Abstract
Growing evidence supports the pivotal role played by periprostatic adipose tissue (PPAT) in prostate cancer (PCa) microenvironment. We investigated whether PPAT can affect response to Docetaxel (DCTX) and the mechanisms associated. Conditioned medium was collected from the in vitro differentiated adipocytes isolated from PPAT which was isolated from PCa patients, during radical prostatectomy. Drug efficacy was studied by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide citotoxicity assay. Culture with CM of human PPAT (AdipoCM) promotes DCTX resistance in two different human prostate cancer cell lines (DU145 and PC3) and upregulated the expression of BCL‐xL, BCL‐2, and TUBB2B. AG1024, a well‐known IGF‐1 receptor inhibitor, counteracts the decreased response to DCTX observed in presence of AdipoCM and decreased TUBB2B expression, suggesting that a paracrine secretion of IGF‐1 by PPAT affect DCTX response of PCa cell. Collectively, our study showed that factors secreted by PPAT elicits DCTX resistance through antiapoptotic proteins and TUBB2B upregulation in androgen independent PCa cell lines. These findings reveal the potential of novel therapeutic strategies targeting adipocyte‐released factors and IGF‐1 axis to overcome DCTX resistance in patients with PCa.
Keywords: adipocytes, docetaxel, drug resistance, periprostatic adipose tissue, prostate cancer
Abbreviations
- AdipoCM
conditioned medium of human PPAT
- AdMSCs
adipocytes mesenchymal stem cells
- ADT
Androgen deprivation therapy
- AT
adipose tissue
- BCL‐2
B‐cell lymphoma 2
- BCL‐xL
B‐cell lymphoma extra large
- BMI
body mass index
- BSA
bovine serum albumin
- C/EBPα
CCAAT/enhancer‐binding protein alpha
- DAB
3,3′‐Diaminobenzidine
- DCTX
docetaxel
- EMT
epithelial–mesenchymal transition
- GAPDH
glyceraldehyde‐3‐phosphate dehydrogenase
- IGF‐1
insulin‐like growth factor‐1
- mCRPC
metastatic castration‐resistant prostate cancer
- MTT
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide
- PBS
Phosphate‐buffered saline
- PCa
prostate cancer
- PPARγ
peroxisome proliferator‐activated receptor gamma
- PPAT
periprostatic adipose tissue
- TBS
tris‐buffered saline
- TME
tumor microenvironment
- TUBB2B
β‐tubulin isoform 2B
1. INTRODUCTION
Cancer progression is the end‐result of a complicated interplay between cancer cell and its microenvironment. 1 Prostate tumors are surrounded by type I collagen‐rich tissue and adipose tissue (AT). Insights in the function of AT highlighted a dynamic organ, releasing growth factors, cytokines, and hormones, 2 supporting the idea that AT acts as a functional player in the cross‐talk between cancer and its microenvironment.
In the last decade, experimental evidence emerged on the effect of adipocyte‐released factors on the therapy resistance of various cancers. 3 , 4 In particular, it has been demonstrated that AT creates a microenvironment that supports resistance to chemotherapy for disseminating cells, 5 through different molecular mechanisms such as sequester of cancer drugs in lipids of adipocytes 6 or metabolic adaptation. 7 Adipocytes differentiate from mesenchymal stromal cells (MSC) which, together with other nonmalignant cells, constitute tumor microenvironment (TME) potentially able to promote cancer progression. 8 TME includes cells able to release fatty acids and bioactive molecules regulating signaling pathways involved in cancer progression. 9 , 10 However, molecular mechanisms at the basis of adipocyte‐prostate cancer (PCa) cell crosstalk driving towards therapy escape remain elusive.
Docetaxel (DCTX) is the mainstay combination with androgen deprivation in earlier stages of PCa, however patients often develop resistance. 11
DCTX binds to diverse sites on tubulin and suppresses microtubule dynamics, blocks mitosis at the metaphase/anaphase transition and induces cell death. 12
DCTX resistance may reflect lack of tubulin engagement, because of mechanisms impairing the ability of taxane to stabilize microtubules. 13
In the present study we investigated the factors regulating adipocyte‐PCa cell interactions in the context of response to DCTX, first‐line therapy in metastatic prostate cancer.
Understanding the tumor‐promoting factors secreted by PPAT and the underlying mechanisms of chemoresistance activated in PCa cells may enrich the list of potential targets for therapy and overcoming chemoresistance in PCa patients.
2. MATERIALS AND METHODS
2.1. Materials
Media, sera and antibiotics for cell culture were from GIBCO (Thermo Fisher Scientific). Antibodies against tubulin β class IIB (TUBB2B) was purchased from Thermo Fisher Scientific, insulin‐like growth factor‐1 receptor (IGF‐1 R), glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH), peroxisome proliferator‐activated receptor gamma (PPARγ), CCAAT/enhancer‐binding protein alpha (C/EBPα), pAKT Ser473, B‐cell lymphoma extra large (BCL‐xL), B‐cell lymphoma 2 (BCL‐2), and actin were obtained from Santa Cruz Biotechnology. Sodium dodecyl sulfate‐polyacrylamide gel electrophoresis reagents were from Bio‐Rad. All the other chemicals were from Sigma‐Aldrich.
2.2. Cell cultures
PC3 and DU145 human prostate cancer cells were cultured in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum and 2 mmol/L glutamine, 100 IU/ml penicillin, and 100 IU/ml streptomycin. Cultures were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37°C. Human periprostatic adipose tissue (PPAT) samples were obtained from men undergoing radical prostatectomy for PCa and prostatic adenectomy for benign disease. All men were free from endocrine diseases. Informed consent was obtained from every study participant before the surgical procedure. The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the Ethics Committee of the University of Naples “Federico II” (project identification code 118/20).
Periprostatic adipose derived Mesenchymal Stem Cells (Ad‐MSCs) were isolated from the Stromal Vascular Fraction and differentiated in mature adipocytes as previously described. 14
2.3. Conditioned media system
Mature adipocytes were washed two times with sterile phosphate‐buffered saline (PBS) and incubated with serum‐free media supplemented with 0.25% albumin bovine serum (BSA). After 24 h, adipocyte‐conditioned media (AdipoCM) were collected, centrifuged to remove cellular debris and placed onto recipient cells.
2.4. Viability assay
PC3 and DU145 cells were plated in 96 well plates (2 × 104 cells/well) in triplicate. AdipoCM, DCTX, and AG1024 were added to cells as described in Results section. Viability was evaluated 48 h following treatment using the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay (CGD1, Sigma) according to the manufacturer's protocol.
2.5. Western blot
For western blot assays, cells were washed with ice‐cold PBS and harvested in Laemmli buffer (with β‐mercaptoethanol) containing a mixture of phosphatase inhibitors (0.5 mM sodium vanadate, 2 mM sodium pyrophosphate, 5 mM β‐glycerolphosphate, and 50 mM sodium fluoride) and the proteases inhibitor phenylmethylsulfonyl fluoride (Sigma–Aldrich). Western blots were carried out as previously reported. 15
2.6. IGF‐1 assay
Insulin‐like growth factor‐1 (IGF‐1) was determined using LIAISON® IGF‐I (DiaSorin). The method for quantitative determination is a single‐stage sandwich chemiluminescence immunoassay and the assay was performed according to manufacturer's instructions.
2.7. Statistical analysis
Comparisons between groups were performed by using one‐way analysis of variance. Statistical analysis was performed by using SPSS version 20 software. Bar and errors flags represent mean ± standard error of the mean of at least three independent experiments. For all statistical tests, differences were considered significant at the 5% level (*p values < .05).
3. RESULTS
3.1. AdipoCM affects cell viability in PCa cells
First, we induced adipocyte differentiation of human mesenchymal stem cells (AdMSCs) isolated from PPAT of patients with benign adenoma and PCa with different Gleason score [G7(3+4); G8 (4+4)]. As shown in Figure 1A, AdMSCs from PPAT expressed PPARγ and C/EBPα protein, the master regulator of adipogenic differentiation.
Figure 1.
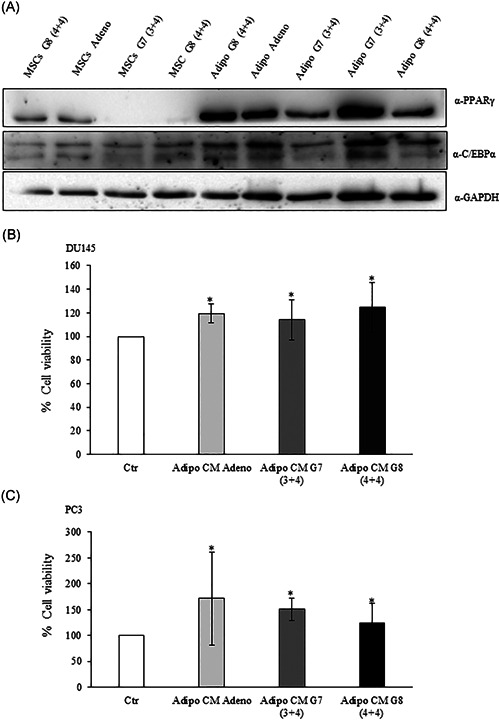
Adipocyte‐conditioned media effect on prostate cancer cell viability. (A) Mesenchymal stem cells from PPAT were isolated and differentiated as described in Section 2. The lysates were analyzed by immunoblotting with PPARγ and C/EBPα antibodies and autoradiography. GAPDH antibody was used for normalization. (B) DU145 and (C) PC3 (2 × 104 cells) cells were plated in 96 well plates and serum starved for 16 h cells. Then, the cells were incubated with 0,25% BSA or PPAT Adipocyte‐CM from Adeno, G7(3 + 4) and G8(4 + 4) adipocytes for 48 h. Cell viability was assessed by the MTT assay. The results were reported as percentage of viable cells compared to control, considered as maximum viability (100%). Data represent the mean ± SD of triplicate samples of three independent experiments. The bars represent the mean ± SD of at least three independent experiments. *p value < .05. BSA, bovine serum albumin; C/EBPα, CCAAT/enhancer‐binding protein alpha; MTT, 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide; PPARγ, peroxisome proliferator‐activated receptor gamma; PPAT, periprostatic adipose tissue; SD, standard deviation
To investigate the impact of adipocyte‐released factors from PPAT on PCa cell phenotype, we used conditioned media (CM) approach. To this aim, we treated PC3 and DU145, two highly aggressive androgen‐independent PCa cell lines, with AdipoCM of PPAT from adenoma and PCa specimens. In both PC acell lines, AdipoCM from adenoma, G7 (3+4) and G8 (4+4) significantly increased cell viability (Figure 1B,C).
3.2. Adipocyte‐released factors affect DCTX sensitivity
DCTX‐based chemotherapy is a first‐line agent in metastatic castration‐resistant PCa (mCRPC). However, most patients eventually develop resistance to this treatment and the key molecular mechanisms are still unknown. 16 Therefore, we wondered if AdipoCM modify the responsiveness of PCa cells to DCTX. As reported in Figure 2 and as already demonstrated, 17 8 nM docetaxel induces a 30% reduction of cell viability in both DU145 and PC3 cells. AdipoCM of PPAT from adenoma, G7 (3+4) and G8 (4+4) specimens induced the reduction in DCTX responsiveness (Figure 2), both in DU145 and PC3 prostate cancer cells.
Figure 2.
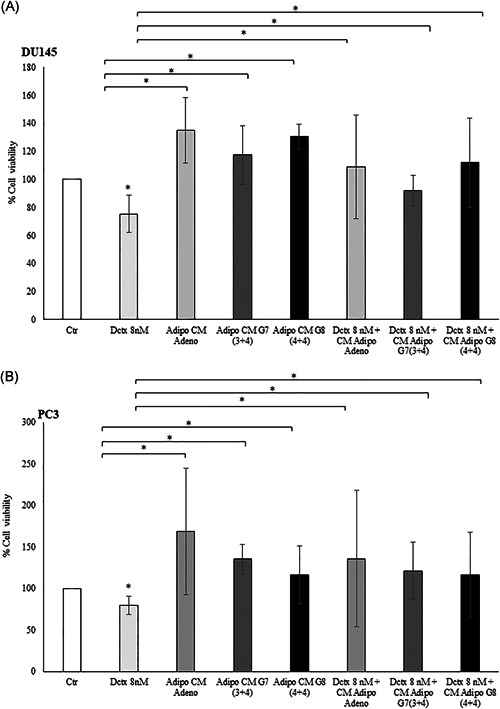
Docetaxel response in prostate cancer cell line upon AdipoCM treatment. DU145 and PC3 (2 × 104 cells) cells were plated in 96 well plates and serum starved for 16 h cells. The cells were incubated with 0.25% BSA or PPAT Adipocyte‐CM from Adeno, G7(3 + 4) and G8(4 + 4) adipocytes for 24 h. Then, the cells were treated with docetaxel 8 nM for 48 h alone or in combination with AdipoCM. Cell viability was assessed by the MTT assay. The results were reported as percentage of viable cells compared to control, considered as maximum viability (100%). Data represent the mean ± SD of triplicate samples of three independent experiments. *p value < .05. BSA, bovine serum albumin; MTT, 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide; PPAT, periprostatic adipose tissue; SD, standard deviation
To address whether AdipoCM or DCTX affected cell death, we evaluated the expression of two antiapoptotic proteins of BCLs family, BCL‐xL, and BCL‐2 in DU145 and PC3 cells upon AdipoCM and DCTX treatment, alone or in combination. As shown in Figure S1, the addition of AdipoCM in cells treated with DCTX counteract the decrease of antiapoptotic proteins in both cell lines.
3.3. AdipoCM upregulate the expression of β‐tubulin isoform TUBB2B
To investigate the molecular mechanisms potentially involved in the reduction of DCTX responsiveness by AdipoCM, we focused on the effect of AdipoCM in the expression of genes involved in DCTX resistance, such as TUBB2B, an alternative β‐tubulin isoform, preventing taxane‐induced microtubule stabilization. In DU145 and PC3 cells, the treatment with AdipoCM from PCa specimens increased expression of TUBB2B (Figure 3), suggesting that its upregulation could be a possible mechanism for DCTX resistance induced by PPAT.
Figure 3.
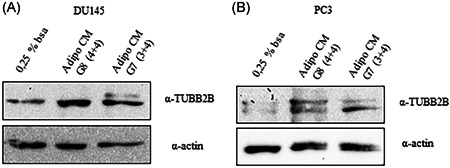
TUBB2B expression in prostate cancer cell. (A) DU145 and (B) PC3 were serum starved for 16 h cells and then incubated with 0,25% BSA or PPAT Adipocyte‐CM from G7(3 + 4) and G8(4 + 4) adipocytes for 48 h. The lysates were analyzed by immunoblotting with TUBB2B antibody and autoradiography. Actin antibody was used for normalization. The autoradiograph shown is representative of three different experiments. BSA, bovine serum albumin; PPAT, periprostatic adipose tissue; TUBB2B, β‐tubulin isoform 2B
3.4. IGF‐1 is a driver of AdipoCM effect on PCa cell malignant phenotype
Preclinical studies demonstrated that IGF‐1 axis modulates PCa phenotype. 18 In addition, epidemiological data revealed that high IGF‐1 circulating levels are associated with increased risk of prostate cancer. 19 Moreover, several authors reported that IGF‐1 is involved in DCTX resistance of PCa cell lines. 20 , 21 Therefore, we evaluated IGF‐1 levels in AdipoCM from PPAT of adenoma and PCa specimens. We found that IGF‐1 concentration ranged from 15 to 45.1 ng/ml (median value 22.0 ng/ml). The detailed results were described in Table 1.
Table 1.
Clinicopathological characteristics of patients
| Low grade patients | Age (years) | BMI (kg/m2) | Grading (Gleason score) | IGF‐1 [ng/ml] |
|---|---|---|---|---|
| 1 | 66 | 25 | 6 (3 + 3) | 24.8 |
| 2 | 61 | 21 | 7 (3 + 4) | 24.5 |
| 3 | 74 | 24 | 7 (3 + 4) | 21.4 |
| 4 | 63 | 31 | 7 (3 + 4) | 22.0 |
| 5 | 63 | 27 | 7 (3 + 4) | 23.3 |
| 6 | 65 | 32 | 7 (3 + 4) | 15.1 |
| 7 | 65 | 32 | 7 (3 + 4) | 15.0 |
| High grade patients | Age (years) | BMI (kg/m 2 ) | Grading (Gleason score) | |
|---|---|---|---|---|
| 8 | 75 | 25 | 7 (4 + 3) | 15.0 |
| 9 | 74 | 23 | 8 (4 + 4) | 15.1 |
| 10 | 75 | 21 | 8 (4 + 4) | 15.0 |
| 11 | 65 | 26 | 8 (4 + 4) | 38.2 |
| 12 | 70 | 26 | 8 (4 + 4) | 45.1 |
| 13 | 68 | 24 | 8 (4 + 4) | 27.5 |
| 14 | 57 | 26 | 8 (4 + 4) | 15.0 |
| 15 | 73 | 29 | 9 (4 + 5) | 23.3 |
| BH patients (controls) | Age (years) | BMI (kg/m 2 ) | ||
|---|---|---|---|---|
| 16 | 70 | 27 | ‐ | 15.0 |
| 17 | 57 | 37 | ‐ | 36.7 |
| 18 | 84 | 21 | ‐ | 22.1 |
Abbreviations: BMI, body mass index; IGF‐1, insulin‐like growth factor‐1.
In addition, we evaluated IGF‐1 receptor expression in PC3 and DU145 cell lines, showing that both cell line expressed IGF‐1 receptor at basal levels and upon AdipoCM incubation (Figure 4).
Figure 4.
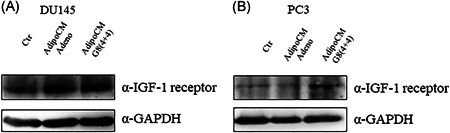
Expression of IGF‐1 receptor in PCa cell lines. (A) DU145 and (B) PC3 were serum starved for 16 h cells and then incubated with 0,25% BSA or PPAT Adipocyte‐CM from adenoma and G8(4 + 4) adipocytes for 48 h. The lysates were analyzed by immunoblotting with IGF‐1 receptor antibody and autoradiography. GAPDH antibody was used for normalization. The autoradiograph shown is representative of three different experiments. BSA, bovine serum albumin; IGF‐1, insulin‐like growth factor‐1; PCa, prostate cancer; PPAT, periprostatic adipose tissue
Next, we pretreated DU145 and PC3 with AG1024 8 µM, a widely used IGF‐1 receptor inhibitor, and then incubated with AdipoCM and DCTX, alone or in combination. As shown in Figure 5, in DU145 (Figure 5A) and PC3 (Figure 5B) the incubation with AdipoCM significantly reduce response to DCTX. Of note, the treatment with AG1024 reduced the increase of cell viability observed in presence of AdipoCM and DCTX (Figure 5).
Figure 5.
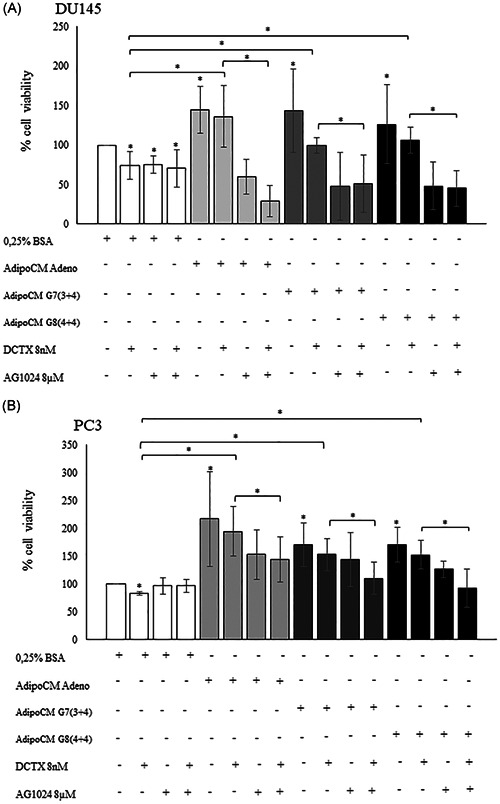
Insulin‐like growth factor inhibitor AG1024 effect on docetaxel sensitivity. (A) DU145 and (B) PC3 (2 × 104 cells) cells were plated in 96 well plates and serum starved for 16 h cells. Then, the cells were incubated with 0,25% BSA or PPAT Adipocyte‐CM from Adeno, G7(3 + 4) and G8(4 + 4) adipocytes for 48 h. Then, the cells were treated with docetaxel 8 nM and AG1024 8 µM alone or in combination with AdipoCM. Cell viability was assessed by the MTT assay. The results were reported as percentage of viable cells compared with control, considered as maximum viability (100%). Data represent the mean ± SD of triplicate samples of three independent experiments. *p value < .05. BSA, bovine serum albumin; MTT, 3‐(4,5‐dimethyl‐2‐thiazolyl)‐2,5‐diphenyl‐2H‐tetrazolium bromide; PPAT, periprostatic adipose tissue; SD, standard deviation
3.5. IGF‐1 inhibition decreases TUBB2B protein expression in PCa cell
To clarify the molecular mechanisms by which IGF‐1 inhibition restore DCTX response, we evaluated TUBB2B protein expression after AG1024 treatment. Interestingly, AdipoCM increased AKT phosphorylation (ser473) compared with control and this effect was blocked by AG1024 treatment (Figure 6A,B). Of note, in both cancer cell lines, DU145 (Figure 6A) and PC3 (Figure 6B) the treatment with AdipoCM from adenoma and G8 (4+4) increased TUBB2B protein expression. Moreover, the pretreatment with AG1024 8 µM reduced TUBB2B expression, suggesting that IGF‐1 is involved in DCTX resistance induced by AdipoCM. To test our hypothesis, we treated DU145 and PC3 cells with recombinant IGF‐1, choosing the lowest concentration found in AdipoCM from PPAT. In both PCa cell lines, IGF‐1 15 ng/ml increased TUBB2B protein expression and the pretreatment with AG1024 reverted this effect (Figure 6C,D).
Figure 6.
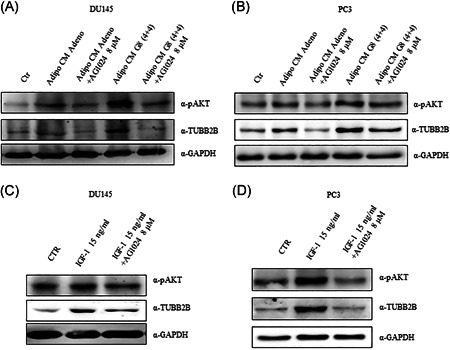
AG1024 effect on TUBB2B expression in prostate cancer cell. (A) DU145 and (B) PC3 were serum starved for 16 h cells and then incubated with 0,25% BSA or PPAT Adipocyte‐CM from Adeno and G8(4 + 4) adipocytes and in combination with AG1024 8 µM. (C) DU145 and (D) PC3 cells were serum starved for 16 h, pretreated with AG1024 8 µM and then stimulated with IGF‐1 recombinant protein for 48 h. The lysates were analyzed by immunoblotting with pAKT and TUBB2B antibodies and autoradiography. GAPDH antibody was used for normalization. The autoradiograph shown is representative of three different experiments. BSA, bovine serum albumin; PPAT, periprostatic adipose tissue; TUBB2B, β‐tubulin isoform 2B
4. DISCUSSION
Prostate gland is covered anteriorly by PPAT. 22 PCa infiltration into the adjacent fat is a risk factor for PCa progression. 23 PPAT thickness has been positively associated with aggressiveness of PCa. 24 , 25 Sasaki et al. 26 recently showed that pretreatment ratio of periprostatic adipose tissue‐to‐subcutaneous adipose tissue thickness on MRI is an independent predictor of survival in men with advanced PCa. Several authors reported that PPAT releases several cytokines as IL6, TNF‐alpha, chemokines as CCL7 and matricellular protein as osteopontin able to favor PCa growth, migration, and invasion. 27 , 28 , 29 In addition, experimental evidence were available indicating that PCa cell stimulate PPAT to release factors promoting their aggressiveness, leading to a positive feedback loop. 27
Less is known about the role of AT in cancer chemoresistance. Recently, Su et al. 30 demonstrated that adipocytes promote both epithelial to mesenchimal transition (EMT) and chemoresistance in PCa cell culture and animal models. 30 Herroon et al. 31 using both in vivo and in vitro coculture systems showed a reduced response of PCa cells to DCTX induced by exposure to adipocytes mediated by lipolysis.
Taxanes (DCTX and cabazitaxel) are still the first line therapy for the treatment of CRPC who have experienced progression after androgen‐deprivation therapy (ADT). 32
The mechanisms of resistance to taxanes included mainly upregulation of efflux transporters as ABCB1 33 and increased expression of β‐tubulin isoforms as Class II, III, and IV. 34 , 35 In particular, Ploussard et al. 34 identified a role for class III β‐tubulin expression as prognostic and predictive biomarker in CRPC. Recently, it has been demonstrated that taxanes resistance in PCa involves lack of drug‐target engagement. 13
In this study using CM approach in two different androgen‐independent PCa cell lines, DU145 and PC3, we demonstrated that exposure to adipocyte‐released factors significantly increases TUBB2B expression, a β‐tubulin isoform previously associated with DCTX resistance in metastatic PCa cells. 36 Notably, we obtained data from GEO data set GDS1439 on microarray expression profile of 13 PCa tumors (seven clinically localized primary PCa and six metastatic PCa) and six benign prostate tissues. Data showed that the expression of TUBB2B in metastatic PCa was higher compared to clinically localized PCa and normal prostate tissues (Figure S2), highlighting that in vivo TUBB2B expression was significantly associated with prostate cancer progression and poor clinical outcome.
We also showed that AG1024, a selective inhibitor of IGF‐1 receptor, reduced the increase of cell viability observed in presence of AdipoCM and DCTX in DU145 and PC3 cell line, suggesting that IGF‐1 released by PPAT could be a key regulator of the effect of PPAT on DCTX efficacy in PCa cell. Our study pointed out that IGF‐1 supplied by PPAT could affect therapeutic response in PCa patients. Moreover, our findings are first to demonstrate that IGF‐1 augments the expression of a β‐tubulin isotype associated with DCTX‐resistant phenotype.
GH/IGF1 axis plays a key role in prostate differentiation and cancer development. 37 In particular, IGF1 axis is upregulated in advanced PCa. 38 Moreover, the association between obesity and cancer has been partly explained by the alterations in the IGF1 axis in PCa and in several forms of cancers. 14 , 37 , 39 , 40 , 41 , 42
In addition, in vivo evidence was available regarding the involvement of IGF‐1 in the development of drug resistance in PCa. 43 Furthermore, several authors reported circulating IGF‐binding proteins (IGFBPs) lower level in patients with high‐grade compared with low‐grade PCa. 44 , 45 High preoperative circulating IGF‐1 levels are associated with an increased risk of advanced prostate cancer. 46 , 47 PCa tissue expression of IGF‐1 receptor was significantly associated with the risk of progression to a lethal disease. 48 Notably, IGF‐1R knockdown reduced prostate cancer cell lines growth. 49
IGF‐1 in PPAT may act in a paracrine manner, regulating function of the surrounding cells, including PCa cells. Accordingly, we showed that inhibition of IGF‐1 receptor by AG1024, decreased the effect of adipocyte‐released factors on DCTX response, thereby indicating that IGF‐1 is a pivotal factor in adipocyte regulation of PCa cell drug response. However, these results are limited to an in vitro interaction model between PCa cells and adipocytes. We used isolated adipocytes rather than whole AT. It is well known that AT is a mixture of different types of cells including mature adipocytes, pre‐adipocytes, fibroblasts, and immune cells and the effects observed in vivo could be due to interactions between these components. In addition, a supplemental source of diversity was the intrinsic heterogeneity of the studied patients. Further studies are needed to assess the relevance of IGF‐1‐released by PPAT in the regulation of DCTX response in PCa. However, our study reinforces the hypothesis that individual AT (e.g., PPAT) may drive cancer progression 24 and chemotherapy efficacy more than BMI. Accordingly, Cushen et al. 50 showed that high volume of visceral fat, but not BMI more than 25 kg/m2 is associated with poorer survival in patients with CRPC treated with DCTX chemotherapy. Accordingly, Wu et al. 51 reported in 333 patients with mCRPC that high BMI was associated with longer survival, conversely the presence of visceral obesity is associated with poorer survival. Collectively, there is growing evidence that BMI does not accurately discriminate between lean and fat tissues and it is unable to mirror the effect of each individual AT (e.g., visceral and subcutaneous). 52 Based on our findings, measuring PPAT thickness should provide more relevant information on PCa prognosis than using general obesity markers as BMI. It could be useful to incorporate PPAT‐related measure into PCa risk calculator to ameliorate PCa prognosis assessment. Our results provided not only more robust molecular basis on the relationship between PPAT and PCa progression, but also it offers relevant insight into the potential use of β‐tubulin isotypes as prognostic biomarkers and IGF‐1 as potential therapeutic target in patients with metastatic PCa.
Collectively, our results demonstrated for the first time in PCa that tumor‐surrounding adipocytes promote resistance to DCTX through a TUBB2B—dependent mechanism. In human PCa tissues, TUBB2B is upregulated in high‐grade, highlighting the clinical relevance of our results.
This study reveals a new role of PPAT in affecting PCa clinical outcome and could provide interesting opportunities to set up specific strategies for the treatment of PCa patients exhibiting aggressive PCa.
5. CONCLUSIONS
Chemoresistance is one of the main barriers in cancer therapy. The present study showed that conditioned media collected from PPAT increased antiapoptotic and TUBB2B proteins expression and consequently led to decreased DCTX efficacy in DU145 and PC3 androgen independent PCa cell lines. IGF‐1 released by PPAT enhanced TUBB2B expression and DCTX resistance (Figure 7).
Figure 7.
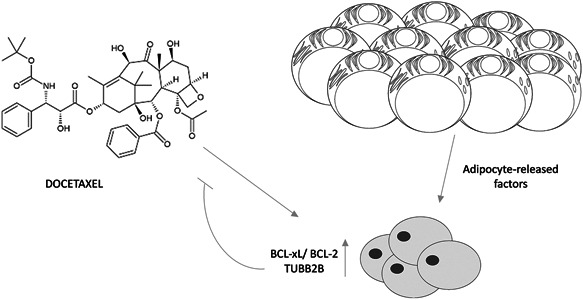
Schematic representation of the proposed role of PPAT in promoting DCTX resistance in PCa cell. (A) Periprostatic mature adipocytes released factors upregulated BCL‐xL, BCL‐2, and TUBB2B expression in PCa cell favoring DCTX resistance. BCL‐2, B‐cell lymphoma‐2; BCL‐xL, B‐cell lymphoma extra large; DCTX, docetaxel; PCa, prostate cancer; PPAT, periprostatic adipose tissue; TUBB2B, β‐tubulin isoform 2B
These findings provide a better understanding of the relevance of AT in PCa microenvironment for the development of chemoresistance and indicate the potential of revealing novel therapeutic strategies to overcome drug resistance in CRPC.
Additional studies are needed to confirm this new proposed model linking PPAT and PCa aggressiveness and thereby recognize novel molecular target to disjoin the cooperating role of PPAT in PCa drug resistance.
CONFLICTS OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization: Antonietta Liotti and Daniela Terracciano. Data curation: Antonietta Liotti, Evelina La Civita, Michele Cennamo, Felice Crocetto, Elia Guadagno, and Pasquale Liguoro. Formal analysis: Antonietta Liotti and Evelina La Civita. Funding acquisition: Pietro Formisano and Francesco Beguinot. Investigation: Antonietta Liotti, Evelina La Civita, and Elia Guadagno. Methodology: Daniela Terracciano; Resources, Felice Crocetto, Luigi Insabato, Ciro Imbimbo, Alessandro Palmieri, and Vincenzo Mirone. Supervision: Pietro Formisano, Francesco Beguinot, and Daniela Terracciano. Validation: Antonietta Liotti. Visualization: Daniela Terracciano. Writing – original draft: Antonietta Liotti and Daniela Terracciano. Writing – review & editing: Matteo Ferro and Daniela Terracciano.
Supporting information
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
We would like to thank Dr Domenico Liguoro for his valuable contributions to our research. We appreciate his technical support to our research related activities.
Liotti A, La Civita E, Cennamo M, et al. Periprostatic adipose tissue promotes prostate cancer resistance to docetaxel by paracrine IGF‐1 upregulation of TUBB2B beta‐tubulin isoform. The Prostate. 2021;81:407–417. 10.1002/pros.24117
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Vibishan B, Watve MG. Context‐dependent selection as the keystone in the somatic evolution of cancer. Sci Rep. 2020;10(1):4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831(10):1533‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nowicka A, Marini FC, Solley TN, et al. Human omental‐derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS One. 2013;8(12):e81859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duong MN, Cleret A, Matera EL, et al. Adipose cells promote resistance of breast cancer cells to trastuzumab‐mediated antibody‐dependent cellular cytotoxicity. Breast Cancer Res. 2015;17:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMillin DW, Negri JM, Mitsiades CS. The role of tumour‐stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov. 2013;12(3):217‐228. [DOI] [PubMed] [Google Scholar]
- 6. Sheng X, Parmentier JH, Tucci J, et al. Adipocytes sequester and metabolize the chemotherapeutic daunorubicin. Mol Cancer Res. 2017;15(12):1704‐1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ye H, Adane B, Khan N, et al. Leukemic stem cells evade chemotherapy by metabolic adaptation to an adipose tissue niche. Cell Stem Cell. 2016;19(1):23‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jung Y, Kim JK, Shiozawa Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev. 2011;32(4):550‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer‐‐mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10(8):455‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crawford ED, Higano CS, Shore ND, Hussain M, Petrylak DP. Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies. J Urol. 2015;194(6):1537‐1547. [DOI] [PubMed] [Google Scholar]
- 12. Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253‐265. [DOI] [PubMed] [Google Scholar]
- 13. Gjyrezi A, Xie F, Voznesensky O, et al. Taxane resistance in prostate cancer is mediated by decreased drug‐target engagement. J Clin Invest. 2020;130(6):3287‐3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D'Esposito V, Passaretti F, Hammarstedt A, et al. Adipocyte‐released insulin‐like growth factor‐1 is regulated by glucose and fatty acids and controls breast cancer cell growth in vitro. Diabetologia. 2012;55(10):2811‐2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ariemma F, D'Esposito V, Liguoro D, et al. Low‐dose bisphenol‐a impairs adipogenesis and generates dysfunctional 3T3‐L1 adipocytes. PLoS One. 2016;11(3):e0150762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marín‐Aguilera M, Codony‐Servat J, Kalko SG, et al. Identification of docetaxel resistance genes in castration‐resistant prostate cancer. Mol Cancer Ther. 2012;11(2):329‐339. [DOI] [PubMed] [Google Scholar]
- 17. Liotti A, Cosimato V, Mirra P, et al. Oleic acid promotes prostate cancer malignant phenotype via the G protein‐coupled receptor FFA1/GPR40. J Cell Physiol. 2018;233(9):7367‐7378. [DOI] [PubMed] [Google Scholar]
- 18. Heidegger I, Massoner P, Sampson N, Klocker H. The insulin‐like growth factor (IGF) axis as an anticancer target in prostate cancer. Cancer Lett. 2015;367(2):113‐121. [DOI] [PubMed] [Google Scholar]
- 19. Wang S, Wang N, Yu B, et al. Circulating IGF‐1 promotes prostate adenocarcinoma via FOXO3A/BIM signaling in a double‐transgenic mouse model. Oncogene. 2019;38(36):6338‐6353. [DOI] [PubMed] [Google Scholar]
- 20. Liang C, Zhou F, Chen X, et al. GSK1838705A, an insulin‐like growth factor‐1 receptor/insulin receptor inhibitor, induces apoptosis and reduces viability of docetaxel‐resistant prostate cancer cells both in vitro and in vivo. Onco Targets Ther. 2015;8:753‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niu XB, Fu GB, Wang L, et al. Insulin‐like growth factor‐I induces chemoresistence to docetaxel by inhibiting miR‐143 in human prostate cancer. Oncotarget. 2017;8(63):107157‐107166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nassar ZD, Aref AT, Miladinovic D, et al. Peri‐prostatic adipose tissue: the metabolic microenvironment of prostate cancer. BJU Int. 2018;121(Suppl 3):9‐21. [DOI] [PubMed] [Google Scholar]
- 23. Taylor RA, Lo J, Ascui N, Watt MJ. Linking obesogenic dysregulation to prostate cancer progression. Endocr Connect. 2015;4(4):R68‐R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dahran N, Szewczyk‐Bieda M, Wei C, Vinnicombe S, Nabi G. Normalized periprostatic fat MRI measurements can predict prostate cancer aggressiveness in men undergoing radical prostatectomy for clinically localised disease. Sci Rep. 2017;7(1):4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vidal AC, Oyekunle T, Howard LE, et al. Obesity, race, and long‐term prostate cancer outcomes. Cancer. 2020;126:3733‐3741. [DOI] [PubMed] [Google Scholar]
- 26. Sasaki T, Sugino Y, Kato M, Nishikawa K, Kanda H. Pre‐treatment ratio of periprostatic to subcutaneous fat thickness on MRI is an independent survival predictor in hormone‐naive men with advanced prostate cancer. Int J Clin Oncol. 2020;25(2):370‐376. [DOI] [PubMed] [Google Scholar]
- 27. Ribeiro RJT, Monteiro CPD, Cunha VFPM, et al. Tumor cell‐educated periprostatic adipose tissue acquires an aggressive cancer‐promoting secretory profile. Cell Physiol Biochem. 2012;29(1‐2):233‐240. [DOI] [PubMed] [Google Scholar]
- 28. Ribeiro R, Monteiro C, Cunha V, et al. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J Exp Clin Cancer Res. 2012;31:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laurent V, Guérard A, Mazerolles C, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun. 2016;7:10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Su F, Ahn S, Saha A, DiGiovanni J, Kolonin MG. Adipose stromal cell targeting suppresses prostate cancer epithelial‐mesenchymal transition and chemoresistance. Oncogene. 2019;38(11):1979‐1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herroon MK, Diedrich JD, Rajagurubandara E, et al. Prostate tumor cell‐derived IL1beta induces an inflammatory phenotype in bone marrow adipocytes and reduces sensitivity to docetaxel via lipolysis‐dependent mechanisms. Mol Cancer Res. 2019;17(12):2508‐2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dong L, Zieren RC, Xue W, de Reijke TM, Pienta KJ. Metastatic prostate cancer remains incurable, why? Asian J Urol. 2019;6(1):26‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug‐resistant cancer. Nat Rev Cancer. 2018;18(7):452‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ploussard G, Terry S, Maillé P, et al. Class III beta‐tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel‐based chemotherapy. Cancer Res. 2010;70(22):9253‐9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Makarovskiy AN, Siryaporn E, Hixson DC, Akerley W. Survival of docetaxel‐resistant prostate cancer cells in vitro depends on phenotype alterations and continuity of drug exposure. Cell Mol Life Sci. 2002;59(7):1198‐1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cánovas V, Puñal Y, Maggio V, et al. Prostate Tumor Overexpressed‐1 (PTOV1) promotes docetaxel‐resistance and survival of castration resistant prostate cancer cells. Oncotarget. 2017;8(35):59165‐59180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. F LL, Sarmento‐Cabral A, Herrero‐Aguayo V, Gahete MD, Castano JP, Luque RM. Obesity and metabolic dysfunction severely influence prostate cell function: role of insulin and IGF1. J Cell Mol Med. 2017;21(9):1893‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Massoner P, Ladurner Rennau M, Heidegger I, et al. Expression of the IGF axis is decreased in local prostate cancer but enhanced after benign prostate epithelial differentiation and TGF‐beta treatment. Am J Pathol. 2011;179(6):2905‐2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tong Y, Wu J, Huang O, et al. IGF‐1 interacted with obesity in prognosis prediction in HER2‐positive breast cancer patients. Front Oncol. 2020;10:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. AsghariHanjani N, Vafa M. The role of IGF‐1 in obesity, cardiovascular disease, and cancer. Med J Islam Repub Iran. 2019;33:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clatici VG, Voicu C, Voaides C, Roseanu A, Icriverzi M, Jurcoane S. Diseases of civilization ‐ cancer, diabetes, obesity and acne ‐ the implication of milk, IGF‐1 and mTORC1. Maedica. 2018;13(4):273‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doyle SL, Donohoe CL, Finn SP, et al. IGF‐1 and its receptor in esophageal cancer: association with adenocarcinoma and visceral obesity. Am J Gastroenterol. 2012;107(2):196‐204. [DOI] [PubMed] [Google Scholar]
- 43. Dayyani F, Varkaris A, Araujo JC, et al. Increased serum insulin‐like growth factor‐1 levels are associated with prolonged response to dasatinib‐based regimens in metastatic prostate cancer. Prostate. 2013;73(9):979‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shariat SF, Lamb DJ, Kattan MW, et al. Association of preoperative plasma levels of insulin‐like growth factor I and insulin‐like growth factor binding proteins‐2 and ‐3 with prostate cancer invasion, progression, and metastasis. J Clin Oncol. 2002;20(3):833‐841. [DOI] [PubMed] [Google Scholar]
- 45. Terracciano D, Bruzzese D, Ferro M, et al. Preoperative insulin‐like growth factor‐binding protein‐3 (IGFBP‐3) blood level predicts gleason sum upgrading. Prostate. 2012;72(1):100‐107. [DOI] [PubMed] [Google Scholar]
- 46. Nimptsch K, Platz EA, Pollak MN, et al. Plasma insulin‐like growth factor 1 is positively associated with low‐grade prostate cancer in the Health Professionals Follow‐up Study 1993‐2004. Int J Cancer. 2011;128(3):660‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Price AJ, Allen NE, Appleby PN, et al. Insulin‐like growth factor‐I concentration and risk of prostate cancer: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1531‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ahearn TU, Peisch S, Pettersson A, et al. Expression of IGF/insulin receptor in prostate cancer tissue and progression to lethal disease. Carcinogenesis. 2018;39(12):1431‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ofer P, Heidegger I, Eder IE, et al. Both IGF1R and INSR knockdown exert antitumorigenic effects in prostate cancer in vitro and in vivo. Mol Endocrinol. 2015;29(12):1694‐1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cushen SJ, Power DG, Murphy KP, et al. Impact of body composition parameters on clinical outcomes in patients with metastatic castrate‐resistant prostate cancer treated with docetaxel. Clin Nutr ESPEN. 2016;13:e39‐e45. [DOI] [PubMed] [Google Scholar]
- 51. Wu W, Liu X, Chaftari P, et al. Association of body composition with outcome of docetaxel chemotherapy in metastatic prostate cancer: a retrospective review. PLoS One. 2015;10(3):e0122047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Donini LM, Pinto A, Giusti AM, Lenzi A, Poggiogalle E. Obesity or BMI paradox? Beneath the tip of the iceberg. Front Nutr. 2020;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


