Abstract
Interleukin (IL)‐10 has anti‐inflammatory and CD8+ T‐cell‐stimulating properties. Pegilodecakin (pegylated recombinant human IL‐10) induces intratumoral antigen‐specific CD8 + T‐cells and upregulates IFNγ and major histocompatibility complexes (MHC) I and II. Pegilodecakin has single‐agent activity with manageable toxicity in advanced renal cell carcinama (aRCC) (data cutoff 24 March 2016). Pegilodecakin with pembrolizumab or nivolumab revealed clinical activity in aRCC (data cutoff 1 July 2018). Here, we report for the first time the results of pegilodecakin+ pazopanib, and final results for monotherapy and long‐term follow‐up with pegilodecakin + anti‐programmed cell death 1 (anti‐PD‐1) inhibitors (data cutoff 19 February 2019). Phase 1/1b multi‐cohort dose escalation IVY study enrolled 353 patients. Sixty‐six patients with aRCC were treated with pegilodecakin alone or with pazopanib or anti‐PD‐1 inhibitor in cohorts A, G, H and I (data cutoff 19 February 2019). Primary endpoints included safety and tolerability. Secondary endpoint was tumor response by immune‐related response criteria (irRC). Pegilodecakin plus nivolumab or pembrolizumab yielded median progression‐free survival (mPFS) of 13.9 months and 6‐month PFS probability of 60%, 76% 1‐year overall survival (OS) probability and 61% 2‐year OS probability. Pegilodecakin monotherapy produced mPFS of 1.8 months, 6‐month PFS probability 25%, 1‐year OS 50%, and 2‐year OS 17%. Median OS was not reached in both combinations. Objective response rates (ORRs) were 33% with pazopanib and 43% with anti‐PD‐1. Most common Grade 3/4 treatment‐related adverse events included anemia, thrombocytopenia and hypertriglyceridemia. In these heavily pretreated renal cell carcinama cohorts of IVY, pegilodecakin+anti‐PD‐1 inhibitor showed promising clinical activity. Safety profile of pegilodecakin alone and with anti‐PD‐1 inhibitors was consistent as previously reported.
Keywords: nivolumab, pegilodecakin, pegylated IL‐10, pembrolizumab, renal cancer
Short abstract
What's new?
Despite recent progress in the treatment of renal cell cancer (RCC), there is still an urgent need for treatments that will further improve the prognosis of patients with advanced RCC. Pegilodecakin is a promising IL‐10 analogue that induces CD8+T‐cell‐mediated immune activation. Does a combination regimen yield better outcomes in heavily pretreated RCC patients than pegilodecakin alone? In this study, the authors found that the most promising clinical activity was obtained using a combination of pegilodecakin plus an anti‐PD‐1 inhibitor. Combination with a tyrosine‐kinase inhibitor of VEGFR was also better than pegilodecakin monotherapy.
Abbreviations
- AE
adverse event
- Anti‐PD‐1
anti‐programmed cell death 1
- aRCC
advanced renal cell carcinoma
- DLT
dose‐limiting toxicity
- ECOG
Eastern Cooperative Oncology Group
- IL‐10
interleukin‐10
- IMDC
International Metastatic Disease Consortium
- irPD
immune‐related progressive disease
- irRC
immune‐related response criteria
- mPFS
median progression‐free survival
- MTD
maximum tolerated dose
- ORR
objective response rate
- RCC
renal cell carcinoma
- RP2D
recommended Phase 2 dose
- TRAE
treatment‐related adverse event
1. INTRODUCTION
Since April 2018, the treatment landscape for advanced renal cell carcinoma (aRCC) has evolved with the approvals of the dual immune checkpoint inhibitors, nivolumab and ipilimumab, and the combinations of pembrolizumab plus axitinib and avelumab plus axitinib in the first‐line setting, with more combinations of a PD‐1 antibody plus a vascular endothelial growth factor receptor tyrosine kinase inhibitor (VEGFR‐TKI) expected to be approved in the near future. 1 , 2 Until recently, the mainstay therapy for patients with metastatic renal cell carcinama (RCC) was a first‐generation VEGFR‐TKI such as sunitinib or pazopanib followed by nivolumab, cabozantinib, axitinib or the combination of lenvatinib plus everolimus. 3 , 4 Despite the progress made in the developmental therapeutics of RCC, there is a substantial unmet need to improve the treatment outcomes of patients with aRCC. 5 In this context, the IVY study was designed and conducted using pegilodecakin as monotherapy, in combination with nivolumab or pembrolizumab, and in combination with pazopanib.
Pegilodecakin, a pegylated recombinant form of human interleukin‐10 (PEG‐hIL‐10), has demonstrated the ability to induce CD8 + T‐cell‐mediated immune activation. 6 , 7 Phase I study IVY explored the benefit of pegilodecakin either as monotherapy or in combination in patients with advanced solid tumors. 8 Patients with metastatic RCC were included and enrolled in cohorts with pegilodecakin alone (Cohort A), in combination with anti‐programmed cell death 1 (anti‐PD‐1) inhibitors (pembrolizumab [Cohort H]; nivolumab [Cohort I]) and in combination with pazopanib (Cohort G). Initial results revealed single‐agent activity in the investigated patient population with heavily pretreated RCC, 6 and objective responses were observed in the combination cohorts with anti‐PD‐1 inhibitors. 8 Here, we report the final results of the RCC cohorts of Phase I study IVY.
2. MATERIALS AND METHODS
Our study is part of a multi‐arm, Phase 1b dose escalation and expansion study (NCT02009449), which enrolled 353 patients in total (Supplemental Figure 1 depicts all cohorts of IVY). Patients diagnosed with histologically or cytologically confirmed advanced metastatic RCC (mRCC; N = 66) were included across three cohorts: cohort A (pegilodecakin monotherapy; N = 24; provided 2.5 μg/kg [n = 3]; 5 μg/kg [n = 1]; 10 μg/kg [n = 1]; or 20 μg/kg [n = 19]); Cohort G (pazopanib; N = 4; provided 10 μg/kg); Cohort H (pembrolizumab; N = 9; provided 10 μg/kg [n = 5] or 20 μg/kg [n = 4]); and Cohort I (nivolumab; N = 29; provided 20 μg/kg). Patients were ≥18 years of age, had ECOG PS 0 or 1, had ≥1 measurable lesion per irRC and had adequate organ function. Patients received study treatment until confirmed progressive disease (irPD) but could continue after confirmed irPD in the absence of clinical deterioration, if the investigator considered that the patient continued to receive benefit from the treatment.
At baseline, all patients underwent baseline investigations. Pegilodecakin was self‐administered subcutaneously daily. Pegilodecakin monotherapy dose escalation included 1 to 20 μg/kg, and the dose expansion phase of the study included 10 to 20 μg/kg in combination with pembrolizumab (2 mg/kg intravenously [IV] every 3 weeks) or with pazopanib (800 mg, po QD) and 20‐40 μg/kg pegilodecakin with nivolumab (3 mg/kg IV every 2 weeks). Tumor assessment per irRC occurred every 8 weeks, following the recommended dosing schedule. Toxicities were graded and recorded according to National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03 and were monitored until 30 days after last dose of treatment. Primary endpoints of the study included safety and tolerability, maximal tolerated dose (MTD) and pharmacokinetics (PK). Secondary endpoints included assessment of tumor response by irRC.
Data were summarized using descriptive statistics, including means, medians and ranges for continuous variables. Evaluable population was defined as patients who had initiated treatment and had an adequate baseline tumor assessment and at least one post‐baseline adequate tumor assessment. The ORR was complete response (CR) + partial response (PR); disease control rate was CR + PR + stable disease. AEs were evaluated in the safety population (all patients who received any study medication). Medical Dictionary for Regulatory Activities (MedDRA) Version 22.0 was used to code and CTCAE 4.03 was used to grade and report AEs. Overall survival (OS) and PFS estimates were determined using the Kaplan‐Meier method (Kaplan), and SAS software Version 9.4 was used to perform the analyses.
3. RESULTS AND DISCUSSION
Data cutoff was 19 February 2019 for the analyses, which included all patients with advanced pretreated RCC (N = 66) who received pegilodecakin either as monotherapy (cohort A: n = 24; 1‐20 μg/kg SC daily), with pazopanib (Cohort G: n = 4; pegilodecakin [10 μg/kg SC daily], pazopanib [800 mg po QD]), or with anti‐PD‐1 inhibitors (Cohort H/I: pegilodecakin [10‐20 μg/kg SC daily], pembrolizumab [Cohort H; n = 9; 2 mg/kg IV], nivolumab [Cohort I; n = 29; 3 mg/kg IV]). Pegilodecakin dosing in these dose expansion cohorts followed previously established recommended Phase II dose (RP2D). 6 The patients' median age was 62.5 years, and the majority were male with an initial diagnosis of Stage IV clear cell renal carcinoma (Table 1). Most patients had an intermediate‐risk per International Metastatic Disease Consortium (IMDC) category (Table 1). Median number of prior therapies ranged between 1.5 and 3, with the majority of patients (43/66; 65%) receiving ≥2 prior therapies (Supplemental Table 1). Tyrosine kinase inhibitors such as sunitinib, pazopanib and axitinib were the most common prior therapies (Supplemental Table 1). At the time of data cutoff, the majority (59/66 [89%]) of patients had discontinued study treatment. The most common reason for discontinuation was progressive disease (Cohort A: 15/24 [63%]; Cohort G: 2/4 [50%]; Cohort H/I: 16/38 [42%]), clinical deterioration (Cohort A: 5/24 [21%]; Cohort G: 1/4 [25%]; Cohort H/I: 4/38 [11%]) and adverse events (AEs) (Cohort A: 2/24 [8%]; Cohort G: [0%]; Cohort H/I: 5/38 [13%]).
TABLE 1.
Baseline characteristics
| Safety population | PEG monotherapy (N = 24) | PEG+pazopanib (N = 4) | PEG+anti‐PD‐1 a (N = 38) |
|---|---|---|---|
| Age, median, years (range) | 61 (22‐71) | 72 (52‐76) | 66 (32‐77) |
| Sex, n (%) | |||
| Male | 16 (67) | 3 (75) | 27 (71) |
| ECOG PS, n (%) | |||
| 0 | 14 (58) | 2 (50) | 12 (32) |
| 1 | 10 (42) | 2 (50) | 26 (68) |
| TNM stage at initial diagnosis, n (%)b | |||
| Stage I/II | 7 (29) | 0 | 9 (24) |
| Stage III | 5 (21) | 2 (50) | 13 (34) |
| Stage IV | 11 (46) | 2 (50) | 15 (40) |
| IMDC risk groups, n (%) | |||
| Favorable | 2 (8) | 1 (25) | 6 (16) |
| Intermediate | 18 (75) | 1 (25) | 29 (76) |
| Poor | 4 (17) | 2 (50) | 3 (8) |
| Prior therapy (median) | 3 | 1.5 | 2 |
| 0 | 1 (4) | 1 (25) | 6 (16) |
| 1 | 4 (17) | 1 (25) | 10 (26) |
| 2 | 6 (25) | 2 (50) | 9 (24) |
| ≥3 | 13 (54) | 0 | 13 (34) |
| Clear cell histology | 15 (63) | 2 (50) | 25 (66) |
Anti‐PD‐1 inhibitors included pembrolizumab and nivolumab. bOne patient in monotherapy and anti‐PD‐1 cohorts had TNM stage classified as “other”. Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; n, number of patients in group.
Of the evaluable patients (N = 58), the ORR was 43% (15/35) in the combination arm with anti‐PD‐1 inhibitors (15 PRs). ORR in the pazopanib combination cohort and monotherapy cohort was 33% (1 of 3) and 20% (4 of 20), respectively. Also, notable tumor reduction was observed in the anti‐PD‐1 combination cohorts (Figure 1A,B). One‐year OS probability was 76% with pegilodecakin+anti‐PD‐1 and 50% in the other cohorts (Figure 1C). Median PFS in Cohorts H and I was 13.9 months and 1.8 months and 3.7 months for Cohorts A and G, respectively (Figure 1D).
FIGURE 1.
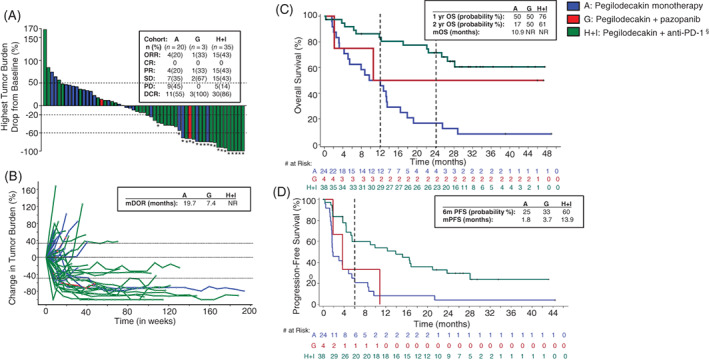
Patient response. A, Waterfall plot depicting change in tumor burden immune‐related response criteria (irRC) from pegilodecakin monotherapy (blue bars), pegilodecakin+pazopanib (red bars) or pegilodecakin+anti‐PD‐1 (green bars) therapy in patients with renal cell carcinoma (RCC). The waterfall plot includes 57 of 58 evaluable patients. One patient from the anti‐PD‐1 cohort had only nonmeasurable lesions and could not be included in the analysis. “*” symbol indicates patients who had a partial response per tumor response assessment based on irRC. § symbol indicates anti‐PD‐1 inhibitors that include pembrolizumab and nivolumab. Best overall response per irRC is displayed in a table inset for each cohort. ORR, overall response rate; CR, complete response; DCR, disease control rate; PD, progressive disease; PR, partial response; SD, stable disease. B, Spider plot depicting change in tumor burden per irRC in pegilodecakin monotherapy (blue), pegilodecakin+anti‐PD‐1 (green) and pegilodecakin+pazopanib (red) cohorts in patients with RCC. Median duration of response (mDOR) in months is displayed in table inset for all cohorts. NR, not reached. C, Kaplan‐Meier plot of overall survival for pegilodecakin monotherapy (blue), pegilodecakin+anti‐PD‐1 (green)and pegilodecakin+pazopanib (red) in all evaluable patients with RCC. Number of patients at risk over time is displayed below the plot. Table inset displays 1‐year, 2‐year and median overall survival probabilities. NR, not reached. D, Kaplan‐Meier plot of progression‐free survival for pegilodecakin monotherapy (blue), pegilodecakin+anti‐PD‐1 (green) and pegilodecakin+pazopanib (red) in all evaluable patients with RCC. Number of patients at risk over time is displayed below the plot. Table inset displays median PFS (mPFS) and 6‐month probability
Toxicity was similar to previously reported with pegilodecakin alone 6 or in combination with anti‐PD‐1 inhibitors. 8 The most common Grade 3/4 treatment‐related adverse events (TRAEs) included anemia (21/66 [32%]), thrombocytopenia (10/66 [15%]) and hypertriglyceridemia (9/66 [14%]; Table 2). Serious AEs were observed in 30/66 (45%) patients, with anemia (4/66 [6.1%]), dyspnea (4/66 [6.1%]), pyrexia (3/66 [4.5%]) and pneumonia (3/66 [4.5%]) as the most frequent.
TABLE 2.
Treatment‐related adverse events
| PEG monotherapy (N = 24) | PEG+pazopanib (N = 4) | PEG+anti‐PD‐1 (N = 38) | ||||
|---|---|---|---|---|---|---|
| n, (%) | Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 |
| Patients with ≥1 TRAE | 22 (92) | 14 (58) | 4 (100) | 4 (100) | 37 (97) | 26 (68) |
| Anemia | 15 (63) | 9 (38) | 0 | 0 | 25 (66) | 12 (32) |
| Thrombocytopenia | 9 (38) | 2 (8) | 0 | 0 | 18 (47) | 8 (21) |
| Neutropenia | 0 | 0 | 0 | 0 | 3 (8) | 3 (8) |
| Pancreatitis | 0 | 0 | 1 (25) | 1 (25) | 0 | 0 |
| Fatigue | 10 (42) | 2 (8) | 4 (100) | 0 | 15 (40) | 1 (3) |
| Platelet count decreased | 1 (4) | 1 (4) | 1 (25) | 1 (25) | 14 (37) | 3 (8) |
| Aspartate aminotransferase increased | 2 (8) | 2 (8) | 1 (25) | 1 (25) | 9 (24) | 2 (5) |
| Alanine aminotransferase increased | 2 (8) | 2 (8) | 1 (25) | 1 (25) | 6 (16) | 1 (3) |
| Lipase increased | 1 (4) | 0 | 1 (25) | 1 (25) | 5 (13) | 2 (5) |
| Blood alkaline phosphatase increased | 1 (4) | 1 (4) | 1 (25) | 1 (25) | 2 (5) | 0 |
| Amylase increased | 1 (4) | 0 | 0 | 0 | 3 (8) | 3 (8) |
| Hypertriglyceridemia | 7 (29) | 3 (13) | 0 | 0 | 14 (37) | 6 (16) |
| Pruritus | 3 (13) | 0 | 0 | 0 | 11 (29) | 2 (5) |
Note: The treatment‐related adverse events displayed in the table included only those with an incidence of ≥5% Grade 3/4/5.
Abbreviations: n, number of patients in group; PEG, pegilodecakin; TRAE, treatment‐related adverse event.
A previous dose‐escalation study investigated self‐administered subcutaneous dosing of pegilodecakin (1‐40 μg/kg once daily, accounting for weight) in sequential cohorts. 6 Although a formal maximum tolerated dose (highest dose <33% of patients experienced dose‐limiting toxicities [DLTs] in the first 28 days of treatment) was not established, the overall tolerability and efficacy results helped guide the RP2D used in subsequent dose expansion cohorts. In that study, a DLT of anemia was observed. 6
Here, we report for the first time the final and complete results of all patients with RCC included in the Phase I study IVY. The extended follow‐up of 7 months provided by this analysis using the final data cutoff of February 2019 further supported the previously observed results (data cutoff: July 2018), although the median OS still not reached. 8 Considering that the patients that were investigated in IVY were a heavily pretreated population, the ORR of 43% (15 of 35) with pegilodecakin plus anti‐PD‐1 inhibitors was promising compared to ORR of 20% in previous results with anti‐PD‐1 inhibitors alone in similar patient populations. 9 In preclinical models, recombinant human IL‐10 (rhIL‐10) has previously been shown to extend the life span of CD8+ T‐cells and enhance its cytotoxic activity, helping form our hypothesis that the combination of anti‐PD‐1 and rhIL‐10 could be synergistic and enhance clinical activity. 7
The observed toxicity profiles were similar to what were previously observed with pegilodecakin, 6 , 8 with anemia and thrombocytopenia being the most prevalent AEs. Importantly, the immune‐related toxicity of pegilodecakin with nivolumab in our study was low, with no observed pruritis or stomatitis and 3% incidence of pneumonitis and peripheral edema (data not shown). These hematologic AEs may be explained in part by pegilodecakin's proposed mechanism, which likely occurs through CD8+ T‐cell stimulation as well as reduction of tumor‐promoting inflammation. 10 Anemia has been previously observed in chronic inflammatory disorders, and cytokines such as IL‐10 may be the key players involved in its pathogenesis. 11 Also, thrombocytopenia has previously been observed in correlation with IL‐10 administration. 12 In the current study, toxicity presented in a dose‐dependent manner and did not show a notable increase with the addition of anti‐PD1 inhibitors or pazopanib, except for grade ≥3 thrombocytopenia (8/38 [21%; anti‐PD‐1 cohort] vs 2/24 [8%; monotherapy cohort]).
The main limitation of our study was the small sample size for efficacy assessment and lack of a comparator arm. However, these final and complete results of the RCC cohorts of IVY point to the clinical activity provided by the administration of pegilodecakin in combination with the anti‐PD‐1 inhibitors, pembrolizumab and nivolumab. Previous exploratory findings with pegilodecakin suggest increased IFN‐γ and IL‐18 may be critical to achieve clinical benefit in patients with metastatic RCC, with direct stimulation of memory CD8+ T‐cell proliferation by IL‐18. 7 Further investigation of pegilodecakin in combination with an anti‐PD‐1 antibody in RCC is warranted.
CONFLICT OF INTEREST
The following represents disclosure information provided by the authors. Nizar M. Tannir: Personal honorarium fees: Eli Lilly and Company; personal fees and/or grants outside the submitted work from Eisai Medical Research, Nektar Therapeutics, Exelixis, Inc., Oncorena, Calithera Bioscience, Surface Oncology, Novartis, and Ipsen. Kyriakos P. Papadopoulos: Research Funding: Abbvie, Amgen, ArQule, ARMO BioSciences, ADC Therapeutics, Anheart, 3D Medicines, Basilia, Bayer, Calithera Biosciences, Daiichi Sankyo, Eli Lilly and Company, EMD Serono, F‐star, Incyte, Jounce Therapeutics, Linnaeus, Mabspace Biosciences, Merck, Mirati Therapeutics, MedImmune, Mersana, Peleton Therapeutics, Regeneron, Syros Pharmaceuticals, Pfizer, Treadwell Therapeutics, and Tempest Therapeutics. Advisory Board Fees: Arqule, Basilia, Bayer. Deborah J. Wong: Grant/research funding paid to institution: ARMO BioSciences, a wholly owned subsidiary of Eli Lilly and Company, and Eli Lilly and Company. Raid Aljumaily: Consulting or Advisory Role outside the submitted work: AstraZeneca and Regeneron. Alexandra Drakaki: No conflict of interest related to this manuscript. Johanna Bendell: Research Funding During the Conduct of the Study: Eli Lilly and Company. Research Grants Outside the Submitted Work: EMD Serono, Koltan, SynDevRex, Forty Seven, Abbvie, Onyx, Takeda, Eisai, Celldex, CytomX, Nektar, Boston Biomedical, Tarveda, Tyrogenex, Marshall Edwards, Pieris, Mersana, Calithera, Blueprint, Merus, Jacobio, Effector, Novocare, Arrys, Tracon, Sierra, Therapeutics, Vyriad, Harpoon, ADC, Millennium, Imclome, Acerta Pharma, Rgenix, Bellicum, Gossamer Bio, Arcus Bio, Tempest Tx, Shattuck Labs, Synthorx, Inc, Revolution Medicines, Inc., Zymeworks, AtlasMedx, Scholar Rock, NGM Biopharma, Treadwell Therapeutics, IGM Biosciences, Mabspace, REPARE Therapeutics, NeoImmune Tech. Research Grants and Other Outside the Submitted Work: Gilead, Genentech/Roche, BMS, Five Prime, Eli Lilly and Company, Merck, MedImmune, Celgene, Taiho, Macrogenics, GSK, Novartis, OncoMed, LEAP, TG Therapeutics, AstraZeneca, BI, Daiichi Sankyo, Bayer, Incyte, Apexigen, Array, Sanofi, Agios, ARMO BioSciences a wholly owned subsidiary of Eli Lilly and Company, Ipsen, Merrimack, Oncogenex, Evelo, FORMA, Innate, Arch Oncology, Prelude Therapeutics, Amgen, Pfizer, Seattle Genetics, Bicycle Therapeutics, Relay Therapeutics. Consulting Fees to Institution: Phoenix Bio, Cyteir, Molecular Partners, Torque, Tizona, Janssen, Tolero, TD2 (Translational Drug Development), Moderna Therapeutics, Tanabe Research Laboratories, Beigene, Continuum Clinical, Amgen, Evelo, Piper Biotech, Samsung Bioepios, Fusion Therapeutics. Annie Hung, Manuel Afable, Jong Seok Kim, David Ferry: Employees of Eli Lilly and Company with stock holdings during the conduct of the study. Aung Naing; Research funding: NCI, EMD Serono, MedImmune, Healios Onc. Nutrition, Atterocor, Amplimmune, ARMO BioSciences, Eli Lilly, Kaeryopharm Therapeutics, Incyte, Novartis, Regeneron, Merck, BMS, Pfizer, CytomX Therapeutics, Neon Therapeutics, Calithera Biosciences, TopAlliance Biosciences, Kymab, PsiOxus, Arcus Biosciences, NeoimmuneTech, ImmuneOcia, Surface Oncology. Advisory board: CytomX Therapeutics, Novartics and Genome & Company, OncoSec KEYNOTE‐695, STCube Pharmaceuticals, Kymab. Travel and accommodation expenses: Armo Biosciences. Spouse: research funding from Immunde Deficiency Foundation, Jeffery Modell Foundation and Chao Physician‐scientist, and Baxalta; Advisory Board for Takeda, CSL Behring, and Horizon Pharma.
ETHICS STATEMENT
All procedures performed in the study involving human participants were in accordance with Good Clinical Practice Guidelines (GCP), the US Code of Federal Regulations governing the protection of human patients (21 CFR 50), International Conference on Harmonization guidelines, local ethical requirements consistent with the current version of the Declaration of Helsinki, and the Institutional Review Board or Independent Ethics Committees (IEC; 21 CFR 56). Informed consent was obtained from all study participants. Clinical trial registration number is NCT02009449.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGEMENTS
Kristi Gruver, employee of Eli Lilly and Company, provided medical writing assistance. We thank all the patients who contributed to this study and to all the staff who worked on this project. This study was a collaboration between researchers from different institutions: MD Anderson Cancer Center, Sarah Cannon Research Institute, University of California Los Angeles (UCLA), University of California San Francisco (UCSF), START Center for Cancer Care, Stephenson Cancer Center of the University of Oklahoma, and Eli Lilly and Company. The work was supported by ARMO BioSciences, a wholly owned subsidiary of Eli Lilly and Company.
Tannir NM, Papadopoulos KP, Wong DJ, et al. Pegilodecakin as monotherapy or in combination with anti‐PD‐1 or tyrosine kinase inhibitor in heavily pretreated patients with advanced renal cell carcinoma: Final results of cohorts A, G, H and I of IVY Phase I study. Int. J. Cancer. 2021;149:403–408. 10.1002/ijc.33556
Funding information Eli Lilly and Company
DATA AVAILABILITY STATEMENT
Lilly provides access to all individual data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org. Further information is available from the corresponding author upon request.
REFERENCES
- 1. Hasanov E, Gao J, Tannir NM. The immunotherapy revolution in kidney cancer treatment: scientific rationale and first‐generation results. Cancer J. 2020;26(5):419‐431. [DOI] [PubMed] [Google Scholar]
- 2. Atkins MB, Tannir NM. Current and emerging therapies for first‐line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev. 2018;70:127‐137. [DOI] [PubMed] [Google Scholar]
- 3. Tannir NM, Pal SK, Atkins MB. Second‐line treatment landscape for renal cell carcinoma: a comprehensive review. Oncologist. 2018;23(5):540‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Motzer RJ, Escudier B, George S, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long‐term follow‐up of the randomized, open‐label, phase 3 CheckMate 025 trial. Cancer. 2020;126(18):4156‐4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chowdhury SMD, Voss MH, Hawkins RE, et al. A phase I/II study to assess the safety and efficacy of pazopanib (PAZ) and pembrolizumab (PEM) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2017;35:4506. [Google Scholar]
- 6. Naing A, Papadopoulos KP, Autio KA, et al. Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin‐10 (AM0010) in patients with advanced solid tumors. J Clin Oncol. 2016;34(29):3562‐3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Naing A, Infante JR, Papadopoulos KP, et al. PEGylated IL‐10 (Pegilodecakin) induces systemic immune activation, CD8(+) T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell. 2018;34(5):775‐791. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naing A, Wong DJ, Infante JR, et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): a multicentre, multicohort, open‐label, phase 1b trial. Lancet Oncol. 2019;20(11):1544‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33(13):1430‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pal S, Hu‐Lieskovan S, Agarwal N. Can pegylated IL‐10 add to a backbone of PD‐1 inhibition for solid tumours? Lancet Oncol. 2019;20(11):1473‐1474. [DOI] [PubMed] [Google Scholar]
- 11. Tilg H, Ulmer H, Kaser A, Weiss G. Role of IL‐10 for induction of anemia during inflammation. J Immunol. 2002;169(4):2204‐2209. [DOI] [PubMed] [Google Scholar]
- 12. Sosman JA, Verma A, Moss S, et al. Interleukin 10‐induced thrombocytopenia in normal healthy adult volunteers: evidence for decreased platelet production. Br J Haematol. 2000;111(1):104‐111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
Lilly provides access to all individual data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org. Further information is available from the corresponding author upon request.


