Abstract
Introduction
Nusinersen was approved as the first treatment for all types of spinal muscular atrophy (SMA), including adults with SMA types 2 and 3. Robust biomarkers of treatment response in SMA adults are lacking. Our aim was to examine cerebrospinal fluid (CSF) amyloid‐β40 (Aβ40) and amyloid‐β42 (Aβ42) peptides as biomarkers of treatment response.
Methods
Eight patients with SMA types 2 and 3 were recruited consecutively in a single‐center study. CSF was sampled at baseline, after a loading dose, and after three maintenance doses. Levels of Aβ42 and Aβ40 were evaluated for each CSF sampling. Wilcoxon matched‐pairs signed‐rank test was used to detect longitudinal changes.
Results
CSF levels of Aβ42 increased from baseline to day 420 (95% confidence interval, P = .018), with a significant increase at days 180 and 420 compared with days 0 and 300, respectively (95% confidence interval, P = .012 and P = .018).
Discussion
The maintenance and promotion of wellness of residual motor neurons mediated by the restored level of SMN protein due to nusinersen could result in an increased level of amyloid peptides.
Keywords: amyloid‐β, nusinersen, spinal muscular atrophy
Abbreviations
- Aβ
amyloid‐β peptide
- Aβ40
amyloid‐β40 peptide
- Aβ42
amyloid‐β42 peptide
- ALS
amyotrophic lateral sclerosis
- sAPP
soluble fragments of β‐amyloid precursor protein
- ALS
amyotrophic lateral sclerosis
- APP
amyloid precursor protein
- CSF
cerebrospinal fluid
- SMA
spinal muscular atrophy
- SMN
survival motor neuron
1. INTRODUCTION
Spinal muscular atrophy (SMA) is an autosomal, neurodegenerative disease caused by a homozygous deletion or point mutation in the survival motor neuron 1 gene (SMN1) on chromosome 5, which results in a reduction of fully functional SMN protein. 1 The antisense oligonucleotide nusinersen was the first disease‐modifying drug approved for the treatment of children and adult SMA patients. 2 Although several studies have shown the prognostic role of some cerebrospinal fluid (CSF) biomarkers, such as neurofilament and tau in children3, 4 and adults5, 6 with SMA treated with nusinersen, recent studies on patients with late‐onset SMA type 3 provided less robust results, due to the wide range of disease onset and disease duration and the highly variable phenotypic spectrum.7, 8 Furthermore, in adult SMA, the evaluation of treatment response is even more difficult considering that clinical improvements by the standardized motor scales (Hammersmith Functional Motor Scale―Expanded version 9 and Revised Upper Limb Module 10 ) are minimal and mainly subjective. 11
Considering their role in other motor neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS),12, 13, 14 we investigated the CSF amyloid‐β40 (Aβ40) and amyloid‐β42 (Aβ42) peptides as biomarkers of treatment response in an adult cohort of patients with SMA types 2 and 3.
2. METHODS
2.1. Subjects
All patients were recruited at the regional Center of Motor Neuron Diseases at the Department of Neurology, University of Bari, Bari, Italy, and were treated from October 2018 to June 2020. All patients fulfilled the inclusion criteria for nusinersen treatment 15 and gave written informed consent. The study was approved by the Interregional Independent Ethical Committee of “Azienda Ospedaliero Universitaria of Bari,” Italy.
2.2. CSF analysis
Nusinersen was administered according to a published protocol. 15 CSF was collected at baseline (T0), after a loading dose at day 63 (T1), and then on days 180 (T2), 300 (T3), and 420 (T4) of maintenance dosing.
CSF was frozen within 1 hour after lumbar puncture and stored at −80°C until analysis.
We used a solid‐phase enzyme immunoassay (INNOTEST; Fujirebio, Ghent, Belgium) for quantitative determination of Aβ40 and Aβ42 in CSF (intra‐assay coefficient of variation <5%, interassay coefficient of variation <10%). Reference in‐house values were Aβ42 >500 pg/mL and Aβ40 4532‐20 116 pg/mL.
2.3. Statistical analysis
For comparing baseline with T1, T2, T3, and T4, we used a Wilcoxon matched‐pairs signed‐rank test. Significance was set at α ≤ 0.05. Statistical analysis was performed using SPSS version 22 (IBM Corp, Armonk, NY).
3. RESULTS
Eight adult patients with SMA types 2 and 3 were included in the study: three were SMA type 2 and five were type 3. Genetic analysis detected three copies of SMN2 in seven patients and four copies in one patient. One patient had undergone spinal fusion surgery due to severe scoliosis. Clinical and demographic characteristics are summarized in Table 1. One patient dropped out at T4 due to the inability to obtain CSF because of severe scoliosis.
TABLE 1.
Clinical and demographic characteristics of the study cohort a
| Age at onset (years) | 7.50 ± 6.02; 6.5 (1.00‐16) |
|---|---|
| Sex (M/F) | 5/3 |
| Age at treatment (years) | 43.13 ± 15.25; 42 (18‐72) |
| Disease duration (years) | 35.88 ± 15.88; 36 (4‐62) |
| Ambulators (yes/no) | 2/6 |
| Revised upper limb module at baseline | 17.9 ± 14.8; 16 (0‐37) |
| Hammersmith Functional Motor Scale―Expanded | 15.63 ± 22.15; 4 (0‐61) |
Data expressed as mean ± standard deviation; median (range).
Aβ42 and Aβ40 levels at T0 were within the normal range. Levels of Aβ42 increased from baseline to T4 with a significant increase at T2 and T4 compared with T1 and T3, respectively (Table 2). In particular, the levels of CSF Aβ42 showed the first significant change at T2, with a stability at T3 and a subsequent significant increase at T4 (Figure 1). No significant changes of Aβ40 levels were found.
TABLE 2.
Levels of Aβ40 and Aβ42 for each administration
| Aβ40 | Aβ42 | |||||
|---|---|---|---|---|---|---|
| Mean ± SD; median (range) | P value vs previous administration | P value vs T0 | Mean ± SD; median (range) | P value vs previous administration | P value vs T0 | |
| T0 | 6437.5 ± 3201; 6000 (3100‐12 300), n = 8 | 577.3 ± 228; 631.5 (278‐915), n = 8 | ||||
| T1 | 5825 ± 1921.9 (3400‐8400), n = 8 | .528 | .528 | 604 ± 253.1; 609 (282‐952), n = 8 | .483 | .483 |
| T2 | 6260.4 ± 2277.9 (5130.5‐9718), n = 8 | .116 | .833 | 634.6 ± 266; 638 (303‐970), n = 8 | .012 a | .068 |
| T3 | 6522.5 ± 2394.1 (5855‐10 500), n = 8 | .401 | .944 | 627.6 ± 50.9 (277‐952; 660), n = 8 | .528 | .263 |
| T4 | 6842.9 ± 1391.5 (7100‐8600), n = 7 | .499 | .498 | 891 ± 462.2; 787 (295‐676), n = 7 | .018 a | .018 a |
Statistically significant (P ≤ .05).
Abbrevations: Aβ40, amyloid‐β40 peptide; Aβ42, amyloid‐β42 peptide; SD, standard deviation.
FIGURE 1.
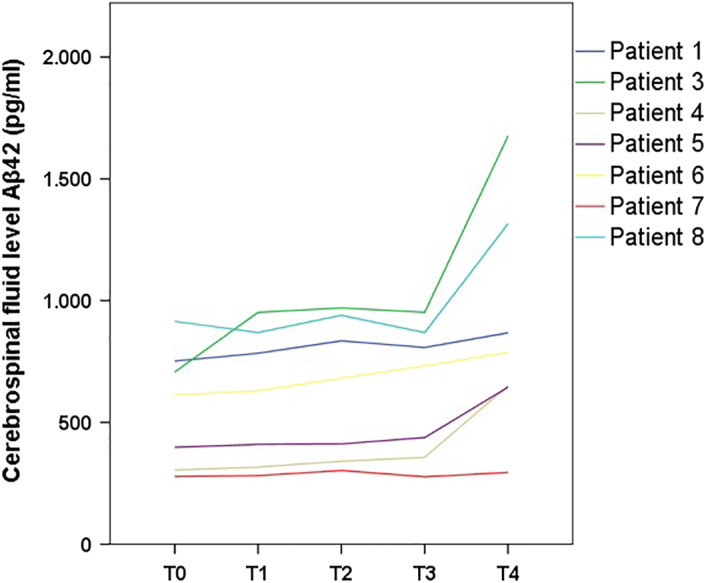
Cerebrospinal fluid levels of amyloid‐β42 peptide over time
4. DISCUSSION
In our small cohort of SMA patients treated with nusinersen, we found a significant increase in levels of CSF Aβ42. Looking at the trend of the change in CSF Aβ42, T3 may be considered as the inflection point after which the actual increase of Aβ42 was evident. In SMA adult patients treated with nusinersen, one study reported no significant changes in Aβ42 and Aβ40 CSF levels. 6 The follow‐up period after dosing in that study was 300 days, but the maximum change in our cohort occurred after than time.
One could speculate that the increased level of Aβ42 is indirect evidence of an effect of nusinersen in promoting the survival of motor neurons in the spinal anterior horn. Aβ42 has been studied in other neurodegenerative diseases involving motor neurons, such as ALS. Increased levels were reported in ALS patients with a short disease duration,12, 16 in contrast to decreased levels related to a longer disease duration or a rapidly progressive course.13, 14 It is speculated that increased levels of Aβ42 could reflect the attempt of motor neurons to survive in the early stage of the disease, whereas, in the later stage, or in rapidly progressive disease, the reduction of Aβ42 could be due to the irreversible loss of functional motor neurons. In support of this hypothesis, Xie et al demonstrated that, in the spinal cord of aged rats, the amyloid precursor protein (APP) and level of Aβs increased in motor neurons that survived after axonal injury, suggesting a beneficial role of these peptides on neuronal survival and wellness. 17 How the Aβs exert their beneficial effects is still unclear. In previous studies, it was demonstrated that Aβs protect neurons after chemical injuries 18 and oxidative stress, 19 regulating neuronal homeostasis; that is, picomolar (but not higher) amounts of Aβs stimulate synaptic plasticity and memory and stimulate presynaptic transmitter release. 20 The restored expression of SMN protein 21 could improve small nuclear ribonucleoprotein (snRNP) biogenesis and pre‐mRNA splicing, 22 leading to increased expression of APP and its secretases and ultimately increasing the level of Aβs. Thus, APP and Aβs could mirror the beneficial role of nusinersen on maintenance and promoting the survival of residual motor neurons.
In contrast, if it is confirmed that an increased level of Aβs occurs after administration of nusinersen, then we should ask whether the potential continuous increase of Aβs does not translate into a detrimental effect due to high concentrations and subsequent deposition of amyloid, such as in Alzheimer pathogenesis. 23 This could be a major concern for treatment continuation, especially in adult SMA patients. Questions that must be addressed are: 1) Do Aβ levels stabilize after reaching a new steady‐state level, or do they increase continuously during nusinersen treatment and therefore give rise ultimately to Aβs oligomers and plaques? 2) If the latter is correct, do adults with SMA types 2 and 3 need careful cognitive follow‐up during nusinersen treatment?
The main limitation of our study is the lack of an age‐matched control group of untreated SMA patients or other neurological patients who underwent repeated lumbar puncture with the same schedule. To fully define the role of APP and the products of its processing as a treatment response to nusinersen, future direction based on our preliminary results will be a study of the level of sAPP and Aβs in the CSF of a larger cohort of adult SMA patients treated with nusinersen for a longer period of observation.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
A.I., G.M., and I.L.S. conceptualized the study, had full access to all data, and take responsibility for the integrity of the data and the accuracy of the data analysis. M.R. analyzed the CSF data. A.I., G.M., and I.L.S. contributed to the data analysis and the writing of the manuscript. All authors contributed to the data interpretation and reviewed and approved the final version. Authors declare that the work described has not been published previously; that it is not under consideration for publication anywhere else; that its publication has been approved by all coauthors, if any, as well as by the responsible authorities―tacitly or explicitly―at the institute where the work was performed.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The authors thank Professor Filomena Puntillo who administered nusinersen.
Introna A, Milella G, D'Errico E, et al. Is cerebrospinal fluid amyloid‐β42 a promising biomarker of response to nusinersen in adult spinal muscular atrophy patients? Muscle & Nerve. 2021;63:905–909. 10.1002/mus.27212
Alessandro Introna, Giammarco Milella contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Darras BT. Spinal muscular atrophies. Pediatr Clin North Am. 2015;62:743‐766. [DOI] [PubMed] [Google Scholar]
- 2. Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later‐onset spinal muscular atrophy. N Engl J Med. 2018;378:625‐635. [DOI] [PubMed] [Google Scholar]
- 3. Winter B, Guenther R, Ludolph AC, Hermann A, Otto M, Wurster CD. Neurofilaments and tau in CSF in an infant with SMA type 1 treated with nusinersen. J Neurol Neurosurg. Psychiatry. 2019;90:1068‐1069. [DOI] [PubMed] [Google Scholar]
- 4. Olsson B, Alberg L, Cullen NC, et al. NFL is a marker of treatment response in children with SMA treated with nusinersen. J Neurol. 2019;266:2129‐2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faravelli I, Meneri M, Saccomanno D, et al. Nusinersen treatment and cerebrospinal fluid neurofilaments: an explorative study on spinal muscular atrophy type 3 patients. J Cell Mol Med. 2020;24:3034‐3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walter MC, Wenninger S, Thiele S, et al. Safety and treatment effects of nusinersen in longstanding adult 5q‐SMA type 3―a prospective observational study. J Neuromuscul Dis. 2019;6:453‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Totzeck A, Stolte B, Kizina K, et al. Neurofilament heavy chain and tau protein are not elevated in cerebrospinal fluid of adult patients with spinal muscular atrophy during loading with nusinersen. Int J Mol Sci. 2019;20:5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wurster CD, Günther R, Steinacker P, et al. Neurochemical markers in CSF of adolescent and adult SMA patients undergoing nusinersen treatment. Ther Adv Neurol Disord. 2019;12:1756286419846058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glanzman AM, O'Hagen JM, McDermott MP, et al. Validation of the expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J Child Neurol. 2011;26:1499‐1507. [DOI] [PubMed] [Google Scholar]
- 10. Mazzone ES, Mayhew A, Montes J, et al. Revised upper limb module for spinal muscular atrophy: development of a new module. Muscle Nerve. 2017;55:869‐874. [DOI] [PubMed] [Google Scholar]
- 11. Sansone VA, Walter MC, Attarian S, et al. Measuring outcomes in adults with spinal muscular atrophy―challenges and future directions―meeting report. J Neuromuscul Dis. 2020;7:523‐534. [DOI] [PubMed] [Google Scholar]
- 12. Lanznaster D, Hergesheimer RC, Bakkouche SE, et al. Aβ1‐42 and tau as potential biomarkers for diagnosis and prognosis of amyotrophic lateral sclerosis. Int J Mol Sci. 2020;21:2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sjögren M, Davidsson P, Wallin A, et al. Decreased CSF‐beta‐amyloid 42 in Alzheimer's disease and amyotrophic lateral sclerosis may reflect mismetabolism of beta‐amyloid induced by disparate mechanisms. Dement Geriatr Cogn Disord. 2002;13:112‐118. [DOI] [PubMed] [Google Scholar]
- 14. Steinacker P, Fang L, Kuhle J, et al. Soluble beta‐amyloid precursor protein is related to disease progression in amyotrophic lateral sclerosis. PLoS One. 2011;6:e23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoy SM. Nusinersen: first global approval. Drugs. 2017;77:473‐479. [DOI] [PubMed] [Google Scholar]
- 16. Ye L‐Q, Li X‐Y, Zhang Y‐B, et al. The discriminative capacity of CSF β‐amyloid 42 and tau in neurodegenerative diseases in the Chinese population. J Neurol Sci. 2020;412:116756. [DOI] [PubMed] [Google Scholar]
- 17. Xie Y, Yao Z, Chai H, Wong W‐M, Wu W. Potential roles of Alzheimer precursor protein A4 and β‐amyloid in survival and function of aged spinal motor neurons after axonal injury. J Neurosci Res. 2003;73:557‐564. [DOI] [PubMed] [Google Scholar]
- 18. Bishop GM, Robinson SR, Liu Q, Perry G, Atwood CS, Smith MA. Iron: a pathological mediator of Alzheimer disease? Dev Neurosci. 2002;24:184‐187. [DOI] [PubMed] [Google Scholar]
- 19. ResearchGate. β‐amyloid helps to protect neurons from oxidative stress. https://www.researchgate.net/publication/247225659_b‐amyloid_helps_to_protect_neurons_from_oxidative_stress. Accessed September 18, 2020.
- 20. Puzzo D, Privitera L, Leznik E, et al. Picomolar amyloid‐beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537‐14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paton DM. Nusinersen: antisense oligonucleotide to increase SMN protein production in spinal muscular atrophy. Drugs Today (Barcelona, Spain: 1998). 2017;53:327‐337. [DOI] [PubMed] [Google Scholar]
- 22. Mourelatos Z, Abel L, Yong J, Kataoka N, Dreyfuss G. SMN interacts with a novel family of hnRNP and spliceosomal proteins. EMBO J. 2001;20:5443‐5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carrillo‐Mora P, Luna R, Colín‐Barenque L. Amyloid beta: multiple mechanisms of toxicity and only some protective effects?. Oxid Med Cell Longev. 2014;2014:1‐15. 10.1155/2014/795375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


