Abstract
In order to improve the diagnosis and treatment of senile thyroid diseases in China and to promote healthy aging, the Endocrine Metabolic Diseases Group of the Chinese Geriatrics Society, and the Thyroid Group of the Chinese Society of Endocrinology jointly drafted the “Expert consensus on diagnosis and treatment for Chinese elderly with thyroid diseases” (referred to as consensus). The Consensus consists of five parts that set 40 recommendations on main clinical issues. The consensus emphasizes clinical focus on the age‐related changes of the hypothalamus‐pituitary‐thyroid axis in the elderly, and it recommends application of comprehensive geriatric assessment to thoroughly evaluate the impact of thyroid diseases and relevant intervention on overall health condition. Meanwhile, it recommends generalization of screening for hypothyroidism during admission to nursing institution or hospital, and routine health check‐ups. Furthermore, it develops individualized stratified management on hyperthyroidism, hypothyroidism, thyroid nodules, and differentiated thyroid carcinoma in the elderly distinguished from the youngers, including treatment regimen, control ranges, and flowcharts for diagnosis and treatment. The Consensus will provide the basis for clinical decisions and health management of thyroid diseases in the elderly by geriatrician, endocrinologist, and general practitioners.
Keywords: clinical decisions, differentiated thyroid carcinoma, elderly, hyperthyroidism, hypothyroidism, thyroid nodules
The consensus develops individualized stratified management on hyperthyroidism, hypothyroidism, thyroid nodules and differentiated thyroid carcinoma in the elderly distinguished from the youngers, including treatment regimen, control ranges and flow charts for diagnosis and treatment.
1. PREAMBLE
Globally, life expectancy has steadily extended and populations are aging at a rapid rate. Thyroid diseases have become one of the most common disorders, reflected in a prevalence of over 50% in aged individuals in China. However, the diagnosis and treatment of senile thyroid diseases are hindered by several factors, including physiological changes in the hypothalamic‐pituitary‐thyroid (HPT) axis, comorbidities, superimpositions of geriatric syndromes, and the decline in multiple organ functions. Standardization of diagnosis and treatment of senile thyroid diseases is of great significance to improve clinical management of senile thyroid diseases in China and to promote healthy aging. In October 2019, the Endocrine Metabolic Diseases Group of the Chinese Geriatrics Society and the Thyroid Group of the Chinese Society of Endocrinology formed a writing group composed of geriatric experts and endocrinologist and jointly drafted the “Expert consensus on diagnosis and treatment for Chinese elderly with thyroid diseases” (referred to as consensus). The final Consensus was finished after three rounds of refinement, including first draft by authors based on the outlines, then reviewed and edited by all corresponding authors via six online and two in‐person discussion, and final proof from members in both groups. The Consensus consists of five parts, including the epidemiology of senile thyroid diseases, laboratory, imaging and cytological examinations and comprehensive geriatric assessment (CGA), and screening, hyperthyroidism, hypothyroidism, and thyroid nodules and differentiated thyroid carcinoma in the elderly. Following the principles of practicability, acceptability, and advancedness, the Consensus absorbs the essence of thyroid diseases guidelines, authoritative books, and evidence‐based medical literatures published both at home and abroad in recent years, and put more emphasis on domestic research findings in this field. In the future, we will update this Consensus in accordance with the research progress, to meet the demands of clinical decisions and health management of thyroid diseases in the elderly by geriatrician, endocrinologist, and general practitioners.
2. EPIDEMIOLOGY OF SENILE THYROID DISEASES
An epidemiological survey of 78 470 people in 31 cities in China showed that the overall prevalence of thyroid diseases was 50.96%, with higher prevalence in the elderly 1 (Table 1). Meanwhile, subclinical hypothyroidism is the most common thyroid diseases in the elderly with the prevalence of nearly 20%. 1 , 2 Most of them were referred as mild subclinical hypothyroidism, only 10% with the level of thyroid stimulating hormone (TSH) above 10 mU/L. A community survey in China showed that total incidence rate of hypothyroidism and subclinical hypothyroidism was 15.07% in women over 65 years old but 9.22% in their male contemporaries. 3 The prevalence of hypothyroidism, subclinical hypothyroidism, and subclinical hyperthyroidism increase with age, while the prevalence of hyperthyroidism decreases. 1 , 4 , 5 The rates of thyroid peroxidase antibody (TPOAb) and thyroid globulin antibody (TgAb) in the elderly over 60 years old were about 11% and 10%, respectively. 1 Thyroid diseases are prevalent in older adults, among which the incidence of subclinical hypothyroidism is the highest.
TABLE 1.
Prevalence of thyroid diseases in different age groups in 31 provinces and cities of China (%; n = 78 470)
|
Age (years old) |
Hyperthyroidism | Subclinical hyperthyroidism | Graves' disease | Hypothyroidism | Subclinical hypothyroidism | Thyroid nodules |
|---|---|---|---|---|---|---|
| Overall | 0.78 | 0.44 | 0.53 | 1.02 | 12.93 | 20.43 |
| 18–29 | 0.82 | 0.34 | 0.55 | 0.45 | 11.77 | 11.30 |
| 30–39 | 0.85 | 0.38 | 0.57 | 0.70 | 10.10 | 15.50 |
| 40‐49 | 0.83 | 0.51 | 0.59 | 1.26 | 12.04 | 20.38 |
| 50–59 | 0.76 | 0.49 | 0.54 | 1.31 | 15.00 | 27.57 |
| 60–69 | 0.65 | 0.40 | 0.46 | 1.42 | 16.13 | 32.23 |
| ≥70 | 0.47 | 0.66 | 0.28 | 2.08 | 19.09 | 36.01 |
| P | .005 | .08 | .01 | <.0001 | .005 | <.0001 |
For every 10 years of age increase between 40–69 years, the upper limit of the normal range of TSH rises by 0.3mU/L, whilst the same limit rises more than 1.6 or 3.2 mIU/L in groups aged between 70–80 years or over 80 years compared with counterparts aged between 60–69 years, respectively. 6 Domestic studies also found that age mainly affected the upper limit of the normal range of TSH. Compared with the normal reference range of TSH, the prevalence of senile subclinical hypothyroidism, clinical hypothyroidism would be reduced from 19.87% to 3.3% and from 2.09% to 1.6%, respectively, in the elderly over 65 via applying the age‐specific TSH reference range, whilst the prevalence of clinical hyperthyroidism might remain. 2 The trend of the prevalence of hyperthyroidism with age is related to the iodine status of the population. 7 In iodine‐deficient areas, the prevalence of hyperthyroidism increases with age, reaching as high as 15% in the elderly over 70 years old. 8 The prevalence of hyperthyroidism in the elderly in iodine‐rich areas is lower than that in the general population, with about 65%–75% being mild. 1 , 9 Aging mainly impacts the upper limit of serum TSH normal range. Iodine status is the main factor affecting the prevalence of thyroid dysfunction in the elderly.
In China, the prevalence of thyroid nodules is 32.23%, 36.01% in the elderly aged 60–69 and over 70, respectively. 1 Approximately, 5%–15% of thyroid nodules are proved as carcinomas. In the United States, the overall incidence of thyroid carcinomas is 7.98/105 from 1974 to 2013. The incidence is highest in the age group of 40–59 years old (13.28/105) compared to the 60–79 group (13.15/105) and over 80 group (8.81/105). 10 Meanwhile, the overall mortality rate is 0.44/105, with the highest ratein the 60–79 age group and a significant decrease in the elderly over 80 years old. The prevalence of thyroid nodules increases with age; meanwhile the prevalence of thyroid carcinoma decreases over 80 years old.
The multimorbidity of thyroid diseases and metabolic syndrome, diabetes mellitus, hypertension, and dyslipidemia are quite common in the elderly, 3 , 11 especially in elderly women. 3 A survey of 24 endocrine and geriatric medical centers in China found that 23.79% of patients with type 2 diabetes mellitus were associated with thyroid dysfunction in the elderly from 2015 to 2016. The survey also indicated that the prevalence of subclinical hypothyroidism, hypothyroidism, subclinical hyperthyroidism, hyperthyroidism was 4.89%, 9.3%, 1.13%, and 3.16%, respectively. Among the patients previously diagnosed with senile type 2 diabetes, the multi‐morbidities of thyroid dysfunction were 20.88% in women and 8.96% in men. Therefore, attention should be paid to the screening of thyroid diseases in elderly diabetic patients.
-
■
Recommendation 1: The elderly are at high risk for thyroid disease and have a high rate of multimorbidity with metabolic diseases. Therefore, more attention should be paid to patients with senile thyroid diseases.
-
■
Recommendation 2: Aging mainly impacts the upper limit of serum TSH normal range. Iodine status is the main factor affecting the prevalence of thyroid dysfunction in the elderly.
3. LABORATORY, IMAGING, AND CYTOLOGICAL EXAMINATIONS AND COMPREHENSIVE GERIATRIC ASSESSMENT AND SCREENING FOR SENILE THYROID DISEASES
3.1. The age‐related changes of the hypothalamus‐pituitary‐thyroid axis
The HPT axis of healthy elderly people changes with age, which is presented as decreased levels of serum total triiodothyronine (TT3) and free triiodothyronine (FT3), slightly increased or remained levels of free thyroxine (FT4), decreased ratio of FT3/FT4, and increased TSH levels. 12 , 13 , 14 A prospective cohort study in Australia selected 908 people with an average age of 45 without thyroid diseases and thyroid antibodies. It was found that TSH increased by an average of 0.32 mU/L in 13 years, which was more obvious in the elderly over 60 years old, while no significant change in FT4. 15 The American Cardiovascular Research Survey (CHS) conducted a follow‐up study including 843 subjects with an average age of 72 for almost 13 years. The study revealed a 13% (0.34 mU/L) increase in TSH, 1.7% (0.02 ng/dL) increase in FT4, and about 13% (−14.9 ng/dL) decrease in TT3. 16 Another cross‐sectional survey found that the levels of serum TSH in centenarians (average age of 98, 1.97 mU/L) were significantly higher than those in controls (average age of 72, 1.55 mU/L). 17 The age‐related changes of HPT axis may be a protective mechanism for the elderly to slow down their catabolism. Due to waning metabolism in the elderly, the conversion of T4 to T3 is reduced, the inhibition of TSH feedback is weakened, and the level of TSH is increased. In addition, it might also be associated with an increase in the tuning of TSH response to thyroid hormones or a decrease in TSH biological activity with aging.
3.2. Laboratory tests
3.2.1. Measurement of thyroid hormone levels
Immunoassay is widely employed in thyroid hormone detection at present. 18 The bound and free T4 and T3 form the TT4 and TT3, which are modulated by thyroglobulin. Hypoproteinemia, which is common in the elderly, can decrease serum thyroxine‐binding globulin (TBG) and TT4 levels. Besides, certain drugs and disease states can also interfere with the measurement of serum TT4 and TT3.
FT4 and FT3, free forms of thyroid hormones, are theoretically better indicators reflecting thyroid hormone activity as they are not affected by TBG.
3.2.2. Measurement of TSH levels
With application of sensitive TSH assay, TSH has become the most sensitive indicator of thyroid function. The level of serum TSH increases with aging. A domestic survey involving 14 985 subjects showed that the upper limit of serum TSH in the elderly (8.86 mU/L) was significantly higher than that in the non‐elderly (6.57 mU/L), while there was no significant difference in the lower limit of normal range. 2 Therefore, the age‐specific serum TSH reference range is recommended for the diagnosis of thyroid diseases in the elderly by some experts. However, to date, there is no convincing evidence to prove the effect of changing the normal range of TSH in the elderly on health outcomes. 19 Thus, the normal range of TSH in non‐elderly is still adopted.
3.2.3. Measurement of thyroid autoantibody titers
Antithyroid peroxidase antibody (TPOAb) and antithyroglobulin antibody (TgAb) titers are major indicators of autoimmune thyroiditis. The positive rate of TgAb in patients with autoimmune thyroiditis is relatively low, and its sensitivity is inferior to TPOAb. The positive rates of TPOAb and TgAb are higher in the elderly. 5 Thyroid‐stimulating hormone receptor antibody (TRAb) is a crucial marker of Graves' disease. TRAb is positive in approximately 60%–90% of patients with first‐onset Graves' disease. TRAb can also be positive in patients with Graves' ophthalmopathy with normal thyroid function. In addition, TRAb has certain significance in predicting recurrence of hyperthyroidism after withdrawal of antithyroid drug therapy. The specificity and sensitivity of positive TRAb to predict recurrence is about 50%, but the prediction of negative TRAb is non‐specific. 20
3.3. Imaging
3.3.1. Ultrasonography
Ultrasonography provides detailed views that help measure the size of the thyroid gland, identify structural abnormalities, and characterize its texture and blood flow status. It plays an important role in the diagnosis and differential diagnosis of thyroid diseases. Diffuse hypoechoic, reticular changes reflect lymphocyte infiltration and interstitial fibrosis in the thyroid gland, while flaky hypoechoic changes with irregular margins are often indicative of thyroid destruction. Color Doppler can display thyroid blood flow. The peak systolic blood flow velocity of internal and inferior thyroid artery is elevated in Graves' disease. The purpose of initial sonographic assessment is to screen, diagnose thyroid nodules, and further to distinguish benign nodules from those with suspicious or malignant features, especially evidenced by physical examination or other imaging. Ultrasonography is also applied in fine‐needle aspiration biopsy (FNAB) of thyroid nodules, assessment of cervical lymph nodes, the follow‐up of thyroid nodules. The significance of thyroid ultrasound is superior to computed tomography (CT) and magnetic resonance imaging (MRI) in distinguishing between benign and malignant nodules.
3.3.2. Scintigraphy
-
■
131I uptake: It is primarily recommended for the differential diagnosis of thyrotoxicosis and the evaluation of 131I therapeutic dose, instead of the diagnosis of hyperthyroidism due to the application of sensitive TSH.
-
■
Radionuclide scintigraphy: The thyroid gland can uptake and concentrate 99mTcO4 − or radioactive iodine (131I or 123I), which can generate images that display the position, size, shape, and radioactivity distribution of the thyroid gland. Radionuclide scintigraphy is indicated for functional nodules or function status of thyroid, and diagnose of ectopic thyroid tissue or thyroid absence. It is usually not used to differentiate between benign and malignant nodules. “Cold nodules” indicates non‐functioning, while “hot nodules” indicate hyperfunctioning nodules or toxic thyroid adenoma. When the radionuclide imaging shows that the reginal thyroid tissue is sparse or defective radionuclide distribution, a pro‐tumor imaging agent can be injected. And further pro‐tumor phenomenon could be confirmed when radionuclide occupies this region, indicating probable malignant tumor. 131I whole‐body diagnostic scintigraphy is indicated for detection of postoperative residual, metastasis, and recurrence of differentiated thyroid carcinoma.
3.3.3. Computed tomography and magnetic resonance imaging
Computed tomography and MRI are important in differential diagnosis of thyroid nodules as they can visualize the contour of thyroid against its surrounding tissues. For thyroid with suspicious features, CT and MRI are preferred to evaluate the extent of lesions and to detect tracheal invasion and metastasis to lymph nodes. CT and MRI are also utilized to discriminate between thyroid and non‐thyroid mediastinal cancers.
It should be noted that thyrotoxicosis might develop in 1–3 months after administration of iodinated contrast media. Therefore, it is recommended that elderly with high risk factors (such as known atrial fibrillation or ischemic heart disease) should perform thyroid function tests before administration of iodinated‐contrast agents.
3.4. Fine‐needle aspiration biopsy
Ultrasound‐guided FNAB remains the most important method for detecting malignancy in the management and monitoring of thyroid nodules. In accordance with the recently developed Bethesda System for Reporting Thyroid Cytopathology, a sampled nodule can be categorized as non‐diagnostic or unsatisfactory, benign, atypia of undetermined significance or follicular lesion of undetermined significance, follicular neoplasm or suspicious for a follicular neoplasm, suspicious for malignancy, and malignant.
Ultrasound‐guided FNAB is generally safe in the elderly when excluded with contraindications, such as hemorrhagic tendency, significantly prolonged bleeding and clotting time, significantly decreased prothrombin activity, and uncooperativeness due to mental disorders or cardiopulmonary insufficiency. For patients with a low risk of cardiovascular events, it is recommended to discontinue aspirin 3–7 days before puncture and then resume aspirin 24–48 hours after this procedure. 20 , 21 Patients with a higher risk of cardiovascular events (such as history of coronary stent implantation or ischemic stroke, etc) are suggested not to discontinue during the procedure, but should be informed of the potential risk of bleeding.
3.5. Comprehensive geriatric assessment
Comorbidity of thyroid diseases with multiple chronic diseases and geriatric syndromes is common in the elderly suffering frailty. In turn, consequent polypharmacy could be a major health hazard. 11 , 22 CGA is recommended to be adopted in both clinic and research to thoroughly evaluate the impacts of senile thyroid diseases and relevant interventions on the overall health condition and further to adjust the management if necessary. 23 The main points are as follows:
Purpose: To maintain the function and to ensure the quality of life, especially to improve the ability of daily living of the elderly.
Object: Elderly patients with complicated conditions, impaired self‐care ability, and psychological disorders, but with a certain potential for recovery.
Timing: Regular assessment, especially after changes in the health status or adjustment of treatment.
Items: Activities of daily living 24 , 25 , 26 (including basic activities of daily living [BADL], instrumental activities of daily living [IADL] and advanced activities of daily living [AADL]), mobility and balance, comprehension/communication (including cognitive assessment), 27 mood and emotion (such as depression anxiety assessment), malnutrition, sarcopenia (grip strength, 6‐meter walking speed, body composition analysis), frailty (Fried's frailty phenotype, FRAIL scale), 28 , 29 , 30 assessment of quality of life.
3.6. Screening for senile thyroid diseases
Despite the features of high prevalence, nonspecific symptoms and signs, and hazard to health condition in senile thyroid diseases, there is still a lack of evidence‐based medical recommendations to screen thyroid diseases in general elderly population. However, generalization of screening for hypothyroidism during admission to nursing institution or hospital, and routine health check‐ups, especially for elderly women is recommended. Active screening is needed for the following situations: history of external cervical radiation or 131I treatment, history of thyroid surgery or abnormal thyroid function, family history of autoimmune thyroid disease, complications of other autoimmune diseases, anemia, dyslipidemia, hypertension, diabetes or other metabolic diseases, mental and cognitive disorders, cardiovascular diseases, pulmonary hypertension, digestive system diseases, osteoporosis or sarcopenia, as well as current medication of amiodarone, ketoconazole, lithium, interferon‐α, interleukin‐2, tyrosine kinase inhibitors, or immune checkpoint inhibitors, etc. Serum TSH should be preferred as screening indicator. Ultrasonography screening for thyroid nodules is not recommended in elderly community dwellers.
-
■
Recommendation 3: The HPT axis changes with age, which is mainly manifested as increased TSH levels and decreased T3 levels.
-
■
Recommendation 4: It recommends application of comprehensive geriatric assessment to thoroughly evaluate the impacts of senile thyroid diseases and relevant intervention on overall health condition, especially in the initial state of disease and during adjustment of treatment, on purpose of individualized stratified management.
-
■
Recommendation 5: It recommends generalization of screening for hypothyroidism during admission to nursing institution or hospital, and routine health check‐ups, especially for elderly women and family history of thyroid diseases. TSH should be preferred as screening indicator.
-
■
Recommendation 6: It is not recommended to generally screen thyroid nodules in elderly community dwellers via ultrasonography.
4. SENILE HYPERTHYROIDISM
4.1. Definition
Hyperthyroidism is defined as thyrotoxicosis due to overproduction of thyroid hormones by an overactive thyroid. Clinically, serum TSH is decreased or even undetectable, whilst levels of free triiodothyronine (FT3) and/or FT4 are elevated over the upper limits of normal ranges.
4.2. Etiology
The common causes of endogenous hyperthyroidism in the elderly are Graves' disease (GD), toxic multinodular goiter (TMNG), and toxic adenomas (TA). Other rare causes include TSH‐secreting pituitary tumors, trophoblastic tumors, and metastatic differentiated thyroid carcinoma. GD is the main cause of senile hyperthyroidism in iodine‐rich areas. Senile hyperthyroidism in iodine deficiency areas is mostly manifested as TMNG. 9 Hyperthyroidism caused by excess iodine is common in the elderly. The risk of iodine‐induced hyperthyroidism is increased in the elderly under exposure of iodinated‐contrast medium. The incidence of Amiodarone‐induced thyrotoxicosis is 6%–10%, which is more frequently described in iodine‐deficiency areas. 31
4.3. Clinical manifestations
Hyperthyroidism in the elderly is occult in onset due to absence of sympathetic symptoms and signs. The initial and most commonly occurring symptoms are cardiovascular‐related, including palpitation, atrial fibrillation, increased systolic blood pressure, wide pulse pressure, heart failure, and induced angina pectoris in patients with coronary heart disease. 32 , 33 Atrial fibrillation is estimated to be present in 30%–60% of hospitalized elderly patients suffering from hyperthyroidism. The cumulative incidence of atrial fibrillation in hyperthyroidism patients aged over 60 reached 13% in a Denmark population‐based study after 20‐year follow‐up. 34 Atrial fibrillation inclines to evoke embolic stroke in senile hyperthyroidism patients. Also, they are prone to suffer from heart failure, half of which result from left ventricular dysfunction. The elderly with a history of atrial fibrillation, hypertension, ischemic heart disease, or risk factors for coronary heart disease, such as smoking and diabetes, are more prone to develop congestive heart failure.
Apathetic hyperthyroidism is more common in the elderly, characterized by symptoms of weight loss, palpitations, diarrhea, anorexia, apathy, somnolence, and even insanity in severe cases. Hyperthyroidism in older adults has been associated with impaired attention and concentration and emotional and cognitive decline. Elevated serum FT4 levels could predict new‐onset dementia in community‐dwelling men aged over 70 years. 35 In addition, overt hyperthyroidism is a well‐recognized risk factor for low bone mineral density and osteoporotic fracture in the elderly.
The peak of Graves ophthalmopathy (GO) is between 50 to 60 years of age. However, older men over 60 years are inclined to develop optic neuropathy. 36 The risk factors for developing GO include smoking, prolonged duration, and high level of FT4. The degree of goiter in elderly patients was commonly lower than that in young patients. Large goiters or retrosternal goiters may occur in elderly patients with TMNG, leading to obstructive symptoms such as dyspnea.
Thyrotoxis crisis, also known as thyroid storm, is characterized by rapid metabolic explosion with multisystem involvement. It mostly occurs in thyrotoxic patients who are untreated or inadequately treated. The common precipitants are infection or other acute illness, non‐thyroid surgery, trauma, and emotional stress, etc. Sympathetic symptoms, such as fever, profuse sweating, and tachycardia, are usually absent in older patients. Instead, more of them present with apathetic crisis, characterized by fatigue, but not high fever. The presentation of apathetic crisis can be varied as congestive heart failure, liver failure, acute abdominal pain, epilepsy, stroke, rhabdomyolysis, coma, and shock. Evaluation of thyrotoxis crisis is needed in elderly patients with multi‐organ involvement or in critical condition, especially when level of thyroid hormone is elevated. 37
4.4. Diagnosis and differential diagnosis
4.4.1. Diagnostic criteria
For overt hyperthyroidism, TSH concentrations are lower than the reference range and serum FT4 and/or FT3 concentration are above the reference range. With mild hyperthyroidism, FT4 and TT4 are still within the reference range, but T3 is elevated with suppressed TSH level, which is also known as “T3 hyperthyroidism.”
4.4.2. Etiological diagnosis
TRAb is one of the major indicators to distinguish the etiology of hyperthyroidism and to diagnose GD. Among newly diagnosed GD, 60%–90% are TRAb positive. The uptake of radioactive iodine increased and the time to peak is advanced in hyperthyroidism. On radionuclide imaging with 123I or 99mTc, GD presents as homogeneous distribution of radionuclide in the thyroid; TMNG as concentration of radionuclide in multiple spots asymmetrically; TA as concentrated radionuclide in one spot and decreased uptake in residual thyroid tissues. Ultrasonography is sometimes used as a cost‐effective scanning. The peak velocity of inferior thyroid artery is increased. Solitary or multiple nodules could be detected in cases of TMNG or TA.
4.4.3. Differential diagnosis
Hyperthyroidism should be distinguished from thyrotoxicosis derived from other causes, including destructive thyroiditis or overdose of thyroid hormone tablets. Radionuclide imaging is the major method for discrimination. Destructive thyroditis refers to release of preformed thyroid hormones via autoimmune mechanism includes subacute thyroiditis and painless thyroiditis. Uptake of radioactive 123I or 99mTc is low in the above conditions. The uptake is also reduced within 1–2 months after the administration of iodinated‐contrast medium or amiodarone. The measurement of inferior thyroid artery peak systolic velocity by Doppler ultrasound is also helpful to distinguish hyperthyroidism from destructive thyrotoxicosis.
4.5. Treatment
Overt hyperthyroidism has been associated with cardiovascular mortality, osteoporosis, and cognitive decline. Thus, interventions are demanded irrespective of age. There are three treatment options: antithyroid drugs (ATDs), radioactive iodine (RAI) ablation of the thyroid gland, and surgical thyroidectomy. For elderly patients with hyperthyroidism, it is very important to control adrenergic symptoms rapidly and avoid recurrence.
4.5.1. Antithyroid drugs
Indications
Antithyroid drugs, including methimazole (MMI) and propylthiouracil (PTU) available in China, are preferred as first‐line therapy for mild cases of senile Graves' hyperthyroidism without cardiovascular complications. ATDs can be administrated as long‐term maintenance therapy for senile TMNG patients with limited survival, or those who cannot follow radioiodine treatment. 38
Initial dose
Elderly patients with hyperthyroidism exhibit a mild increase in thyroid hormone. Besides, the side effects of ATDs are dose‐related. Therefore, it is suggested that the initial ATDs dose in the elderly not be set too high. The recommended daily dose of MMI is 5–20 mg or PTU 50–300 mg.
Duration of treatment
The standard duration of ATDs therapy for GD is 12–18 months. A meta‐analysis showed a higher remission rate and lower recurrence rate in elderly patients taking ATDs than that in young patients. 39 However, relapse rate of senile hyperthyroidism after the conventional duration of ATD treatment ranges from 40% to 60%. Long‐term maintenance therapy with low‐dose MMI can effectively improve the thyroid function with a high tolerance for older patients with frailty, recurrent GD symptoms, refusal to or with surgical contraindications, in case of cardiovascular side effects. 40
Adverse effects of ATDs
Mild adverse effects of ATDs include rash, pruritus, arthralgia, and gastrointestinal symptoms. Major rare toxic reactions include agranulocytosis and hepatitis/cholestasis, mostly within 3 months of ATDs initiation. Antineutrophil cytoplasmic antibodies (ANCA)‐positive vasculitis rarely occur in elderly patients. Patients taking ATDs should be closely monitored and be informed about the adverse drug reaction. Additionally, for elderly patients with cognitive impairment, written information about this serious adverse event and its associated symptoms should be provided for their families or guardians.
The incidence of agranulocytosis caused by ATDs ranges from 0.2% to 0.5%. Agranulocytosis is more likely and more fatal in older patients than in younger adults. According to a survey in the UK, the fatality rate of agranulocytosis and granulocytopenia caused by ATDs in patients over 65 years old is significantly higher than that in younger patients (13.8% vs 1.2%). 41 Whenever fever and/or pharyngitis is developed in patients on ATDs, a prompt differential blood count should be obtained. Once granulocytopenia is confirmed, ATDs should be discontinued; moreover, broad spectrum antibiotic and the granulocyte colony‐stimulating factor should be prescribed in symptomatic patients. In the setting of agranulocytosis induced by one ATD, switching to the other ATD is contraindicated. Baseline blood cell count before initiation of ATDs and routine cell count monitoring is recommended for early detection of asymptomatic granulocytopenia.
The most common feature of ATDs‐induced hepatotoxicity is elevated transaminase and bilirubin, while acute liver failure is a serious and rare manifestation. ATD‐induced hepatoxicity is more common in adults aged over 65 years. 42 Liver function test should be obtained immediately in patients who experience symptoms of liver injury including jaundice, acholic stool, or dark urine. Baseline liver enzymes and follow‐up monitoring is advised for all patients.
Drug interaction
Methimazole and PTU are both rapidly absorbed by the gastrointestinal tract. Then, 75%–80% of MMI is excreted in the urine in the form of prototype or metabolites, while 40% of PTU is detected in urine. MMI or PTU rarely interact with other drugs. 43
4.5.2. Radioactive iodine ablation
Indication
For senile hyperthyroidism with heart diseases such as atrial fibrillation, tachyarrhythmia, and heart failure, TMNG, and TA, the first therapeutic choice is radioactive iodine ablation (RAI). 9
Precautions
The elderly are insensitive to 131I; as a result, higher dose of 131I should be preferred for these patients. Transient thyrotoxicosis deterioration may occur within a short term after RAI. Studies have shown that thyroxine levels could increase by about 36% three weeks after RAI, and the risk of complications might increase even in asymptomatic elderly patients with hyperthyroidism. For patients with severe thyrotoxicosis, atrial fibrillation, cardiac dysfunction, pulmonary hypertension, lung disease, cerebrovascular disease, or uncontrolled hyperglycemia, it is recommended to take MMI and β‐adrenoceptor blockers (β‐blockers) before RAI and restart these drugs 3–7 days after RAI. For those unable to take ATDs due to serious side effects, lithium can control thyroid function to a certain extent without influencing the effectiveness of RAI. Permanent hypothyroidism might be developed after RAI treatment. Hence, thyroid function should be monitored after RAI, and thyroid hormone replacement should start in the early stages of hypothyroidism.
4.5.3. Surgery
Indication
Thyroidectomy is not preferred for elderly hyperthyroidism. Instead, it is the original treatment in patients with large goiters, and even associated obstructive symptoms, or in patients with suspected malignancy, or coexisting hyperparathyroidism. 44
Precautions
A high‐volume thyroid surgeon is preferred for thyroidectomy in elderly. Ideally, patients should be prescribed with ATDs and β‐blockers preoperatively to achieve a euthyroid state and cardiac rate control. The range of thyroid resection should be as thorough as possible to avoid hyperthyroidism or tumor recurrence.
4.5.4. Therapy of senile hyperthyroidism accompanied with special clinical manifestations
Cardiovascular events
Evaluation of cardiac function should be obtained via testing serum B‐type natriuretic peptide (BNP) or N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), electrocardiogram, echocardiography, and dynamic 24‐hour Holter. β‐adrenergic blockade is used in patients with moderate to severe hyperthyroidism to prevent or immediately control heart rate. Anticoagulation therapy is required in patients with atrial fibrillation. It is a challenge to treat congestive heart failure in elderly patients with hyperthyroidism. The priority is to maintain cardiac hemodynamics, controlling heart rate, and prevent thromboembolism during atrial fibrillation. Treatment modalities include immediate restoration of euthyroid function, control of heart rate by β‐blockers, diuretics to reduce volume overload, and management of hypokalemia.
Graves ophthalmopathy
Assessment of ophthalmopathy in routine clinical practice includes visual acuity, visual field, intraocular pressure, exophthalmos, fundus, extraocular muscle function, and optic nerve function. CT and MRI are used to evaluate involvement of extraocular muscle and to exclude exophthalmos due to other causes. For patients with active GO, smoking cessation is suggested, while RAI treatment is not recommended. Besides, euthyroidism should be maintained during treatment. For patients with active moderate to severe GO treated with high‐dose glucocorticoid, gastrointestinal‐protective agents, calcium, and vitamin D should be administered concomitantly. Bisphosphonates could be used for patients with osteoporosis.
Thyrotoxic crisis
Thyrotoxic crisis has a high mortality rate. Early detection and multidisciplinary collaboration are crucial. The therapeutic goal is to suppress overproduction of thyroid hormone and further release of thyroid hormone into circulation, block peripheral conversion of T4‐T3, control rising adrenergic tone, remove precipitants, and treat coexisting issues. ATDs should be administrated as soon as possible. The preferred medicine is PTU. The loading dose for PTU or MMI is 600 mg/d or 60 mg/d, respectively. An hour after the administration of ATDs, give five drops of supersaturated potassium iodine by mouth every 6 hours, or sodium iodide 1.0 g dissolved in 500 mL liquid intravenously at a total dosage of 1–3 g in the first 24 hours and then be reduced gradually after control of thyrotoxicosis. Glucocorticoids should be administered, such as intravenous dexamethasone 2 mg every 6–8 hours, or hydrocortisone 50‐100 mg every 6–8 hours. Patients without or having recovered from heart failure could be treated with propranolol 20–40 mg every 6 hours, or other selective β1‐blockers. Patients with heart failure are contraindicated. Therapeutic plasmapheresis should be considered if clinical improvement is not noted within initial treatment of ATDs, inorganic iodine, corticosteroids, or β‐blockers.
4.6. Prognosis
For hyperthyroidism patients aged over 65 years, who remained untreated for more than 6 months, the risk of cardiovascular events increased significantly even after controlling other risk factors such as hyperglycemia, dyslipidemia, and hypertension. 44 Sixty percent of patients with coexisting atrial fibrillation and new‐onset hyperthyroidism could restore normal heart rhythm while maintaining euthyroidism. However, the remission rate of senile hyperthyroidism is rather low. Atrial fibrillation is an independent risk of mortality in senile hyperthyroidism. A cohort study revealed that all patients who suffer atrial fibrillation during ATD therapy were aged over 65 years. 45 Another study showed that only one‐third of patients aged over 60 could restore normal left ventricular diastolic function with achieved euthyroid function.
4.7. Flowchart for diagnosis and treatment of senile hyperthyroidism
Figure 1.
-
■
Recommendation 7: The common causes of senile hyperthyroidism are GD, TMNG, and TA. Serum TRAb, thyroid ultrasound, and static radionuclide scan help determine the causes of thyrotoxicosis.
-
■
Recommendation 8: Elderly patients often present with apathetic hyperthyroidism, and are more prone to suffer with cardiovascular complications such as atrial fibrillation and cardiac dysfunction. The classic manifestations, like high fever, are usually absent in older adults with thyroid crisis, making the diagnosis challenging. Comprehensive geriatric assessment before and after treatment is recommended, to promptly detect frailty for subsequent individualized management.
-
■
Recommendation 9: ATDs are preferred for mild cases of senile Graves' hyperthyroidism with no cardiac complications. ATDs can also be used as long‐term maintenance therapy for senile TMNG or TA patients with limited survival or contraindicated for RAI therapy.
-
■
Recommendation 10: The initial ATDs administration in older adults have a lower dose requirement. The recommended daily dose of MMI is 5‐20 mg or PTU 50‐300 mg. ATDs should be prescribed for 12‐18 months, or serve as long‐term maintenance therapy in low‐dose regimes.
-
■
Recommendation 11: Severe adverse effects of ATDs include agranulocytosis and hepatoxicity, for which the elderly are at higher risks. Total blood cell count and differential blood count and liver function test should be monitored before and throughout ATDs administration. Verbal and written information about these severe adverse effects and precautions should be provided for patients and their families.
-
■
Recommendation 12: For Graves' hyperthyroidism, TMNG, and TA accompanied by cardiovascular changes, including atrial fibrillation, tachyarrhythmia, and heart failure, the first therapeutic modality is RAI. If necessary, to suppress thyrotoxicosis, MMI can be administrated before RAI and 3‐7 days after RAI.
-
■
Recommendation 13: Thyroidectomy is preferred for patients with large goiters, and even associated obstructive symptoms, suspected malignancy, or coexisting hyperparathyroidism.
-
■
Recommendation 14: thyroid crisis in elderly patients requires early detection and multiple modalities, including ATDs, inorganic iodide, glucocorticoids, β‐blockers, removal of precipitants, and management of other comorbidities.
FIGURE 1.
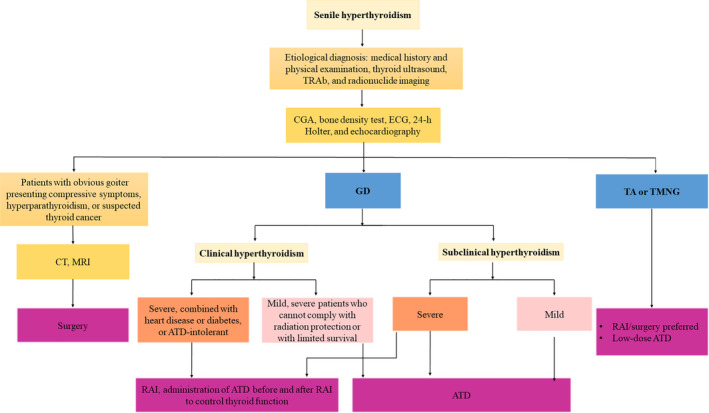
Flowchart for diagnosis and treatment of senile overt hyperthyroidism and subclinical hyperthyroidism. ATD, antithyroid drug; CGA, comprehensive geriatric assessment; CT, computed tomography; ECG, electrocardiogram; GD, Graves' disease; MRI, magnetic resonance imaging; RAI, radioactive iodine; TA, toxic adenomas; TMNG, toxic multinodular goiter
5. SENILE SUBCLINICAL HYPERTHYROIDISM
5.1. Definition
Subclinical hyperthyroidism refers to low or undetectable serum TSH with normal levels of serum FT4, TT3, and/or FT.
5.2. Classification
The classification schemes of senile subclinical hyperthyroidism are consistent with that in adults: (a) according to the serum TSH concentration, it can be divided into categories of mild (0.1 mU/L ‐ lower limit of normal) and severe (<0.1 mU/L). (b) according to the course of disease, it can be divided into categories of continuous (≥3 months) and temporary (<3 months). (3) according to the etiology, it can be divided into endogenous and exogenous types. Exogenous subclinical hyperthyroidism is caused by excessive replacement of levothyroxine or intake of iodine‐rich foods.
5.3. Etiology
Common causes of endogenous subclinical hyperthyroidism are identical with overt hyperthyroidism, including GD, TA, and TMNG. Exogenous subclinical hyperthyroidism often due to excessive replacement or TSH suppression by levothyroxine. Transient subclinical thyrotoxicosis commonly occurs during treatment of ATDs or RAI for hyperthyroidism, subacute thyroiditis or painless (silent) thyroiditis. In addition, a clinical history can distinguish subclinical hyperthyroidism from other causes of low TSH but unrelated thyroid overactivity, such as medication of certain drugs (dopamine, dobutamine, high‐dose glucocorticoids, bromocriptine, somatostatin analogs, gonadotropin‐releasing hormone analogs, amphetamine, and bexarotene, etc), pituitary or hypothalamic deficiencies, psychiatric diseases, and severe non‐thyroidal illness (sick euthyroid syndrome). 46
5.4. Clinical manifestations
-
Atypical or asymptomatic
The elderly with subclinical hyperthyroidism usually present asymptomatic or only non‐specific symptoms, such as fatigue and weakness, insomnia, palpitation, weight loss, and anorexia, all of which are superimposed with signs of neurasthenia or geriatric syndromes. Goiters might be observed, and attention should be paid to the presence of thyroid nodules or GO.
-
Cardiovascular effects
The absolute risk of cardiovascular events in elderly patients with subclinical hyperthyroidism increases markedly with aging, but the relative risk is not increased significantly. HUNT study evaluating a total of 26 707 participants showed that subclinical hyperthyroidism is associated with increased cardiovascular mortality. 47 The risk of cardiovascular events, especially atrial fibrillation, inversely correlates with TSH levels. 46 Based on a prospective observation study 48 and a Meta‐analysis, 49 subclinical hyperthyroidism is strongly associated with increased atrial fibrillation, arrhythmia, heart failure, major cardiovascular adverse events, cardiovascular mortality, and all‐cause mortality.
-
Osteoporosis and fracture
A meta‐analysis of 13 prospective cohort studies revealed severe subclinical hyperthyroidism (TSH <0.10 mIU/L) was associated with an increased risk of osteoporotic fracture, but no association was found between mild subclinical hyperthyroidism and fracture risk. 50 Subclinical hyperthyroidism results in an elevated risk of osteoporosis, which is often affected by sarcopenia, frailty, slowness, weakness, low activity, and the duration of subclinical hyperthyroidism. 28
-
Cognitive decline and dementia
Severe subclinical hyperthyroidism is associated with an increased risk of cognitive decline and dementia. 51 TEARS study demonstrated that endogenous subclinical hyperthyroidism was associated with an increased risk of dementia, but no evidence showed the association with serum TSH levels. 52
5.5. Diagnosis
Subclinical hyperthyroidism is diagnosed by laboratory tests as low or even undetectable TSH level with normal serum FT4, TT3, and/or FT3. It should be noted to find the causes and conduct risk assessments in elderly patients.
5.6. Treatment
The therapeutic methods of endogenous subclinical hyperthyroidism with various underlying etiologies are similar to that of overt hyperthyroidism. Individualized management is suggested based on stratification of severity of subclinical hyperthyroidism‐associated risks, which includes etiology, age and serum TSH level.
5.6.1. Indications
Antithyroid therapy is recommended for patients with severe subclinical hyperthyroidism.
For patients with mild subclinical hyperthyroidism, treatment could be considered if they have atrial fibrillation. It recommends considering treatment for patients with heart disease, diabetes, renal failure, history of stroke or transient ischemic attacks, or cardiovascular risks like atherosclerotic cardiovascular disease, heart failure, and valvular heart disease.
5.6.2. Treatment modalities
ATDs are preferred for patients over 65 years old with mild GD while TSH levels of 0.1‐0.4 mIU/L, while RAI is a definitive treatment for patients with ATDs intolerance, relapse, or heart disease.
ATDs or RAI is recommended for patients over 65 years old with subclinical hyperthyroidism due to GD when heart disease is present, who are at a higher risk of cardiovascular events.
For patients with TMNG or TA when low TSH is persistent, RAI or surgery is preferred. If patients are contraindicated to RAI, life‐long therapy of low‐dose ATDs therapy could also be considered.
Surgical Thyroidectomy is recommended for patients with large goiters and obstructive symptoms, suspected malignancy or coexisting hyperparathyroidism.
5.7. Prognosis
Senile subclinical hyperthyroidism may progress to overt hyperthyroidism or return to euthyroidism, or remained with a low TSH level. The risk of progression to clinical hyperthyroidism is related to the degree and duration of serum TSH suppression, etiology, age, and iodine ingestion.
The rate of progression to clinical hyperthyroidism inversely correlates with baseline TSH level. A 7‐year prospective follow‐up study found that only 0.5%‐0.7% of patients with mild subclinical hyperthyroidism progressed to clinical hyperthyroidism, while 25%‐50% returned to euthyroidism. About 5%‐8% of severe subclinical hyperthyroidism progressed to clinical hyperthyroidism annually. The 5‐year follow‐up data in China revealed that 71.74% of the subclinical hyperthyroidism returned to euthyroidism, 19.57% maintained the low TSH levels, and 5.43% developed into overt hyperthyroidism. Patients with low TSH level, positive TPOAb or goiter tend to progress to overt hyperthyroidism. 53
The disease course is less predictable by etiology, especially in subclinical hyperthyroidism caused by GD than TMNG. Progression to overt hyperthyroidism caused by GD often occurs within 1 year, and the progression rate is higher after stress. Patients with TMNG tend to have a lower remission. In iodine‐deficient areas, progression rate to overt hyperthyroidism within 4‐5 years is approximately 10%. The risk of progression increases especially after iodine exposure, such as the administration of iodine‐contrast agents or iodine‐containing drugs.
5.8. Flowchart for diagnosis and treatment of senile subclinical hyperthyroidism
Figure 1.
-
■
Recommendation 15: For the elderly with low level of serum TSH but normal levels of thyroid hormone, subclinical hyperthyroidism is diagnosed by repeated screening tests.
-
■
Recommendation 16: Risk assessment of cardiovascular effects, osteoporosis and fracture, cognitive decline and dementia should be performed in elderly patients with subclinical hyperthyroidism. Comprehensive geriatric assessment is recommended for early detection of frailty to guide individual intervention.
-
■
Recommendation 17: Individualized management is suggested based on stratification of severity of subclinical hyperthyroidism‐associated risks, which includes etiology, age, and serum TSH level.
-
■
Recommendation 18: Antithyroid therapy is recommended for elderly patients with severe subclinical hyperthyroidism. For patients with mild subclinical hyperthyroidism, treatment could be considered if necessary; it recommend considering treatment for patients with cardiovascular risks, including heart disease, diabetes and heart failure. The therapeutic options for endogenous subclinical hyperthyroidism are similar to those of overt hyperthyroidism.
6. SENILE HYPOTHYROIDISM
6.1. Definition
Hypothyroidism is a metabolic condition caused by inadequate production of thyroid hormone or inadequate action of thyroid hormone in target tissues. Hypothyroidism is mainly divided into overt hypothyroidism and subclinical hypothyroidism (SCH). The prevalence and incidence of hypothyroidism is related to the threshold of TSH range, age, sex, race, and other factors (see Chapter 2 and 3).
6.2. Etiology
Most hypothyroidism in the elderly (more than 99%) arise as primary from the thyroid gland. Chronic autoimmune (Hashimoto) thyroiditis is the most common cause of hypothyroidism, followed by antithyroid treatment with 131I or thyroid surgery.
Central hypothyroidism is rare in the elderly, mainly caused by a deficiency of TSH due to hypothalamic and/or pituitary diseases. Symptoms of central hypothyroidism might be ignored due to deficiencies of other pituitary hormones (mainly adrenocorticotropic hormone).
Drug‐induced hypothyroidism is relatively common in the elderly. It can be caused by ATDs, amiodarone, lithium, cytokines, or tumor‐targeted drugs. Amiodarone, an iodine‐rich antiarrhythmic drug, causes hypothyroidism in up to 20% of cases, which is the most common cause of drug‐induced hypothyroidism in the elderly.
Thyroid hormone resistance syndrome (RTH) is a type of hypothyroidism caused by impaired function of thyroid hormone in peripheral tissue.
6.3. Clinical manifestations
Hypothyroidism in the elderly is an insidious condition with slow progression. The symptoms and signs are often non‐specific, including cold intolerance, fatigue, dry skin, puffiness, drowsiness or insomnia, depression, impaired memory, gait instability, weight gain, constipation, joint and muscle pain, all of which resembles symptoms of frailty, cognitive or psychological disorders in the elderly. Hypothyroidism can lead to pericardial effusion and increased risk of cardiovascular diseases and is strongly correlated with morbidity and progression of heart failure. In addition, hypothyroidism will result in obstructive sleep apnea–hypopnea syndrome, anemia and renal dysfunction, aggravating chest distress, shortness of breath, and edema. Myxedema coma is an extremely life‐threatening complication of hypothyroidism, usually seen in elderly patients induced by coexisting diseases. Its main manifestations are lethargy, psychosis, stupor, coma, pale skin, hypothermia, bradycardia, respiratory failure, or heart failure, with poor prognosis and high mortality.
6.4. Diagnosis and differential diagnosis
6.4.1. Diagnosis
The diagnosis of hypothyroidism is based on laboratory test of serum levels of TSH and FT4. Overt hypothyroidism is diagnosed by an elevated TSH level and a decreased FT4 level. T3 is not a necessary indicator for the diagnosis as it mainly comes from the peripheral conversion of T4.
6.4.2. Differential diagnosis
Central hypothyroidism should be considered when serum TT4 and FT4 levels are below the lower reference limit but TSH level is reduced or inappropriately elevated. Imaging of the hypothalamus and pituitary gland and laboratory screening of other hypothalamus‐pituitary axis are required for patients in whom central hypothyroidism is suspected. Collection of medication history is important to screen suspected drug‐induced hypothyroidism as polypharmacy is common among elderly patients. Hypothyroidism in the elderly should be distinguished from frailty, cognitive impairment, depression, loss of appetite, constipation, or other gastrointestinal diseases.
6.5. Treatment
6.5.1. Pre‐treatment assessment
Comprehensive geriatric assessment before treatment of hypothyroidism is recommended in the elderly. Assessment of current cardiac situation and performing coronary revascularization when necessary are suggested in patients with angina pectoris before thyroid hormone replacement.
6.5.2. Treatment target for thyroid hormone replacement in the elderly
The therapeutic purpose is to relieve the symptoms and avoid progression to myxedema coma. The target TSH levels should be individualized based on age, the prevalence and risks of cardiac diseases, osteoporosis, and fracture. Patients aged between 60 and 70 years old, without evidence of cardiac diseases or related risks, maintain TSH levels above 50% of the upper limit of reference range, which is consistent with younger adults. The reference range of TSH for patients over 70 years should be 4–6 mU/L, and that of 6–7 mU/L for patients with high risk of arrhythmia or osteoporotic fracture.
6.5.3. Titration of levothyroxine therapy
Levothyroxine (L‐T4) is the mainstay of treating hypothyroidism. Initial dosage as low as 0.5–1.0 μg/kg/d and up‐titration at a slower pace are recommended for elderly patients, and the maintenance dose may also have to be lower. A starting dose of 12.5–25 μg/d and slower is recommended in those with ischemic heart diseases, in order to prevent induction of angina pectoris or aggravation of myocardial ischemia. The maintenance dosage should also be lower than younger adults. L‐T4 has a plasma half‐life of 7 days, and it is suggested to take L‐T4 1 hour before breakfast every morning with an interval of over 2–4 hours before administration of other drugs or food. Dose adjustments are required based on the monitoring indices as co‐administered food and concomitant medications may affect absorption. 54 Lifelong replacement is usually required, yet there are cases of spontaneous remission of hypothyroidism caused by Hashimoto's thyroiditis.
Consume of l‐triiodothyronine (L‐T3) alone is not recommended as an alternative replacement therapy. As Desiccated thyroid hormone derived from animals, it is unsuitable for elderly patients due to its unstable bioavailability and high T3 content.
6.5.4. Post‐treatment monitoring
The dose of L‐T4 can be titrated every 4–6 weeks based on monitored levels of TSH and FT4 function. Adjust dose in increments of 12.5–25 μg/d till clinically euthyroid or achieving target TSH levels. Once stabilized, evaluate biochemical response every 6 to 12 months.
6.5.5. Drug interactions
Absorption and effect of L‐T4 could be suppressed by concomitant intake of caffeine, calcium carbonate, ferrous sulfate, aluminum hydroxide, and magnesium hydroxide. In addition, bioavailability of L‐T4 would be enhanced by 25% when co‐administered with rifampicin. 43
6.5.6. Risk of overtreatment and management
L‐T4 replacement therapy is associated with iatrogenic thyrotoxicosis. Mortality is increased in both over‐ and under‐treatment, and the risk of mortality is higher with overtreatment. 54 Major adverse consequences of long‐term over‐treatment include atrial fibrillation, osteoporosis, sarcopenia, and frailty. Therefore, close monitor of thyroid function is required with initial L‐T4 replacement, as well as routine comprehensive evaluation of disease status, especially progression of myocardial ischemia, atrial fibrillation, heart failure, osteoporosis, sarcopenia, and frailty. To maintain the best function and life quality of elderly patients, the control target of TSH and the dosage of L‐T4 should be adjusted in time.
6.5.7. Treatment for myxedema coma
Myxedema coma must be treated promptly due to the severity and lethality. Treatment involves:
Removal of induced factors. Infection, which accounts for 35% of all the precipitants, needs to be treated with antibiotics.
Thyroid hormone replacement. Intravenous injection of T3 is preferred, followed by oral intake until patients are recovered from comatose with improved clinical signs. Lower dose should be considered for patients with coronary heart disease or arrhythmia. An initial L‐T4 dose of 200–300 μg administered intravenously should be followed by 50 μg administered intravenously daily until the patient is able to take oral replacement. Intravenous administration could be replaced by nasogastric feeding of L‐T4 powder.
Glucocorticoid supplementation. Hydrocortisone should be administered intravenously at a dosage of 200–400 mg daily intermittently for 3–7 days. Lastly, supportive treatment. It includes mechanical ventilation, passive warming, maintenance of electrocyte levels, correction of anemia, and stabilization of hemodynamics. Warming blankets should be avoided as the resulting peripheral dilation may cause and insufficient blood volume.
6.6. Euthyroid sick syndrome
Euthyroid sick syndrome (ESS), also known as low T3 syndrome or non‐thyroidal illness syndrome (NTIS), is characterized by low thyroid hormone level with a variety of nonthyroidal systemic illness, such as severe illness, starvation, malnutrition, major operation, or trauma. ESS is considered as an adaptive response to preserve energy, which is usually seen in the elderly, with the main feature of decreased FT3 and TT3 levels, elevated trans‐triiodothyronine (rT3) level, normal or slightly elevated TT4 level, and normal TSH level. The prevalence of ESS in the hospitalized elderly patients is 17.7%–31.86%, with 60%–90% of them in critical condition that is positively related to the mortality. 55 The severity of ESS is usually associated with the degree of TT3 reduction. Patients with severe illness may have decreased TT4 and FT4 levels but normal TSH level, which is also known as low T3‐T4 syndrome. Most patients can achieve normal TSH level after treating the underlying diseases. A transient increase of TSH may occur in recovery phase, which needs to be distinguished from primary hypothyroidism. Thyroid hormone replacement is not recommended for ESS.
6.7. Flowchart for diagnosis and treatment of senile hypothyroidism
Figure 2.
-
■
Recommendation 19: L‐T4 replacement is the mainstay of treating hypothyroidism in the elderly. Consume of L‐T3 or desiccated thyroid extract alone is not recommended as an alternative replacement therapy.
-
■
Recommendation 20: Comprehensive geriatric assessment before treatment of hypothyroidism is recommended in the elderly for early detection of frailty. Management should be individualized based on age, risks of cardiac diseases, osteoporosis, and fracture.
-
■
Recommendation 21: The therapeutic targets of hypothyroidism in the elderly should be individualized. Patients aged between 60 and 70 years, without evidence of cardiac diseases or related risks, maintain TSH levels above 50% of the upper limit of reference range. The reference range of TSH for patients over 70 years should be 4–6 mU/L, and that of 6–7 mU/L for patients with high risk of arrhythmia or osteoporotic fracture.
-
■
Recommendation 22: Initial dosage as low as 0.5–1.0 μg/kg/d and up‐titration at a slower pace of 12.5–25 μg are recommended for elderly patients. Initial dosage and titration should be even lower in elderly patients with ischemic heart disease.
-
■
Recommendation 23: Monitor of thyroid function required every 4–6 weeks and then 6–12 months intervals until euthyroidism.
-
■
Recommendation 24: Drug interaction of the risk of over‐treatment should be emphasized during L‐T4 replacement.
-
■
Recommendation 25: Thyroid hormone replacement is not recommended for elderly patients with ESS.
FIGURE 2.
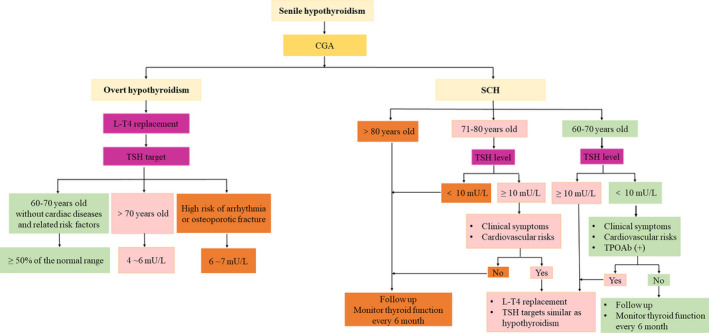
Flowcharts for diagnosis and treatment of senile overt hypothyroidism and subclinical hypothyroidism. CGA, comprehensive geriatric assessment
7. SENILE SUBCLINICAL HYPOTHYROIDISM
7.1. Definition
Subclinical hypothyroidism (SCH) is characterized by an elevated serum TSH level but normal T4 levels. SCH is classified as mild and severe based on TSH level. Mild SCH is more common in the elderly.
7.2. Etiology
Consistent with overt hypothyroidism, autoimmune thyroiditis is the major cause of SCH in the elderly, followed by prior 131I therapy or thyroid surgery.
7.3. Clinical manifestations
Signs and symptoms of SCH are usually non‐specific or absent, which may be confused with those of geriatric syndromes or mental disorders.
Patients with a higher TSH level (above 10 mU/L) are at higher risk of cardiovascular events, fracture, and mortality. A prospective cohort study targeting older patients aged between 70 and 82 years with SCH reported correlation between high TSH level (above 10mU/L) with increased incidence of heart failure. 56 A meta‐analysis also described an increased risk of heart failure in SCH patient with TSH level higher than 10 mU/L. 57 Waring et al. reported a higher risk of cardiovascular mortality in older participants with SCH only when TSH was above 10mU/L. 58 A retrospective study that included 162 369 participants with SCH described a higher risk of heart failure, all fractures, fragility fractures, and mortality with TSH above 10 mU/L in patients aged over 65 years old. 59 A recent meta‐analysis included five observational studies and two randomized controlled trials with 21 055 subjects. Subgroup analyses showed that thyroid hormone therapy was not significantly associated with all‐cause or cardiovascular mortality aged over 65–70 years old. However, a significant association between thyroid hormone therapy and a lower all‐cause or cardiovascular mortality aged under 65–70 years old was observed. 60
The influence of SCH on cognitive function and quality of life among elderly patients remains unclear. A study conducted by Aubert et al. of participants with SCH aged 70–79 years old, demonstrated no relationship between SCH and the risk of dementia and cognitive impairment. 51 Another meta‐analysis found that the association between SCH and cognitive impairment only existed in participants under the age of 75 years, and the risk of cognitive impairment was positively correlated with the increment of TSH level. 61 Additionally, a cross‐sectional study of participants with SCH aged over 65 years old showed that there were no significant differences between SCH and euthroidism in symptoms (fatigue, constipation, weight gain, lack of energy), cognition, neuropsychological function, emotion, and quality of life. 62 , 63 Another study reported that higher quality of life in elderly patients with SCH than in those with euthyroidism. 64 , 65
7.4. Diagnosis and differential diagnosis
7.4.1. Diagnosis
The diagnosis of SCH is assigned after repeated measurement of elevated TSH level but normal TT4 and FT4 levels within 2–3 months. A level of TSH between the upper reference limit and 10 mU/L is a mild SCH, while levels above 10 mU/L represent severe SCH.
7.4.2. Differential diagnosis
An elevation of TSH level could due to other causes, which requires discrimination: (a) False‐positive results due to thyroid autoantibodies; (b) a transient elevation in the recovery stage of ESS; (c) about 20% of patients with central hypothyroidism have a mild increase of TSH level; (d) about 10.5% of patients with end‐stage renal disease have an elevated TSH level; (e) glucocorticoid deficiency may lead to a mild increase of TSH level. (f) adaptive response to long‐term cold exposure (9 months), which raises TSH level by 30%–50%; (g) recovery phase of subacute thyroiditis; (h) drugs, including amiodarone and lithium.
7.5. Prognosis
The outcomes of SCH includes spontaneous euthyroidism, maintenance of SCH condition, and progression to overt hypothyroidism. Baseline level of TSH and TPOAb are major factors affecting the outcomes of SCH. A study from Japan reported that 50% of elderly patients with SCH regained normal TSH levels, and 7% of them progressed to overt hypothyroidism after a mean follow‐up of 4.2 years. It also showed that euthyroidism was not achieved in patients whose TSH level was above 8 mU/L. 66 Another cohort study showed that 46% of patients with SCH regained a normal TSH level with a baseline TSH of 4.5–6.9 mU/L, while only 7% of patients with SCH achieved euthyroidism when baseline TSH was above 10 mU/L after 2 years. 67 It was also noticed that positive TPOAb strongly correlates with high risk of progression to overt hypothyroidism.
7.6. Treatment
7.6.1. Treatment controversies
There is controversy surrounding the benefit of thyroid hormone replacement in elderly patients with SCH on cardiovascular risks. In a randomized, controlled, double‐blind study on elderly patients with SCH after median treatment of L‐T4 for 18.4 months, no differences were observed in the average thickness of carotid intima media, minimum carotid intima‐media and maximum carotid plaque between the treatment group and the placebo group. 68 In another randomized controlled study on patients with SCH over 40 years old with a median follow‐up of 7.6 years, L‐T4 treatment was reported to correlate with lower risks of ischemic heart disease events, while no evident association was observed in patients over 70 years old. 69
It also remains controversial whether cognitive function will be benefited from thyroid hormone therapy. A randomized controlled trial recruited 94 patients with SCH aged over 65 years reported no significant improvement of cognitive function in patients receiving L‐T4 replacement for 12 months. 70 In TRUST study, 737 patients with SCH (TSH: 6.40 ± 2.0 mU/L) aged over 65 years were included. No beneficial effects were seen on the Hypothyroid Symptoms score and Tiredness score with a median follow‐up of 1 year. 71 So far, most of the studies suggested L‐T4 provided no apparent benefits in cognitive function and symptoms in elderly people with SCH.
7.6.2. Risk of overtreatment
L‐T4 replacement is associated with iatrogenic thyrotoxicosis, especially in elderly persons with SCH. The principal adverse consequences of long‐term over‐treatment are new‐onset atrial fibrillation, heart failure, osteoporosis, all‐cause and cardiovascular mortality. 71 , 72 It can lead to the decline in activities of daily living, mobility/balance ability, comprehension/interaction (including cognitive function). Besides, it can also cause psychological and emotional disorders such as depression and anxiety, the progression of malnutrition, sarcopenia and frailty, and a lower quality of life. 24 , 25 , 26 , 27 , 28 , 29 , 30
Grossman et al. 73 found that L‐T4 treatment significantly increased the mortality of individuals aged over 65 years with SCH. Jabbar et al. 74 reported that L‐T4 treatment had no significant benefit on left ventricular ejection fraction in elderly patients with SCH and coexisting acute myocardial infarction. Benefits of L‐T4 replacement remain unclear, while adverse effects of over‐treatment are noted. Therefore, therapeutic decisions in elderly persons with SCH need to be made with caution. Comprehensive geriatric assessment is especially required regarding the progression of atrial fibrillation, osteoporosis, sarcopenia, frailty, and malnutrition. The control target of TSH and the dosage of L‐T4 should be adjusted promptly.
7.6.3. Therapeutic decisions
Individualized L‐T4 replacement for elderly patients with SCH should be considered based on the degree of TSH elevation, age, life expectancy, potential risks, and comorbidity.
7.7. Flowchart for diagnosis and treatment of senile SCH
Figure 2.
For elderly patients with SCH over 80 years old, no benefits were proved with L‐T4 treatment, and elevated TSH level was even associated with lower mortality in some studies. 25 , 75 Therefore, routine replacement is not recommended. Instead, monitor of thyroid function every 6 months is advised.
For elderly patients with SCH aged 70–80 years old, it is accepted that those with TSH levels above as well as with clinical symptoms and cardiovascular risks, should be treated. Conversely, a close follow‐up and thyroid function examination is recommended in patients whose TSH is below 10 mU/L.
For elderly patients with SCH aged between 60 and 70 years but TSH levels below 10 mU/L, treatment is only considered when they have clinical symptoms, cardiovascular risks, or positive TPOAb. Otherwise, routine monitor of thyroid function is recommended every 6 months. If no clinical improvements observed or even adverse effect developed after 3–4 months later once achieving euthyroidism, treatment should be terminated gradually. L‐T4 replacement is recommended in those with TSH levels above 10 mU/L, L‐T4 replacement is recommended as those with overt hypothyroidism.
Diagnosis and treatment process of SCH for elderly patients, as shown in Figure 2.
-
■
Recommendation 26: The diagnosis of SCH is assigned after repeated measurement of elevated TSH level but normal TT4 and FT4 levels within 2‐3 months.
-
■
Recommendation 27: Elderly patients with a higher TSH level (above 10 mU/L) are at higher risk of cardiovascular events. However, the benefits of L‐T4 replacement on cardiovascular outcomes and cognition in elderly patients with SCH remains unclear.
-
■
Recommendation 28: Therapeutic decisions in elderly persons with SCH need to be made with caution. Comprehensive geriatric assessment is especially required for early detection of frailty. Individualized L‐T4 replacement for elderly patients with SCH should be considered based on the degree of TSH elevation, age, life expectancy, potential risks, and comorbidity.
8. THYROID NODULES AND DIFFERENTIATED THYROID CARCINOMA IN THE ELDERLY
8.1. Definition
Thyroid nodule is defined as an abnormal growth of thyroid cells that forms a lump within the thyroid gland. Thyroid nodules are determined as benign or cancerous. The incidence of malignant nodules in the elderly is about 10%, equal to or lower than that in the young. Benign nodules include nodular goiter, thyroid cyst, follicular adenoma, and eosinophilic adenoma. Thyroid follicular carcinoma include a couple of pathological types: papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), Hurthle cell carcinoma (HCC) and poor differentiated/anaplastic thyroid carcinoma (PDTC or ATC), the first three of which are collectively known as differentiated thyroid carcinoma (DTC). There are also medullary thyroid carcinoma (MTC) originating from parafollicular cells, lymphoma and other metastatic cancers.
8.2. Etiology and risk factors
According to the epidemiological study of thyroid diseases in 31 provinces and cities in China, iodine deficiency is a risk factor for thyroid nodules. The incidence of thyroid nodules in women is significantly higher than that in men. Age, being overweight, and family history are also risk factors for thyroid nodules. 1 The following signs suggest increased risk of malignancy in thyroid nodules: history of head and neck radiotherapy; family history of medullary thyroid carcinoma, multiple endocrine neoplasia type 2(MEN2) or thyroid papillary carcinoma; age <14 years old or >70 years old; male; a rapidly growing nodule with firm, swelling, and fixed feature; persistent hoarseness, dysphagia, or dyspnea.
The histologic subtypes of thyroid carcinoma change with age. Lin et al. 76 A retrospective analysis conducted by Lin et al. 76 (n = 204) reported 68% DTC, 29% other types (including ATC, lymphoma, and metastatic cancer from other sources). Further among DTC, PTC accounts for only 68%, FTC takes up 30%, and the other 2% is HCC. Girardi et al. 77 analyzed 596 cases of adult thyroid carcinoma. Compared with other age groups, the proportion of PTC in patients over 65 years with thyroid cancer was the lowest (71.9%), and the proportion of FTC, MTC and ATC were the highest among all age groups (12.3%, 5.6%, and 7.8%, respectively). In addition, compared with younger adults, older patients aged over 65 have larger tumor volume with higher proportion of stage IV, and higher proportion of extra glandular invasion and non‐PTC histological types. 78 In 2018, the SEER (The National Cancer Institute's Surveillance Epidemiology and End Results) database tracks survival rates of 59 892 patients and concluded factors contributing to poor prognosis in group over 65 years, including higher proportion of male gender, advanced tumor grade, FTC subtypes and advanced tumor stages. 79
8.3. Clinical manifestations
Most patients with thyroid nodules have no symptoms. Symptomatic patients usually present hoarseness due to the obstructiveness by nodules.
8.4. Laboratory and auxiliary diagnosis
8.4.1. Laboratory parameters
(1) TSH: Studies have confirmed that elevated serum TSH levels are associated with thyroid malignancies. TSH levels should be detected in all elderly patients with thyroid nodules. The possibility of autonomously functioning thyroid nodules should be considered when TSH is suppressed.
(2) Thyroglobulin (Tg), TgAb, TPOAb: Positive TPOAb increased the risk of malignancies of thyroid nodules. 80 Elevated Tg levels do not help to distinguish benign from malignant thyroid nodules. Tg and TgAb can predict the recurrence of DTC. It should be noted that measurement of Tg levels might be affected by positive TgAb.
(3) Calcitonin: Calcitonin should be measured when medullary thyroid carcinoma or type 2 multiple endocrine neoplasia type 2 is suspected, or when there is a relevant family history.
8.4.2. Imaging parameters
(1) Ultrasonography: Ultrasonography with high resolution is preferred in assessment of thyroid nodules. Malignant nodules are indicated by sonographic patterns of hypoechogenicity, border irregularity, microcalcification, taller‐than‐wide shape (anteroposterior/transverse diameter ratio ≥1), and abnormal cervical lymph nodes are patterns. Thyroid imaging reporting and data system (TI‐RADS) is now widely used to report the classification of thyroid nodules.
(2) Others: Thyroid scintigraphy provides functioning information for assessment of nodules >1 cm in diameter with low TSH levels. The significance of CT and MRI are not superior to ultrasound in distinguishing between benign and malignant thyroid nodules. The role of preoperative CT or MRI scanning is to visualize the contour of thyroid against its surrounding tissues as well as to localize suspicious nodes. If necessary, CT scanning with contrast is optional. 18FDG‐PET cannot accurately distinguish between benign and malignant nodules. Therefore, CT, MRI and PET are not routinely recommended for the evaluation of thyroid nodules.
8.4.3. Fine‐needle aspiration cytology
FNAB has become a routine method for detecting malignancies of thyroid nodules. The Bethesda system for reporting thyroid cytopathology (TBSRTC) is widely employed to guide cytological diagnoses. Current research shows that fine needle aspiration of thyroid nodules (≥1 cm) in the elderly over 70 years is safe and beneficial to identify malignant nodules. Only 1.5% of the results are confirmed as high‐risk thyroid carcinoma. 81 Therefore, cytology is cost‐effective and devoid of unnecessary thyroid surgery.
8.4.4. Molecular marker test
For thyroid nodules that cannot be determined by FNAB, molecular markers of thyroid carcinoma can be detected by the puncture specimens. Current studies have shown that high specificity of BRAFV600E mutation in the diagnosis of adult PTC. BRAFV600E mutation is also present in a small number of patients with ATC, which are more common in older adults than in the general population.
8.5. Flowchart for diagnosis and management of thyroid nodules and differentiated thyroid carcinoma in the elderly
Figure 3.
FIGURE 3.
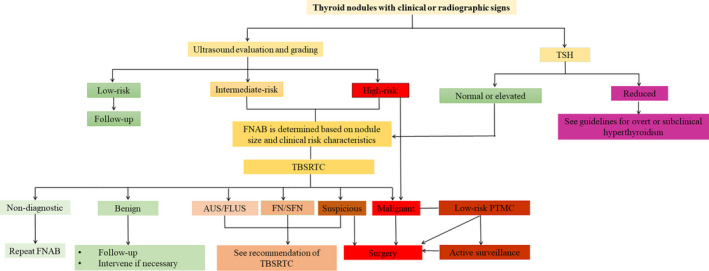
Flowchart of clinical diagnosis and treatment of thyroid nodules and differentiated thyroid carcinoma in the elderly. AUS, atypia of undetermined significance; FLUS, follicular lesion of undetermined significance; FN, follicular neoplasm; FNAB, fine needle aspiration biopsy; PTMC, papillary thyroid microcarcinoma; SFN, suspicious for follicular neoplasm; TSH, thyrotropic hormone
8.6. Treatment for benign thyroid nodules
8.6.1. Follow‐up
Elderly patients with benign thyroid nodules can be followed up for a long time. The general follow‐up period is 12 years. 82 , 83 During the follow‐up, FNAB should be considered if the nodule grows rapidly significantly or presents new sonographic patterns of malignancies.
8.6.2. Non‐surgical treatment
(1) TSH suppression therapy: L‐T4 is generally used to suppress TSH levels and then to reduce the size of nodules. Data showed that only 5%–10% of the nodule decreased in volume by more than 75% after L‐T4 treatment, and about 30% of the nodules had a 50% reduction in volume. Overt or subclinical thyrotoxicosis induced by long‐term TSH suppression by L‐T4 may lead to side effects, including osteoporosis and arrhythmias. Therefore, TSH suppression is not routinely recommended in therapies of benign thyroid nodules in the elderly.
(2) Percutaneous ethanol injection (PEI): PEI is a safe and effective technique for thyroid cysts or cystic‐solid nodules that mainly consist of cysts. 83 , 84 FNAB should be performed on solid components of cystic‐solid lesions before PEI. The common side effects are pain, transient dysphonia, dizziness, fever, and intracystic hemorrhage.
(3) Thermal ablation: Thermal ablation mainly includes laser ablation, radiofrequency ablation, microwave ablation, and high‐energy focused ultrasound, etc. Laser or radiofrequency ablation is recommended in patients with progressively growing solid/mixed nodules that can cause compression symptoms and/or cosmetic concerns, but not in asymptomatic patients. Domestic studies on elderly patients have found that ultrasound‐guided laser ablation or microwave ablation is safe and cost‐effective for thyroid nodule. 84 , 85 However, thermal ablation is contraindicated in elderly patients with retrosternal goiter, contralateral vocal cord dysfunction, hemorrhagic tendency, or cardiovascular insufficiency. The incidence of treatment complications is very low, and major side effects include mild to moderate neck pain and/or burning sensation, fever, hematoma, transient dysphoria, skin burns, and temporary thyroid dysfunction.
(4) Radioiodine therapy: Radioiodine therapy is considered for elderly patients with autonomic functioning nodules and toxic nodular goiter. Its purpose is to eliminate autonomic functioning lesions, restore euthyroidism, and reduce goiter in size. Radioiodine therapy is safe. More than 5% patients developed transient thyrotoxicosis 2–4 weeks after radioiodine treatment. After 20 years of follow‐up, up to 60% of the patients may develop hypothyroidism, which is related to radioiodine dose, responsiveness to radioiodine, and autoimmune thyroiditis.
8.6.3. Surgery
Surgical indications for benign thyroid nodules include 86 : ① The presence of local compression‐related symptoms due to nodules; ② The nodules extend in the mediastinum or posterior to the sternum; ③ The nodules are growing progressively with suspicion of malignancy. To reduce the incidence of complications related to surgery and anesthesia, comprehensive preoperative assessment and cardiovascular risk stratification are recommended for adults over 70 years old, which will provide a basis for individualized management of surgery, anesthesia and cares. 22 , 87
8.7. Treatment for differentiated thyroid carcinoma
8.7.1. Active surveillance
Active surveillance can be an alternative to immediate surgery in some elderly patients with low‐risk DTC, especially those with low‐risk papillary thyroid microcarcinoma (PTMC). In 2014, Ito et al. 88 conducted an observation analysis on 1235 diagnosed with low‐risk PTMC who adopted active surveillance for 18–227 months (average 75 months). They reported lower proportion of older patients progressed. e. The rates of tumor enlargement, newly developed lymph nodes metastasis, and clinical disease progression were 2.2%, 0.4%, and 1.6%, respectively, in patients aged over 60 years, which were significantly lower than other age groups. Tuttle et al. 89 found younger age (<40 years old) at diagnosis was correlated with tumor growth rates of patients diagnosed. A recent meta‐analysis, including five studies from Japan, South Korea, and the United States, showed that the risk of tumor enlargement and lymph node metastasis of patients with PTMC diagnosed over 40 years old under active surveillance were lower than that of patients younger than 40 years. 90 Overall, the progression rate of low‐risk PTMC in the elderly is lower than that in adults. Therefore, older patients with PTMC are good candidates for active surveillance. Due to poor prognosis in older patients with DTC, patients with relevant family history, lymph nodes metastasis, extrathyroidal invasion or aggressive histology should be excluded, while information on consent should be provided for patients and their guardians. The initial observation period of active surveillance is 3–6 months, and then the follow‐up period is determined by disease condition. Patients under active surveillance may still have surgery as an option after progression.
8.7.2. Surgery
Individualized assessment of comorbidity and life expectancy in elderly patients with DTC is required for consideration of surgery and its extent. Key factors that determine the benefits and risks of this approach include biological features of thyroid carcinoma, course of disease, indications and contraindications for surgery, surgeon experience, and expectations from patient.
A meta‐analysis showed significantly higher risk of postoperative recurrence, distant metastasis, and postoperative complications in elderly patients with DTC. 91 Therefore, total thyroidectomy is a reasonable option for most elderly patients with DTC. SEER data analysis showed that the 5‐ and 10‐year disease‐specific survival (DSS) rate of patients aged 66 years or older with stage T1N0M0 who received total thyroidectomy and lobectomy were 98.9% and 98.3%, respectively, and neither total thyroidectomy or 131I treatment could improve DSS. 92 Therefore, lobectomy is preferred for older patients with low‐risk DTC, especially those with poor overall status and tumor size <2 cm.
8.7.3. Radioiodine ablation
131I therapy should be considered for DTC patients at medium and high risk of recurrence after surgery. The indications for elderly patients are similar to those for younger adults. It should be noted physiologically gradual decline in renal function may affect 131I clearance. Radioiodine dose usually remains during adjuvant therapy. However, lower dose should be considered over the treatment of metastatic disease, which requires high dose of131I. Elderly patients with DTC usually have a good tolerance to 131I therapy but may have the risk of nausea, salivary gland dysfunction, and secondary tumor.
8.7.4. TSH suppression therapy
(1) TSH goals: Thyroid hormone therapy is generally required after surgery to avoid hypothyroidism and to prevent recurrence of DTC by suppressing TSH. The course of TSH suppression therapy is divided into initial and follow‐up stage based on TSH goals. In 2015, the American Thyroid Society (ATA) set TSH control goals of 0.5–2.0, 0.1–0.5 and <0.1 mU/L, respectively, according to the low, medium, and high risk of recurrence after DTC operation. 83 Before starting L‐T4 therapy, we should evaluate the cardiac function and bone mineral density status of elderly DTC patients, as well as perform the comprehensive geriatric assessment. Postoperative TSH <0.35 mU/L is significantly associated with higher risk of arrhythmias in elderly DTC. Mazziott et al. 93 found that during L‐T4 treatment, prevalence of vertebral fracture was significantly higher in patients with TSH <0.5 mU/L than patients with TSH at 0.5–1 or >1 mU/L. Prevalence of side effects are even higher in patients with coexisting arrhythmia or osteoporosis. According to the dual‐risk assessment system, TSH at the initial treatment stage could be maintained at or slightly below the lower limit of the reference range. For low‐risk elderly patients, postoperative L‐T4 replacement may be the only therapy required. In the long‐term course, risk of tumor growth/recurrence should be assessed dynamically based on response to therapy, and concurrently risks of TSH suppression‐related side effects need to be taken into consideration, for purpose of TSH goals adjustment.
8.7.5. Postoperative evaluation
Postoperative risk stratification for DTC includes dynamic assessment of risk of death from thyroid cancer, risk of persistence/recurrence, and response to therapy. Since the age at diagnosis of DTC is an independent predictor of DSS, which affects the risk of postoperative mortality, the American Joint Committee on Cancer (AJCC) TNM staging raised the cut‐off age from 45 years old to 55 years old. 94 Tam et al. 95 showed that the 10‐year DSS of DTC patients ≥55 years old was 93%, and the risk of cancer‐related death was four times that of DTC patients <55 years old. A retrospective study of 1235 PTMC patients conducted by Wang et al. showed that patients over 65 years old have poorer recurrence‐free survival. Age, central cervical lymph node metastases, and tumor >5 cm in diameter are independent risk factors for PTMC recurrence in the elderly. 96 Once the elderly DTC patients are identified with local recurrence and/or distant metastases, the risk of tumor‐specific mortality can reach 20%. Therefore, the risk of disease persistence/recurrence after initial treatment should be evaluated according to the degree of local invasion, aggressive histology, local or distant metastases, surgical modalities, postoperative serum Tg level, and whether or not to undergo remnant ablation.
8.7.6. Postoperative follow‐up
Similar to adult patients, serum markers (Tg, TgAb) and imaging methods (neck ultrasound, scintigraphy, CT, MRI, and PET‐CT) are adopted for routine monitoring of DTC in older patients during long‐term follow‐up. Highly sensitive Tg assay makes Tg a good candidate to assess response to therapy. Routine monitor of Tg and/or TgAb are recommended 3–4 weeks after operation or 131I treatment, to evaluate the risk of tumor recurrence. Patients with non‐stimulated Tg above 0.2 ng/mL, stimulated Tg above 2–5 ng/mL, continued elevated Tg or TgAb under TSH suppression, are indicated for indicating further evaluation. In this case, diagnostic scanning, like neck ultrasound, scintigraphy, or PET‐CT is required for detection of remnant thyroid or incomplete surgical resection.
-
■
Recommendation 29: For elderly patients with thyroid nodules, collection of detailed medical history and complete physical examination are required, as well as serum assay of TSH and neck ultrasound.
-
■
Recommendation 30: For elderly patients with low TSH levels, scintigraphy is considered to exclude autonomously functioning thyroid adenoma.
-
■
Recommendation 31: For elderly patients with normal or elevated TSH levels, ultrasound and cytology should be considered. Meanwhile, cytology is interpreted based on the Bethesda System.
-
■
Recommendation 32: For elderly with benign nodules, routine monitoring is preferred. The frequency of follow‐up is determined regarding the ultrasound pattern, symptoms, and signs. TSH suppression therapy should be avoided.
-
■
Recommendation 33: For elderly with thyroid nodules comprised of purely cyst or major cystic component, PEI is recommended. Ultrasound‐guided thermal ablation is considered in patients with benign nodules that cause compression symptoms and/or cosmetic concerns and/or recurrence.
-
■
Recommendation 34: Elderly patients with benign thyroid nodules that cause obstructive symptoms or extend in the mediastinum or posterior to the sternum, or are suspicious for malignancy, are surgical candidates.
-
■
Recommendation 35: Comprehensive geriatric assessment and evaluation of survival expectancy, and individualized risk assessment of DTC should be emphasized before treating DTC elderly.
-
■
Recommendation 36: Limited numbers of PTMC patients could adopt active surveillance by high‐volume medical team after evaluation of benefits and risks.
-
■
Recommendation 37: Total thyroidectomy is preferred for most elderly patients with DTC. Some low‐risk DTC patients or poor health condition may choose lobectomy.
-
■
Recommendation 38: Radioiodine remnant ablation after thyroidectomy in DTC elderly is considered based on recurrence risk. Assessment of renal function is required before 131I treatment. Dose of therapeutic 131I could be reduced in high‐dose therapy.
-
■
Recommendation 39: Postoperative TSH goals in DTC elderly are based on a dual‐risk assessment, namely the risk of persistence/recurrence and the risk of side effects to TSH inhibition. Geriatric comprehensive assessment is required before and after treatment, for early detection of frailty. Cardiovascular status and bone mineral density should be followed up, and TSH levels could be maintained at or slightly below the lower limit of the reference range if necessary.
-
■
Recommendation 40: Levels of Tg and TgAb should be routinely monitored in elderly patients with DTC after surgery to evaluate response to therapy.
9. CONFLICTS OF INTEREST
Nothing to disclose.
APPENDIX 1.
Academic Board
Chairman: Youshuo Liu (Endocrine Metabolic Diseases Group of the Chinese Geriatrics Society), Zhongyan Shan (Thyroid Group of the Chinese Society of Endocrinology,Chinese Medical Association)
Geriatric Consultant (Listed in alphabetic order by last name in Chinese Pinyin): Xiao‐Ying Li (Department of Geriatric Cardiology, the Second Medical Center, Chinese PLA General Hospital); Er‐Yuan Liao (Hunan Kangya Hospital); Jian‐Ye WANG (National Geriatric Center of Beijing Hospital); Pu‐Lin Yu (National Geriatric Center of Beijing Hospital); Cun‐Tai Zhang (Department of Geriatrics, Tongji Hospital, Tongji Medical College of HUST)
Endocrinology Consultant (Listed in alphabetic order by last name in Chinese Pinyin): Wei‐Ping Teng (Institute of Endocrinology, China Medical University); Jia‐Jun Zhao (Department of Endocrinology, Affiliated Provincial Hospital of Shandong First Medical University); Zhi‐Guang Zhou (National Clinical Research Center for Metabolic Diseases (the Second Xiangya Hospital of Central South University))
Writing Group (Geriatrics): You‐Shuo Liu (Department of Geriatrics/Department of Geriatric Endocrinology, the Second Xiangya Hospital of Central South University); Guo‐Xian Ding (Department of Geriatrics/Department of Geriatric Endocrinology, the First Affiliated Hospital with Nanjing Medical University (Jiangsu Province Hospital)); Zhen Liang (Department of Geriatrics, Shenzhen People's Hospital); Jun‐Kun Zhan (Department of Geriatrics/Department of Geriatric Endocrinology, the Second Xiangya Hospital of Central South University); Yan‐Jiao Wang (Department of Geriatrics/Department of Geriatric Endocrinology, the Second Xiangya Hospital of Central South University); Jie‐Yu He (Department of Geriatrics/Department of Geriatric Endocrinology, the Second Xiangya Hospital of Central South University); Shuang Li (Department of Geriatrics/Department of Geriatric Endocrinology, the Second Xiangya Hospital of Central South University).
Writing Group (Endocrinology): Zhong‐Yan Shan (Department of Endocrinology and Metabolism, the First Hospital of China Medical University); Zhao‐Hui Lv (Department of Endocrinology, the First Medical Center, Chinese PLA General Hospital); Yu‐Shu Li (Department of Endocrinology and Metabolism, the First Hospital of China Medical University); Hai‐Qing Zhang (Department of Endocrinology, Affiliated Provincial Hospital of Shandong First Medical University); Shu‐Hang Xu (Department of Endocrinology, Affiliated Hospital of Integrated Traditional Chinese and Western Medicine of Nanjing University of Chinese Medicine)
Secretaries: Yi Wang (Department of Geriatrics/Department of Geriatric Endocrinology, the Second Xiangya Hospital of Central South University); Wei‐Wei Wang (Department of Endocrinology and Metabolism, the First Hospital of China Medical University)
Members of geriatrics expert group (Listed in alphabetic order by last name in Chinese Pinyin): Mei Cheng (Department of geriatrics/Department of Geriatric Endocrine, Qilu Hospital, Shandong University); Cai‐Xia Cao (Department of Geriatric Endocrinology, Affiliated Hospital of Medical College of Qingdao University); Xiao‐Pei Cao(Department of Endocrinology, the First Affiliated Hospital of Sun Yat‐sen University); Ping Chen (Department of Geriatrics, Sichuan Provincial People's Hospital); Shu‐Zhi Feng (Department of Geriatrics, General Hospital of Tianjin Medical University); Ji‐Rui He (Department of Geriatrics, the Second Hospital of Lanzhou University); Wu Huang (Department of Geriatrics/Department of Geriatric Endocrinology, the Second Xiangya Hospital of Central South University); Dong‐Mei Kang (Department of Geriatrics, the First Affiliated Hospital of USTC (Anhui Provincial Hospital)); Jian Kong (Department of Geriatrics, Bethune First Hospital of Jilin University); Cai‐Ping Li (General Medical Department, Tongji Hospital, Tongji Medical College of HUST); Hua‐Hua Li (Department of Geriatrics, Mawangdui District, Hunan Provincial People's Hospital); Gan‐Xiong Liang (Department of Endocrinology, Zhongshan People's Hospital); Ming‐Zhu Lin (Department of Endocrinology, the First Affiliated Hospital of Xiamen University); Juan Liu (Department of Geriatrics/Department of Geriatric Endocrinology, the First Affiliated Hospital with Nanjing Medical University (Jiangsu Province Hospital); Hui‐Xia Liu (Department of Geriatrics/Department of Geriatric Endocrinology, Xiangya Hospital, Central South University); Li‐Bin Liu (Department of Endocrinology, Union Hospital, Fujian Medical University); Yu Liu (Department of Endocrinology, Sir Run Run Hospital, Nanjing Medical University); Zheng‐tang Liu (Department of Endocrinology, Xiyuan Hospital, China Academy of Chinese Medical Sciences); Lan Luo (Department of Geriatrics, Affiliated Hospital of Nantong University); Hui Ma (Department of Geriatrics, Zhongshan Hospital, Fudan University); Hui‐Juan Ma (Department of Endocrinology, Hebei Provincial People's Hospital).
Members of endocrinology expert group (List in alphabetic order by last name in Chinese Pinyin): Xiao‐Pin Cai (Department of Endocrinology, China‐Japan Friendship Hospital); Zhi‐Feng Cheng (Department of Endocrinology and Metabolism, The Fourth Affiliated Hospital of Harbin Medical University); Xiao‐Yun Feng (Department of Endocrinology & Metabolism, Shanghai General Hospital, Shanghai Jiaotong University); Ying Gao (Department of Endocrinology, Peking University First Hospital); Hong‐Yu Guan (Department of Endocrinology and Diabetes Center, The First Affiliated Hospital of Sun Yat‐sen University); Lan‐Jie He [(Department of Endocrinology, Qilu Hospital of Shandong University (Qingdao)]; Jun‐Xiu Hou (Department of Endocrinology, Affiliated Hospital of Inner Mongolia Medical University); Ling Hu (Department of Endocrinology, The Tenth Affiliated Hospital of Sun Yat‐sen University); Li‐Zhen Lan (Department of General Practice, The First Hospital of Shanxi Medical University); Cheng‐Jiang Li (Department of Endocrinology and Metabolic Disease, The First Affiliated Hospital, Zhejiang University School of Medicine); Yan‐Bo Li (Department of Endocrinology, Pinghu Hospital of Shenzhen University); Xiao‐Miao Li (Department of Endocrinology, The First Affiliated Hospital of Air Force Medical University; Xiao‐Lan Lian (Department of Endocrinology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College); Ai‐Hua Liu (Department of Endocrinology and Metabolism, Peking University Third Hospital); Dong‐Fang Liu (Department of Endocrinology and Metabolism, The Second Affiliated Hospital of Chongqing Medical University); Xiao‐Yun Liu (Department of Endocrinology, The First Affiliated Hospital of Nanjing Medical University); Nian‐Chun Peng (Department of Endocrinology, The Affiliated Hospital of Guizhou Medical University); Chun‐Jun Sheng (Department of Endocrinology and Metabolism, Shanghai Tenth People's Hospital, Tongji University School of Medicine); Jie Shen [Department of Endocrinology, Shunde Hospital of Southern Medical University (The First People's Hospital of Shunde)]; Yong‐Quan Shi (Department of Endocrinology, Shanghai Changzheng Hospital, Second Military Medical University); Xiao‐Guang Shi (Department of Endocrinology and Metabolism, The First Affiliated Hospital of China Medical University); Huai‐Dong Song (Department of Endocrinology, Shanghai Ninth People's Hospital, Shanghai Jiaotong University School of Medicine); Nan‐Wei Tong (Department of Endocrinology and Metabolism, West China Hospital of Sichuan University); Hong‐Yan Wei (Department of Endocrinology and Metabolism, Tianjin Medical University General Hospital); Qiong Wei (Department of Endocrinology, ZhongDa Hospital, School of Medicine, Southeast University); Jun‐Ping Wen (Department of Endocrinology, Fujian Provincial Hospital, Fujian Medical University); Mu‐Chao Wu (Department of Endocrinology, Sun Yat‐sen Memorial Hospital, Sun Yat‐sen University); Fei Wang (Department of Endocrinology, The Affiliated Hospital of Qingdao University); Guang Wang (Department of Endocrinology, Beijing Chao‐yang Hospital, Capital Medical University); Hai‐Ning Wang (Department of Endocrinology and Metabolism, Peking University Third Hospital); Yue Wang (Department of Endocrinology, The First Affiliated Hospital of Xi'an JiaoTong University); Shu Wang (Department of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine); Xiao‐Xia Wang (Department of Endocrinology, Beijing Hospital); Qian Xing (Department of Endocrinology, The First Affiliated Hospital of Dalian Medical University); Jiao‐Yue Zhang (Department of Endocrinology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology); Jin‐An Zhang (Department of Endocrinology, Shanghai University of Medicine & Health Sciences Affiliated Zhoupu Hospital); Xiang‐Hai Zhou (Department of Endocrinology and Metabolism, Peking University People's Hospital); Chun‐Lin Zuo (Department of Endocrinology, Institute of Endocrinology and Metabolism, The First Affiliated Hospital of Anhui Medical University).
Youshuo Liu, Zhongyan Shan; Endocrine Metabolic Diseases Group of the Chinese Geriatrics Society ; Thyroid Group of the Chinese Society of Endocrinology, Chinese Medical Association . Expert consensus on diagnosis and treatment for elderly with thyroid diseases in China (2021). Aging Med. 2021;4:70–92. 10.1002/agm2.12165
Members of the groups are listed in Appendix 1 section.
Contributor Information
Youshuo Liu, Email: liuyoushuo@csu.edu.cn.
Endocrine Metabolic Diseases Group of the Chinese Geriatrics Society:
Mei Cheng, Cai‐Xia Cao, Xiao‐Pei Cao, Ping Chen, Shu‐Zhi Feng, Ji‐Rui He, Wu Huang, Dong‐Mei Kang, Jian Kong, Cai‐Ping Li, Hua‐Hua Li, Gan‐Xiong Liang, Ming‐Zhu Lin, Juan Liu, Hui‐Xia Liu, Li‐Bin Liu, Yu Liu, Lan Luo, Hui Ma, Hui‐Juan Ma, Chao Meng, Yue Nie, Qi Pan, Wen Peng, Li‐Ya Shen, Lin‐Hui Shen, Hui Su, Zhen‐Li Su, Shuang Wang, Song‐Lin Wu, Shuang‐Tong Yan, Chun‐Yu Zhang, Hai‐Yan Zhang, Ke‐Xiang Zhao, Xin‐Lan Zhao, Yan Zhou, Ying‐Sheng Zhou, Xiao‐Pin Cai, Zhi‐Feng Cheng, Xiao‐Yun Feng, Ying Gao, Hong‐Yu Guan, Lan‐Jie He, Jun‐Xiu Hou, Ling Hu, Li‐Zhen Lan, Cheng‐Jiang Li, Yan‐Bo Li, Xiao‐Miao Li, Xiao‐Lan Lian, Ai‐Hua Liu, Dong‐Fang Liu, Xiao‐Yun Liu, Nian‐Chun Peng, Chun‐Jun Sheng, Jie Shen, Yong‐Quan Shi, Xiao‐Guang Shi, Huai‐Dong Song, Nan‐Wei Tong, Hong‐Yan Wei, Qiong Wei, Jun‐Ping Wen, Mu‐Chao Wu, Fei Wang, Guang Wang, Hai‐Ning Wang, Yue Wang, Shu Wang, Xiao‐Xia Wang, Qian Xing, Jiao‐Yue Zhang, Jin‐An Zhang, Xiang‐Hai Zhou, and Chun‐Lin Zuo
REFERENCES
- 1. Li Y, Teng D, Ba J, et al. Efficacy and safety of long‐term universal salt iodization on thyroid disorders: epidemiological evidence from 31 provinces of mainland China. Thyroid. 2020;30(4):568‐579. 10.1089/thy.2019.0067 [DOI] [PubMed] [Google Scholar]
- 2. Zhai X, Zhang L, Chen L, et al. An age‐specific serum thyrotropin reference range for the diagnosis of thyroid diseases in older adults: a cross‐sectional survey in China. Thyroid. 2018;28(12):1571‐1579. 10.1089/thy.2017.0715 [DOI] [PubMed] [Google Scholar]
- 3. Meng Z, Liu M, Zhang Q, et al. Gender and age impacts on the association between thyroid function and metabolic syndrome in Chinese. Medicine (Baltimore). 2015;94(50):e2193. 10.1097/MD.0000000000002193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laurberg P, Andersen S, Bulow Pedersen I, et al. Hypothyroidism in the elderly: pathophysiology, diagnosis and treatment. Drugs Aging. 2005;22(1):23‐38. 10.2165/00002512-200522010-00002 [DOI] [PubMed] [Google Scholar]
- 5. Bensenor IM, Olmos RD, Lotufo PA. Hypothyroidism in the elderly: diagnosis and management. Clin Interv Aging. 2012;7:97‐111. 10.2147/CIA.S23966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489‐499. 10.1210/jcem.87.2.8182 [DOI] [PubMed] [Google Scholar]
- 7. Laurberg P, Cerqueira C, Ovesen L, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. 2010;24(1):13‐27. 10.1016/j.beem.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 8. Biondi B, Bartalena L, Cooper DS, et al. The 2015 European thyroid association guidelines on diagnosis and treatment of endogenous subclinical hyperthyroidism. Eur Thyroid J. 2015;4(3):149‐163. 10.1159/000438750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boelaert K. Thyroid dysfunction in the elderly. Nat Rev Endocrinol. 2013;9(4):194‐204. 10.1038/nrendo.2013.30 [DOI] [PubMed] [Google Scholar]
- 10. Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017;317(13):1338‐1348. 10.1001/jama.2017.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu Y, Xu F, Shen J, et al. Prevalence of thyroid dysfunction in older Chinese patients with type 2 diabetes‐A multicenter cross‐sectional observational study across China. PLoS One. 2019;14(5):e0216151. 10.1371/journal.pone.0216151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strich D, Karavani G, Edri S, et al. TSH enhancement of FT4 to FT3 conversion is age dependent. Eur J Endocrinol. 2016;175(1):49‐54. 10.1530/EJE-16-0007 [DOI] [PubMed] [Google Scholar]
- 13. Atzmon G, Barzilai N, Surks MI, et al. Genetic predisposition to elevated serum thyrotropin is associated with exceptional longevity. J Clin Endocrinol Metab. 2009;94(12):4768‐4775. 10.1210/jc.2009-0808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheng Y, Ma D, Zhou Q, et al. Association of thyroid function with sarcopenia in elderly Chinese euthyroid subjects. Aging Clin Exp Res. 2019;31(8):1113‐1120. 10.1007/s40520-018-1057-z [DOI] [PubMed] [Google Scholar]
- 15. Bremner AP, Feddema P, Leedman PJ, et al. Age‐related changes in thyroid function: a longitudinal study of a community‐based cohort. J Clin Endocrinol Metab. 2012;97(5):1554‐1562. 10.1210/jc.2011-3020 [DOI] [PubMed] [Google Scholar]
- 16. Waring AC, Arnold AM, Newman AB, et al. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all‐stars study. J Clin Endocrinol Metab. 2012;97(11):3944‐3950. 10.1210/jc.2012-2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atzmon G, Barzilai N, Hollowell JG, et al. Extreme longevity is associated with increased serum thyrotropin. J Clin Endocrinol Metab. 2009;94(4):1251‐1254. 10.1210/jc.2008-2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chinese Society of Endocrinology, Group of Chinese Guidelines for Diagnosis and Treatment of Thyroid Disease . Chinese guidelines for diagnosis and treatment of thyroid diseases‐laboratory and auxiliary examinations of thyroid diseases. Chin J Intern Med. 2007;46(8):697‐702. 10.3760/j.issn:0578-1426.2007.08.038 [DOI] [Google Scholar]
- 19. Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390(10101):1550‐1562. 10.1016/S0140-6736(17)30703-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu XY, Xu SH, Wu HX, et al. Standardizing management of fine needle aspiration for thyroid nodules. Chin J Endocrinol Metab. 2019;35(10):819‐824. 10.3760/cma.j.issn.1000-6699.2019.10.001 [DOI] [Google Scholar]
- 21. MacGinley R, Champion De Crespigny PJ, Gutman T, et al. KHA‐CARI Guideline recommendations for renal biopsy. Nephrology. 2019;24(12):1205‐1213. 10.1111/nep.13662 [DOI] [PubMed] [Google Scholar]
- 22. Chen Z. Guidelines for the Management of Geriatric Syndromes. Beijing: Peking Union Medical College Publisher; 2010. [Google Scholar]
- 23. Wang YJ, Liu YS. Longevity and thyroid dysfunctions in the elderly. Chin J Endocrinol Metab. 2014;30(11):1031‐1034. 10.3760/cma.j.issn.1000-6699.2014.11.021 [DOI] [Google Scholar]
- 24. Virgini VS, Wijsman LW, Rodondi N, et al. Subclinical thyroid dysfunction and functional capacity among elderly. Thyroid. 2014;24(2):208‐214. 10.1089/thy.2013.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gussekloo J, van Exel E, de Craen AJ, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292(21):2591‐2599. 10.1001/jama.292.21.2591 [DOI] [PubMed] [Google Scholar]
- 26. Silva SO, Chan IT, Lobo Santos MA, et al. Impact of thyroid status and age on comprehensive geriatric assessment. Endocrine. 2014;47(1):255‐265. 10.1007/s12020-013-0077-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rieben C, Segna D, da Costa BR, et al. Subclinical thyroid dysfunction and the risk of cognitive decline: a meta‐analysis of prospective cohort studies. J Clin Endocrinol Metab. 2016;101(12):4945‐4954. 10.1210/jc.2016-2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Virgini VS, Rodondi N, Cawthon PM, et al. Subclinical thyroid dysfunction and frailty among older men. J Clin Endocrinol Metab. 2015;100(12):4524‐4532. 10.1210/jc.2015-3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dent E, Morley JE, Cruz‐Jentoft AJ, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. 2019;23(9):771‐787. 10.1007/s12603-019-1273-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bano A, Chaker L, Schoufour J, et al. High circulating free thyroxine levels may increase the risk of frailty: The Rotterdam Study. J Clin Endocrinol Metab. 2018;103(1):328‐335. 10.1210/jc.2017-01854 [DOI] [PubMed] [Google Scholar]
- 31. Cohen‐Lehman J, Dahl P, Danzi S, et al. Effects of amiodarone therapy on thyroid function. Nat Rev Endocrinol. 2010;6(1):34‐41. 10.1038/nrendo.2009.225 [DOI] [PubMed] [Google Scholar]
- 32. Nordyke RA, Gilbert FI Jr, Harada AS. Graves' disease. Influence of age on clinical findings. Arch Intern Med. 1988;148(3):626‐631. 10.1001/archinte.148.3.626 [DOI] [PubMed] [Google Scholar]
- 33. Boelaert K, Torlinska B, Holder RL, et al. Older subjects with hyperthyroidism present with a paucity of symptoms and signs: a large cross‐sectional study. J Clin Endocrinol Metab. 2010;95(6):2715‐2726. 10.1210/jc.2009-2495 [DOI] [PubMed] [Google Scholar]
- 34. Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population‐based study. Arch Intern Med. 2004;164(15):1675‐1678. 10.1001/archinte.164.15.1675 [DOI] [PubMed] [Google Scholar]
- 35. Yeap BB, Alfonso H, Chubb SA, et al. Higher free thyroxine levels predict increased incidence of dementia in older men: the Health in Men Study. J Clin Endocrinol Metab. 2012;97(12):E2230‐2237. 10.1210/jc.2012-2108 [DOI] [PubMed] [Google Scholar]
- 36. Laurberg P, Berman DC, Bulow Pedersen I, et al. Incidence and clinical presentation of moderate to severe graves' orbitopathy in a Danish population before and after iodine fortification of salt. J Clin Endocrinol Metab. 2012;97(7):2325‐2332. 10.1210/jc.2012-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ross DS, Burch HB, Cooper DS, et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343‐1421. 10.1089/thy.2016.0229 [DOI] [PubMed] [Google Scholar]
- 38. Takats KI, Szabolcs I, Foldes J, et al. The efficacy of long term thyrostatic treatment in elderly patients with toxic nodular goitre compared to radioiodine therapy with different doses. Exp Clin Endocrinol Diabetes. 1999;107(1):70‐74. 10.1055/s-0029-1212076 [DOI] [PubMed] [Google Scholar]
- 39. Allahabadia A, Daykin J, Holder RL, et al. Age and gender predict the outcome of treatment for Graves' hyperthyroidism. J Clin Endocrinol Metab. 2000;85(3):1038‐1042. 10.1210/jcem.85.3.6430 [DOI] [PubMed] [Google Scholar]
- 40. Kim YA, Cho SW, Choi HS, et al. The second antithyroid drug treatment is effective in relapsed Graves' disease patients: a median 11‐year follow‐up study. Thyroid. 2017;27(4):491‐496. 10.1089/thy.2016.0056 [DOI] [PubMed] [Google Scholar]
- 41. Pearce SH. Spontaneous reporting of adverse reactions to carbimazole and propylthiouracil in the UK. Clin Endocrinol (Oxf). 2004;61(5):589‐594. 10.1111/j.1365-2265.2004.02135.x [DOI] [PubMed] [Google Scholar]
- 42. Wang MT, Lee WJ, Huang TY, et al. Antithyroid drug‐related hepatotoxicity in hyperthyroidism patients: a population‐based cohort study. Br J Clin Pharmacol. 2014;78(3):619‐629. 10.1111/bcp.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. China Geriatric Health Care Medical Research Association, geriatric endocrine and metabolic diseases branch, Clinical Toxicology Committee of Chinese Society of Toxicology . Expert consensus on safety management of multi drug use in the elderly. Chin J Diabetes. 2018;26(9):705‐717. 10.3969/j.issn.1006-6187.2018.09.001 [DOI] [Google Scholar]
- 44. Raffaelli M, Bellantone R, Princi P, et al. Surgical treatment of thyroid diseases in elderly patients. Am J Surg. 2010;200(4):467‐472. 10.1016/j.amjsurg.2009.12.020 [DOI] [PubMed] [Google Scholar]
- 45. Lillevang‐Johansen M, Abrahamsen B, Jorgensen HL, et al. Duration of hyperthyroidism and lack of sufficient treatment are associated with increased cardiovascular risk. Thyroid. 2019;29(3):332‐340. 10.1089/thy.2018.0320 [DOI] [PubMed] [Google Scholar]
- 46. Selmer C, Olesen JB, Hansen ML, et al. The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ. 2012;345:e7895. 10.1136/bmj.e7895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Asvold BO, Bjoro T, Platou C, et al. Thyroid function and the risk of coronary heart disease: 12‐year follow‐up of the HUNT study in Norway. Clin Endocrinol (Oxf). 2012;77(6):911‐917. 10.1111/j.1365-2265.2012.04477.x [DOI] [PubMed] [Google Scholar]
- 48. Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295(9):1033‐1041. 10.1001/jama.295.9.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Collet TH, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172(10):799‐809. 10.1001/archinternmed.2012.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blum MR, Bauer DC, Collet TH, et al. Subclinical thyroid dysfunction and fracture risk: a meta‐analysis. JAMA. 2015;313(20):2055‐2065. 10.1001/jama.2015.5161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aubert CE, Bauer DC, da Costa BR, et al. The association between subclinical thyroid dysfunction and dementia: the health, aging and body composition (Health ABC) study. Clin Endocrinol (Oxf). 2017;87(5):617‐626. 10.1111/cen.13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vadiveloo T, Donnan PT, Cochrane L, et al. The thyroid epidemiology, audit, and research study (TEARS): the natural history of endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab. 2011;96(1):E1‐8. 10.1210/jc.2010-0854 [DOI] [PubMed] [Google Scholar]
- 53. Teng XC, Teng D, Shan ZY, et al. Impact of iodine intake on thyroid diseases—a five‐year prospective epidemiological study. Chin J Endocrinol Metab. 2011;96(1):E1‐8. 10.1210/jc.2010-0854 [DOI] [Google Scholar]
- 54. Lillevang‐Johansen M, Abrahamsen B, Jorgensen HL, et al. Over‐ and under‐treatment of hypothyroidism is associated with excess mortality: a register‐based cohort study. Thyroid. 2018;28(5):566‐574. 10.1089/thy.2017.0517 [DOI] [PubMed] [Google Scholar]
- 55. Chen SF, Bi JY, Song HM, et al. Analysis of influential factors of non‐thyroidal illness syndrome in elderly inpatients. Chin J Geriatr. 2018;37(5):532‐535. 10.3760/cma.j.issn.0254-9026.2018.05.012 [DOI] [Google Scholar]
- 56. Nanchen D, Gussekloo J, Westendorp RG, et al. Subclinical thyroid dysfunction and the risk of heart failure in older persons at high cardiovascular risk. J Clin Endocrinol Metab. 2012;97(3):852‐861. 10.1210/jc.2011-1978 [DOI] [PubMed] [Google Scholar]
- 57. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126(9):1040‐1049. 10.1161/CIRCULATIONAHA.112.096024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Waring AC, Harrison S, Samuels MH, et al. Thyroid function and mortality in older men: a prospective study. J Clin Endocrinol Metab. 2012;97(3):862‐870. 10.1210/jc.2011-2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thayakaran R, Adderley NJ, Sainsbury C, et al. Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: longitudinal study. BMJ. 2019;366:l4892. 10.1136/bmj.l4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peng CC, Huang HK, Wu BB, et al. Association of thyroid hormone therapy with mortality in subclinical hypothyroidism: a systematic review and meta‐analysis. J Clin Endocrinol Metab. 2021;106(1):292‐303. 10.1210/clinem/dgaa777 [DOI] [PubMed] [Google Scholar]
- 61. Pasqualetti G, Pagano G, Rengo G, et al. Subclinical hypothyroidism and cognitive impairment: systematic review and meta‐analysis. J Clin Endocrinol Metab. 2015;100(11):4240‐4248. 10.1210/jc.2015-2046 [DOI] [PubMed] [Google Scholar]
- 62. Lindeman RD, Schade DS, LaRue A, et al. Subclinical hypothyroidism in a biethnic, urban community. J Am Geriatr Soc. 1999;47(6):703‐709. 10.1111/j.1532-5415.1999.tb01593.x [DOI] [PubMed] [Google Scholar]
- 63. Park YJ, Lee EJ, Lee YJ, et al. Subclinical hypothyroidism (SCH) is not associated with metabolic derangement, cognitive impairment, depression or poor quality of life (QoL) in elderly subjects. Arch Gerontol Geriatr. 2010;50(3):e68‐73. 10.1016/j.archger.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 64. Simonsick EM, Newman AB, Ferrucci L, et al. Subclinical hypothyroidism and functional mobility in older adults. Arch Intern Med. 2009;169(21):2011‐2017. 10.1001/archinternmed.2009.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pearce SH, Razvi S, Yadegarfar ME, et al. Serum thyroid function, mortality and disability in advanced old Age: the Newcastle 85+ study. J Clin Endocrinol Metab. 2016;101(11):4385‐4394. 10.1210/jc.2016-1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Imaizumi M, Sera N, Ueki I, et al. Risk for progression to overt hypothyroidism in an elderly Japanese population with subclinical hypothyroidism. Thyroid. 2011;21(11):1177‐1182. 10.1089/thy.2010.0411 [DOI] [PubMed] [Google Scholar]
- 67. Somwaru LL, Rariy CM, Arnold AM, et al. The natural history of subclinical hypothyroidism in the elderly: the cardiovascular health study. J Clin Endocrinol Metab. 2012;97(6):1962‐1969. 10.1210/jc.2011-3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Blum MR, Gencer B, Adam L, et al. Impact of thyroid hormone therapy on atherosclerosis in the elderly with subclinical hypothyroidism: a randomized trial. J Clin Endocrinol Metab. 2018;103(8):2988‐2997. 10.1210/jc.2018-00279 [DOI] [PubMed] [Google Scholar]
- 69. Razvi S, Weaver JU, Butler TJ, et al. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172(10):811‐817. 10.1001/archinternmed.2012.1159 [DOI] [PubMed] [Google Scholar]
- 70. Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community‐living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95(8):3623‐3632. 10.1210/jc.2009-2571 [DOI] [PubMed] [Google Scholar]
- 71. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376(26):2534‐2544. 10.1056/NEJMoa1603825 [DOI] [PubMed] [Google Scholar]
- 72. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331(19):1249‐1252. 10.1056/NEJM199411103311901 [DOI] [PubMed] [Google Scholar]
- 73. Grossman A, Feldhamer I, Meyerovitch J. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur J Intern Med. 2018;50:65‐68. 10.1016/j.ejim.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 74. Jabbar A, Ingoe L, Junejo S, et al. Effect of levothyroxine on left ventricular ejection fraction in patients with subclinical hypothyroidism and acute myocardial infarction: a randomized clinical trial. JAMA. 2020;324(3):249‐258. 10.1001/jama.2020.9389 [DOI] [PubMed] [Google Scholar]
- 75. Mooijaart SP, Du Puy RS, Stott DJ, et al. Association between levothyroxine treatment and thyroid‐related symptoms among adults aged 80 years and older with subclinical hypothyroidism. JAMA. 2019;322:1‐11. 10.1001/jama.2019.17274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lin JD, Chao TC, Chen ST, et al. Characteristics of thyroid carcinomas in aging patients. Eur J Clin Invest. 2000;30(2):147‐153. 10.1046/j.1365-2362.2000.00598.x [DOI] [PubMed] [Google Scholar]
- 77. Girardi FM. Thyroid carcinoma pattern presentation according to age. Int Arch Otorhinolaryngol. 2017;21(1):38‐41. 10.1055/s-0036-1585095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Park HS, Roman SA, Sosa JA. Treatment patterns of aging Americans with differentiated thyroid cancer. Cancer. 2010;116(1):20‐30. 10.1002/cncr.24717 [DOI] [PubMed] [Google Scholar]
- 79. Shi LY, Liu J, Yu LJ, et al. Clinic‐pathologic features and prognostic analysis of thyroid cancer in the older adult: a SEER based study. J Cancer. 2018;9(15):2744‐2750. 10.7150/jca.24625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Silva de Morais N, Stuart J, Guan H, et al. The impact of Hashimoto thyroiditis on thyroid nodule cytology and risk of thyroid cancer. J Endocr Soc. 2019;3(4):791‐800. 10.1210/js.2018-00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang Z, Vyas CM, Van Benschoten O, et al. Quantitative analysis of the benefits and risk of thyroid nodule evaluation in patients ≥70 years old. Thyroid. 2018;28(4):465‐471. 10.1089/thy.2017.0655 [DOI] [PubMed] [Google Scholar]
- 82. Tessler FN, Middleton WD, Grant EG, et al. ACR thyroid imaging, reporting and data system (TI‐RADS): white paper of the ACR TI‐RADS committee. J Am Coll Radiol. 2017;14(5):587‐595. 10.1016/j.jacr.2017.01.046 [DOI] [PubMed] [Google Scholar]
- 83. Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1‐133. 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhou B, Zou DZ, Shao Q. Ultrasound‐guided percutaneous laser ablation for benign thyroid nodules in senior patients. Chin J Endocr Surg. 2016;10(1):83‐84. 10.3760/cma.j.issn.1674-6090.2016.01.022 [DOI] [Google Scholar]
- 85. Liu YH, Sun J, Wang ST, et al. Surgical resection and microwave ablation of thyroid nodules in aged patients: comparative analysis of clinical efficacy and safety. J Intervent Radiol. 2016;25(1):44‐47. 10.3969/j.issn.1008-794X.2016.01.010 [DOI] [Google Scholar]
- 86. Chinese Society of Endocrinology , Endorcinology Group Surgery Branch Chinese Medical Association , Head and Neck Cancer Committee of China Anti‐Cancer Association , et al. Guidelines for the diagnosis and treatment of thyroid nodules and differentiated thyroid cancer. Chin J Endocrinol Metab. 2012;28(10):779‐797. 10.3760/cma.j.issn.1000-6699.2012.10.002 [DOI] [Google Scholar]
- 87. Li S, Nie Y, Zhan J, et al. The analysis of correlation between frailty index and postoperative complications of aged patients with nodular goiter. Aging Med (Milton). 2018;1(1):18‐22. 10.1002/agm2.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24(1):27‐34. 10.1089/thy.2013.0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tuttle RM, Fagin JA, Minkowitz G, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. 2017;143(10):1015‐1020. 10.1001/jamaoto.2017.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Koshkina A, Fazelzad R, Sugitani I, et al. Association of patient age with progression of low‐risk papillary thyroid carcinoma under active surveillance: a systematic review and meta‐analysis. JAMA Otolaryngol Head Neck Surg. 2020;146(6):552‐560. 10.1001/jamaoto.2020.0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Joseph KR, Edirimanne S, Eslick GD. Thyroidectomy for thyroid cancer in the elderly: a meta‐analysis. Eur J Surg Oncol. 2019;45(3):310‐317. 10.1016/j.ejso.2018.07.055 [DOI] [PubMed] [Google Scholar]
- 92. Zambeli‐Ljepovic A, Wang F, Dinan MA, et al. Low‐risk thyroid cancer in elderly: total thyroidectomy/RAI predominates but lacks survival advantage. J Surg Res. 2019;243:189‐197. 10.1016/j.jss.2019.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mazziotti G, Formenti AM, Frara S, et al. High prevalence of radiological vertebral fractures in women on thyroid‐stimulating hormone‐suppressive therapy for thyroid carcinoma. J Clin Endocrinol Metab. 2018;103(3):956‐964. 10.1210/jc.2017-01986 [DOI] [PubMed] [Google Scholar]
- 94. Amin MBES, Greene FL, et al. AJCC Cancer Staging Manual, 8th edn[M]. New York, NY: Springer; 2017. [Google Scholar]
- 95. Tam S, Boonsripitayanon M, Amit M, et al. Survival in differentiated thyroid cancer: comparing the AJCC cancer staging seventh and eighth editions. Thyroid. 2018;28(10):1301‐1310. 10.1089/thy.2017.0572 [DOI] [PubMed] [Google Scholar]
- 96. Wang X, Lei J, Wei T, et al. Clinicopathological characteristics and recurrence risk of papillary thyroid microcarcinoma in the elderly. Cancer Manag Res. 2019;11:2371‐2377. 10.2147/CMAR.S198451 [DOI] [PMC free article] [PubMed] [Google Scholar]


