, 0.6–1.0, or >1 cm. Molecular subtypes were derived from Agilent 4x44k tumor mRNA expression profiles and categorized as mesenchymal (MES) or non-mesenchymal (non-MES).
Results.
Operative and molecular data was available for 334 patients. Median OS was shorter in patients with MES compared to non-MES subtypes (34.2 vs 44.6 months; P = 0.009). Patients with MES subtype were more likely to have upper abdominal/miliary disease compared to non-MES subtype (90% vs. 72%, P < 0.001). For patients with upper abdominal/miliary disease, complete resection (RD0) was less common in MES compared to non-MES subtypes (11% vs. 27%, P = 0.004). On multivariable analysis, RD was the only factor associated with OS(P < 0.001). In patients with upper abdominal/miliary disease, though less commonly achieved, RD0 improved survival irrespective of molecular subtype (median OS of 69.2 and 57.9 months for MES and non-MES subtype).
Conclusions.
Our results support a paradigm in which molecular subtype is an important driver of dissemination pattern; this in turn impacts resectability and ultimately survival. Consequently mesenchymal subtype is associated with much lower rates of complete resection, though RD0 remains the most important independent predictor of survival.
Keywords: High-grade serous ovarian cancer, Epithelial ovarian cancer, Mesenchymal, Residual disease, TCGA subtype, Molecular subtype
1. Introduction
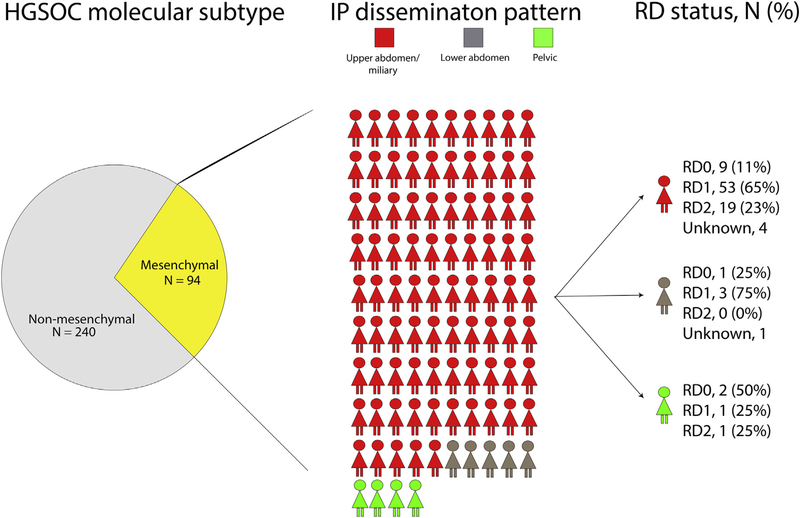
Molecular classification of high-grade serous ovarian cancer (HGSOC) using tumor mRNA profiling was first described by Tothill et al. [1] and has been independently confirmed by multiple studies, including our own [2, 3]. We subsequently demonstrated that molecular subtypes are associated with intraperitoneal (IP) disease dissemination patterns and surgical outcomes in advanced HGSOC [2–4]. The relationship between molecular subtype, dissemination patterns, and residual disease (RD) is complex. Patients with mesenchymal (MES) subtype are more likely to present with disease in the upper abdomen [4] and, in turn, disease in the upper abdomen is more difficult to resect [4–7]. Irrespective of dissemination pattern, MES tumors are more difficult to completely resect (RD0) than non-MES tumors (Fig. 1). Even among patients with upper abdominal/miliary disease, the rate of complete resection (RD0) is lower in patients with MES compared to non-MES tumors [4]. This appears to reflect, in part, both the driving effect of molecular characteristics on disease spread, and the complex interaction between tumor biology and resectability of disease in advanced HGSOC.
Fig. 1.
Breakdown of intraperitoneal (IP) disease dissemination pattern and residual disease (RD) among patients with high-grade serous ovarian cancer (HGSOC) and mesenchymal subtype. RD0, completely resected; RD1, 0.1–1 cm; RD2, >1 cm.
HGSOCs that present with upper abdominal disease or are MES subtype have worse OS [2, 3, 5, 8]. Both of these factors appear to impact residual disease (RD) at primary surgery: RD is a consistently important factor across molecular subtype and disease pattern [3, 9–11]. Collectively this implies that while subsets of MES HGSOC will benefit from aggressive primary debulking surgery (PDS), many will not, owing to limitations of disease spread and resectability. As we approach the era of preoperative molecular tumor testing [12], it will be important to understand which subsets of patients may benefit from alternative approaches.
Few studies are available to examine the independent association between IP disease dissemination pattern, molecular subtype, and RD on OS. Most of the large cohort studies with molecular profiling lack detailed data or primary surgical factors such as initial volume of disease and residual disease [13]. Our primary hypothesis is that all three factors (molecular subtype, IP disease dissemination pattern, and RD) independently impact survival in advanced HGSOC. In addition, among patients with upper abdominal or miliary disease and MES subtype, minimizing RD remains an important goal to improve OS when feasible. As improved molecular characterization becomes available and is obtainable preoperatively [12], understanding these complex relationships becomes more important to individualize treatment of patients with advanced HGSOC.
2. Methods
The Mayo Clinic Institutional Review Board approved this single institution, retrospective study. Perioperative patient characteristics and surgical outcome variables were collected from prospectively maintained databases of patients undergoing PDS from 1994 to 2011. Inclusion criteria were high-grade (grade 2–4) serous or mixed serous histology, ovarian, fallopian, or primary peritoneal cancer, and operable stages III–IV with molecular profiling. Patients with borderline tumors, those who were treated with neoadjuvant chemotherapy, and those without research consent or molecular profiling were excluded.
IP disease dissemination patterns among eligible patients with stage III and stage IV HGSOC were defined into four categories using our previously published criteria [4]: pelvic disease, lower abdominal disease, upper abdominal disease, and miliary disease (Supplementary Table S1). Four RD groups were defined, RD0, RD 0.1–0.5 cm, RD 0.6–1.0 cm, RD >1 cm, based on the largest residual tumor diameter. Surgical complexity was assigned using previously published methods and classified as low, intermediate, or high complexity surgery [10]. Since patients with upper abdominal or miliary disease have similar surgical outcomes [4], we combined the two into one IP disease dissemination pattern for all statistical comparisons.
Gene expression profiles were measured using Agilent Whole Human Genome 4x44K Expression Arrays. Expression data normalization and molecular subtype assignment was done as described in past publications [2, 3]. Patients with molecular profiling data were assigned to one of four advanced HGSOC molecular subtypes: MES, immunoreactive (IMM), proliferative (PRO), or differentiated (DIFF). Since patients with IMM, PRO, and DIFF subtype have significantly better OS compared to MES subtype [2, 3], we grouped them into one category (non-MES) for the statistical comparisons by molecular subtype.
Demographic, preoperative, and intraoperative characteristics were summarized for all patients undergoing PDS. Overall survival following the date of the surgery was estimated using the Kaplan-Meier method. Univariate and multivariable Cox proportional hazards regression models were fit to evaluate associations with death due to any cause; associations were summarized by calculating the hazard ratio (HR) and corresponding 95% confidence interval (CI). Variables included in the multivariable models were based on well-described clinical variables associated with overall survival (age at surgery, American Society of Anesthesiologists (ASA) score, preoperative albumin level, International Federation of Gynecology and Obstetrics (FIGO) stage, and RD) [14]. IP adjuvant chemotherapy or first-line maintenance therapy was not used often enough to justify including route of chemotherapy or maintenance therapy in the multivariable model. Statistical analysis was performed using the SAS version 9.3 software package (SAS Institute, Inc.; Cary, NC). All calculated P values were two-sided, and P values < 0.05 were considered statistically significant.
3. Results
Between 1994 and 2011, 741 patients with stage III or IV HGSOC underwent PDS with curative intent. Among these patients, 334 had molecular profiling available on their primary tumor. Baseline patient and tumor characteristics of the cohort are summarized in Table 1.
Table 1.
Patient characteristics among 334 advanced HGSOC patients.
| Characteristic | |
|---|---|
| Age at surgery (years), mean (SD) | 63.5 (11.4) |
| ASA score | |
| <3 | 174(52.1%) |
| ≥3 | 160 (47.9%) |
| Preoperative albumin (% of 179) | |
| ≥3.5 g/dL | 140 (78.2%) |
| <3.5 g/dL | 39 (21.8%) |
| FIGO stage | |
| IIIA/B | 27 (8.1%) |
| IIIC | 228 (68.3%) |
| IV | 79 (23.7%) |
| Residual disease (% of 323) | |
| 0 cm | 101 (31.3%) |
| 0.1–0.5 cm | 131 (40.6%) |
| 0.6–1.0 cm | 36 (11.1%) |
| >1.0 cm | 55 (17.0%) |
| Surgical complexity (% of333) | |
| Low | 67 (20.1%) |
| Intermediate | 157 (47.1%) |
| High | 109 (32.7%) |
| Intraperitoneal dissemination pattern | |
| Pelvic | 29 (8.7%) |
| Lower abdominal | 48 (14.4%) |
| Upper abdominal/miliary | 257 (76.9%) |
| Molecular subtype | |
| Proliferative | 92 (27.5%) |
| Differentiated | 73 (21.9%) |
| Mesenchymal | 94 (28.1%) |
| Immunoreactive | 75 (22.5%) |
Abbreviations: ASA, American Society of Anesthesiologists; FIGO, International Federation of Gynecology and Obstetrics; HGSOC, high-grade serous ovarian cancer.
As summarized in Table 1, 28% of our cohort had MES subtype. Patients with MES subtype were more likely to have upper abdominal/miliary disease compared to non-MES subtype (90% vs. 72%, P < 0.001). Fig. 1 illustrates the impact of the MES subtype on disease dissemination pattern and in turn, on resectability. As illustrated, 90% of the patients with MES subtype had upper abdominal/miliary disease. The RD0 rate in this subgroup of patients was 11% (in comparison to 27% with non-MES subtype and upper abdominal/miliary disease, P = 0.004). Only nine patients had disease limited to the pelvis and lower abdomen; thus, it is not clear how much of an impact MES subtype had in patients with lower disease burden.
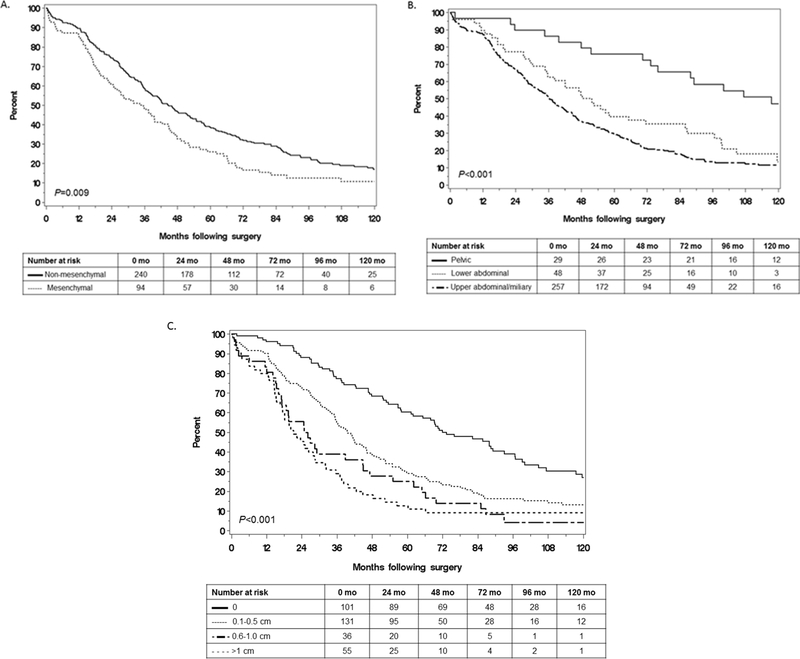
We evaluated the impact of each variable (molecular subtype, IP disease dissemination pattern, and RD) on OS by univariate analysis. Median OS was shorter for, i) patients with MES vs. non-MES subtype (34.2 vs 44.6 months; P = 0.009; Fig. 2A) and ii) patients with upper abdominal/miliary vs. lower abdominal disease vs. pelvic disease (36.3, 50.1, and 117.5 months, respectively; P < 0.001; Fig. 2B). Not surprisingly, median OS was longer in patients with complete resection (RD0) compared to those with 0.1–0.5, 0.6–1.0, and >1 cm of residual disease (72.0, 39.6, 25.4, and 21.2 months, respectively; P < 0.001; Fig. 2C).
Fig. 2.
Comparison of overall survival by (A) molecular subtype, (B) intraperitoneal dissemination pattern, and (C) residual disease.
We next evaluated the relative value of the 3 factors (subtype, IP dissemination pattern, and RD) by testing a series of multivariable models. Inthe first model we utilized factors potentially available preoperatively (including molecular characterization). We observed that IP disease dissemination pattern, and not molecular subtype, was significantly associated with OS (Table 2, Model A). In particular, the adjusted hazard ratio for upper abdominal/miliary disease (vs. pelvic disease) was 2.02 (95% CI 1.17, 3.51). Thus, it appears that molecular subtype as a preoperative factor is less relevant than disease pattern. In our final multivariable model we included RD in addition to the other previously analyzed variables (Table 2, Model B). RD was the only independent predictor of OS. Furthermore, after adjusting for time period (categorized into five 3-year periods) the magnitude of the hazard ratios for the seven factors reported in Model B in Table 2 did not change appreciably (data not shown). These data re-enforce the complex relationship between inherent biology (e.g. subtype) impacting disease spread and resectability, which in turn negatively impacts OS.
Table 2.
Multivariable analysis of factors evaluated for an association with overall survival, based on 334 patients with molecular subtype available.
| Characteristic | Univariate analyses |
Multivariable Model A. |
Multivariable Model B. |
|||
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | |
| Age at surgery (years)a | 1.15 (1.03,1.28) | 0.01 | 1.10 (0.98,1.23) | 0.11 | 1.10 (0.98,1.24) | 0.09 |
| ASA score | 0.07 | 0.26 | 0.57 | |||
| <3 (N = 174) | Reference | Reference | Reference | |||
| ≥3 (N = 160) | 1.24(0.98,1.57) | 1.15 (0.90,1.46) | 1.07 (0.84,1.37) | |||
| Preoperative albumin | 0.01 | 0.03 | 0.12 | |||
| ≥3.5 g/dL (N = 140) | Reference | Reference | Reference | |||
| <3.5 g/dL (N = 39) | 1.75 (1.20, 2.56) | 1.70 (1.15, 2.49) | 1.51 (1.02, 2.23) | |||
| Not available (N = 155) | 1.06 (0.83,1.37) | 1.16 (0.90,1.50) | 1.12 (0.87,1.45) | |||
| FIGO stage | <0.001 | 0.08 | 0.12 | |||
| IIIA/B (N = 27) | Reference | Reference | Reference | |||
| IIIC (N = 228) | 2.28 (1.40, 3.70) | 1.39 (0.79, 2.46) | 1.28 (0.72, 2.29) | |||
| IV (N = 79) | 3.06 (1.82, 5.14) | 1.80 (0.98,3.30) | 1.65 (0.89, 3.06) | |||
| Intraperitoneal dissemination pattern | <0.001 | 0.04 | 0.36 | |||
| Pelvic (N = 29) | Reference | Reference | Reference | |||
| Lower abdominal (N = 48) | 2.00 (1.16, 3.44) | 1.74 (0.95,3.19) | 1.38 (0.73, 2.62) | |||
| Upper abdominal/miliary (N = 257) | 2.73 (1.72,4.34) | 2.02 (1.17,3.51) | 1.54 (0.84, 2.80) | |||
| Molecular subtype | 0.009 | 0.13 | 0.47 | |||
| Non-mesenchymal (N = 240) | Reference | Reference | Reference | |||
| Mesenchymal (N = 94) | 1.41 (1.09,1.82) | 1.23 (0.94, 1.60) | 1.10 (0.84,1.45) | |||
| Residual disease (cm) | <0.001 | <0.001 | ||||
| 0 (N = 101) | Reference | Reference | ||||
| 0.1–0.5 (N = 131) | 1.83 (1.36, 2.46) | 1.34 (0.96,1.88) | ||||
| 0.6–1.0 (n = 36) | 2.84(1.89,4.26) | 2.07 (1.34, 3.22) | ||||
| >1.0 (N = 55) | 3.40 (2.35,4.91) | 2.59 (1.74, 3.86) | ||||
Abbreviations: ASA, American Society of Anesthesiologists; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio.
Hazard ratio per 10-year increase in age.
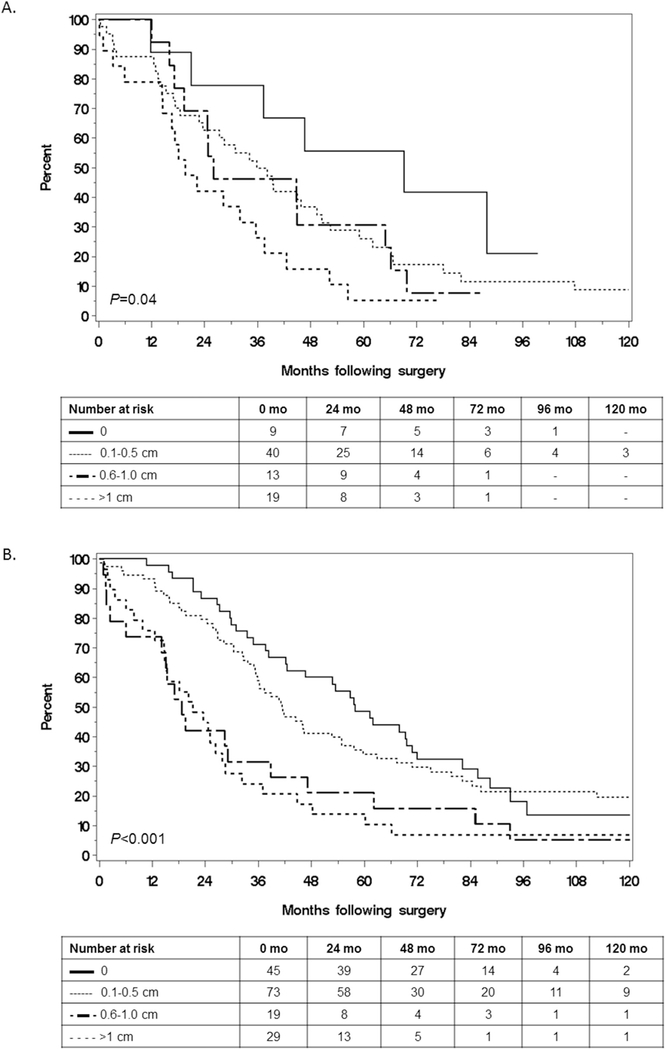
Patients with upper abdominal/miliary disease had the lowest RD0 resection with a RD0 rate of 22% (54/247), compared to 43% (20/47) among those with lower abdominal disease and 93% (27/29) among those with pelvic disease (P < 0.001). We therefore focused on the question of resectability among the subset with upper abdominal/miliary disease. Despite RD0 being less often achievable in this subset, the median OS was significantly longer in those with RD0 compared to those with less optimal resections, an effect that was observed in both MES and non-MES tumors (Fig. 3A and B respectively). Therefore, the benefit of complete resection when feasible persisted regardless of molecular subtype, which confirms the multivariable model.
Fig. 3.
Comparison of overall survival by extent of residual disease among patients with upper abdominal or miliary disease and (A) mesenchymal subtype or (B) non-mesenchymal subtype.
4. Discussion
In the current study we investigated the association between important molecular and clinical factors and OS in advanced HGSOC. As expected, patients with worse initial volume of disease, specifically upper abdominal or miliary disease, had shorter median OS. While the MES molecular subtype is associated with disease dissemination pattern, surprisingly, we did not find that it was an independent predictor of survival. On multivariable analysis controlling for age, ASA score, preoperative albumin, stage, disease dissemination pattern, molecular subtype, and RD, RD was the only variable independently associated with OS: an effect that persists even in patients with the worst prognosis (e.g. MES subtype and upper abdominal/miliary disease). Notwithstanding the benefits of lower RD, specific molecular subtypes appear to be reasonably consistently associated with disease dissemination patterns and could either be impacting or predictive of resectability of disease. These observations may be useful in decision making models that include preoperative tumor analysis.
Consistent with previous studies [5–8], patients with upper abdominal disease have the shortest median OS compared to other disease patterns. Our results provide a biological basis to explain the reported survival differences. Specifically, patients with MES subtype are more likely to have upper abdominal disease which translates to lower rates of complete resection [3, 4]; in turn, this is associated with shorter median OS (Fig. 1). Our lower rate of complete resection in patients with upper abdominal disease occurred despite a relatively aggressive surgical approach to ovarian cancer with high surgical complexity. Surgical complexity of surgery used and rates of complete resection likely differ over time and among centers. Our own rates of complete resection have increased [11] reflecting the increased awareness of the importance of complete resection, and the results of this present study should be validated in other centers.
Horowitz et al. investigated the impact of disease spread, RD and surgery in a secondary analysis from a randomized GOG trial. Similar to this present study, they also observed the independent importance of RD and disease spread on survival. They found that even among the RD0 subtype, those with the highest disease burden had shorter OS and PFS [8]. Our cohort of patients differed in that it was a single institution study with higher rates of stage IV disease (24% vs. 11%) and high surgical complexity scores (33% vs. 16%) to achieve similar rates of complete resection (31% vs. 32%).
Despite the benefits of RD0 even in patients with upper abdominal/miliary disease and MES subtype, our data does not support that all such patients should be triaged to PDS. For some categories the rate of successful resection is very low and morbidity likely high. Unfortunately, current limitations exist to successful triage. Relevant to the present study, molecular subtype is not available preoperatively, and preoperative imaging models have limited ability to accurately predict RD for most cases of advanced stage ovarian cancer when tested in multiple centers [15–18]. Further studies are needed on this subgroup of patients to develop more effective tools and approaches to triage to maximize benefit and minimize harm.
The major strength of this study is the use of a large single institution surgical database with detailed information on disease burden and resectability, including location and size of disease at the beginning and end of PDS. To our knowledge, it is the most detailed surgical database of patients with molecular subtyping. Detailed operative reports were used to assign patients to one of four mutually exclusive IP disease dissemination patterns. Our IP disease dissemination patterns were previously published and are reproducible [4]. Molecular profiling was performed as previously described and our technique has been validated in public cohorts [2, 3]. The study also provides a sensible picture which links molecular characteristics, disease spread, and resectability of disease to survival.
Limitations of this study include the retrospective design, particularly the use of operative reports to define IP disease dissemination patterns. This highlights the need to prospectively document disease spread, as recommended by the National Comprehensive Cancer Network (NCNN) [19]. Our data should also be validated in other centers and with a narrower range of surgical dates to reflect changes in surgical practice (e.g. rates of successful surgical resection have improved over time) [11]. Another potential weakness is the lumping of upper abdominal and miliary disease IP dissemination patterns. We reasoned that patients with upper abdominal and miliary disease have similar surgical outcomes and molecular profiles: both are more likely to be MES, and both more often have incomplete resections compared to lower abdominal and pelvic disease. Previous studies have reported on the prognostic significance of miliary disease [20]. However, we observed similar OS between patients with upper abdominal and miliary disease. Given the resemblance in tumor biology and oncologic outcomes between the two [4], we justified grouping the two IP disease dissemination patterns in our analyses. Although we statistically evaluated two-way interactions between mesenchymal subtype, IP dissemination pattern, and resectability and did not identify any significant interaction effects, the statistical power to evaluate interactions was limited given that 90% of the patients with MES subtype had upper abdominal/miliary disease and just 9 of these patients were resected to RD0 (Fig. 1).
In summary, IP disease dissemination pattern, molecular subtype, and RD are all accepted as important factors in predicting outcomes in HGSOC. Our findings confirm the importance of lowest RD, but underscore the challenge in obtaining complete resection in the HGSOC MES subtype. Accurate triage of those patients likely to benefit as well as the majority of MES patients unlikely to benefit from aggressive surgical attempts should be a future goal. As the paradigm to individualize the surgical approach to ovarian cancer continues to evolve [12], preoperative molecular profiling may become useful in assisting clinicians tailor cancer care among women presenting with advanced disease and aggressive tumor biology.
Supplementary Material
HIGHLIGHTS.
Median OS is shortest in patients with upper abdominal/miliary disease and mesenchymal subtype
Median OS is shortest in patients with RD >1cm
RD is the only predictor of OS in multivariable analysis
Among patients with upper abdominal/miliary disease, there is a survival benefit of achieving RD0, irrespective of tumor biology
Among patients with upper abdominal/miliary disease, there is a survival benefit of achieving RD0, irrespective of subtype.
Footnotes
Conflicts of interest
None of the authors has any conflicts of interest to declare.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2018.06.002.
References
- [1].Tothill RW, et al. , Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome, Clin. Cancer Res. 14 (16) (2008) 5198–5208. [DOI] [PubMed] [Google Scholar]
- [2].Konecny GE, et al. , Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer, J. Natl. Cancer Inst. 106 (10) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang C, et al. , Pooled clustering of high-grade serous ovarian cancer gene expression leads to novel consensus subtypes associated with survival and surgical outcomes, Clin. Cancer Res. 23 (15) (2017) 4077–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Torres D, et al. , Intraperitoneal disease dissemination patterns are associated with residual disease, extent of surgery, and molecular subtypes in advanced ovarian cancer, Gynecol. Oncol. 147 (3) (2017) 503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aletti GD, et al. , Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment, Gynecol. Oncol. 120 (1) (2011) 23–28. [DOI] [PubMed] [Google Scholar]
- [6].Hamilton CA, et al. , The impact of disease distribution on survival in patients with stage III epithelial ovarian cancer cytoreduced to microscopic residual: a Gynecologic Oncology Group study, Gynecol. Oncol. 122 (3) (2011) 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sehouli J, et al. , Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer, J. Surg. Oncol. 99 (7) (2009) 424–427. [DOI] [PubMed] [Google Scholar]
- [8].Horowitz NS, et al. , Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182, J. Clin. Oncol. 33 (8) (2015) 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chi DS, et al. , Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm, Gynecol. Oncol. 114 (1) (2009) 26–31. [DOI] [PubMed] [Google Scholar]
- [10].Aletti GD, et al. , Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer, Am. J. Obstet. Gynecol. 197 (6) (2007) 676 e1–7. [DOI] [PubMed] [Google Scholar]
- [11].Wallace S, et al. , Efforts at maximal cytoreduction improve survival in ovarian cancer patients, even when complete gross resection is not feasible, Gynecol. Oncol. 145 (1) (2017) 21–26. [DOI] [PubMed] [Google Scholar]
- [12].Nick AM, et al. , A framework for a personalized surgical approach to ovarian cancer, Nat. Rev. Clin. Oncol. 12 (4) (2015) 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cancer Genome Atlas Research, N, Integrated genomic analyses of ovarian carcinoma, Nature 474 (7353) (2011) 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kumar A, et al. , Risk-prediction model of severe postoperative complications after primary debulking surgery for advanced ovarian cancer, Gynecol. Oncol. 140 (1) (2016) 15–21. [DOI] [PubMed] [Google Scholar]
- [15].Axtell AE, et al. , Multi-institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoreduction in patients with advanced ovarian cancer, J. Clin. Oncol. 25 (4) (2007) 384–389. [DOI] [PubMed] [Google Scholar]
- [16].Gerestein CG, et al. , Nomogram for suboptimal cytoreduction at primary surgery for advanced stage ovarian cancer, Anticancer Res. 31 (11) (2011) 4043–4049. [PubMed] [Google Scholar]
- [17].Suidan RS, et al. , A multicenter assessment of the ability of preoperative computed tomography scan and CA-125 to predict gross residual disease at primary debulking for advanced epithelial ovarian cancer, Gynecol. Oncol. 145 (1) (2017) 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Suidan RS, et al. , A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer, Gynecol. Oncol. 134 (3) (2014) 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].N.C.C. Network, Ovarian Cancer (Version 2.2018), Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf March 18, 2018.
- [20].Eng KH, et al. , Prognostic value of miliary versus non-miliary sub-staging in advanced ovarian cancer, Gynecol. Oncol. 146 (1) (2017) 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.