Abstract
The effects of the combination of bis (α‐furancarboxylato) oxovanadium (IV) (BFOV) and metformin (Met) on hepatic steatosis were investigated in high‐fat diet‐induced obese C57BL/6J mice (HFC57 mice) for 6 weeks. Oral glucose tolerance test was performed to evaluate glucose metabolism. Moreover, blood and hepatic biochemical and histological indices were detected. Besides, Affymetrix‐GeneChip analysis and Western blot of the liver were performed. Comparing to the monotherapy group, BFOV + Met showed more effective improvement in glucose metabolism, which decreased the fasting blood glucose, insulin levels and improved insulin sensitivity in HFC57 mice. BFOV + Met significantly decreased serum ALT and AST activities and reduced hepatic triglyceride content and iNOS activities, accompanied by ameliorating intrahepatic fat accumulation and hepatocellular vacuolation. Enhanced hepatic insulin signalling transduction and attenuated inflammation pathway were identified as the major pathways in the BFOV + Met group. BFOV + Met significantly down‐regulated the protein expression levels of MMPs, NF‐κB, iNOS and up‐regulated phosphorylation of AKT and AMPK levels. We concluded that a combination of BFOV and metformin ameliorates hepatic steatosis in HFC57 mice via alleviating hepatic inflammation and enhancing insulin signalling pathway, suggesting that the combination of BFOV and metformin is a potential treatment for hepatic steatosis.
Keywords: bis (α‐furancarboxylato) oxovanadium (IV), combination therapy, hepatic steatosis, inflammation, metformin
1. INTRODUCTION
Non‐alcoholic fatty liver disease (NAFLD) is the most common type of hepatic disorder worldwide, which refers to a pathological condition of hepatic steatosis. 1 NAFLD is a series of stages that comprise a continuum of liver conditions, including hepatic steatosis (fatty liver) and NASH, which is defined as a more serious process with inflammation and hepatocyte damage (steatohepatitis). 2
Considering that obesity, insulin resistance and type 2 diabetes mellitus (T2DM) are strongly associated with the development of hepatic steatosis, so far lifestyle interventions, such as dietary restriction and exercise, are currently the principal therapy for hepatic steatosis. 3 Many antidiabetic drugs have been tested in NAFLD patients during these years. 4 Among these, pioglitazone seems to improve some histological features of NASH and has been approved for NAFLD treatment, but it has no clear effect on fibrosis. 5 However, there have been no specific medications to the intervention of NAFLD. 6 Hepatic steatosis (fatty liver), which is caused by the accumulation of excessive fat in the liver, is strongly associated with the development of hepatic insulin resistance and T2DM. Lipid accumulation and inflammation have been implicated in the pathogenesis of insulin resistance and hepatic steatosis. 7 Thus, novel antidiabetic drugs, which ameliorate insulin sensitivity and reduce excess fatty acids in the circulation system, will become candidates for the treatment of hepatic steatosis, even the NAFLD.
Vanadium (V), a potent non‐selective inhibitor of protein tyrosine phosphatases, has been known to possess an insulin‐enhancing effect. 8 , 9 When administered at lower doses in diabetic animals, vanadium showed potent stress alleviating effects but toxic adverse effects, such as liver and kidney toxicity, which have limited the further application of V. 10 However, vanadium organic complexes have acceptable pharmacokinetic properties and exhibit an excellent hypoglycaemic effect. In our previous studies, we have reported a novel oral antidiabetic candidate vanadyl complex, bis (α‐furancarboxylato) oxovanadium (IV) (BFOV), which displayed a potential antidiabetic effect in type 2 diabetic KKAy (KK Cg‐Ay/J) mice and also ameliorated hepatic lipid accumulation, which indicates that there might be a potential role of BFOV on hepatic steatosis. 11 Metformin is a commonly applied oral hypoglycaemic agent in monotherapy or in combination treatment, which is generally recommended as first‐line therapy for type 2 diabetes. Metformin has recently been extensively studied, and emerging evidence suggests metformin decreases hepatocyte triglyceride accumulation. However, there is insufficient clinical evidence data, suggesting metformin to be used for NAFLD. 12 , 13
According to pieces of evidence, we hypothesized that the combination of BFOV and metformin might exhibit a synergetic role in the improvement of fatty liver in obesity and insulin‐resistant mice. In our previous study, we have investigated and found that the combination of BFOV and metformin synergistically improved hyperglycaemia and glucose intolerance in alloxan‐induced type 1 diabetic mice by two‐way ANOVA analysis. Using Q value analysis, we found that the combination of BFOV (20 mg/kg) and metformin (100 mg/kg) possesses the highest synergism. Then, in the present study, we further verified the effects of a combination of BFOV and metformin on hepatic steatosis and the related insulin resistance in a high‐fat diet‐induced obese C57BL/6J mouse model. Our data showed a potential synergy in vivo with a combination of BFOV and metformin significantly improving glucose homeostasis and liver lipid accumulation compared with monotherapy and ameliorated hepatic steatosis through down‐regulation of inflammatory factors and activation of hepatic phosphorylated AMPK and AKT signalling pathways.
2. MATERIALS AND METHODS
2.1. Animals
12‐week‐old C57BL/6J male mice were purchased from the Experimental Animal Center, Chinese Academy of Medical Science, Beijing, China. The animals were housed in a constant 12‐hours light/ dark cycle in a temperature‐controlled central facility (22 ± 3°C) with free access to tap water and chow. C57BL/6J mice were fed standard laboratory chow as Normal group (Nor) or fed with a high‐calorie diet (RD12492, Research Diets Inc) to induce the development or progression of obesity and diabetes. The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies. 14 All animal experiments were carried out in strict accordance with the Standards for Laboratory Animals (GB14925‐2001) and the Guideline on the Humane Treatment of Laboratory Animals (MOST 2006a) established by the People's Republic of China, and all animal procedures were approved by Beijing Administration Office of Laboratory Animal (approval number: SCXKBeijing‐2009‐0004).
2.2. Animal experimental procedures
C57BL/6J mice with an average body‐weight of 45 g were reared with a high‐fat diet for 12 weeks and randomly divided into four groups (n = 9/group) as Con, Met, BFOV and BFOV + Met based on fasting blood glucose, triglyceride and total cholesterol, body‐weight and decreasing percentage of plasma glucose at 40 min in insulin tolerance test (0.4 IU/kg, ITT). The Con group was orally administered by gavage with normal saline, and BFOV and Met group with 20 mg/kg BFOV alone and 100 mg/kg metformin suspended or dissolved in normal saline for 6 weeks, respectively. BFOV + Met group was orally administered by gavage with 20 mg/kg BFOV plus 100 mg/kg metformin. BFOV (C 41.52%, H 2.08%, V 17.61%, purity > 98.5% by HPLC) was synthesized according to the method described previously. 15 The standard diet‐fed C57BL/6J mice (n = 9, average body‐weight 25) were selected as the Nor group and administered with normal saline.
During the 6‐week treatment, fasting blood glucose, insulin tolerance tests (ITTs) and oral glucose tolerance tests (OGTTs) were monitored monthly after 2‐week treatment. The circulating markers of metabolic disturbances including insulin, ALT and NO were measured after 4 weeks of treatment and at the end of the experiment. Animals from each group were euthanized, and the liver was excised, and one part was fixed for haematoxylin and eosin (HE) staining. The others were stored for hepatic biochemical indices, liver mRNA GeneChip and protein expression level assay.
2.3. Oral glucose tolerance test(OGTT) and insulin tolerance test (ITT)
The mice were fasted for 4 hours, and blood was sampled from the tail for glucose assay by the glucose oxidase method at baseline (as 0 minute) and 30, 60 and 120 minutes after glucose (2 g/kg) loading in OGTT or 40 and 90 minutes after insulin (0.4 IU/kg) subcutaneous injection in ITT. The area under the curve (AUC) was calculated by the data collected during the OGTT and ITT.
2.4. Haematoxylin and eosin (HE) staining and biochemical assay of liver tissue
The liver was immediately isolated from the mice and weighed and then cut into four fractions. One fraction was fixed in 4% paraformaldehyde overnight and was then processed for paraffin embedding. Multiple 5‐μm sections from each liver were stained with HE and photographed (using the Olympus CX41RF system, Olympus, Tokyo, Japan). The left three fractions were stored at −80°C for further analysis. Liver samples (100 mg) were homogenized in ice‐cold physiological saline and centrifugated. The supernatants were collected for the assessment of lipid levels and other parameters.
2.5. Biochemical analysis
Glucose was monitored by the glucose oxidase method as previously reported, 16 and levels of triglycerides (TG), total cholesterol (TC), malondialdehyde (MDA), nitric oxide (NO), inducible nitric oxide synthase (iNOS) and aspartate aminotransferase (ALT) were determined by enzymatic colorimetric methods with commercial kits (BioSino Biotechnology & Science Inc Beijing, China). Blood insulin level (FINS) was measured by ELISA Kit (ALPCO Inc USA). Protein content was assayed in the BCA protein assay kit (P1511, Applygen Inc Beijing, China), using bovine serum albumin (BSA) as standard.
2.6. Western blot
Liver tissue samples were homogenized in lysis buffer (50 mmol/L Tris–HCl, 2% SDS, 10% glycerol) supplemented with protease inhibitor cocktail (P1265, Applygen Inc Beijing, China). The homogenate was centrifuged at 12 000 x g for 10 minutes, and the supernatant was taken to determine the protein concentrations. Equal amounts of protein samples were resolved electrophoretically by a 10% sodium dodecyl polyacrylamide gel and transferred to PVDF membranes. Information of the antibodies are as follows: anti‐AMPKα (2532s, 62KD, CST, USA), anti‐ Phospho‐AMPKα (Thr172) (2531, 62KD,CST, USA), anti‐AKT (4685, 60KD, CST, USA), anti‐ Phospho‐Akt (Ser473) (4060, 60KD, CST, USA), anti‐NF‐κB p65 (D14E12) (8242, 65KD, CST, USA), anti‐ MMP‐2 (D2O4T) (87809, 72KD, CST, USA) and anti‐iNOS (D6B6S) (13120, 130KD, CST, USA). β‐Actin antibody (C1313) and secondary antibodies were from Applygen Technologies Inc, China. The membranes were probed using standard procedures, and the signal was visualized by using an enhanced chemiluminescence detection system (ChemiScope2850, CLiNX science Instruments). Protein band densities were analysed using Gel‐Pro‐Analyzer 3.1 software. 17
2.7. Microarray and bioinformatics analysis using the Affymetrix‐GeneChip
For microarray analysis, we used the Affymetrix‐GeneChip MouseWG‐6 v2, which is designed specifically to monitor gene expression in mice. We used RNA extracted from 3 liver tissues for each group. All procedures of the GeneChip arrays, as well as data collection, were performed at the Beijing Compass Biotechnology company. In brief, cDNAs were synthesized from RNA samples, then fragmented and labelled with biotin. Subsequently, fragmented cDNAs were hybridized onto the Affymetrix‐GeneChip MouseWG‐6 v2. Illumina Bead studio Application software was used to extract raw data. To understand the function of mRNAs associated with the antidiabetic effects, we established the role of differentially expressed mRNA through KEGG analyses. Venn diagram was applied to display the co‐regulated mRNAs. Transcript detection was selected for differential gene expression of ≥ 5‐fold relative to the Con group.
2.8. Statistical analysis
In this study, data were analysed by one‐way ANOVA with Bonferroni's correction by GraphPad Prism 6.0, and the results were presented as means ± standard error of mean (SEM); P <.05 was considered as statistically significant.
3. RESULTS
3.1. The combination of BFOV and metformin ameliorates impaired glucose homeostasis and insulin sensitivity in HFC57 mice
As shown in Figure 1, compared to those of standard diet‐fed mice (Nor), high‐fat diet induced obese C57BL/6J mice (Con) characterized as elevated fasting blood glucose (FBG), hyperinsulinaemia, impaired oral glucose tolerance (AUCOGTT) and insulin resistance (AUCITT). After 2‐week treatment, BFOV + Met displayed more effective effects on the normalization of the FBG (Figure 1A), while the BFOV or metformin monotherapy showed no effects until 4‐week treatment. BFOV + Met exhibited a sustained anti‐hyperglycaemic effect during 6‐week treatment. We conducted ITT after 2‐week treatment; BFOV + Met displayed significant effects on decreasing AUCITT and more effective than BFOV or metformin monotherapy (Figure 1D and 1E). Fasting blood insulin level and HOMA‐IR were also significantly lower in the BFOV + Met group than the BFOV or metformin monotherapy after 42 days of treatment (Figure 1B and 1C). At the end of treatment, BFOV + Met displayed more effective effects on impaired glucose tolerance and decreasing AUCOGTT (Figure 1F) than BFOV or metformin monotherapy.
FIGURE 1.
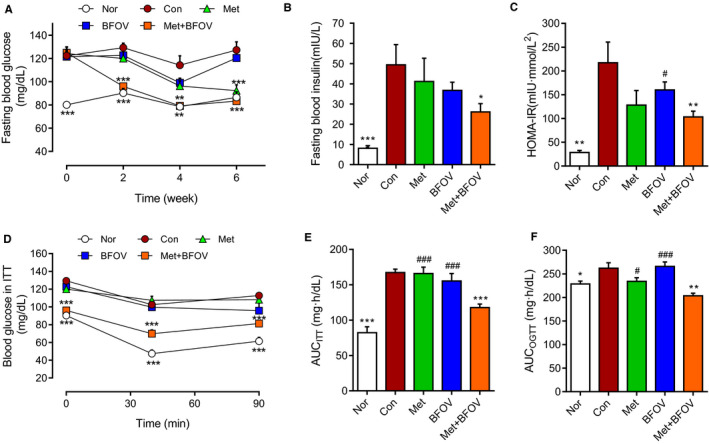
The combination of BFOV and metformin improves impaired glucose homeostasis and insulin sensitivity in HFC57 mice. (A) Fasting blood glucose during the treatment. (B) Fasting blood insulin levels on 42‐days of treatment. (C) Homeostatic (HOMA) model based on fasting glucose and fasting insulin on 42‐days of treatment, HOMA‐IR = [glucose] (mmol/L) × [insulin] (µIU/mL)/22.5). (D, E) Blood glucose levels and area under the curve (AUC) in the ITT after 2‐weeks treatment. (F) AUC in the OGTT after 6‐weeks treatment. Nor, saline‐treated C57BL/6J mice. The statistics were performed with each single time point compared to Con group in Figure 1A and 1D. Con, saline‐treated HFC57 mice. BFOV, 20 mg/kg BW BFOV‐treated HFC57 mice. Met, 100 mg/kg BW metformin‐treated HFC57 mice. BFOV + Met, 20 mg/kg BW BFOV and 100 mg/kg BW metformin treated HFC57 mice. n = 8‐9/group. Values are means ± SEM. One‐way ANOVA: * P <.05, ** P <.01, *** P <.001 vs. Con. # P <.05, ## P <.01, ### P <.001 vs. BFOV + Met
3.2. The combination of BFOV and metformin improves hepatic steatosis in HFC57 mice
As shown in Figure 2A‐2D, BFOV + Met markedly decreased serum ALT and AST levels after 6‐week treatment, accompanied by lowered hepatic TG and CHO contents significantly and more effective than that of BFOV or metformin monotherapy. To evaluate the pathologic morphology, liver sections were stained with haematoxylin‐eosin (HE). In Figure 2E, it is shown that there was extensive micro‐ and macrovesicular hepatocyte vacuolation, reflecting intrahepatic fat accumulation and diffuse fatty degeneration in HFC57 mice (Con group), in comparison with the Nor group. In contrast, the mass of hepatocellular vacuolation was significantly decreased in the BFOV + Met group after 6 week of treatment, less than that in BFOV or metformin monotherapy.
FIGURE 2.
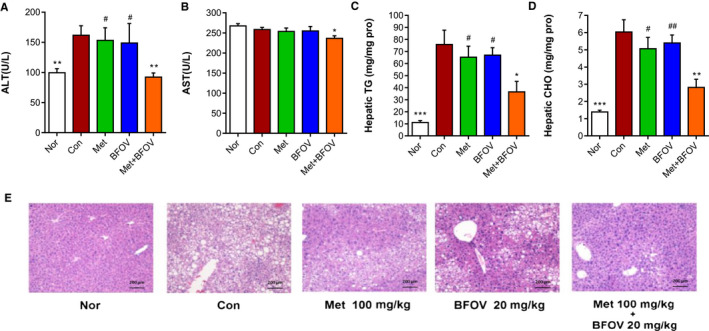
The combination of BFOV and metformin ameliorates hepatic steatosis in HFC57 mice. (A, B) Serum ALT and AST determined after 6‐weeks of treatment. (C, D) Hepatic TG and CHO content. (E) Typical liver sections stained with H&E prepared at the end of treatment and captured under a light microscope (Olympus CX41RF, Olympus, Tokyo, Japan. ×100). Scale bar, 200μm. Nor, saline‐treated C57BL/6J mice. Con, saline‐treated HFC57 mice. BFOV, 20 mg/kg BW BFOV‐treated HFC57 mice. Met, 100 mg/kg BW metformin‐treated HFC57 mice. BFOV + Met, 20 mg/kg BW BFOV and 100 mg/kg BW metformin‐treated HFC57 mice. n = 5/group. Values are means ± SEM. One‐way ANOVA: * P <.05, *** P <.001 vs. Con. # P <.05, ## P <.01, ### P <.001 vs. BFOV + Met
3.3. Comparison of hepatic differential gene expression after a combination of BFOV and metformin treatment
Based on the beneficial effect of treatment with a combination of BFOV and metformin in vivo, especially the improved hepatic steatosis in the BFOV + Met group compared with the Con group, we conducted gene chip analysis to investigate the changes in gene expression in the liver. Differential gene expression fold changes ≥ 5 were selected for further analysis. As shown in Figure 3A, in all differentially expressed genes, Venn diagram analysis identified 628 genes differentially expressed in all three groups, while the BFOV + Met group showed a differential gene expression pattern more similar to the BFOV group than to the metformin group (containing the same changed 541 genes vs. 330 genes, respectively, common to the data sets). In Figure 3B, the functional classes of differentially expressed transcripts in the BFOV + Met group were analysed, the most genetically dynamic functional class was the genes associated with metabolic pathways (13.7%).
FIGURE 3.
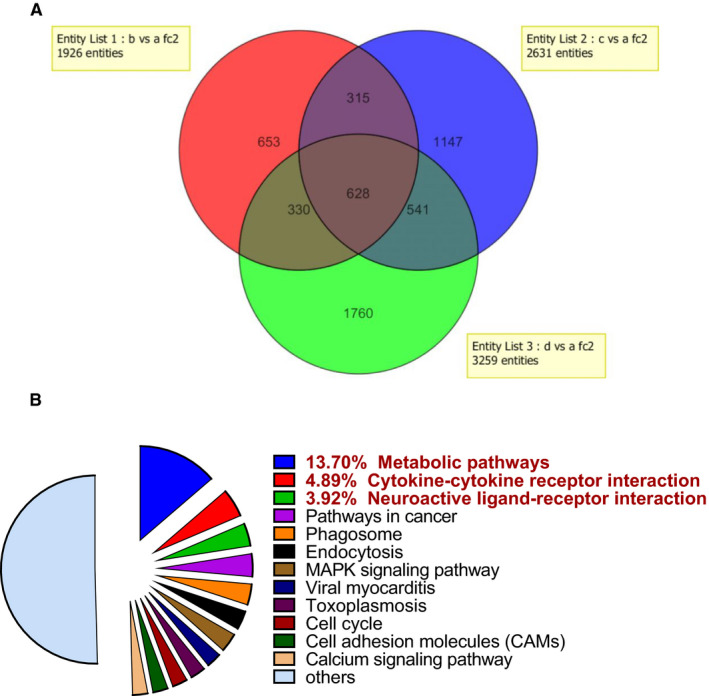
Comparison of hepatic differential gene expression by gene chip analysis. (A) Venn diagram of differentially expressed genes in the liver of HFC57 mice across the three treated groups with either 100 mg/kg metformin alone (Met, Red part, b), 20 mg/kg BFOV alone (BFOV, Blue part, c), or 100 mg/kg metformin and 20 mg/kg BFOV in combination (BFOV + Met, Green part, d). Transcript detection was selected for differential expression of ≥ 5‐folds relative to the Con group (a). (B) Pie charts of the functional classes of differentially expressed transcripts from BFOV + Met group. Transcript detection was selected for differential gene expression of ≥ 5‐folds relative to the Con group. Differentially expressed genes belonging to various functional categories were annotated from the KEGG pathways
Differentially regulated transcripts in the BFOV + Met group are summarized in Table 1. BFOV + Met significantly down‐regulated gene expression of MMPs, NF‐κB, IL1β and RBP4, and up‐regulated gene expression of IR, IRS, PI3K and GCK in the liver, suggesting attenuated inflammatory pathway, enhanced insulin signalling and glucose metabolic pathway after BFOV + Met treatment.
TABLE 1.
Different hepatic gene expressions in the BFOV + Met group compared with the Con group in the microarray analysis
| Probe ID | GeneBank No. | Gene name | Gene symbol | Fold changes |
|---|---|---|---|---|
| Lipid metabolism | ||||
| ILMN_2699880 | NM_147220.1 | ATP‐binding cassette, sub‐family A member 9 | Abca9 | +5.6 |
| ILMN_2874104 | NM_007988.3 | Fatty acid synthase | Fasn | −2.9 |
| ILMN_1221255 | NM_010700.2 | Low‐density lipoprotein receptor | Ldlr | +2.2 |
| ILMN_3111326 | NM_001039507.1 | Lipase, hormone‐sensitive | Lipa | −3.2 |
| Insulin signalling transduction | ||||
| ILMN_2753272 | NM_009167.2 | Src homology 2 containing transforming protein C3 | Shc3 | +2.4 |
| ILMN_2628026 | NM_010568.1 | Insulin receptor | Insr | +2.5 |
| ILMN_2755312 | NM_010571.3 | Insulin receptor substrate 3 | Irs3 | +3.0 |
| 1426690_a_at | AI326423 | Phosphoinositide‐3‐kinase, catalytic, gamma | Pik3cg | +3.2 |
| Inflammatory signalling pathway | ||||
| ILMN_2642239 | NM_019408.1 | Nuclear factor of kappa polypeptide gene enhancer 2 | Nfkb2 | −2.1 |
| ILMN_3103896 | NM_011593.2 | Tissue inhibitor of metalloproteinase 1 | Timp1 | −4.2 |
| ILMN_3077034 | NM_032006.2 | Matrix metallopeptidase 1a (interstitial collagenase) | Mmp1a | −5.7 |
| ILMN_2678218 | NM_008610.2 | Matrix metallopeptidase 2 | Mmp2 | −1.6 |
| ILMN_2753809 | NM_010809.1 | Matrix metallopeptidase 3 | Mmp3 | −6.5 |
| ILMN_2799267 | NM_008607.1 | Matrix metallopeptidase 13 | Mmp13 | −2.6 |
| ILMN_1233678 | NM_008364.1 | Interleukin 1 receptor accessory protein | Il1rap | −3.2 |
| ILMN_2777498 | NM_008361 | interleukin 1 beta | Il1b | −4.6 |
| ILMN_2854497 | NM_011608.1 | Tumour necrosis factor receptor superfamily, 17 | Tnfrsf17 | −4.7 |
| Others | ||||
| ILMN_2813859 | NM_009034.2 | Retinol‐binding protein 2, cellular | Rbp4 | −5.4 |
| ILMN_2697615 | NM_013871.2 | Mitogen‐activated protein kinase 12 | Mapk12 | +3.2 |
| ILMN_2736578 | NM_146146.1 | Leptin receptor | Lepr | +2.3 |
| 1425303_at | L38990 | Glucokinase | Gck | +1.8 |
Transcript detection was selected for differential expression of ≥ 5‐fold relative to the Con group.
3.4. The combination of BFOV and metformin improves hepatic inflammatory state and energy metabolism via down‐regulating inflammatory signalling pathway and activating the insulin signalling pathway in HFC57 mice
To assess the inflammatory state in the liver, enzyme activity and protein expression levels of a certain factor involved in the inflammatory pathway were detected. As shown in Figure 4A and 4B, the HFC57 mice showed elevated hepatic NO levels and iNOS activity compared with the Nor group, indicating elevated inflammatory state in the liver after high‐fat diet‐induced obesity. BFOV + Met markedly reduced the hepatic iNOS activity and more effectively than BFOV or metformin monotherapy. To assess the combined therapeutic effect of BFOV + Met treatment on hepatic inflammation state, we detected the expression of key factors involved in the inflammatory signalling pathway in the liver. According to the results of gene chip analysis, the expression levels of NF‐κB‐p65, MMP2 and iNOS were detected. As shown in Figure 4C‐4E, the expressions of iNOS and MMP2 were elevated in the liver of HFC57 mice, while they were all markedly down‐regulated in the BFOV + Met group, compared to the Con group.
FIGURE 4.
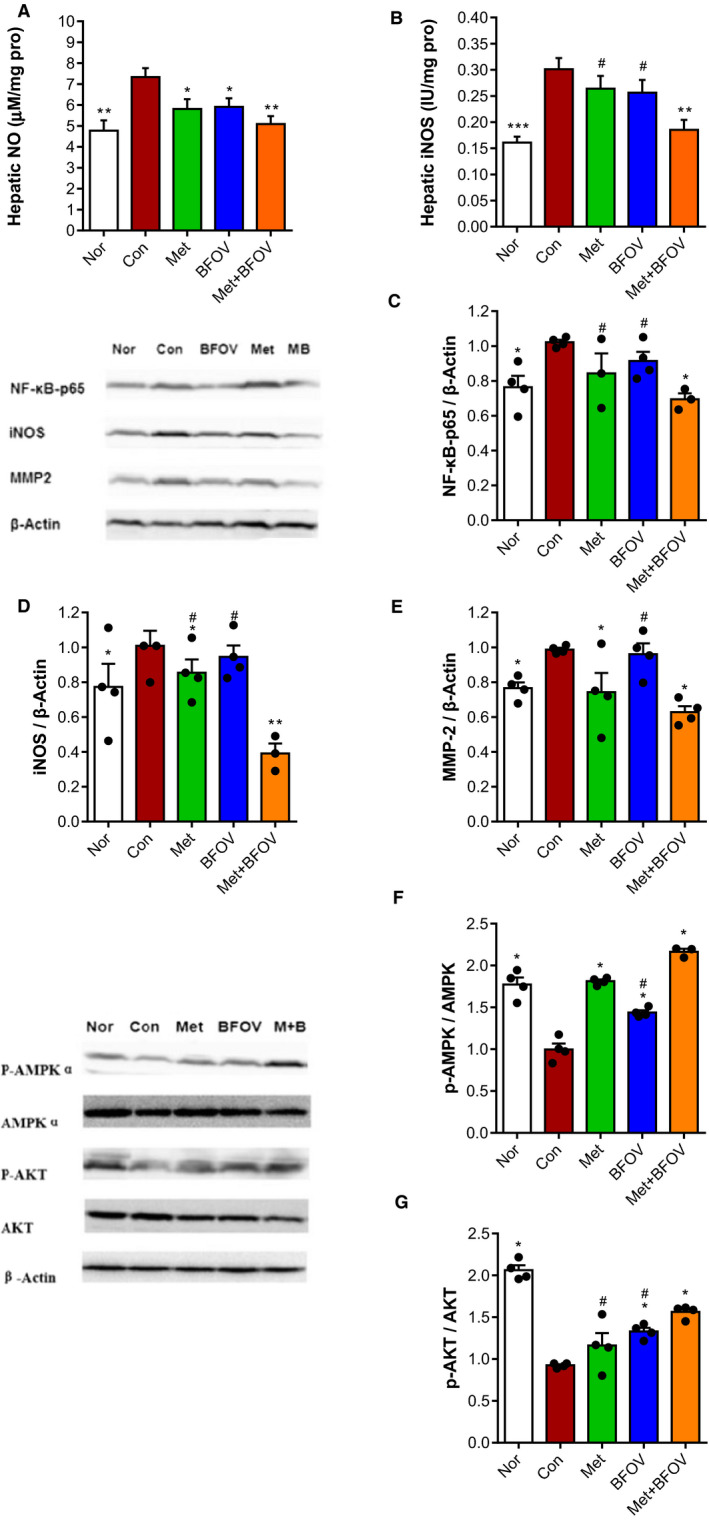
The combination of BFOV and metformin improved hepatic inflammatory state and energy metabolism via downregulating inflammatory signaling pathways and activating phosphorylation of AMPK and AKT in the liver of HFC57 mice. (A) Hepatic NO content. (B) Hepatic iNOS activity. (C‐E) Representative bands and quantitative analysis of main factors involving in the inflammatory pathway in the liver by Western blot, including (C) NFκB‐p65 (65KD), (D) iNOS (130KD) and (E) MMP‐2 (72KD). (F, G) Representative bands and quantitative analysis of ratio of phosphorylation AMPKα (62KD) and phosphorylation AKT (60KD) expression in liver by Western blot, each phosphorylation was normalized by the total amount of (F) AMPK (62KD), (G) AKT (60KD). β‐Actin served as a loading control. Data represented the mean of at least three independent experiments ± SEM. One‐way ANOVA: * P <.05, ** P <.01, *** P <.001 vs. Con. # P <.05, ## P <.01, ### P <.001 vs. BFOV + Met
Besides, to investigate whether the improved glucose metabolism in the BFOV + Met group is relative to activated energy metabolism and insulin signalling pathway in the liver, we detected the phosphorylation levels of hepatic AMPK and AKT, the key factors involved in those pathways. As shown in Figure 4F and 4G, it was shown that the phosphorylation ratio of both AMPK and AKT significantly decreased in the HFC57 mice, compared to the Nor group. A combination of BFOV and metformin markedly enhanced the phosphorylation ratio of both AMPK and AKT, compared to the Con group.
4. DISCUSSION
We have reported a new orally active antidiabetic vanadyl complex, bis (a‐furancarboxylato) oxovanadium (IV) (BFOV), which displayed a potent antidiabetic potential both in type 1 and type 2 diabetic animals in our previous studies. 15 , 18 BFOV reduced FFA release from isolated rat adipocytes treated with epinephrine and enhanced the uptake of 2‐deoxy‐D‐[3H]‐glucose in dexamethasone‐induced insulin resistance 3T3‐L1 adipocyte. 19 Because the BFOV and metformin (Met) have different roles in regulating glucose homeostasis, including improving the insulin sensitivity of adipose tissue and reducing hepatic glucose production, respectively, we hypothesized the combination of these two agents would provide a combined effect on insulin resistance and related hepatic steatosis in a high‐fat diet‐induced obese animal model. Therefore, the present study is the first to examine the effects of combination therapy with BFOV and Met on type 2 diabetes and hepatic steatosis in high‐fat diet‐induced obese C57 mice (HFC57 mice). We conducted a combined application of the two in alloxan‐induced hyperglycaemic mice and explored the best ratio of the synergistic effect between the two, BFOV (5 mg/kg, 10mg/kg or 20 mg/kg) and Met (100 mg/kg). The probability sum test showed that when BFOV (20 mg/kg) and Met (100 mg/kg) were used in combination, the antidiabetic synergistic effect was the greatest. According to these results, in the present study, we chose the best dosing ratio, being BFOV (20 mg/kg) and Met (100 mg/kg) in the HFC57 mice.
Consistent with our previous findings in diabetic KKAy mice, a combination of BFOV and metformin (BFOV + Met) displayed synergistic antidiabetic effects in HFC57 mice, including decreased FBG and FINS after 4 weeks of treatment (Figure 1), and also improved glucose tolerance and insulin sensitivity. Interestingly, BFOV + Met potentiated the effects of BFOV or metformin on improving glucose homeostasis, respectively. As shown above, the combined therapy of BFOV and Met synergistically improved whole‐body insulin sensitivity, with decreased AUC and increased decreasing percentage of blood glucose at 40 minutes in ITT (Figure 1C). Different mechanisms may be involved in the improvement of BFOV + Met on glucose homeostasis. To explain the mechanisms of BFOV + Met on a metabolic pathway, a microarray analysis using the Affymetrix‐GeneChip MouseWG‐6 v2 was conducted in the liver at the end of treatment. It was shown that the BFOV + Met group showed more similar to the BFOV group (628 + 541) than to the metformin group (628 + 330) in gene expression pattern (Figure 3A). Compared to the Con group, the differentially expressed genes of up‐regulated and down‐regulated genes identified (total 3259) in the BFOV + Met group were functionally annotated and subsequently classified into over ten functional categories (Figure 3B). These categories mainly included metabolism (14%), cytokine and receptor interaction (5%) and so on.
The AMP‐activated protein kinase (AMPK) system acts as a sensor of cellular metabolic status that is conserved in all eukaryotic cells. 20 Metformin inhibits the mitochondrial respiratory chain in the liver, leading to activation of AMPK and improved insulin sensitivity. 21 It is already clear that AMPK is one of the main targets of metformin. It has also been revealed that vanadium compounds exert insulin‐like properties effects via multiple mechanisms involving activation of PPARs‐AMPK signalling and several key components of insulin signalling pathways, 22 , 23 including the mitogen‐activated protein kinases (MAPKs) extracellular signal‐regulated kinase 1/2 (ERK1/2) and p38MAPK, and phosphatidylinositol 3‐kinase (PI3‐K)/AKT. 24 Based on this evidence, we explored whether the synergistic antidiabetic effects of a combination of BFOV and metformin in HFC57 mice depend on the activation of AMPK and enhanced insulin signalling pathway in the liver. The key elements of these two signalling cascade phosphorylation levels of AMPK and AKT were detected in the liver by Western blot. BFOV + Met treatment synergistically up‐regulated the phosphorylation of AMPK and AKT levels, more effectively than BFOV or metformin monotherapy, suggesting a significant enhanced AMPK and insulin signalling pathway due to the synergistic effects.
Also, BFOV + Met treatment also decreased hepatic TG and CHO content and alleviative AST and ALT levels at the end of treatment. In the liver, sustained TG accretion leads to non‐alcoholic fatty liver disease (NAFLD). Pharmacological interventions pursued to prevent lipid accumulation in hepatocytes would ameliorate the associated pathophysiological conditions. 25 Furthermore, the most expected finding of this study was the robust effect of BFOV + Met on liver pathology, and decreased mass of hepatocellular lipid vacuolation in BFOV + Met group was observed by histological staining with HE, compared to the Con group, which indicates a decrease in liver lipid accumulation and improved hepatic steatosis. Thus, the present findings showed that the combination of BFOV and metformin provided better control not only on hyperglycaemia than the individual component monotherapy, but also on markedly improved hepatic steatosis in the HFC57 mice.
Recent studies have found when excess hepatic lipid accumulates, it often causes insulin resistance and chronic inflammation on increasing the risk of progressive liver disease. As shown in Table 1, GeneChip assay has shown that BFOV + Met ameliorated the hepatic lipid metabolism via up‐regulating Abca9 and Ldlr and down‐regulating Fasn gene expression levels (Table 1). Moreover, many inflammatory factors are involved in hepatic insulin resistance and steatosis has been also down‐regulated after BFOV + Met treatment in the GeneChip assay. NF‐κB is the main transcription factor involved in the inflammatory pathway. The activation of NF‐κB has been demonstrated in several liver diseases both in humans and in mice, including patients with fatty liver. 26 As expected, mRNA levels of NF‐κB and IL1β gene expression levels in the liver were down‐regulated over twofold after BFOV + Met treatment, compared to the Con group. MMPs are known to play vital roles in the degradation of basement membranes and extracellular matrix (ECM). Many studies also show that MMPs can be induced in multiple cells by inflammatory cytokines including IL1β and TNF‐α. 27 , 28 , 29 As expected, mRNA levels of MMP1a, MMP2, MMP3, MMP13, NF‐κB and IL1β in the liver were down‐regulated over twofold after BFOV + Met treatment, compared to the Con group.
According to these results from the GeneChip assay, we subsequently detected the enzyme activities and protein expression levels of MMP2, iNOS and NF‐κB in the liver. It can be summarized as follows (Figure 4A‐E): hepatic MMP2, iNOS and NF‐κB expression was increased in HFC57 and also elevated NO content and iNOS activity, indicating chronic inflammatory states in the liver. BFOV + Met treatment synergistically reduces the MMP2, iNOS and NF‐κB expression and also NO content and iNOS activity in the liver. These results indicate that prolonged exposure to an inflammatory state led to impaired altered insulin signalling and oxidative stress in the liver in HFC57 mice. Treatment with BFOV + Met significantly improved the hepatic inflammatory state, more effectively than BFOV or metformin monotherapy. It was indicated that there must be additional mechanism involved in the BFOV + Met group. Actually, we further investigated the underlying mechanism of the combination of BFOV + Met treatment in the HepG2 cell line in vitro (data not shown). These results indicated that Met might enhance the efficacy of BFOV by further activating insulin signalling and co‐affecting a multitude of enzymatic processes and thus synergistically improving the insulin resistance state.
In summary, NAFLD is becoming a public health burden. While many antidiabetic drug agents have shown to improve biochemical parameters, the effects on improvement of liver histology are limited. In this study, we have shown that a combination of BFOV and metformin improved hyperglycaemia and hepatic steatosis above and beyond what could be expected from monotherapy, which provides a new promising approach of complementary therapy in NAFLD.
5. CONCLUSIONS
In conclusion, our study provides profound evidence that a combination of bis (α‐furancarboxylato) oxovanadium (IV) (BFOV) and metformin (Met) improves hepatic steatosis in high‐fat diet‐induced obese C57BL/6J mice. BFOV + Met corrected diet‐induced glucose intolerance, insulin resistance and hepatic steatosis through enhancing insulin signalling pathways and down‐regulating inflammatory state. Hence, the development of a pharmacological combination of metformin and the vanadium organic complexes may be a useful strategy to combat obesity‐related disorders such as IR and NAFLD.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENT
This study is supported by grants from the National Natural Science Foundation of China (Grant no. 81973379, 81260502 and 81900480), Natural Science Foundation of Beijing Municipality (Grant no. 7202137 and 7204281) and Yunnan Provincial Science Foundation (Grant no. 2012FB021), partly supported by grants from the Drug Innovation Major Project of the National Major Science and Technology Projects of China (Grant nos. 2018ZX09711001‐003‐011 and 2018ZX09711001‐009‐014) and the CAMS Innovation Fund for Medical Sciences (Grant no. 2017‐I2M‐1‐010).
Liu Q, Li L, Gao L, et al. Combination of bis (α ‐furancarboxylato) oxovanadium (IV) and metformin improves hepatic steatosis through downregulating inflammatory pathways in high‐fat diet‐induced obese C57BL/6J mice. Basic Clin Pharmacol Toxicol. 2021;128:747–757. 10.1111/bcpt.13573
Contributor Information
Shuainan Liu, Email: liusn@imm.ac.cn, Email: shenzhf@imm.ac.cn.
Zhufang Shen, Email: shenzhf@imm.ac.cn.
REFERENCES
- 1. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47‐64. [DOI] [PubMed] [Google Scholar]
- 2. Sumida Y, Yoneda M, Tokushige K, et al. Antidiabetic therapy in the treatment of nonalcoholic steatohepatitis. Int J Mol Sci. 2020;21:1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perumpail BJ, Cholankeril R, Yoo ER, Kim D, Ahmed A. An overview of dietary interventions and strategies to optimize the management of non‐alcoholic fatty liver disease. Diseases. 2017;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cholankeril R, Patel V, Perumpail BJ, et al. Anti‐diabetic medications for the pharmacologic management of NAFLD. Diseases. 2018;6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boeckmans J, Natale A, Rombaut M, et al. Anti‐NASH drug development hitches a lift on PPAR agonism. Cells 2019;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blazina I, Selph S. Diabetes drugs for nonalcoholic fatty liver disease: a systematic review. Syst Rev. 2019;8:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finck BN. Targeting metabolism, insulin resistance, and diabetes to treat nonalcoholic steatohepatitis. Diabetes 2018;67:2485‐2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Willsky GR, Chi LH, Godzala M 3rd, et al. Anti‐diabetic effects of a series of vanadium dipicolinate complexes in rats with streptozotocin‐induced diabetes. Coord Chem Rev. 2011;255:2258‐2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haratake M, Fukunaga M, Ono M, Nakayama M. Synthesis of vanadium(IV, V) hydroxamic acid complexes and in vivo assessment of their insulin‐like activity. J Biol Inorg Chem. 2005;10:250‐258. [DOI] [PubMed] [Google Scholar]
- 10. Domingo JL, Gomez M. Vanadium compounds for the treatment of human diabetes mellitus: A scientific curiosity? A review of thirty years of research. Food Chem Toxicol. 2016;95:137‐141. [DOI] [PubMed] [Google Scholar]
- 11. Li L, Gao L, Liu S, et al. Bis(alpha‐furancarboxylato)oxovanadium(IV) exerts durable antidiabetic effects and suppresses matrix metalloproteinase‐2 activity in spontaneous type 2 diabetic KKAy mice. Biol Trace Elem Res. 2013;153:329‐339. [DOI] [PubMed] [Google Scholar]
- 12. Brandt A, Hernandez‐Arriaga A, Kehm R, et al. Metformin attenuates the onset of non‐alcoholic fatty liver disease and affects intestinal microbiota and barrier in small intestine. Sci Rep. 2019;9:6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Handzlik G, Holecki M, Kozaczka J, et al. Evaluation of metformin therapy using controlled attenuation parameter and transient elastography in patients with non‐alcoholic fatty liver disease. Pharmacol Rep. 2019;71:183‐188. [DOI] [PubMed] [Google Scholar]
- 14. Tveden‐Nyborg P, Bergmann TK, Lykkesfeldt J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol. 2018;123:233‐235. [DOI] [PubMed] [Google Scholar]
- 15. Xie M, Gao L, Li L, Liu W, Yan S. A new orally active antidiabetic vanadyl complex–bis(alpha‐furancarboxylato)oxovanadium(IV). J Inorg Biochem. 2005;99:546‐551. [DOI] [PubMed] [Google Scholar]
- 16. Liu Q, Liu S, Gao L, et al. Anti‐diabetic effects and mechanisms of action of a Chinese herbal medicine preparation JQ‐R in vitro and in diabetic KK(Ay) mice. Acta Pharm Sin B. 2017;7:461‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu SN, Liu Q, Li LY, Huan Y, Sun SJ, Shen ZF. Long‐term fenofibrate treatment impaired glucose‐stimulated insulin secretion and up‐regulated pancreatic NF‐kappa B and iNOS expression in monosodium glutamate‐induced obese rats: is that a latent disadvantage? J Transl Med. 2011;9:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niu Y, Liu W, Tian C, et al. Effects of bis(alpha‐furancarboxylato)oxovanadium(IV) on glucose metabolism in fat‐fed/streptozotocin‐diabetic rats. Eur J Pharmacol. 2007;572:213‐219. [DOI] [PubMed] [Google Scholar]
- 19. Zuo YQ, Liu WP, Niu YF, et al. Bis(alpha‐furancarboxylato)oxovanadium(IV) prevents and improves dexamethasone‐induced insulin resistance in 3T3‐L1 adipocytes. J Pharm Pharmacol. 2008;60:1335‐1340. [DOI] [PubMed] [Google Scholar]
- 20. Garcia D, Shaw RJ. AMPK: Mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell. 2017;66:789‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duca FA, Cote CD, Rasmussen BA, et al. Metformin activates a duodenal Ampk‐dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21:506‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu Y, Huang M, Zhao P, Yang X. Vanadyl acetylacetonate upregulates PPARgamma and adiponectin expression in differentiated rat adipocytes. J Biol Inorg Chem. 2013;18:623‐631. [DOI] [PubMed] [Google Scholar]
- 23. Zhang L, Huang Y, Liu F, Zhang F, Ding W. Vanadium(IV)‐chlorodipicolinate inhibits 3T3‐L1 preadipocyte adipogenesis by activating LKB1/AMPK signaling pathway. J Inorg Biochem. 2016;162:1‐8. [DOI] [PubMed] [Google Scholar]
- 24. Gallardo‐Vera F, Tapia‐Rodriguez M, Diaz D, Fortoul van der Goes T, Montano LF, Rendon‐Huerta EP. Vanadium pentoxide increased PTEN and decreased SHP1 expression in NK‐92MI cells, affecting PI3K‐AKT‐mTOR and Ras‐MAPK pathways. J Immunotoxicol. 2018;15:1‐11. [DOI] [PubMed] [Google Scholar]
- 25. Quiroga AD, Lehner R. Pharmacological intervention of liver triacylglycerol lipolysis: The good, the bad and the ugly. Biochem Pharmacol. 2018;155:233‐241. [DOI] [PubMed] [Google Scholar]
- 26. Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naim A, Pan Q, Baig MS. Matrix Metalloproteinases (MMPs) in Liver Diseases. J Clin Exp Hepatol. 2017;7:367‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Munsterman ID, Kendall TJ, Khelil N, et al. Extracellular matrix components indicate remodelling activity in different fibrosis stages of human non‐alcoholic fatty liver disease. Histopathology 2018;73:612‐621. [DOI] [PubMed] [Google Scholar]
- 29. Yokomori H, Oda M, Ando W, Inagaki Y, Okazaki I. Hepatic progenitor cell expansion in early‐stage nonalcoholic steatohepatitis: evidence from immunohistochemistry and immunoelectron microscopy of matrix metalloproteinase‐1. Med Mol Morphol. 2017;50:238‐242. [DOI] [PubMed] [Google Scholar]


