Abstract
Aim
To investigate the association between dietary inflammatory potential and poor periodontal health.
Material and Methods
A cross‐sectional analysis of a nationally representative sample of participants was performed. NHANES 2011–2014 (n = 7081) and NHANES 2001–2004 (n = 5098) were used as discovery and validation datasets, respectively. The energy‐adjusted dietary inflammatory index (E‐DII) score was calculated for each participant based on 24‐h dietary recalls to assess diet‐associated inflammation. Periodontitis was defined by the CDC/AAP using clinical periodontal parameters. Natural cubic spline was applied to identify any non‐linear associations of the E‐DII score with moderate/severe periodontitis. Furthermore, interaction analyses were performed by age, gender, and race/ethnicity to explore the moderating roles of these factors.
Results
In the discovery dataset, a non‐linear positive relationship with periodontitis was identified for the E‐DII score (p non‐linearity < .001) after adjustment for potential confounders. Compared with those individuals in the lowest tertile of E‐DII, participants in the highest tertile who consumed a pro‐inflammatory diet were 53% more likely to be periodontitis (OR tertile3vs1 = 1.53, 95% CI: 1.33–1.77). The validation dataset showed similar associations. Relatively stronger associations were seen in older adults and males.
Conclusion
Consuming a pro‐inflammatory diet indicated by the E‐DII score is associated with periodontal disease in the U.S. general adult population.
Keywords: dietary inflammatory index, inflammation, NHANES, periodontal health
Clinical Relevance.
Scientific rationale for the study: Single nutrients may influence periodontal health, but its overall association with the inflammatory dietary pattern (measured by the energy‐adjusted dietary inflammatory index [E‐DII]) is still unclear.
Principal finding: A non‐linear positive association with periodontitis was identified for the E‐DII score. More pro‐inflammatory diets were associated with an increased risk of periodontitis. These associations could be replicated in the validation dataset. Relatively stronger associations were observed between E‐DII and periodontitis among adults >60 years old and males.
Practical implications: Individuals subsisting on a pro‐inflammatory diet are more likely to have poor periodontal health, suggesting the possible utility of the modulation of dietary inflammatory potential in strategies intended to prevent and treat periodontal disease.
1. INTRODUCTION
Periodontitis is a prevalent oral inflammatory disease associated with complex interactions among plaque bacteria, environmental factors, and the host's immune system (Peres et al., 2019; Zhang et al., 2020). The host‐modulation imbalance between microbial factors and the immune–inflammatory response causes periodontitis onset and progression (Van der Velden et al., 2011). Conventional treatment and prevention of periodontitis mainly involve mechanical removal of the dental biofilm and calculus (i.e. professional cleaning and oral hygiene improvement). In complex cases, antibiotics and surgical treatment may be applied (Sanz et al., 2020). Additionally, healthy lifestyle changes should be incorporated in managing periodontal disease, including smoking cessation, stress reduction, and engaging in robust physical activity. Beyond these factors, adherence to a healthy diet plays an integral role in maintaining and promoting periodontal health (Kaye, 2012).
A dietary index can be used to identify the synergistic effects among various nutrients and determine the overall healthy qualities of an individual's nutritional patterns (Hu, 2002; Liese et al., 2015; Schwingshackl et al., 2018), which is distinct from focusing solely on a specific macronutrient or micronutrient. Recent studies on various dietary indices have shown that unhealthy dietary patterns are associated with gingival bleeding and periodontal disease, including the Mediterranean diet, the Western diet, the Healthy Eating Index (HEI), and the Alternative HEI (Bawadi et al., 2011; Salazar et al., 2018; Laiola et al., 2020; Wright et al., 2020; Alhassani et al., 2021). Nevertheless, these index‐based dietary patterns were not designed to capture the inflammatory potential of diets. The energy‐adjusted dietary inflammatory index (E‐DII) was developed to measure an individual's dietary inflammatory potential (Shivappa et al., 2014). There have been an increasing number of studies reporting the associations between the E‐DII score and a variety of inflammatory diseases and conditions, including diabetes mellitus (Laouali et al., 2019), cardiovascular disease (Khan et al., 2020), metabolic syndrome (Kim et al., 2018), cognitive impairment (Frith et al., 2018), and chronic kidney disease (Mazidi et al., 2018). In a cross‐sectional study conducted in the U.S. population, the E‐DII score was positively associated with the number of missing teeth (Kotsakis et al., 2018). In the subgroup analysis, the significant association remained in persons aged ≥50 years who have suffered tooth loss primarily due to periodontitis. Adherence to a pro‐inflammatory diet could result in a low‐grade sustained inflammation, which could, in turn, exacerbate the progress of periodontitis through the regulation of immunity.
This study aimed to examine the relationship between the E‐DII score and clinically determined periodontitis after adjusting for potential confounders (including sociodemographic factors, lifestyle, and periodontitis‐related diseases). We also explore differences in this association by age, gender, and race/ethnicity to determine the potential existence of subpopulations for dietary intervention.
2. METHODS
2.1. Study design and population
The current cross‐sectional study uses two waves of survey data acquired from the National Health and Nutrition Examination Survey (NHANES). The NHANES is a stratified, multistage, clustered probability sampling study focusing on U.S. civilians’ health and nutritional status; the NHANES is administered by the National Center for Health Statistics (NCHS). The survey consists of an in‐home interview followed by physical examinations and biological sample collections at a mobile examination centre. The NCHS Ethics Review Board approved all NHANES protocols and testing procedures, and all participants provided written informed consent (Curtin et al., 2012). Additional information including protocol numbers are available here (Protocol #98‐12, https://www.cdc.gov/nchs/nhanes/irba98.htm).
NHANES 2011–2012 and 2013–2014 were merged as a discovery dataset for primary analysis; NHANES 2001–2002 and 2003–2004 were combined as a validation dataset to replicate the findings. The present study excluded individuals below 30 years of age, edentulous, and no data available on dietary or periodontal measurements. We also excluded individuals with extreme total energy intakes outside the range of 500–5000 Kcal/day (Shin et al., 2017).
2.2. Exposure variable
Dietary information was used to calculate the dietary inflammatory index score for each subject as an exposure variable. Trained interviewers conducted 24‐h dietary recalls to obtain dietary information using methods developed by the United States Department of Agriculture (Moshfegh et al., 2008). Dietary data consisted of all foods consumed and the quantities eaten. The dietary inflammatory index methodology was developed based on a systematic review of approximately 2000 published research articles (Shivappa et al., 2014). The dietary inflammatory index estimates dietary inflammatory potential based on the balance of pro‐ and anti‐inflammatory properties of 45 different food components (mostly micro‐ and macronutrients). For each food component, an inflammatory effect score was derived based on its pro‐inflammatory effect (set to +1), anti‐inflammatory effect (set to −1), or null effect (set to 0). These scores were weighted based on study design (i.e. randomized control trials received the greatest weight and cell culture studies received the lowest weight). The dietary inflammatory index score was standardized to a representative world database from 11 populations worldwide, allowing the score to be used across different dietary patterns and cultures (Shivappa et al., 2014).
NHANES applies a total of 27 food components to calculate the E‐DII score: carbohydrate; protein; total fat; dietary fibre; cholesterol; saturated, monounsaturated, and polyunsaturated fatty acids; omega‐3 and omega‐6 polyunsaturated fatty acids; vitamins A, B1, B2, B3 (niacin), B6, B9 (folic acid), B12, C, D, and E; alcohol; beta‐carotene; caffeine; iron; magnesium; zinc; and selenium. A z‐score for each food parameter was computed by subtracting the world standard means and then dividing it by its standard deviation. Thereafter, each food parameter's z‐score was converted to a centred percentile score and multiplied by the literature‐derived inflammatory effect score. All of the specific food parameters’ dietary inflammatory index scores were then summed to create each participant's overall score. To control the total energy intake effect, the dietary inflammatory index was calculated per 1000 calories of food consumed (i.e. E‐DII). Higher E‐DII scores correspond to more pro‐inflammatory diets, whereas more negative values are more anti‐inflammatory (Shivappa et al., 2014).
2.3. Outcome variable
The primary outcome of interest was moderate or severe periodontitis. In the discovery dataset (NHANES 2011–2014), the full mouth (four quadrants) was evaluated in each participant. Trained and calibrated dentists examined the periodontal status of each participant. Probing pocket depth (PPD) and clinical attachment loss (CAL) have shown good reliability, with Inter‐class Correlation Coefficients (ICCs) ranging from 0.80–0.90 and 0.79–0.86, respectively (Dye et al., 2019). The periodontal examination included probing assessments at six sites per tooth (mesio‐, mid‐, disto‐buccal and mesio‐, mid‐, disto‐lingual). As the third molars were excluded, a maximum of 28 teeth and 168 sites per individual could be examined to assess periodontal status.
We used the CDC/AAP case definition (Centers for Disease Control & Prevention and American Academy of Periodontology) to categorize periodontal disease (Page & Eke, 2007). No or mild periodontitis was defined as no evidence of moderate, or severe periodontitis; Moderate: ≥2 inter‐proximal site with ≥4 mm CAL (not on the same tooth), or ≥2 interproximal site with ≥5 mm PPD; Severe: ≥2 interproximal site with ≥6 mm CAL (not on the same tooth) and ≥1 interproximal site with ≥5 mm PPD. The participants were dichotomized as no/mild periodontitis and moderate/severe periodontitis (Weintraub et al., 2019). The periodontal examination protocol and periodontitis definition in the validation dataset were provided in the Method S1.
2.4. Potential confounders
Sociodemographic variables were age, gender, race/ethnicity, educational level, and annual household income. We categorized race/ethnicity into non‐Hispanic Whites and others. Educational level and annual household income were grouped into three categories: less than high school, some college, or college graduate or more and <20,000$, 20,000–75,000$, or >75,000$, respectively. Behavioural variables included smoking status (current, former, or never), the average number of alcoholic drinks consumed in past year (0, <1, 1–8, and >8 drinks per week) (Gay et al., 2018), level of physical activity (low, moderate, or high), and time since last dental visit (<1, 1–3, >3 years). Moreover, smoking intensity was quantitated among current smokers (in the last 30 days) and former smokers (before quitting) by calculating the average number of cigarettes smoked per day (Aoki et al., 2017).
Systemic biomarkers of inflammation, including C‐reactive protein (CRP, only for NHANES 2001–2004) and white blood cell (WBC) count, were measured using blood samples taken from the participants. As done in a previous study (Siegel et al., 2016), we used the following eight variables to characterize systemic diseases: abdominal adiposity, obesity, hypertension, dyslipidaemia, dysglycaemia, cardiovascular diseases, and arthritis (Table S1). Since both periodontal disease and E‐DII could be related to the above covariates, we decided to adjust our analyses for these potential confounders.
2.5. Statistical analysis
Descriptive statistics were calculated to describe the characteristics of participants overall and by the E‐DII tertiles. The Kolmogorov‐Smirnov test was used to test the normality of the distribution of the continuous variables. Continuous variables with normal distribution were reported as mean with standard deviation and compared among tertile groups using a one‐way ANOVA test. Non‐normal variables were presented as median with interquartile range and compared using an independent‐samples Kruskal–Wallis test. Categorical variables were described as numbers with frequencies and compared using a Chi‐square test.
In the discovery dataset, a multivariable logistic model was used to study the association between the E‐DII score and moderate/severe periodontitis after adjusting for age, gender, race/ethnicity, education, income, smoking habit, alcohol consumption, physical activity, dental visit, abdominal adiposity, obesity, hypertension, dyslipidaemia, dysglycaemia, WBC count, cardiovascular, arthritis, total energy intake, and the number of teeth present. To avoid arbitrary categorization, we explored the relationship between continuous E‐DII and periodontitis. Natural cubic splines with two knots equally spaced within the range of E‐DII were used to examine the potential non‐linear relationships. Secondly, we examined the association between the E‐DII tertiles and periodontitis. We performed interaction analyses to determine whether the association between the E‐DII score tertiles and periodontitis differed by age group (≤60 and >60), gender, and race/ethnicity (Whites and others). Furtherly, the robustness of the total and subgroup associations between E‐DII tertiles and moderate/severe periodontitis was validated using data from the NHANES 2001–2004.
We performed a sensitivity analysis in the discovery dataset to investigate effect modification by total energy intake (<median and ≥median). In additional sensitivity analyses using data from the NHANES 2001–2004, we also considered a definition of periodontal health newly proposed by the Joint Workshop of the European Federation of Periodontology and the American Academy of Periodontology (EFP/AAP) (Chapple et al., 2018). Periodontal health was defined as no sites with PPD >3 mm and <10% of sites with BOP in an intact periodontium (CAL <3 mm); the others were set as a reference. Given the small percentage of missing data, a complete case analysis was used in this study. All the analyses were conducted with the R Project for Statistical Computing (version 3.6.0) and SPSS 25 (SPSS Inc.). A p value of <.05 was considered statistically significant.
3. RESULTS
3.1. Characteristics of the study sample
As depicted in Figure 1, data on 7081 participants of the NAHENS 2011–2014 were used as a discovery dataset, and data on 5098 participants of the NHANES 2001–2004 were used as a validation dataset. Table 1 presents the characteristics of participants overall and across the E‐DII tertiles in the discovery dataset. On average, participants were 53.3 years of age, with the sample being almost equally divided by gender (50.6% female). The overall percentage of people with moderate/severe periodontitis was 41.8%. The E‐DII score ranged from −5.45 to 4.74: the first tertile (n = 2360; E‐DII = −5.45 to −0.85), the second tertile (n = 2362; E‐DII = −0.85 to 2.81), and the third tertile (n = 2359; E‐DII = 2.81–4.74). Compared with those in the first tertile of E‐DII score (i.e. those who consumed the most anti‐inflammatory diet), participants in the third tertile were more likely to be female, less educated, and lower income. They also had a higher prevalence of abdominal adiposity, obesity, hypertension, dyslipidaemia, dysglycaemia, and cardiovascular disease than those in the first E‐DII tertile (all p < .05). Additionally, the baseline characteristics of the validation dataset (NHANES 2001–2004) are displayed in Table S2.
FIGURE 1.
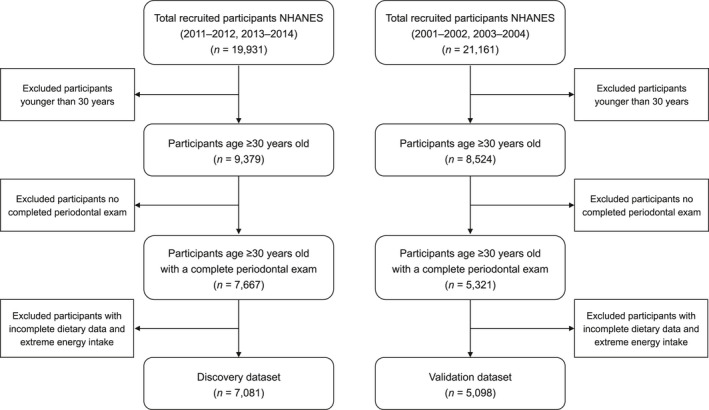
Flow chart indicating the discovery and validation datasets of participants included for the analysis from the National Health and Nutrition Examination Survey 2011–2014 and 2001–2004 dataset
TABLE 1.
Characteristics of 7081 adults aged ≥30 years in the discovery dataset (NHANES 2011–2014) overall and by tertile of E‐DII
| Energy‐adjusted Dietary Inflammatory Index a | |||||
|---|---|---|---|---|---|
|
Overall n = 7081 |
Tertile 1 n = 2360 |
Tertile 2 n = 2362 |
Tertile 3 n = 2359 |
p value | |
| Sociodemographic variables | |||||
| Age, year, mean (SD) | 53.31 (14.66) | 52.51 (14.96) | 52.74 (14.57) | 54.67 (14.36) | <.001 |
| Age group, n (%) | |||||
| <60 years | 4687 (66.2) | 1605 (68.0) | 1506 (63.7) | 1576 (66.9) | .429 |
| >60 years | 2394 (33.8) | 755 (32.0) | 860 (36.3) | 779 (33.1) | |
| Gender, n (%) | |||||
| Male | 3495 (49.4) | 1289 (54.6) | 1342 (56.7% | 864 (36.7) | <.001 |
| Female | 3586 (50.6) | 1071 (45.4) | 1024 (43.3% | 1491 (63.3) | |
| Race/ethnicity, n (%) | |||||
| Non‐Hispanic Whites | 4073 (57.5) | 987 (41.8) | 1104 (46.7) | 917 (38.9) | .046 |
| Others | 3008 (42.5) | 1373 (58.2) | 1262 (53.3) | 1438 (61.1) | |
| Education, n (%) b | |||||
| <High school | 3171 (44.8) | 952 (40.4) | 1066 (45.1) | 1153 (48.9) | <.001 |
| College | 2003 (28.3) | 637 (27.0) | 687 (29.1) | 679 (28.8) | |
| >College | 1904 (26.9) | 768 (32.6) | 609 (25.8) | 527 (22.2) | |
| Annual household income, n (%) b | |||||
| <20,000$ | 1456 (21.4) | 319 (13.6) | 608 (26.3) | 529 (24.8) | <.001 |
| 20,000–75,000$ | 3399 (50.1) | 1185 (50.4) | 1109 (48.0) | 1105 (52.0) | |
| >75,000$ | 1934 (28.5) | 846 (36.0) | 595 (25.7) | 493 (23.2) | |
| Lifestyle variables | |||||
| Smoking habit, n (%) b | |||||
| Non smoker | 3841 (54.3) | 1391 (59.0) | 1287 (54.5) | 1163 (49.3) | <.001 |
| Former smoker | 1850 (26.1) | 620 (26.3) | 621 (26.3) | 609 (25.8) | |
| Current smoker | 1386 (19.6) | 345 (14.6) | 454 (19.2) | 587 (24.9) | |
| Smoking intensity, median (IQR) c | 10 (15) | 10 (16) | 10 (15) | 10 (16) | <.001 |
| Average alcohol use in past 12 months | |||||
| 0 drink/week | 2602 (36.8) | 969 (41.1) | 810 (34.4) | 823 (34.9) | <.001 |
| <1 drink/week | 1934 (27.3) | 633 (26.9) | 723 (30.7) | 578 (24.5) | |
| 1–8 drinks/week | 1719 (24.3) | 545 (23.1) | 570 (24.2) | 604 (25.6) | |
| >8 drinks/week | 817 (11.6) | 208 (8.8) | 255 (10.8) | 354 (15.2) | |
| Physical activity level, n (%) b | |||||
| Low | 2889 (40.8) | 968 (41.0) | 990 (41.9) | 931 (39.5) | .410 |
| Moderate | 2396 (33.8) | 781 (33.1) | 789 (33.4) | 826 (35.0) | |
| High | 1796 (25.4) | 611 (25.9) | 583 (24.7) | 602 (25.5) | |
| Time since the last dental visit, n (%) b | |||||
| Less than 1 year | 3961 (56.0) | 1529 (64.8) | 1459 (61.8) | 973 (41.3) | <.001 |
| 1–3 years | 1368 (19.3) | 363 (15.4) | 307 (13.0) | 698 (29.6) | |
| More than 3 years | 1746 (24.7) | 467 (19.8) | 593 (25.1) | 686 (29.1) | |
| Systemic disease and related factors | |||||
| Abdominal adiposity, n (%) b | |||||
| Tertile 1st of WHtR | 2290 (33.4) | 843 (36.9) | 760 (33.6) | 687 (29.5) | <.001 |
| Tertile 2nd of WHtR | 2288 (33.3) | 763 (33.4) | 706 (31.2) | 819 (35.2) | |
| Tertile 3rd of WHtR | 2288 (33.3) | 678 (29.7) | 795 (35.2) | 815 (35.1) | |
| Obesity, n (%) b | |||||
| Normal | 1921 (27.3) | 692 (29.5) | 600 (25.6) | 629 (26.9) | .044 |
| Overweight | 2408 (34.3) | 771 (32.9) | 832 (35.5) | 805 (34.4) | |
| Obese | 2699 (38.4) | 881 (37.6) | 911 (38.9) | 907 (38.7) | |
| Hypertension, n (%) b | |||||
| Normal | 2179 (31.2) | 1100 (47.3) | 612 (26.2) | 467 (20.1) | <.001 |
| Prehypertension | 1457 (20.8) | 388 (16.7) | 516 (22.1) | 553 (23.7) | |
| Hypertension | 3354 (48.0) | 837 (36.0) | 1208 (51.7) | 1309 (56.2) | |
| Dyslipidaemia, n (%) b | |||||
| Normal | 2738 (40.5) | 974 (42.9) | 881 (39.4) | 883 (39.1) | .003 |
| Intermediate dyslipidaemia | 1922 (28.4) | 636 (28.0) | 671 (30.0) | 615 (27.2) | |
| Dyslipidaemia | 2108 (31.1) | 661 (29.1) | 685 (30.6) | 762 (33.7) | |
| Dysglycaemia, n (%) b | |||||
| Normal | 3741 (54.3) | 1403 (60.7) | 1419 (62.4) | 919 (39.9) | <.001 |
| Intermediate dysglycaemia | 2137 (31.0) | 582 (25.2) | 520 (22.9) | 1035 (44.9) | |
| Diagnosed diabetes | 1010 (14.7) | 326 (14.1) | 334 (14.6) | 350 (15.2) | |
| Cardiovascular disease, n (%) b | |||||
| No physician's diagnosis | 6316 (89.4) | 2139 (90.8) | 2125 (90.2) | 2052 (87.3) | <.001 |
| Physician's diagnosis | 747 (10.6) | 217 (9.2) | 231 (9.8) | 299 (12.7) | |
| Arthritis, n (%) b | |||||
| No physician's diagnosis | 5032 (71.2) | 1658 (70.3) | 1676 (71.1) | 1698 (72.1) | .409 |
| Physician's diagnosis | 2037 (28.8) | 699 (29.6) | 681 (28.9) | 657 (27.9) | |
| White blood cell count, mean (SD) | 7.10 (2.22) | 6.99 (2.08) | 7.12 (2.20) | 7.20 (2.38) | .007 |
| Total energy intake (Kcal/day), mean (SD) | 2053 (853) | 2077 (873) | 2058 (847) | 2035 (825) | .037 |
| Total energy intake group, n (%) | |||||
| < median | 3541 (50.0) | 1048 (44.4) | 1176 (49.7) | 1317 (55.9) | <.001 |
| > median | 3540 (50.0) | 1312 (55.6) | 1190 (50.3) | 1038 (44.1) | |
| Periodontal disease variables | |||||
| Number of the teeth present, median (IQR) | 25 (9) | 25 (7) | 25 (11) | 25 (9) | <.001 |
| CDC/AAP case definition, n (%) | |||||
| Non/mild periodontitis | 4124 (58.2) | 1427 (60.5) | 1449 (61.2) | 1248 (53.0) | <.001 |
| Moderate/severe periodontitis | 2957 (41.8) | 933 (39.5) | 917 (38.8) | 1107 (47.0) | |
All p values were calculated with a two‐sided significance level of .05.
Abbreviations: AAP, American Academy of Periodontology; CDC, Centers for Disease Control and Prevention; E‐DII, energy‐adjusted dietary inflammatory index; IQR, interquartile range; NHANES, National Health and Nutrition Examination Survey; SD, standard deviation; WHtR, waist‐to‐height ratio.
E‐DII ranges for Tertile 1, Tertile 2, and Tertile 3 were −5.45 to −0.85, −0.85 to 2.81, and 2.81 to 4.74.
Missing values for total study: education (n = 3; 0.1%), income (n = 292; 4.1%), smoking status (n = 4; 0.1%), Smoking intensity (n = 98; 1.4%), alcohol (n = 9; 0.1%), dental visit (n = 6; 0.1%), abdominal adiposity (n = 215; 3%), obesity (n = 53; 0.7%), hypertension (n = 91; 1.3%), dyslipidaemia (n = 313; 4.4%), dysglycaemia (n = 193; 2.7%), cardiovascular disease (n = 18; 0.3%), and arthritis (n = 12; 0.2%).
Average number of cigarettes smoked per day was used as a measure of smoking intensity.
3.2. Diet‐borne inflammation and periodontal disease
In the discovery dataset, the E‐DII score has a non‐linear positive relationship with moderate/severe periodontitis (p non‐linearity < .001, Figure 2). The risk of periodontitis was relatively flat before the E‐DII score was around 2 and then increased rapidly afterward. Based on this, the E‐DII score was grouped into tertiles in the further analyses. Figure 3 shows the results with moderate/severe periodontitis as the outcome for the overall samples in the discovery and validation datasets. After adjustment for potential confounders, participants from the discovery population in the most pro‐inflammatory E‐DII group (i.e. the third tertile) had a significantly increased risk of periodontitis compared to those in the first tertile (adjusted OR for the third vs. the first tertile of E‐DII [OR tertile3vs1]: 1.53, 95% CI: 1.33–1.77). Similar trend and direction of adjusted odds ratios for moderate/severe periodontitis were obtained in the validation dataset (Figure 3): OR tertile3vs1: 2.66, 95% CI: 2.17–3.27.
FIGURE 2.
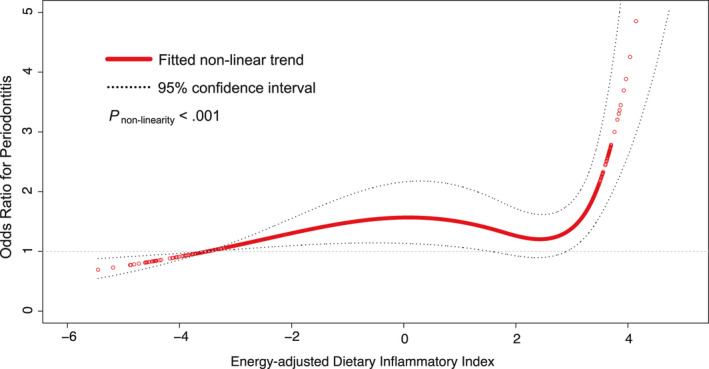
Natural spline curve indicates the non‐linear association between the E‐DII score and moderate/severe periodontitis in participants aged >30 years in the discovery dataset (NHANES 2011–2014, n = 7081). The red hollow‐circle line and dotted lines represent the estimated values and their corresponding 95% CI. Odds ratios are based on logistic regression models adjusted for age, gender, race/ethnicity, education, income, smoking habit, alcohol consumption, physical activity, dental visit, abdominal adiposity, obesity, hypertension, dyslipidaemia, dysglycaemia, WBC count, cardiovascular disease, arthritis, total energy intake, and the number of teeth present. Periodontitis was defined by CDC/AAP case definition. Knots are at the 33.3th and 66.7th percentiles for the E‐DII score. Abbreviations: AAP, American Academy of Periodontology; CDC, Centers for Disease Control and Prevention; CI, confidence interval; E‐DII, energy‐adjusted dietary inflammatory index; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; WBC, white blood cell
FIGURE 3.
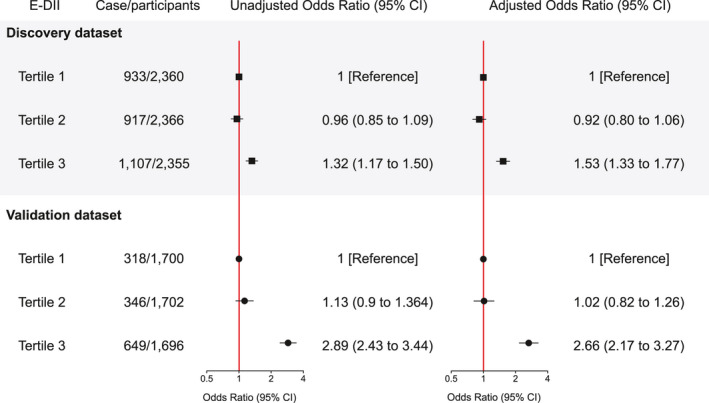
Associations between the tertiles of E‐DII and moderate/severe periodontitis in participants aged >30 years in the discovery dataset (NHANES 2011–2014, n = 7081) and the validation dataset (NHANES 2001–2004, n = 5098). Association is deemed significant if the 95% CI does not include zero. All p values were calculated with a two‐sided significance level of .05. A multivariable logistic regression model was adjusted for potential confounders (see legend of Figure 2). Periodontitis was defined by CDC/AAP case definition. Abbreviations: E‐DII, energy‐adjusted dietary inflammatory index; NHANES, National Health and Nutrition Examination Survey; CI, confidence interval; OR, odds ratio; CDC, Centers for Disease Control and Prevention; AAP, American Academy of Periodontology
3.3. Subgroup analyses
When stratified by age and gender in the discovery dataset, relatively stronger associations were observed between E‐DII tertiles and periodontitis among older adults (OR tertile3vs1 = 1.84; 95% CI: 1.45–2.35) and males (OR tertile3vs1 = 2.69; 95% CI: 2.17–3.32), with significant interactions (both p interaction < .001, Table 2). There was no evidence in the discovery population supporting the modified effect of race/ethnicity on the association between the E‐DII tertiles and periodontitis (p interaction = .146, Table 2). During the validation analysis in the NHANES 2001–2004, no statistically significant modification effects were observed (p interaction = .228 for age, .243 for gender, and .113 for race/ethnicity, Table S3).
TABLE 2.
Subgroup analyses of the associations between the tertiles of E‐DII and moderate/severe periodontitis by age, gender, and race/ethnicity in the discovery dataset (NHANES 2011–2014; n = 7081)
| Subgroup | Energy‐adjusted DIETARY INFLAMMATORY INDEX | p for interaction | ||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| Age | ||||
| <60 years | ||||
| Cases/participants | 574/1605 | 578/1506 | 648/1576 | <.001 |
| Odds Ratio (95% CI) | 1 [Reference] | 1.18 (0.99 to 1.40) | 1.36 (1.15 to 1.61) | |
| >60 years | ||||
| Cases/participants | 359/755 | 339/860 | 459/779 | |
| Odds Ratio (95% CI) | 1 [Reference] | 0.66 (0.52 to 0.83) | 1.84 (1.45 to 2.35) | |
| Gender | ||||
| Male | ||||
| Cases/participants | 581/1289 | 543/1342 | 589/864 | <.001 |
| Odds Ratio (95% CI) | 1 [Reference] | 0.78 (0.65 to 0.93) | 2.69 (2.17 to 3.32) | |
| Female | ||||
| Cases/participants | 352/1071 | 374/1024 | 518/1491 | |
| Odds Ratio (95% CI) | 1 [Reference] | 1.27 (1.02 to 1.56) | 1.02 (0.84 to 1.24) | |
| Race/ethnicity | ||||
| Whites | ||||
| Cases/participants | 285/987 | 355/1104 | 363/917 | .146 |
| Odds Ratio (95% CI) | 1 [Reference] | 1.09 (0.88 to 1.35) | 1.75 (1.40 to 2.18) | |
| Others | ||||
| Cases/participants | 648/1373 | 562/1262 | 744/1438 | |
| Odds Ratio (95% CI) | 1 [Reference] | 0.87 (0.72 to 1.04) | 1.35 (1.13 to 1.61) | |
All logistic regression models were adjusted for potential confounders (see legend of Figure 1) unless the covariate was tested as an effect modifier, in which case it was also entered as an interaction term.
Periodontitis was defined by CDC/AAP case definition.
Abbreviations: AAP, American Academy of Periodontology; CDC, Centers for Disease Control and Prevention; CI, confidence interval; E‐DII, energy‐adjusted dietary inflammatory index; NHANES, National Health and Nutrition Examination Survey.
3.4. Sensitivity analyses
We conducted a sensitivity analysis in the discovery dataset to determine whether the total energy intake affected the relationship between E‐DII tertiles and periodontitis. No significant effect modification by the total energy intake was found for periodontitis (p interaction = .240, Table S4). Sensitivity analysis was also performed using an alternate definition of periodontal health in the validation dataset (NHANES 2001–04). The E‐DII score had a non‐linear relationship with periodontal health (p non‐linearity < .001, Figure S1). The odd of periodontal health slowly decreased as E‐DII increased until 1.5 and rapidly decreased afterward. Participants who consumed pro‐inflammatory diets had worse periodontal health compared with those with anti‐inflammatory diets (OR tertile3vs1: 0.58, 95% CI: 0.49–0.69) after adjusting for potential confounders (Table S5). Interaction analyses yielded a stronger association between the E‐DII tertiles and periodontal health among older adults, although the interaction was not statistically significant (p interaction = .159, Table S5). No significant modification effect by gender and race/ethnicity was found (p interaction = .482 for gender and .322 for race/ethnicity, Table S5).
4. DISCUSSION
The consumption of more pro‐inflammatory diets was associated with moderate/severe periodontitis among U.S. adults in the cross‐sectional study using data from NHANES 2011–2014 and NHANES 2001–2004. This study revealed that the E‐DII score has a non‐linear positive relationship with periodontitis; the association was more marked when E‐DII was higher than approximate 2. The relatively stronger associations of the E‐DII tertiles with periodontitis were observed in the elderly and males from the discovery population. However, the modification effects were not replicated in the validation dataset. The primary findings persisted after adjusting for a range of potential confounders and were robust in various validation and sensitivity analysis.
Dietary and nutritional interventions have a favourable effect on periodontal therapy outcomes and have gradually become essential tools for regulating host immunity to prevent periodontitis (Kaye, 2012; Né et al., 2019). In a study on experimental gingivitis, the participants lived under Stone Age conditions (e.g. reduced intake of processed carbohydrates and refined sugars) for 4 weeks (Baumgartner et al., 2009). The Stone Age diet significantly decreased gingival bleeding (from 34.8% to 12.6%), although this decrease was accompanied by plaque accumulation. A randomized controlled trial recently indicated that using an anti‐inflammatory diet (plant‐based whole‐foods) –characterized by a higher intake of omega‐3, vitamin C, vitamin D, and fibre – as an intervention significantly reduced gingivitis among individuals living on a Western diet, although without changes in plaque (Woelber et al., 2019). These findings from the small‐scale clinical trials were confirmed by the current large‐scale observational study that pro‐inflammatory diets are associated with periodontal disease in a general population.
The inflammatory potential of different diets could be attributed to different nutritional components. Typically, a pro‐inflammatory dietary pattern is characterized by an increased intake of processed and red meat, saturated fats, and simple carbohydrates. A recent prospective study reported that the Western diet (enrich with red meat, processed meat, eggs, butter, and refined grains) was significantly associated with incident periodontitis among obese individuals (Alhassani et al., 2021). In contrast, an anti‐inflammatory pattern is characterized by frequent intake of vegetables and fruits, whole grains, legumes, nuts, and fish (Ahluwalia et al., 2013). Using a data‐driven approach in the NHANES population, the dietary pattern (rich in salad, fruit and vegetables, poultry, and seafood) was negatively associated with the extent of periodontitis (Wright et al., 2020). These epidemiological studies reported that periodontal status might be affected by inflammatory eating patterns defined using principal components analysis (Alhassani et al., 2021) and treelet transformation analysis (Wright et al., 2020). Both analyses derive a summary score for each pattern through statistical modelling of dietary data at hand (e.g. food frequency questionnaires or 24‐h dietary records) (Hu, 2002). Thus, principal components analysis and treelet transformation analysis are considered “a posteriori” diet pattern that is a data‐driven approach based on subjective methods and reported dietary intake (Trichopoulos & Lagiou, 2001; Steffen & Hootman, 2016). In contrast, dietary inflammatory index is “a priori” diet pattern because the indices are created on the basis of current knowledge and understanding of the diet–disease relationship (Hu, 2002). Even though the defining methods may vary, the association of pro‐inflammatory dietary patterns on periodontitis in the reported studies is consistent with our findings.
Numerous pathways exist through which pro‐inflammatory diets can influence periodontal health. The dietary inflammatory index was used to assess overall dietary quality in terms of inflammation. The findings were validated using several different inflammatory markers (e.g. CRP, interleukin [IL]‐1, IL‐2, IL‐6, interferon‐gamma, and tumour necrosis factor‐alpha) (Shivappa, Hebert, et al., 2017; Corley et al., 2019). Periodontal health may be influenced by dietary inflammation via systemic regulation of immunity. The newly proposed definition of periodontal health can accurately reflect a patient's current inflammation level by taking into account gingival bleeding (Chapple et al., 2018). Recent studies have explored the association between active periodontitis with chronic conditions through systemic inflammatory burden (Li et al., 2020; Pietropaoli et al., 2020). Since periodontal inflammation might result from a higher intake of a pro‐inflammatory diet or the lack of an anti‐inflammatory diet, a focus on dental plaque control alone might overlook primary prevention and cause‐oriented therapy opportunities (Woelber & Tennert, 2020).
The positive association between the E‐DII score and moderate/severe periodontitis was non‐linear when E‐DII was used as continuous. To be more specific, when E‐DII is above a certain critical threshold, the likelihood of being periodontitis rapidly increases, suggesting some compensatory immunomodulation may exist. Of note, the non‐linear relationship was replicated in the NHANES 2001–2004 when the new definition of periodontal health was regarded as an outcome. The J‐shaped relationship was consistent with a previous study in which dietary inflammatory index was grouped into quartile groups (Kotsakis et al., 2018). Compared to the 1st quartile, the association of dietary inflammatory index and periodontal tooth loss was statistically significant in the 4th quartile (p = .02) but not in the intermediate quartile groups. This result would have clinical implications in periodontal prevention and treatment that dietary intervention is more important for the target population who consume a more pro‐inflammatory diet.
Moreover, we identified a trend towards relatively stronger associations in older people and males through interaction analyses. Previous studies on the association between the E‐DII score and the presence of other chronic diseases have obtained inconsistent results concerning age and gender, with the associations being stronger for the elderly (Cho et al., 2016) and for men (Harmon et al., 2017; Zhang et al., 2017). Elderly individuals and males at the upper end of the E‐DII score scale may benefit the most from diet and lifestyle interventions if the results concerning causal association presented in this paper can be confirmed in future randomized controlled trials.
Several limitations of this study should be considered. The NHANES 2001–2004 employed a random half‐mouth protocol, which underestimates the prevalence and severity of periodontal disease compared to full‐mouth examination of six sites on all teeth (Albandar, 2011). It might explain why some results could not be replicated in the validation dataset. Besides, the E‐DII score was calculated based on 27 of 45 food parameters. The selection of these parameters may have affected the findings. However, the previous study showed no change in the predictive ability of the E‐DII food parameters available in NHANES data in terms of assessing inflammation compared with the full list (Shivappa et al., 2017). Similarly, a single 24‐h dietary recall interview is not ideal for estimating long‐term habitual intake, given that it may not account for intra‐individual (i.e. day‐to‐day) variability in diet, leading to imprecise estimates (Basiotis et al., 1987). Finally, it is not possible to identify causal relationships between pro‐inflammatory diet and periodontitis using cross‐sectional designs. Future large‐scale randomized controlled trials are needed to elucidate the potential causal association and thus help confirm the beneficial effect of reducing pro‐inflammatory foods’ consumption or adopting an anti‐inflammatory diet on periodontal health.
5. CONCLUSION
It was found that the consumption of a pro‐inflammatory diet may affect periodontal health in a representative population of U.S. adults. The benefits of consuming a less inflammatory diet in terms of periodontal health may be more pronounced for adults >60 years old and males. In addition to conventional periodontal treatment, dentists could recommend an anti‐inflammatory diet (e.g. reduced intake of fats, red meat, and processed foods and accordingly increased consumption of green leafy vegetables, fruits, whole grains, and fish) to their patients.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Ethical approval was not required.
AUTHOR CONTRIBUTIONS
A.L. was responsible for study conception and design, statistical analyses, data interpretation, and the drafted manuscript; Y.C., a statistical consultant, contributed to the study design, statistical analyses, and the drafted manuscript; A.A. contributed to the data interpretation and critically reviewed manuscript; L.S. contributed to the data interpretation and critical revision of the manuscript; and G.‐H.T. contributed to the study conception and design, data interpretation, and critically reviewing manuscript. All authors gave final approval and agreed to be accountable for all aspects of this work to ensure integrity and accuracy.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the National Health and Nutrition Examination Survey (NHANES) staff and investigators. A special thanks to all who participated in the NHANES survey for making this study possible through their participation.
An Li and Yuntao Chen contributed equally to the manuscript.
Funding information
A.L. and Y.C. are Ph.D. students conducting the research in the UMCG with funding from the China Scholarship Council studentship (No. 201708440245 and No. 201706100193). And the authors acknowledge funding support from the University of Groningen
Contributor Information
An Li, Email: anli05081024@gmail.com.
Geerten‐Has E. Tjakkes, Email: g.h.e.tjakkes@umcg.nl.
DATA AVAILABILITY STATEMENT
This research uses data from the National Health and Nutrition Examination Survey (NHANES). The data sets generated and analysed during the current study are publicly available in the NHANES repository, https://wwwn.cdc.gov/nchs/nhanes/.
REFERENCES
- Ahluwalia, N. , Andreeva, V. A. , Kesse‐Guyot, E. , & Hercberg, S. (2013). Dietary patterns, inflammation and the metabolic syndrome. Diabetes and Metabolism, 39(2), 99–110. 10.1016/j.diabet.2012.08.007 [DOI] [PubMed] [Google Scholar]
- Albandar, J. M. (2011). Underestimation of periodontitis in NHANES surveys. Journal of Periodontology, 82(3), 337–341. 10.1902/jop.2011.100638 [DOI] [PubMed] [Google Scholar]
- Alhassani, A. A. , Hu, F. B. , Li, Y. , Rosner, B. A. , Willett, W. C. , & Joshipura, K. J. (2021). The associations between major dietary patterns and risk of periodontitis. Journal of Clinical Periodontology, 48(1), 2–13. 10.1111/jcpe.13380 [DOI] [PubMed] [Google Scholar]
- Aoki, Y. , Yee, J. , & Mortensen, M. E. (2017). Blood cadmium by race/hispanic origin: The role of smoking. Environmental Research, 155, 193–198. 10.1016/j.envres.2017.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basiotis, P. P. , Welsh, S. O. , Cronin, F. J. , Kelsay, J. L. , & Mertz, W. (1987). Number of days of food intake records required to estimate individual and group nutrient intakes with defined confidence. Journal of Nutrition, 117(9), 1638–1641. 10.1093/jn/117.9.1638 [DOI] [PubMed] [Google Scholar]
- Baumgartner, S. , Imfeld, T. , Schicht, O. , Rath, C. , Persson, R. E. , & Persson, G. R. (2009). The impact of the stone age diet on gingival conditions in the absence of oral hygiene. Journal of Periodontology, 80(5), 759–768. 10.1902/jop.2009.080376 [DOI] [PubMed] [Google Scholar]
- Bawadi, H. A. , Khader, Y. S. , Haroun, T. F. , Al‐Omari, M. , & Tayyem, R. F. (2011). The association between periodontal disease, physical activity and healthy diet among adults in Jordan. Journal of Periodontal Research, 46(1), 74–81. 10.1111/j.1600-0765.2010.01314.x [DOI] [PubMed] [Google Scholar]
- Chapple, I. L. C. , Mealey, B. L. , Van Dyke, T. E. , Bartold, P. M. , Dommisch, H. , Eickholz, P. , Geisinger, M. L. , Genco, R. J. , Glogauer, M. , Goldstein, M. , Griffin, T. J. , Holmstrup, P. , Johnson, G. K. , Kapila, Y. , Lang, N. P. , Meyle, J. , Murakami, S. , Plemons, J. , Romito, G. A. , … Yoshie, H. (2018). Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Periodontology, 89(Suppl 1), S74–s84. 10.1002/jper.17-0719 [DOI] [PubMed] [Google Scholar]
- Cho, Y. A. , Lee, J. , Oh, J. H. , Shin, A. , & Kim, J. (2016). Dietary inflammatory index and risk of colorectal cancer: A case‐control study in Korea. Nutrients, 8(8), 469. 10.3390/nu8080469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley, J. , Shivappa, N. , Hébert, J. R. , Starr, J. M. , & Deary, I. J. (2019). Associations between dietary inflammatory index scores and inflammatory biomarkers among older adults in the Lothian birth cohort 1936 study. The Journal of Nutrition, Health and Aging, 23(7), 628–636. 10.1007/s12603-019-1221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin, L. R. , Mohadjer, L. K. , Dohrmann, S. M. , Montaquila, J. M. , Kruszan‐Moran, D. , Mirel, L. B. , Carroll, M. D. , Hirsch, R. , & Schober, S. & Johnson, C. L. (2012). The National Health and Nutrition Examination Survey: Sample Design, 1999–2006. Vital Health Statistics, 2(155), 1–39. [PubMed] [Google Scholar]
- Dye, B. A. , Afful, J. , Thornton‐Evans, G. , & Iafolla, T. (2019). Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2011–2014. BMC Oral Health, 19(1), 2011–2014. 10.1186/s12903-019-0777-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith, E. , Shivappa, N. , Mann, J. R. , Hébert, J. R. , Wirth, M. D. , & Loprinzi, P. D. (2018). Dietary inflammatory index and memory function: population‐based national sample of elderly Americans. British Journal of Nutrition, 119(5), 552–558. 10.1017/s0007114517003804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay, I. C. , Tran, D. T. , & Paquette, D. W. (2018). Alcohol intake and periodontitis in adults aged ≥30 years: NHANES 2009–2012. Journal of Periodontology, 89(6), 625–634. 10.1002/jper.17-0276 [DOI] [PubMed] [Google Scholar]
- Harmon, B. E. , Wirth, M. D. , Boushey, C. J. , Wilkens, L. R. , Draluck, E. , Shivappa, N. , Steck, S. E. , Hofseth, L. , Haiman, C. A. , Le Marchand, L. , & Hébert, J. R. (2017). The dietary inflammatory index is associated with colorectal cancer risk in the multiethnic cohort. Journal of Nutrition, 147(3), 430–438. 10.3945/jn.116.242529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, F. B. (2002). Dietary pattern analysis: A new direction in nutritional epidemiology. Current Opinion in Lipidology, 13(1), 3–9. 10.1097/00041433-200202000-00002 [DOI] [PubMed] [Google Scholar]
- Kaye, E. K. (2012). Nutrition, dietary guidelines and optimal periodontal health. Periodontology 2000, 58(1), 93–111. 10.1111/j.1600-0757.2011.00418.x [DOI] [PubMed] [Google Scholar]
- Khan, I. , Kwon, M. , Shivappa, N. , Hébert, J. R. , & Kim, M. K. (2020). Positive association of dietary inflammatory index with incidence of cardiovascular disease: Findings from a Korean population‐based prospective study. Nutrients, 12(2), 588. 10.3390/nu12020588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. Y. , Lee, J. , & Kim, J. (2018). Association between dietary inflammatory index and metabolic syndrome in the general Korean Population. Nutrients, 10(5), 648. 10.3390/nu10050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsakis, G. A. , Chrepa, V. , Shivappa, N. , Wirth, M. , Hébert, J. , Koyanagi, A. , & Tyrovolas, S. (2018). Diet‐borne systemic inflammation is associated with prevalent tooth loss. Clinical Nutrition, 37(4), 1306–1312. 10.1016/j.clnu.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiola, M. , De Filippis, F. , Vitaglione, P. , & Ercolini, D. (2020). A Mediterranean diet intervention reduces the levels of salivary Periodontopathogenic bacteria in overweight and obese subjects. Applied and Environment Microbiology, 86(12), e00777‐20. 10.1128/aem.00777-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouali, N. , Mancini, F. R. , Hajji‐Louati, M. , El Fatouhi, D. , Balkau, B. , Boutron‐Ruault, M.‐C. , Bonnet, F. , & Fagherazzi, G. (2019). Dietary inflammatory index and type 2 diabetes risk in a prospective cohort of 70,991 women followed for 20 years: The mediating role of BMI. Diabetologia, 62(12), 2222–2232. 10.1007/s00125-019-04972-0 [DOI] [PubMed] [Google Scholar]
- Li, A. , Chen, Y. , van der Sluis, L. W. M. , Schuller, A. A. , & Tjakkes, G. H. (2020). White blood cell count mediates the association between periodontal inflammation and cognitive performance measured by digit symbol substitution test among older U.S. Adults. The Journal of Gerontology: Series A, 1–7. 10.1093/gerona/glaa223 [DOI] [PubMed] [Google Scholar]
- Liese, A. D. , Krebs‐Smith, S. M. , Subar, A. F. , George, S. M. , Harmon, B. E. , Neuhouser, M. L. , Boushey, C. J. , Schap, T. R. E. , & Reedy, J. (2015). The Dietary Patterns Methods Project: Synthesis of findings across cohorts and relevance to dietary guidance. Journal of Nutrition, 145(3), 393–402. 10.3945/jn.114.205336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazidi, M. , Shivappa, N. , Wirth, M. D. , Hebert, J. R. , & Kengne, A. P. (2018). Greater Dietary Inflammatory Index score is associated with higher likelihood of chronic kidney disease. British Journal of Nutrition, 120(2), 204–209. 10.1017/s0007114518001071 [DOI] [PubMed] [Google Scholar]
- Moshfegh, A. J. , Rhodes, D. G. , Baer, D. J. , Murayi, T. , Clemens, J. C. , Rumpler, W. V. , Paul, D. R. , Sebastian, R. S. , Kuczynski, K. J. , Ingwersen, L. A. , Staples, R. C. , & Cleveland, L. E. (2008). The US Department of Agriculture Automated Multiple‐Pass Method reduces bias in the collection of energy intakes. American Journal of Clinical Nutrition, 88(2), 324–332. 10.1093/ajcn/88.2.324 [DOI] [PubMed] [Google Scholar]
- Né, Y. G. S. , Martins, B. V. , Castro, M. M. L. , Alvarenga, M. O. P. , Fagundes, N. C. F. , Magno, M. B. , Maia, L. C. , & Lima, R. R. (2019). Is nutritional intervention an improvement factor in the management of periodontitis? A systematic review. Clinical Nutrition, 39(9), 2639–2646. 10.1016/j.clnu.2019.12.016 [DOI] [PubMed] [Google Scholar]
- Page, R. C. , & Eke, P. I. (2007). Case definitions for use in population‐based surveillance of periodontitis. Journal of Periodontology, 78(7 Suppl), 1387–1399. 10.1902/jop.2007.060264 [DOI] [PubMed] [Google Scholar]
- Peres, M. A. , Macpherson, L. M. D. , Weyant, R. J. , Daly, B. , Venturelli, R. , Mathur, M. R. , Listl, S. , Celeste, R. K. , Guarnizo‐Herreño, C. C. , Kearns, C. , Benzian, H. , Allison, P. , & Watt, R. G. (2019). Oral diseases: a global public health challenge. Lancet, 394(10194), 249–260. 10.1016/s0140-6736(19)31146-8 [DOI] [PubMed] [Google Scholar]
- Pietropaoli, D. , Monaco, A. , D’Aiuto, F. , Muñoz Aguilera, E. , Ortu, E. , Giannoni, M. , Czesnikiewicz‐Guzik, M. , Guzik, T. J. , Ferri, C. , & Del Pinto, R. (2020). Active gingival inflammation is linked to hypertension. Journal of Hypertension, 38(10), 2018–2027. 10.1097/hjh.0000000000002514 [DOI] [PubMed] [Google Scholar]
- Salazar, C. R. , Laniado, N. , Mossavar‐Rahmani, Y. , Borrell, L. N. , Qi, Q. , Sotres‐Alvarez, D. , Morse, D. E. , Singer, R. H. , Kaplan, R. C. , Badner, V. , & Lamster, I. B. (2018). Better‐quality diet is associated with lower odds of severe periodontitis in US Hispanics/Latinos. Journal of Clinical Periodontology, 45(7), 780–790. 10.1111/jcpe.12926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz, M. , Herrera, D. , Kebschull, M. , Chapple, I. , Jepsen, S. , Berglundh, T. , Sculean, A. , Tonetti, M. S. , Merete Aass, A. , Aimetti, M. , Kuru, B. E. , Belibasakis, G. , Blanco, J. , Bol‐van den Hil, E. , Bostanci, N. , Bozic, D. , Bouchard, P. , Buduneli, N. , Cairo, F. , … Wennström, J. (2020). Treatment of stage I‐III periodontitis‐The EFP S3 level clinical practice guideline. Journal of Clinical Periodontology, 47(Suppl 22), 4–60. 10.1111/jcpe.13290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl, L. , Bogensberger, B. , & Hoffmann, G. (2018). Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: An updated systematic review and meta‐analysis of cohort studies. Journal of the Academy of Nutrition and Dietetics, 118(1), 74–100.e111. 10.1016/j.jand.2017.08.024 [DOI] [PubMed] [Google Scholar]
- Shin, D. , Hur, J. , Cho, E.‐H. , Chung, H.‐K. , Shivappa, N. , Wirth, M. D. , Hébert, J. R. , & Lee, K. W. (2017). Pre‐pregnancy body mass index is associated with dietary inflammatory index and C‐reactive protein concentrations during pregnancy. Nutrients, 9(4), 351. 10.3390/nu9040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa, N. , Hebert, J. R. , Marcos, A. , Diaz, L.‐E. , Gomez, S. , Nova, E. , Michels, N. , Arouca, A. , González‐Gil, E. , Frederic, G. , González‐Gross, M. , Castillo, M. J. , Manios, Y. , Kersting, M. , Gunter, M. J. , De Henauw, S. , Antonios, K. , Widhalm, K. , Molnar, D. , … Huybrechts, I. (2017). Association between dietary inflammatory index and inflammatory markers in the HELENA study. Molecular Nutrition and Food Research, 61(6), 1600707. 10.1002/mnfr.201600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa, N. , Steck, S. E. , Hurley, T. G. , Hussey, J. R. , & Hébert, J. R. (2014). Designing and developing a literature‐derived, population‐based dietary inflammatory index. Public Health Nutrition, 17(8), 1689–1696. 10.1017/s1368980013002115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa, N. , Wirth, M. D. , Hurley, T. G. , & Hébert, J. R. (2017). Association between the dietary inflammatory index (DII) and telomere length and C‐reactive protein from the National Health and Nutrition Examination Survey‐1999‐2002. Molecular Nutrition and Food Research, 61(4), 1999–2002. 10.1002/mnfr.201600630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, K. R. , McKeever Bullard, K. , Imperatore, G. , Kahn, H. S. , Stein, A. D. , Ali, M. K. , & Narayan, K. M. (2016). Association of higher consumption of foods derived from subsidized commodities with adverse cardiometabolic risk among US adults. JAMA Internal Medicine, 176(8), 1124–1132. 10.1001/jamainternmed.2016.2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen, L. M. , & Hootman, K. C. (2016). A posteriori data‐derived dietary patterns and incident coronary heart disease: Making sense of inconsistent findings. Current Nutrition Reports, 5(3), 168–179. 10.1007/s13668-016-0176-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichopoulos, D. , & Lagiou, P. (2001). Dietary patterns and mortality. British Journal of Nutrition, 85(2), 133–134. 10.1079/bjn2000282 [DOI] [PubMed] [Google Scholar]
- Van der Velden, U. , Kuzmanova, D. , & Chapple, I. L. (2011). Micronutritional approaches to periodontal therapy. Journal of Clinical Periodontology, 38(Suppl 11), 142–158. 10.1111/j.1600-051X.2010.01663.x [DOI] [PubMed] [Google Scholar]
- Weintraub, J. A. , Lopez Mitnik, G. , & Dye, B. A. (2019). Oral diseases associated with nonalcoholic fatty liver disease in the United States. Journal of Dental Research, 98(11), 1219–1226. 10.1177/0022034519866442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelber, J. P. , Gärtner, M. , Breuninger, L. , Anderson, A. , König, D. , Hellwig, E. , Al‐Ahmad, A. , Vach, K. , Dötsch, A. , Ratka‐Krüger, P. , & Tennert, C. (2019). The influence of an anti‐inflammatory diet on gingivitis. A randomized controlled trial. Journal of Clinical Periodontology, 46(4), 481–490. 10.1111/jcpe.13094 [DOI] [PubMed] [Google Scholar]
- Woelber, J. P. , & Tennert, C. (2020). Chapter 13: Diet and periodontal diseases. Monographs in Oral Science, 28, 125–133. 10.1159/000455380 [DOI] [PubMed] [Google Scholar]
- Wright, D. M. , McKenna, G. , Nugent, A. , Winning, L. , Linden, G. J. , & Woodside, J. V. (2020). Association between diet and periodontitis: a cross‐sectional study of 10,000 NHANES participants. American Journal of Clinical Nutrition, 112(6), 1485–1491. 10.1093/ajcn/nqaa266 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Yu, N. , & Arce, R. M. (2020). Periodontal inflammation: Integrating genes and dysbiosis. Periodontology 2000, 82(1), 129–142. 10.1111/prd.12267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z.‐Q. , Cao, W.‐T. , Shivappa, N. , Hebert, J. R. , Li, B.‐L. , He, J. , Tang, X.‐Y. , Liang, Y.‐Y. , & Chen, Y.‐M. (2017). Association between diet inflammatory index and osteoporotic hip fracture in elderly Chinese population. Journal of the American Medical Directors Association, 18(8), 671–677. 10.1016/j.jamda.2017.02.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
This research uses data from the National Health and Nutrition Examination Survey (NHANES). The data sets generated and analysed during the current study are publicly available in the NHANES repository, https://wwwn.cdc.gov/nchs/nhanes/.


