Abstract
Background
Women with endometriosis are prescribed opioids for pain relief but may be vulnerable to chronic opioid use given their comorbidity profile.
Methods
A cohort study was conducted in the Clinformatics™ DataMart database between 2006 and 2017 comparing women aged 18–50 years with endometriosis (N = 36 373) to those without (N = 2 172 936) in terms of risk of chronic opioid use, opioid dependence diagnosis, and opioid overdose. Chronic opioid use was defined as ≥120 days' supply dispensed or ≥10 fills of an opioid during any 365‐day interval. Among women with endometriosis, we evaluated factors associated with higher risk of chronic opioid use and quantified the risk of complications associated with the use of opioids.
Results
Women with endometriosis were at greater risk for chronic opioid use (OR: 3.76; 95%CI: 3.57–3.96), dependence (OR: 2.73, 95%CI: 2.38–3.13) and overdose (OR: 4.34, 95%CI: 3.06–6.15) compared to women without. Chronic users displayed dose escalation and increase in days supplied over time, as well as co‐prescribing with benzodiazepines and sedatives. Approximately 34% of chronic users developed constipation, 20% experienced falls, and 8% reported dizziness. Among endometriosis patients, women in younger age groups, those with other comorbidities associated with pain symptoms, as well as those with depression or anxiety were at a higher risk of developing chronic opioid use.
Conclusions
Women with endometriosis had a four times greater risk of chronic opioid use compared to women without. Multimorbidity among these patients was associated with the elevated risk of chronic opioid use and should be taken into account during treatment selection.
Keywords: chronic opioid use, chronic pain management, endometriosis‐associated pain, healthcare utilization database, opioid utilization patterns
Key Points.
Studies evaluating the risk of chronic opioid use and its complications among women with endometriosis are limited. Using a large, US‐based claims database, the 2‐year risk for chronic opioid use was 4.4% in women with endometriosis, a 4‐fold greater risk compared to women without endometriosis.
Women with endometriosis displayed multimorbidity, including other pain‐related conditions, contributing to the elevated risk of chronic opioid use.
Chronic opioid users displayed an escalation in dose and days supplied per fill. Injurious co‐prescribing patterns (e.g., benzodiazepine and sedatives prescriptions), were also common.
These findings contribute to the overall understanding of the disease burden among endometriosis patients and may help to inform benefit–risk decision making in disease management.
1. INTRODUCTION
Endometriosis affects 6%–10% of women of reproductive age 1 , 2 and up to 60% of women with endometriosis experience significant chronic pain including dysmenorrhea, non‐menstrual pelvic pain, and dyspareunia. 3 , 4 While first line therapy for endometriosis pain includes oral contraceptives and nonsteroidal anti‐inflammatory drugs, many women have residual pain 5 or acute post‐procedural pain and may be prescribed opioids.
Recent studies have demonstrated that women with endometriosis were more likely to fill a prescription for opioids, take higher doses, for a longer time, and concomitantly with benzodiazepines than women without endometriosis. 6 , 7 Further, women prescribed opioids had significantly higher healthcare burden, resource utilization and costs compared to non‐opioid users over 2 years. 8 , 9 However the risk of chronic opioid use has not been quantified.
Over the past decade, the incidence of opioid use disorders and opioid‐related deaths among women in the United States has risen substantially and disproportionately, increasing the associated morbidity, mortality and economic burden. 10 Much of the increase can be attributed to increased prescribing. Further, chronic opioid use and dependence is a risk factor for heroin initiation, 11 and among women in the US, the highest mortality rates due to opioid‐related overdoses were observed in those 25–44 years old. 12 Women with endometriosis have a higher prevalence of known risk factors for chronic opioid use, including young age, other pain‐related comorbidities, mental health conditions, and substance use disorders. 11 , 12 , 13 Thus the main objectives of this study were to estimate the risk of chronic opioid use and associated complications in a large cohort of commercially insured women with endometriosis and to evaluate patient characteristics associated with chronic opioid use.
2. METHODS
2.1. Data source
The study was conducted using the de‐identified Clinformatics™ DataMart database, which includes healthcare utilization and reimbursement claims (inpatient, outpatient, pharmacy) data for a large, nationwide commercial insurer (2006–2017). Only beneficiaries with both medical and prescription drug coverage were included. The Quorum Review Institutional Review Board (Seattle, Washington) determined that this study did not constitute research involving human subjects and was therefore exempt from review.
2.2. Study population, exposure, and study period
The study population included women aged 18–50 years with continuous insurance enrollment of at least 6 months prior to cohort entry (baseline period) and a minimum of 2 years following cohort entry (follow‐up period) (Figure 1). Women with prior hysterectomy or oophorectomy as well as heavy chronic opioid use at baseline, defined as ≥30 days' supply of an opioid dispensed in the 6‐months baseline period, were excluded.
FIGURE 1.
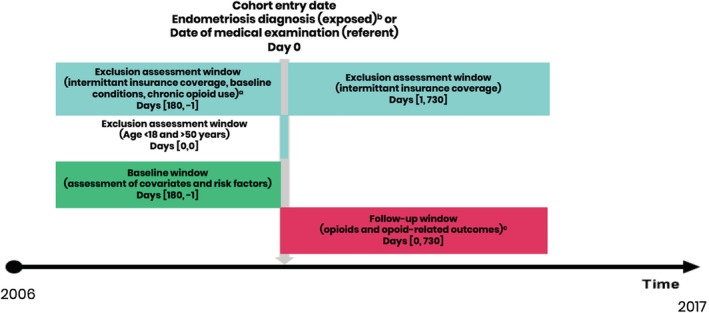
Study design. a Excluded in evidence of the following baseline conditions: hysterectomy or oophorectomy. Chronic opioid use at baseline defined as >=30 days supply of an opioid dispensed in 6‐months baseline period. b Endometriosis was identified by either (1) ≥2 inpatient or outpatient claims with endometriosis‐related diagnoses codes (ICD‐9 codes 617.X or ICD‐10 codes N80.X), with the cohort entry date being the date of the second claim with endometriosis code; or (2) one inpatient or outpatient claim with an endometriosis code preceded by a laparoscopic procedure within the 30 days prior, with the cohort entry date being the date of the endometriosis claim. c Opioid use was defined as a dispensing of any of the following drugs: methadone, fentanyl, oxycodone, codeine, morphine, hydromorphone, oxymorphone, meperidine, opium, hydrocodone and tramadol. Chronic opioid use was defined as ≥120 days supply or ≥10 fills of an opioid during any 365‐day interval in the 2‐year follow‐up. Opioid dependence/abuse and opioid overdose were defined with ICD‐9 or ICD‐10 code [Colour figure can be viewed at wileyonlinelibrary.com]
Women with endometriosis were identified based on one of two criteria: (1) ≥2 inpatient or outpatient claims with endometriosis‐related diagnoses codes (ICD‐9 codes 617.X or ICD‐10 codes N80.X), with the cohort entry date being the date of the second claim with endometriosis code; or (2) one inpatient or outpatient claim with an endometriosis code preceded by a laparoscopic procedure within the 30 days prior, with the cohort entry date being the date of the endometriosis claim. 13 , 14 The referent category included women without an endometriosis claim who had a general medical examination or annual gynecological examination to ensure that the referent population had an interaction with the healthcare system minimizing the possibility of detection bias that arises due to the increase in the number of clinical encounters. The patient selection flow chart is presented in Figure 2.
FIGURE 2.
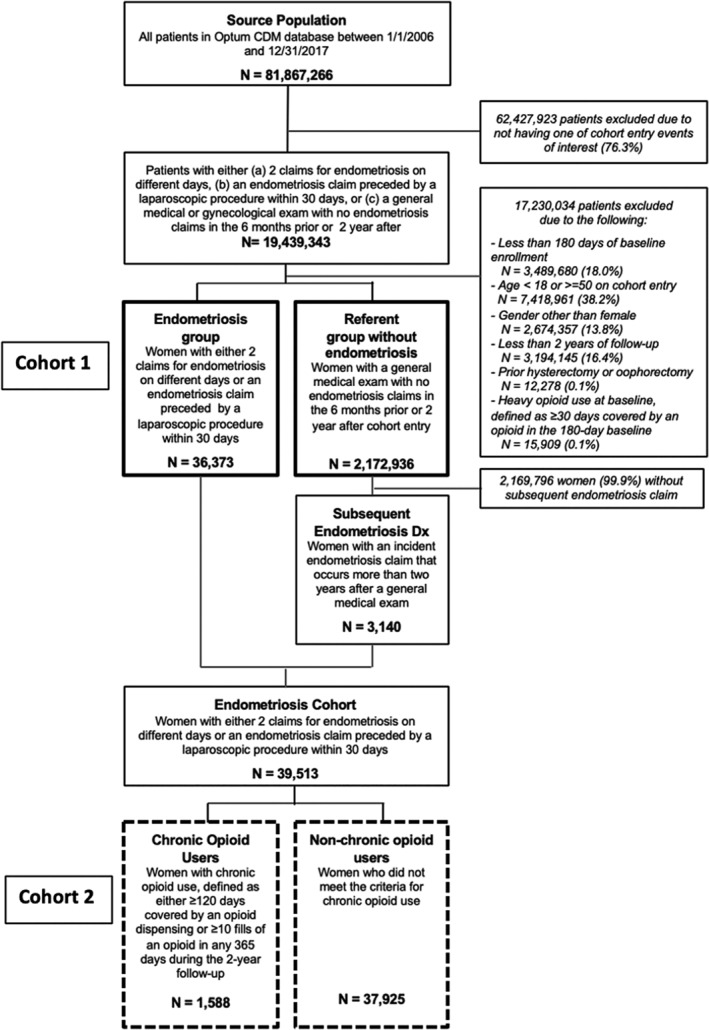
Patient flow chart for study of chronic opioid use among women with endometriosis. Optum CDM data, 2006–2017. Two sets of study populations were used for the analysis. Cohort 1 (solid lines) was used in the analyses to quantify the trends in opioid initiation and chronic use and compare the 2‐year risk of chronic opioid use in women with endometriosis to the risk in women without endometriosis. Cohort 2 (dotted lines) includes women who have endometriosis only and was used in the analyses to characterize opioid use and dose trajectories in chronic compared to non‐chronic users, identify risk factors for chronic opioid use and quantify the association of chronic opioid use with its sequalae
2.3. Ascertainment of opioids and opioid‐related outcomes
Opioid use was defined as a dispensing of any of the following drugs: methadone, fentanyl, oxycodone, codeine, morphine, hydromorphone, oxymorphone, meperidine, opium, hydrocodone, and tramadol. 15 The dose for each opioid dispensed was converted to Morphine Milligram Equivalents (MME) and reported as total dose per fill and daily prescribed dose (calculated as total dose prescribed/days supplied). Chronic opioid use was defined as ≥120 days supply or ≥10 fills of an opioid during any 365 day interval in the 2‐year follow‐up, as described by Sun et al. 16 , 17 Opioid dependence/abuse and opioid overdose were defined with ICD‐9 or ICD‐10 codes, as defined in eTable 1.
2.4. Risk factors, complications, and other variables
ICD‐9 and ICD‐10 diagnosis codes were used to identify comorbidities, including conditions associated with pain, mental health and immunologic disorders, history of substance abuse, and cardiovascular risk factors as a marker of general health. Published validated algorithms were used to define the presence of comorbidities in the baseline period, when available. For all other variables, comorbidities were considered present if there was at least 1 ICD code in the baseline period. Women were considered users of each medication if they filled at least 1 prescription in the pharmacy claims during the baseline period.
2.5. Statistical methods
Descriptive statistics were used to characterize the patient population in baseline. Standardized differences were used to compare women with endometriosis to those without.
Due to the fixed follow‐up, we used multivariable logistic regression models to estimate a 2‐year risk and odds ratios (OR) (95%CI) for chronic opioid use and related diagnoses (opioid dependence/abuse diagnosis, and opioid overdose) in women with endometriosis compared to women without. Models were adjusted for age, race, region, other prevalent comorbidities associated with pain, mental health disorders, history of substance use/abuse, cardiovascular risk factors, and baseline use of antidepressants, antipsychotics, benzodiazepines, and stimulants.
Among women with endometriosis, we evaluated baseline characteristics associated with chronic use using logistics regression models. Potential risk factors were considered based on existing literature and evaluated in baseline, prior to endometriosis diagnosis. 18 , 19 , 20 , 21 We also quantified the risk of known adverse outcomes associated with opioid use including constipation, dizziness, falls, as well as the risk of dangerous co‐prescribing with benzodiazepines or other sedatives among chronic users.
All analyses were performed using the Aetion Evidence Platform™ version 3.7, which has previously been validated. 22 , 23 p values were two‐tailed, and significance was set at p < 0.05. Definitions of all study covariates can be found in the Appendix S1 (eTable 1).
2.6. Sensitivity analysis
To evaluate the robustness of our results, we conducted two sets of sensitivity analyses by (1) restricting our definition of endometriosis to patients with a laparoscopic procedure only; and (2) repeating the main analysis on a population without the 2‐year follow‐up requirement and using a rate model (Cox Proportional Hazard).
3. RESULTS
3.1. Baseline patient characteristics
Our analysis included 36 373 women with endometriosis and 2 172 936 women without (Figure 2). The mean age was approximately 35 years in both groups. The prevalence of most comorbidities, including other conditions associated with pain, was higher in women with endometriosis compared to women without. Baseline use of medications including prior opioid use, non‐opioid analgesics and benzodiazepines among endometriosis patients was almost double the use among patients without endometriosis (Table 1).
TABLE 1.
Baseline characteristics of women with endometriosis and a referent population ages 18–50 years
| Characteristics | Endometriosis cohort | Referent cohort | Standardized difference |
|---|---|---|---|
| N | 36 373 | 2 172 936 | |
| Demographic characteristics | |||
| Age (years) | |||
| Mean (SD) | 35.2 ± 7.3 | 34.6 ± 9.3 | 6.9% |
| Median (IQR) | 35.0 [30.0, 41.0] | 35.0 [27.0, 43.0] | |
| Race | 2.8% | ||
| White | 23 167 (63.7%) a | 1 412 068 (65.0%) | |
| Asian | 1700 (4.7%) | 98 911 (4.6%) | |
| Black | 3572 (9.8%) | 201 897 (9.3%) | |
| Hispanic | 3598 (9.9%) | 210 279 (9.7%) | |
| Geographic region | 7.7% | ||
| Northeast | 3316 (9.1%) | 245 893 (11.3%) | |
| South | 17 349 (47.7%) | 989 417 (45.5%) | |
| Midwest | 9059 (24.9%) | 541 792 (24.9%) | |
| West | 6591 (18.1%) | 391 549 (18.0%) | |
| Provider type, on index date | |||
| Obstetrician/gynecologist | 70% | 38% | |
| Primary care, internal medicine or family medicine | 13% | 44% | |
| Other | 17% | 18% | |
| Conditions associated with pain | |||
| Pelvic pain | 15 421 (42.4%) | 167 150 (7.7%) | 81.5% |
| Fibromyalgia | 1480 (4.1%) | 54 085 (2.5%) | 8.9% |
| Chronic low back pain | 5010 (13.8%) | 195 909 (9.0%) | 15.0% |
| Chronic headaches/migraines | 2004 (5.5%) | 62 778 (2.9%) | 13.1% |
| Mental health | |||
| Depression | 2775 (7.6%) | 113 500 (5.2%) | 9.8% |
| Anxiety | 2554 (7.0%) | 100 152 (4.6%) | 10.3% |
| Fatigue | 4372 (12.0%) | 144 594 (6.7%) | 18.5% |
| Substance abuse (other than opioids) | 67 (0.2%) | 2390 (0.1%) | 1.9% |
| Immunologic | |||
| Irritable bowel syndrome (IBS) | 999 (2.7%) | 18 870 (0.9%) | 14.1% |
| Rheumatoid arthritis (RA) | 193 (0.5%) | 9607 (0.4%) | 1.3% |
| Lupus | 157 (0.4%) | 5301 (0.2%) | 3.2% |
| Cardiovascular risk factors | |||
| Diabetes mellitus, type II | 1762 (4.8%) | 68 022 (3.1%) | 8.8% |
| Hypertension | 4408 (12.1%) | 232 376 (10.7%) | 4.5% |
| Hyperlipidemia | 2943 (8.1%) | 130 200 (6.0%) | 8.2% |
| Obesity | 1405 (3.9%) | 56 570 (2.6%) | 7.1% |
| Medication use | |||
| Benzodiazepines | 4136 (11.4%) | 133 104 (6.1%) | 18.6% |
| Opioid analgesics | 12 460 (34.3%) | 321 107 (14.8%) | 46.5% |
| Non‐opioid analgesics | 7156 (19.7%) | 199 858 (9.2%) | 30.1% |
| Antidepressants | 6923 (19.0%) | 319 929 (14.7%) | 11.5% |
| Stimulants | 693 (1.9%) | 47 283 (2.2%) | 1.9% |
| Antipsychotics | 634 (1.7%) | 25 201 (1.2%) | 4.9% |
Abbreviations: IQR, interquartile range; SD, standard deviation.
n (%), all such values.
3.2. Risk of chronic opioid use
The 2‐year risk for chronic opioid use was 4.4% in women with endometriosis. After adjusting for age, race and geographic region, the OR for chronic opioid use among women with endometriosis compared to those without was 3.76 (95% CI 3.57–3.96). Women with endometriosis had a higher risk of having a diagnosis of opioid dependence/abuse (OR 2.73, 95% CI 2.38–3.13) and opioid overdose (OR 4.34, 95% CI 3.06–6.15) relative to the referent group. After adjusting for comorbidities and use of other medications, the OR for chronic opioid use was 2.88 (95% CI 2.72–3.04) (Table 2).
TABLE 2.
Risk and relative risk of chronic opioid use and dependence among women with endometriosis compared to women without
| Outcome | Endometriosis cohort (N = 36 373) N (%) | Referent cohort (N = 2 172 936) N (%) | Unadjusted odds ratio (95% CI) | Adjusted odds ratio, Model 1 (95%CI) a | Adjusted odds ratio, Model 2 (95%CI) b |
|---|---|---|---|---|---|
| Chronic opioid use c | 1587 (4.4%) | 24 872 (1.1%) | 3.94 (3.74–4.15) | 3.76 (3.57–3.96) | 2.88 (2.72–3.04) |
| Opioid dependence or abuse diagnosis a | 217 (0.6%) | 5312 (0.2%) | 2.45 (2.14–2.81) | 2.73 (2.38–3.13) | 1.86 (1.61–2.15) |
| Opioid overdose diagnosis | 34 (0.09%) | 545 (0.03%) | 3.73 (2.64–5.27) | 4.34 (3.06–6.15) | 2.88 (2.02–4.12) |
Abbreviations: CI, confidence interval; OR, odds ratio.
Adjusted for age categories, race, geographic region.
Adjusted for age categories, race, geographic region, fibromyalgia, chronic low back pain, migraines, irritable bowel syndrome, rheumatoid arthritis, depression, anxiety, substance abuse disorders, alcohol abuse, tobacco use, use of benzodiazepines, use of antidepressants, use of stimulants, use of antipsychotics, and cardiovascular risk factors.
Defined as ≥10 fills or ≥120 days covered by an opioid in any given year.
Results were similar using an alternative definition of chronic opioid use defined as ≥6 fills of an opioid in any given year 24 [adjusted OR 3.77, 95% CI 3.62–3.91] (Table 2). In sensitivity analysis, comparing women with endometriosis with a laparoscopic procedure compared to women without endometriosis, the adjusted OR for chronic opioid use was 3.58 (95% CI 3.32, 3.87) (eTable 3). Additionally, the adjusted hazard ratio (HR) for chronic opioid use in a population without the 2‐year follow‐up requirement was 4.09 (95%CI: 3.93, 4.26) (eTable 5).
3.3. Opioid use patterns and adverse effects of opioid use among endometriosis patients
Of the 2 172 936 women in the referent population without evidence of endometriosis, 3140 women subsequently had a claim for endometriosis outside of the 2‐year follow‐up. These women were included in the analysis of risk factors for chronic opioid use bringing a total number of women with endometriosis eligible for this analysis to n = 39 513. Of these women, 1588 (4.0%) met the criteria for chronic opioid use (Figure 2) with evident upward trajectories of days supplied and dose per prescription over 2 years of follow‐up; the mean number of days covered increased by 41 days from the first to second year of follow‐up (eTable 2) and the total mean dose per prescription dispensed increased from 302 (SD 580) to 740 (SD 5823) MME over 2 years (Figure 3). The mean prescribed daily dose among chronic opioid users (40.70 MME in year 1 and 41.39 MME in year 2) was not higher than among non‐chronic users (41.39 MME in year 1 and 45.11 MME in year 2) (eTable 2). Among chronic users, 80% of women continued to fill a prescription 20–24 months after the initial fill, compared to 20% of women among the non‐chronic users (Figure 3(B)).Chronic opioid users had a high risk of experiencing side effects of opioid use during the 2‐years of follow‐up, including constipation (34.2%), dizziness (19.6%), falls (8.2%). Further, chronic opioid users were commonly prescribed high‐dose opioids (daily prescribed dose of ≥90 MME) (49%), benzodiazepine (66.1%) and other sedatives (23.1%) compared to non‐chronic users. More than 6% of chronic users had a claim for opioid dependence and 0.8% had a claim for overdose (Table 3).
FIGURE 3.
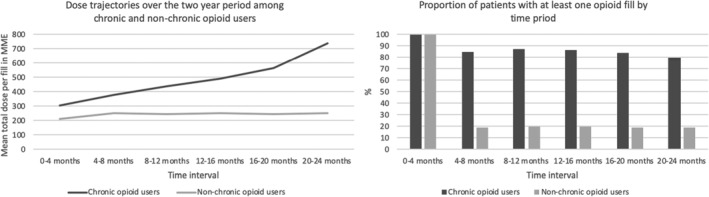
Proportion of patients with at least one fill and dose trajectories over the two‐year period among chronic and non‐chronic users of opioids among endometriosis patients identified in Optum CDM. Mean total dose per period is calculated among those patients with at least one opioid fill during the period of interest. Follow‐up begins during the first fill. MME = Morphine Milligram Equivalents
TABLE 3.
Risk and relative risk of complications and dangerous prescribing in chronic users of opioids compared to non‐chronic users of opioids among women with endometriosis
| Chronic opioid user a | Non‐chronic opioid user | |||
|---|---|---|---|---|
| Outcomes | Events‐n | Risk (%) b | Events‐n | Risk (%) |
| Complications | ||||
| Opioid dependence | 104 | 6.6 | 132 | 0.4 |
| Opioid overdose | 12 | 0.8 | 21 | 0.1 |
| Agonist‐based Medication Assisted Treatment | 121 | 7.7 | 283 | 1.0 |
| Constipation | 538 | 34.2 | 5595 | 15.0 |
| Osteoporosis | 37 | 2.4 | 441 | 1.2 |
| Altered mental status | 44 | 2.8 | 200 | 1.0 |
| Dizziness | 308 | 19.6 | 3829 | 10.3 |
| Falls | 129 | 8.2 | 719 | 1.9 |
| Dangerous prescribing patterns | ||||
| High‐dose opioid c | 772 | 49.0 | 4656 | 12.5 |
| Co‐prescribed benzodiazepines | 1041 | 66.1 | 9193 | 24.7 |
| Co‐prescribed sedatives | 363 | 23.1 | 2627 | 7.1 |
Abbreviation: CI, confidence interval.
Defined as ≥10 fills of ≥120 days supply in 1 year.
2‐year risk per 1000 patients.
Defined as a daily dose of ≥90 MME.
3.4. Risk factors for chronic opioid use
Among endometriosis patients, baseline opioid use was associated with a 4‐fold greater risk of developing chronic use (OR 3.95 95% CI 3.29–4.74). Other risk factors associated with increase in risk included concomitant chronic conditions associated with pain, including fibromyalgia, chronic back pain, migraines, irritable bowel syndrome, rheumatoid arthritis, and use of psychiatric medications (except stimulants) each associated with a significant OR of >1.5. (Table 4). Characteristics associated with lower risk of chronic opioid use included older age (OR for age 35–50 v age 18–25: 0.72 95%CI 0.55–0.94) and Hispanic ethnicity (OR for Hispanic v non‐Hispanic women: 0.56 95%CI 0.39–0.81).
TABLE 4.
Predictors of chronic opioid use among women with endometriosis
| Risk factor | Chronic opioid user a | Non‐chronic opioid user | Odds Ratio (95% CI) |
|---|---|---|---|
| N | 1588 | 37 925 | |
| Age‐years | |||
| <25 | 186 (11.7%) | 2905 (7.7%) | 1.0 (ref) |
| 25–35 | 671 (42.3%) | 13 417 (35.4%) | 0.99 (0.75–1.30) |
| 35–50 | 731 (46.0%) | 21 602 (57.0%) | 0.72 (0.55–0.94) |
| Race/Ethnicity | |||
| White | 1113 (70.1%) | 23 592 (62.2%) | 1.0 (ref) |
| Asian | 19 (1.2%) | 1861 (4.9%) | 0.39 (0.20–0.77) |
| Black | 153 (9.6%) | 3726 (9.8%) | 1.04 (0.79–1.36) |
| Hispanic | 97 (6.1%) | 3812 (10.1%) | 0.56 (0.39–0.81) |
| Geographic region | |||
| Northeast | 94 (5.9%) | 3508 (9.2%) | 1.0 (ref) |
| South | 761 (47.9%) | 17 849 (47.1%) | 1.22 (0.87–1.71) |
| Midwest | 410 (25.8%) | 9142 (24.1%) | 1.31 (0.92–1.86) |
| West | 318 (20.0%) | 6976 (18.4%) | 1.37 (0.95–1.98) |
| Pain conditions | |||
| Fibromyalgia | 133 (8.4%) | 1481 (3.9%) | 1.49 (1.12–1.99) |
| Chronic back pain | 438 (27.6%) | 5048 (13.3%) | 1.55 (1.28–1.88) |
| Chronic headaches/migraines | 226 (14.2%) | 1929 (5.1%) | 1.49 (1.16–1.92) |
| Irritable bowel syndrome | 103 (6.5%) | 974 (2.6%) | 1.61 (1.16–2.25) |
| Rheumatoid arthritis | 16 (1.0%) | 194 (0.5%) | 2.52 (1.28–4.97) |
| Substance abuse | |||
| Alcohol abuse | 16 (1.0%) | 72 (0.2%) | 0.95 (0.31–2.90) |
| Tobacco use | 233 (14.7%) | 1443 (3.8%) | 1.87 (1.44–2.42) |
| Other substance abuse | 16 (1.0%) | 50 (0.1%) | 1.33 (0.55–3.21) |
| Medication use | |||
| Antidepressants | 676 (42.6%) | 6734 (17.8%) | 2.07 (1.74–2.47) |
| Antipsychotics | 97 (6.1%) | 595 (1.6%) | 1.66 (1.17–2.36) |
| Benzodiazepines | 523 (32.9%) | 4149 (10.9%) | 1.87 (1.55–2.27) |
| Stimulants | 68 (4.3%) | 742 (2.0%) | 1.02 (0.66–1.57) |
| Baseline opioid use | 1243 (78.3%) | 12 385 (32.7%) | 3.95 (3.29–4.74) |
Note: N (%), all such values.
Abbreviation: CI, 95% confidence interval.
Defined as ≥10 fills or ≥120 days covered by an opioid in any given year.
4. DISCUSSION
4.1. Principal findings
Despite the lack of evidence to support the long‐term benefits of opioids for non‐cancer chronic pain, 25 , 26 4.4% of women became chronic opioid users within 2 years following a claim for endometriosis ‐ a rate that is four times that of women without endometriosis. The elevated risk for chronic opioid use among was only partially explained by high prevalence of other pain‐related comorbidities and mental health conditions. Further, chronic opioid use was associated with an escalation in dose, driven by an increase in number of days supplied per prescription, and dangerous opioid prescribing patterns, including prescriptions for benzodiazepine and sedatives.
4.2. Results in context
Recent studies in administrative claims databases have demonstrated that more than half of women fill a prescription for opioids, primarily short‐acting opioids, after receiving a diagnosis code for endometriosis. 7 Further, women with endometriosis are more likely to fill at least one prescription for opioids than women without endometriosis, to be prescribed opioids at higher doses, for a greater number of days and concomitantly with benzodiazepines for up to 90 days than women without endometriosis, 6 leading to greater healthcare utilization and costs. 8 , 9 The current study expands these findings by demonstrating that there is an elevated risk of chronic opioid use. Importantly, the results from this study or prior studies 6 , 7 , 8 , 9 cannot attribute opioid use directly to the management of endometriosis‐associated pain. However, irrespective of the indication for opioid initiation, women with endometriosis are at greater risk for becoming chronic users.
The association between endometriosis and risk of chronic opioid use among women remained significant but was attenuated after adjustment for factors such as age, mental health conditions, and other conditions associated with pain. Given the circumspect number of predictors available in this analysis, it is likely that while endometriosis is associated with chronic opioid use, the combination of comorbidities among women with endometriosis may contribute to this increased risk.
Our study suggests that it may be possible to identify endometriosis patients who are at higher risk for becoming chronic opioid users based on their comorbidity profile and history of medication use. Younger age and the presence of other conditions associated with pain, such as back pain, depression, and prior opioid use may put these patients at an increased risk. Although this study was not able to evaluate the indication for opioid prescribing, given the previously reported lag between pain symptom onset and diagnosis of endometriosis, this finding may reflect a population with uncontrolled endometriosis pain prior to formal diagnosis. Further, in this setting, prior opioid use may be a marker of more severe disease, which is not captured adequately in claims. These risk factors are consistent with predictors of chronic use among patients with other chronic pain conditions, such as chronic pelvic pain 15 and rheumatoid arthritis, 18 , 27 as well as opioid‐naïve surgical patients, 19 , 20 , 21 including gynecological surgeries for the treatment of endometriosis. 28
Among women with endometriosis, 60% of chronic opioid users were prescribed benzodiazepines, which is consistent with the co‐prescribing pattern seen in another commercially‐insured population described by Sun and colleagues. 29 The use of benzodiazepines may exacerbate complications among chronic opioid users. In the Sun et al study, benzodiazepine use was associated with a 2‐fold increased risk of opioid overdose in chronic opioid users. 29 Among US veterans identified as opioid users, the hazard ratio for opioid‐related overdose was 2.33 (95% CI 2.05–2.64) for individuals with a past prescription for benzodiazepine and 3.86 (95% CI 3.49–4.26) for those with a current prescription, compared to individuals with no benzodiazepine prescription. 30
Recognizing the risks and complications of opioid use in chronic pain management, it is critical to note the limited long‐term, effective medical therapy for treating chronic endometriosis‐associated pain. Approximately 25%–33% of women with endometriosis do not respond to first line therapies. 5 In a prior analysis in administrative claims data, over half of the women with endometriosis filled a prescription for an opioid within 1 year of the initial endometriosis diagnosis code. 7 In the current study, after excluding heavy users of opioids, 34% of women with endometriosis had some exposure to opioids 6‐months prior to their initial endometriosis claim. Surgical ablation or excision of endometrial lesions can be effective, but recurrence of pain symptoms is common 31 and may lead to chronic opioid use. 28
The strengths of this study are the large sample size, data accuracy on filled opioid prescriptions, and geographic diversity in the patient population. However, there are limitations that warrant discussion. First, these results were derived from commercially insured patients and may not generalize to populations with different demographics, such as those uninsured. The ability to define a cohort of women with incident endometriosis in administrative data is challenging due to the chronic nature of the disease and the lack of first line therapy that is specific for the treatment of endometriosis or its symptoms. Thus women in this analysis identified using diagnosis codes for endometriosis may include women who have had endometriosis for varying lengths of time, including those with more severe disease that is has not been well managed by first line therapies. The documented long delay between symptom onset and disease diagnosis (on average 7 years), 32 the high frequency of opioid prescriptions within the year after the first ICD diagnosis code 7 and the average age at cohort entry of 35 years in the current study supports that the ICD diagnosis in claims data is not the initial diagnosis of endometriosis.
Another limitation is the potential for selection bias that arises when only a population with long follow‐up is selected into the study. To address this limitation, we repeated the analysis for risk of chronic use in a population without a follow‐up requirement and using a Cox proportional hazards model. Results indicate that women without any follow‐up requirement were similar based on baseline characteristics, including demographics, comorbidity profile and use of medications, and the hazard ratios for chronic use and overdose were unchanged, pointing to a 4‐fold increase in risk of chronic use among endometriosis patients (eTables 3 and 4).
Studies using administrative claims are prone to exposure misclassification; women with endometriosis and no corresponding medical claim may have been included in the referent population, leading to attenuation in the association between endometriosis and risk of chronic opioid use. However, an attenuation is likely minimal due to the large sample size in the referent group. Additionally, the comparator population included women with at least one general wellness visit, which ensured that women in both groups have interactions with the healthcare system. Finally, because opioid use is ascertained based on prescription fills, we quantified daily dose of opioids based on the MME per day as prescribed and not as taken by the patient, which is a more accurate method to estimate dose and dose escalation.
Despite the stated limitations, our study provides strong evidence that may help inform the benefit–risk ratio of opioid use for pain management in women with endometriosis and which characteristics may increase a woman's risk for developing chronic opioid use.
5. CONCLUSIONS
In conclusion, women with endometriosis are more likely to become chronic opioid users and experience harmful opioid‐related complications compared to women without endometriosis, a risk that is partially mediated through endometriosis‐associated comorbidities. The identification of concomitant risk factors for chronic opioid use may aid clinicians in screening women with endometriosis that may be susceptible to chronic opioid use and its consequences and prompt close monitoring in women for whom opioid treatment is selected. These insights contribute to the existing knowledge of the potential risks associated with opioid use among women with endometriosis 25 and may help to inform benefit–risk decision making for this population.
CONFLICT OF INTEREST
Stephanie E. Chiuve, Ryan D. Kilpatrick, Lani R. Wegrzyn, and Michael C. Snabes are employees of AbbVie receiving stock and/or stock options. Natalia Petruski‐Ivleva and Elizabeth C. Dabrowski, are employees of and holds stock at Aetion, Inc., a software and data analytics company that provides services to the healthcare industry. Priscilla Velentgas was an employee at Aetion at the time the work was completed and is currently an employee at IQVIA. Mark D. Hornstein and Brian T. Bateman conducted this work as paid consultants to Aetion, are publishing in that capacity. Mark D. Hornstein and Brian T. Bateman did not receive payment for authorship. Aetion received funding from AbbVie for conducting the study. These data were presented at the Annual Clinical and Scientific Meeting of the American College of Obstetricians and Gynecologists, May 3–6, 2019, Nashville, TN.
AUTHOR CONTRIBUTIONS
Stephanie E. Chiuve and Brian T. Bateman were responsible for conception and design of this study, analysis and interpretation of the data, along with drafting the manuscript. Ryan D. Kilpatrick, Mark D. Hornstein, Natalia Petruski‐Ivleva, Lani R. Wegrzyn, and Priscilla Velentgas were involved in the design of the study and analysis and interpretation of the data. Elizabeth C. Dabrowski and Michael C. Snabes provided substantial contributions with respect data analysis and interpretation. Stephanie E. Chiuve, Ryan D. Kilpatrick, Mark D. Hornstein, Natalia Petruski‐Ivleva, Lani R. Wegrzyn, Elizabeth C. Dabrowski, Priscilla Velentgas, WCS and Brian T. Bateman all critically revised the manuscript and gave final approval for publication.
ETHICS STATEMENT
The Quorum Review institutional review board (Seattle, Washington) determined that this study does not constitute research involving human subjects and was therefore exempt from review.
Supporting information
Appendix S1. Supporting information.
ACKNOWLEDGMENTS
This study was funded by AbbVie Inc.
Chiuve SE, Kilpatrick RD, Hornstein MD, et al. Chronic opioid use and complication risks in women with endometriosis: A cohort study in US administrative claims. Pharmacoepidemiol Drug Saf. 2021;30:787–796. 10.1002/pds.5209
Funding information AbbVie
REFERENCES
- 1. Kennedy S, Bergqvist A, Chapron C, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698‐2704. [DOI] [PubMed] [Google Scholar]
- 2. Rogers PA, Adamson GD, Al‐Jefout M, et al. Research priorities for endometriosis. Reprod Sci. 2017;24:202‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Graaff AA, D'Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross‐sectional survey. Hum Reprod. 2013;28:2677‐2685. [DOI] [PubMed] [Google Scholar]
- 4. Schliep KC, Mumford SL, Peterson CM, et al. Pain typology and incident endometriosis. Hum Reprod. 2015;30:2427‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400‐412. [DOI] [PubMed] [Google Scholar]
- 6. Lamvu G, Soliman AM, Manthena SR, Gordon K, Knight J, Taylor HS. Patterns of prescription opioid use in women with endometriosis: evaluating prolonged use, daily dose, and concomitant use with benzodiazepines. Obstet Gynecol. 2019;133:1120‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. As‐Sanie S, Soliman AM, Evans K, Erpelding N, Lanier RK, Katz NP. Short‐acting and long‐acting opioids utilization among women diagnosed with endometriosis in the United States: a population‐based claims study. J Minim Invasive Gynecol. 2020;28:297‐306. [DOI] [PubMed] [Google Scholar]
- 8. Estes SJ, Soliman AM, Zivkovic M, Chopra D, Zhu X. Healthcare resource use and costs associated with opioid initiation among patients with newly diagnosed endometriosis with commercial insurance in the USA. Adv Ther. 2020;37:2777‐2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. As‐Sanie S, Soliman AM, Evans K, Erpelding N, Lanier R, Katz NP. Healthcare utilization and cost burden among women with endometriosis by opioid prescription status in the first year after diagnosis: a retrospective claims database analysis. J Med Econ. 2020;23:371‐377. [DOI] [PubMed] [Google Scholar]
- 10. VanHouten JP, Rudd RA, Ballesteros MF, Mack KA. Drug overdose deaths among women aged 30–64 years ‐ United States, 1999–2017. MMWR Morb Mortal Wkly Rep. 2019;68:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carlson RG, Nahhas RW, Martins SS, Daniulaityte R. Predictors of transition to heroin use among initially non‐opioid dependent illicit pharmaceutical opioid users: a natural history study. Drug Alcohol Depend. 2016;160:127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilson N, Kariisa M, Seth P, Smith HT, Davis NL. Drug and opioid‐involved overdose deaths ‐ United States, 2017‐2018. MMWR Morb Mortal Wkly Rep. 2020;69:290‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu CY, Chang WP, Chang YH, Li CP, Chuang CM. The risk of irritable bowel syndrome in patients with endometriosis during a 5‐year follow‐up: a nationwide population‐based cohort study. Int J Color Dis. 2015;30:907‐912. [DOI] [PubMed] [Google Scholar]
- 14. Soliman AM, Taylor HS, Bonafede M, Nelson JK, Castelli‐Haley J. Incremental direct and indirect cost burden attributed to endometriosis surgeries in the United States. Fertil Steril. 2017;107:1181‐1190.e1182. [DOI] [PubMed] [Google Scholar]
- 15. Chen JH, Humphreys K, Shah NH, Lembke A. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid‐naive patients in the postoperative period. JAMA Intern Med. 2016;176:1286‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Connell C, Azad TD, Mittal V, et al. Preoperative depression, lumbar fusion, and opioid use: an assessment of postoperative prescription, quality, and economic outcomes. Neurosurg Focus. 2018;44:E5. [DOI] [PubMed] [Google Scholar]
- 18. Cichowski SB, Rogers RG, Komesu Y, et al. A 10‐yr analysis of chronic pelvic pain and chronic opioid therapy in the women veteran population. Mil Med. 2018;183:e635–e640. [DOI] [PubMed] [Google Scholar]
- 19. Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid‐naive women. Am J Obstet Gynecol. 2016;215:353 e351–353 e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As‐Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219:486.e1‐486.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang SV, Verpillat P, Rassen JA, Patrick A, Garry EM, Bartels DB. Transparency and reproducibility of observational cohort studies using large healthcare databases. Clin Pharmacol Ther. 2016;99:325‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim SC, Solomon DH, Rogers JR, et al. Cardiovascular safety of Tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis: a multi‐database cohort study. Arthritis Rheumatol. 2017;69:1154‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deyo RA, Hallvik SE, Hildebran C, et al. Association between initial opioid prescribing patterns and subsequent long‐term use among opioid‐naive patients: a statewide retrospective cohort study. J Gen Intern Med. 2017;32:21‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA. 2016;315:1624‐1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long‐term opioid therapy for chronic pain: a systematic review for a National Institutes of Health pathways to prevention workshop. Ann Intern Med. 2015;162:276‐286. [DOI] [PubMed] [Google Scholar]
- 27. Lee YC, Kremer J, Guan H, Greenberg J, Solomon DH. Chronic opioid use in rheumatoid arthritis: prevalence and predictors. Arthritis Rheumatol. 2018;71:670‐677. [DOI] [PubMed] [Google Scholar]
- 28. Wright JD, Huang Y, Melamed A, et al. Use and misuse of opioids after gynecologic surgical procedures. Obstet Gynecol. 2019;134:250‐260. [DOI] [PubMed] [Google Scholar]
- 29. Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case‐cohort study. BMJ. 2015;350:h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389‐2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366‐373 e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


