Abstract
Social interaction is a complex and highly conserved behavior that safeguards survival and reproductive success. Although considerable progress has been made regarding our understanding of same‐sex conspecific and non‐aggressive interactions, questions regarding the precise contribution of sensory cues in social approach and their specific neurobiological correlates remain open. Here, by designing a series of experiments with diverse social and object stimuli manipulations in custom‐made enclosures, we first sought to deconstruct key elements of social preference as assessed by the three‐chamber task. Our results highlight the importance of social olfactory cues in approach behavior. Subsequently, we interrogated whether a social odor would activate dopaminergic neurons of the Ventral Tegmental Area in the same way as a juvenile conspecific would. Employing in vivo recordings in freely behaving mice, we observed an increase of the firing only during the transition toward the juvenile mouse and not during the transition toward the object impregnated with social odor, suggesting that these two experiences are distinct and can be differentiated at the neuronal level. Moreover, using a four‐choice task, we further showed that mice prefer to explore complex social stimuli compared to isolated sensory cues. Our findings offer insights toward understanding how different sensory modalities contribute to the neurobiological basis of social behavior which can be essential when studying social deficits observed in autism‐, depression‐, anxiety‐, or schizophrenia‐related mouse models.
Keywords: dopamine, sensory cues, sociability, three‐chamber test, VTA
Mice preferred a juvenile conspecific and an object impregnated with social odors (Os) when respectively compared to an object. Employing in vivo recordings, we observed an increase in VTA pDA firing when experimental mice entered in the proximity of the social stimulus or the Os. On the other hand, we noticed an increase of firing only when the mice transitioned toward the juvenile, suggesting that these two perceptual experiences are distinct and can be differentiated at the neuronal level.

1. INTRODUCTION
Although considerable progress has been made regarding our understanding of same‐sex conspecific and non‐aggressive interactions (Gunaydin et al., 2014; Puglisi‐Allegra & Cabib, 1997; Robinson et al., 2002, 2011), questions regarding the contribution of sensory cues in social approach and their specific neurobiological correlates remain open. When individuals encounter their conspecifics for the first time, they need to process social information in order to decide how to react and adapt their behavior. More specifically, depending on the partner's identity, as well as on environmental and sensory cues, the individual can choose whether to interact or avoid conspecifics. Each individual will then store information about the experience in order to be able to recognize a familiar conspecific during a second encounter and to adapt the behavior accordingly. Understanding how the brain processes social information is fundamental in order to uncover the neurobiological basis of social behavior.
Social interaction involves the detection and integration of multiple sensory modalities (Chen & Hong, 2018). Although olfactory, visual, auditory, and somatosensory cues independently can be detected by an individual and transformed into a behavioral output, multiple senses are often employed during social interaction. Furthermore, impairments in sensory perception and/or multisensory integration could partly explain social deficits in psychiatric disorders such as Autism Spectrum Disorders (ASDs; Tavassoli et al., 2018). In order to understand whether and how sensory processes contribute to social impairments, we need first to identify how sensory modalities contribute to social behavior.
Given the complexity and the universality of social behavior, rodent models have been indispensable in helping to dissect the essential components of social behavior in a systematic way. Although the relative contribution of sensory modalities can be different in humans and rodents, commonalities seem to exist between species. Mice can indeed, recognize and discriminate between individual conspecifics and this ability is vital for the survival of the species. In a laboratory setting, recognition and discrimination can be observed and investigated using a three‐chamber interaction behavior. Specifically, during this task mice spend more time investigating a social stimulus over an inanimate object and display a preference toward a novel conspecific over a familiar one (Moy et al., 2004). This behavior in rodents relays mostly on chemosensory cues released by the stimuli and perceived by the experimental animals. It has been shown that lesion of the olfactory bulb and anosmia impairs the ability of rodents to recognize conspecifics (Popik et al., 1991). Notably, although mice rely upon olfactory cues during typical social interaction, visual, auditory, and tactile senses are all senses that are employed during affiliative interactions (Portfors, 2007; Ryan et al., 2008; Strasser & Dixon, 1986). Indeed, it has been shown that the intensity of touch determines the rewarding properties of social interaction. While olfaction and audition do not induce conditioned place preference, taction is needed during conditioning (Kummer et al., 2011). Although several studies analyzed how different neurons within the central nervous system represent social information, it is still unknown whether the same neurons may be differently activated by individual social cues.
Social interaction is rewarding (Bariselli et al., 2018; Dölen et al., 2013; Panksepp & Lahvis, 2007) and it has been shown that dopamine (DA) neurons of the Ventral Tegmental Area (VTA) are activated by conspecific interaction (Prévost‐Solié et al., 2020) and moreover, that this activity is necessary for social novelty exploration (Bariselli, Hörnberg, et al., 2018). Furthermore, alterations in synaptic properties of VTA DA neurons have been observed in autism mouse models and they have been linked with impairments in maintaining social interest (Bariselli et al., 2016) and socially conditioned place preference (Bariselli, Contestabile, et al., 2018).
Here, we used modified enclosures to dissect the contribution of sensory cues in driving sociability using the three‐chamber task. Our results highlight and provide proof for the importance of olfactory cues in approach behavior. Subsequently, using in vivo recordings in freely moving mice we interrogated whether a social odor would activate DA neurons of the VTA in the same way as a juvenile conspecific would. In fact, our data demonstrate that complex social stimuli and decomposed social odor cues result in different activation patterns of VTA DA neurons. Moreover, using a four‐choice task, we further show that mice prefer to explore complex social stimuli compared to isolated sensory cues.
2. MATERIALS AND METHODS
2.1. Animals
The experimental procedures described here were conducted in accordance with the Swiss laws and previously approved by the Geneva Cantonal Veterinary Authority. Male and female C57Bl/6j (experimental animals) were purchased from Charles River Laboratories and housed in the institutional animal facility under standard 12 hr/12 hr light/dark cycles with food and water ad libitum. Experimental adult animals were group‐housed (4–5 per cage) and were behaviorally tested as stated in the corresponding sections in the following paragraphs. Younger non‐familiar male mice (3.5–4 weeks; sex‐matched) were used as stimuli animals in the three‐chamber social interaction assay. Behavioral experiments were conducted in a room with fixed low illumination (10–15 Lux) and with controlled humidity (40%) and temperature (22–24°C). The experiments were always performed within a time frame that started approximately 2 hr after the end of the dark circle and ended 2 hr before the start of the next dark circle.
2.2. Three‐chamber test
A social interaction assay was used, comprising a rectangular Plexiglas arena (60 × 40×22 cm; Ugo Basile) divided into three‐chambers (each 20 × 40 × 22 cm). The walls of the central chamber had doors that could be lifted to allow free access to all chambers. The social preference test was performed similarly as published by (Moy et al., 2004): each mouse was placed in the arena for a habituation period of 10 min and it was allowed to freely explore the empty arena. At the end of the habituation, the experimental mouse was blocked in the central chamber and two enclosures were randomly placed in the center of the two outer chambers. The test started immediately after the opening of the doors when the experimental mouse was allowed to freely explore the apparatus and the enclosures for 10 min. Except for the in vivo recording experiments, the experimental mice performed only one three‐chamber test and different cohorts of animals were used for each experiment. For the in vivo recording experiment, implanted mice performed both conditions in a counterbalanced way. The stimuli mice were always novel for the experimental mouse but were used several times (always in the same condition, and between the third and fourth week of life). Several kinds of typical and custom‐made enclosures were used in the study (Figure 2a, all the enclosures measured the same size: 16 cm × 9 cm): (1) the typical enclosures, provided with the arena by the manufacturer (Ugo Basile, Varese, Italy), with metal vertical bars allowing visual, auditory, olfactory and tactile contact between the experimental mice and the mice acting as social stimuli; (2) transparent enclosures with large openings (diameter = 1 cm) which also allowed visual, auditory, olfactory and tactile contact between mice, but were made of Plexiglas in order to allow comparisons with the other custom‐made enclosures; (3) transparent Plexiglas enclosures with smaller openings (diameter = 0.3 cm) which allowed visual, auditory, olfactory but not tactile contact between mice; (4) opaque dark plastic enclosures with small openings (diameter = 0.3 cm) which allowed only auditory and olfactory but not visual and tactile cues between mice; 5. transparent Plexiglas enclosures without openings which allowed only visual and presumably auditory but not olfactory and tactile contact between mice. The enclosures were made in such a way that ensured equal total open surface between enclosures with small openings and enclosures with larger openings. The enclosures could contain an inanimate object (a locker), a small robot (8 × 4 cm) that alternately rotates his arms in a semi‐random manner (described as a moving object in the manuscript), an inanimate object (locker) impregnated with social odors, an inanimate object (locker) impregnated with lime odor, an anesthetized juvenile conspecific or an awake juvenile conspecific depending on the test. The juvenile conspecifics (sex‐matched mice between 3.5 and 4 weeks old) in the enclosures were habituated to the apparatus and the enclosures on the 3 days preceding the experiment. An inanimate object was left for 7 days in a home‐cage with 4–5 juvenile sex‐matched conspecifics in order to impregnate the object with social odors. Lime aroma was directly applied to an object in order to obtain the inanimate object impregnated with lime odor. Animals that their total exploration time for both the enclosures was <10 s were excluded from the analysis. Every session was video‐tracked and recorded using Ethovision XT (Noldus, Wageningen, the Netherlands), which provided an automated recording of the time around the enclosures (with virtual zones designed around them), the distance moved, and the velocity. The time spent around each enclosure was assessed and then used to determine the preference index:
The arena was cleaned with 70% ethanol solution and dried between trials.
FIGURE 2.
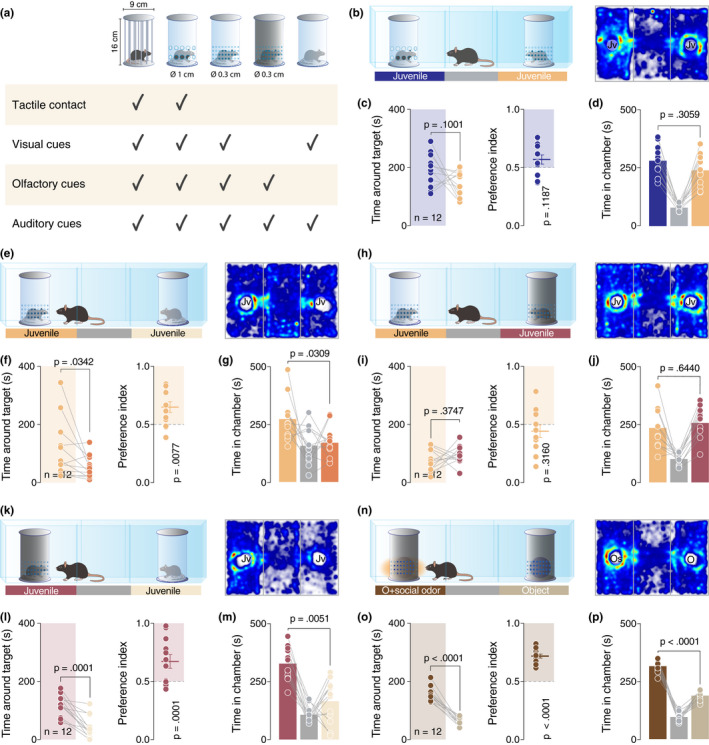
Social odors are sufficient to develop the preference. (a) Schematic representation of the type of enclosures used in this study. (b, e, h, k and n) Left: schematic representation of the three‐chamber test. Right: heatmap reporting the mean occupancy of the batch of mice during the test. (c, f, i, l and o) Left: time around the enclosures containing the stimuli (paired t‐test. c: t 11 = 1.795, p = .1001. f: p = .0342 (Wilcoxon matched‐pairs signed rank test). i: t 11 = 0.9252, p = .3747. l: t 11 = 5.736, p = .0001. o: t 11 = 10.82, p < .0001). Right: calculated preference index (one‐sample t‐test against theoretical mean = 0.5. c: t 11 = 1.692, p = .1187. f: t 11 = 3.255, p = .0077. i: t 11 = 1.05, p = .3160. l: t 11 = 5.786, p = .0001. o: t 11 = 13.09, p < .0001). (d, g, j, m and p) Time spent in each chamber (paired t‐test. d: t 11 = 1.074, p = .3059. g: t 11 = 2.473, p = .0309. j: t 11 = 0.4751, p = .6440. M: t 11 = 3.482, p = .0051. P: t 11 = 9.921, p < .0001). Jv, juvenile conspecific; O, object; Os = object impregnated with social odors and n = number of mice that performed the experiment
2.3. Drugs
In cases where juvenile conspecifics were reversibly anesthetized, a mixture of Ketamine‐Xylazine with an injection volume of 20 ml/kg was used. More specifically, the compound contained Ketamine (Ketalar®) 60 mg/kg with a final concentration of 3 mg/ml and Xylazine (Rompun 2%®) 12 mg/kg with a final concentration of 0.6 mg/ml. The IP injection was made 20 min before the experiment. The duration of the anesthesia was approximately 1 hr.
2.4. Spike‐unit recording
Male mice of 6–8 weeks old were anesthetized with 1%–3% isoflurane vaporized in oxygen (1 L/min) and placed on a stereotaxic apparatus. Craniotomies were performed to implant 4 tetrodes (Nickel‐Chrome of 18 µm with an average impedance of 300 kOhms defined by gold plating) in the right VTA (AP: −3.2, ML: 0.5, DV: −4.25) and 2 references (stainless steel of 127 µm) on the cerebellar surface. The electrodes were connected to an Electrode Interface Board (EIB‐16, Neuralynx, Bozemann, MT) and assembled to a homemade microdrive. The position of the electrodes was confirmed post‐mortem.
At least 1 week after surgery, mice performed the 3‐chamber task and neuronal activity was recorded with a Digital Lynx System (Neuralynx, Bozemann, MT) to amplify, band‐pass filter the signal between 600–6000 Hz and digitize the recording at a sampling rate of 32 kHz. The single spike units were extracted and spike sorted with a homemade Matlab code based on PCA (principal component analysis) of the waveform and then clustering by EMGM (Expectation Maximization of Gaussian Mixture). Clusters were then visually inspected and eventually excluded due to insufficient separation from other clusters, poor waveform appearance, or the presence of spike in the refractory period defined by auto‐correlation.
The identification of pDA or pGABA VTA neurons was performed using the same approach as Prévost‐Solié et al. (2020 ) using a dataset consisting of pDA neurons, pGABA neurons, and DA neurons identified by opto tagging. Briefly, a cluster analysis with a PCA followed by an EMGM was performed on 58 electrophysiological features based on activity pattern and waveform extracted during the first 5 min of each recording. To confirm these observations, the membership of each single unit recording was scored using a classification model (ensemble of bagged classification trees 5× cross‐validated reaching 99.6% of accuracy). Only 1 neuron visually identified as pGABA was included in the pDA cluster. Only single‐unit recordings with a confidence score higher than 0.9 for the pDA neuron cluster were kept.
For the analysis of the spiking activity evoked by events, the neuronal activity was aligned, centered on the events to make a peri‐event time histogram (PETH), and then averaged across the trials for each neuron. Spiking activity was normalized by subtracting the mean frequency of the record from the mean frequency of a sliding window (average window of 1 s used for quantification and Peri Event Time Histogram (PETH) of enclosure interaction events and average window of 5 s for PETH of transition events). For PETH, the spiking activity is then filtered by convolution with a 400 ms Gaussian window.
Heatmaps of neuronal activity were obtained by averaging the neuronal activity recorded for each x and y position tracked with Ethovision and then filtered with a 2 cm2 convolution. Significant neuronal activity changes were calculated for each coordinate by calculating the p‐value from t‐test by comparing the activity of each x and y position with the average activity. In case of camera occlusion, the missing coordinates were replaced by the last available coordinates.
The events were obtained from the analysis of specific behaviors from the synchronized video:
Interactions with the enclosures are defined by the introduction of the experimental mouse within a 5 cm perimeter around the enclosure and realigned with the activity peak in a given interval (4 s for sessions with object + odor vs. object and 2.5 s for sessions with social vs. object). Baseline quantification is calculated by analyzing activity between 10 and 4 s before and after the event. Event quantification is calculated by analyzing activity 1 s before and after the event.
Transitions are defined as the transition of a mouse from one stimulus chamber to another passing through the middle chamber. The transitions taken into account in the analysis presented in Figure 3, have a distance of <35 cm without backward orientation. Baseline quantification is calculated by analyzing activity between −6 and −2 s before the event and 10 and 14 s after the event. Event quantification is calculated by analyzing activity between 0 and 4 s after the event.
The events used for velocity control are defined by velocity peaks outside the transition events and higher than 20 cm/sec (corresponding to a speed higher than the average transition speed). Baseline quantification is calculated by analyzing activity between 10 and 4 s before and after the event. Event quantification is calculated by analyzing activity 1 s before and after the event.
FIGURE 3.
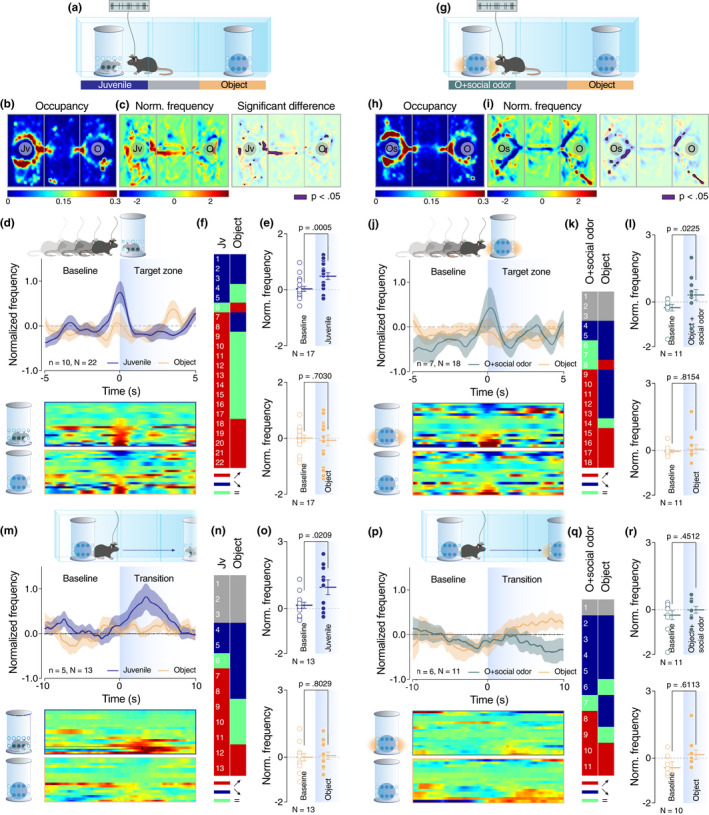
VTA dopaminergic neurons behave differently according to the perception of sensory cues. (a and g) Schematic representation of the three‐chamber test. (b and h) Heatmap reporting the mean occupancy of the batch of mice during the test. (c and i) Left: heatmap reporting the normalized VTA pDA activity regarding the occupancy of the batch of mice during the test. Right: same heatmap overlaid by the occupancy with a significant difference of VTA‐pDA activity compared to overall activity (p < .05). (d, j, m, and p) Top: schematic representation of the behavior analyzed (enter of experimental mice in the proximity of the enclosure (target zone) for d and j; initiation of the transition from one chamber to the opposite one for m and p). Middle: PETH of normalized VTA pDA activity centered on the behavior described above. Down: a heatmap of the corresponding PETH of normalized VTA pDA activity for each neuron recorded. (e, k, n and q) Table of activity response of individual VTA pDA neurons for each event and stimuli (red, positive response; blue, negative response; green, no response for given stimuli; grey, no response for any stimuli). (f and l) Comparison of normalized VTA DA activity for positive responding neurons between baseline and the enter in the target zone (f top: paired t‐test (t 16 = 4.311); f down: paired t‐test (t 16 = 0.3882); l top: Wilcoxon test (W = 50); l down: Wilcoxon test (W = −6)). (o and r) Comparison of normalized VTA DA activity for positive responding neurons between baseline and direct transition (o top: paired t‐test (t 12 = 2.656); o down: paired t‐test (t 12 = 0.2552); r top: Wilcoxon test (W = 18); r down: Wilcoxon test (W = 11). Jv = juvenile conspecific, O = object, n = number of mice that performed the experiment, and N = number of neurons recorded during the experiment
The individual neuronal response was determined by calculating the p‐value from the t‐test for each neuron by comparing the basal activity and the distribution of activity from each trial during each event analyzed (1 s before and 1 s after the interaction and velocity control events; 4 s after the start of the transition events). Every significant t‐test determined if a neuron was a responder or not. The average activity of the neuron during events (below or above the baseline) determined the positive or negative response. Neurons without response for any stimuli were considered as non‐responders and neurons with the response in only a subset of stimuli were considered as neutral for the stimuli without response. Only cells with at least one positive response during interaction events were processed to determine the average neuronal activity and compare the baseline period with the period of the events analyzed (see Figure 3f,l). Only mice that performed a transition were considered in the analysis showed in Figure 3o,r.
2.5. Four choices test
The test was performed in a cubic arena (41 cm × 41 cm × 41 cm). Similarly to the three‐chamber test, each experimental mouse was placed in the arena for a habituation period of 10 min and it was allowed to freely explore the empty arena. At the end of the habituation, four different enclosures (transparent with large openings, transparent with small openings, transparent without openings, and black with small openings) each containing a juvenile sex‐matched conspecific were placed in the corners of the arena. The experimental mouse was then allowed to freely explore the apparatus and the enclosures for 20 min. Every session was video‐tracked and recorded using Ethovision XT (Noldus), which provided an automated recording of the time around the enclosures (with virtual zones designed around them), the distance moved, and the velocity. The time spent around each enclosure was assessed. The arena was cleaned with 70% ethanol solution and dried between trials.
2.6. Statistical analysis
Statistical analysis was conducted with GraphPad Prism 7 and 8. Statistical outliers were identified with the ROUT method (Q = 1) and excluded from the analysis. The normality of sample distributions was assessed with the Shapiro–Wilk criterion and when violated non‐parametric tests were used. When normally distributed, the data were analyzed with independent t‐tests, one‐sample t‐tests, one‐way ANOVA, and repeated measures (RM) ANOVA as appropriate. When normality was violated, the data were analyzed with Mann–Whitney test, while for multiple comparisons, Kruskal–Wallis or Friedman test was followed by Dunn's test. For the analysis of variance with two factors (two‐way ANOVA, RM two‐way ANOVA, and RM two‐way ANOVA by both factors), normality of sample distribution was assumed, and followed by Bonferroni post hoc test. Data are represented as the mean ± s.e.m. and the significance was set at 95% of confidence interval.
3. RESULTS
3.1. Active social reciprocity is not a necessary component of preference expression in the three‐chamber task
The three‐chamber test is a widely‐used social behavioral task that allows an experimental mouse to freely explore two stimuli, each situated in different enclosures in opposite chambers of the arena. This test assumes that mice are rational agents which make choices aligned with a hierarchical outcome totally dependent on their preference. Typically, the time spent exploring a sex‐matched juvenile conspecific is compared to the time spent exploring an inanimate object (Figure 1a). As expected according to previously published results (Bariselli et al., 2016; Moy et al., 2004), mice showed a clear preference for the juvenile conspecific and the chamber where it was located (Figure 1b,c), resulting in a social preference index significantly higher than 0.5 (Figure 1b).
FIGURE 1.
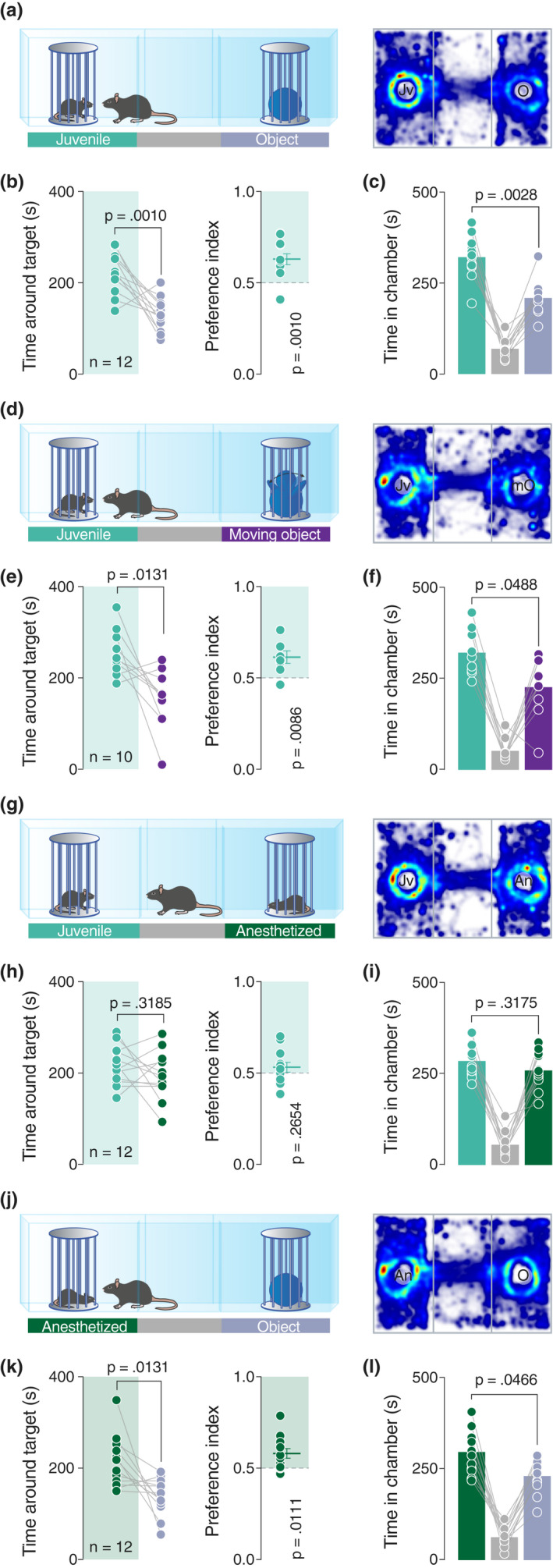
The movements are not essentials to drive sociability in mice. (a, d, g and j) Left: schematic representation of the three‐chamber test. Right: heatmap reporting the mean occupancy of the batch of mice during the test. (b, e, h and k) Left: time around the enclosures containing the stimuli (paired t‐test. b: t 11 = 4.444, p = .0010. e: t 9 = 3.081, p = .0131. h: t 11 = 1.112, p = .3185. k: t 11 = 2.956, p = .0131). Right: calculated preference index (one‐sample t‐test against theoretical mean = 0.5. b: t 11 = 4.41, p = .0010. e: t 9 = 3.346, p = .0086. h: t 11 = 1.173, p = .2654. k: t 11 = 3.048, p = .0111). (c, f, i, and l) Time spent in each chamber (paired t‐test. c: t 11 = 3.823, p = .0028. f: p = .0488, W = −39 (Wilcoxon matched‐pairs signed rank test). i: t 11 = 1.047, p = .3175. l: t 11 = 2.241, p = .0466). Jv, juvenile conspecific; O, object; mO, moving object; An, anesthetized juvenile; n, number of mice that performed the experiment
We next investigated whether the movement of an inanimate object can drive preference in mice. Comparing the time spent exploring a small robot characterized by the semi‐random movement of his arms (moving object) or a juvenile conspecific (Figure 1d), we observed that mice still preferred the social stimulus (Figure 1e,f). We then questioned whether reciprocal exploratory behaviors displayed by the stimulus mouse can be an important movement‐related factor that determines preference. We, thus, exposed experimental mice to a setup with an enclosure containing a juvenile conspecific as usual, and an enclosure containing an anesthetized mouse (Figure 1g). Interestingly, no preference was expressed for any of the enclosures (Figure 1h–I), with experimental mice exploring both the awake and the anesthetized mouse. Taken together, these results indicate that the movement and/or reciprocity are not the key determining factors of preference in mice.
In order to verify if the hierarchical preference of the mice is preserved, we applied the assumptions of the rational choice theory:
where , and . According to this prediction and data collected thus far, we assumed that in a direct comparison between an anesthetized juvenile conspecific and an inanimate object, the experimental mice should prefer the anesthetized conspecific (Figure 1j). Indeed, our data confirmed this prediction, with mice spending more time around the anesthetized mouse compared to the object, suggesting that social preference is expressed even when the stimulus mouse is not engaged in active social reciprocity (Figure 1k–l).
In these experiments (Figure 1a,d,g and j), we have used as experimental mice 6 males and 6 females. Interestingly, males expressed a higher preference index compared to females which according to our results showed no preference for a specific stimulus (Figure S1A–P). Although further investigations regarding sex‐specific effects are warranted, in order to better control our experimental conditions and minimize the number of animals needed, from this point onwards all the experiments were performed with male mice.
3.2. Social odors are sufficient to develop preference
As active reciprocity is not a necessary element for expressing a social preference, what are then the essential properties of social stimuli that determine such preference? To answer this question, we decided to further dissect the contribution of sensory modalities to social preference by using custom‐made enclosures that allowed us to selectively manipulate sensorial aspects (Figure 2a). We first validated the enclosures by ensuring that social preference under a typical three‐chamber setup was still observed as before (Figure S2A–C). We then tested whether lack of tactile cues, when compared to an enclosure permitting taction, as usual, would be associated with reduced preference. More specifically, the mice were exposed to two juvenile conspecifics, enclosed in transparent enclosures, with one having large openings (∅ 1 cm) allowing perception of all sensory cues and the other having small openings (∅ 0.3 cm), thus preventing tactile contact (Figure 2b). Interestingly, we observed no significant difference between time spent exploring the enclosures or chambers (Figure 2c,d), suggesting that lack of tactile cues is not preventing the mice from expressing social preference behavior.
As the access to tactile communication does not seem to be a necessary component for expressing a preference in the three‐chamber task, we tested the role of olfactory and visual cues. For this purpose, we first exposed the mice to two juveniles, one in an enclosure with small openings preventing tactile cues as before, and one in an enclosure with no openings allowing mainly visual cues (Figure 2e). Mice spent less time near the enclosure that prevented odor perception and in the associated chamber, compared to the enclosure with small openings allowing for olfactory and visual cues (Figure 2f–g).
In order to directly interrogate the role of visual cues when olfactory cues are available in both chambers, we exposed the mice to two juveniles, one contained in a transparent enclosure with small openings as before and the other in a black opaque enclosure with the same size of openings (Figure 2h). Mice showed no clear preference for any of the enclosures or chambers (Figure 2h–j). We then proceeded to compare the preference between a social stimulus and an object, first in absence of odors in both chambers and then in absence of visual cues using the opaque enclosures for both juvenile and object (Figure S3A,D). According to the rational choice theory presented above and the results obtained until now, we hypothesized that experimental mice would prefer the juvenile in absence of visual cues and would not show a preference in absence of odor cues. Experimental results verified our expectations (Figure S3B–C and E–F). Moreover, a direct comparison of the preference for two juvenile conspecifics in two different enclosures with the absence of visual or odor cues respectively (Figure 2k) revealed a significant preference for the odor‐permitting enclosure (Figure 2l–m). This evidence suggests a fundamental role of the olfactory than the visual or tactile system in the development and expression of the social preference in the three‐chamber task.
Finally, to further establish the key role of olfactory cues in determining preference in the three‐chamber task, we exposed mice to two black opaque enclosures containing two identical inanimate objects. One of them was impregnated with social odors (Figure 2n). Mice showed a clear preference for the social odor‐releasing object (Figure 2o–p). In order to verify that this preference was induced only by social and no other type of odors, we repeated the experiment comparing an object impregnated with social odors as before and an object impregnated with lime aroma (Figure S4A). The results confirmed the preference for the object impregnated with social odors (Figure S4B–C).
3.3. VTA dopaminergic neurons behave differently according to the perception of sensory cues
Our findings so far suggest that social olfactory cues are necessary and sufficient to induce preference behavior in mice. Moreover, direct comparison of a juvenile conspecific and an object impregnated with social odors did not reveal any preference between the two stimuli during the three‐chamber task (Figure S4D–F). However, as social interaction is a composite behavior with the participation of all sensory modalities and given previously published data that show the importance of other modalities, such as tact (Kummer et al., 2011), in social interactions, we asked how the neuronal representation of isolated social olfactory cues (i.e., social odor‐impregnated object) compares to the representation of sensory cues corresponding to a more complex social experience (i.e., juvenile conspecific still in the presence of an enclosure but with no sensory restrictions). We recorded the activity of putative dopamine neurons of the VTA during the three‐chamber test when mice were exposed to the abovementioned conditions (Figure 3a,g). VTA pDA neurons were identified with the help of a classification model based on the waveform and firing pattern of recorded neurons as previously described by Prévost‐Solié et al., 2020; see their Figure S1l–N). Post‐hoc localization of the electrode's tip was verified for each mouse used in this in vivo recording experiment (Figure S5A). As expected, mice showed a preference for the social stimulus and social odor‐impregnated object respectively when compared to the object (Figure 3b–c and 3h–I; Figure S5B–E). A significant increase in global VTA pDA firing was observed both when the experimental mouse entered in the proximity of the enclosure containing the social stimulus (Figure 3d) and in the proximity of the social odor‐impregnated object (Figure 3j). Regarding individual cell responses, enclosure proximity was associated with an increase of firing activity in 73% of recorded neurons when the enclosure contained a juvenile conspecific (Figure 3e–f). Similarly, 59% of recorded neurons increased the firing activity when the experimental animal entered in the proximity of the enclosure containing the object with social odors (Figure 3k–l). On the other hand, proximity to inanimate object was constantly associated with activity increase in only 1/3 of recorded neurons (Figure 3e–f and 3k–l).
Moreover, we analyzed the VTA pDA firing during the transition of the experimental animal from one stimulus chamber to another passing through the middle chamber. More precisely, we aligned the normalized firing when the mice start a direct transition by leaving a stimulus chamber to go to the opposite one without backward movement (time = 0 in Figure 3m,p). Remarkably, at a population level, we noticed an increase of the firing only during the transitions toward the conspecific and not during the transition toward the object or the object impregnated with social odors (Figure 3m,p). It is important to note that the peak of activity observed during the transition is not related to the entrance of the experimental mouse in the target zone. We can affirm this fact because mice needed an average of 14.04 s (std 21.24) for the transition and for reaching the opposite target zone, a time period solidly higher than the time corresponding to the peak of frequency in Figure 3m (3.50 s).
As a control, we analyzed neuronal activity during velocity peak randomly selected during the sessions, similar to velocity peak occurring during the transition events (Figure S5F–G). While some individual cells (35%) responded positively by increased firing during velocity peak (Figure S5H–I), strengthening the hypothesis of multiplexed information encoded by VTA pDA activity (Kremer et al., 2020), no changes were observed at the population level (Figure S5J). This data suggests a differential encoding in VTA pDA neurons depending on the target stimulus being approached, that is, toward the combined social experience offered by the juvenile versus the isolated social odor of the impregnated object. This difference was consistent in individual cell responses with 50% of recorded neurons responding positively during transitions toward juvenile conspecific (Figure 3n–o). On the other hand, only 36% of recorded neurons showed increased activity during the transitions toward the object impregnated with social odors (Figure 3q–r) and even less (1/6) during the transitions toward the object (Figure 3n–o and q–r). Overall, these findings strongly support the hypothesis that approach‐triggered neuronal representation of the combined and multi‐sensory social experiences is distinct from the one corresponding to the isolated olfactory mono‐sensory cues.
3.4. Mice prefer to explore complex stimuli which influence multi‐sensory modalities
Single‐unit recording of the VTA pDA neurons revealed differences in the brain representation of mono‐ and multi‐sensory stimuli. In order to further dissect whether these differences can be detected behaviorally, we directly compared the preference of the mice for enclosures that allowed mono‐ versus multi‐sensory contact with the stimuli mice (Figure 4a). Remarkably, we observed that mice spent less time near the enclosure that allowed only odor perception as compared to a more complex stimulus (i.e., enclosure allowing for communication via multiple sensory cues; Figure 4a–b). No significant difference was observed in the time in a chamber (Figure 4c). In order to directly assess the preference of the mice when given more than two choices, as it is the case with the three‐chamber test, we performed a four‐choice test, where the animals were exposed to four different enclosure types simultaneously, each containing a juvenile mouse. This experimental setup allowed us to compare among different conditions: access to all sensory cues, visual and odor cues, only visual or only odor cues (Figure 4d). Results revealed that the least preferred enclosure was the one allowing only for visual cues (Figure 4e). Interestingly, for the other conditions, the time around the enclosures did not indicate a stimulus that is more attractive (Figure 4e). On the other hand, Markov process analysis conducted on the transitions between the stimuli revealed a higher probability for the experimental animal to return near the enclosure which allowed for multi‐sensory cues (Figure 4f; red color‐coded loop).
FIGURE 4.
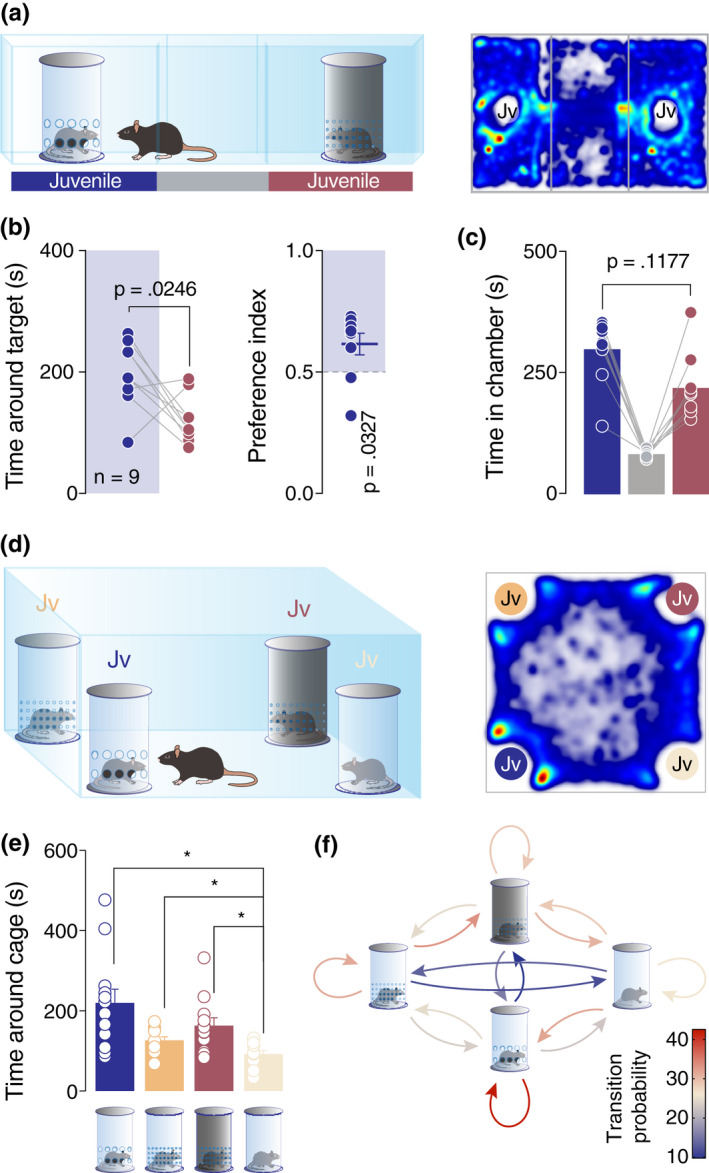
Mice prefer to explore complex stimuli which influence multi‐sensory modalities. (a) Left: schematic representation of the three‐chamber test. Right: heatmap reporting the mean occupancy of the batch of mice during the test. (b) Left: time around the enclosures containing the stimuli (paired t‐test. t 8 = 2.762, p = .0246). Right: calculated preference index (one‐sample t‐test against theoretical mean = 0.5. t 8 = 2.579, p = .0327). (c) Time spent in each chamber (paired t‐test. t 8 = 1.753, p = .1177). (d) Left: schematic representation of the Four‐choices test. Right: heatmap reporting the mean occupancy of the batch of mice during the test. (e) Time around the enclosures containing the stimuli (RM one‐way ANOVA: F 1.436,15.8 = 6.906, p = .0116, followed by Bonferroni's multiple comparison post hoc test). (f) A diagram representing a two‐state Markov process. The heatmaps report the probability of the Markov process changing from one state to another state, with the direction indicated by the arrow. Jv, juvenile conspecific and n, number of mice that performed the experiment
4. DISCUSSION
Here we showed that olfactory cues from conspecifics are sufficient to induce a preference in mice. However, in terms of neuronal encoding, approach toward a juvenile mouse versus toward a social odor were distinct events, with an increase of neuron firing only during the transition toward the juvenile mouse and not during the transition toward the object impregnated with social odor. Overall, our data indicate that although mice are motivated to approach social odor cues, these isolated olfactory cues do not recapitulate the entire complexity of social interaction. These data clearly suggest that multiple sensory cues from conspecifics are integrated with odor cues and result in a dynamically distinct pDA neuron activation. We then sought to corroborate this conclusion behaviorally by giving the animals the opportunity to explore four juveniles simultaneously but caged in enclosures allowing varying levels of sensory information. With this experimental setup, we showed an increased probability for the mice to return to the enclosure that permitted the highest level of social cues complexity.
Approach behavior assessed with the three‐chamber task has its limitations. Indeed, it mainly reflects the motivation of the experimental animal to interact with a caged conspecific without considering the complexity and full syllabus of social behavior that can be expressed during free reciprocal interactions (Chen & Hong, 2018). Behavioral readouts in the three‐chamber task can also be influenced by other factors, such as movement and anxiety‐like behavior (Lukas & de Jong, 2017). Anxious animals, specifically, can express either a decrease in social preference when they are too anxious to explore their surroundings or they can increase their social approach in an attempt to socially buffer their anxiety (Kikusui et al., 2006; Lukas & de Jong, 2017). Moreover, under our experimental conditions, female mice did not show a preference for the social stimulus and this should, thus, be investigated separately in future studies. Although Moy et al. (2004) report a clear preference for a conspecific by the same strain of female mice, there are potential factors that could explain this discrepancy. One possibility could be that age at testing could influence the expression of social preference. Moy et al. used younger, 6‐week‐old female mice (i.e., around P42), whereas in our case, mice were between 8 and 10 weeks of age. It is, thus, plausible that any hormonal or possible estrous cycle variations could differentially affect the observed behavior in younger versus older mice. And while we cannot attribute this lack of preference to estrous cycle phases at this point, this possibility cannot be completely excluded. Another possibility could be that the number of female mice tested here (n = 6) was not adequate under the particular experimental conditions to allow us to observe a significant social preference. Future studies will need to address this matter by systematically assessing female social preference under the same conditions and by increasing the number of females tested. Despite these constraints that warrant careful interpretation of results obtained with the three‐chamber task, this experimental setup allowed us to dissect the contribution of different sensory modalities to the expression of approach behavior in a controlled and systematic manner.
Social behavior in rodents relies mostly on chemosensory cues released by the stimuli and perceived by the experimental animals. More specifically, it has been shown that lesion of the olfactory bulb and anosmia impairs the ability of rodents to recognize their conspecific (Popik et al., 1991). However, although olfaction is a very important sensory modality during social interactions in mice, vision, audition, and taction can also play a role during affiliative interactions (Portfors, 2007; Ryan et al., 2008; Strasser & Dixon, 1986). In particular, it has been shown while olfactory and auditory cues do not induce a conditioned place preference, taction is needed to determine the rewarding properties of social interaction and during conditioning processes (Kummer et al., 2011). One important point to emphasize here is that the contribution of sensory modalities can vary not only among species, but also within the species for different types of social behavior. For example, it is conceivable that social approach and social recognition behavior can principally rely on different modalities and correspond to different firing patterns. Similarly, other mechanisms and sensory modality contributions can be crucial to determine the expression and strength of socially conditioned place preference. One possible limitation of our study is that the contribution of auditory cues could not be controlled in an optimal way with our enclosures. While it is logical to hypothesize that the auditory cues were minimized when using the enclosure with no openings, we have not conducted a systematic control to understand whether ultrasonic vocalizations could be exchanged when stimuli mice were caged in this type of enclosure. These aspects will need to be addressed explicitly in future studies.
Despite the recent advances regarding the neural circuit dynamics linked to social behavior, many relevant questions remain unanswered. Here we show a significant increase in global VTA pDA firing both when the experimental mouse entered in the proximity of the enclosure containing the social stimulus and in the proximity of the social odor‐impregnated object. These data are in line with previously published evidence that associates dopamine with affiliative same‐sex social behaviors (Gunaydin et al., 2014; Puglisi‐Allegra & Cabib, 1997; Robinson et al., 2002, 2011). Interestingly, we further analyzed the DA neurons activity during transitions between two chambers of the arena. We observed that neurons increase their firing only during the transitions toward the social stimulus and not during the transition toward the object or the object impregnated with social odors. Altogether our data indicate that although social odor per se is sufficient to drive approach behavior, neurons encode differently complex conspecific versus a social odor.
The data obtained here highlight social olfactory cues as crucial components that drive approach in mice. Nevertheless, we also present evidence that the synthesis of multiple sensory modalities seems to represent a more salient stimulus that is associated with increased exploration probability and corresponds to distinct neuronal firing in vivo. Our findings can have important implications on data interpretation from animal models of disease where social impairment is a fundamental feature. Most importantly, our results can offer insights toward understanding different sensory modality contributions in social deficits observed in autism‐related and other animal models of disease that involve sociability deficits and, ultimately, aid to gain knowledge regarding the inter‐relationships between core disease symptoms.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
AC, GC, ST and CB conceived and designed the experiments. AC and GC performed and analyzed the three‐chamber and the four choices experiments. BG performed and analyzed the spikes‐unit recording. AC, BG, ST and CB wrote the manuscript and AC prepared the figures.
STATEMENT ON DATA SHARING
Raw data, videos, and Matlab codes are available on request (Camilla.Bellone@unige.ch).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15179.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
ACKNOWLEDGMENTS
CB is supported by the Swiss National Science Foundation, Pierre Mercier Foundation, NCCR Synapsy and ERC consolidator grant. We thank Lorena Jourdain, Jérémy Pantone and Clement Prévost‐Solié for technical support.
Contestabile A, Casarotto G, Girard B, Tzanoulinou S, Bellone C. Deconstructing the contribution of sensory cues in social approach. Eur J Neurosci. 2021;53:3199–3211. 10.1111/ejn.15179
Alessandro Contestabile, Giulia Casarotto and Benoit Girard contributed equally.
Edited by: Mathias Schmidt
Contributor Information
Stamatina Tzanoulinou, Email: stamatina.tzanoulinou@unil.ch.
Camilla Bellone, Email: camilla.bellone@unige.ch.
REFERENCES
- Bariselli, S. , Contestabile, A. , Tzanoulinou, S. , Musardo, S. , & Bellone, C. (2018). SHANK3 downregulation in the ventral tegmental area accelerates the extinction of contextual associations induced by juvenile non‐familiar conspecific interaction. Frontiers in Molecular Neuroscience, 11, 360. 10.3389/fnmol.2018.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariselli, S. , Hörnberg, H. , Prévost‐Solié, C. , Musardo, S. , Hatstatt‐Burklé, L. , Scheiffele, P. , & Bellone, C. (2018). Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction. Nature Communications, 9, 3173. 10.1038/s41467-018-05382-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariselli, S. , Tzanoulinou, S. , Glangetas, C. , Prévost‐Solié, C. , Pucci, L. , Viguié, J. , Bezzi, P. , O'Connor, E. C. , Georges, F. , Lüscher, C. , & Bellone, C. (2016). SHANK3 controls maturation of social reward circuits in the VTA. Nature Neuroscience, 19, 926–934. 10.1038/nn.4319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P. , & Hong, W. (2018). Neural circuit mechanisms of social behavior. Neuron, 98, 16–30. 10.1016/j.neuron.2018.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen, G. , Darvishzadeh, A. , Huang, K. W. , & Malenka, R. C. (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501, 179–184. 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin, L. A. , Grosenick, L. , Finkelstein, J. C. , Kauvar, I. V. , Fenno, L. E. , Adhikari, A. , Lammel, S. , Mirzabekov, J. J. , Airan, R. D. , Zalocusky, K. A. , Tye, K. M. , Anikeeva, P. , Malenka, R. C. , & Deisseroth, K. (2014). Natural neural projection dynamics underlying social behavior. Cell, 157, 1535–1551. 10.1016/j.cell.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui, T. , Winslow, J. T. , & Mori, Y. (2006). Social buffering: Relief from stress and anxiety. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 361, 2215–2228. 10.1098/rstb.2006.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer, I. , Flakowski, J. , Rohner, C. , & Luscher, C. (2020). Context‐dependent multiplexing by individual VTA dopamine neurons. Journal of Neuroscience, 40, 7489–7509. 10.1523/JNEUROSCI.0502-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer, K. , Klement, S. , Eggart, V. , Mayr, M. J. , Saria, A. , & Zernig, G. (2011). Conditioned place preference for social interaction in rats: Contribution of sensory components. Frontiers in Behavioural Neurosciences, 5, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas, M. , & de Jong, T. R. (2017). Conspecific interactions in adult laboratory rodents: Friends or foes? Current Topics in Behavioral Neurosciences, 30, 3–24. [DOI] [PubMed] [Google Scholar]
- Moy, S. S. , Nadler, J. J. , Perez, A. , Barbaro, R. P. , Johns, J. M. , Magnuson, T. R. , Piven, J. , & Crawley, J. N. (2004). Sociability and preference for social novelty in five inbred strains: An approach to assess autistic‐like behavior in mice. Genes, Brain, and Behavior, 3, 287–302. 10.1111/j.1601-1848.2004.00076.x [DOI] [PubMed] [Google Scholar]
- Panksepp, J. B. , & Lahvis, G. P. (2007). Social reward among juvenile mice. Genes, Brain, and Behavior, 6, 661–671. 10.1111/j.1601-183X.2006.00295.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik, P. , Vetulani, J. , Bisaga, A. , & Van Ree, J. M. (1991). Recognition cue in the rat's social memory paradigm. Journal of Basic and Clinical Physiology and Pharmacology, 2, 315–327. 10.1515/JBCPP.1991.2.4.315 [DOI] [PubMed] [Google Scholar]
- Portfors, C. V. (2007). Types and functions of ultrasonic vocalizations in laboratory rats and mice. Journal of the American Association for Laboratory Animal Science, 46, 28–34. [PubMed] [Google Scholar]
- Prévost‐Solié, C. , Girard, B. , Righetti, B. , Tapparel, M. , & Bellone, C. (2020). Dopamine neurons of the VTA encode active conspecific interaction and promote social learning through social reward prediction error. bioRxiv 2020.05.27.118851. 10.1101/2020.05.27.118851 [DOI]
- Puglisi‐Allegra, S. , & Cabib, S. (1997). Psychopharmacology of dopamine: The contribution of comparative studies in inbred strains of mice. Progress in Neurobiology, 51, 637–661. 10.1016/S0301-0082(97)00008-7 [DOI] [PubMed] [Google Scholar]
- Robinson, D. L. , Heien, M. L. A. V. , & Wightman, R. M. (2002). Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. Journal of Neuroscience, 22, 10477–10486. 10.1523/JNEUROSCI.22-23-10477.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, D. L. , Zitzman, D. L. , Smith, K. J. , & Spear, L. P. (2011). Fast dopamine release events in the nucleus accumbens of early adolescent rats. Neuroscience, 176, 296–307. 10.1016/j.neuroscience.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, B. C. , Young, N. B. , Moy, S. S. , & Crawley, J. N. (2008). Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behavioural Brain Research, 193, 235–242. 10.1016/j.bbr.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser, S. , & Dixon, A. K. (1986). Effects of visual and acoustic deprivation on agonistic behaviour of the albino mouse (M. musculus L.). Physiology & Behavior, 36, 773–778. [DOI] [PubMed] [Google Scholar]
- Tavassoli, T. , Miller, L. J. , Schoen, S. A. , Jo Brout, J. , Sullivan, J. , & Baron‐Cohen, S. (2018). Sensory reactivity, empathizing and systemizing in autism spectrum conditions and sensory processing disorder. Developmental Cognitive Neuroscience, 29, 72–77. 10.1016/j.dcn.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5


