Background: Thrombosis with thrombocytopenia has been reported after vaccination against SARS-CoV-2 with 2 vaccines based on recombinant adenovirus vectors—the ChAdOx1 vaccine from AstraZeneca (1, 2) and the Ad26.COV2.S vaccine from Johnson & Johnson/Janssen (3). This syndrome is similar to heparin-induced thrombocytopenia (HIT), and it is known as vaccine-induced thrombosis with thrombocytopenia (VITT) or thrombocytopenia with thrombosis syndrome (TTS). To our knowledge, there are no reports of VITT or TTS after a SARS-CoV-2 vaccine based on messenger RNA (mRNA) technology (4).
Objective: To describe a patient with VITT or TTS after administration of the mRNA-1273 vaccine from Moderna.
Case Report: A 65-year-old man with chronic hypertension and hyperlipidemia presented to our hospital with 1 week of bilateral lower-extremity discomfort, intermittent headaches, and 2 days of dyspnea. He had received a second dose of the mRNA-1273 vaccine 10 days before the onset of symptoms. He had no known prior heparin exposure.
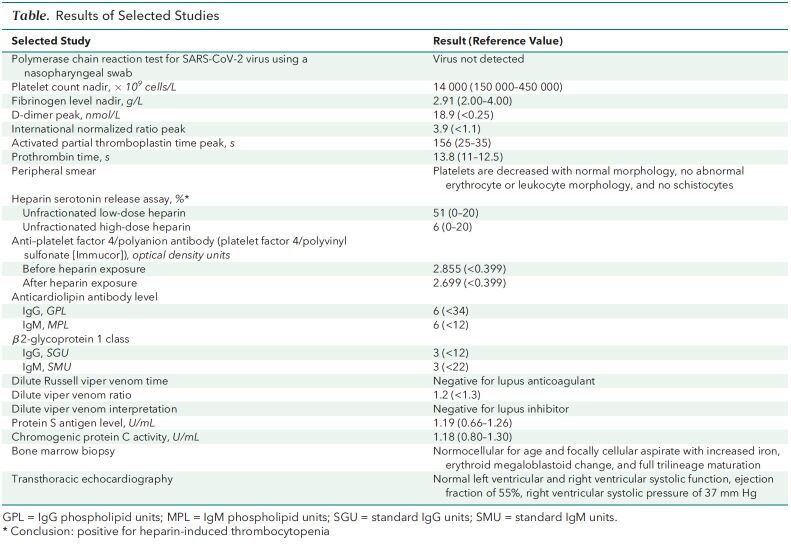
A computed tomography angiogram of the chest showed large, bilateral, acute pulmonary emboli with right ventricular strain. Doppler studies of the lower extremities revealed acute deep venous thromboses in both lower extremities. The patient had severe thrombocytopenia (14 × 109 cells/L) (Table and Figure) (171 × 109 cells/L had been documented 18 months earlier).
Table.
Results of Selected Studies
Figure. Summary of clinical events.
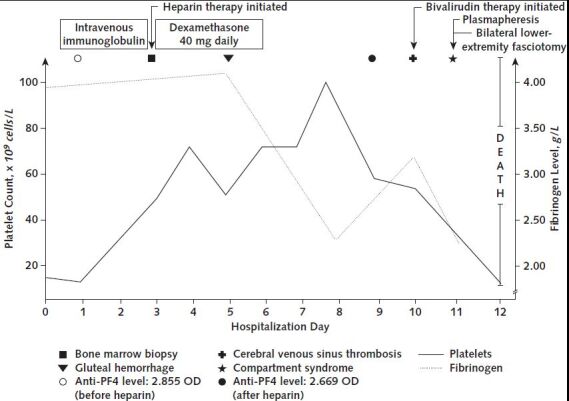
Anti-PF4 = anti–platelet factor 4; OD = optical density.
We decided against systemic anticoagulation because of the thrombocytopenia and placed an inferior vena cava filter. We also did a bone marrow biopsy. We administered 2 doses of intravenous immunoglobulin followed by 40 mg of dexamethasone intravenously for 4 days for presumed immune-mediated thrombocytopenia. Unfractionated heparin therapy was initiated after platelet transfusions elevated his platelet count to 69 000 × 109 cells/L.
Three days later, the patient developed an acute gluteal hematoma requiring withdrawal of heparin. The thrombocytopenia persisted, and we evaluated him for HIT. An enzyme-linked immunosorbent assay for anti–platelet factor 4/heparin IgG was strongly positive (2.669 optical density; reference value, <0.399 optical density). A platelet activation assay using patient serum and normal platelets showed the release of serotonin at a low concentration of heparin that was inhibited by a high concentration, which was consistent with HIT. Twelve hours later, he developed acute encephalopathy. A computed tomography angiogram of the head and neck showed cerebral venous sinus thrombosis, which was confirmed with a computed tomography venogram. Progression of the lower-extremity deep venous thromboses and a new upper-extremity deep venous thrombosis were noted. We initiated bivalirudin treatment at a dose of 0.02 mg/kg of body weight per hour and started plasmapheresis. Nevertheless, the patient continued to deteriorate with shock, lactic acidemia, and compartment syndrome of the lower extremities, requiring emergent bilateral fasciotomies. The same day, blood cultures were obtained, and we initiated vancomycin and cefepime treatment. Within 24 hours, blood cultures grew methicillin-sensitive Staphylococcus aureus. He continued to deteriorate, his family elected to pursue comfort measures, and he died after compassionate extubation. After his death, serum collected during admission, but before he received heparin, was strongly positive for anti–platelet factor 4/heparin IgG (2.855 optical density).
Discussion: In retrospect, this patient met the criteria for VITT or TTS (5). He developed thrombocytopenia and thrombosis within 5 to 10 days after vaccine administration. The distribution of thrombosis, especially the cerebral venous sinus thrombosis, was characteristic of VITT or TTS. Most of his clotting and other relevant work-up were consistent with the syndrome. We were unable to identify other causes, including SARS-CoV-2 infection, other infections, immune thrombocytopenia, or thrombotic thrombocytopenic purpura. These findings fulfill the interim case definition of VITT or TTS from the Centers for Disease Control and Prevention and the Brighton Collaboration. Further, the positive platelet factor 4 enzyme-linked immunosorbent assay of the blood drawn before heparin administration strengthens the likelihood of VITT or TTS. Although we believe the evidence supporting VITT or TTS in this case is robust, we cannot rule out atypical HIT or HIT with unrecorded heparin administration.
Had we suspected VITT or TTS earlier, we would have treated the patient differently. Following current guidelines (5), in addition to intravenous immunoglobulin, and dexamethasone, we would have administered bivalirudin (or another recommended nonheparin anticoagulant) earlier; avoided platelet transfusions; and done more extensive serologic testing of platelet-activating antibodies (2).
In summary, we believe it is important to note that many millions of people have received COVID-19 vaccines that use mRNA technology. This is the only report to date of possible VITT or TTS in those recipients, and such a rare event, even if confirmed by additional reports, should not prevent persons from receiving the benefits of these vaccines. In addition, this report complicates hypotheses that implicate adenoviral vectors as the sole cause of VITT or TTS.
Footnotes
This article was published at Annals.org on 29 June 2021.
References
- 1. Schultz NH , Sørvoll IH , Michelsen AE , et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. [PMID: ] doi: 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greinacher A , Thiele T , Warkentin TE , et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092-2101. [PMID: ] doi: 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muir KL , Kallam A , Koepsell SA , et al. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination [Letter]. N Engl J Med. 2021;384:1964-1965. [PMID: ] doi: 10.1056/NEJMc2105869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cines DB , Bussel JB . SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia [Editorial]. N Engl J Med. 2021;384:2254-2256. [PMID: ] doi: 10.1056/NEJMe2106315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Society of Hematology. Thrombosis with thrombocytopenia syndrome (also termed vaccine-induced thrombotic thrombocytopenia). Updated 29 April 2021. Accessed at www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia on 26 May 2021.