Abstract
Objective
To investigate the efficacy of 16‐week treatment with etanercept (ETN) in patients with suspected nonradiographic axial spondyloarthritis (SpA).
Methods
Tumor necrosis factor inhibitor–naive patients with inflammatory back pain with at least 2 SpA features and high disease activity (Bath Ankylosing Spondylitis Disease Activity Index score ≥4), without the requirement of a positive finding on magnetic resonance imaging (MRI) of the sacroiliac (SI) joint and/or elevated C‐reactive protein (CRP) level, were randomized (1:1) to receive ETN (n = 40) or placebo (n = 40) for 16 weeks and subsequently were followed up for a further 8 weeks (to 24 weeks from baseline) without study medication. The primary end point was the Assessment of SpondyloArthritis international Society 20 (ASAS20) response at 16 weeks. Secondary end points included the Ankylosing Spondylitis Disease Activity Score (ASDAS) and changes in disease parameters, including the Bath Ankylosing Spondylitis Metrology Index (BASMI), CRP level, erythrocyte sedimentation rate (ESR), and Spondyloarthritis Research Consortium of Canada index scores (MRI of the SI joint), after 16 and 24 weeks.
Results
Patient characteristics at baseline were comparable between the ETN and placebo groups. At 16 weeks, there was no significant difference in the percentage of patients exhibiting ASAS20 response between the ETN group (6 patients [16.7%]) and the placebo group (4 patients [11.1%]) (relative risk 0.7 [95% confidence interval 0.2–2.2], P = 0.5). Only the ESR showed more improvement in the ETN group compared to the placebo group at 16 weeks (decreases of 2.2 mm/hour and 1.4 mm/hour, respectively), but the difference did not reach statistical significance. Between 16 and 24 weeks, without study medication, the BASMI, CRP level, and ESR had worsened to a greater extent in the ETN group compared to the placebo group, with the difference being significant for the CRP level.
Conclusion
This study shows that in patients with suspected nonradiographic axial SpA with high disease activity but without the requirement of a positive finding on SI joint MRI and/or elevated CRP level, treatment with ETN is not effective.
INTRODUCTION
According to the Assessment of SpondyloArthritis international Society (ASAS) classification criteria, axial spondyloarthritis (SpA) can be divided in 2 groups: patients with radiographic signs of sacroiliitis (radiographic axial SpA; ankylosing spondylitis [AS]) and patients without radiographic sacroiliitis (nonradiographic axial SpA) (1). Despite the availability of these classification criteria, there is still a lack of understanding of disease presentation and progression, especially in patients with nonradiographic axial SpA (2, 3, 4, 5).
Inflammatory back pain is present in ~70% of patients diagnosed as having axial SpA, and therefore is an important clinical symptom of axial involvement (5, 6). An algorithm described by Rudwaleit et al showed a high probability that AS could be diagnosed at the preradiographic stage in patients with chronic back pain with inflammatory back pain as the primary presenting symptom (7). Based on this algorithm, the probability that the patient has AS is at least 90% if inflammatory back pain plus 2 or 3 other features are present (8).
Currently, the ASAS classification criteria are widely accepted for use in clinical practice, although they were developed for the purpose of classification for study eligibility and not for clinical diagnosis (1, 9, 10, 11). In order to classify axial SpA at an early stage of the disease, the ASAS classification criteria divide patients in 2 groups: patients who meet the “clinical arm” and patients who meet the “imaging arm.” The “clinical arm” includes patients who are HLA–B27 positive and have 2 additional features of SpA, and the “imaging arm” includes patients with active inflammatory lesions of the sacroiliac (SI) joints as seen on magnetic resonance imaging (MRI) along with 1 additional feature of SpA (1, 10).
In many reported studies, a positive SI joint finding on MRI has been one of the prerequisites for starting tumor necrosis factor inhibitor (TNFi) treatment in patients with nonradiographic axial SpA. The other criteria for starting TNFi therapy in nonradiographic axial SpA are unsuccessful treatment with at least 2 different nonsteroidal antiinflammatory drugs (NSAIDs) and increased C‐reactive (CRP) levels in the setting of negative MRI findings (12, 13). Increased CRP levels, however, were found in only 30% of patients with nonradiographic axial SpA (9, 14), and in 59–64% of patients with nonradiographic axial SpA with high disease activity, inflammatory lesions of the SI joint are not detected on MRI. A patient population selected based on the ASAS classification criteria may therefore be different from the population seen in daily clinical practice (6, 14).
In addition, in several studies MRI has shown false‐positive results of bone marrow edema at the SI joint (not related to axial SpA disease). This is the case in ~23% of healthy individuals, 57% of postpartum women, and in recreational runners, professional athletes, and military recruits undergoing physical training. In all of these cases the MRI component of the ASAS classification criteria was fulfilled (15, 16, 17).
In most studies a higher rate of response to TNFi was observed in nonradiographic axial SpA patients who had elevated CRP levels and/or MRI‐detected inflammatory lesions compared to patients without these factors (13, 18, 19, 20). Only 2 randomized clinical trials on the efficacy of TNFi included patients with nonradiographic axial SpA without the abovementioned requirements (21, 22), 1 of which did not include an objective scoring method for MRI‐detected SI joint lesions (21). Both studies revealed a significant difference in response according to the ASAS criteria for 40% improvement (ASAS40) (23) between the placebo group (12.5–15%) and the treatment group (36–54.5%).
Data are lacking on the indications for biologic treatment in patients with suspected nonradiographic axial SpA who have low CRP levels and do not have active lesions seen on SI joint MRI. Therefore, a double blind, placebo‐controlled clinical trial with the TNFi etanercept (ETN) was initiated. The primary aim of this proof‐of‐concept study was to assess the short‐term efficacy of TNFi treatment, according to the ASAS20 response over 16 weeks, in patients with inflammatory back pain and suspected nonradiographic axial SpA with high disease activity, regardless of the CRP level or the presence of SI joint inflammation seen on MRI. “Disease activity” was used in this study as terminology by default, since there is no validation for the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (24) in this patient group (25); we actually investigated the “level of symptoms” at 16 and 24 weeks. Secondary aims were to investigate the number of AS Disease Activity Score (ASDAS) (26) responders after 16 weeks, change from baseline in mean disease status, and the proportion of patients with inflammatory lesions of the SI joints seen on MRI at 16 and 24 weeks.
PATIENTS AND METHODS
Study population
Patients with chronic back pain and a suspicion of nonradiographic axial SpA were recruited from November 11, 2009 through August 29, 2014 at the rheumatology outpatient clinics of the VU University Medical Center (VUMC) and Reade/Jan van Breemen Research Institute, and via the website of the Dutch AS patient society (Dutch Axial Spondyloarthritis Foundation). Patients were eligible for inclusion if they were at least 18 years of age and fulfilled the Calin criteria for inflammatory back pain (27). Patients were enrolled based on the algorithm of Rudwaleit et al (7), with at least 2 SpA features according to the European Spondylarthropathy Study Group classification criteria (28) if HLA–B27 negative, and at least 1 SpA feature if B27 positive. In addition, patients had to have a high disease activity score (BASDAI ≥4) and insufficient response to at least 2 different NSAIDs. Patients were excluded if they had definite AS according to the modified New York criteria (29) or had received biologic treatment in the past (Table 1). Detailed descriptions of the inclusion and exclusion criteria have been published previously (30). Because of a slow enrollment rate in the first study period, adaptations of the inclusion criteria were made in the second half of 2011, allowing patients without inflammatory lesions seen on MRI to be included in the study. The study was approved by the local ethical review board, and all patients provided written informed consent prior to screening.
Table 1.
PrevAS study inclusion and exclusion criteria*
| Inclusion criteria | Exclusion criteria |
|---|---|
|
Age ≥18 years Inflammatory back pain meeting the Calin criteria (27)† |
Diagnosis of radiographic axial SpA/AS according to the modified New York criteria (29) |
| HLA–B27 positive with ≥1 SpA feature or HLA–B27 negative with ≥2 SpA features (1)‡ | Previous treatment with a biologic agent |
| High disease activity score (BASDAI ≥4) | Contraindications to treatment with a TNFi |
| Insufficient response to ≥2 different NSAIDs |
PrevAS = Prevention of the Progression of Very Early Symptoms in Ankylosing Spondylitis; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index; TNFi = tumor necrosis factor inhibitor.
Back pain with an insidious onset before the age of 45 years, chronic back pain persistence for at least 3 months, morning stiffness, improvement with exercise, pain at night.
Spondyloarthritis (SpA) features include asymmetric arthritis, alternating buttock pain, dactylitis, enthesitis of the Achilles tendon or the plantar fascia, presence or history of psoriasis, inflammatory bowel disease (IBD), or acute anterior uveitis (AAU), first‐ or second‐degree relative with ankylosing spondylitis (AS)/psoriasis/AAU/IBD, positive response to nonsteroidal antiinflammatory drugs (NSAIDs), and increased C‐reactive protein level (≥10.0 mg/liter) or erythrocyte sedimentation rate (≥15 mm/hour).
Treatment allocation and methods
The Prevention of the Progression of Very Early Symptoms in Ankylosing Spondylitis study was a randomized, double blind, placebo‐controlled trial performed at VUMC (EudraCT number 2009‐015515‐40). Patients were randomly assigned (1:1) to receive ETN (25 mg twice weekly) or placebo. After 16 weeks, patients were followed up without study treatment for up to 3 years. Radiographs were obtained at baseline and after 1 year and 3 years of follow‐up (only baseline data provided in the present report).
The study drug ETN was supplied by Pfizer. Placebo ETN was developed and validated at the clinical pharmacology department of VUMC. The study medication was labeled at the VUMC central pharmacy and distributed to the VUMC outpatient pharmacy department for dispensing to study subjects. The pharmacist randomized the patients and provided the masked study medication to the study personnel. All investigators, including the study physician and research nurse, remained blinded with regard to the treatment until the last patient had completed the study. The study drug was self‐administrated twice weekly with a subcutaneous injection that contained 25 mg of ETN or placebo. The normally distributed injections of ETN, in a dose of 50 mg administered once a week, were not feasible for this study because the placebo could only be produced in a 25‐mg formulation.
Patients were allowed to continue taking analgesics, NSAIDs, disease‐modifying antirheumatic drugs (DMARDs), and/or oral glucocorticoids (≤10 mg/day). The dosage had to be stable for 2 weeks prior to the baseline evaluation in the case of NSAIDs and oral glucocorticoids, and 4 weeks in the case of DMARDs; during the study, the dosage could be reduced or the treatment temporarily discontinued. Patients were allowed to receive intraarticular glucocorticoid injections. Treatment with any cytotoxic drugs, investigational drugs, or agents targeted at reducing TNF was not allowed during the first 16 weeks. All concomitant medication used during the study or changes in medication dosage were reported during each study visit.
Assessments
All study personnel and patients were blinded with regard to the randomization schedule and to treatment assignments until the last patient had completed the 3‐year follow‐up. In order to prevent influencing the study visit assessments (due to events caused by the medication such as injection site reactions), assessors who were not involved in the study performed the physical examinations and evaluated laboratory results.
Data collection
Demographic data were recorded, and disease‐specific variables were assessed, including disease duration (duration of back pain at baseline), inflammatory back pain, SpA features, family history and presence of extraarticular manifestations such as uveitis, inflammatory bowel disease (IBD), and psoriasis, and use of concomitant medication (NSAIDs, DMARDs). Questionnaires on pain, overall well‐being (Bath Ankylosing Spondylitis patient global score [31]), and quality of life (Short Form 36 health survey [32]) were administered during each visit. In addition, physical examinations were performed during each visit, including assessments to determine the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) (33) and swollen and tender joint counts (of 44 joints).
Time on study treatment (ETN or placebo) and compliance were determined. Compliance was calculated based on the number of injections taken divided by the number of injections expected. Safety parameters, such as adverse events, were registered during the follow‐up visits. Safety analyses included all patients who had received ≥1 dose of study treatment.
Clinical efficacy parameters
Clinical efficacy was assessed based on the numbers of patients who met the ASAS20/40 response criteria and the ASDAS using the C‐reactive protein level (ASDAS‐CRP) response criteria (with clinically important improvement defined as a decrease in the ASDAS‐CRP to ≤1.1 and major improvement defined as a decrease in the ASDAS‐CRP to ≤2.0) (34) and based on the frequency of low disease activity (ASDAS‐CRP <2.1) and inactive disease status according to the ASDAS‐CRP criteria (ASDAS‐CRP <1.3). Efficacy was measured throughout the first 16 weeks of treatment and at 24 weeks, i.e., 8 weeks after study‐related treatment was discontinued. Other clinical outcome parameters were the Bath AS Functional Index (35), the Bath AS Metrology Index (BASMI [35]), global pain, and measures of inflammation, i.e., CRP (median value and proportion of patients with values exceeding the upper limit of normal [10.0 mg/liter]) and erythrocyte sedimentation rate (ESR).
MRI outcome measures
MR images were independently assessed by 2 expert readers (RBML and JJHdW), who were blinded with regard to treatment, patient characteristics, and sequence of the different MRIs. Potential reader discrepancies were resolved by consultation with a third reviewer (BJHB).
SI joint MRIs were assessed according to the ASAS definition (37, 38). To quantify the extent of and evaluate the changes in active inflammation seen on SI joint MRI, the Spondyloarthritis Research Consortium of Canada (SPARCC) MRI Indices for Assessment of Spinal and SI Joint Inflammation in AS (SPARCC scores) (39, 40) were used. A score of ≥2 was considered an indicator of SI joint inflammation shown on MRI. The mean score from the 2 independent readers (or 3 readers if a third observer was needed) was used. Minimally important change in the SI joints was defined as a change in the SPARCC score of ≥2.5 (40). Intraclass correlation coefficients (ICCs) were calculated for change scores and presented as scores for absolute agreement.
Statistical analysis
The primary outcome measure of this trial was the ASAS20 response. It was assumed that 50% of the patients treated with ETN (intervention group) and 20% of the patients treated with placebo (control group) would experience an ASAS20 response. In order to statistically support a real difference of 30%, 40 patients per arm were required (α = 0.05, β = 0.20). Data were presented as the mean ± SD or, in cases of skewed distribution, as the median and interquartile range (IQR).
The analysis included the intent‐to‐treat population, with baseline, 16‐week, and 24‐week clinical outcome measurements. All patients who received at least 1 dose of the study drug were included in the intent‐to‐treat analysis. Data up to the last known data point for a study patient were included for analyses.
The primary outcome ASAS20 response and secondary outcomes ASAS40 and ASDAS‐CRP response according to clinically important improvement and major improvement were assessed by chi‐square test or, if the data were skewed, by nonparametric tests, such as the Mann‐Whitney U test. Categorical data were assessed by chi‐square test. Post hoc analyses were performed for ASAS20 and ASDAS‐CRP response at 16 weeks, according to baseline CRP levels (normal CRP/elevated CRP [>10.0 mg/liter]), SI joint inflammation based on SPARCC score (yes/no), HLA–B27 status (positive/negative), sex (male/female), and NSAID use (yes/no). ICC between readers’ scores for change in inflammation parameters on MRI, between baseline and 16 weeks and between 16 weeks and 24 weeks, was calculated. Relative risks (RRs) and 95% confidence intervals (95% CIs) were calculated.
All statistical analyses were performed using SPSS for Windows version 26.0. Two‐sided P values less than 0.05 were considered significant.
RESULTS
Patients and baseline characteristics
One hundred six consecutive patients were screened for this 16‐week placebo‐controlled trial. Twenty‐six patients (24.5%) did not meet entry criteria, and 80 were enrolled (40 in the ETN group and 40 in the placebo group) (Figure 1).
Figure 1.
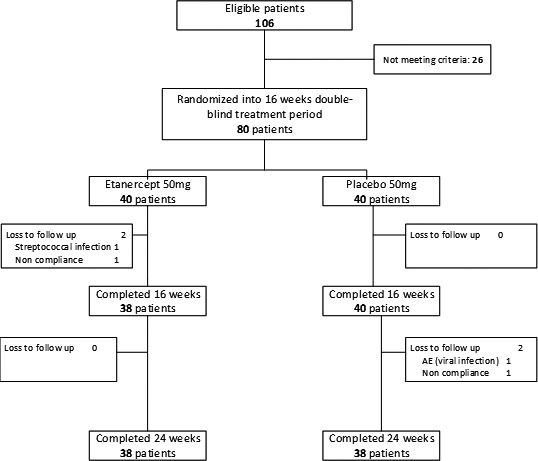
Flow chart showing disposition of the patients. AE = adverse event.
HLA–B27 status, number of SpA features, and other baseline patient characteristics were comparable between the ETN and placebo groups (Tables 2 and 3). The majority of the patients were female (63.8%). The mean ± SD age was 34.5 ± 9.6 years, and 60.0% (48 of 80) were HLA–B27 positive. NSAIDs were used by 67.5% and DMARDs by 11.3% (most commonly methotrexate [n = 3] and sulfasalazine [n = 3]). The median CRP level was 2.5 mg/liter (IQR 2.5–6.0). The mean ± SD BASDAI was 5.1 ± 2.4, and the mean ± SD ASDAS‐CRP was 2.8 ± 1.1, which indicates moderate‐to‐severe disease activity. The median SPARCC‐SI joint score at baseline was 0.0 (IQR 0.0–3.1).
Table 2.
Baseline demographic and clinical characteristics of the study population (n = 80)*
| Total |
Etanercept (n = 40) |
Placebo (n = 40) |
|
|---|---|---|---|
| Demographics | |||
| Female | 51 (64) | 27 (68) | 24 (60) |
| Age, mean ± SD years | 34 ± 10 | 36 ± 10 | 33 ± 9 |
| Clinical characteristics and extraarticular manifestations | |||
| Disease duration, median (IQR) years | 4.0 (2–9) | 5.0 (2.5–14) | 3.5 (2–8) |
| HLA–B27 positive | 48 (60) | 25 (63) | 23 (58) |
| No. of SpA features, mean ± SD† | 3 ± 1 | 3 ± 1 | 3 ± 2 |
| Uveitis | 15 (19) | 10 (25) | 5 (13) |
| Psoriasis | 30 (38) | 15 (38) | 15 (38) |
| IBD | 30 (38) | 13 (33) | 17 (43) |
| Concomitant medications | |||
| NSAIDs | 54 (68) | 26 (65) | 28 (70) |
| DMARDs | 9 (11) | 6 (15) | 3 (8) |
There were no statistically significant differences between groups. Except where indicated otherwise, values are the number (%). IQR = interquartile range; DMARDs = disease‐modifying antiinflammatory drugs.
Spondyloarthritis (SpA) features include asymmetric arthritis, alternating buttock pain, dactylitis, enthesitis of the Achilles tendon or the plantar fascia, presence or history of psoriasis, inflammatory bowel disease (IBD), or acute anterior uveitis (AAU), first‐ or second‐degree relative with ankylosing spondylitis (AS)/psoriasis/AAU/IBD, positive response to nonsteroidal antiinflammatory drugs (NSAIDs), and increased C‐reactive protein level (≥10.0 mg/liter) or erythrocyte sedimentation rate (≥15 mm/hour).
Table 3.
Baseline data on the disease outcomes assessed in the study population (n = 80)*
| Total |
Etanercept (n = 40) |
Placebo (n = 40) |
|
|---|---|---|---|
| ASDAS‐CRP | 2.8 ± 1.1 | 2.8 ± 0.8 | 2.8 ± 1.4 |
| BASDAI, 0–10 NRS | 5.1 ± 2.4 | 4.8 ± 2.2 | 5.4 ± 2.3 |
| BASFI, 0–10 NRS | 3.8 ± 2.5 | 3.8 ± 2.6 | 3.9 ± 2.4 |
| CRP, median (IQR) mg/liter | 2.5 (2.5–6.0) | 2.5 (2.5–5.5) | 2.5 (2.5–6.5) |
| CRP >ULN, no. (%) | 9.0 (13) | 6 (17) | 3 (9) |
| ESR, median (IQR) mm/hour | 6.0 (2.0–11) | 8.0 (2.5–14) | 4.5 (2.0–9.0) |
| BASMIlin, 0–10 NRS | 2.6 ± 1.1 | 2.4 ± 1.1 | 2.7 ± 0.9 |
| MASES, 0–13 | 7.9 ± 3.1 | 7.9 ± 2.6 | 7.9 ± 2.6 |
| SJC (44 joints), median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| TJC (44 joints), median (IQR) | 2.0 (0.0–5.0) | 1.0 (0.0–4.0) | 2.5 (0.0–6.8) |
| TJC >1, no. (%) | 42 (53) | 18 (45) | 24 (60) |
| Patient global well‐being, NRS | 5.4 ± 2.4 | 5.2 ± 2.4 | 5.5 ± 2.5 |
| Patient pain, NRS | 5.2 ± 2.4 | 5.4 ± 2.5 | 5.1 ± 2.3 |
| SF‐36 PCS, 0–100 NRS | 40.8 ± 6.6 | 40.6 ± 6.9 | 41.0 ± 6.4 |
| SF‐36 MCS, 0–010 NRS | 40.0 ± 6.9 | 40.1 ± 7.0 | 39.9 ± 6.8 |
| MRI | |||
| SPARCC SI joint score (0–72), median (IQR) | 0.0 (0.0–3.1) | 0.0 (0.0–3.0) | 0.0 (0.0–3.3) |
| SPARCC SI joint positive (≥2.0), no. (%) | 18 (23) | 8 (21) | 10 (26) |
| ASAS positive, no. (%) | 14 (18) | 8 (21) | 6 (15) |
| Conventional radiography | |||
| BASRI (0–8), median (IQR) | 0.3 (0.0–0.5) | 0.3 (0.0–0.8) | 0.1 (0.0–0.5) |
| BASRI positive (≥2.0), no. (%) | 2 (3) | 2 (5) | 0 (0) |
| mSASSS (0–72), median (IQR) | 2.0 (0.0–3.0) | 2.0 (0.0–2.0) | 2.0 (1.0–5.0) |
| mSASSS positive (≥2.0), no. (%) | 41 (60) | 23 (68) | 18 (53) |
There were no statistically significant differences between groups. For all parameters except the swollen joint count (SJC) and tender joint count (TJC), data were not available from all 80 patients, as follows: For the Ankylosing Spondylitis Disease Activity Score using the C‐reactive protein level (ASDAS‐CRP), n = 67 (35 etanercept, 32 placebo). For the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), n = 78 (39 etanercept, 39 placebo). For the Bath Ankylosing Spondylitis Functional Index (BASMIlin), n = 74 (38 etanercept, 36 placebo). For CRP, n = 69 (36 etanercept, 33 placebo). For erythrocyte sedimentation rate (ESR), n = 69 (33 etanercept, 36 placebo). For the Bath Ankylosing Spondylitis Metrology Index (linear measure) (BASMIlin), (BASMI), n = 77 (38 etanercept, 39 placebo). For the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES), n = 29 (9 etanercept, 20 placebo). For patient global well‐being and patient pain assessments, n = 78 (39 etanercept, 39 placebo). For the Short Form 36 (SF‐36) physical component score (PCS) and mental component score (MCS), n = 78 (39 etanercept, 39 placebo). For magnetic resonance imaging (MRI)–based Spondyloarthritis Research Consortium of Canada (SPARCC) sacroiliac (SI) joint findings and positive diagnosis of spondyloarthritis according to the Assessment of SpondyloArthritis international Society (ASAS) criteria, n = 78 (39 etanercept, 39 placebo). For the Bath Ankylosing Spondylitis Radiology Index (BASRI), n = 77 (39 etanercept, 38 placebo). For the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS), n = 68 (34 etanercept, 34 placebo). Except where indicated otherwise, values are the mean ± SD. NRS = numerical rating scale; IQR = interquartile range; ULN = upper limit of normal.
Exposure and compliance
Within the 16‐week double‐blind period, 2 patients, both in the ETN group, discontinued the study (1 had an unrelated adverse event [AE] [streptococcal infection] and the other patient was lost to follow‐up) and 78 (97.5%) completed the treatment. Compliance with the study medication, i.e., the percentage of patients who took the medication according to the study protocol in the first 16 weeks, was 72.1%. There were no significant differences between the 2 treatment groups. In the follow‐up period without treatment (week 16 to week 24), 2 patients, both in the placebo group, discontinued (1 had a viral infection and the other found the study visits too burdensome), which resulted in an analyzable population of 76 patients (95.0%) at 24 weeks (Figure 1). No patients initiated or restarted ETN or another biologic treatment during the week 16–24 follow‐up period without study medication.
Clinical efficacy
At week 16, 10 of 72 patients (13.9%) had achieved an ASAS20 response: 6 (16.7%) in the ETN group and 4 (11.1%) in the placebo group. This difference was not statistically significant (RR 0.7 [95% CI 0.2–2.2], P = 0.5) (Table 4). ASAS40 response at 16 weeks was achieved in 6 of 72 patients (8.3%): 3 (8.3%) in each treatment group. An ASDAS‐CRP response (clinically important improvement and major improvement) was achieved in 12 of 62 patients (19.4%): 8 (25.0%) in the ETN group and 4 (13.3%) in the placebo group. This difference was also not statistically significant (RR 0.5 [95% CI 0.2–1.6], P = 0.2) (Table 4). Separate assessments of clinically important improvement and major improvement showed no significant differences between the 2 treatments at 16 weeks (RR 0.3 [95% CI 0.1–1.4], P = 0.1 and RR 2.1 [95% CI 0.2–22], P = 0.5, respectively) (Table 4). Low disease activity according to the ASDAS‐CRP at 16 weeks was achieved in 30 of 70 patients (42.9%): 44.1% in the ETN group and 41.7% in the placebo group (RR:0.9 [95% CI 0.6–1.6], P = 0.8). Inactive disease according to the ASDAS‐CRP was achieved in 13 of 70 patients (18.6%): 20.6% in the ETN group and 16.7% in the placebo group (RR 0.8 [95% CI 0.3–2.2], P = 0.7). During the first 16 weeks, both the ESR and the pain score showed more improvement in the ETN group than in the placebo group (mean ± SD change −2.2 ± 5.2 mm/hour versus −1.4 ± 7.4 mm/hour and −1.4 ± 2.7 versus −0.8 ± 2.7, respectively).
Table 4.
Rates of treatment response at 16 weeks*
|
Etanercept group, no. (%) |
Placebo group, no. (%) |
RR (95% CI) |
|
|---|---|---|---|
| Clinical response | |||
| ASAS20 | 6 (17) | 4 (11) | 0.7 (0.2–2.2) |
| ASAS40 | 3 (8) | 3 (8) | 1.00 (0.2–4.6) |
| ASDAS‐CRP response (CII and MI) | 8 (25) | 4 (13) | 0.5 (0.2–1.6) |
| ASDAS‐CRP (CII ≤1.1) | 7 (22) | 2 (7) | 0.3 (0.1–1.4) |
| ASDAS‐CRP (MI ≤2.0) | 1 (3) | 2 (7) | 2.1 (0.2–22) |
| ASDAS‐CRP (LDA <2.1) | 15 (44) | 15 (42) | 0.9 (0.6–1.6) |
| ASDAS‐CRP (ID <1.3) | 7 (21) | 6 (17) | 0.8 (0.3–2.2) |
| Imaging response | |||
| SPARCC SI joint MRI response (change ≥2.5) | 8 (24) | 7 (19) | 0.8 (0.3–2.0) |
None of the relative risk (RR) values were statistically significant. For ASAS response, n = 72 (36 etanercept, 36 placebo). For ASDAS‐CRP clinically important improvement (CII) and major improvement (MI), n = 62 (32 etanercept, 30 placebo). For ASDAS‐CRP low disease activity (LDA) and inactive disease (ID), n = 70 (34 etanercept, 36 placebo). For SPARCC SI joint MRI response, n = 72 (35 etanercept, 37 placebo). 95% CI = 95% confidence interval (see Table 3 for other definitions).
Between 16 weeks and 24 weeks, without study medication, the mean BASMI, CRP level, and ESR worsened more in the ETN group compared to the placebo group (mean ± SD change 1.6 ± 9.9 versus −0.3 ± 1.6, 1.8 ± 5.3 mg/liter versus −0.4 ± 2.7 mg/liter [P = 0.02], and 3.2 ± 9.8 mm/hour versus 0.03 ± 8.2 mm/hour, respectively) (see Supplementary Table 1, on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41607/abstract). Mean disease activity scores are presented in Figure 2.
Figure 2.
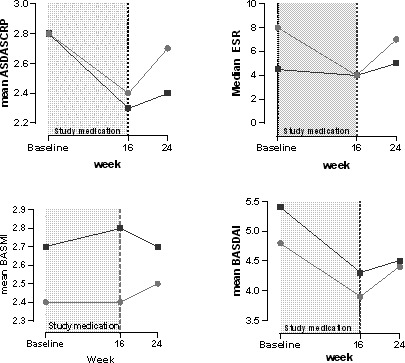
Disease activity by treatment group. Patients were treated for 16 weeks with etanercept (light gray circles) or placebo (dark gray squares). ASDAS‐CRP = Ankylosing Spondylitis Disease Activity Score using the C‐reactive protein level; ESR = erythrocyte sedimentation rate (mm/hour); BASMI = Bath Ankylosing Spondylitis Metrology Index; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index.
MRI findings
Reliability of results between MRI readers was confirmed by ICC analysis. ICCs at baseline, 16 weeks, and 24 weeks were 0.76, 0.72, and 0.70, respectively. Positive SI joint MRI findings according to the ASAS definition were observed in 14 of 78 patients (17.9%) at baseline, 7 of 72 (9.7%) at 16 weeks, and 9 of 73 (12.3%) at 24 weeks. Comparison of the percentage of patients with positive SI joint MRI findings by treatment group revealed no significant differences (at baseline 20.5% in the ETN group versus 15.4% in the placebo group, at 16 weeks 8.6% in the ETN group versus 10.8% in the placebo group, and at 24 weeks 14.3% in the ETN group versus 10.5% in the placebo group; RR [95% CI] 0.8 [0.3–2.0], 1.3 [0.3–5.2], and 0.7 [0.2–2.5], respectively).
Median SPARCC scores in the total study population were 0.0 (IQR 0.0–3.1), 0.0 (IQR 0.0–0.0), and 0.0 (IQR 0.0–1.5) at baseline, 16 weeks, and 24 weeks, respectively. Differences between groups were negligible and not statistically significant at baseline and 16 weeks. At 24 weeks, the difference in the change in SPARCC score (median 0.0 [IQR 0.0–1.0] in the ETN group versus 0.0 [IQR 0.0–0.0] in the placebo group) appeared statistically significant (P = 0.03). Positive SI joint MRI findings according to the SPARCC score (score ≥2.0) were observed in 18 of 78 patients (23.1%) at baseline, 10 of 72 (13.9%) at 16 weeks, and 9 of 73 (12.3%) at 24 weeks. Comparison of the percentage of patients with positive SI joint MRI findings according to SPARCC score by treatment group revealed no significant differences (at baseline 20.5% in the ETN group versus 25.6% in the placebo group, at 16 weeks 8.6% in the ETN group versus 10.8% in the placebo group, and at 24 weeks 10.5% in the ETN group versus 14.3% in the placebo group; RR [95% CI] 1.3 [0.6–2.8], 0.9 [0.8–1.2], and 0.7 [0.2–2.5], respectively).
At 16 weeks, a minimally important change in the SPARCC‐score (decrease of ≥ 2.5) had been achieved in 8 patients (23.5%) in the ETN group and 7 patients (19.4%) in the placebo group (RR 0.8 [95% CI 0.3–2.0], P = 0.7) (Table 4).
Subgroup analyses
Fulfillment of the ASAS20 response after 16 weeks was not related to an elevated CRP level (≥10.0 mg/liter) or a positive finding on SI joint MRI according to the SPARCC score or ASAS definition, or to both an elevated CRP level and a positive finding on SI joint MRI. Of the 10 patients who had achieved an ASAS20 response at 16 weeks, 2 had an elevated CRP level at baseline: 1 of 4 in the placebo group (25.0%) and 1 of 6 in the ETN group (16.7%). Comparison between patients with a positive finding on SI joint MRI according to the ASAS definition revealed no differences between treatment groups. Three patients with a positive finding on SI joint MRI according to the ASAS definition achieved an ASAS20 response: 1 of 4 in the placebo group (25.0%) and 2 of 6 in the ETN group (33.3%). Results were similar when a positive finding on SI joint MRI was assessed using the SPARCC score. In both the ETN group and the placebo group, only 1 patient with both an elevated CRP level and a positive finding on SI joint MRI achieved an ASAS20 response. In addition, ASAS20 response at 16 weeks was not influenced by sex, age, NSAID use, DMARD use, HLA–B27 status, or history of IBD, uveitis, or psoriasis.
Safety
At 16 weeks, AEs were reported in 30 of 78 patients (38.5%): 15 of 38 (39.5%) in the ETN group and 15 of 40 (37.5%) in the placebo group. Observed AEs were mainly diarrhea (20.0%), colds, and flu (both 10.0%). One patient was diagnosed as having a serious infection (streptococcal infection) and was withdrawn from the study. In 8 of 30 cases (26.7%) a possible relationship to the study drug was considered, and in 4 of 30 cases (13.3%) the AE was classified as being probably related to the drug. A higher proportion of patients in the ETN group had an AE that was possibly or probably related to the study drug compared to the placebo group (7 [50%] versus 5 [31.0%]). In 6 of 30 cases (20.0%), treatment for the AE was needed. Study drug was temporarily stopped in only 2 of the 30 cases (6.7%), both in the placebo group. At 24 weeks, 21 of 76 patients (27.6%) reported having had an AE; these were probably not related to study treatment since no patient received a biologic between week 16 and week 24. However, 1 patient experienced a viral infection that was serious enough for the patient to discontinue study participation. One patient experienced an exacerbation of IBD.
DISCUSSION
In this study, 16 weeks of treatment with ETN in patients with suspected nonradiographic axial SpA and reportedly high disease activity, but without the requirement of a positive MRI finding and/or elevated CRP level, did not result in significant improvement of disease activity compared to placebo. To date there have been only 2 other published placebo‐controlled trials that included patients with nonradiographic axial SpA with high disease activity without the requirement of elevated CRP level and/or inflammatory lesions seen on SI joint MRI (21, 22). Both studies had a slightly higher proportion of HLA–B27–positive patients compared to our study (75% versus 60%). One study had a high percentage of patients with active MRI lesions at baseline (63% of the 46 patients) whereas the other had a lower percentage of patients with positive findings (32% of 200 patients), as in our study (23% of 80 patients). The numbers of patients with an increased CRP level are difficult to compare between studies, as each study used a different definition of elevated CRP level, ranging from ≥6 mg/liter to ≥10 mg/liter.
In the earlier studies a significantly higher rate of ASAS20 response was observed in the groups treated with TNFi compared to placebo (54.5–71.1% versus 12.5–40.0%), which contrasts with our present findings. In our study, the frequency of improvement in disease activity scores (ASAS20 and ASDAS‐CRP) was not significantly different in the ETN group compared to the placebo group. Comparison of the ASAS20 and ASDAS‐CRP response within the ETN group showed a slightly higher percentage of responders according to the ASDAS‐CRP (25%) than according to the ASAS20 (17%). This might reflect the influence of TNFi on CRP levels rather than on other outcome parameters.
In the study by Haibel and colleagues (21), the proportion of patients with positive SI joint MRI findings at baseline was much higher than was observed in the present study (63% versus 32%). Being HLA–B27 positive and/or having active inflammatory lesions seen on SI joint MRI at baseline were predictors of an ASAS20 response in previous studies (20, 21, 41, 42). Subanalyses by HLA–B27 status in our study revealed that B27‐positive patients slightly more frequently had a positive SI joint MRI result according to the SPARCC score (26% versus 19%) and/or an elevated CRP level (≥10.0 mg/liter) compared to B27‐negative patients. Due to the small number of patients in our study who had positive SI joint MRI findings at baseline, we were unable to detect differences in treatment efficacy between patients with and those without a positive SI joint MRI result and/or increased CRP levels. The relatively low number of patients with either a positive SI joint MRI finding (23%) and/or elevated CRP level (13%) at baseline in our study could be an explanation for the absence of an observed treatment effect in favor of ETN.
In addition, features of the patients included in this study might have more overlap with the “axial SpA group with peripheral signs,” as described in a recent publication by Sepriano and colleagues (43), than with “pure axial SpA.” This assumption is based on the low prevalence of positive SI joint MRI findings, high MASES scores, high number of patients with a tender joint count of >1 (52%), high proportion of female patients (64%), and high prevalence of psoriasis (38%) and IBD (38%), which are often associated with peripheral symptoms.
Our study cohort was relatively unique compared to most populations used in clinical trials of biologic treatments in axial SpA. With this unique study population some limitations emerged. For example, according to the algorithm described by Rudwaleit et al (7), many of the patients in our study population had a high probability (up to 90%) of developing a form of axial SpA, and this may be one of the reasons we did not demonstrate significant results regarding efficacy of ETN treatment. A longer‐term study (3‐year follow‐up) is underway, which should allow us to further characterize the disease progression in this population. Questions could be raised as to whether our inclusion and exclusion criteria captured patients with true nonradiographic axial SpA. Although our patients are typical of those commonly seen in clinical practice, published scientific data are scant. This study adds to the body of evidence and provides some direction with regard to prescription of TNFi treatment in this patient group. The terminology “disease activity” was used by default, although we realize the disease activity outcome measures used have not been validated for this study population, and what we actually measured was the “level of symptoms” (25). Another limitation is that we learned, during analysis of the data, that the study was underpowered to compare patients with versus those without a positive SI joint MRI finding and/or elevated CRP level, although the data were sufficient to analyze differences in disease activity between the 2 treatment groups. Furthermore, there was a long period of enrollment, due to the use of only one study center. The fact that we included only one center might, however, increase the reliability of the results by limiting the number of observers, and we have no reason to believe a faster enrollment rate would have influenced the study results.
In conclusion, the present results indicate that early treatment with ETN is not effective in patients with suspected nonradiographic axial SpA without the requirement of a positive MRI result or increased CRP level. It would be of interest to know whether our findings can be replicated in future investigations with comparable study populations and equal proportions of patients with and without positive SI joint MRI findings and elevated CRP levels.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Ms Rusman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Van der Weijden, Bet, van der Horst‐Bruinsma.
Acquisition of data
Rusman, van der Weijden, Landewé, de Winter, Boden, van der Bijl, van der Horst‐Bruinsma.
Analysis and interpretation of data
Rusman, van der Weijden, Nurmohamed, Landewé, de Winter, Boden, van der Laken, van der Horst‐Bruinsma.
Supporting information
Supplementary Material
EudraCT database no. 2009‐015515‐40.
Supported by an unrestricted grant from Pfizer and ReumaNederland.
Dr. Landewé has received consulting fees and/or honoraria from AbbVie, Bristol Myers Squibb, Celgene, GlaxoSmithKline, UCB, Amgen, Lilly, Pfizer, Novartis, Galápagos, Gilead, and Janssen (less than $10,000 each) and research grants from AbbVie, Centocor, Novartis, Pfizer, and UCB. Dr. van der Horst‐Bruinsma has received consulting fees or speaking fees from AbbVie, Bristol Myers Squibb, Pfizer, UCB, MSD, Lilly, and Novartis (less than $10,000 each) and unrestricted research grants from MSD, Pfizer, and AbbVie for investigator‐initiated studies. No other disclosures relevant to this article were reported.
References
- 1. Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis. Part II. Validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 2. Deodhar A, Reveille JD, van den Bosch F, Braun J, Burgos‐Vargas R, Caplan L, et al. The concept of axial spondyloarthritis: joint statement of the Spondyloarthritis Research and Treatment Network and the Assessment of SpondyloArthritis international Society in response to the US Food and Drug Administration’s comments and concerns. Arthritis Rheumatol 2014;66:2649–56. [DOI] [PubMed] [Google Scholar]
- 3. Kiltz U, Baraliakos X, Karakostas P, Igelmann M, Kalthoff L, Klink C, et al. Do patients with non‐radiographic axial spondylarthritis differ from patients with ankylosing spondylitis? Arthritis Care Res (Hoboken) 2012;64:1415–22. [DOI] [PubMed] [Google Scholar]
- 4. Robinson PC, Bird P, Lim I, Saad N, Schachna L, Taylor AL, et al. Consensus statement on the investigation and management of non‐radiographic axial spondyloarthritis (nr‐axSpA). Int J Rheum Dis 2014;17:548–56. [DOI] [PubMed] [Google Scholar]
- 5. Rudwaleit M, Sieper J. Referral strategies for early diagnosis of axial spondyloarthritis [review]. Nat Rev Rheumatol 2012;8:262–8. [DOI] [PubMed] [Google Scholar]
- 6. Rudwaleit M, Haibel H, Baraliakos X, Listing J, Märker‐Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717–27. [DOI] [PubMed] [Google Scholar]
- 7. Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis 2004;63:535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sieper J, Rudwaleit M. Early referral recommendations for ankylosing spondylitis (including pre‐radiographic and radiographic forms) in primary care. Ann Rheum Dis 2005;64:659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Proft F, Poddubnyy D. Ankylosing spondylitis and axial spondyloarthritis: recent insights and impact of new classification criteria. Ther Adv Musculoskelet Dis 2018;10:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rudwaleit M, van der Heijde D, Landewe R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31. [DOI] [PubMed] [Google Scholar]
- 11. Gazeau P, Cornec D, Timsit MA, Dougados M, Saraux A. Classification criteria versus physician’s opinion for considering a patient with inflammatory back pain as suffering from spondyloarthritis. Joint Bone Spine 2018;85:85–91. [DOI] [PubMed] [Google Scholar]
- 12. Deodhar A, Reveille JD, van den Bosch F, Braun J, Burgos‐Vargas R, Caplan L, et al. The concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the Assessment of SpondyloArthritis international Society in response to the US Food and Drug Administration’s comments and concerns. Arthritis Rheumatol 2014;66:2649–56. [DOI] [PubMed] [Google Scholar]
- 13. Sieper J, van der Heijde D, Dougados M, Mease PJ, Maksymowych WP, Brown MA, et al. Efficacy and safety of adalimumab in patients with non‐radiographic axial spondyloarthritis: results of a randomised placebo‐controlled trial (ABILITY‐1). Ann Rheum Dis 2013;72:815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Braun J, Baraliakos X, Kiltz U, Heldmann F, Sieper J. Classification and diagnosis of axial spondyloarthritis: what is the clinically relevant difference? J Rheumatol 2015;42:31–8. [DOI] [PubMed] [Google Scholar]
- 15. De Winter J, de Hooge M, van de Sande M, de Jong H, van Hoeven L, de Koning A, et al. Magnetic resonance imaging of the sacroiliac joints indicating sacroiliitis according to the Assessment of SpondyloArthritis international Society definition in healthy individuals, runners, and women with postpartum back pain. Arthritis Rheumatol 2018;70:1042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Varkas G, de Hooge M, Renson T, De Mits S, Carron P, Jacques P, et al. Effect of mechanical stress on magnetic resonance imaging of the sacroiliac joints: assessment of military recruits by magnetic resonance imaging study [published erratum appears in Rheumatology (Oxford) 2018;57:588]. Rheumatology (Oxford) 2018;57:508–13. [DOI] [PubMed] [Google Scholar]
- 17. Weber U, Jurik AG, Zejden A, Larsen E, Jørgensen SH, Rufibach K, et al. Frequency and anatomic distribution of magnetic resonance imaging features in the sacroiliac joints of young athletes: exploring "background noise" toward a data‐driven definition of sacroiliitis in early spondyloarthritis. Arthritis Rheumatol 2018;70:736–45. [DOI] [PubMed] [Google Scholar]
- 18. Moltó A, Paternotte S, Claudepierre P, Breban M, Dougados M. Effectiveness of tumor necrosis factor α blockers in early axial spondyloarthritis: data from the DESIR cohort. Arthritis Rheumatol 2014;66:1734–44. [DOI] [PubMed] [Google Scholar]
- 19. Van der Heijde D, Baraliakos X, Hermann KA, Landewé RB, Machado PM, Maksymowych WP, et al. Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4‐year imaging outcomes from the RAPID‐axSpA phase III randomised trial. Ann Rheum Dis 2018;77:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gulfe A, Kapetanovic MC, Kristensen LE. Efficacy and drug survival of anti‐tumour necrosis factor‐α therapies in patients with non‐radiographic axial spondyloarthritis: an observational cohort study from Southern Sweden. Scand J Rheumatol 2014;43:493–7. [DOI] [PubMed] [Google Scholar]
- 21. Haibel H, Rudwaleit M, Listing J, Heldmann F, Wong RL, Kupper H, et al. Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve‐week randomized, double‐blind, placebo‐controlled trial followed by an open‐label extension up to week fifty‐two. Arthritis Rheum 2008;58:1981–91. [DOI] [PubMed] [Google Scholar]
- 22. Sieper J, van der Heijde D, Dougados M, Maksymowych WP, Scott BB, Boice JA, et al. A randomized, double‐blind, placebo‐controlled, sixteen‐week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2015;67:2702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson JJ, Baron G, van der Heijde D, Felson DT, Dougados M. Ankylosing Spondylitis Assessment Group preliminary definition of short‐term improvement in ankylosing spondylitis. Arthritis Rheum 2001;44:1876–86. [DOI] [PubMed] [Google Scholar]
- 24. Garrett S, Jenkinson T, Kennedy LG, Whiteloc H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 25. Kilic E, Kilic G, Akgul O, Ozgocmen S. Discriminant validity of the Ankylosing Spondylitis Disease Activity Score (ASDAS) in patients with non‐radiographic axial spondyloarthritis and ankylosing spondylitis: a cohort study. Rheumatol Int 2015;35:981–9. [DOI] [PubMed] [Google Scholar]
- 26. Lukas C, Landewé R, Sieper J, Dougados M, Davis J, Braun J, et al, for the Assessment of SpondyloArthritis international Society . Development of an ASAS‐endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18–24. [DOI] [PubMed] [Google Scholar]
- 27. Calin A, Porta J, Fries JF, Schurman DJ. Clinical history as a screening test for ankylosing spondylitis. JAMA 1977;237:2613–4. [PubMed] [Google Scholar]
- 28. Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991;34:1218–27. [DOI] [PubMed] [Google Scholar]
- 29. Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 30. Rusman T, John MB, van der Weijden MA, Boden BJ, van der Bijl CM, Bruijnen ST, et al. Presence of active MRI lesions in patients suspected of non‐radiographic axial spondyloarthritis with high disease activity and chance at conversion after a 6‐month follow‐up period. Clin Rheumatol 2020;39:1521–9. [DOI] [PubMed] [Google Scholar]
- 31. Jones SD, Steiner A, Garrett SL, Calin A. The Bath Ankylosing Spondylitis Patient Global Score. Rheumatology (Oxford) 1996;35:66–71. [DOI] [PubMed] [Google Scholar]
- 32. Ware JE Jr, Snow KK, Kosinski M, Gandek B. SF‐36 health survey: manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 33. Heuft‐Dorenbosch L, Spoorenberg A, van Tubergen A, Landewé R, van der Tempel H, Mielants H, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 2003;62:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Machado P, Landewé R, Lie E, Kvien TK, Braun J, Baker D, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut‐off values for disease activity states and improvement scores. Ann Rheum Dis 2011;70:47–53. [DOI] [PubMed] [Google Scholar]
- 35. Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- 36. Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS): the Bath AS Metrology Index. J Rheumatol 1994;21:1694–8. [PubMed] [Google Scholar]
- 37. Maksymowych WP, Lambert RG, Ostergaard M, Pedersen SJ, Machado PM, Weber U, et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann Rheum Dis 2019;78:1550–8. [DOI] [PubMed] [Google Scholar]
- 38. Rudwaleit M, Jurik AG, Hermann KG, Landewe R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. [DOI] [PubMed] [Google Scholar]
- 39. Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Williams M, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:703–9. [DOI] [PubMed] [Google Scholar]
- 40. Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Williams M, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:703–9. [DOI] [PubMed] [Google Scholar]
- 41. Marzo‐Ortega H, McGonagle D, O’Connor P, Hensor EM, Bennett AN, Green MJ, et al. Baseline and one year magnetic resonance imaging of the sacroiliac joint and lumbar spine in very early inflammatory back pain: relationship between symptoms, HLA‐B27, and disease extent and persistence. Ann Rheum Dis 2008;68:1721–7. [DOI] [PubMed] [Google Scholar]
- 42. Van Onna M, Jurik AG, van der Heijde D, van Tubergen A, Heuft‐Dorenbosch L, Landewé R. HLA‐B27 and gender independently determine the likelihood of a positive MRI of the sacroiliac joints in patients with early inflammatory back pain: a 2‐year MRI follow‐up study. Ann Rheum Dis 2011;70:1981–5. [DOI] [PubMed] [Google Scholar]
- 43. Sepriano A, Ramiro S, van der Heijde D, van Gaalen F, Hoonhout P, Molto A, et al. What is axial spondyloarthritis? A latent class and transition analysis in the SPACE and DESIR cohorts [published erratum appears in Ann Rheum Dis 2020;79:e78]. Ann Rheum Dis 2020;79:324–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


