Abstract
Background and Objectives
The histological growth pattern (HGP) represents a strong prognostic factor in patients undergoing surgery for colorectal liver metastases (CRLM). We evaluated whether the combination of HGP with clinico‐metabolic parameters could improve its prognostic value.
Methods
In a series of 108 patients undergoing resection of CRLM, the HGP of CRLM was scored according to international guidelines. Baseline clinico‐metabolic clinical status was evaluated using a metabolic‐Clinical Risk Score (mCRS), combining traditional Memorial Sloan Kettering‐CRS parameters with the tumor‐to‐liver glucose uptake ratio as measured with 18Fluorodeoxyglucose/positron emission tomography.
Results
In patients with desmoplastic HGP (DHGP) CRLM (20% of all patients), 5‐ and 10‐years overall survival (OS) and disease free survival (DFS) were 66% and 43% and 37% and 24.5%, as compared with 35% and 21% and 11% and 11% in the non‐DHGP group (p = 0.07 and 0.054). Among DHGP patients, those with a low‐risk mCRS had improved postoperative outcomes, 5‐ and 10‐years OS and DFS reaching 83.3% and 62.5% and 50% and 33%, as compared with 18% and 0% and 0% and 0% in high‐risk mCRS patients (p = 0.007 and 0.003). In contrast, mCRS did not influence postoperative survivals in non‐DHGP patients.
Conclusions
Combining the clinico‐metabolic characteristics with the HGP may improve prognostication in patients undergoing surgery for CRLM.
Keywords: colorectal, liver metastasis, prognostic model, surgery
1. INTRODUCTION
Currently, surgery, whether or not associated with perioperative chemotherapy, is almost systematically recommended for patients with technically resectable colorectal liver metastases (CRLM), leading to prolonged survival in 35%–50% and cure in 20%–35% of the cases. 1 , 2 , 3 , 4 Although justified by the fact that surgery still represents the only potentially curative option for these patients, this therapeutic decision‐making remains poorly individualized as the majority of the patients recur postoperatively, including a substantial proportion with rapid and aggressive relapses. 5 , 6 Accordingly, the identification of better selection criteria for surgery in patients with CRLM still represents a major objective, in particular, to prevent futile interventions in patients with resectable disease but aggressive tumor biology. Several clinical risk models have indeed been established for prognostication and stratification of these patients. 7 , 8 , 9 , 10 However, these scoring systems remain poorly predictive at the individual level, 11 underlying the need for identifying more accurate biomarkers of tumor biology and metastatic behavior. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19
The histopathological growth pattern (HGP) of liver metastases has now repeatedly been demonstrated as a reproducible, independent, and strong prognostic factor in patients who underwent resection of CRLM. 20 , 21 , 22 Two main HGPs have been described in CRLM: (1) Desmoplastic HGP (DHGP), characterized by a fibrous or connective tissue rim surrounding the metastasis and separating tumor from liver cells, with numerous immune cells at the tumor–liver interface (TLI) and angiogenesis and, (2) Replacement HGP (RHGP), characterized by cancer cells growing into the liver parenchyma resulting in direct contact between cancer cells and liver cells, minimal to absent immune cell infiltrate at the TLI and tumor vascularization relying on the use of pre‐existing hepatic vessels, that is, vessel cooption. 23 , 24 A third, but uncommon HGP pattern has been observed, defined as the pushing HGP (PHGP), in which the metastasis pushes away the liver tissue, without desmoplastic rim and without invasion of cancer in the liver cell plates. Multiple studies have shown that patients have a significantly improved outcome when resected CRLM present a DHGP compared to CRLM with an RHGP. 25 , 26 , 27 In particular, postoperative survivals are significantly increased in patients with pure DHGP CRLM, that is, when 100% of the TLI displays desmoplastic features, as compared with CRLM with any RHGP component.
In parallel, tumor glucose uptake, as measured by 18Fluorodeoxyglucose‐positron emission tomography (18F‐FDG/PET‐CT), had shown its prognostic value in different cancers. 28 , 29 , 30 , 31 , 32 , 33 Generally, glucose uptake of cancer cells reflects the tumor metabolism that is increased by diverse mechanisms associated with its aggressiveness. 34 Specifically in colorectal cancer and in CRLM, high preoperative glucose uptake is associated with a poor prognosis. 30 , 35 , 36 We recently reported that the baseline tumor‐to‐liver glucose uptake ratio, as measured with 18F‐FDG/PET‐CT at diagnosis of CRLM, combined with the parameters of the Memorial Sloan Kettering Clinical Risk Score (CRS), a model that is widely used for stratification of patients with CRLM, 7 may improve the prediction of the postoperative outcome in these patients. On this basis, we proposed a new prognostic score, defined as the metabolic CRS (mCRS). 37
In this study, we analyzed whether combining HGP with preoperative metabolic parameters measured by 18F‐FDG/PET‐CT associated with CRS (so‐defined mCRS) would improve the prediction of postoperative outcome in patients undergoing surgery for CRLM. To that purpose, we analyzed a retrospective series of patients operated for CRLM and related survival to HGP and mCRS.
1.1. Patients and methods
1.1.1. Study population
A consecutive series of 357 patients who underwent curative‐intent liver resection for CRLM at Institut Jules Bordet and Hôpital Erasme, Université Libre de Bruxelles, Belgium, between 2005 and 2017 was reviewed. Patients with extrahepatic disease were excluded. Hematoxylin and eosin (H&E) stained tissue sections of all resected CRLM were available and suitable for HGP scoring in 263/357 patients. Pathological samples could not be used for HGP scoring when tissue preservation was poor, in case of complete pathological response to preoperative chemotherapy, or when all CRLM had been treated with radiofrequency destruction. Patients with R2‐resection of the CRLM were also excluded. In the remaining patient cohort, we identified 108 patients with baseline 18F‐FDG/PET‐CT available, that is, when performed at the time of the diagnosis of CRLM, before any eventual preoperative treatment. This study has been approved by ethical committees of both institutions (P2019/232 and CE2953).
1.1.2. Clinicopathologic and surgical data
Demographic and clinicopathologic parameters were collected in a prospective database. CRS was calculated by assigning 1 point for the presence of each of the following conditions: node‐positive primary tumor, CRLM diagnosis ≤ 12 months after resection of the primary, number of CRLM lesions > 1, size of the largest CRLM ≥ 50 mm, and preoperative carcinoembryonic antigen (CEA) ≥ 200 ng/ml7. The CRS was divided into low‐risk (0–2 points) and high‐risk (3–5 points). Hepatectomy was defined as minor or major when less than 3 and greater than or equal to 3 segments were resected, respectively. Postoperative complications were recorded within 90 postoperative days and graded according to Clavien Dindo classification. 38 Overall survival (OS) was defined as the time between liver surgery for CRLM and the last follow‐up or death. Disease free survival (DFS) was defined as the time between liver surgery for CRLM and the first hepatic or extrahepatic recurrence, last follow‐up or death.
1.1.3. HGP scoring
HGPs were evaluated according to international consensus guidelines published by the Liver Metastasis Research Network in 2017. 39 In each patient, all available H&E‐stained sections of the TLI of all available metastases were evaluated using light microscopy. All tissue sections were examined by one experienced pathologist, together with one surgeon and a medical student, blinded to individual patient's data. Individual H&E‐stained sections were excluded when less than 20% of the TLI was available. Results were indicated as average HGP‐scores that represent the mean percentage of each HGP per metastasis (in case of multiple slides per metastasis) or per patient (in case of multiple metastases per patient). According to the international consensus guidelines, when a fibrotic rim surrounded the tumor and no contact between cancer cells and hepatocytes was present, DHGP was noted. In the absence of a peritumor fibrotic rim and when cancer cells grew into the liver parenchyma with close contact with hepatocytes, RHGP was noted. In the absence of a peritumoral fibrotic rim and when no direct contact between cancer cells and hepatocytes within the liver cell plates existed, PHGP was noted. Patients were categorized as DHGP when the desmoplastic HGP represented 100% of the TLI. All other patients were categorized as non‐DHGP (i.e., any presence of RHGP or PHGP).
1.1.4. 18F‐FDG/PET‐CT imaging procedure and analyses
18FDG‐PET/CT was performed with four different cameras (GE Discovery LightSpeed, GE Discovery 690, Philips Gemini‐16P, and Philips Gemini GXL). Whole‐body imaging (skull base or apex of the skull to mid‐thigh) was performed according to the standardized practice guidelines. Imaging started 60–120 min after 18F‐FDG administration. Images were analyzed using dedicated commercial software (PET VCAR v.4.6; Advantage Workstation; GE Healthcare). Maximum standardized uptake values (SUVmax) were computed for all observable CRLM. Additionally, reference SUV of the background liver specific to each patient was calculated by measuring the average SUV in a spherical region of interest of 3 cm of diameter in nontumor liver tissue (SUVmean(liver)), similarly to PERCIST criteria 1.0. 40 When no lesion was observable, the lesion's SUVmax value were considered equal to SUVmean(liver). Ratios SUVmax/SUVmean(liver) were calculated to reduce the variability of the measurements due to differences between cameras. Images were analyzed by three experienced nuclear physicians blinded to individual patient's data.
1.1.5. Metabolic CRS
As previously reported, 37 we defined a mCRS by expanding the CRS with metabolic data derived from 18F‐FDG/PET‐CT. One point was added to the CRS when the highest SUVmax/SUVmean(liver) ratio of the metastatic hepatic lesion was greater than 4.3. 41 The mCRS was calculated for each patient and divided into low‐risk (0–3 points) and high‐risk (4–6 points).
1.1.6. Statistical analysis
The data were analyzed with the statistical software SPSS (IBM) version 27. Values are expressed as medians (interquartile range [IQR]), mean (SD) or the number of patients and percentages. Descriptive analysis of all of the clinical and histological parameters was done. Survival curves were generated using the Kaplan–Meier method. Survival was compared between the groups (DHGP vs. non‐DHGP) using the Log rank test. The factors affecting survival were evaluated using a univariate and multivariate cox regression analysis. Factors with a p < 0.1 in univariate analysis were entered to a multivariate cox regression model. A p < 0.05 was considered statistically significant.
2. RESULTS
2.1. Patient characteristics and postoperative outcomes
The demographic and clinicopathological characteristics of the 108 patients are described in Table 1. The mean age was 64 years and 62% of the patients were male. Forty‐two patients (38.9%) had a body mass index (BMI) between 25 and 29.9 kg/m2, 18 patients (16.6%) had a BMI > 30 kg/m2, and 35% of the patients were classified ASA III. Forty percent of the patients had a left colon or sigmoid primary tumor and a third a rectal tumor. Seventy‐five percent had a stage III or IV primary tumor and 44% a mutated KRAS status. Approximately half of the patients received adjuvant chemotherapy after colorectal surgery. CRLM were synchronous and multiple in 70% of the cases. The majority of the patients (80%) received chemotherapy before liver resection and 16% received chemotherapy after liver surgery. Almost half of the patients underwent major liver resection and 20% had high‐grade postoperative complications. No postoperative mortality was recorded. After a median follow‐up of 66 ± 14 months, the 3‐, 5‐ and 10‐years OS in the entire series was 54.2%, 41.9%, and 25.3%, respectively, with a median OS of 45 ± 12.6 months (Figure 1A). The 3‐, 5‐ and 10‐years DFS was 18.4%, 16.6%, and 12.4%, respectively, with a median DFS of 9 ± 1.3 months (Figure 1B).
Table 1.
Patient, tumor, and treatment characteristics
| Number of patients | 108 |
| Age (mean ± SD) years | 64.3 ± 12.3 |
| Male sex, n (%) | 67 (62%) |
| Primary tumor, n (%) | |
| Right‐sided | 19 (17.6%) |
| Transverse | 7 (6.5%) |
| Left‐sided | 46 (42.6%) |
| Rectum | 36 (33.3%) |
| KRAS mutated | 42/96 (43.8%) |
| N + status | 75 (69.4%) |
| Stage III–IV | 81 (75%) |
| Chemotherapy for primary tumor, n (%) | |
| Neoadjuvant | 42 (38.8%) |
| Adjuvant | 56 (51.9%) |
| CRLM, n (%) | |
| Synchronous < 12 months after primary tumor | 74 (68.5%) |
| Number of LM, median (IQR) | 2 (4) |
| Size of the largest CRLM (mm), median (IQR) | 29 (25) |
| Size of the largest CRLM > 50 mm, mean (%) | 26 (24.1%) |
| CEA (ng/ml), median (IQR) | 15 (31) |
| CEA > 200 ng/ml, n (%) | 11 (10.2%) |
| Clinical risk score, n (%) | |
| 0 | 4 (3.7%) |
| 1 | 15 (13.9%) |
| 2 | 38 (35.2%) |
| 3 | 38 (35.2%) |
| 4 | 10 (9.3%) |
| 5 | 3 (2.8%) |
| Low risk (0–2) | 57 (52.8%) |
| High risk (3–5) | 51 (47.2%) |
| Metabolic chracteristics CRLM | |
| SUVmax/SUVmean(liver), median (IQR) | 3.9 (2.3) |
| SUVmax/SUVmean(liver) > 4.3, n (%) | 46 (42.6%) |
| Metabolic Clinical Risk Score, n (%): | |
| 0 | 4 (3.7%) |
| 1 | 10 (9.3%) |
| 2 | 26 (24.1%) |
| 3 | 40 (37%) |
| 4 | 19 (17.6%) |
| 5 | 6 (5.6%) |
| 6 | 3 (2.8%) |
| Low risk (0–3) | 80 (74.1%) |
| High risk (4–6) | 28 (25.9%) |
| Liver surgery, n (%): | |
| Portal vein embolization | 13 (12%) |
| Two‐steps hepatectomy | 19 (17.6%) |
| Major hepatectomy | 53 (49.1%) |
| Complication clavien Dindo, n (%) | |
| 0 | 64 (59.3%) |
| I | 10 (9.3%) |
| II | 12 (11.1%) |
| IIIa | 17 (15.7%) |
| IVa | 5 (4.6%) |
| V | 0 |
| Chemotherapy for CRLM, n (%): | |
| Preoperative | 85 (78.7%) |
| Postoperative | 17 (15.7%) |
| HGP CRLM, n (%): | |
| DHGP | 22 (20%) |
| non‐DHGP | 86 (80%) |
Abbreviations: CEA, carcino embryonic antigen; CRLM, colorectal liver metastasis; DHGP, pure desmoplastic histologic growth pattern; HGP, histologic growth pattern; KRAS, Kirsten rat sarcoma 2 viral oncogene homolog; LM, liver metastasis; N+, lymph node; non‐DHGP, nondesmoplastic histologic growth pattern; SUV, standardized uptake values.
Figure 1.
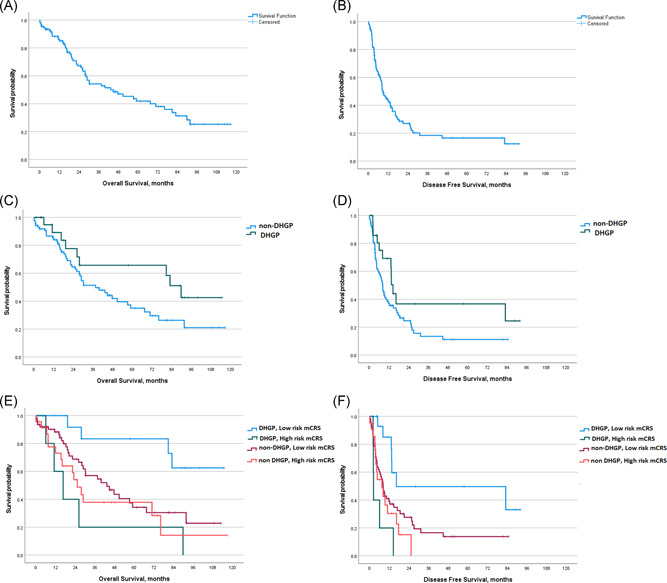
Kaplan–Meier survival plots of overall survival and disease‐free survival, in the whole cohort (A) and (B), stratified by the histological growth pattern (as DHGP and non‐DHGP) (C) and (D) and by the histological growth pattern associated with metabolic CRS (E) and (F). CRS, clinical risk score; DHGP, desmoplastic histological growth pattern
2.2. Histopathological growth patterns
The individual HGP scores are detailed in Figure 2. Twenty percent of the patients were categorized as DHGP and 80% as non‐DHGP (Table 1 and Figure 2).
Figure 2.
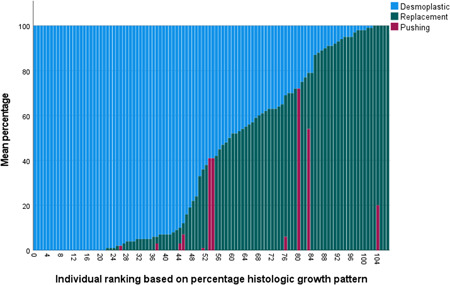
Distribution of HGP: Ranking based on percentage desmoplastic HGP. HGP, histologic growth patterns
2.3. Primary tumor characteristics, preoperative treatments, CRS, metabolic characteristics and mCRS in relation with HGP
There was no correlation between KRAS mutational status and the DHGP or non‐DHGP presentations (Table 2). Similarly, we found no association between the administration of preoperative chemotherapy and the DHGP or non‐DHGP presentations (Tables 2). In the entire series, almost half of the patients presented with a high‐risk CRS at the time of liver surgery (Table 1). Forty‐six patients (42.6%) had at least 1 CRLM with a SUVmax/SUVmean(liver) > 4.3, and 80 patients (74%) has a low‐risk mCRS (Table 1).
Table 2.
Clinical risk score, metabolic characteristics and metabolic clinical risk score in DHGP and non‐DHGP groups
| DHGP (n = 22) | Non‐DHGP (n = 86) | P value | |
|---|---|---|---|
| KRAS mutation | 9 (47.4%) | 33 (42.9%) | 0.799 |
| Clinical risk score, n (%) | 0.585 | ||
| 0 | 1 (4.5%) | 3 (3.5%) | |
| 1 | 2 (9.1%) | 13 (15.1%) | |
| 2 | 8 (36.4%) | 30 (34.9%) | |
| 3 | 7 (31.8%) | 31 (36%) | |
| 4 | 4 (18.2%) | 6 (7%) | |
| 5 | 0 | 3 (3.5%) | |
| High risk clinical risk score, n (%) | 11 (50%) | 40 (46.5%) | 0.814 |
| SUVmax/SUVmean(liver) | 3.16 (1.74) | 4.26 (2.5) | 0.009 |
| SUVmax/SUVmean(liver) > 4.3 | 3 (13.6%) | 43 (50%) | 0.003 |
| Metabolic clinical risk score | 0.94 | ||
| 0 | 1 (4.5%) | 3 (3.5%) | |
| 1 | 2 (9.1%) | 8 (9.3%) | |
| 2 | 7 (31.8%) | 19 (22.1%) | |
| 3 | 7 (31.8%) | 33 (38.4%) | |
| 4 | 4 (18.2%) | 15 (17.4%) | |
| 5 | 1 (4.5%) | 5 (5.8%) | |
| 6 | 0 | 3 (3.5%) | |
| High risk metabolic clinical risk score | 5 (22.7%) | 23 (26.7%) | 0.791 |
| Neoadjuvant chemotherapy | 19 (86.4%) | 66 (76.7%) | 0.396 |
| Immunotherapy | 0.835 | ||
| Bevacizumab | 2 (9.1%) | 8 (9.3%) | |
| Cetuximab | 3 (13.6%) | 8 (9.3%) |
There was no correlation between CRS, mCRS categories and low‐ and high‐risk mCRS and the DHGP or non‐DHGP presentations (Table 2). A higher baseline glucose uptake of CRLM was observed in the non‐DHGP patients as compared with DHGP, mean SUVmax/SUVmean(liver) being of 4.5 ± 2 and 3.4 ± 1.7 and (p = 0.009), and significantly more patients in the non‐DHGP group had at least 1 CRLM with SUVmax/SUVmean(liver) > 4.3, representing 50%, as compared with 13.6% in the DHGP group (p = 0.003) (Table 2). However, a considerable overlap of these values was observed between DHGP and non‐DHGP, as witnessed by the confidence intervals. There was no significant difference in the mCRS categories and ratios of low‐ versus high‐risk mCRS between DHGP and non‐DHGP groups (Table 2).
2.4. Risk factors associated with postoperative survivals
The size of the largest CRLM > 50 mm, high‐risk mCRS, and major hepatectomy were significantly associated with poorer OS in univariate analysis (Table 3). A statistical trend for an improved OS was associated with DHGP when compared with non‐DHGP, median OS being respectively 89 ± 7.5 months versus 37 ± 7.5 (p = 0.075) (Figure 1C and Table 4). In multivariate analysis, none of these factors remained significant (Table 3). Positive lymph node status of primary tumor, more than 1 CRLM, high‐risk CRS, high‐risk mCRS and major hepatectomy, were all significantly associated with poorer DFS in univariate analysis (Table 3). A tendency for a better DFS was observed in DHGP as compared with non‐DHGP, median DFS being respectively of 14.4 months versus 8.3 (p = 0.057) (Figure 1D and Table 4). All these factors have been tested for colinearity and, as no significant colinearity was found, all of the factors were entered into a multivariable model. In multivariate analysis, more than 1 CRLM (hazardous ratio [HR] = 1.93, 95% confidence interval [CI] = 1.06–3.52, p = 0.031) and non‐DHGP (HR = 2.375, 95%CI = 1.187–4.75, p = 0.014) were significant for poorer DFS (Table 4). When HGP and mCRS were considered together, a significantly better outcome is observed in patients with DHGP and low‐risk mCRS as compared with all other groups for OS (p = 0.007) (Figure 1E) and for DFS (p = 0.003) (Figure 1F). In particular, in patients with DHGP and low‐risk mCRS, 3‐, 5‐ and 10‐years OS were 83%, 83% and 62.5%, as compared to 18%, 18% and 0%, in patients with DHGP and high‐risk mCRS (p = 0.003) (Table 4 and Figure 1E), and 3‐ and 5‐years DFS were 50% and 33%, as compared with 0% and 0%, in patients with DHGP and high‐risk mCRS (p < 0.001) (Table 4 and Figure 1F). Of note, OS and DFS in DHGP with high‐risk mCRS were similar to patients with non‐DHGP independent of mCRS. (Table 4, Figures 1E and 1F).
Table 3.
Survival depending to both HGP and metabolic clinical risk score
| DHGP (n = 22) | Non‐DHGP (n = 86) | p | |||
|---|---|---|---|---|---|
| Low risk mCRS (n = 17) | High risk mCRS (n = 5) | Low risk mCRS (n = 63) | High risk mCRS (n = 23) | ||
| OS | 0.007 | ||||
| 3 years | 83.3% | 17.9% | 57% | 37.9% | |
| 5 years | 83.3% | 17.9% | 34.3% | 37.9% | |
| 10 years | 62.5% | 0% | 22.8% | 14.2% | |
| Median OS | 16.6 months | 43.3 months | 25 months | ||
| DFS | 0.003 | ||||
| 3 years | 49.7% | 0% | 16.6% | 0% | |
| 5 years | 33.1% | 0% | 13.8% | 0% | |
| 10 years | |||||
| Median DFS | 72 months | 10 months | 28 months | 7.4 | |
Abbreviations: DFS, disease free survival; mCRS, metabolic clinical risk score; OS, overall survival.
Table 4.
Univariate and multivariate cox regression analysis of factors affecting OS and DFS
| OS | DFS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Node‐positive primary tumor, n (%) | 1.654 | 0.884–3.096 | 0.115 | 1.717 | 0.997–2.957 | 0.051 | 1.66 | 0.88–3.11 | 0.117 | |||
| Disease‐free interval < 12 months, n (%) | 1.034 | 0.587–1.819 | 0.909 | 1.126 | 0.688–1.841 | 0.637 | ||||||
| Number of CRLM: >1 liver metastasis, n (%) | 1.141 | 0.637–2.044 | 0.657 | 1.982 | 1.180–3.329 | 0.01 | 1.93 | 1.06‐3.52 | 0.031 | |||
| Size of CRLM: Largest >50 mm, n (%) | 1.86 | 1.036–3.339 | 0.038 | 1.08 | 0.51–2.31 | 0.835 | 1.246 | 0.714–2.173 | 0.438 | |||
| CEA > 200 | 0.715 | 0.285–1.797 | 0.476 | 1.075 | 0.492–2.349 | 0.857 | ||||||
| KRAS mutation | 1.437 | 0.814–2.535 | 0.211 | 1.065 | 0.651–1.743 | 0.803 | ||||||
| Major hepatectomy (≥3 segments) | 1.755 | 1.024–3.007 | 0.041 | 1.57 | 0.84–2.95 | 0.158 | 1.583 | 0.997–2.513 | 0.052 | 1.51 | 0.88–2.57 | 0.13 |
| CRS Categories (high risk vs. low risk) | 1.647 | 0.965–2.811 | 0.067 | 1.12 | 0.50‐2.53 | 0.787 | 2.114 | 1.290–3.464 | 0.003 | 1.01 | 0.52–2.31 | 0.807 |
| SUVmax/SUVmean(liver) >4.3 | 1.637 | 0.961–2.786 | 0.07 | 0.899 | 0.40–2.03 | 0.797 | 1.289 | 0.812–2.047 | 0.281 | |||
| Metabolic risk score (high risk vs. low risk) | 1.968 | 1.133–3.421 | 0.016 | 1.45 | 0.50–1.19 | 0.489 | 1.83 | 1.089–3.075 | 0.022 | 1.186 | 0.46–1.94 | 0.873 |
| Desmoplastic vs. nondesmoplastic | 1.93 | 0.93–4.1 | 0.075 | 1.96 | 0.86–4.46 | 0.11 | 1.836 | 0.98–3.44 | 0.057 | 2.375 | 1.19–4.75 | 0.014 |
| Neoadjuvant chemotherapy before primary surgery | 1.433 | 0.837–2.454 | 0.19 | 1.229 | 0.761–1.985 | 0.4 | ||||||
| Adjuvant chemotherapy after primary surgery | 1.196 | 0.703–2.035 | 0.509 | 1.366 | 0.852–2.189 | 0.195 | ||||||
| Neoadjuvant chemotherapy before LM surgery | 1.209 | 0.591–2.473 | 0.604 | 0.87 | 0.499–1.518 | 0.625 | ||||||
| Adjuvant chemotherapy after LM surgery | 0.594 | 0.287–1.232 | 0.162 | 0.642 | 0.338–1.221 | 0.177 | ||||||
Abbreviations: DFS, disease free survival; LM, liver metastasis; mCRS, metabolic clinical risk score; OS, overall survival.
3. DISCUSSION
The challenge faced when selecting patients for CRLM surgery is in fact to distinguish between the patients with restricted metastatic capacity, or oligometastatic disease, 42 who may benefit from metastasis‐targeted therapies, and those with aggressive metastatic spreading, for whom surgery will ultimately be ineffective. It is expected that these different types of metastatic progressions depend on both the intrinsic tumor cell characteristics and the host responses. HGPs of CRLM reflect these complex interactions of cancer cells and the specific microenvironment of the liver and might thus represent an attractive candidate biomarker in this setting. At present, the prognostic value of HGP has been reproducibly demonstrated in patients undergoing resection of CRLM. 20 , 26 , 27 , 43 , 44 Furthermore, similar observations have been made in patients operated for liver metastases from other origins, such as breast cancer 45 or melanoma. 46 , 47 This supports the potential value of HGP as a (surrogate) marker predominantly related to the individual tumor biology and its metastatic profile, rather than to its primary origin.
The present study confirms the independent prognostic value of the HGP of CRLM with a hazard ratio of 2.375 for developing recurrent disease in patients with non‐DHGP metastases. Of note, the use of other cut‐offs for HGP categorization, such as desmoplastic features at more than 95%, more than 66% or more than 50% of the TLI, is not associated with an improvement of the prognostic value (data not shown). This result should be interpreted taking into account the fact that a high proportion of patients in the current series received preoperative chemotherapy, a factor that has been shown to increase the ratio of DHGP, while decreasing its intrinsic favorable prognostic value. 48 , 49 This selection bias in our patient population is due to the fact that we routinely use 18F‐FDG/PET‐CT to evaluate the response to chemotherapy. The limited number of patients without chemotherapy before liver resection does not allow comparing the respective prognostic value of HGPs in patients with or without preoperative systemic treatment. Furthermore, the absence of an evaluable effect of preoperative chemotherapy on HGP in this series was verified when using other cut‐offs for desmoplastic and replacement patterns categorization. For instance, when patients were identified as desmoplastic‐dominant (such as greater than 50% of replacement features at the TLI) or replacement‐dominant (such as greater than 50% of desmoplastic features at the TLI), 83% and 73.5%, respectively, had received preoperative chemotherapy (data not shown). The results of this survival analysis still has a broad impact, given that many groups worldwide advocate the use of preoperative chemotherapy in patients undergoing surgery for CRLM. 3 , 50 The only other factor that independently predicted DFS was multinodularity of CRLM. It is well‐known that this factor is predictive for postoperative relapse, but it poorly discriminates the patients who will or will not benefit from surgery. 1 , 7
The main finding of our study is that, among the patients with resected liver metastases with the most favorable desmoplastic pattern, clinico‐metabolic characterization may be applied to refine the prognostic value of this HGP. To evaluate the clinico‐metabolic status in each patient, we relied on the mCRS that we previously described, 37 which combines the traditional CRS parameters 7 with the tumor glucose metabolism assessed with 18F‐FDG/PET‐CT. An advantage of the mCRS, and in contrast with HGP, is the availability of this score at the time of diagnosis of CRLM, not influenced by any preoperative systemic treatment. When patients undergoing resection of DHGP CRLM are divided into low or high‐risk mCRS groups, highly significant differences in postoperative survivals were observed. In patients with DHGP CRLM and low‐mCRS, 5‐years OS and DFS reached 83% and 33%, as compared with 18% and 0%, respectively, in patients with DHGP CRLM and high‐mCRS. In contrast, we found no impact of the mCRS in patients with non‐DHGP. Furthermore, the metabolic and the clinico‐metabolic characteristics, including the SUVmax/SUVmean(liver) ratio, the SUVmax/SUVmean(liver) ratio > 4.3, the mCRS categories and low‐ versus high‐risk CRS, were not prognostic when considered independently of the HGP. An additional caveat of our study is that chemotherapy can change the HGP of a CRLM as has been suggested by several authors. 22 , 48 Given that the assessment of the mCRS occurs before and the HGP scoring after the administration of chemotherapy, the analyses of the association between these two parameters (Table 2) might have been affected by HGP conversion, for example, from replacement to desmoplastic, in some patients. The elevated preoperative FDG uptake by liver metastases with a postoperative non‐DHGP is, however, to be expected, as the replacement HGP is composed of less‐differentiated carcinoma with an efficient blood supply by vessel co‐option. 24 , 51 Still, large overlaps between uptake values in DHGP and non‐DHGP CRLM preclude the use of FDG‐uptake for predicting HGP in the present series.
HGP assessment on surgical resection specimen represents a snapshot of the tumor's histological organization that may change as a natural disease course or as a consequence of therapy, such as chemotherapy. In that sense, having access to noninvasive means, such as CT‐ or magnetic resonance imaging (MRI)‐imaging and radiomics to repeatedly identify the HGP of CRLM in a patient would be of significantly added value, both to improve its reliability as a biomarker and to better understand tumor biology, including response to chemotherapy. 52 , 53 More particularly, the MRI‐technique may allow now to precisely explore the tumor and the peripheral liver parenchyma compartments, offering promising perspectives in this respect. 54 In parallel, recent developments in molecular imaging techniques using specific PET tracers could provide qualitative, quantitative and dynamic evaluation on the nature of intra‐ and peri‐tumoral cellular infiltrates, such as lymphocytes or fibroblasts. 55 , 56 Such information could be of major interest to predict and to follow the evolution of different HGPs. This would create an attractive tool, integrating HGP into new therapeutic decision algorithms to predict the benefit (or not) of surgery for CRLM.
The main limitations of our study are the small number of patients, its retrospective nature, and the absence of a significant group of patients that were chemo‐naive at the time of surgery. This emphasizes the need for further validation of our main finding, the significantly improved DFS of patients with DHGP CRLMs with a low‐risk mCRS in larger cohorts that will also include patients without preoperative chemotherapy.
In conclusion, the addition of baseline clinico‐metabolic characteristics may significantly improve the prognostic value of HGP. When noninvasive methods able to predict HGP are available, the resulting parameters could be integrated into new risk models to improve the precision of the therapeutic decisions in patients with CRLM candidate for surgery.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENT
This study has been funded by “Le Fonds Ithier”, “Les Amis de Bordet and a grant from Roche.
Bohlok A, Duran Derijckere I, Azema H, et al. Clinico‐metabolic characterization improves the prognostic value of histological growth patterns in patients undergoing surgery for colorectal liver metastases. J Surg Oncol. 2021;123:1773–1783. 10.1002/jso.26466
Ali Bohlok and Ivan Duran Derijckere are co‐first authors.
Peter Vermeulen and Vincent Donckier are co‐last authors.
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10‐year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575‐4580. 10.1200/JCO.2007.11.0833 [DOI] [PubMed] [Google Scholar]
- 2. LiverMetSurvey ARCAD . https://livermetsurvey-arcad.org/. Accessed May 8, 2019.
- 3. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386‐1422. 10.1093/annonc/mdw235 [DOI] [PubMed] [Google Scholar]
- 4. Nordlinger B, Guiguet M, Vaillant J‐C, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77(7):1254‐1262. [DOI] [PubMed] [Google Scholar]
- 5. Viganò L, Capussotti L, Lapointe R, et al. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey‐based study of 6,025 patients. Ann Surg Oncol. 2014;21(4):1276‐1286. 10.1245/s10434-013-3421-8 [DOI] [PubMed] [Google Scholar]
- 6. Malik HZ, Gomez D, Wong V, et al. Predictors of early disease recurrence following hepatic resection for colorectal cancer metastasis. Eur J Surg Oncol. 2007;33(8):1003‐1009. 10.1016/j.ejso.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 7. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309‐318. http://www.ncbi.nlm.nih.gov/pubmed/10493478. Accessed May 8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zakaria S, Donohue JH, Que FG, et al. Hepatic resection for colorectal metastases. Value Risk Scoring Syst? 2007;246:183‐191. 10.1097/SLA.0b013e3180603039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dupré A, Rehman A, Jones RP, et al. Validation of clinical prognostic scores for patients treated with curative‐intent for recurrent colorectal liver metastases. J Surg Oncol. 2018;117(6):1330‐1336. 10.1002/jso.24959 [DOI] [PubMed] [Google Scholar]
- 10. Reissfelder C, Rahbari NN, Koch M, et al. Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16(12):3279‐3288. 10.1245/s10434-009-0654-7 [DOI] [PubMed] [Google Scholar]
- 11. Domchek SM, Friebel TM, Singer CF, et al. Association of risk‐reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967. 10.1001/jama.2010.1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brudvik KW, Jones RP, Giuliante F, et al. RAS mutation clinical risk score to predict survival after resection of colorectal liver metastases. Ann Surg. 2019;269(1):120‐126. 10.1097/SLA.0000000000002319 [DOI] [PubMed] [Google Scholar]
- 13. Liu W, Wang K, Han Y, Liang JY, Li YH, Xing BC. Nomogram predicted disease free survival for colorectal liver metastasis patients with preoperative chemotherapy followed by hepatic resection. Eur J Surg Oncol. 2019;45(11):2070‐2077. 10.1016/j.ejso.2019.06.033 [DOI] [PubMed] [Google Scholar]
- 14. Paredes AZ, Hyer JM, Tsilimigras DI, et al. A novel machine‐learning approach to predict recurrence after resection of colorectal liver metastases. Ann Surg Oncol. 2020;27(13). 10.1245/s10434-020-08991-9 [DOI] [PubMed] [Google Scholar]
- 15. Margonis GA, Sasaki K, Kim Y, et al. Tumor biology rather than surgical technique dictates prognosis in colorectal cancer liver metastases. J Gastrointest Surg. 2016;20(11):1821‐1829. 10.1007/s11605-016-3198-8 [DOI] [PubMed] [Google Scholar]
- 16. Margonis GA, Kim Y, Spolverato G, et al. Association between specific mutations in KRAS codon 12 and colorectal liver metastasis. JAMA Surg. 2015;150(8):722. 10.1001/jamasurg.2015.0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brudvik KW, Mise Y, Chung MH, et al. RAS mutation predicts positive resection margins and narrower resection margins in patients undergoing resection of colorectal liver metastases. Ann Surg Oncol. 2016;23(8):2635‐2643. 10.1245/s10434-016-5187-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta‐analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015;102(10):1175‐1183. 10.1002/bjs.9870 [DOI] [PubMed] [Google Scholar]
- 19. Vauthey J‐N, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258(4):619‐627. 10.1097/SLA.0b013e3182a5025a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galjart B, Nierop PMH, van der Stok EP, et al. Angiogenic desmoplastic histopathological growth pattern as a prognostic marker of good outcome in patients with colorectal liver metastases. Angiogenesis. 2019;22(2):355‐368. 10.1007/s10456-019-09661-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barnhill R, Dam PJ, Vermeulen P, et al. Replacement and desmoplastic histopathological growth patterns in cutaneous melanoma liver metastases: frequency, characteristics, and robust prognostic value. J Pathol Clin Res. 2020;6(3):195‐206. 10.1002/cjp2.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frentzas S, Simoneau E, Bridgeman VL, et al. Vessel co‐option mediates resistance to anti‐angiogenic therapy in liver metastases. Nat Med. 2016;22(11):1294‐1302. 10.1038/nm.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Dam PJ, Van Der Stok EP, Teuwen LA, et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer. 2017;117(10):1427‐1441. 10.1038/bjc.2017.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vermeulen PB, Colpaert C, Salgado R, et al. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol. 2001;195(3):336‐342. 10.1002/path.966 [DOI] [PubMed] [Google Scholar]
- 25. Moro F. Growth patterns of colorectal cancer liver metastases and their impact on prognosis: a systematic review. BMJ Open Gastro. 2018;5:217. 10.1136/bmjgast-2018-000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eefsen RL, Vermeulen PB, Christensen IJ, et al. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin Exp Metastasis. 2015;32(4):369‐381. 10.1007/s10585-015-9715-4 [DOI] [PubMed] [Google Scholar]
- 27. Nierop PMH, Galjart B, Höppener DJ, et al. Salvage treatment for recurrences after first resection of colorectal liver metastases: the impact of histopathological growth patterns. Clin Exp Metastasis. 2019;36(2):109‐118. 10.1007/s10585-019-09960-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nockel P, El Lakis M, Gaitanidis A, et al. Preoperative 18F‐FDG PET/CT in pheochromocytomas and paragangliomas allows for precision surgery. Ann Surg. 2019;269(4):741‐747. 10.1097/SLA.0000000000002671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lerut T, Flamen P, Ectors N, et al. Histopathologic validation of lymph node staging with FDG‐PET scan in cancer of the esophagus and gastroesophageal junction: a prospective study based on primary surgery with extensive lymphadenectomy. Ann Surg. 2000;232(6):743‐752. 10.1097/00000658-200012000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strasberg SM, Dehdashti F, Siegel BA, Drebin JA, Linehan D. Survival of patients evaluated by FDG‐PET before hepatic resection for metastatic colorectal carcinoma: a prospective database study. Ann Surg. 2001;233(3):293‐299. 10.1097/00000658-200103000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beasley GM, Parsons C, Broadwater G, et al. A multicenter prospective evaluation of the clinical utility of F‐18 FDG‐PET/CT in patients with AJCC stage IIIB or IIIC extremity melanoma. Ann Surg. 2012;256(2):350‐356. 10.1097/SLA.0b013e318256d1f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pedrazzoli S, Sperti C, Pasquali C, Bissoli S, Chierichetti F. Comparison of international consensus guidelines versus 18‐FDG PET in detecting malignancy of intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2011;254(6):971‐976. 10.1097/SLA.0b013e3182383137 [DOI] [PubMed] [Google Scholar]
- 33. Schwarzbach MHM, Hinz U, Dimitrakopoulou‐Strauss A, et al. Prognostic significance of preoperative [18‐F] fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging in patients with resectable soft tissue sarcomas. Ann Surg. 2005;241(2):286‐294. 10.1097/01.sla.0000152663.61348.6f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wittig R, Coy JF. The role of glucose metabolism and glucose‐associated signalling in cancer. Perspect Medicin Chem. 2008;1:64‐82. http://www.ncbi.nlm.nih.gov/pubmed/19812737. Accessed November 25, 2020. [PMC free article] [PubMed] [Google Scholar]
- 35. Shim JR, Lee SD, Han SS, et al. Prognostic significance of 18F‐FDG PET/CT in patients with colorectal cancer liver metastases after hepatectomy. Eur J Surg Oncol. 2018;44(5):670‐676. 10.1016/j.ejso.2018.01.243 [DOI] [PubMed] [Google Scholar]
- 36. Nemeth Z, Wijker W, Lengyel Z, Hitre E, Borbely K. Metabolic parameters as predictors for progression free and overall survival of patients with metastatic colorectal cancer. Pathol Oncol Res. 2020;26(4):2683‐2691. 10.1007/s12253-020-00865-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duran Derijckere I, Levillain H, Bohlok A, et al. The metabolic clinical risk score as a new prognostic model for surgical decision‐making in patients with colorectal liver metastases. J Surg Oncol. 2020;121(2):350‐356. 10.1002/jso.25763 [DOI] [PubMed] [Google Scholar]
- 38. Dindo D, Demartines N, Clavien P‐A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205‐213. http://www.ncbi.nlm.nih.gov/pubmed/15273542. Accessed May 8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Dam P‐J, van der Stok EP, Teuwen L‐A, et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer. 2017;117(10):1427‐1441. 10.1038/bjc.2017.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl. 1):122S‐150S. 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shim JR, Lee SD, Han SS, et al. Prognostic significance of 18F‐FDG PET/CT in patients with colorectal cancer liver metastases after hepatectomy. Eur J Surg Oncol. 2018;44(5):670‐676. 10.1016/j.ejso.2018.01.243 [DOI] [PubMed] [Google Scholar]
- 42. Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8(6):378‐382. 10.1038/nrclinonc.2011.44 [DOI] [PubMed] [Google Scholar]
- 43. Nierop PMH, Galjart B, Höppener DJ, et al. Salvage treatment for recurrences after first resection of colorectal liver metastases: the impact of histopathological growth patterns. Clin Exp Metastasis. 2019;36(2):109‐118. 10.1007/s10585-019-09960-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Höppener DJ, Nierop PMH, Herpel E, et al. Histopathological growth patterns of colorectal liver metastasis exhibit little heterogeneity and can be determined with a high diagnostic accuracy. Clin Exp Metastasis. 2019;36(4):311‐319. 10.1007/s10585-019-09975-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bohlok A, Vermeulen P, Leduc S, et al. Association between the histopathological growth patterns of liver metastases and survival after hepatic surgery in breast cancer patients. NPJ Breast Cancer. 2020;6(1):64. 10.1038/s41523-020-00209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barnhill R, Vermeulen P, Daelemans S, et al. Replacement and desmoplastic histopathological growth patterns: a pilot study of prediction of outcome in patients with uveal melanoma liver metastases. J Pathol Clin Res. 2018;4(4):227‐240. 10.1002/cjp2.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barnhill R, Dam PJ, Vermeulen P, et al. Replacement and desmoplastic histopathological growth patterns in cutaneous melanoma liver metastases: frequency, characteristics, and robust prognostic value. J Pathol Clin Res. 2020;6(3):195‐206. 10.1002/cjp2.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buisman FE, van der Stok EP, Galjart B, et al. Histopathological growth patterns as biomarker for adjuvant systemic chemotherapy in patients with resected colorectal liver metastases. Clin Exp Metastasis. 2020;37(5):593‐605. 10.1007/s10585-020-10048-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frentzas S, Simoneau E, Bridgeman VL, et al. Vessel co‐option mediates resistance to anti‐angiogenic therapy in liver metastases. Nat Med. 2016;22(11):1294‐1302. 10.1038/nm.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long‐term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208‐1215. 10.1016/S1470-2045(13)70447-9 [DOI] [PubMed] [Google Scholar]
- 51. Eefsen RL, Van Den Eynden GG, Høyer‐Hansen G, et al. Histopathological growth pattern, proteolysis and angiogenesis in chemonaive patients resected for multiple colorectal liver metastases. J Oncol. 2012;2012:12. 10.1155/2012/907971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Han Y, Chai F, Wei J, et al. Identification of predominant histopathological growth patterns of colorectal liver metastasis by multi‐habitat and multi‐sequence based radiomics analysis. Front Oncol. 2020;10:1363. 10.3389/fonc.2020.01363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Latacz E, van Dam PJ, Vanhove C, et al. Can medical imaging identify the histopathological growth patterns of liver metastases? Semin Cancer Biol. 2020. 10.1016/j.semcancer.2020.07.002 [DOI] [PubMed] [Google Scholar]
- 54. Nakai Y, Gonoi W, Kurokawa R, et al. MRI findings of liver parenchyma peripheral to colorectal liver metastasis: a potential predictor of long‐term prognosis. Radiology. 2020;297(3):584‐594. 10.1148/radiol.2020202367 [DOI] [PubMed] [Google Scholar]
- 55. Pandit‐Taskar N, Postow MA, Hellmann MD, et al. First‐in‐humans imaging with 89Zr‐Df‐IAB22M2C anti‐CD8 minibody in patients with solid malignancies: preliminary pharmacokinetics, biodistribution, and lesion targeting. J Nucl Med. 2020;61(4):512‐519. 10.2967/jnumed.119.229781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kratochwil C, Flechsig P, Lindner T, et al. 68Ga‐FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60(6):801‐805. 10.2967/jnumed.119.227967 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


