Abstract
Objective
Lip skin dryness and chapping are major concerns related to lip skin care in many populations. The distinctive features of lip skin, such as the low water‐holding capacity and weak skin barrier, are strongly associated with these problems; however, few studies have examined lip skin characteristics and the mechanisms underlying these issues. This study was conducted to identify the biophysical properties of dry lip skin and molecular targets affecting lip skin physiology.
Methods
Skin hydration, transepidermal water loss and lip skin scaling were evaluated in 40 female subjects. Skin scaling was assessed as a percentage area divided into five categories (G0, G1, G2, G3 and G4) according to the thickness level of tape‐stripped corneocytes. The activities and amounts of proteases, cathepsin D and bleomycin hydrolase were measured as markers for the desquamation process and skin hydration, respectively.
Results
Skin hydration showed a significantly positive correlation with the percentage area of evenly thin corneocytes (G0) and negative correlations with the percentage areas of slightly thick to severely thick corneocytes (G1‐G4). The corneocyte unevenness ratio (CUR) was calculated by dividing the sum of the G1, G2, G3 and G4 values with the G0 value. The CUR was significantly negatively correlated with skin hydration, suggesting that CUR is a new parameter representing the severity of lip scaling. Subjects with lower hydration and higher CUR had higher bleomycin hydrolase activity and lower cathepsin D activity, respectively, than subjects with higher hydration and lower CUR.
Conclusion
Our study revealed a correlation between lip skin hydration and severity of lip scaling and verified the association of protease activity with the hydration and chapping state of lip skin. These observations provide a basis for further studies of the persistent problem of lip skin dryness and chapping.
Keywords: lip scaling, protease activity, skin barrier, skin dryness, skin physiology, statistics, structure
Résumé
Objectif
La sécheresse et la gerçure de la peau des lèvres sont des préoccupations majeures liées aux soins de la peau des lèvres chez de nombreuses populations. Les caractéristiques distinctives de la peau des lèvres, telles que la faible capacité de rétention d’eau et la faible barrière cutanée, sont fortement associées à ces problèmes ; cependant, peu d’études ont examiné les caractéristiques de la peau des lèvres et les mécanismes sous‐jacents à ces problèmes. Cette étude a été menée dans le but d’identifier les propriétés biophysiques de la peau sèche des lèvres et les cibles moléculaires affectant la physiologie de la peau des lèvres.
Méthodes
L’hydratation cutanée, la perte d’eau transépidermique et la desquamation de la peau des lèvres ont été évaluées chez 40 sujets de sexe féminin. La desquamation cutanée a été évaluée en tant que pourcentage de surface, divisée en cinq catégories (G0, G1, G2, G3 et G4) en fonction du niveau d’épaisseur des cornocytes sur la bande adhésive. Les activités et quantités des protéases, de la cathepsine D et de la bléomycine hydrolase ont été mesurées comme marqueurs du processus de desquamation et de l’hydratation cutanée, respectivement.
Résultats
L’hydratation cutanée a montré une corrélation significativement positive avec le pourcentage de surface avec cornocytes uniformément minces (G0), et des corrélations négatives avec les pourcentages de surface avec cornocytes légèrement épais à très épais (G1‐G4). Le rapport d’irrégularité des cornocytes (Corneocyte Unevenness Ratio, CUR) a été calculé en divisant la somme des valeurs de G1, G2, G3 et G4 par la valeur de G0. Le CUR était significativement corrélé négativement avec l’hydratation de la peau, ce qui suggère que le CUR est un nouveau paramètre représentant la gravité de la desquamation des lèvres. Les sujets avec une hydratation plus faible et un CUR plus élevé présentaient une activité de la bléomycine hydrolase plus élevée et une activité de la cathepsine D plus faible, respectivement, par rapport aux sujets avec une hydratation plus élevée et un CUR plus faible.
Conclusion
Notre étude a révélé une corrélation entre l’hydratation de la peau des lèvres et la gravité de la desquamation des lèvres, et a vérifié l’association de l’activité de la protéase avec l’état d’hydratation et de gerçure de la peau des lèvres. Ces observations fournissent une base pour d’autres études sur le problème persistant de la sécheresse et de la gerçure de la peau des lèvres.
INTRODUCTION
The lip skin has unique characteristics because it is located at the boundary of the mucous membrane and facial skin. Unlike the skin on the rest of the body, the lip skin comprises a thin stratum corneum (SC) and no hair or sweat glands. Additionally, the non‐keratinized epithelium of the lip skin is directly exposed to the external environment. These features make the lip skin more susceptible to dry conditions than skin on the other areas of the body. The lip skin has a higher transepidermal water loss (TEWL) and lower water content than skin on the cheeks [1, 2].
The water‐holding capacity of the lip skin is strongly associated with lip function. Dry and chapped lips accompanied by flaking and peeling of corneocytes (scales) are among the most common problems in the population, with lips showing a low water‐holding capacity. Even the population with normal skin often experiences these issues in extreme climatic conditions.
However, previous studies of the lip skin mostly focused on age‐related changes rather than functional problems. Some studies revealed changes in biophysical features and found that the hydration (capacitance) of the lip increases, whereas the TEWL and redness decrease with age [3, 4]. In morphological studies, young subjects showed a higher lip thickness than that in elder subjects, and an increase in the width of the lips and flattened vermilion border has been observed with age [5, 6].
A few groups have attempted to identify the key physiological factors affecting lip skin dryness. Tamura et al. [7] demonstrated that the degree of lip skin roughness is related to ceramide profiles, including their amount, species and carbon numbers. Additionally, Hikima et al. [8] reported that cathepsin D (CTSD) activity is related to chapping of the lips.
Proteases such as CTSD are one of the biological factors involved in various activities in the skin, including the desquamation process [9]. They also control generation of the natural moisturizing factor (NMF) by regulating their precursor, filaggrin. Thus, altered activities or expression of proteases are associated with dry skin.
CTSD, an aspartic acid protease, is involved in desmosomal degradation and activation of transglutaminase 1 in the SC. CTSD is essential for the epidermis desquamation process [10], and the role of CTSD in repairing the photo‐damaged skin barrier has been reported [11].
Bleomycin hydrolase (BH) is a neutral cysteine protease involved in the final steps of NMF generation by degradation of the filaggrin monomer. The expression level of BH decreases in the dry skin of healthy humans [12]; impaired BH activity in the lesions of atopic dermatitis has been reported [13]. Additionally, conflicting reports describe elevated BH activity in sun‐exposed areas of the skin [14] and during the winter [15].
Several studies have investigated the skin characteristics related to dryness of the face and body, but few have focused on the characteristics of the lip skin. Additionally, the relationship between quantitative parameters related to dry and chapped lip skin has not been examined using in vivo and ex vivo methods.
Hence, this study was conducted to determine the relationship between lip dryness and chapping‐related biophysical parameters and to verify the role of CTSD and BH according to the lip skin condition.
MATERIALS AND METHODS
Subjects
Forty healthy female subjects from Korea, aged 30.40 ± 4.66 years, participated in this study. The study was performed under the Declaration of Helsinki and approved by the Institutional Review Board of LG Household & Health Care. Before participation, all subjects were informed of the aims of the study and provided written informed consent. The participants applied no topical agents on their lips for at least 12 hour before the measurements. During the measurement, all activities that could affect lip skin characteristics such as drinking, lip licking and other activities were restricted. The measurements were conducted in April 2020.
Biophysical measurements
The lips of the subjects were wiped using paper towels and acclimated for 20 minutes in an air‐conditioned room (temperature: 22 ± 2°C; relative humidity: 50 ± 10%). All measurements were performed on the lower lip vermilion. Skin hydration was measured using a capacitance based Corneometer® CM 825 (C + K electronic GmbH, Köln, Germany) and expressed as arbitrary units (a.u.). TEWL was measured using a Tewameter® TM nano (C + K electronic GmbH), which is an open‐chamber device suitable for measuring water flux density diffusing from the small skin sites such as lips, nails and scalp. Skin corneocytes produced during the desquamation process were collected by stripping the surface of the lips twice with black D‐squame® tape (CuDerm, Dallas, TX, USA) and assessed using the Visioscan® VC 98 (C + K electronic GmbH) and its software (Visioscan 2000 FW).
The captured image of corneocytes using Visioscan® VC 98 was shown in 256 grey levels (0‐255 distributions) depending on the difference in ultraviolet light absorption due to the thickness of skin flakes (Figure 1a). To eliminate unnecessary noise and analyse the corneocytes clearly, the grey‐level threshold was manually adjusted from 0 to 130. The grey levels of corneocyte images showing a range of 130 to 255 were classified into five categories based on the degree of flake thickness [16], including G0, G1, G2, G3 and G4, and each category was expressed in a different colour. These values in each category reflect the percentage area of corneocytes corresponding to a specific range of grey levels: G0 (130‐155), G1 (155‐180), G2 (180‐205), G3 (205‐230) and G4 (230‐255) (Figure 1b‐c). For instance, G0 and G4 represent the percentages of even layers of corneocytes and thick flakes for the total analysis area, respectively.
Figure 1.
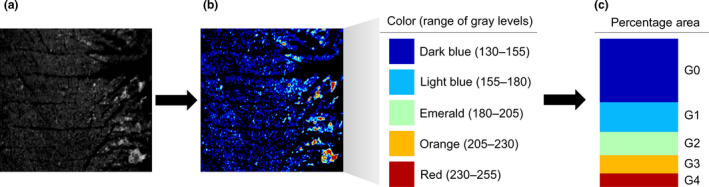
Analysis of corneocyte‐related parameters (G0, G1, G2, G3 and G4). (a) Images of tape‐stripped corneocytes from the lip skin using the Visioscan® VC 98. (b) Classification and colour assignment into five categories based on the thickness of lip skin flakes. (c) Calculation of percentage area in each category (G0, G1, G2, G3 and G4)
Protein extraction from the lip skin
To quantify proteases in the samples, proteins were extracted as described with slight modification [17]. The stripping tape containing the corneocytes from the lower lip skin was immersed in Mammalian Protein Extraction Reagent (M‐PER™, 78503; Thermo Fisher Scientific, Waltham, MA, USA) followed by 15 minutes of sonication in an iced‐cooled (0‐4°C) ultrasonic water bath (Bransonic® 2510, Danbury, CT, USA) within 10 minutes. The total protein extracted from a sample was measured using the Pierce bicinchoninic acid assay kit (23227; Thermo Fisher Scientific) according to the manufacturer’s protocol. All samples were immediately stored at −80°C until analysis.
Measurement of protease activity
CTSD activity was measured using a CTSD activity assay kit (ab65302; Abcam, Cambridge, UK) according to the manufacturer’s instructions. Briefly, pre‐chilled CD cell lysis buffer was added to protein extracts from the lip skin. The samples were mixed with the substrate suspended in the reaction buffer. Fluorescence from the cleaved substrate was measured with the VICTOR multi‐label plate reader (PelkinElmer, Waltham, MA, USA) at an excitation/emission of 328/460 nm.
BH activity was quantified as described [18, 19]. Briefly, protein extracts from the lip skin were treated with 0.1 mM H‐citrulline‐AMC fluorescent substrate (4019017; Bachem, Bubendorf, Switzerland) and buffer [50 mM 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid, 5 mM ethylenediaminetetraacetic acid and 10 mM dithiothreitol dissolved in distilled water]. Fluorescence intensity was measured at an excitation/emission of 328/460 nm using the VICTOR multi‐label plate reader.
Quantification of protease concentrations
The expression levels of both markers were determined using a commercial enzyme‐linked immunosorbent assay kit according to the manufacturer's instructions; CTSD levels were measured using the Human CTSD enzyme‐linked immunosorbent assay kit (ab119586, Abcam), and BH levels were measured using the LS‐FS6623 assay kit (LS‐F6623, LSbio, Seattle, WA, USA). Absorbance was measured at a wavelength of 450 nm using an Epoch™ microplate spectrophotometer (BioTek, Winooski, VT, USA).
Statistical analysis
All statistical analyses were performed using SPSS Statistics, version 25 (IBM Corp., Armonk, NY, USA). The normality of data was tested by the Shapiro‐Wilk test. The relationships between biophysical parameters were analysed by Pearson’s correlation test. Statistical comparisons of the features of each biomarker between groups were performed using the Mann‐Whitney U test. p‐values < 0.05 were significant.
RESULTS
Correlations between lip dryness‐related biophysical parameters
Table 1 shows the correlation coefficients between the skin biophysical parameters related to lip dryness. There were significant correlations between skin hydration and corneocyte‐related parameters. Skin hydration had a significant positive correlation with G0, the percentage area of thin corneocytes. However, significant negative correlations between skin hydration and the other four corneocyte‐related parameters (G1‐G4) were observed. G1‐G4 represent corneocytes that are thicker than those of G0. Thus, lip skins showing more hydration had a higher percentage area of uniform corneocytes and lower percentage of tangled corneocytes than lip skins showing lower hydration.
Table 1.
Correlation coefficients between skin biophysical parameters
| Corneocyte‐related parameters | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Hydration | TEWL | G0 | G1 | G2 | G3 | G4 |
| Hydration | ‐ | −0.065 | 0.518*** | −0.532*** | −0.606*** | −0.601*** | −0.568*** |
| TEWL | ‐ | −0.271 | −0.275 | −0.064 | 0.075 | 0.074 | |
Values of **p < 0.01 and ***p < 0.001 were determined by the Pearson’s correlation test. TEWL, transepidermal water loss; G0, grey levels 130‐155; G1, grey levels 155‐180; G2, grey levels 180‐205; G3, grey levels 205‐230; G4, grey levels 230‐255.
Based on the results, a ratio of all thick overlapped corneocytes (G1‐G4) to uniformly desquamated corneocytes (G0), the corneocyte unevenness ratio (CUR), was calculated as a new integrated parameter representing the severity of lip scaling using the following equation:
A significant negative correlation between skin hydration and CUR was obtained (Figure 2). Figure 3 shows the difference in lip corneocytes between relatively high‐ and low‐CUR values. There were no significant correlations between TEWL and the other parameters.
Figure 2.
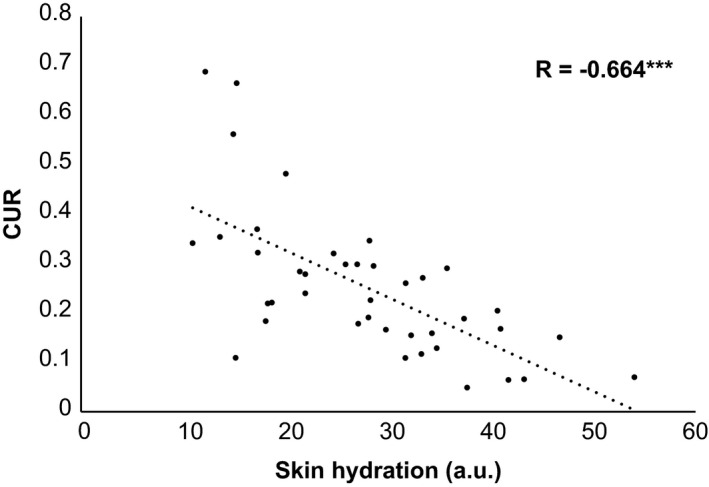
Correlation between skin hydration and CUR values. Skin hydration in the lower lip vermilion was measured using the Corneometer® CM 825 and expressed as a.u. Pearson’s correlation coefficient test (***p < 0.001) was used for statistical analysis. R indicates correlation coefficients. CUR, corneocyte unevenness ratio; a.u.; arbitrary units
Figure 3.
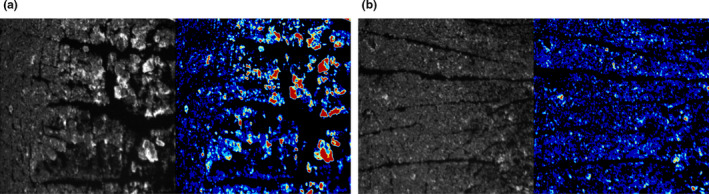
Images of tape‐stripped corneocytes showing different values of CUR. (a) Images of corneocytes showing relatively high CUR values. (b) Images of corneocytes showing relatively low‐CUR values. CUR, corneocyte unevenness ratio
Differences in activities and amounts of proteases related to lip skin hydration
To verify the relationship between lip skin hydration and the biomarkers by comparing relatively dry and non‐dry lip skin, each subject was classified according to the median of skin hydration values (28.12 a.u.) into the following two groups: Group I included subjects with a higher water content, whereas Group II comprised subjects with a lower water content.
There was a significant difference in BH activity between Groups I and II. BH activity in Group I was lower than in Group II (p < 0.05). The CTSD activity of Group I was marginally higher than Group II (p = 0.12) (Figure 4). There were no significant differences in the amounts of both proteases between Groups I and II (data not shown).
Figure 4.
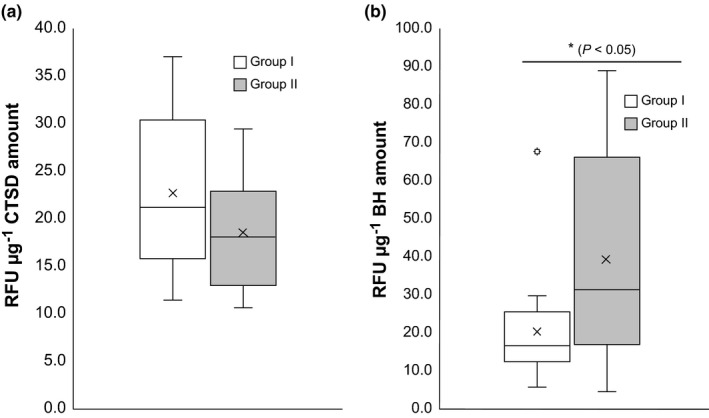
Activities of (a) CTSD and (b) BH in corneocytes from subjects of Groups I and II. Group I included subjects showing a water content higher than 28.12 a.u., whereas Group II comprised subjects showing a water content lower than 28.12 a.u., as determined with a Corneometer®. Significance between each group was determined using the Mann‐Whitney U test (*p < 0.05, n = 20 per group). CTSD, cathepsin D; BH, bleomycin hydrolase; a.u., arbitrary units; RFU, relative fluorescence unit
Differences in activities and amounts of proteases related to chapping of lip skin
To confirm the relationship between lip scaling and the biomarkers by distinguishing the relative lip scaling and non‐scaling levels, we assigned the subjects into two groups based on their median of CUR values (0.22) in the same method described previously; Group III included subjects with a lower CUR, and Group IV comprised subjects with a higher CUR.
A significant difference in CTSD activity between Groups III and IV was observed. The activity of CTSD was significantly higher in Group III (p < 0.05) than in Group IV. BH activity was slightly lower in Group III than in Group IV (p = 0.06) (Figure 5). The expression levels of both biomarkers were not considerably different between Groups III and IV (data not shown).
Figure 5.
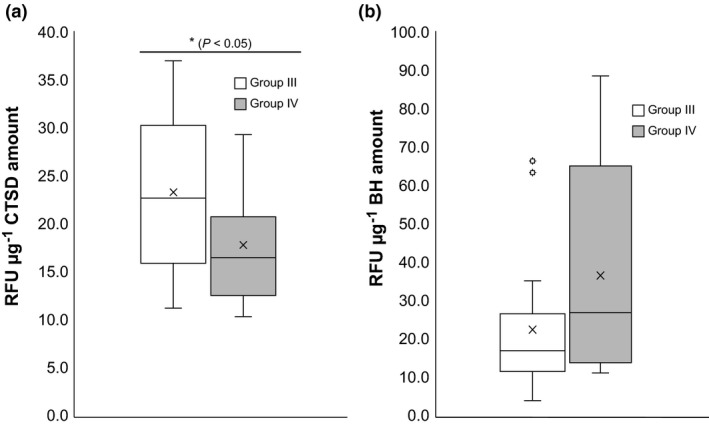
Activities of (a) CTSD and (b) BH in corneocytes from subjects of Groups III and IV. Group III included subjects with a CUR value lower than 0.22, whereas Group IV comprised subjects with a CUR value higher than 0.22. Significance between each Group was determined by the Mann‐Whitney U test (*p < 0.05, n = 20 per group). CTSD, cathepsin D; BH, bleomycin hydrolase; CUR, corneocyte unevenness ratio; RFU, relative fluorescence unit
DISCUSSION
In the present study, we analysed the biophysical and biochemical factors related to lip dryness and chapping using non‐invasive techniques and molecular analysis. Dry and chapped lip skin is a major concern for several people. However, little data and research are available on lip skin compared to facial skin for identifying the cause of these problems.
The relationship between lip skin hydration and scaling (G0‐G4) was studied. The correlation of skin scaling with hydration differed between G0 (positive) and the other four parameters (negative). Additionally, based on the results, the CUR showed a strong negative correlation with skin hydration, suggesting that the dryness of lip skin adversely affects the normal desquamation mechanism. A few studies have reported a high percentage of overlapped corneocytes compared to total corneocytes, suggesting an irregularity in the desquamation process [20], and it is often observed in rough skin with low hydration. [21] Additionally, the hydration level of the SC is lower in chapped lips because of abnormal desquamation [8]. Therefore, hydration plays a critical role in affecting the function of lip skin, and the CUR may be a new comprehensive parameter for determining an abnormality of desquamation, reflecting lip skin dryness and chapping conditions.
Far from our prediction, the TEWL values of the lip skin showed no significant correlations with any other parameters. Based on the characteristics of lip skin which has the lowest water content and highest TEWL compared to other regions [2], we hypothesized that a negative relationship would be observed between TEWL and other parameters. However, as previously reported by Tamura et al. [7], the increase in SC thickness could be due to abnormal desquamation ‐ a phenomenon occurring in dry lips [8] ‐ thus causing the decrease in TEWL. Besides, the relationship between TEWL and skin hydration state is still being debated. While Thune et al. [22] reported that TEWL is negatively correlated with SC hydration, other studies have revealed no correlations between TEWL and skin hydration, except in certain areas such as the hand and sole [23, 24]. Accordingly, our results, which show that TEWL has no significant relationship with skin hydration and scaling, may supported by these studies.
We validated that the properties of enzymes in the lips vary depending on the parameters related to skin hydration and scaling severity. We found that CTSD regulating the skin desquamation procedure was related to lip scaling. The activity of CTSD was decreased in subjects with high CUR values. These results agree with those reported by Hikima et al. [8]. As the activity of CTSD can be affected by multiple environmental conditions such as skin pH [9], it can be altered in a more rapid and delicate manner than alteration at the expression level. Therefore, this result suggests that CTSD activity contributes more to the physiological status of the lip skin than the level of its expression.
We also found that BH, a protease that regulates filaggrin processing, was related to skin hydration. The subject group with a lower hydration value showed higher BH activity than the group with a higher value. The elevated activity of BH in dry lips appeared to contradict our prediction, which was based on the NMF‐producing function of BH [25]. However, Shibata et al. [14] reported that normal skin, compared to atopic dermatitis skin, exhibits seasonal changes in BH, producing NMF to improve epidermal water retention. Raj et al. [15] proposed that a feedback mechanism can increase the levels of NMF‐producing proteases to repair the skin barrier. Based on these observations, our data suggest that a reverse pattern of BH activity reflects the natural compensating mechanism for restoring hydration of the lip skin. Overall, these differences in protease activities suggest that differences in the biophysical parameters of lip skin are derived from the different actions of proteases.
One limitation of our study is that we validated the activity of a few well‐known molecular targets only compared to various molecular targets studied for the facial skin. The two markers may not explain the detailed mechanism underlying lip skin dryness and chapping. However, our study provides a foundation for future research of lip skin, which has unique characteristics.
In conclusion, there is an interrelationship between the biophysical characteristics associated with lip skin dryness and chapping. Particularly, CUR may be developed as a novel quantitative parameter for studying the lip skin. Moreover, the activity of BH and CTSD is related to dry and chapped lip skin conditions. These results will be useful for future studies of the lip skin physiology to develop fundamental solutions for improving lip skin conditions.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGEMENTS
The authors would like to thank all members of the skin efficacy development team in LG Household & Health Care for their invaluable assistance. All research in this article was funded by LG Household & Health Care.
J. Kim and H. Yeo should be considered joint first co‐authors.
References
- 1. Tagami H., Location‐related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int. J. Cosmet. Sci. 30, 413–414 (2008). [DOI] [PubMed] [Google Scholar]
- 2. Tagami H., Kobayashi H., Functional properties of the surface of the vermilion border of the lips are distinct from those of the facial skin. Br. J. Dermatol. 150, 563–567 (2004). [DOI] [PubMed] [Google Scholar]
- 3. Tamura E., Ishikawa J., Sugata K., Tsukahara K., Yasumori H., Yamamoto T., Age‐related differences in the functional properties of lips compared with skin. Skin Res. Technol. 24, 472–478 (2018). [DOI] [PubMed] [Google Scholar]
- 4. Kim H., Lee M., Park S. Y., Kim Y. M., Han J., Kim E., Age‐related changes in lip morphological and physiological characteristics in Korean women. Skin Res. Technol. 25, 277–282 (2019). [DOI] [PubMed] [Google Scholar]
- 5. Gibelli D., Codari M., Rosati R., Dolci C., Tartaglia G. M., Cattaneo C., Sforza C., A quantitative analysis of lip aesthetics: the influence of gender and aging. Aesth. Surg. J. 39, 771–776 (2015). [DOI] [PubMed] [Google Scholar]
- 6. Chong Y., Dong R., Liu X., Wang X., Yu N., Long X., Stereophotogrammetry to reveal age‐related changes of labial morphology among Chinese women aging from 20 to 60. Skin Res. Technol. 27,1–8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tamura E., Ishikawa J., Naoe A., Yamamoto T., The roughness of lip skin is related to the ceramide profile in the stratum corneum. Int. J. Cosmet. Sci. 38, 615–621 (2016). [DOI] [PubMed] [Google Scholar]
- 8. Hikima R., Igarashi S., Ikeda N., Matsumoto M., Hanyama A., Egawa Y., Horikoshi T., Hayashi S., Development of lip treatment on the basis of desquamation mechanism. Int. J. Cosmet. Sci. 26, 165–167 (2004). [Google Scholar]
- 9. Rawlings A. V., Voegeli R., Stratum corneum proteases and dry skin conditions. Cell. Tissue. Res. 351, 217–235 (2013). [DOI] [PubMed] [Google Scholar]
- 10. Horikoshi T., Igarashi S., Uchiwa H., Brysk H., Brysk M. M., Role of endogenous cathepsin D‐like and chymotrypsin‐like proteolysis in human epidermal desquamation. Br. J. Dermatol. 141, 453–459 (1999). [DOI] [PubMed] [Google Scholar]
- 11. Zheng Y., Chen H., Lai W., Xu Q., Liu C., Wu L., Maibach H. I., Cathepsin D repairing role in photodamaged skin barrier. Skin Pharmacol. Physiol. 28, 97–102 (2015). [DOI] [PubMed] [Google Scholar]
- 12. Son E. D., Kim Y., Joo K. M., Kim H. J., Lee E., Nam G. W., Cho E. G., Noh M., Chung J. H., Byun S. Y. et al., Skin dryness in apparently healthy human skin is associated with decreased expression of bleomycin hydrolase in the stratum corneum. Clin. Exp. Dermatol. 40, 247–253 (2015). [DOI] [PubMed] [Google Scholar]
- 13. Chiba T., Activity of natural moisturizing factor forming enzyme bleomycin hydrolase in atopic dermatitis affected by disease control status and seasonal change. J. Allergy Clin. Immunol. 135, AB262 (2015). [Google Scholar]
- 14. Shibata M., Miyai M., Morita K., Chiba T., Ohya Y., Hibino T., A seasonal change of bleomycin hydrolase activity in the extract of human stratum corneum. J. Dermatol. Sci. 84, e41 (2016). [Google Scholar]
- 15. Raj N., Voegeli R., Rawlings A. V., Summers B., Munday M. R., Lane M. E., Variation in the activities of late stage filaggrin processing enzymes, calpain‐1 and bleomycin hydrolase, together with pyrrolidone carboxylic acid levels, corneocyte phenotypes and plasmin activities in non‐sun exposed and sun‐exposed facial stratum corneum of different ethnicities. Int. J. Cosmet. Sci. 38, 567–575 (2016). [DOI] [PubMed] [Google Scholar]
- 16. Quatresooz P., Piérard G. E., 26 The visioscan‐driven ULEV and SELS methods. Cosmetic Sci. Technol. 283‐290(2009). [Google Scholar]
- 17. Clausen M. L., Slotved H. C., Krogfelt K. A., Agner T., Tape stripping technique for stratum corneum protein analysis. Sci. Rep. 6, 19918 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riise R., Odqvist L., Mattsson J., Monkley S., Abdillahi S. M., Tyrchan C., Muthas D., Yrlid L. F., Bleomycin hydrolase regulates the release of chemokines important for inflammation and wound healing by keratinocytes. Sci. Rep. 9, 20407 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamata Y., Yamamoto M., Kawakami F., Tsuboi R., Takeda A., Ishihara K., Hibino T., Bleomycin hydrolase is regulated biphasically in a differentiation‐ and cytokine‐dependent manner: relevance to atopic dermatitis. J. Biol. Chem. 286, 8204–8212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishida‐Yamamoto A., Igawa S., Kishibe M., Order and disorder in corneocyte adhesion. J. Dermatol. 38, 645–654 (2011). [DOI] [PubMed] [Google Scholar]
- 21. Masaki H., Yamashita Y., Kyotani D., Honda T., Takano K., Tamura T., Mizutani T., Okano Y., Correlations between skin hydration parameters and corneocyte‐derived parameters to characterize skin conditions. J. Cosmet. Dermatol. 18, 308–314 (2018). [DOI] [PubMed] [Google Scholar]
- 22. Thune P., Nilsen T., Hanstad I. K., Gustavsen T., Lövig Dahl H., The water barrier function of the skin in relation to the water content of stratum corneum, pH, and skin lipids. The effect of alkaline soap and syndet on dry skin in elderly, non‐atopic patients. Acta Derm. Venereol. 68, 277–283 (1988). [PubMed] [Google Scholar]
- 23. Conti A., Schiavi M., Seidenari S., Capacitance, transepidermal water loss, and causal level of sebum in healthy subjects in relation to site, sex, and age. Int. J. Cosmet. Sci. 17, 77–85 (1995). [DOI] [PubMed] [Google Scholar]
- 24. Loden M., Olsson H., Axell T., Linde Y. W., Friction, capacitance, and transepidermal water loss (TEWL) in dry atopic and normal skin. Br. J. Dermatol. 126, 137–141 (1992). [DOI] [PubMed] [Google Scholar]
- 25. Kamata Y., Taniguchi A., Yamamoto M., Nomura J., Ishihara K., Takahara H., Hibino T., Takeda A., Neutral cysteine protease bleomycin hydrolase is essential for the breakdown of deiminated filaggrin into amino acids. J. Biol. Chem. 284, 12829–12836 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]


