Abstract
Background
Systemic therapy (ST) can be deferred in patients who have metastatic renal cell carcinoma (mRCC) and slow‐growing metastases. Currently, this subset of patients managed with active surveillance (AS) is not well described in the literature.
Methods
This was a prospective observational study of patients with mRCC across 46 US community and academic centers. The objective was to describe baseline characteristics and demographics of patients with mRCC initially managed by AS, reasons for AS, and patient outcomes. Descriptive statistics were used to characterize demographics, baseline characteristics, and patient‐related outcomes. Wilcoxon 2‐sample rank‐sum tests and χ2 tests were used to assess differences between ST and AS cohorts in continuous and categorical variables, respectively. Kaplan‐Meier survival curves were used to assess survival.
Results
Of 504 patients, mRCC was initially managed by AS (n = 143) or ST (n = 305); 56 patients were excluded from the analysis. Disease was present in 69% of patients who received AS, whereas the remaining 31% had no evidence of disease. At data cutoff, 72 of 143 patients (50%) in the AS cohort had not received ST. The median overall survival was not reached (95% CI, 122 months to not estimable) in patients who received AS versus 30 months (95% CI, 25‐44 months) in those who received ST. Quality of life at baseline was significantly better in patients who were managed with AS versus ST.
Conclusions
AS occurs frequently (32%) in real‐world clinical practice and appears to be a safe and appropriate alternative to immediate ST in selected patients.
Keywords: active surveillance, metastatic, observational study, renal cell carcinoma
Short abstract
This prospective observational study followed patients with metastatic renal cell carcinoma who were managed by active surveillance before treatment. The approach is used frequently in clinical practice and appears to be a safe and appropriate option for some patients.
Introduction
Metastatic renal cell carcinoma (mRCC) is a heterogeneous disease with wide variations in its clinical presentation, natural history, and response to therapy. Multivariate prognostic models, based on timing of the development of metastases (eg, whether the patient presents with metastases de novo, recurs with metastases early after nephrectomy [<1 year], or recurs later after nephrectomy [>1 year]), patient performance status, and circulating biomarkers of inflammation and disease biology, can predict survival for patients with mRCC. 1 , 2 , 3
New treatment approaches for mRCC are focusing on combining agents, and preliminary results suggest improved outcomes with some combination therapies compared with single‐agent therapy. 4 , 5 , 6 , 7 , 8 , 9 , 10 These newer approaches are producing greater clinical benefit for patients, which may increase the pressure to treat patients earlier. 11 , 12 However, clinical benefit may vary by prognosis and must be weighed against the expected cumulative and chronic adverse events many patients will experience with treatment, some of which could be serious or life‐threatening. Because real‐world patients generally have greater comorbidity burdens than those in clinical trials, 13 practicing clinicians must consider a wider range of treatment approaches than those offered in a trial and must also consider the relative sequencing of treatments for patients who have mRCC.
Among patients with mRCC, there is a subset with slow‐growing metastases for whom systemic therapy (ST) can be safely delayed and active surveillance (AS) pursued, sparing treatment toxicity while preserving both quality of life (QoL) and quantity of life. These patients have not been well defined because contemporary mRCC trials do not include an arm without treatment. Retrospective studies suggest that patients may defer ST for various reasons, including metastasectomy (or other metastasis‐directed treatment), intercurrent illness, and comorbidity, or simply may choose AS. 14 , 15 , 16 Metastasectomy is relatively well described in the literature, and the best available evidence suggests that approximately 30% of these patients may remain disease free at 5 years. 17 , 18 , 19
Rini and colleagues reported the only prospective study of AS in mRCC to date. 20 Their cohort included 48 treatment‐naive patients with asymptomatic mRCC who were accrued over 5 years at 5 US and European academic centers. The median time on AS was 14.9 months (95% CI, 10.6‐25.0 months), and the median overall survival (OS) from the start of AS was 44.5 months (95% CI, 37.6 months to not reached). These data support AS for a subset of patients with mRCC before starting ST, and the authors conclude that more study of AS is necessary.
No prospective study reported to date has compared the outcomes of patients with mRCC who undergo AS versus those who are treated early. Furthermore, the proportion of patients with mRCC who delay ST is unknown. There are also no studies of AS in the community setting. Therefore, we created the Metastatic Renal Cell Cancer (MaRCC) Registry, a prospective observational study of patients with mRCC accrued in both community and academic centers, and followed these patients for treatment selection and outcomes. 21 Knowing that a proportion of patients may have ST deferred and that these patients are not well represented in the literature, we specifically aimed to capture the outcomes of these patients. In the current report, we use the MaRCC Registry to characterize patients with mRCC who delay ST with a focus on those who undergo AS, including the reasons for AS, and we compare these patients with those undergoing immediate ST.
Materials and Methods
Study Population
MaRCC is a prospective observational study that was designed to enroll approximately 500 patients with mRCC from both academic and community sites in the United States. The study design and methodology have been previously described. 21 Briefly, patients aged ≥18 years who were diagnosed with mRCC and received no prior ST for mRCC were eligible. Also, patients who had surgery, radiation therapy, and prior neoadjuvant or adjuvant therapy for nonrenal malignancies were eligible. As mentioned above, patients with mRCC who did not receive ST but currently were under observation were purposefully included. Patients were excluded if they were currently receiving ST for active malignancies other than mRCC unless ST was completed ≤3 months before enrollment. All study participants provided written informed consent. The study was approved by the Duke University School of Medicine Institutional Review Board and by the central or local institutional review boards for each participating research site.
Statistical Analysis
Descriptive statistics were used to characterize demographics, baseline characteristics, and patient‐reported outcomes (PROs). Wilcoxon 2‐sample rank‐sum tests and χ2 tests were used to assess differences between the ST and AS cohorts in continuous and categorical variables, respectively. Kaplan‐Meier survival curves were used to assess survival. All statistical analyses were conducted using SAS software, version 9.4 (SAS Institute Inc).
Selection of Cohort and Data Elements
We defined the AS cohort based on the Physician Treatment Selection Assessment Survey (see Supporting Fig. 1), which was prospectively completed by the treating physician at study entry. In total, 168 patients were categorized in Section A: No Systemic Therapy Selected for the Patient with a primary reason as active surveillance (dichotomized as either with no evidence of disease following a procedure or disease present). After excluding 25 patients who received ST within 90 days of a metastatic diagnosis (because such immediate ST is not consistent with a strategy of AS), the final AS cohort included 143 patients (Fig. 1).
Figure 1.
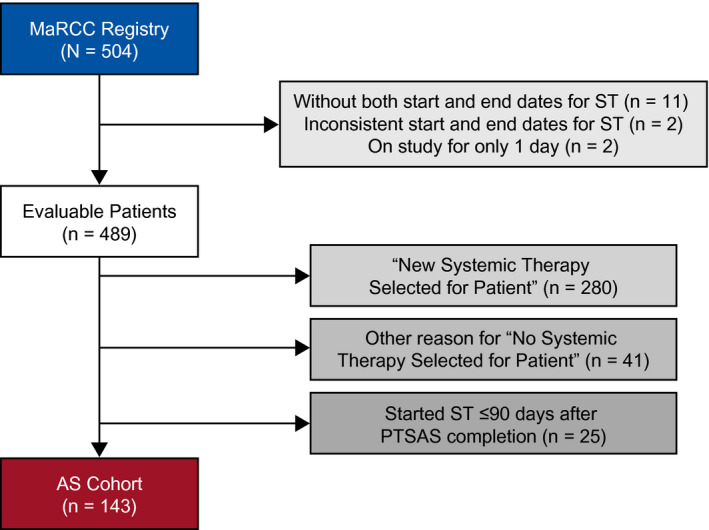
This chart describes the active surveillance (AS) cohort. MaRCC Registry indicates the Metastatic Renal Cell Carcinoma Registry; PTSAS, Physician Treatment Selection Assessment Survey; ST, systemic therapy.
Information collected at baseline included demographic characteristics, tumor and prior treatment history, laboratory tests, performance status, physician treatment selection (including AS), and PROs, ie, National Comprehensive Cancer Network (NCCN)/Functional Assessment of Cancer Therapy (FACT)–Kidney Symptom Index 19 (NCCN–FACT FKSI‐19) and the FACT–General (FACT‐G). Variables, which were updated at subsequent visits, included laboratory tests, performance status, physician treatment selection (reasons for starting ST, stopping ST, or starting AS), and the same PROs that were assessed at baseline. Patients were followed for OS (for further methodological details, see Supporting Figs. 1‐3).
Results
Patients
Between March 24, 2014 and December 22, 2016, 504 evaluable patients with mRCC were enrolled in the MaRCC study from 46 sites, including 26 community centers (n = 124) and 20 academic centers (n = 380). Of these, 448 patients were classified at enrollment as having been selected to receive either AS (n = 143) or ST (n = 305). Demographic and clinical characteristics of the AS and ST cohorts in the MaRCC study are typical of populations with advanced RCC, 22 with a few caveats (Table 1). Approximately 11% of patients had an Eastern Cooperative Oncology Group performance status of 2 or 3, and these patients typically are excluded from trials. 13 , 23 A lower proportion of patients had undergone prior nephrectomy across both cohorts (approximately 56% vs >75% in recent front‐line mRCC treatment trials). 6 , 22
TABLE 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | No. of Patients (%) | ||
|---|---|---|---|
| AS, n = 143 | ST, n = 305 | Total, N = 488 a | |
| Age: Median (IQR: 25%, 75%), y | 65 (58, 72) | 62 (55, 70) | 63 (56, 71) |
| Sex: Men | 103 (72) | 217 (71) | 320 (71) |
| Ethnicity: White | 131 (92) | 249 (82) | 380 (85) |
| ECOG PS b | |||
| 0 | 76 (53) | 109 (36) | 185 (41) |
| 1 | 43 (30) | 120 (39) | 163 (36) |
| 2 | 3 (2) | 37 (12) | 40 (9) |
| 3 | 1 (1) | 10 (3) | 11 (3) |
| Missing | 20 (14) | 29 (10) | 49 (11) |
| Histology type: Clear cell | 125 (87) | 232 (76) | 357 (80) |
| No. of metastatic sites b | |||
| 1 | 99 (69) | 175 (57) | 274 (61) |
| 2 | 33 (23) | 79 (26) | 112 (25) |
| ≥3 | 11 (8) | 51 (17) | 62 (14) |
| Site of metastasis | |||
| Liver | 11 (8) | 40 (13) | 51 (11) |
| Bone | 26 (18) | 83 (27) | 109 (24) |
| Brain/CNS | 6 (4) | 22 (7) | 28 (6) |
| Lung | 73 (51) | 173 (57) | 246 (55) |
| Lymph node | 32 (22) | 86 (28) | 118 (26) |
| Adrenal gland | 23 (16) | 25 (8) | 48 (11) |
| Prior nephrectomy | 83 (58) | 168 (55) | 251 (56) |
| Missing | 2 (1) | 9 (3) | 11 (3) |
| No. of IMDC risk factors b | |||
| 0, Favorable risk | 86 (60) | 42 (14) | 128 (29) |
| 1‐2, Intermediate risk | 54 (38) | 197 (65) | 251 (56) |
| ≥3, Poor risk | 3 (2) | 66 (22) | 69 (15) |
Abbreviations: AS, active surveillance; CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; IMDC, International Metastatic Renal Cell Database Consortium; IQR, interquartile range; ST, systemic therapy.
Forty‐one patients who were not classified as receiving AS or ST were not included. Other reason for "No Systemic Therapy Selected for Patient", see Figure 1.
P < .05.
There were some differences in baseline prognostic factors between the cohorts (Table 1). Patients who received AS versus ST had a slightly better Eastern Cooperative Oncology Group performance status (2‐3: 2.8% [95% CI, 0.8‐7.0%] vs 15.4% [95% CI, 11.6‐20.0%], respectively), a more favorable International Metastatic Renal Cell Database Consortium (IMDC) risk profile (favorable risk, 60% vs 14%; intermediate risk, 38% vs 65%; poor risk, 2% vs 22%), fewer metastatic sites (1 site, 69% vs 57%; 2 sites, 23% vs 26%; ≥3 sites, 8% vs 17%), a higher percentage of adrenal metastasis (16% vs 8%), a lower rate of bone metastasis (18% vs 27%), and a lower proportion who received ST within 1 year of an initial RCC diagnosis (13% vs 70%). In the AS cohort, 34% (n = 49), 30% (n = 43), and 36% (n = 51) were <3 months, 3 to 12 months, and ≥12 months from their mRCC diagnosis, respectively, to the start of the MaRCC study; and median time from diagnosis of mRCC to the study start was 5.5 months. For the AS cohort, the time from initial RCC diagnosis to the development of metastatic disease among 143 patients was <1 year for 43% (n = 62) and ≥1 year for 57% (n = 81).
Management Decisions and Breakdown of AS Patients
At each time point when a management decision was made, including choosing to initiate (or not initiate) ST, a Physician Treatment Selection Assessment Survey was completed by the treating physician regarding the reason for this decision (Table 2). Of patients in the AS cohort, 98 (69%) had disease present, and 45 (32%) had no evidence of disease present. (Although prior radiographic or pathologic evidence of metastatic disease was a requirement for enrollment in the MaRCC study, current measurable disease was not.) Compared with community sites, a higher proportion of patients at academic sites were categorized as AS with disease present (72% vs 54%). Other than higher rates of lymph node metastasis (28% vs 11%) and lung metastasis (57% vs 38%) in the group with disease present, there were no differences in baseline demographics according to whether patients had disease present or no evidence of disease present.
TABLE 2.
Physician Treatment Selection Survey: Primary Reason for Active Surveillance, By Center
| Primary Reason, n (%) | Academic Center, n = 115 | Community Center, n = 28 | Total, N = 143 |
|---|---|---|---|
| Disease present | 83 (72) | 15 (54) | 98 (69) |
| No evidence of disease after procedure | 32 (28) | 13 (46) | 45 (32) |
Outcomes
Patients in the AS cohort were followed for a median of 33 months (95% CI, 29‐35 months) from enrollment. Their median duration of follow‐up from metastatic diagnosis to the end of the study was 42 months (95% CI, 39‐47 months), and the median time from initial diagnosis to the end of the study was 79 months (95% CI, 55‐91 months). Patients in the ST cohort were followed for a median of 29 months (95% CI, 27‐31 months) from enrollment. Their median duration of follow‐up from metastatic diagnosis to the end of the study was 32 months (95% CI, 29‐34 months), and the median time from initial diagnosis to the end of the study was 46 months (95% CI, 41‐50 months).
The Kaplan‐Meier curve for ST‐free survival is shown in Figure 2. At the time of data cutoff (March 13, 2019), 72 of 143 patients (50%) in the AS cohort had not received ST, which represented 72 of 504 patients (14%) in the entire MaRCC study. The duration of time on AS is illustrated in Supporting Figure 2. There were fewer deaths in the AS cohort (n = 25; 17%) versus the ST cohort (n = 157; 52%). The median OS from metastatic disease diagnosis was not reached (95% CI, 122 months to not estimable) in the AS cohort and 30 months (95% CI, 25‐44 months) in the ST cohort (Fig. 3A). The Kaplan‐Meier estimate of the proportion surviving at 3 years was 0.84 for the AS cohort and 0.45 for the ST cohort. When the AS cohort was dichotomized for the presence of disease, the median OS from metastatic disease diagnosis was 122 months (95% CI, 97 months to not estimable) for those with disease present and not reached (not estimable) for those with no evidence of disease (see Supporting Fig. 3). The Kaplan‐Meier estimate of the proportion surviving at 3 years was 0.79 for the AS cohort for those with disease present and 0.97 for those with no evidence of disease. When stratified for whether the time between initial diagnosis and metastatic disease was <1 or ≥1 year, the OS curves in the AS cohort were similar (Fig. 3B), whereas the curves in the ST cohort diverged (Fig. 3C). Similarly, when analyzing by IMDC risk group, there was less clear and early separation of the OS curves in the AS cohort (Fig. 3D) compared with the definite early separation of the OS curves in the ST cohort (Fig. 3E). However, there were only 3 patients in the poor‐risk group in the AS cohort.
Figure 2.
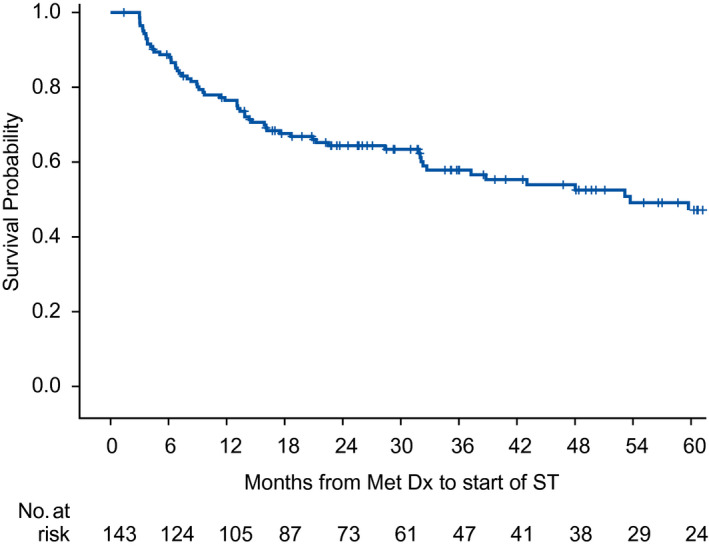
This Kaplan‐Meier curve illustrates systemic therapy (ST)–free survival in the active surveillance cohort. Dx indicates diagnosis; met, metastatic.
Figure 3.
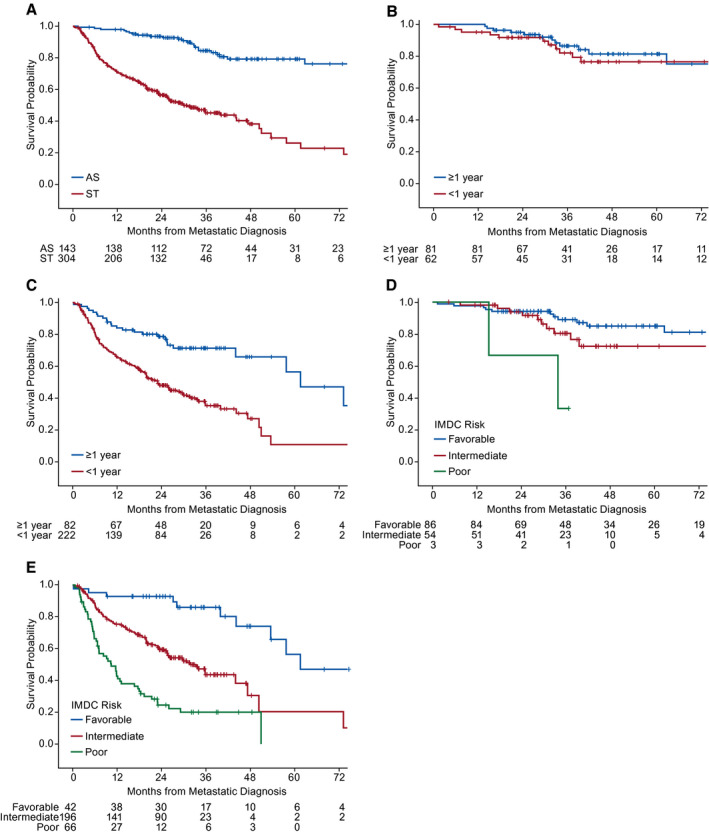
Kaplan‐Meier curves illustrate overall survival in (A) the active surveillance (AS) versus systemic therapy (ST) cohorts, (B) the AS cohort according to the time from initial diagnosis to metastatic diagnosis <1 year versus ≥1 year, (C) the ST cohort according to the time from initial diagnosis to metastatic diagnosis <1 year versus ≥1 year, (D) the AS cohort comparing those with International Metastatic Renal Cell Database Consortium (IMDC) favorable‐risk versus intermediate‐risk versus poor‐risk scores, and (E) the ST cohort comparing those with IMDC favorable‐risk versus intermediate‐risk versus poor‐risk scores.
Patient‐Reported QoL
The completion of PRO questionnaires was optional; 120 of 143 patients (84%) in the AS cohort and 239 of 305 patients (78%) in the ST cohort completed both the NCCN‐FACT FKSI‐19 and the FACT‐G questionnaires. At baseline, QoL, measured by the NCCN‐FACT FKSI‐19 (P < .0001) and the FACT‐G (P < .0001), was significantly better in patients who received AS versus those who received ST (Table 3). Scores on all subscales of the FACT‐G, except social well‐being, were significantly higher (P < .01) in the AS cohort versus ST cohort, indicating higher baseline QoL.
TABLE 3.
Baseline Patient‐Reported Outcomes in the Active Surveillance and Systemic Therapy Cohorts
| Initial Treatment Assignment | AS, n = 143 | ST, n = 305 | P |
|---|---|---|---|
| NCCN‐FACT FKSI‐19 score, no. | 120 | 239 | |
| Median (IQR: 25%, 75%) | 59 (55, 65) | 55 (46, 63) | <.0001 |
| FACT‐G score, no. | 120 | 239 | |
| Median (IQR: 25%, 75%) | 88 (76, 98) | 79 (64, 92) | <.0001 |
| Physical well‐being (IQR: 25%, 75%) | 25 (23, 27) | 23 (18, 26) | .0001 |
| Social well‐being (IQR: 25%, 75%) | 26 (22, 28) | 25 (21, 27) | .1050 |
| Emotional well‐being (IQR: 25%, 75%) | 19 (15, 21) | 17 (13, 20) | .0062 |
| Functional well‐being (IQR: 25%, 75%) | 21 (16, 26) | 17 (11, 23) | <.0001 |
Abbreviations: AS, active surveillance; FACT‐G, Functional Assessment of Cancer Therapy–General; IQR, interquartile range; NCCN‐FACT FKSI‐19, National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Kidney Symptom Index 19; ST, systemic therapy.
Discussion
We report the largest prospective, multicenter experience of AS in patients with mRCC to date. To our knowledge, there is only 1 previously reported prospective study of AS in the postcytokine era, comprising 48 patients enrolled at 5 academic centers over almost 5 years. 20 By comparison, in the MaRCC study, we were surprised to find that AS was the initial management strategy in >25% (n = 143) of enrolled patients. With a median follow‐up of 33 months from enrollment, most of these patients remained on AS. Although follow‐up is currently too short to assess long‐term outcomes, the median OS has still not been reached in the AS cohort compared with a median OS of 30 months from metastatic diagnosis in the ST cohort. This suggests that, in carefully selected patients, AS is a justifiable management option for the treatment of mRCC. Likewise, because our cohort is representative of both academic and community practice, as well as a broad range of patient demographics, we suspect AS for mRCC is a real‐world practice that may be associated with better QoL in a subgroup of patients who are destined to have excellent OS.
Our observation that patients who were receiving ST—but not AS—could be stratified for OS by a known prognostic factor (a time from initial RCC diagnosis to the development of mRCC of <1 year or ≥1 year) (Fig. 3C; see Supporting Fig. 3) suggests that those selected for AS may have a different tumor biology than those undergoing immediate ST. This also suggests that using mRCC prognostic tools, such as the IMDC risk score, derived from patient cohorts on active treatment, may not be appropriate for selecting patients for AS. Our data also suggest that AS is common in both community and academic settings and across a broad range of demographics. Furthermore, AS based on the treating physician and patient's shared decision‐making in real‐world practice may be associated with better baseline QoL.
Although there were more IMDC favorable‐risk patients in the AS cohort, intermediate‐risk and poor‐risk patients also were represented. Perhaps most surprisingly, nearly one‐half (43%) of the AS cohort included patients who developed metastases within 1 year of initial diagnosis, something that historically has been considered an indicator of a very poor prognosis and counts as an adverse attribute for formal risk stratification. However, we found that this subgroup had an OS similar to that of patients who developed metastases much later, which suggests that the time to metastasis may not be important in some patient subgroups. One potential reason for this observation is that patients who defer therapy in favor of AS are more likely to have low‐volume or oligometastatic and asymptomatic disease.
We acknowledge that further studies will be required to determine the optimal selection of patients with mRCC for AS. New prognostic models, perhaps incorporating biologic factors such as loss of polybromo 1 (PBRM1) or BRCA1‐associated protein‐1 (BAP1) status, 24 and more precise measures of tumor volume and numbers of metastases may be necessary. Prospective biomarker collection and radiographic image analysis might also be useful in future prospective observational studies.
The current data should be interpreted in the context of other management options for mRCC. The landscape of treatment options for previously untreated mRCC is changing. During the accrual period, ST options for favorable‐risk or intermediate‐risk patients included high‐dose interleukin‐2, sunitinib, pazopanib, or clinical trial participation. Currently, the NCCN guidelines list AS as a level 2A recommendation in selected patients, but with limited supportive evidence. 25 Recently approved and emerging treatment options include combination immunotherapy with nivolumab plus ipilimumab, combinations of immune checkpoint inhibitors plus VEGFR tyrosine kinase inhibitors, or cabozantinib. 6 , 8 , 26 , 27 , 28 Although these systemic options have clear disease activity and possible survival benefits, they are associated with significant cost and adverse event profiles. Rarely do these therapies lead to complete responses that allow for permanent treatment discontinuation, which suggests that most patients receiving ST will be treated indefinitely, sequencing from 1 therapy to the next. Recognizing that some of these patients may not require ST for months or years is an important and missing observation that our current study brings to the field.
This analysis has several limitations. First, this is an observational study and, although prospective, the decision to defer ST and pursue AS or to immediately start ST was left to the judgment of the treating physician and patient. Our intent was to observe physician decision making in real‐world clinical practice and not to dictate management. Second, a centralized assessment of tumor volume at baseline is missing from the data. We collected radiology reports at key time points, including at baseline, and plan to examine these data in a subsequent analysis. Third, the data are not fully mature because the median follow‐up is relatively short, and the median time to initiation of therapy in the AS cohort was not reached. However, in the context of patients undergoing immediate ST, we have already observed a clinically significant difference in OS. Finally, although we believe the MaRCC study represents a broad cross‐section of patients with mRCC, it was not mandated that consecutive patients be enrolled at each site. Therefore, we cannot rule out some element of selection bias, leading to an artificial increase or decrease in the rate of AS in the MaRCC study. We have attempted to address the potential for lead‐time bias by reporting the median time from diagnosis with metastatic disease.
Conclusions
AS occurs frequently in real‐world clinical practice and appears to be a safe and appropriate alternative to immediate ST in selected patients. Our large prospective MaRCC study is a unique resource to define this population and their outcomes for future prospective populations. Further study of these findings is warranted, including the identification of relevant clinical and laboratory factors and biomarkers, to more accurately clarify the characteristics of these patients. Given the totality of the evidence, 20 including our large prospective study, AS should become more of a standard approach for selected patients with mRCC.
Funding Support
This study was sponsored by Pfizer. Academic advisers and representatives of the study sponsor designed the study. The sponsor conducted the study and collected the data. Data were analyzed by a statistician employed by the funder. Medical writers contracted by the funder assisted in preparing the manuscript under the direction of the authors.
Conflict of Interest Disclosures
Michael R. Harrison reports personal fees and honoraria from Argos, AstraZeneca, Bayer, Exelixis Inc, Genentech, and Pfizer; and institutional research funding from Acerta, Argos, Bristol‐Myers Squibb, Exelixis Inc, Genentech, Merck, and Pfizer, outside the submitted work. Nrupen A. Bhavsar reports institutional research funding from Pfizer, outside the submitted work. Ulka Vaishampayan reports grants and personal fees from Astellas Inc, Bristol‐Myers Squibb, and Exelixis Inc, and personal fees from Bayer, Pfizer, and Genentech, outside the submitted work. Sumanta K. Pal reports personal fees from Astellas, Aveo, Bristol‐Myers Squibb, Eisai, Exelixis Inc, Genentech, Ipsen, Novartis, Pfizer, and Roche, outside the submitted work. Yousef Zakharia reports personal fees from Amgen, Castle Bioscience, Eisai, Exelixis Inc, Novartis, Pfizer, and Roche Diagnostics; and personal fees and other support from Janssen, outside the submitted work. Heather S. L. Jim reports institutional research funding from Kite and personal fees from Redhill BioPharma, Janssen Scientific Affairs, and Merck, outside the submitted work. Mayer N. Fishman reports grants from Acceleron, Alkermes, AstraZeneca, Nektar, and Prometheus; personal fees from EMD Serrono and Ipsen; and grants and personal fees from Bristol‐Myers Squibb, Eisai, Exelixis Inc, Merck, and Pfizer, outside the submitted work. Ana M. Molina reports personal fees from Novartis and Exelixis Inc, outside the submitted work. Christos E. Kyriakopoulos reports grants from Sanofi Genzyme, Pfizer, Incyte, and Merck; and personal fees and other support from Exelixis Inc, outside the submitted work. Che‐Kai Tsao reports personal fees from Pfizer, Eisai, and Boehringer Ingelheim, outside the submitted work. Leonard J. Appleman reports grants from Pfizer, outside the submitted work. Benjamin A. Gartrell reports personal fees from Exelixis Inc, Pfizer, Janssen, Genomic Health, and EMD Serono, outside the submitted work. Arif Hussain reports personal fees from Novartis, Bayer, Bristol‐Myers Squibb, AstraZeneca, and Pfizer, outside the submitted work. Walter M. Stadler reports grants from Calithera, Exelixis Inc, Merck, Novartis, and X4 Pharmaceuticals; grants and personal fees from AstraZeneca, Bayer, Bristol‐Myers Squibb, Eisai, Genentech, and Pfizer; and personal fees from Caremark‐CVS, outside the submitted work. Neeraj Agarwal reports personal fees from Astellas, AstraZeneca, Bayer, Bristol‐Myers Squibb, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis Inc, Foundation Medicine, Genentech, Janssen, Merck, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics, outside the submitted work. Russell K. Pachynski reports institutional research funding from Ferring; institutional research funding and personal fees from Janssen; and personal fees from Argos, AstraZeneca, Bristol‐Myers Squibb, Dendreon, EMD Serrono, Exelixis Inc, Jounce, Sanofi‐Genzyme, Genentech/Roche, Genomic Health, and Merck, outside the submitted work. Thomas E. Hutson reports grants and personal fees from Bristol‐Myers Squibb, Exelixis Inc, Pfizer, Novartis, Aveo, Astellas, Genentech/Roche, and Merck, outside the submitted work. Hans Joerg Hammers reports personal fees from Bristol‐Myers Squibb, Merck, SFJ Pharmaceuticals, Novartis, Armo Biosciences, and Pfizer, outside the submitted work. Christopher W. Ryan reports institutional research funding from Argos Therapeutics, Bristol‐Myers Squibb, CytRx Corporation, Daiichi‐Sankyo, Genentech, GlaxoSmithKline/Novartis, Janssen, Karyopharm Therapeutics, MabVax Therapeutics, Merck, Morphotek, Threshold Pharmaceuticals, and TRACON Pharma; institutional research funding and personal fees from Eisai, and Exelixis Inc; and personal fees from Genentech/Roche, Novartis, and Pfizer, outside the submitted work. Brant A. Inman reports grants from Dendreon, FKD Therapies, QED Therapeutics, Abbott Laboratories, and Anchiano Therapeutics; grants and personal fees from Genentech/Roche, Combat Medical, Nucleix, and Taris Biomedical; and personal fees from Fergene, outside the submitted work. Jack Mardekian was an employee of Pfizer during the conduct of the study. Azah Borham is an employee of Pfizer and holds shares in the company. Daniel J. George reports grants from Genentech/Roche, Novartis, Astellas, Celldex, and Acerta; grants and personal fees from Exelixis Inc, Janssen, Pfizer, Innocrin Pharma, and Bristol‐Myers Squibb; and personal fees from Sanofi, Bayer, and Merck, outside the submitted work. Brian A. Costello made no disclosures.
Author Contributions
Michael R. Harrison: Conceptualization, data curation, formal analysis, investigation, methodology, supervision, validation, writing– original draft, and writing–review and editing. Brian A. Costello: Conceptualization, data curation, formal analysis, investigation, methodology, writing–original draft, and writing–review and editing. Nrupen A. Bhavsar: Conceptualization, formal analysis, investigation, methodology, writing–original draft, and writing–review and editing. Ulka Vaishampayan: Conceptualization, data curation, formal analysis, investigation, methodology, writing–original draft, and writing–review and editing. Sumanta K. Pal: Data curation, investigation, writing–original draft, and writing–review and editing. Yousef Zakharia: Conceptualization, formal analysis, investigation, methodology, writing–original draft, and writing–review and editing. Heather S. L. Jim: Conceptualization, data curation, investigation, methodology, writing–original draft, and writing–review and editing. Mayer N. Fishman: Conceptualization, data curation, formal analysis, investigation, methodology, writing–original draft, and writing–review and editing. Ana M. Molina: Data curation, investigation, writing–original draft, and writing–review and editing. Christos E. Kyriakopoulos: Data curation, formal analysis, investigation, writing writing–original draft, and writing–review and editing. Che‐Kai Tsao: Data curation, formal analysis, investigation, writing–original draft, and writing–review and editing. Leonard J. Appleman: Conceptualization, data curation, formal analysis, investigation, methodology, writing–original draft, and writing–review and editing. Benjamin A. Gartrell: Conceptualization, data curation, writing–original draft, and writing–review and editing. Arif Hussain: Data curation, writing–original draft, and writing–review and editing. Walter M. Stadler: Data curation, writing–original draft, and writing–review and editing. Neeraj Agarwal: Data curation, formal analysis, investigation, writing–original draft, and writing–review and editing. Russell K. Pachynski: Data curation, writing–original draft, and writing–review and editing. Thomas E. Hutson: Data curation, writing–original draft, and writing–review and editing. Hans J. Hammers: Data curation, writing–original draft, and writing–review and editing. Christopher W. Ryan: Data curation, writing–original draft, and writing–review and editing. Brant A. Inman: Conceptualization, writing–original draft, and writing–review and editing. Jack Mardekian: Data curation, formal analysis, validation, writing–original draft, and writing–review and editing. Azah Borham: Conceptualization, data curation, formal analysis, investigation, methodology, supervision, validation, writing–original draft, and writing–review and editing. Daniel J. George: Conceptualization, methodology, supervision, validation, writing–original draft, and writing–review and editing. Academic advisers and representatives of the study sponsor designed the study. The sponsor conducted the study and collected the data. Data were analyzed by a statistician employed by the funder. Medical writers contracted by the funder assisted in preparing the article under the direction of the authors. All authors helped prepare, review, and approve the article.
Supporting information
Fig S1‐S3
Harrison MR, Costello BA, Bhavsar NA, Vaishampayan U, Pal SK, Zakharia Y, Jim HSL, Fishman MN, Molina AM, Kyriakopoulos CE, Tsao C‐K, Appleman LJ, Gartrell BA, Hussain A, Stadler WM, Agarwal N, Pachynski RK, Hutson TE, Hammers HJ, Ryan CW, Inman BA, Mardekian J, Borham A, George DJ. Active surveillance of metastatic renal cell carcinoma: Results from a prospective observational study (MaRCC). Cancer. 2021. 10.1002/cncr.33494
See editorial on pages 2184‐2186, this issue.
Editorial and writing support was provided by Vardit Dror, PhD, and Philip Matthews, PhD, CMPP, of Engage Scientific Solutions, and funded by Pfizer.
Data Availability
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information), Pfizer will provide access to individual de‐identified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices: 1) for indications that have been approved in the United States and/or the European Union, or 2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de‐identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- 1. Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530‐2540. [DOI] [PubMed] [Google Scholar]
- 2. Mekhail TM, Abou‐Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan‐Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23:832‐841. [DOI] [PubMed] [Google Scholar]
- 3. Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor‐targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794‐5799. [DOI] [PubMed] [Google Scholar]
- 4. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal‐cell carcinoma. N Engl J Med. 2007;356:115‐124. [DOI] [PubMed] [Google Scholar]
- 5. Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061‐1068. [DOI] [PubMed] [Google Scholar]
- 6. Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35:591‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atkins MB, McDermott DF, Powles T, et al. IMmotion150: a phase II trial in untreated metastatic renal cell carcinoma (mRCC) patients (pts) of atezolizumab (atezo) and bevacizumab (bev) vs and following atezo or sunitinib (sun) [abstract]. J Clin Oncol. 2017;35(15 suppl):4505. doi: 10.1200/JCO.2017.35.15_suppl.4505 [DOI] [Google Scholar]
- 8. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N Engl J Med. 2018;378:1277‐1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med. 2019;380:1116‐1127. [DOI] [PubMed] [Google Scholar]
- 10. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med. 2019;380:1103‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marchioni M, Bandini M, Pompe RS, et al. Survival of metastatic renal cell carcinoma patients continues to improve over time, even in targeted therapy era. Int Urol Nephrol. 2017;49:2143‐2149. [DOI] [PubMed] [Google Scholar]
- 12. Lindskog M, Wahlgren T, Sandin R, et al. Overall survival in Swedish patients with renal cell carcinoma treated in the period 2002 to 2012: update of the RENCOMP study with subgroup analysis of the synchronous metastatic and elderly populations. Urol Oncol. 2017;35:541.e15‐541.e22. [DOI] [PubMed] [Google Scholar]
- 13. Heng DY, Choueiri TK, Rini BI, et al. Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol. 2014;25:149‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitchell AP, Hirsch BR, Harrison MR, Abernethy AP, George DJ. Deferred systemic therapy in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer. 2015;13:e159‐e166. [DOI] [PubMed] [Google Scholar]
- 15. Matsubara N, Mukai H, Naito Y, Itoh K, Komai Y, Sakai Y. First experience of active surveillance before systemic target therapy in patients with metastatic renal cell carcinoma. Urology. 2013;82:118‐123. [DOI] [PubMed] [Google Scholar]
- 16. Park I, Lee JL, Ahn JH, et al. Active surveillance for metastatic or recurrent renal cell carcinoma. J Cancer Res Clin Oncol. 2014;140:1421‐1428. [DOI] [PubMed] [Google Scholar]
- 17. Dabestani S, Marconi L, Hofmann F, et al. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol. 2014;15:e549‐e561. [DOI] [PubMed] [Google Scholar]
- 18. Zaid HB, Parker WP, Safdar NS, et al. Outcomes following complete surgical metastasectomy for patients with metastatic renal cell carcinoma: a systematic review and meta‐analysis. J Urol. 2017;197:44‐49. [DOI] [PubMed] [Google Scholar]
- 19. Oliver RT, Nethersell AB, Bottomley JM. Unexplained spontaneous regression and alpha‐interferon as treatment for metastatic renal carcinoma. Br J Urol. 1989;63:128‐131. [DOI] [PubMed] [Google Scholar]
- 20. Rini BI, Dorff TB, Elson P, et al. Active surveillance in metastatic renal‐cell carcinoma: a prospective, phase 2 trial. Lancet Oncol. 2016;17:1317‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhavsar NA, Harrison MR, Hirsch BR, et al. Design and rationale of the Metastatic Renal Cell Carcinoma (MaRCC) Registry: a prospective academic and community‐based study of patients with metastatic renal cell cancer. Cancer Invest. 2017;35:333‐344. [DOI] [PubMed] [Google Scholar]
- 22. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal‐cell carcinoma. N Engl J Med. 2013;369:722‐731. [DOI] [PubMed] [Google Scholar]
- 23. Mitchell AP, Harrison MR, Walker MS, George DJ, Abernethy AP, Hirsch BR. Clinical trial participants with metastatic renal cell carcinoma differ from patients treated in real‐world practice. J Oncol Pract. 2015;11:491‐497. [DOI] [PubMed] [Google Scholar]
- 24. Miao D, Margolis CA, Gao W, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359:801‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Motzer RJ, Jonasch E, Michaelson MD, et al. NCCN Guidelines Insights: Kidney Cancer, Version 2.2020. J Natl Compr Canc Netw. 2019;17:1278‐1285. [DOI] [PubMed] [Google Scholar]
- 26. Escudier B, Tannir NM, McDermott DF, et al. CheckMate 214: efficacy and safety of nivolumab plus ipilimumab vs sunitinib for treatment‐naive advance or metastatic renal cell carcinoma, including IMDC risk and PD‐L1 expression subgroups [abstract]. Ann Oncol. 2017;28(suppl 5):LBA5. [Google Scholar]
- 27. Motzer RJ, Atkins MB, Escudier B, et al. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC) [abstract]. J Clin Oncol. 2018;36(suppl 6):578. [Google Scholar]
- 28. Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non‐randomised, open‐label, dose‐finding, and dose‐expansion phase 1b trial. Lancet Oncol. 2018;19:405‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S3
Data Availability Statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information), Pfizer will provide access to individual de‐identified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices: 1) for indications that have been approved in the United States and/or the European Union, or 2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de‐identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.


