Abstract
Background and Aims
Platelet‐stored serotonin critically affects liver regeneration in mice and humans. Selective serotonin reuptake inhibitors (SSRIs) and serotonin noradrenalin reuptake inhibitors (SNRIs) reduce intraplatelet serotonin. As SSRIs/SNRIs are now one of the most commonly prescribed drugs in the United States and Europe and given serotonin’s impact on liver regeneration, we evaluated whether perioperative use of SSRIs/SNRIs affects outcome after hepatic resection.
Approach and Results
Consecutive patients undergoing hepatic resection (n = 754) were retrospectively included from prospectively maintained databases from two European institutions. Further, an independent cohort of 495 patients from the United States was assessed to validate our exploratory findings. Perioperative intake of SSRIs/SNRIs was recorded, and patients were followed up for postoperative liver dysfunction (LD), morbidity, and mortality. Perioperative intraplatelet serotonin levels were significantly decreased in patients receiving SSRI/SNRI treatment. Patients treated with SSRIs/SNRIs showed a higher incidence of morbidity, severe morbidity, LD, and LD requiring intervention. Associations were confirmed in the independent validation cohort. Combined cohorts documented a significant increase in deleterious postoperative outcome (morbidity odds ratio [OR], 1.56; 95% confidence interval [CI], 1.07‐2.31; severe morbidity OR, 1.86; 95% CI, 1.22‐2.79; LD OR, 1.96; 95% CI, 1.23‐3.06; LD requiring intervention OR, 2.22; 95% CI, 1.03‐4.36). Further, multivariable analysis confirmed the independent association of SSRIs/SNRIs with postoperative LD, which was closely associated with postoperative 90‐day mortality and 1‐year overall survival.
Conclusions
We observed a significant association of perioperative SSRI/SNRI intake with adverse postoperative outcome after hepatic resection. This indicates that SSRIs/SNRIs should be avoided perioperatively in patients undergoing hepatic resections.
Abbreviations
- CCC
cholangiocellular carcinoma
- CI
confidence interval
- CRLM
colorectal cancer liver metastasis
- HCC
hepatocellular carcinoma
- INR
international normalized ratio
- ISGLS
International Study Group of Liver Surgery
- LD
liver dysfunction
- OR
odds ratio
- POD
postoperative day
- PT
prothrombin time
- PVE
portal vein embolization
- SB
serum bilirubin
- SNRI
serotonin and noradrenaline reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
Liver resection is considered the only potentially curative treatment option for several neoplastic entities of the liver.( 1 ) Accordingly, hepatectomies are performed for eligible patients based on the resection of all radiological and macroscopic detectable tumor while preserving at least 25% of healthy total liver volume.( 2 ) Despite substantial improvements in surgical techniques and perioperative care, postoperative morbidity and mortality remain important concerns after liver resection.( 3 ) The most significant factor determining morbidity and mortality following hepatectomy is the ability of the remnant liver to regenerate. Platelets have been found to substantially contribute to liver regeneration, likely by releasing their granule content.( 4 , 5 , 6 , 7 , 8 ) Several clinical studies have now documented that circulating platelet counts per se are associated with poor clinical outcome after liver resection.( 9 , 10 , 11 ) A well‐defined mechanism of how platelets affect liver regeneration is represented by intraplatelet serotonin. In particular, extensive experimental evidence has documented that platelet‐derived serotonin promotes liver regeneration in rodent models.( 12 , 13 , 14 ) Recently, we were able to provide evidence that platelet‐stored serotonin is also a relevant promoter of liver regeneration in humans, by documenting that patients suffering from adverse postoperative outcome had significantly lower levels of intraplatelet serotonin prior to liver resection.( 5 )
Serotonin is produced by the enterochromaffin cells in the intestine and then taken up and stored by platelets, where 95% of biologically available serotonin is stored. Of interest, the process of serotonin packing into platelets is negatively affected by serotonin reuptake inhibitors such as selective serotonin or serotonin and noradrenaline reuptake inhibitors (SSRIs/SNRIs).( 15 , 16 ) Accordingly, we provided exploratory evidence that SSRI‐treated patients undergoing liver surgery do indeed demonstrate reduced intraplatelet serotonin levels.( 17 ) To date, SSRIs/SNRIs are among the most commonly prescribed medications in Western countries. Approximately 12.5% of the US population is treated with SSRIs, and within Europe, documented SSRI/SNRI intake can reach 15.7% in certain countries.( 18 , 19 ) This makes SSRIs/SNRIs one of the most commonly prescribed medications in the United States and Europe per se and prior to liver surgery.
Given the established experimental and translational evidence that serotonin represents a critical regulator of postoperative liver regeneration and that SSRIs/SNRIs might critically reduce intraplatelet serotonin levels, we set out to define if and to what extent perioperative SSRI/SNRI use would negatively affect postoperative outcomes in patients undergoing hepatic resection.
Patients and Methods
Study Cohorts
Elective hepatic resections from 2000 to 2018 were included in this study from a total of three independent institutions (Medical University of Vienna, Vienna, Austria; Medical University of Innsbruck, Innsbruck, Austria; Mayo Clinic, Rochester, MN). The exploration cohort did represent consecutive patients from the two Austrian/European centers. To increase the incidence of postoperative outcome events as well as limit heterogeneity and concomitantly increase the power of our analyses, the validation cohort from the Mayo Clinic only included major resections and only patients suffering from one of the three most frequent neoplastic entities (colorectal cancer liver metastasis [CRLM], hepatocellular carcinoma [HCC], or cholangiocellular carcinoma [CCC]; as summarized in Supporting Fig. S1; Current Procedural Terminology codes were used to identify patients in this cohort). In a subgroup of 70 patients from the exploration cohort, we assessed perioperative intraplatelet serotonin levels with regard to SSRI/SNRI intake. Serum and plasma preparations were performed immediately prior to surgery and on postoperative day 1 (POD1) as well as on POD5 after liver resection for intraplatelet serotonin assessment. Results of this cohort with respect to the relevance of intraplatelet serotonin contents and postoperative outcome have previously been reported on by our group.( 17 ) Basic characteristics were recorded including age, sex, fibrosis grade/cirrhosis, diabetes, obesity (body mass index >30 kg/m2), as well as cardiac, pulmonary, and renal comorbidities. Extent of resection (according to the Brisbane 2000 Terminology),( 20 ) preoperative portal vein embolization (PVE), intraoperative intermittent pedicle clamping (Pringle maneuver), and intraoperative administration of red blood cell concentrations were recorded.
The analysis of blood samples and patient data was approved by the institutional ethics committee (no. 424/2010); all patients gave written informed consent for serotonin analyses. Institutional review board approval (Mayo Clinic, Rochester) was obtained for the validation cohort (no. 18‐011747).
Definition of SSRI/SNRI intake
Patients were classified as SSRI/SNRI intake–positive if they received preoperative treatment (of any duration). If patients only received a postoperative treatment, they were not classified as SSRI/SNRI‐positive as for sufficient platelet depletion of serotonin preoperative treatment for several days is necessary. In the event that SSRIs/SNRIs were paused during or immediately after surgery, patients were still classified as SSRI/SNRI‐positive as intraplatelet serotonin depletion was still present during the first days after surgery.
Definition and Classification of Postoperative Outcome Measures
International Study Group of Liver Surgery (ISGLS) criteria were used to define and grade postoperative liver dysfunction (LD). Accordingly, LD was defined by an abnormal serum bilirubin (SB) level and prothrombin time (PT) or international normalized ratio (INR) on or after POD5 based on the threshold values of the local laboratory. If a patient presented with abnormal preoperative SB or PT, a postoperative aggravation on or after POD5 (compared to the previous day) was identified as postoperative LD. Of note, patients who reached normal SB or PT/INR values prior to POD5 (and hence no further blood collection was performed on or after POD5) were considered as “no LD.” If a patient died in fulminant liver failure prior to POD5, the patient was classified as LD.
To evaluate postoperative morbidity, the classification described by Dindo et al.( 21 ) was applied, and the severity of postoperative complications was recorded as grade I to V. In case of multiple complications per patient, the most serious one was considered for statistical analysis. Postoperative hemorrhage was defined as intervention requiring (surgical or radiological) bleeding episode according to grade C ISGLS criteria.( 22 )
Optimized Blood Sample Preparation to Measure Intraplatelet Serotonin
For a subgroup of 70 patients at the Medical University of Vienna, perioperative serum and plasma samples were collected. An optimized plasma preparation technique was applied as described.( 23 ) Briefly, blood was drawn into prechilled tubes containing citrate, theophylline, adenosine, and dipyridamole; it was immediately placed on ice and further processed within 30 minutes. After an initial centrifugation step at 1,000g and 4°C for 10 minutes, the plasma supernatant was subjected to further centrifugation at 10,000g and 4°C for 10 minutes (to remove remaining platelets). The supernatant was stored in aliquots at −80°C. Serum samples were retrieved by blood collection without the addition of anticoagulants and by centrifugation (at 1,000g and room temperature for 10 minutes) 30 minutes after collection. The supernatant was stored in aliquots at −80°C.
Quantification of Intraplatelet Serotonin
Plasma and serum samples were analyzed by commercially available enzyme‐linked immunosorbent assay tests for human serotonin (Serotonin ELISA; IBL, Hamburg, Germany) according to the manufacturer’s instructions. To calculate the actual intraplatelet serotonin pool, plasma serotonin levels, reflecting the actual circulating amount of serotonin, were subtracted from serum serotonin levels, which contain all serotonin released by platelet activation during blood clotting.
Statistical Analysis
Statistical analyses were carried out with SPSS 20 Software (SPSS, Inc., Chicago, IL) and R, version 3.6.1 (R Project for Statistical Computing, Vienna, Austria; https://www.R‐project.org). Nonparametric tests (Wilcoxon rank‐sum test) were used to compare serotonin levels. The chi‐squared test was used to evaluate frequencies between two groups (or, where stated, Fisher’s exact test for limited sample size with expected frequencies smaller than 5), and logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs). P < 0.05 was considered statistically significant. Univariate logistic regression analyses were performed in the exploration cohort using baseline parameters as candidate variables. A multivariable logistic regression model including all variables with P < 0.1 in the univariate model was used to assess the effect of the respective parameters on LD status. Multicollinearity was ruled out by determination of the variance inflation factor (R package car), which was for all included variables smaller than 2 (Supporting File S1). Subgroup analyses were used to account for potential confounders caused by inclusion of different tumor entities. Finally, by performing sensitivity analyses (using R package obsSens), we observed that an unmeasured confounder would have to be very strongly associated with postoperative outcome measures and highly imbalanced between SSRI/SNRI users and nonusers (i.e., OR, <0.25 or >4.0, with >40% difference in prevalence) to fully attenuate the relationship with SSRIs/SNRIs.
Results
Patient Demographics
A total of 1,249 patients over 18 years of age undergoing elective liver resection from 2000 to 2018 were included in this study. Of the 754 consecutive patients from two independent Austrian institutions serving as the exploration cohort (Medical University of Vienna and Medical University of Innsbruck; Table 1), no statistically significant differences in baseline characteristics were observed between patients with and without SSRI/SNRI treatment, with the exception of an increased degree of steatosis in patients without SSRI/SNRI treatment. Subsequently, 495 patients were assessed as a validation cohort from Mayo Clinic (Rochester, MN; Table 2). To increase the incidence of postoperative outcome events, limit heterogeneity, and concomitantly increase the power of our analyses, the validation cohort only included major resections in those with a formal diagnosis of one of the three most frequent neoplastic entities for liver surgery (CRLM, HCC, and CCC). No statistically significant differences in baseline characteristics were observed between patients with and without SSRI/SNRI treatment, with the exception of an increased median bilirubin levels in patients without SSRI/SNRI treatment (Table 2). Details on type of SSRIs/SNRIs are listed in Supporting Table S1.
Table 1.
Patient Demographics: Exploration Cohort
| Parameter | Total (n = 754) | SSRI/SNRI+ (n = 71) | SSRI− (n = 683) | P |
|---|---|---|---|---|
| Median (Range), n (%) | Median (Range), n (%) | Median (Range), n (%) | ||
| Gender | 0.124 | |||
| Male | 457 (60.6%) | 37 (52.1%) | 420 (61.5%) | |
| Female | 297(39.4%) | 34 (47.9%) | 263 (38.5%) | |
| Age (years) | 63 (20‐89) | 65 (25‐83) | 63 (20‐89) | 0.628 |
| Hepatic resection | 0.586 | |||
| Minor (<3 segments) | 363 (48.1%) | 32 (45.1%) | 331 (48.5%) | |
| Major (≥3 segments) | 391 (51.9%) | 39 (54.9%) | 352 (51.5%) | |
| Tumor type | 0.117 | |||
| CRLM | 473 (62.7%) | 36 (50.7%) | 437 (64.0%) | |
| HCC | 99 (13.1%) | 10 (14.1%) | 89 (13.0%) | |
| CCC | 123 (16.3%) | 14 (19.7%) | 109 (16.0%) | |
| Benign | 30 (4.0%) | 2 (6.7%) | 28 (4.1%) | |
| Other | 29 (3.8%) | 9 (12.7%) | 20 (2.9%) | |
| Cofactors | ||||
| Steatosis (%) | 5.00 (0‐100) | 0.00 (0‐80) | 5.00 (0‐100) | 0.024 |
| Fibrosis | 0.304 | |||
| F0 | 312 (46.4%) | 25 (39.1%) | 287 (47.1%) | |
| F1 | 216 (32.1%) | 20 (31.3%) | 196 (32.2%) | |
| F2 | 73 (10.8%) | 11 (17.2%) | 62 (10.2%) | |
| F3 | 30 (4.5%) | 2 (3.1%) | 28 (4.6%) | |
| F4/cirrhosis | 42 (6.2%) | 6 (9.4%) | 36 (5.9%) | |
| Intraoperative RBC | 0 (0‐28) | 0 (0‐14) | 0(0‐28) | 0.537 |
| Pringle applied | 82 (12.5%) | 7 (12.5%) | 75 (12.5%) | 0.563 |
| PVE | 45 (7.3%) | 6 (11.1%) | 39 (6.9%) | 0.260 |
| Comorbidities | ||||
| Cardiovascular | 72 (10.9%) | 8 (13.6%) | 64 (10.6%) | 0.488 |
| Pulmonary | 43 (6.4%) | 4 (6.5%) | 39 (6.4%) | 0.994 |
| Renal | 13 (1.9%) | 3 (4.8%) | 10 (1.6%) | 0.078 |
| Diabetes | 82 (12.0%) | 6 (9.5%) | 76 (12.2.%) | 0.527 |
| Obesity | 159 (22.7%) | 9 (14.3%) | 150 (23.5%) | 0.095 |
| Preoperative parameters | ||||
| SB (mg/dL) | 0.62 (0.14‐18.5) | 0.58 (0.14‐2.01) | 0.62 (0.15‐18.5) | 0.215 |
| PT (%) | 103 (39‐150) | 101.5 (61‐150) | 104 (39‐150) | 0.441 |
| AP (U/L) | 98.5 (14‐2,005) | 92.0 (38‐936) | 99.5 (38‐936) | 0.209 |
| GGT (U/L) | 58 (7‐2,055) | 53.5 (7‐1,163) | 58 (8‐2,055) | 0.752 |
| AST (U/L) | 30 (5‐615) | 32 (8‐297) | 29 (5‐615) | 0.510 |
| ALT (U/L) | 26 (2‐497) | 30 (5‐279) | 26 (2‐497) | 0.367 |
| Albumin (g/L) | 42 (21‐54) | 41.0 (31‐48) | 42.0 (21‐54) | 0.175 |
| Platelets (G/L) | 201 (43‐678) | 211 (60‐471) | 200 (43‐679) | 0.988 |
| Morbidity | ||||
| None | 445 (59.4%) | 37 (52.1%) | 408 (60.2%) | |
| Any grade | 304 (40.6%) | 34 (47.9%) | 270 (39.8%) | 0.188 |
| Severe | 163 (21.8%) | 22 (31.0%) | 141 (20.8%) | 0.048 |
| Posthepatectomy hemorrhage | ||||
| ISGLS C | 24 (3.5%) | 3 (4.8%) | 21 (3.4%) | 0.380 |
| LD ISGLS | ||||
| No LD | 661 (87.7%) | 56 (78.9%) | 605 (88.6%) | |
| All grades | 93 (12.3%) | 15 (21.1%) | 78 (11.4%) | 0.018 |
| Grade C | 29 (3.8%) | 6 (8.5%) | 23 (3.4%) | 0.034 |
Bold indicates significance.
Abbreviations: ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transpeptidase; RBC, red blood cells.
Table 2.
Patient Demographics: Validation Cohort
| Parameter | Total (n = 495) | SSRI/SNRI+ (n = 50) | SSRI− (n = 445) | P |
|---|---|---|---|---|
| Median (Range), n (%) | Median (Range), n (%) | Median (Range), n (%) | ||
| Gender | 0.445 | |||
| Male | 258 (52.1%) | 23 (46.0%) | 235 (52.8%) | |
| Female | 237 (47.9%) | 27 (52.9%) | 210 (47.3%) | |
| Age (years) | 62 (18‐84) | 60 (28‐79) | 62 (18‐84) | 0.628 |
| Hepatic resection | ||||
| Major | 495 (100%) | 50 (100%) | 444 (100%) | |
| Tumor type | 0.180 | |||
| CRLM | 188 (38.0%) | 22 (44.0%) | 166 (37.3%) | |
| HCC | 122 (24.6%) | 15 (30.0%) | 107 (24.1%) | |
| CCC | 185 (37.4%) | 13 (26.0%) | 172 (38.7%) | |
| Preoperative parameters | ||||
| SB (mg/dL) | 0.60 (0.10‐25.5) | 0.50 (0.10‐7.70) | 0.60 (0.10‐25.5) | 0.003 |
| INR | 1.0 (0.8‐4.0) | 1.0 (0.8‐4.0) | 1.0 (0.8‐2.0) | 0.694 |
| ALT (U/L) | 51 (10‐2420) | 40 (10‐301) | 52 (13‐2420) | 0.0.71 |
| Albumin (g/L) | 41 (23‐45) | 41.0 (31‐48) | 42.0 (21‐54) | 0.665 |
| Platelets (G7l) | 244 (29‐969) | 255 (78‐649) | 243 (29‐969) | 0.234 |
| Morbidity | ||||
| None | 151 (33.0%) | 10 (20.0%) | 141 (31.7%) | |
| Any grade | 307 (67.0%) | 39 (78.0%) | 268 (60.2%) | 0.048 |
| Severe | 95 (20.7%) | 17 (34.0%) | 78 (17.5%) | 0.011 |
| LD ISGLS | ||||
| No LD | 410 (82.8%) | 37 (74.0%) | 373 (84.0%) | |
| All grades | 85 (17.2%) | 13 (26.0%) | 71 (16.0%) | 0.040 |
| Grade C | 25 (5.1%) | 5 (10.0%) | 20 (4.5%) | 0.102 |
Bold indicates significance.
Abbreviation: ALT, alanine aminotransferase.
Perioperative Time Course of 5‐Hydroxytryptamine Levels in Patients Receiving Perioperative SSRI/SNRI Treatment
In a subgroup of 70 patients intraplatelet serotonin contents were measured. This included 7 patients treated with SSRI/SNRI. Figure 1 illustrates perioperative intraplatelet serotonin levels in patients with SSRI/SNRI intake. Among patients with SSRI/SNRI intake intraplatelet serotonin levels were significantly reduced at all perioperative time points (median intraplatelet serotonin: preoperative SSRI/SNRI+, 17.35 ng/mL; preoperative SSRI/SNRI−, 111.49 ng/mL; P < 0.001; POD1 SSRI/SNRI+, 14.13 ng/mL; POD1 SSRI/SNRI−, 70.38 ng/mL; P < 0.001; POD5 SSRI/SNRI+, 20.69 ng/mL; POD5 SSRI/SNRI−, 78.43 ng/mL; P = 0.024).
FIG. 1.
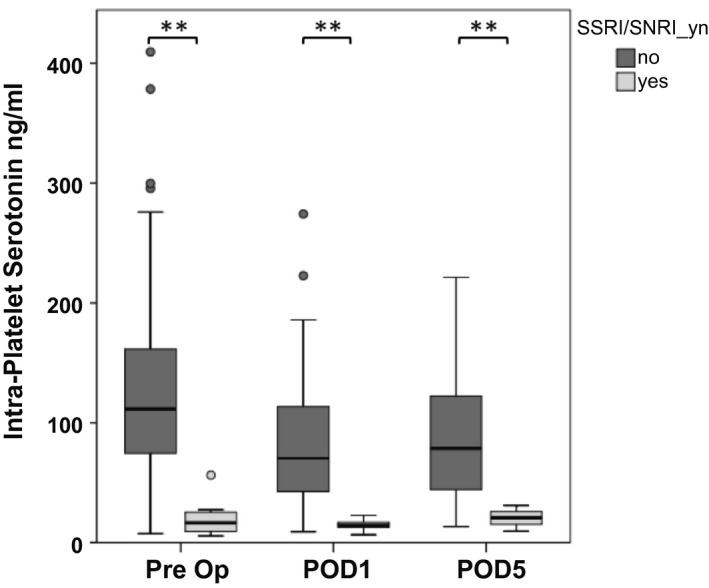
SSRI/SNRI intake reduces intraplatelet serotonin during the perioperative period. Perioperative time course of intraplatelet serotonin levels with respect to SSRI/SNRI intake. Optimized plasma and serum preparation was performed 1 day prior to surgery, on POD1, and on POD5. **P < 0.005, *P < 0.05. Abbreviation: Pre Op, preoperative.
SSRI/SNRI Use and Postoperative Outcome After Hepatic Resection
Within the exploration cohort, patients perioperatively treated with SSRIs/SNRIs had an elevated OR of 2.08 for developing postoperative LD (95% CI, 1.09‐3.77), of 2.65 for developing grade C LD (95% CI, 0.95‐6.36), of 1.39 for developing any grade morbidity (95% CI, 0.85‐2.27), and of 1.73 for developing severe morbidity (95% CI, 0.99‐2.92) (Fig. 2A; absolute incidences are illustrated in Table 1). Grade C bleeding complications did not seem to be associated with SSRI/SNRI intake (OR, 1.63; 95% CI, 0.38‐4.92). Table 3 illustrates the results of multivariable analysis, demonstrating an independent association of SSRIs/SNRIs with postoperative LD.
FIG. 2.
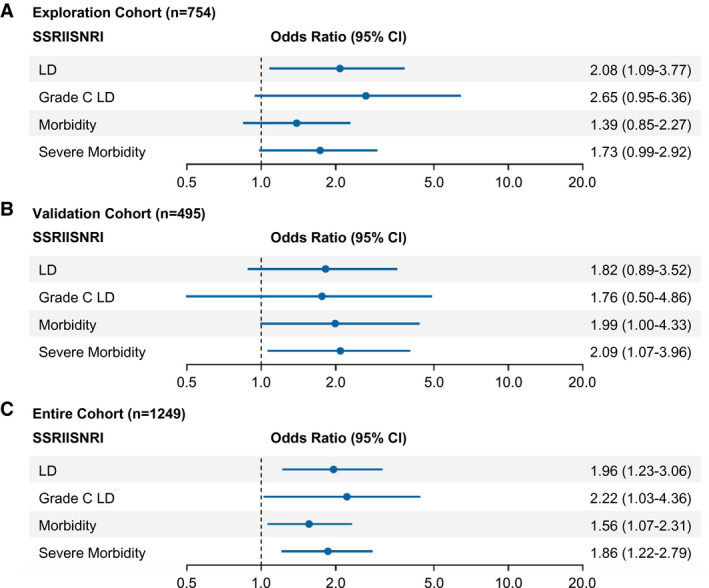
Risk of postoperative adverse outcome according to SSRI/SNRI intake. ORs and 95% CIs are presented to show the risk of any grade LD, grade C liver dysfunction, morbidity, and severe morbidity among patients with perioperative exposure to SSRIs/SNRIs compared to the risk among patients without such exposure. (A) Exploration cohort, (B) analysis of the validation cohort, and (C) analysis of the combined cohorts.
Table 3.
Predictors of Postoperative LD
| Explanatory variable | n | Univariate Logistic Regression | Multivariable Logistic Regression | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Treatment | |||||||
| SSRI|SNRI | 754 | 2.08 | 1.09‐3.77 | 0.020 | 2.40 | 1.00‐5.40 | 0.040 |
| Aspirin | 752 | 1.83 | 0.87‐3.56 | 0.090 | 0.97 | 0.35‐2.43 | 0.950 |
| Patient characteristics | |||||||
| Gender (female vs. male) | 754 | 0.87 | 0.55‐1.36 | 0.551 | — | — | — |
| Age (years) | 754 | 1.02 | 1.00‐1.05 | 0.021 | 1.03 | 1.00‐1.06 | 0.074 |
| Resection (major vs. minor) | 754 | 8.49 | 4.63‐17.13 | <0.001 | 8.38 | 3.90‐20.86 | <0.001 |
| Tumor CRLM | 754 | 0.76 | 0.18‐2.27 | 0.222 | — | — | — |
| HCC | 754 | 1.20 | 0.19‐2.37 | 0.558 | — | — | — |
| CCC | 754 | 1.48 | 0.99‐1.19 | 0.150 | — | — | — |
| Benign | 754 | 0.78 | 0.82‐3.02 | 0.692 | — | — | — |
| Other | 754 | 0.81 | 0.83‐4.28 | 0.740 | — | — | — |
| Intraoperative RBC (no.) | 749 | 1.09 | 0.99‐1.19 | 0.056 | 1.05 | 0.93‐1.15 | 0.369 |
| Pringle maneuver | 658 | 1.62 | 0.82‐3.02 | 0.146 | — | — | — |
| PVE | 616 | 1.99 | 0.83‐4.28 | 0.097 | 0.97 | 0.36‐2.28 | 0.943 |
| Steatosis (%) | 680 | 1.00 | 0.99‐1.01 | 0.985 | — | — | — |
| Cirrhosis | 673 | 1.51 | 0.60‐3.32 | 0.344 | — | — | — |
| Comorbidities | |||||||
| Cardiac | 662 | 2.26 | 1.15‐4.20 | 0.013 | 1.80 | 0.76‐4.06 | 0.166 |
| Pulmonal | 671 | 0.79 | 0.23‐2.03 | 0.658 | — | — | — |
| CKD | 678 | 2.59 | 0.57‐8.71 | 0.156 | — | — | — |
| Diabetes | 684 | 1.17 | 0.54‐2.28 | 0.669 | — | — | — |
| Obesity | 701 | 1.09 | 0.62‐1.86 | 0.758 | — | — | — |
Bold indicates significance.
Abbreviations: CKD, chronic kidney disease; RBC, red blood cells.
Validation of Results of SSRI/SNRI Use and Postoperative Outcome
After explorative analysis on the association of SSRIs/SNRIs and clinical outcome, we aimed to validate our results in an independent cohort. Figure 1B demonstrates that in our validation cohort SSRIs/SNRIs were again associated with an increased OR of 1.82 for developing postoperative LD (95% CI, 0.89‐3.52), of 1.76 for developing grade C LD (95% CI, 0.50‐4.86), of 1.99 for developing any grade morbidity (95% CI, 1.00‐4.33), and of 2.09 for developing severe morbidity (95% CI, 1.07‐3.96) (Fig. 2B; absolute incidences are illustrated in Table 2). In analysis of combined cohorts, a very strong association with clinical outcome parameters was observed: an OR in SSRI/SNRI patients of 1.96 for developing postoperative LD (95% CI, 1.23‐3.06), of 2.22 for developing grade C LD (95% CI, 1.03‐4.36), of 1.56 for developing any grade morbidity (95% CI, 1.07‐2.31), and of 1.86 for developing severe morbidity (95% CI, 1.22‐2.79) (Fig. 2C). Similar results were obtained when our three major subgroups (CRLM, HCC, and CCC) were assessed separately (Supporting Fig. S2) as well as when we assessed SSRI therapy alone (Supporting Fig. S3).
Consequences of Postoperative LD on Overall Disease Outcome
Given the significant increase in postoperative LD, we aimed to define long‐term clinical effects of this outcome variable in our combined cohort. We found that patients with postoperative LD had significantly worse postoperative outcome in terms of postoperative morbidity (OR, 3.73; 95% CI, 2.60‐5.47), severe morbidity (OR, 4.29; 95% CI, 3.05‐6.02), particularly 90‐day mortality (OR, 9.11; 95% CI, 4.91‐17.2), and 1‐year overall survival (OR, 2.56; 95% CI, 1.69‐3.82) (Fig. 3).
FIG. 3.
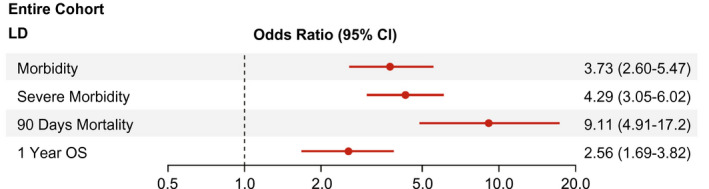
Risk of postoperative adverse outcome according to postoperative LD. ORs and 95% CIs are presented to show the risk of postoperative morbidity, severe morbidity, 90‐day mortality, and 1‐year overall survival among patients suffering from postoperative LD. Abbreviation: OS, overall survival.
Discussion
Patients undergoing hepatic resection, while being on SSRI/SNRI treatment, are depleted of intraplatelet serotonin, leading to an increase in postoperative LD, grade C LD, morbidity, and (severe) morbidity, even after adjustment through multivariable analysis. The observed 10% increase in postoperative LD additionally impacted postoperative mortality as well as 1‐year overall survival. Thus, we document a substantial effect of perioperative SSRI/SNRI intake on postoperative outcome after hepatic resection, which indicates that SSRIs/SNRIs should be paused in patients undergoing hepatic surgery.
Clinical and preclinical studies suggest that platelets critically regulate the process of liver regeneration.( 9 , 12 ) In particular, serotonin has indisputably been shown to act as one of the major effectors of platelet‐mediated liver regeneration.( 12 , 14 , 24 ) In recent years, we have been able to document that platelet‐derived serotonin seems to play a role in human liver regeneration and found that it is closely associated with postoperative LD and morbidity after partial hepatectomy.( 5 , 7 , 17 ) In particular, reduced intraplatelet serotonin levels, preoperatively as well as on POD1 and POD5, were found to be associated with poor postoperative outcome.( 7 ) Given the fact that SSRIs/SNRIs are capable of reducing serotonin in platelets, we now document that SSRI/SNRI‐treated patients did indeed have significantly lower intraplatelet serotonin levels during the entire perioperative period. This striking reduction of intraplatelet serotonin by SSRIs/SNRIs might account for the association we found with increased postoperative LD and morbidity. However, serotonin depletion in platelets also affects platelet function per se.( 16 ) Accordingly, SSRI/SNRI intake has been associated with an increased perioperative bleeding risk in experimental as well as human settings. In a retrospective analysis of more than 530,000 patients undergoing major surgical procedures, SSRI treatment was found to increase the risk of adverse events.( 25 ) It is important to note that only 3.5% of the patients underwent gastrointestinal surgery, so the minority of these patients underwent liver resections. Further, in their propensity score–matched analysis the risk of bleeding increased from 2.51% to 2.66%, suggesting that the actual increase in bleeding complications is probably minimal. Indeed, when we assessed the incidence of bleeding complications requiring intervention in our cohort, we did not observe a significant increase in bleeding‐associated complications with SSRI/SNRI treatment. Given the very low incidence of this complication, there is a risk that we were underpowered to detect this difference. However, it is important to note that the increase of postoperative LD far exceeded bleeding complications. Indeed, SSRI/SNRI treatment doubled the likelihood of all grades of LD; and the rate of severe, intervention‐requiring LD increased even more, which in turn was associated with 100% severe morbidity and an increase in 90‐day mortality from 2% to 38% in our exploration cohort (validation cohort, 3.9%‐44.4% including only major resections). This renders postoperative LD and grade C LD clinically very relevant outcome parameters in patients undergoing liver surgery and suggests that the negative effects of SSRIs/SNRIs on liver function recovery might be the prime mechanism of action for adverse postoperative outcome after hepatic resection.
SSRIs/SNRIs are among the most frequently prescribed drugs in the United States and Europe. Among the first 25 most commonly prescribed medications in the United States, four are SSRIs,( 19 ) leading to an overall prevalence of 12.5% of SSRI intake in the United States.( 26 ) In Europe, SSRI/SNRI prescription rates vary significantly between countries, averaging about 9% in Austria.( 18 ) In line with these results, we found that 10.1% of the US and 9.4% of the European patients undergoing hepatic resection took SSRIs/SNRIs. With this incidence, SSRIs/SNRIs are among the most commonly encountered medications in patients undergoing hepatic resections.
Despite the significant impact on clinical outcome after hepatic resection, SSRI/SNRI‐induced excess morbidity will also have significant impact on health care resource use. Per a recent cost–effectiveness analysis, a major complication after liver resection added a mean of $33,855 (USD) per patient,( 27 ) illustrating the substantial financial burden caused by a 10% increase of major complications. Given the fact that in the United States about 10,000 liver resections are performed per year, an increase of 10% in severe complications after hepatic resection generates a total of almost $34,000,000 each year, exemplifying the magnitude of financial consequences of perioperative SSRI/SNRI treatment.
Cessation of SSRI/SNRI prior to surgery might not be without side effects. Indeed, abrupt discontinuation of SSRIs/SNRIs might cause anxiety, agitation, nausea, irritability, sleep disturbances, and decreased energy levels.( 28 ) While incidences vary between reports, they seem to reach up to 27%. However, these symptoms are often mild, and the benefit from pausing SSRI/SNRI treatment prior to hepatic surgery seems to clearly outweigh the risks of discontinuation syndrome. The more challenging question, however, remains how long SSRIs/SNRIs should be stopped prior to surgery. While released serotonin in the circulation is rapidly metabolized within minutes, the half‐life of intraplatelet serotonin equals that of platelets.( 29 ) Accordingly, given the median half‐life of platelets of 8‐9 days,( 30 ) a 2‐week cessation period should represent a sufficient time frame to allow intraplatelet serotonin levels to recover until hepatic resection. However, it should be mentioned that some SSRIs/SNRIs, such as fluoxetine and norfluoxetine, have a longer half‐life, which should be taken into account when timing surgery to allow for sufficient recovery of intraplatelet serotonin levels.( 28 ) Of note, this time frame usually equals the required planning period of elective hepatic surgery and can therefore easily be integrated into clinical practice.
Besides the proregenerative effects of platelet‐derived serotonin, it has also been documented as a potent inducer of tumor cell growth. Indeed, we recently provided evidence that the absolute intraplatelet serotonin content did correlate with early disease recurrence also in humans.( 17 ) Accordingly, while we observed a trend toward reduced rates of early tumor recurrences in patients receiving SSRI/SNRI treatment, we were unable to confirm this trend in our validation cohort (data not shown). One possible reason for this lack of association is the heterogeneity of tumor types, stages, and molecular tumor subtypes included, which might significantly affect tumor recurrence. Furthermore, neoadjuvant as well as adjuvant treatment schedules (including systemic and local treatments) and their duration might have introduced a relevant bias that we are unable to account for within our analyses. While we still consider the detected trend in our exploration cohort an interesting and promising observation suggesting a potential benefit of SSRI/SNRI treatment to reduce postoperative early disease recurrences, we are unable to provide conclusive evidence to support this hypothesis. Further studies in larger patient cohorts, with detailed oncological information and more clearly defined patient groups, will be necessary to provide high‐quality evidence concerning perioperative/postoperative SSRI/SNRI treatment and an associated decrease in early tumor recurrences.
Based on strong experimental as well as translational evidence that intraplatelet serotonin affects postoperative liver regeneration in rodents and humans, we have demonstrated that SSRI/SNRI treatment not only effectively reduces perioperative intraplatelet serotonin concentrations but also significantly increases postoperative LD and morbidity. Accordingly, given the risk versus benefit profile of SSRI/SNRI cessation prior to liver resection, we recommend cessation of SSRI/SNRI treatment prior to liver resection to avoid postoperative LD, morbidity, and associated mortality and ultimately to decrease health care costs.
Author Contributions
All authors have: (1) substantially contributed to conception and design, acquisition of data, or analysis and interpretation of data; (2) helped drafting the article or revising it critically for important intellectual content; and (3) provided final approval of the version to be published.
Supporting information
Supplementary Material
Supinfo
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Van Cutsem E, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G, et al. Towards a pan‐European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 2006;42:2212‐2221. [DOI] [PubMed] [Google Scholar]
- 2. Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27:3677‐3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854‐862. [DOI] [PubMed] [Google Scholar]
- 4. Lisman T, Porte RJ. Mechanisms of platelet‐mediated liver regeneration. Blood 2016;128:625‐629. [DOI] [PubMed] [Google Scholar]
- 5. Starlinger P, Assinger A, Haegele S, Wanek D, Zikeli S, Schauer D, et al. Evidence for serotonin as a relevant inducer of liver regeneration after liver resection in humans. Hepatology 2014;60:257‐266. [DOI] [PubMed] [Google Scholar]
- 6. Pereyra D, Starlinger P. Shaping the future of liver surgery: implementation of experimental insights into liver regeneration. Eur Surg 2018;50:132‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Starlinger P, Brostjan C, Gruenberger T. Evidence for serotonin as an inducer of liver regeneration in humans—further investigations. Hepatology 2014;60:257‐266. [DOI] [PubMed] [Google Scholar]
- 8. Starlinger P, Haegele S, Offensperger F, Oehlberger L, Pereyra D, Kral JB, et al. The profile of platelet alpha‐granule released molecules affects postoperative liver regeneration. Hepatology 2016;63:1675‐1688. [DOI] [PubMed] [Google Scholar]
- 9. Alkozai EM, Nijsten MW, de Jong KP, de Boer MT, Peeters PM, Slooff MJ, et al. Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann Surg 2010;251:300‐306. [DOI] [PubMed] [Google Scholar]
- 10. Kaneko K, Shirai Y, Wakai T, Yokoyama N, Akazawa K, Hatakeyama K. Low preoperative platelet counts predict a high mortality after partial hepatectomy in patients with hepatocellular carcinoma. World J Gastroenterol 2005;11:5888‐5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soubrane O, Brouquet A, Zalinski S, Terris B, Brezault C, Mallet V, et al. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin‐based chemotherapy for colorectal liver metastases: correlation with post‐hepatectomy outcome. Ann Surg 2010;251:454‐460. [DOI] [PubMed] [Google Scholar]
- 12. Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, et al. Platelet‐derived serotonin mediates liver regeneration. Science 2006;312:104‐107. [DOI] [PubMed] [Google Scholar]
- 13. Matondo RB, Punt C, Homberg J, Toussaint MJ, Kisjes R, Korporaal SJ, et al. Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration. Am J Physiol Gastrointest Liver Physiol 2009;296:G963‐G968. [DOI] [PubMed] [Google Scholar]
- 14. Papadimas GK, Tzirogiannis KN, Panoutsopoulos GI, Demonakou MD, Skaltsas SD, Hereti RI, et al. Effect of serotonin receptor 2 blockage on liver regeneration after partial hepatectomy in the rat liver. Liver Int 2006;26:352‐361. [DOI] [PubMed] [Google Scholar]
- 15. Maurer‐Spurej E, Pittendreigh C, Solomons K. The influence of selective serotonin reuptake inhibitors on human platelet serotonin. Thromb Haemost 2004;91:119‐128. [DOI] [PubMed] [Google Scholar]
- 16. de Abajo FJ. Effects of selective serotonin reuptake inhibitors on platelet function: mechanisms, clinical outcomes and implications for use in elderly patients. Drugs Aging 2011;28:345‐367. [DOI] [PubMed] [Google Scholar]
- 17. Padickakudy R, Pereyra D, Offensperger F, Jonas P, Oehlberger L, Schwarz C, et al. Bivalent role of intra‐platelet serotonin in liver regeneration and tumor recurrence in humans. J Hepatol 2017;67:1243‐1252. [DOI] [PubMed] [Google Scholar]
- 18. Lewer D, O'Reilly C, Mojtabai R, Evans‐Lacko S. Antidepressant use in 27 European countries: associations with sociodemographic, cultural and economic factors. Br J Psychiatry 2015;207:221‐226. [DOI] [PubMed] [Google Scholar]
- 19. Fuentes AV, Pineda MD, Venkata KCN. Comprehension of top 200 prescribed drugs in the US as a resource for pharmacy teaching, training and practice. Pharmacy (Basel) 2018;6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005;12:351‐355. [DOI] [PubMed] [Google Scholar]
- 21. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, et al. Post‐hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford) 2011;13:528‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Starlinger P, Alidzanovic L, Schauer D, Brugger P, Sommerfeldt S, Kuehrer I, et al. Platelet‐stored angiogenesis factors: clinical monitoring is prone to artifacts. Dis Markers 2011;31:55‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sulaiman P, Joseph B, Kaimal SB, Paulose CS. Decreased hepatic 5‐HT1A receptors during liver regeneration and neoplasia in rats. Neurochem Res 2008;33:444‐449. [DOI] [PubMed] [Google Scholar]
- 25. Auerbach AD, Vittinghoff E, Maselli J, Pekow PS, Young JQ, Lindenauer PK. Perioperative use of selective serotonin reuptake inhibitors and risks for adverse outcomes of surgery. JAMA Intern Med 2013;173:1075‐1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pratt LA, Brody DJ, Gu Q. Antidepressant use among persons aged 12 and over: United States, 2011‐2014. NCHS Data Brief 2017;1‐8. [PubMed] [Google Scholar]
- 27. Idrees JJ, Johnston FM, Canner JK, Dillhoff M, Schmidt C, Haut ER, et al. Cost of major complications after liver resection in the United States: are high‐volume centers cost‐effective? Ann Surg 2019;269:503‐510. [DOI] [PubMed] [Google Scholar]
- 28. Renoir T. Selective serotonin reuptake inhibitor antidepressant treatment discontinuation syndrome: a review of the clinical evidence and the possible mechanisms involved. Front Pharmacol 2013;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zinner MJ, Kasher F, Jaffe BM. The hemodynamic effects of intravenous infusions of serotonin in conscious dogs. J Surg Res 1983;34:171‐178. [DOI] [PubMed] [Google Scholar]
- 30. Toghill PJ, Green S, Ferguson F. Platelet dynamics in chronic liver disease with special reference to the role of the spleen. J Clin Pathol 1977;30:367‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supinfo


