Abstract
Microalgae have shown great potential as a source of biofuels, food, and other bioproducts. More recently, microfluidic devices have been employed in microalgae‐related studies. However, at small fluid volumes, the options for controlling flow conditions are more limited and mixing becomes largely reliant on diffusion. In this study, we fabricated magnetic artificial cilia (MAC) and implemented them in millimeter scale culture wells and conducted growth experiments with Scenedesmus subspicatus while actuating the MAC in a rotating magnetic field to create flow and mixing. In addition, surface of MAC was made hydrophilic using plasma treatment and its effect on growth was compared with untreated, hydrophobic MAC. The experiments showed that the growth was enhanced by ten and two times with hydrophobic and hydrophilic MAC, respectively, compared with control groups which contain no MAC. This technique can be used to investigate mixing and flow in small sample volumes, and the enhancement in growth can be beneficial for the throughput of screening studies. Moreover, the methods used for creating and controlling MAC can be easily adopted in labs without microfabrication infrastructures, and they can be mastered by people with little prior experience in microfluidics.
Keywords: artificial cilia, microalgae, microbioreactor, mixing
Magnetically actuated microscopic artificial cilia were implemented in millimeter‐scale culture wells for Scenedesmus subspicatus, and they were able to increase the growth rate of the microalgae by nearly ten times. Hydrophobicity of the cilia surface was also found to significantly influence the enhancement of growth. The technique of creating and controlling the artficial cilia can be easily mastered and adopted in labs without microfabrication infrastructure.

1. INTRODUCTION
Cilia and flagella are microscopic cell protrusions that are ubiquitously found in nature (Gardiner, 2005; Gibbons, 1981). Motile cilia perform essential functions such as generating fluid flow, locomotion, and transportation, usually at a scale where inertial effects can be neglected, under so‐called low Reynolds number conditions (Ibanez‐Tallon, 2003). For more than a decade, researchers have designed and manufactured various kinds of artificial cilia systems that mimick the function of natural cilia (den Toonder & Onck, 2013). Different materials and techniques have been used to create them, making them responsive to various types of stimuli, such as electric field (den Toonder et al., 2008), light (van Oosten et al., 2009), and magnetic fields (Babataheri et al., 2011; Evans et al., 2007; Khaderi et al., 2011; Zhang et al., 2018). These artificial cilia have been demonstrated to perform nature‐mimicking functions such as mixing (Shields et al., 2010; den Toonder et al., 2008), fluid pumping (Belardi et al., 2011; Wang et al., 2013; Wang et al., 2014; Zhang et al., 2018), particle transportation (Zhang et al., 2020), locomotion (McGary et al., 2006), and antibiofouling (Zhang et al., 2020), usually in low Reynolds number, microfluidic environments. The potential of artificial cilia have also been shown in various other scientific and engineering applications, for example, in making microrobots (Kim et al., 2016).
Microalgae, with their high efficiency in energy conversion and relatively low requirement on land and fresh water for production, have shown great potential as a carbon‐neutral and renewable source of biofuels (Maity et al., 2014; Schenk et al., 2008). They are also a great source of food and other bioproducts, such as bioantibiotics and vitamins (Borowitzka, 1995; Khan et al., 2018). For production, microalgae are usually cultivated at scale, in open ponds or closed photobioreactors (Murthy, 2011). More recently, various microfluidic devices and microchambers have been employed in microalgae‐related studies (Kim et al., 2018), for example, in screening for optimum growth conditions such as the light cycle, wavelengths, and intensity (Kim et al., 2014; Shih et al., 2014), pH (Gebhardt et al., 2011), temperature (Saad et al., 2019), and for studying growth kinetics (Dewan et al., 2012), culture purification (Godino et al., 2015; Syed et al., 2018), and harvesting (Hønsvall et al., 2016).
While combining microfluidics with algae research offers the possibilities for high‐throughput screening, easier observation, and single‐cell level analysis, which are demonstrated in the studies above, the characteristics associated with small fluid volumes also bring the intrinsic challenges of controlling flow conditions and mixing (Ottino & Wiggins, 2004; Stroock & Whitesides, 2003; Whitesides, 2006), which are important for the growth and other bioactivities of microalgae (Barbosa et al., 2003; Qiang & Richmond, 1996). Mixing in centimeter‐scale bioreactors or normal flasks can be easily facilitated by conventional stirrers, bubble injection (Barbosa et al., 2003), or static mixers (Huang et al., 2014). However, in bioreactors with (sub)millimeter, or micro‐ and nanoliter scales, for example in microfluidic chambers, droplets, and flow channels, the options for mixing are more limited and mixing becomes largely reliant on diffusion. There are a few examples of mixing in microbioreactors in the literature including using digital microfluidics (Au et al., 2011), flow focusing devices (Johnson‐Chavarria et al., 2014), and static structures in microchannels to either directly mix (Qu et al., 2012) or allow cell trapping for fluid refreshment (Luke et al., 2016). However, most of these devices require special equipment to fabricate, and to make and use them properly also requires a significant amount of experience and know‐how.
In this article, we report using magnetic artificial cilia (MAC) to enhance microalgae Scenedesmus subspicatus growth in microliter‐scale open wells by nearly 10 times, and also examine the effect of surface hydrophobicity of MAC on the growth enhancement. S. subspicatus are nonmotile, colonial fresh water microalgae, and are often used in ecotoxicology and biofuel production research (Christenson & Sims, 2011), as well as in microfluidic based studies (Kwak, Kim, Na, et al., 2016; Kwak, Kim, Woo, et al., 2016). We chose a fabrication technique for MAC and a magnetic actuation method that can be easily adopted in almost every lab, and propose devices that can be setup by people with limited prior knowledge of MAC or in microfluidics.
2. MATERIALS AND METHODS
This section first provides an overview of experimental procedure, followed by details of the fabrication method of MAC, surface treatment, the magnetic actuation, culturing of microalgae, and cell counting method.
2.1. Overview of experimental procedure
The manufacturing method of our MAC is based on magnetic fiber drawing, in which MAC are drawn from a thin layer of liquid polydimethylsiloxane (PDMS) containing carbonyl iron powder (CIP), using a permanent magnet (Figure 1a), as described in detail by Wang et al. (2014). This method allows MAC to be quickly and cheaply fabricated from widely available material and with minimal requirements on infrastructure. However, although some degree of control is possible (Wang et al., 2014), the dimension and distribution of drawn cilia are variable and random (Figure 1c,d), compared with other methods such as molding (S. Zhang et al., 2018). Nevertheless, they can be suitable for applications that do not require such control, such as in this study. The fabricated MAC patches were then placed in open wells made from modified petri dishes for later use (Figure 1a).
Figure 1.
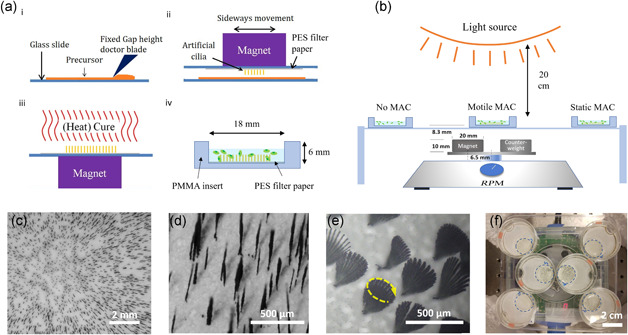
Magnetic artificial cilia (MAC) fabrication and the setup for microalgae experiments. (a) MAC fabrication steps (modified from Wang et al., 2014). i: applying a PDMS‐CIP precursor layer with a fixed thickness; ii: MAC made by magnetic fiber‐drawing on a PES filter paper substrate (see Movie S1); iii: thermal curing of the PDMS; iv: ciliated substrate removed from the supporting glass slide and fixed on the bottom of an 18 mm culturing well by placing a polymethylmethacrylate (PMMA) insert in a 35 mm petri dish. (b) Microalgae culture setup: a modified magnetic stirrer actuates the sample placed above the rotating magnet, while the samples on the sides are not actuated. (c) A representative image of MAC on a substrate. (d) Enlarged view of MAC, showing inhomogeneous distribution and lengths. (e) A superimposed time‐lapse image showing a rotation cycle of MAC in water at 2000 rpm, with a movement trace resembling a cone. In this case the cone is tilted with respect to the surface (see Movie S2). (f) A typical example of sample placement: left‐bottom and right‐top ones without MAC, middle ones with motile MAC and the left‐top and right‐bottom ones with static MAC. The 18 mm diameter wells (blue dotted lines) are created by placing lasercut 6 mm thick PMMA inserts in the 35 mm petri dishes. PES, polyethersulfone; PDMS‐CIP, polydimethylsiloxane containing carbonyl iron powder [Color figure can be viewed at wileyonlinelibrary.com]
A modified magnetic mixer with a neodymium magnet replacing the original one was used for actuating the MAC (Figures 1b and 1f). The rotation of the magnetic field induces conical motions of the cilia, which creates flow and mixing in the fluid (Figure 1e). The centroids of the cone from the bases of the MAC can be tilted with respect to the surface. The tilting angle depends on the position of the particular cilium with respect to the magnet (Wang et al., 2014). At a rotational frequency of 2000 rpm used in this study, the tip speed of MAC can reach about 30 mm/s, inducing a strong local shear rate in the order of 102 s−1, and the local Reynolds number in the vicinity of each cilium is about 10 (see Supplementary Information SI for the calculation). Note that macroscopic flows with similar scale in characteristic numbers were found to enhance the growth of microalgae in much larger containers (Hosaka et al., 1995; Leupold et al., 2013).
For microalgae growth experiments, S. subspicatus were cultured in MAC‐integrated and control petri dishes (Figure 1b) for multiple days under the same light condition (12–12 h day–night cycle). The photosynthetic active radiation (PAR) intensity at the wells position was estimated to be around 104 µmol·m−2s−1, based on manufacturer's data and an estimated 85° light cone. It is believed to be a reasonable light condition, compared to PAR under natural conditions for these fresh water green algae (Mõttus et al., 2012).
For each set of experiments, control groups were added as references. For all experiments, two control groups were used, namely wells without MAC and wells with static MAC (Figures 1b and 1f). In this way, any surface or material induced effects on growth can be filtered out.
2.2. Fabrication of MAC
The MAC manufacturing method used in this project is based on magnetic fiber drawing, in which MAC are drawn from a thin layer of a liquid precursor using a permanent magnet (Wang et al., 2014). The liquid precursor is a mixture of thermally curable polydimethysiloxane (PDMS, Sylgard 184, Dow Corning, base to curing agent weight ratio 10:1) and magnetic microparticles, carbonyl iron powder (CIP, 99:5%, Sigma‐Aldrich). The PDMS base was first mixed by hand with CIP in 10:1 weight ratio, then curing agent was added and mixed right before MAC fabrication. A thin film of this mixture was deposited on a 50 × 75 mm glass slide using a fixed‐height doctor blade (Erichsen Quadruple Film Applicator Model 360, gap size 100 or 150 µm). On another glass slide, a piece of polyethersulfone (PES) filter paper (Merck Millipore Express PLUS 0.45 µm) was taped with the dull side up. PES membrane was chosen to be the substrate because of its biocompatibility and porous nature, which is beneficial for the attachment of MAC. A magnet (20 × 20 × 10 mm N48 Neodymium, Supermagnete) was taped to the other side of this glass slide. The MAC were then drawn from the precursor layer by bringing the two glass slides (filter facing precursor layer) close to each other. This step is a manual process, controlled by changing the distance between the glass slides and sideways movement while observing through a stereo microscope. The sideways movement is a combination of larger movements to position the magnet to a different location with respect to the thin film, and more local, faster sideways motion. The thickness, length and number density of MAC are influenced by the precursor layer mixture and thickness, and the time duration and distance of the drawing process. By applying sideways movement, the MAC can be drawn with a more homogeneous distribution on the filter. Without it, a higher density of MAC would be obtained around the edge of the magnet area, because the magnetic field gradient is highest there. After MAC were successfully drawn, they were thermally cured for 1 h at 65°C while still under the magnetic field. Finally, the substrate was carefully removed from the glass slide for use. A schematic overview is provided in Figure 1a.
2.3. Plasma treatment
A plasma asher (Emitech K1050X) was used to make hydrophilic MAC after they were cured (15 Torr with continuous air flow at 12 ml/min, 30 W for 1 min). After sample treatment, the sample was immediately immersed in algae culture medium. The effect of the treatment slowly fades away but can be retained to a large degree for the duration of the experiments. Contact angle (CA) measurements on cured flat CIP‐PDMS samples show that the CA for DI water on plasma treated surfaces changed from 0 on Day 1 to 31° on Day 35, while the untreated surface kept the contact angle at 97 ± 2°.
2.4. Actuation of MAC
A commercial magnetic stirrer (color squid, IKA) was modified by removing the top plate and the original magnetic and adding a rotating arm with a new Neodymium magnet (20 × 20 × 10 mm, N48 Neodymium, Supermagnete) placed 6.5 mm off‐center to the rotation axis. Replacing the original magnet increases the magnetic field to provide better actuation of MAC at higher frequencies (Wang et al., 2016). The rotation of the magnet imposes a time‐varying magnetic field on the MAC placed above. As a result, a conical MAC motion is induced, and the tilting angle of the cone can be different depending on the exact location of the individual MAC with respect to the rotation axle. The bottom plane of the samples is about 8.3 mm above the top plane of the magnet, and the rotation frequency can reach up to 2000 rpm.
2.5. Microalgae culture
Microalgae strain S. subspicatus was used in the growth experiments. This microalgae belongs to Scenedesmaceae family, which can be used for biofuel production, animal feed, cosmetics, biofertilizer, and wastewater treatment (Ishaq et al., 2016; Renuka et al., 2016; Rodolfi et al., 2009; Xin et al., 2010). Restricted by the size of the magnet during MAC fabrication, only about 18 mm diameter area on the surface of the PES filter was covered by MAC. To fully examine the effect of MAC on microalgae growth, lasercut polymethylmethacrylate (PMMA) inserts were placed over the substrates (with or without MAC) in 35 mm Petri dishes to form wells of 18 mm in diameter and 6 mm in height. A light source for photosynthesis (Philips GreenPower LED flowering lamp deep red/white/far red) was fixed 20 cm above the culture wells and the light intensity was calculated to be 104 µmol·m−2s−1 (taking an 85° light cone angle and the manufacturer's PAR value of 20 µmol/s). The wells were filled with 1.2 ml S. subspicatus (CCAP, SAMS Limited, strain 276/20) at a concentration of 103 cells/µl by dilution from the main culture with nutrient medium. The wells were illuminated at a 12–12 h on‐off cycle for the entire duration of all experiments and pictures are taken using a Keyence VHX‐5000 digital microscope on a daily basis. The motile MAC samples were actuated continuously at 25 or 33 Hz (1500 or 2000 rpm) by the magnetic actuator, and the control samples were placed away from the magnet so that there is no perceivable movement of MAC. The medium used for culturing was 3N‐BBM + V (CCAP, SAMS Ltd.), a Bold Basal Medium with three‐fold nitrogen and added vitamins. Medium slowly evaporates during the experiments, and the wells were refilled with nutrient medium daily.
2.6. Cell counting
Cell counting was performed before inoculation and at the end of experiments. A Marienfeld Thoma counting chamber was used with an inverted microscope in phase contrast imaging mode. First, a glass coverslip was placed over the counting chamber such that there is 100 µm between the counting grid and the glass slide. Then, 10 µl of control‐diluted samples was injected under the coverslip with a precision pipette. The Thoma chamber has 16 large squares in the counting grid in which the cells are counted per square and summed up. Each sample was counted three times and the average is used to calculate the original concentration.
On the last day of the culture experiments, samples are extracted from the wells. They were first actuated at 2000 rpm for 1 min to resuspend microalgae into the medium. All content was then extracted with a sterile syringe and stored in a 0.5 ml vial. To remove the remaining attached algae as thoroughly as possible, 0.5 ml nutrient medium was used to wash the well with repeated in and out motion using a pipette and is also stored in a 0.5 ml vial. Finally, the same counting procedure was applied to determine the final microalgae concentration in each well.
3. RESULTS
The overall effect of actuated MAC on growth of S. subspicatus was first evaluated by comparing with the results from control groups. Then the difference with plasma treated MAC was examined to show the effect of surface hydrophobicity of MAC.
3.1. Enhanced microalgae growth rate by MAC
First, the effect of MAC on microalgae growth was assessed. Figure 2a shows a macroscopic overview of a typical experiment with no MAC, static and motile MAC with an actuation frequency of 33 Hz (2000 rpm) over 11 days. Note that 2000 rpm is the speed of the magnet, and since the MAC are small in size, the shear rate is much smaller than larger bioreactors with rotors at such high rotation speeds. The shear effect is discussed later in this section. It can be seen from Figure 2a that the color of the culture starts to become much greener for samples with motile MAC from around Day 6, suggesting a growth enhancing effect.
Figure 2.
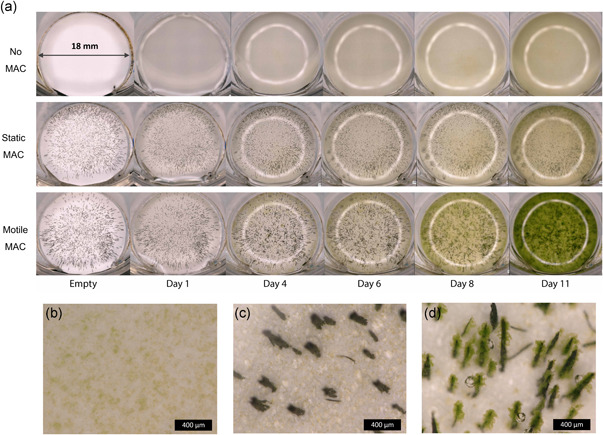
Effect of motile magnetic artificial cilia (MAC) on microalgae growth. (a) Overview images of wells with a plain substrate, static MAC and motile MAC during 11 days of culture. The bright rings are the refection of the epi‐illumination source. (b–d) Microscopic images of samples with (b) no MAC,(c) static MAC, and (d) motile MAC on Day 6 [Color figure can be viewed at wileyonlinelibrary.com]
A closer look with a microscope reveals an interesting difference in growth patterns. On Day 6 (Figure 2b–d) for example, microalgae spread evenly when cultured on a plain substrate or with static MAC. However, clusters of microalgae formed around motile MAC (the change of the clusters over time is shown in Supplementary Information). Formation of oxygen bubbles as a consequence of photosynthesis is also visible on the sides of the motile MAC, much more pronounced than in the other two situations.
The enhancement of microalgae growth overall can be expected, since the motion of MAC creates strong mixing, therefore allowing faster exchange of gas, nutrients, and metabolic products between the microalgae surface and the bulk of fluid, as well as promoting gas exchange on the fluid‐air interface. The formation of clusters, however, is less straightforward to explain, and there might be several reasons. First, various green algae strains have been found to have a critical shear stress level in the range between 0.45 and 0.9 Pa during cell division and 88 Pa at rest (C. Wang & Lan, 2018). Above those critical stress levels, negative effects can occur such as decrease in cell viability, reduction in cell growth, or cell lysis. The shear stress around the surface of the MAC is the highest in the entire culture, at about 0.1 Pa (calculation in the Supplementary Information SI), which is still below those limits, potentially providing the best region for growth regarding flow conditions. Note that there also seems to be more microalgae in the body of fluid when MAC is actuated (see Day 11 in Figure 2a), suggesting that the enhancement of growth happened in the entire well. Second, surface roughness has been found to be an important factor for initial attachment of microalgae on surfaces as well as subsequent biofilm formation, due to the stagnant zone forming in the concaved areas (Q. Zhang et al., 2020). The MAC created in this study have a high surface roughness due to the magnetic alignment of iron particle clusters during fabrication (see Supplementary Information SII), which can be beneficial for microalgae attachment within a strong flow field. Last but not least, adhesion strength of microalgae to surfaces, which is dependent on the species and the chemical properties of the surface, can be high enough to withstand the shear stress and prevent detachment. Surface hydrophobicity, for example, has been found to have an positive effect on S. subspicatus attachment and growth (Deantes‐Espinosa et al., 2019). Similarly, Chlorella vulgaris was found to attach strongly to a hydrophobic surface, but not to a hydrophilic surface, under shear rates of 100–700 s−1 (Ozkan & Berberoglu, 2013). In those studies, attachment of microalgae has been found to have similar enhancement on their growth. The PES filter on which the MAC were manufactured is hydrophilic and the PDMS is hydrophobic (more details in the following section), and this difference likely played a role in the preferential growth on the MAC surface as well.
All these hypotheses on microalgae cluster formation on the surface of MAC can be tested. For example, the critical point where the rate might become too high for growth can be found by increasing the actuation rate of the setup. To do this, however, would require a different setup, since 2000 rpm is already the maximum for this actuation device. Moreover, at very high actuation frequency, the motion of the MAC can diminish (Wang et al., 2014), limiting the shear stress it can reach. The surface roughness of MAC is a result of the fabrication process and is not easy to change. However, there are other fabrication methods, such as molding, that can create much smoother surface. Indeed, earlier experiments have observed less attachment and cluster formation on micromoulded MAC, which can even be used for antifouling applications (S. Zhang et al., 2020). The hydrophobicity of the MAC surfaces, however, can be easily modified through plasma treatment, which will be examined in the next section.
3.2. Effect of surface hydrophobicity of MAC on growth
The surface of freshly prepared MAC is PDMS, which has a low surface energy and is hydrophobic. Treating it with oxygen plasma introduces hydroxy groups (−OH), which makes the surface highly hydrophilic. This process is therefore commonly used for bonding, improving perfusion, and preventing bubble entrapment in microfluidics (Bhattacharya et al., 2005). As mentioned earlier, hydrophobicity has an effect on the adhesion of microalgae, which can influence their growth (Ozkan & Berberoglu, 2013).
Figure 3a shows a macroscopic overview of a typical set of experiments with no MAC, motile hydrophilic MAC (plasma treated) and motile hydrophobic MAC (not treated) with an actuation frequency of 25 Hz (1500 rpm). Similar to the result shown in Figure 2, implementing motile, hydrophobic MAC (same as the third row of Figure 2a) drastically increased the speed of the color change, indicating a rapid growth of microalgae. With the actuation of hydrophilic MAC, there is also a noticeable effect in growth enhancement. However, the less pronounced color change indicates that the level of enhancement is not as high as with hydrophobic MAC.
Figure 3.
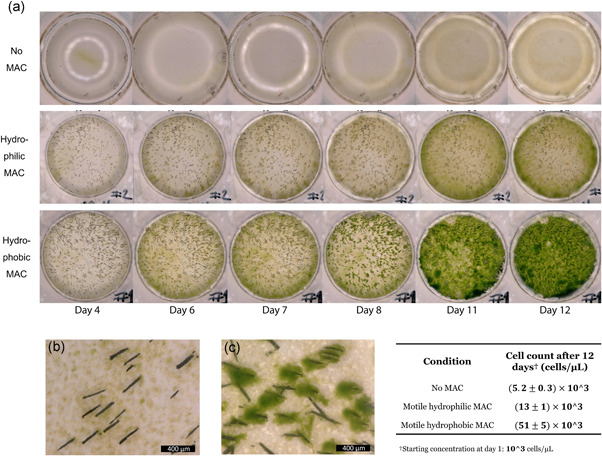
Effect of hydrophobicity of magnetic artificial cilia (MAC) on microalgae growth. (a) Overview images of wells with a plain substrate, motile hydrophilic (plasma treated) MAC and motile hydrophobic (untreated) MAC during 12 days of culture, selectively showing from Day 4 when differences start to become apparent. (b) and (c) Microscopic images of samples with (b) hydrophilic MAC and (c) hydrophobic MAC on Day 8, respectively. The table shows cell counts from different samples using a Thoma counting chamber after 12 days of culture [Color figure can be viewed at wileyonlinelibrary.com]
The difference in growth patterns between hydrophilic and hydrophobic MAC offers a possible explanation for the apparent difference in growth rate. Figure 3b,c shows microscopic images of hydrophilic and hydrophobic MAC samples on Day 8, respectively. It can be clearly seen that no microalgae clusters formed on the surface of hydrophilic MAC, in contrast to hydrophobic MAC, where large clusters formed, and the number density of microalgae was higher as a result.
Quantitative cell counting was performed (table in Figure 3), and the results show that, compared with using no MAC, the density of microalgae after 12 days of culture was doubled with motile hydrophilic MAC, and with hydrophobic MAC the density was 10 times higher.
The result suggests that, even without microalgae attachment and large clusters formation, the flow and mixing created by MAC motion alone can have a positive impact on their growth. On the other hand, for this particular microalgae strain, the attachment on MAC surfaces seems to have even more impact on the growth enhancement. However, the effect of attachment only works in combination with the motion of MAC, as Figure 2 shows that, without motion, there was neither attachment nor enhancement of growth.
4. DISCUSSION AND CONCLUSION
It needs to be pointed out that although MAC can be useful for applications that use small volumes (up to a few milliliters) of fluids, they are not suitable for processes that require large, field level volumes, for example the production of biofuels. This limitation comes from their small sizes and the intrinsic difficulty in generating sufficient magnetic field in large volumes. The production of MAC, however, can be scaled to larger area, if appropriate methods are applied (Wang et al., 2016). Therefore, it is possible to perform experiments on relatively large surface areas, possibly with multiple actuators. Moreover, although the MAC are robust against the forces created by magnetic actuation, they are not strong enough to withstand aggressive mechanical cleaning. Therefore, at least for the samples made in this study, they are unlikely to be reusable. However, the method of fabricating MAC used in this study can be easily adopted for testing and can be applied in small scales for various purposes, for example in screening studies.
For example, MAC can be used to study the influence of flow conditions on microalgae. At small scales, it is easier to obtain high shear flow without having to generate a very high flow speed (which means lower power consumption and a simpler setup), and shear rate is known to be an important factor for various biological process and behavior of microalgae (C. Wang & Lan, 2018). Indeed, as shown in this study, S. subspicatus tend to attach and proliferate on the surface of hydrophobic MAC, where the shear rate is highest (in the order of 100 s−1). However, there must be an upper limit of shear rate, above which the attachment is no longer possible. It was also reported in literature that at high Reynolds numbers, there is a diminishing gain or even reverse in productivity and photosynthetic efficiency (Hondzo & Wang, 2002; Kliphuis et al., 2010). To study these phenomena more quantitatively, series of experiments with actuation frequency sweepings and flow analyses need to be done, combined with biological observations and photosynthetic and metabolic measurements. Also, more experiments could be done to examine the effect of shear independent of mixing and adhesion of the microalgae to the MAC surfaces. These more detailed research, although interesting, were beyond the scope of this study.
The growth enhancement effect can be useful in increasing the efficiency of studies performed in small volumes. For example, selecting the best strain of microalgae for energy, food or pharmaceutical use is an important but complicated and time consuming task, not the least because there are an estimated 44,000 (or significantly more) algae species (Christenhusz & Byng, 2016; Guiry, 2012). To screen for the best culture conditions for biomass and other useful products, light, nutrient, gas, and other factors need to be studied as well. One can easily appreciate the staggering complexity when all the variables are combined. That is why researchers have been spending effort on improving the throughput of microalgae screenings by parallelization and reducing sample volumes, most notably by using microfluidics (H. S. Kim et al., 2018). Improving the growth rate can help in shortening the timespan needed between inoculation and final readout for each experimental unit, which therefore contributes to further boosting the throughput. Of course, introducing flow and mixing by using MAC will also affect the physiology of the microalgae being studied, which requires control and validation studies. In addition to screening and optimization for production, microfluidics can enable studies on algae response to controlled environmental changes, for example, chemotaxis (Choi et al., 2016), phototaxis (De Maleprade et al., 2020), and their interaction with bacteria (Peaudecerf et al., 2018), or other organisms (Shapiro et al., 2016). The ability to control the growth rate with locally generated flow using MAC can add flexibility in designing such studies, and improving fluidic mixing in such studies can provide a more realistic mimicry of the natural environment.
In conclusion, we fabricated MAC and implemented them in millimeter scale culture wells, and we showed that the motile MAC enhance the growth of the microalgae S. subspicatus. In addition, the surface hydrophobicity of MAC was modified to a hydrophilic state using plasma treatment. The experiments showed that the total cell density increased with the introduction of actuated MAC compared with control groups, by a factor of 10 for hydrophobic MAC and by a factor of 2 for hydrophilic ones. Clusters of microalgae were observed forming around the surface of hydrophobic MAC but not the hydrophilic ones. The attachment of microalgae on the surface of hydrophobic MAC and the subsequent formation of clusters apparently contributed to the growth enhancement effect on top of the mixing created by the motion of MAC. This technique of manufacturing and actuation of MAC can be used to investigate the effect of mixing and flow on microalgae in small sample volumes, and the enhancement in microalgae growth can be beneficial for the throughput of screening studies. Moreover, the method used for creating MAC can be easily adopted in labs without microfabrication infrastructure, and it can be mastered by people with little prior experience in microfluidics.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conception and design: Thijn Verburg, Allison Schaap, and Ye Wang. Acquisition of data: Thijn Verburg. Analysis and interpretation of data: Thijn Verburg, Allison Schaap, Shuaizhong Zhang, Jaap den Toonder, and Ye Wang. Writing: Thijn Verburg, Allison Schaap, Shuaizhong Zhang, Jaap den Toonder, and Ye Wang. Final proof reading: Ye Wang. Providing resources: Allison Schaap, Shuaizhong Zhang, Jaap den Toonder, and Ye Wang.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
The authors thank Jaap de Hullu and Irene Dobbelaer for the organization of the laboratory and the assistance in the experiments. The research leading to these results has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program under grant agreement no. 833214.
Verburg, T. , Schaap, A. , Zhang, S. , den Toonder, J. , & Wang, Y. (2021). Enhancement of microalgae growth using magnetic artificial cilia. Biotechnology Bioengineering. 118, 2472–2481. 10.1002/bit.27756
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Au, S. H. , Shih, S. C. C. , & Wheeler, A. R. (2011). Integrated microbioreactor for culture and analysis of bacteria, algae and yeast. Biomedical Microdevices, 13, 41–50. 10.1007/s10544-010-9469-3 [DOI] [PubMed] [Google Scholar]
- Babataheri, A. , Roper, M. , Fermigier, M. , & Du Roure, O. (2011). Tethered fleximags as artificial cilia. Journal of Fluid Mechanics, 678, 5–13. 10.1017/S002211201100005X [DOI] [Google Scholar]
- Barbosa, M. J. , Albrecht, M. , & Wijffels, R. H. (2003). Hydrodynamic stress and lethal events in sparged microalgae cultures. Biotechnology and Bioengineering, 83, 112–120. 10.1002/bit.10657 [DOI] [PubMed] [Google Scholar]
- Barbosa, M. J. , Janssen, M. , Ham, N. , Tramper, J. , & Wijffels, R. H. (2003). Microalgae cultivation in air‐lift reactors: Modeling biomass yield and growth rate as a function of mixing frequency. Biotechnology and Bioengineering, 82, 170–179. 10.1002/bit.10563 [DOI] [PubMed] [Google Scholar]
- Belardi, J. , Schorr, N. , Prucker, O. , & Rühe, J. (2011). Artificial cilia: Generation of magnetic actuators in microfluidic systems. Advanced Functional Materials, 21(17), 3314–3320. 10.1002/adfm.201100787 [DOI] [Google Scholar]
- Bhattacharya, S. , Datta, A. , Berg, J. M. , & Gangopadhyay, S. (2005). Studies on surface wettability of poly(dimethyl) siloxane (PDMS) and glass under oxygen‐plasma treatment and correlation with bond strength. Journal of Microelectromechanical Systems, 14, 590–597. 10.1109/JMEMS.2005.844746 [DOI] [Google Scholar]
- Borowitzka, M. A. (1995). Microalgae as sources of pharmaceuticals and other biologically active compounds. Journal of Applied Phycology, 7, 3–15. 10.1007/BF00003544 [DOI] [Google Scholar]
- Choi, H. I. l. , Kim, J. Y. H. , Kwak, H. S. , Sung, Y. J. , & Sim, S. J. (2016). Quantitative analysis of the chemotaxis of a green alga, Chlamydomonas reinhardtii, to bicarbonate using diffusion‐based microfluidic device. Biomicrofluidics, 10, 014121. 10.1063/1.4942756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenhusz, M. J. M. , & Byng, J. W. (2016). The number of known plants species in the world and its annual increase. Phytotaxa, 261, 201. 10.11646/phytotaxa.261.3.1 [DOI] [Google Scholar]
- Christenson, L. , & Sims, R. (2011). Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnology Advances, 29, 686–702. 10.1016/j.biotechadv.2011.05.015 [DOI] [PubMed] [Google Scholar]
- De Maleprade, H. , Moisy, F. , Ishikawa, T. , & Goldstein, R. E. (2020). Motility and phototaxis of Gonium, the simplest differentiated colonial alga. Physical Review E, 101, 022416. 10.1103/PhysRevE.101.022416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deantes‐Espinosa, V. M. , Zhang, T. Y. , Wang, X. X. , Wu, Y. , Dao, G. H. , & Hu, H. Y. (2019). Attached cultivation of Scenedesmus sp. LX1 on selected solids and the effect of surface properties on attachment. Frontiers of Environmental Science and Engineering, 13, 57. 10.1007/s11783-019-1141-4 [DOI] [Google Scholar]
- Den Toonder, J. , & Onck, P. (2013). Artificial cilia. The Royal Society of Chemistry. 10.1039/9781849737098 [DOI] [Google Scholar]
- Dewan, A. , Kim, J. , Mclean, R. H. , Vanapalli, S. A. , & Karim, M. N. (2012). Growth kinetics of microalgae in microfluidic static droplet arrays. Biotechnology and Bioengineering, 109, 2987–2996. 10.1002/bit.24568 [DOI] [PubMed] [Google Scholar]
- Evans, B. A. , Shields, A. R. , Carroll, R. L. , Washburn, S. , Falvo, M. R. , & Superfine, R. (2007). Magnetically actuated nanorod arrays as biomimetic cilia. Nano Letters, 7(5), 1428–1434. 10.1021/nl070190c [DOI] [PubMed] [Google Scholar]
- Gardiner, M. B. (2005). The importance of being cilia. HHMI Bulletin, 1–6. http://www.hhmi.org/bulletin/sept2005/features/cilia.html%5Cnpapers2://publication/uuid/F76AF892-4AE9-421B-AAD4-6D221D8870E2 [Google Scholar]
- Gebhardt, G. , Hortsch, R. , Kaufmann, K. , Arnold, M. , & Weuster‐Botz, D. (2011). A new microfluidic concept for parallel operated milliliter‐scale stirred tank bioreactors. Biotechnology Progress, 27, 684–690. 10.1002/btpr.570 [DOI] [PubMed] [Google Scholar]
- Gibbons, I. R. (1981). Cilia and flagella of eukaryotes. The Journal of Cell Biology, 91(3 Pt 2), 107s–124s. 10.1083/jcb.91.3.107s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godino, N. , Jorde, F. , Lawlor, D. , Jaeger, M. , & Duschl, C. (2015). Purification of microalgae from bacterial contamination using a disposable inertia‐based microfluidic device. Journal of Micromechanics and Microengineering, 25, 084002. 10.1088/0960-1317/25/8/084002 [DOI] [Google Scholar]
- Guiry, M. D. (2012). How many species of algae are there? In. Journal of Phycology, 48, 1057–1063. 10.1111/j.1529-8817.2012.01222.x [DOI] [PubMed] [Google Scholar]
- Hondzo, M. , & Wang, H. (2002). Effects of turbulence on growth and metabolism of periphyton in a laboratory flume. Water Resources Research, 38, 13‐1–13‐9. 10.1029/2002wr001409 [DOI] [Google Scholar]
- Hønsvall, B. K. , Altin, D. , & Robertson, L. J. (2016). Continuous harvesting of microalgae by new microfluidic technology for particle separation. Bioresource Technology, 200, 360–365. 10.1016/j.biortech.2015.10.046 [DOI] [PubMed] [Google Scholar]
- Hosaka, K. , Hioki, T. , Furuune, H. , & Tanishita, K. (1995). Augmentation of microalgae growth due to hydrodynamic activation. Energy Conversion and Management, 36, 725–728. 10.1016/0196-8904(95)00107-O [DOI] [Google Scholar]
- Huang, J. , Li, Y. , Wan, M. , Yan, Y. , Feng, F. , Qu, X. , Wang, J. , Shen, G. , Li, W. , Fan, J. , & Wang, W. (2014). Novel flat‐plate photobioreactors for microalgae cultivation with special mixers to promote mixing along the light gradient. Bioresource Technology, 159, 8–16. 10.1016/j.biortech.2014.01.134 [DOI] [PubMed] [Google Scholar]
- Ibanez‐Tallon, I. (2003). To beat or not to beat: Roles of cilia in development and disease. Human Molecular Genetics, 12(90001), 27R–35R. 10.1093/hmg/ddg061 [DOI] [PubMed] [Google Scholar]
- Ishaq, A. G. , Matias‐Peralta, H. M. , & Basri, H. (2016). Bioactive compounds from green microalga Scenedesmus and its potential applications: A brief review. Pertanika Journal of Tropical Agricultural Science, 39, 1–15. [Google Scholar]
- Johnson‐Chavarria, E. M. , Agrawal, U. , Tanyeri, M. , Kuhlman, T. E. , & Schroeder, C. M. (2014). Automated single cell microbioreactor for monitoring intracellular dynamics and cell growth in free solution. Lab on a Chip, 14, 2688–2697. 10.1039/c4lc00057a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaderi, S. N. , Craus, C. B. , Hussong, J. , Schorr, N. , Belardi, J. , Westerweel, J. , Prucker, O. , Rühe, J. , den Toonder, J. M. J. , & Onck, P. R. (2011). Magnetically‐actuated artificial cilia for microfluidic propulsion. Lab on a Chip, 11(12), 2002–2010. 10.1039/c0lc00411a [DOI] [PubMed] [Google Scholar]
- Khan, M. I. , Shin, J. H. , & Kim, J. D. (2018). The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microbial Cell Factories, 17, 36. 10.1186/s12934-018-0879-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. S. , Devarenne, T. P. , & Han, A. (2018). Microfluidic systems for microalgal biotechnology: A review. In Algal Research, 30, 149–161. 10.1016/j.algal.2017.11.020 [DOI] [Google Scholar]
- Kim, H. S. , Weiss, T. L. , Thapa, H. R. , Devarenne, T. P. , & Han, A. (2014). A microfluidic photobioreactor array demonstrating high‐throughput screening for microalgal oil production. Lab on a Chip, 14, 1415. 10.1039/c3lc51396c [DOI] [PubMed] [Google Scholar]
- Kim, S. , Lee, S. , Lee, J. , Nelson, B. J. , Zhang, L. , & Choi, H. (2016). Fabrication and manipulation of ciliary microrobots with non‐reciprocal magnetic actuation. Scientific Reports, 10.1038/srep30713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliphuis, A. M. J. , de Winter, L. , Vejrazka, C. , Martens, D. E. , Janssen, M. , & Wijffels, R. H. (2010). Photosynthetic efficiency of Chlorella sorokiniana in a turbulently mixed short light‐path photobioreactor. Biotechnology Progress, 26, 687–696. 10.1002/btpr.379 [DOI] [PubMed] [Google Scholar]
- Kwak, H. S. , Kim, J. Y. H. , Na, S. C. , Jeon, N. L. , & Sim, S. J. (2016). Multiplex microfluidic system integrating sequential operations of microalgal lipid production. Analyst, 141, 1218–1225. 10.1039/c5an02409a [DOI] [PubMed] [Google Scholar]
- Kwak, H. S. , Kim, J. Y. H. , Woo, H. M. , Jin, E. S. , Min, B. K. , & Sim, S. J. (2016). Synergistic effect of multiple stress conditions for improving microalgal lipid production. Algal Research, 19, 215–224. 10.1016/j.algal.2016.09.003 [DOI] [Google Scholar]
- Leupold, M. , Hindersin, S. , Gust, G. , Kerner, M. , & Hanelt, D. (2013). Influence of mixing and shear stress on Chlorella vulgaris, Scenedesmus obliquus, and Chlamydomonas reinhardtii . Journal of Applied Phycology, 25, 485–495. 10.1007/s10811-012-9882-5 [DOI] [Google Scholar]
- Luke, C. S. , Selimkhanov, J. , Baumgart, L. , Cohen, S. E. , Golden, S. S. , Cookson, N. A. , & Hasty, J. (2016). A microfluidic platform for long‐term monitoring of algae in a dynamic environment. ACS Synthetic Biology, 5, 8–14. 10.1021/acssynbio.5b00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity, J. P. , Bundschuh, J. , Chen, C. Y. , & Bhattacharya, P. (2014). Microalgae for third generation biofuel production, mitigation ofgreenhouse gas emissions and wastewater treatment: Present andfuture perspectives: A mini review. Energy, 78, 104–113. 10.1016/j.energy.2014.04.003 [DOI] [Google Scholar]
- McGary, P. D. , Tan, L. , Zou, J. , Stadler, B. J. H. , Downey, P. R. , & Flatau, A. B. (2006). Magnetic nanowires for acoustic sensors (invited). Journal of Applied Physics, 99(8), 08B310.6. 10.1063/1.2167332 [DOI] [Google Scholar]
- Mõttus, M. , Sulev, M. , Baret, F. , Lopez‐Lozano, R. , & Reinart, A. (2012). Photosynthetically active radiation: Measurementphotosynthesis/photosynthetic(ally)active radiation (PAR)measurementand Modelingphotosynthesis/photosynthetic(ally)active radiation (PAR)modeling. In Meyers R. A. (Ed.), Encyclopedia of sustainability science and technology (pp. 7902–7932). Springer. 10.1007/978-1-4419-0851-3_451 [DOI] [Google Scholar]
- Murthy, G. S. (2011). Overview and assessment of algal biofuels production technologies. Biofuels, 415–437. 10.1016/B978-0-12-385099-7.00019-X [DOI] [Google Scholar]
- Ottino, J. M. , & Wiggins, S. (2004). Introduction: Mixing in microfluidics. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 362, 923–935. 10.1098/rsta.2003.1355 [DOI] [PubMed] [Google Scholar]
- Ozkan, A. , & Berberoglu, H. (2013). Adhesion of algal cells to surfaces. Biofouling, 29, 469–482. 10.1080/08927014.2013.782397 [DOI] [PubMed] [Google Scholar]
- Peaudecerf, F. J. , Bunbury, F. , Bhardwaj, V. , Bees, M. A. , Smith, A. G. , Goldstein, R. E. , & Croze, O. A. (2018). Microbial mutualism at a distance: The role of geometry in diffusive exchanges. Physical Review E, 97, 022411. 10.1103/PhysRevE.97.022411 [DOI] [PubMed] [Google Scholar]
- Qiang, H. , & Richmond, A. (1996). Productivity and photosynthetic efficiency of Spirulina platensis as affected by light intensity, algal density and rate of mixing in a flat plate photobioreactor. Journal of Applied Phycology, 8, 139–145. 10.1007/BF02186317 [DOI] [Google Scholar]
- Qu, B. , Eu, Y. J. , Jeong, W. J. , & Kim, D. P. (2012). Droplet electroporation in microfluidics for efficient cell transformation with or without cell wall removal. Lab on a Chip, 12, 4483. 10.1039/c2lc40360a [DOI] [PubMed] [Google Scholar]
- Renuka, N. , Prasanna, R. , Sood, A. , Ahluwalia, A. S. , Bansal, R. , Babu, S. , Singh, R. , Shivay, Y. S. , & Nain, L. (2016). Exploring the efficacy of wastewater‐grown microalgal biomass as a biofertilizer for wheat. Environmental Science and Pollution Research, 23, 6608–6620. 10.1007/s11356-015-5884-6 [DOI] [PubMed] [Google Scholar]
- Rodolfi, L. , Zittelli, G. C. , Bassi, N. , Padovani, G. , Biondi, N. , Bonini, G. , & Tredici, M. R. (2009). Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low‐cost photobioreactor. Biotechnology and Bioengineering, 102, 100–112. 10.1002/bit.22033 [DOI] [PubMed] [Google Scholar]
- Saad, M. G. , Selahi, A. , Zoromba, M. S. , Mekki, L. , El‐Bana, M. , Dosoky, N. S. , Nobles, D. , & Shafik, H. M. (2019). A droplet‐based gradient microfluidic to monitor and evaluate the growth of Chlorella vulgaris under different levels of nitrogen and temperatures. Algal Research, 44, 101657. 10.1016/j.algal.2019.101657 [DOI] [Google Scholar]
- Schenk, P. M. , Thomas‐Hall, S. R. , Stephens, E. , Marx, U. C. , Mussgnug, J. H. , Posten, C. , Kruse, O. , & Hankamer, B. (2008). Second generation biofuels: High‐efficiency microalgae for biodiesel production. BioEnergy Research, 1, 20–43. 10.1007/s12155-008-9008-8 [DOI] [Google Scholar]
- Shapiro, O. H. , Kramarsky‐Winter, E. , Gavish, A. R. , Stocker, R. , & Vardi, A. (2016). A coral‐on‐a‐chip microfluidic platform enabling live‐imaging microscopy of reef‐building corals. Nature Communications, 7, 10860. 10.1038/ncomms10860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields, A. R. , Fiser, B. L. , Evans, B. A. , Falvo, M. R. , Washburn, S. , & Superfine, R. (2010). Biomimetic cilia arrays generate simultaneous pumping and mixing regimes. Proceedings of the National Academy of Sciences of the United States of America, 107(36), 15670–15675. 10.1073/pnas.1005127107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, S. C. C. , Mufti, N. S. , Chamberlain, M. D. , Kim, J. , & Wheeler, A. R. (2014). A droplet‐based screen for wavelength‐dependent lipid production in algae. Energy and Environmental Science, 7, 2366. 10.1039/c4ee01123f [DOI] [Google Scholar]
- Stroock, A. D. , & Whitesides, G. M. (2003). Controlling flows in microchannels with patterned surface charge and topography. Accounts of Chemical Research, 36, 597–604. 10.1021/ar0202870 [DOI] [PubMed] [Google Scholar]
- Syed, M. S. , Rafeie, M. , Vandamme, D. , Asadnia, M. , Henderson, R. , Taylor, R. A. , & Warkiani, M. E. (2018). Selective separation of microalgae cells using inertial microfluidics. Bioresource Technology, 252, 91–99. 10.1016/j.biortech.2017.12.065 [DOI] [PubMed] [Google Scholar]
- Toonder, J. , den Bos, F. , Broer, D. , Filippini, L. , Gillies, M. , de Goede, J. , Mol, T. , Reijme, M. , Talen, W. , Wilderbeek, H. , Khatavkar, V. , & Anderson, P. (2008). Artificial cilia for active micro‐fluidic mixing. Lab on a Chip, 8(4), 533–541. 10.1039/b717681c [DOI] [PubMed] [Google Scholar]
- van Oosten, C. L. , Bastiaansen, C. W. M. , & Broer, D. J. (2009). Printed artificial cilia from liquid‐crystal network actuators modularly driven by light. Nature Materials, 8(8), 677–682. 10.1038/nmat2487 [DOI] [PubMed] [Google Scholar]
- Wang, C. , & Lan, C. Q. (2018). Effects of shear stress on microalgae: A review. Biotechnology Advances, 36, 986–1002. 10.1016/j.biotechadv.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , den Toonder, J. , Cardinaels, R. , & Anderson, P. (2016). A continuous roll‐pulling approach for the fabrication of magnetic artificial cilia with microfluidic pumping capability. Lab Chip, 16(12), 2277–2286. 10.1039/C6LC00531D [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Gao, Y. , Wyss, H. , Anderson, P. , & den Toonder, J. (2013). Out of the cleanroom, self‐assembled magnetic artificial cilia. Lab on a Chip, 13(17), 3360–3366. 10.1039/c3lc50458a [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Gao, Y. , Wyss, H. M. , Anderson, P. D. , & den Toonder, J. M. J. (2014). Artificial cilia fabricated using magnetic fiber drawing generate substantial fluid flow. Microfluidics and Nanofluidics, 18(2), 167–174. 10.1007/s10404-014-1425-8 [DOI] [Google Scholar]
- Whitesides, G. M. (2006). The origins and the future of microfluidics. Nature, 442(7101), 368–373. 10.1038/nature05058 [DOI] [PubMed] [Google Scholar]
- Xin, L. , Hong‐ying, H. , & Jia, Y. (2010). Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga, Scenedesmus sp. LX1, growing in secondary effluent. New Biotechnology, 27, 59–63. 10.1016/j.nbt.2009.11.006 [DOI] [PubMed] [Google Scholar]
- Zhang, Q. , Yu, Z. , Jin, S. , Liu, C. , Li, Y. , Guo, D. , Hu, M. , Ruan, R. , & Liu, Y. (2020). Role of surface roughness in the algal short‐term cell adhesion and long‐term biofilm cultivation under dynamic flow condition. Algal Research. 10.1016/j.algal.2019.101787 [DOI] [Google Scholar]
- Zhang, S. , Wang, Y. , Lavrijsen, R. , Onck, P. R. , & den Toonder, J. M. J. (2018). Versatile microfluidic flow generated by moulded magnetic artificial cilia. Sensors and Actuators, B: Chemical, 263, 614–624. 10.1016/j.snb.2018.01.189 [DOI] [Google Scholar]
- Zhang, S. , Zhang, R. , Wang, Y. , Onck, P. R. , & Den Toonder, J. M. J. (2020). Controlled multidirectional particle transportation by magnetic artificial cilia. ACS Nano. 10.1021/acsnano.0c03801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Zhang, S. , Zuo, P. , Wang, Y. , Wang, Y. , Onck, P. , & Toonder, J. M. J. D. (2020). Anti‐biofouling and self‐cleaning surfaces featured with magnetic artificial cilia. ACS Applied Materials and Interfaces, 12(24), 27726–27736. 10.1021/acsami.0c05403 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


