Foreword

Foreword – antimicrobial prescribing guidelines for poultry
Antimicrobials are essential to modern medicine for treating a range of infections in humans and animals. Importantly, antimicrobial resistance (AMR) is a growing global threat that presents a serious risk to human and animal health. Inappropriate and/or unrestrained use of antimicrobials in humans and animals exerts a strong selection pressure on microbial populations to evolve resistant traits. As a result, antimicrobials have become less effective over time leading to treatment complications and failures, and increased healthcare costs for people and animals. Resistant organisms spread between people, animals and the environment. Globalisation and international travel facilitates this spread between countries.
Here in Australia, the veterinary profession and food‐producing animal industries have a long history of addressing AMR. Their previous and ongoing work – a result of partnerships across the animal sector – has resulted in demonstrated low levels of AMR in our food‐producing animals. Over the past 5 years, the veterinary profession has consolidated its partnership with industry and government by helping to successfully implement Australia's First National Antimicrobial Resistance Strategy 2015–19. With the recent release of Australia's National Antimicrobial Resistance Strategy – 2020 and Beyond (2020 AMR Strategy), the veterinary profession will continue to play a critical role in how we minimise AMR.
One of the seven key objectives of the 2020 AMR Strategy relates to appropriate antimicrobial usage and antimicrobial stewardship practices. Resistance to antimicrobials occurs naturally in microorganisms, but it is significantly amplified by antimicrobial overuse, growth promotion use, and poor husbandry and management.
The antimicrobial prescribing guidelines for poultry directly addresses the fourth objective of the 2020 AMR Strategy, and in particular, Priority Area for Action 4.1, that seeks to ‘ensure that coordinated, evidence‐based antimicrobial prescribing guidelines and best‐practice supports are developed and made easily available, and encourage their use by prescribers’.
These guidelines for Australian poultry veterinarians are sure to be a ready resource. They have been developed specifically for the Australian poultry industry and contain best‐practice prescribing information to help clinical veterinarians in their day‐to‐day use of antimicrobials. The guidelines encourage veterinarians to first pause and consider the need to use antimicrobials in that circumstance: Are there effective non‐antimicrobial alternatives? Prevention and control of infections through strict on‐farm biosecurity is a recognised approach to minimising disease entry and the need to use antimicrobials. Vaccination may also be available to control several important poultry diseases. If antimicrobial use is indicated, have you considered the five rights: right drug, right time, right dose, right duration and right route? Using a lower rating or narrow‐spectrum antimicrobial is the preferred approach, and you can also refer to the Australian Antibacterial Importance Ratings to help with these decisions.
I commend the work of all involved in the development of these guidelines, and urge every poultry veterinarian to use this advice. In doing so, you will help safeguard the ongoing, long‐term efficacy of antimicrobials, deliver the best possible veterinary service to the Australian poultry industry, and play your role in the global response to AMR.
Dr Mark Schipp
Australian Chief Veterinary Officer
President of the OIE World Assembly
Expert panel members

Dr Peter Gray BVSc
Peter Gray graduated with a Bachelor of Veterinary Science from Sydney University in 1983. He spent 2 years in a pet and aviary bird‐focused private practice in western Sydney, and started with Inghams Enterprises in 1986 as a poultry veterinarian. During his ongoing work life at Inghams, Peter has had technical and veterinary roles that have involved him in all aspects of a poultry operation from importation, export, breeding, feed mills, hatching, growing, processing and further processing. His work has covered veterinary work in both chicken and turkey species, as well as welfare and food safety. He has always valued the learnings from many experienced colleagues both from within Inghams and the wider Australian Veterinary Poultry Association community. He has been a representative on industry and government committees, and is a qualified poultry welfare auditor with the Professional Animal Auditor Certification Organization (PAACO). Over the course of his working life, he has seen great change in the Australian industry where genetics, biosecurity, new vaccine strategies and improved management practices have seen an extensive reduction in antibiotic use in the poultry industry. He hopes these guidelines can play a part in continuing that positive trend while maintaining good welfare outcomes for the birds under our care.

Dr Rod Jenner BVSc
Rod Jenner is a consultant poultry veterinarian consulting to both the chicken meat and egg industries, and conducting projects on behalf of Agrifutures Australia and Australian Eggs Ltd. He has been in the poultry industry since graduation.
Rod has served on a number of industry representative committees over the years, including the RIRDC chicken meat advisory committee, and has also served as President of the Australian Veterinary Poultry Association (AVPA), member of Therapeutics Subcommittee and Welfare Subcommittee of the AVPA, Queensland executive of the AVA, and divisional committee of the WPSA. Of recent years, Rod has progressed into teaching veterinary students in the area of commercial poultry medicine at the University of Queensland and James Cook University.

Professor Jacqueline Norris BVSc MVS, PhD, FASM, MASID Grad Cert Higher Ed.
Jacqueline Norris is Professsor of Veterinary Microbiology and Infectious Diseases, and Associate Head of Research at the Sydney School of Veterinary Science, at the University of Sydney. She is a registered practicing veterinarian and is passionate about practical research projects and education programs for veterinary professionals, animal breeders and animal owners. Her main research areas include (1) development of diagnostics and treatments for companion animal viral diseases; (2) Q fever; (3) multidrug‐resistant (MDR) Staphylococcus species; (4) infection prevention and control in veterinary practices; (5) chronic renal disease in domestic and zoo Felids and (6) factors influencing antimicrobial prescribing behaviour of vets and health professionals.

Dr Stephen Page BSc(Vet)(Hons) BVSc(Hons) DipVetClinStud MVetClinStudMAppSci(EnvTox) MANZCVS(Pharmacology)
Stephen Page is a consultant veterinary clinical pharmacologist and toxicologist and founder and sole director of Advanced Veterinary Therapeutics – a consulting company that provides advice on appropriate use of veterinary medicines to veterinarians, veterinary organisations (Australian Veterinary Association, World Veterinary Association, World Organisation for Animal Health), state and national government departments and statutory bodies (APVMA, Department of Agriculture, Department of Health, US Environmental Protection Agency), and global organisations (OIE, FAO, Chatham House).
He is a member of the AVA Antimicrobial of Resistance Advisory Group (ARAG), a member of the ASTAG committee on antimicrobial prioritisation. In 2017, he became the President of the ANZCVS Chapter of Pharmacology, and is a member the World Veterinary Association Pharmaceutical Stewardship Committee.
He has more than 100 publications on which he is author or editor, including chapters on antimicrobial stewardship, clinical pharmacology, adverse drug reactions, use of antimicrobial agents in livestock, and antimicrobial drug discovery and models of infection.
Stephen Page has been a teacher and facilitator of courses at the University of Sydney on food safety, public health and antimicrobial resistance since 2003.
He is regularly invited to speak nationally and internationally at a broad range of conferences and symposiums, especially on the subjects of antimicrobial use, antimicrobial stewardship and risk assessment. He gave his first presentation on veterinary antimicrobial resistance and stewardship at the AVA Conference in Perth in 2000 and remains passionate about improving the use and effective life span of antimicrobial agents.

Professor Glenn Browning BVSc (Hons I) DipVetClinStud, PhD, FASM
Glenn Browning is Professor in Veterinary Microbiology, Director of the Asia‐Pacific Centre for Animal Health and Acting Head of the Melbourne Veterinary School at the University of Melbourne. He completed the Bachelor of Veterinary Science with First Class Honours at the University of Sydney in 1983, a postgraduate Diploma of Veterinary Clinical Studies in Large Animal Medicine and Surgery at the University of Sydney in 1984 and a PhD in Veterinary Virology at the University of Melbourne in 1988.
He was a Veterinary Research Officer at the Moredun Research Institute in Edinburgh from 1988 to 1991, investigating viral enteritis in horses, then joined the staff of the Faculty of Veterinary Science at the University of Melbourne, and has been a member of teaching and research staff there since 1991.
Professor Browning teaches in veterinary and agricultural microbiology. He is a Life Fellow of the Australian Veterinary Association, a Fellow of the Australian Society for Microbiology and Chair Elect of the International Organisation for Mycoplasmology.
He has co‐authored 235 peer‐reviewed research papers and book chapters, has edited two books on recent progress in understanding the mycoplasmas and has co‐supervised 50 research higher degree students. His research interests include the molecular pathogenesis and epidemiology of bacterial and viral pathogens of animals, the development of novel vaccines and diagnostic assays to assist in control of infectious diseases, and antimicrobial stewardship in veterinary medicine.
The 5R framework for good antimicrobial stewardship

Derived from: Page S, Prescott J and Weese S. Veterinary Record 2014;175:207‐208. Image courtesy of Trent Hewson, TKOAH.
Contents
| Core principles of appropriate use of antimicrobial agents | 4 |
| Introduction | 6 |
| Interactions contributing to pharmacokinetic variability | 9 |
| Conclusion | 11 |
| Disease investigation – general approach | 11 |
| Diseases of the digestive tract | 12 |
| Diseases of the respiratory system | 34 |
| Diseases of the locomotory system | 37 |
| Systemic diseases | 39 |
| Diseases of the reproductive system | 41 |
| Diseases of the nervous system | 44 |
| Immunosuppressive diseases | 45 |
| Diseases of the young chick | 46 |
| Treatment | 48 |
| Diseases of turkeys, ducks and other poultry | 48 |
| Considerations for choice of first priority antimicrobials | 49 |
| References | 49 |
Core principles of appropriate use of antimicrobial agents
While the published literature is replete with discussion of misuse and overuse of antimicrobial agents in medical and veterinary situations there has been no generally accepted guidance on what constitutes appropriate use. To redress this omission, the following principles of appropriate use have been identified and categorised after an analysis of current national and international guidelines for antimicrobial use published in the veterinary and medical literature. Independent corroboration of the validity of these principles has recently been provided by the publication (Monnier et al 2018) of a proposed global definition of responsible antibiotic use that was derived from a systematic literature review and input from a multidisciplinary international stakeholder consensus meeting. Interestingly, 22 elements of responsible use were also selected, with 21 of these 22 elements captured by the separate guideline review summarised below.
Pre‐treatment principles
-
Disease prevention
Apply appropriate biosecurity, husbandry, hygiene, health monitoring, vaccination, nutrition, housing, and environmental controls. Use Codes of Practice, Quality Assurance Programmes, Herd Health Surveillance Programmes and Education Programmes that promote responsible and prudent use of antimicrobial agents.
-
Professional intervention
Ensure uses (labelled and extra‐label) of antimicrobials meet all the requirements of a bona fide veterinarian‐client‐patient relationship.
-
Alternatives to antimicrobial agents
Efficacious, scientific evidence‐based alternatives to antimicrobial agents can be an important adjunct to good husbandry practices.
Diagnosis
-
4
Accurate diagnosis
Make clinical diagnosis of bacterial infection with appropriate point of care and laboratory tests, and epidemiological information.
Therapeutic objective and plan
-
5
Therapeutic objective and plan
Develop outcome objectives (for example clinical or microbiological cure) and implementation plan (including consideration of therapeutic choices, supportive therapy, host, environment, infectious agent and other factors).
Drug selection
-
6
Justification of antimicrobial use
Consider other options first; antimicrobials should not be used to compensate for or mask poor farm or veterinary practices.
Use informed professional judgement balancing the risks (especially the risk of AMR selection & dissemination) and benefits to humans, animals & the environment.
-
7
Guidelines for antimicrobial use
Consult disease‐ and species‐specific guidelines to inform antimicrobial selection and use.
-
8
Critically important antimicrobial agents
Use all antimicrobial agents, including those considered important in treating refractory infections in human or veterinary medicine, only after careful review and reasonable justification.
-
9
Culture and susceptibility testing
Utilise culture and susceptibility (or equivalent) testing when clinically relevant to aid selection of antimicrobials, especially if initial treatment has failed.
-
10
Spectrum of activity
Use narrow‐spectrum in preference to broad‐spectrum antimicrobials whenever appropriate.
-
11
Extra‐label (off‐label) antimicrobial therapy
Must be prescribed only in accordance with prevailing laws and regulations.
Confine use to situations where medications used according to label instructions have been ineffective or are unavailable and where there is scientific evidence, including residue data if appropriate, supporting the off‐label use pattern and the veterinarian's recommendation for a suitable withholding period and, if necessary, export slaughter interval (ESI).
Drug use
-
12
Dosage regimens
Where possible optimise regimens for therapeutic antimicrobial use following current pharmacokinetic and pharmacodynamic (PK/PD) guidance.
-
13
Duration of treatment
Minimise therapeutic exposure to antimicrobials by treating only for as long as needed to meet the therapeutic objective.
-
14
Labelling and instructions
Ensure that written instructions on drug use are given to the end user by the veterinarian, with clear details of method of administration, dose rate, frequency and duration of treatment, precautions and withholding period.
-
15
Target animals
Wherever possible limit therapeutic antimicrobial treatment to ill or at‐risk animals, treating the fewest animals possible.
-
16
Record keeping
Keep accurate records of diagnosis (indication), treatment and outcome to allow therapeutic regimens to be evaluated by the prescriber and permit benchmarking as a guide to continuous improvement.
-
17
Compliance
Encourage and ensure that instructions for drug use are implemented appropriately
-
18
Monitor response to treatment
Report to appropriate authorities any reasonable suspicion of an adverse reaction to the medicine in either treated animals or farm staff having contact with the medicine, including any unexpected failure to respond to the medication.
Thoroughly investigate every treated case that fails to respond as expected.
Post‐treatment activities
-
19
Environmental contamination
Minimise environmental contamination with antimicrobials whenever possible.
-
20
Surveillance of antimicrobial resistance
Undertake susceptibility surveillance periodically and provide the results to the prescriber, supervising veterinarians and other relevant parties.
-
21
Continuous evaluation
Evaluate veterinarians' prescribing practices continually, based on such information as the main indications and types of antimicrobials used in different animal species and their relation to available data on antimicrobial resistance and current use guidelines.
-
22
Continuous improvement
Retain an objective and evidence guided assessment of current practice and implement changes when appropriate to refine and improve infection control and disease management.
Each of the core principles is important but CORE PRINCIPLE 11 Extra‐label (off‐label) Antimicrobial Therapy can benefit from additional attention as veterinarians, with professional responsibility for prescribing and playing a key role in residue minimisation, must consider the tissue residue and withholding period (WHP) and, if necessary, export slaughter interval (ESI) implications of off‐label use before selecting this approach to treatment of animals under their care (Reeves 2010; APVMA 2018).
The subject of tissue residue kinetics and calculation of WHPs is very complex requiring a detailed understanding of both pharmacokinetics (PK) and statistics, as both these fields underpin the recommendation of label WHPs. Some key points to consider when estimating an off‐label use WHP include the following:
The new estimate of the WHP will be influenced by (1) the off‐label dose regimen (route, rate, frequency, duration); (2) the elimination rate of residues from edible tissues and (3) the maximum residue limit (MRL).
Approved MRLs are published in the MRL Standard which is linked to the following A PVMA website page: https://apvma.gov.au/node/10806
If there is an MRL for the treated species, then the WHP recommended following the proposed off label use must ensure that residues have depleted below the MRL at the time of slaughter.
If there is no MRL for the treated species, then the WHP recommendation must ensure that no detectable residues are present at the time of slaughter.
Tissue residue kinetics may be quite different to the PK observed in plasma – especially the elimination half‐life and rate of residue depletion. The most comprehensive source of data on residue PK is that of Craigmill et al 2006.
WHP studies undertaken to establish label WHP recommendations are generally undertaken in healthy animals. Animals with infections are likely to have a longer elimination half‐life.
There are many factors that influence variability of the PK of a drug preparation, including the formulation, the route of administration, the target species, age, physiology, pathology and diet.
The following figure provides a summary of typical effects on elimination rates associated with drug use at higher than labelled rates and in animals with infections (Figure 1).
Figure 1.
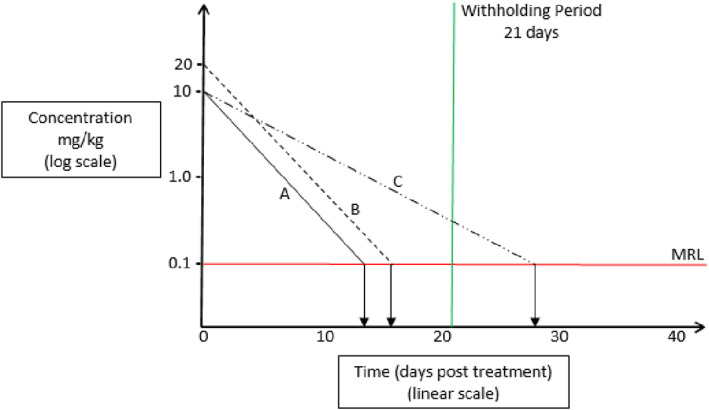
An example of the relationship between the maximum residue limit (MRL) and tissue depletion following administration of a veterinary medicine. In a healthy animal (A), tissue depletion to the MRL often occurs at a time point shorter than the WHP that has been established for the 99/95th percentile of the population. In such an individual animal, if the dose is doubled, tissue depletion (B) should only require one more half‐life and would most likely still be within the established WHP. However, if the half‐life doubles due to disease or other factors, depletion (C) would now require double the normal WHP and may still result in residues exceeding the MRL (adapted from Riviere and Mason, 2011).
References
APVMA. Residues and trade risk assessment manual. Version 1.0 DRAFT. Australian Pesticides and Veterinary Medicines Authority, Kingston, ACT, 2018.
Craigmill AL, Riviere JE, Webb AI. Tabulation of FARAD comparative and veterinary pharmacokinetic data. Ames, Iowa, Wiley‐Blackwell, 2006.
Monnier AA, Eisenstein BI, Hulscher ME, Gyssens IC, Drive‐AB. WP1 Group. Towards a global definition of responsible antibiotic use: results of an international multidisciplinary consensus procedure. Journal of Antimicrobial Chemotherapy 2018;73:3‐16.
Reeves PT. Drug Residues. In: Cunningham F, Elliott J, Lees P, editors. Comparative and Veterinary Pharmacology. Springer Berlin Heidelberg, Berlin, Heidelberg, 2010;265‐290.
Riviere JE, Mason SE. Tissue residues and withdrawal times. In: Riviere JE, editor. Comparative Pharmacokinetics Principles, Techniques, and Applications. second edn. Wiley‐Blackwell, Oxford, UK, 2011;413‐424.
Introduction
Management of disease outbreaks on a commercial poultry farm
Commercial poultry veterinary medicine is a unique stream of veterinary science that focuses strongly on preventive medicine. Infectious disease outbreaks are most commonly the result of lapses in biosecurity, which are not always totally preventable and should never be unexpected. Biosecurity in this context is more than quarantine. It has external, internal and resilience components, which include vaccination, preventive medication, optimal nutrition, appropriate genetics, good husbandry and exemplary management.
The methods used for diagnostic investigation are quite diverse, even though they are being applied to a single animal species, and often to the relatively uniform context of a commercial farm. Animal behaviour, or ethology, is the most frequently used diagnostic tool, and probably the least acknowledged skill used by a field veterinarian. Gross pathology, histopathology, epidemiology, microbiology, and serology are all important diagnostic tools, while the disciplines of immunology, pharmacology, therapeutics and veterinary medicine in public health are employed by commercial poultry veterinarians in the conduct of their role.
Disease treatment considerations
Food industry
The number one consideration is always that the veterinarian is operating within a food production system. Every decision about treatment must incorporate considerations about the wholesomeness of the animal or product as a human food source.
Food safety considerations are paramountTreatment options are severely limited.
Broiler chickens have a very short lifespan relative to antimicrobial treatment regimens. The prescribing veterinarian must be cognisant of the likely slaughter date of the flock before recommending treatments. The use and consequences of antimicrobial therapies must be clearly communicated with both the farmer and the owner/processor of the chickens to ensure that treatment will not contravene the advised WHP.
Egg laying flocks are in constant production, so advice on WHPs precludes the sale or supply of eggs into the food sector for the duration of the WHP for any medication that has a WHP longer than 0 days (NIL).
Backyard poultry flocks are commonly kept for enjoyment, egg production and occasionally for their meat. In most instances, movement of animals, eggs and meat is confined to the primary household. However, it is not uncommon for surplus eggs and chickens to be sold or given away to neighbours and work colleagues. Additionally, certain fancy varieties of backyard poultry may be extensively traded and sold between individuals. Therefore, the prescribing veterinarian must be aware of the medications that can be used safely in poultry and their associated WHPs. Inappropriate advice may have far‐reaching implications.
Treating a flock, not an individual
Treatments are generally applied to an entire flock, rather than to an individual bird. It is cost‐prohibitive to consider hospital pens in large‐scale operations, but this can be feasible in smaller niche farms, or with high value stock (e.g. rare breeds, genetically superior stock, or during situations of severe shortage). However, even high value commercial stocks are generally replaceable, so it is unusual to treat an individual commercial bird.
In contrast, in small backyard poultry flocks, it is common for owners to have a strong bond with their birds. In such instances, the birds may have become part of the family and the owners may be willing to go to extensive lengths to ensure their birds receive individual veterinary medical attention.
When treating a flock with an antimicrobial agent, consideration needs to be given to the long‐term commercial return, as well as the short‐term response.
Valid grounds for antimicrobial medication include animal welfare, managing the risks of disease in susceptible flocks, the zoonotic potential of the disease and true economic loss when there is a no more effective way to control the disease.
Medication is not justified when it will be ineffective, for example for viral or nutritional diseases.
Medication is often not the best approach to disease control, even though in theory, it may be effective. It may be best to process birds early or, in mild cases, let the disease run its course.
Medication can sometimes be counter‐productive, for example, when it may have an impact on live bacterial vaccines.
Medication is unwarranted if the intention is solely to provide non‐specific cover over stressful periods, to be seen to be doing something, to bring peace of mind, or to use up excess drug stocks.
Prudent use
It is important to remember that if antimicrobial therapy is being considered, mass medication in water or feed will not only target sick birds, but will be consumed by healthy birds. In addition, sick chickens tend to have reduced feed and water consumption, limiting their antimicrobial intake. Thus, mass antimicrobial therapy is not targeted therapy, but rather, is largely a preventive approach to limiting the spread of bacteria to healthy individuals.
Treatment options are severely limited in Australia by the restricted number of registered veterinary medicines available for administration in feed or water, and by food safety considerations, placing more emphasis on the importance of preventive measures.
Non‐steroidal anti‐inflammatory drugs are not registered for use in poultry and are never used in poultry medicine, so therapeutic options are limited to antimicrobials. There are relatively few alternatives to preventive antimicrobial therapy, but options include (with variable evidence of efficacy) medium‐chain fatty acids, probiotics, prebiotics (for example, mannan oligosaccharide derivatives), acidifiers, essential oil extracts and many more.
The use of antimicrobials in commercial poultry production is under considerable pressure and can be influenced by major customers, with a growing expectation to demonstrate good antimicrobial stewardship, and an emphasis on strategies to reduce use. Veterinary intervention is closely scrutinised, and there is an increasing requirement to justify approaches to flock health when they involve the use of antimicrobial agents.
Backyard poultry: It can be difficult to design appropriate treatment regimens for backyard poultry due to the limited number of registered veterinary medicines available. Clients may place pressure on the prescribing veterinarian to provide medications that are not approved for use in poultry. This largely occurs when the birds are kept primarily as pets and their eggs are not consumed. It is important for the prescribing veterinarian to be aware that backyard poultry are classified as food‐producing species and to investigate which antimicrobial agents can be used safely and legally. If unregistered medicines or off‐label uses are prescribed, then the prescribing veterinarian must determine and recommend an appropriate WHP for eggs and meat.
Practical considerations
Diagnosis
It is essential that a diagnosis, even if only presumptive, is made before considering medication.
-
2
Drug susceptibility and resistance
All infectious organisms have an inherent pattern of susceptibility and resistance to specific drugs. Resistance to certain drugs may also be acquired. Acquired resistance may be determined by laboratory susceptibility tests or inferred by prior clinical experience and previous response to therapy on a particular farm, although it should always be remembered that prior clinical experience can be misleading, as clinical improvement of a flock may not have been a result of successful antimicrobial therapy. Sampling for susceptibility testing prior to antimicrobial use is essential.
-
3
Bactericidal vs bacteriostatic
Bactericidal antimicrobials kill bacteria, thereby reducing the number of organisms, whereas bacteriostatic antimicrobials inhibit the metabolism, growth or multiplication of bacteria, thereby preventing an increase in the number of organisms. In practice, this generally makes little difference, as a functional immune system is essential for resolution of all infectious diseases, regardless of the mode of action of the drug used to treat them.
-
4
Site of infection
Choosing a drug that will reach the site of infection at an effective concentration for enough time is an important consideration.
-
5
Dose rate
Having selected a drug that is likely to be effective, an appropriate dose rate must be determined. Dose rates should be selected and calculated using the following guidelines:
Water and feed consumption can vary considerably, and is affected by flock health, ambient temperature, species, physiological status and management practices. Therefore, where information is available, antimicrobial dose rates based on bodyweight, in conjunction with known current water or feed consumption, provide the most accurate dosages. The exception is in young rapidly growing birds, where dose rate expressed as a concentration in feed or water provides a more practical calculation method.
Treatment should always commence at maximum recommended dose rates for the greatest efficacy.
Dose rate may need to be adjusted to allow for spillage or wastage, which can be considerable, especially in ducks.
When calculating a dose to be delivered in water, it is necessary to know the:
Bodyweight of the flock (determined by weighing a representative sample of birds)
Amount of water expected to be consumed during the medication period
Required dose rate
Concentration of the active ingredient in the selected antimicrobial product
-
6
Onset of medication
Normally treatment should commence as soon as a presumptive diagnosis is available when disease is acute and a high mortality rate is expected, for example, in fowl cholera (infection with Pasteurella multocida).
For more chronic disease, it is appropriate to wait for the results of susceptibility testing.
-
7
Frequency of medication
In theory, for time‐dependent antimicrobial agents, the minimum inhibitory concentration (MIC) of a drug should be maintained or exceeded at the site of infection throughout the course of treatment to ensure that the infecting organism remains suppressed and is less likely to acquire resistance. It is critical to ensure that the amount of medicated water supplied each day is sufficient to eliminate the risk of birds running out of water during times when the manager is not on the farm (e.g. overnight).
-
8
Duration of medication
In acute disease outbreaks, medication should continue until mortalities stop and clinical signs are no longer apparent in the flock. Usually this takes at least 3 days, and mortalities may continue to rise for the first few days as severely affected birds succumb, especially if they are too sick to consume any medication. However, acute diseases are usually under control within 5–7 days, and if no response is apparent within 3–5 days, the diagnosis and treatment regimen should be reassessed.
Some diseases may require ongoing medication in feed or water to suppress clinical disease and potential spread to other flocks.
-
9
Routes of administration
Oral administration is most effective for infections involving the digestive tract. Drinking water medication is usually more effective than in‐feed medication, as it can be commenced and altered more quickly, and because sick birds may continue drinking even when they have ceased eating. There is also less risk of consumption by non‐target birds/species. It is important that, as the medicated water is consumed, the dose is not diluted with fresh water. Birds should have no access to other water sources.
The efficacy of many antimicrobials can be affected by the route of administration. Once powders are dissolved in solution, or liquids diluted, the drug can lose its activity. As a rule, medications should be prepared daily. Antimicrobials should not be mixed or administered concurrently, as one may interfere with the solubility, absorption or activity of another.
The pharmacology of antimicrobial agents in poultry
Within the critical context of antimicrobial stewardship, it is important to select drug and dosage regimens that reflect the five rights – right drug, right time, right dose, right duration and right route. 1 There are many physiological, pathological and pharmacological sources of variation in antimicrobial drug exposure within and between birds of the same and different species (e.g. chickens, ducks and turkeys), to which can be added sources of variation within and between routes of administration.
There have been several recent reviews of antimicrobial use in poultry 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 and key findings are presented in this summary.
The potential for distribution of antimicrobial agents into the eggs of laying birds is an important consideration when developing treatment plans for laying birds and this subject has been comprehensively evaluated. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 As seen in Appendix 2, there are very few drugs approved for use in birds and even fewer for birds currently producing eggs for human consumption. This is primarily a consequence of the presence, often for prolonged periods, of residues of the antimicrobial agent or its metabolites in meat and/or eggs.
The antimicrobial agents approved for use in birds in Australia represent well‐established and aged classes that were developed for use from the 1940s to the 1970s. With the exception of avilamycin, the antimicrobial agents listed in Appendix 2 with antibacterial indications (amoxicillin, apramycin, bacitracin, chlortetracycline, erythromycin, flavophospholipol, lincomycin, neomycin, oxytetracycline, spectinomycin, sulfadiazine, sulfadimidine, tiamulin, trimethoprim, tylosin, virginiamycin) were available for use in poultry in Australia in 1989. Because of the age of the antimicrobial agents available for use and their availability in most cases from a range of generic sources, there has been very little recent investigation of their pharmacology 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 or efficacy, or optimal dosage regimens 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 for these agents.
When these antimicrobial agents were first approved for use in Australia, it was only necessary to establish the dose regimen based on clinical response to treatment in infection challenge studies and field confirmation studies. The trend in recent decades to define dosage regimens is much more sophisticated and frequently involves an integration of the pharmacokinetic (PK) behaviour of the drug in the target bird species with the pharmacodynamic (PD) response of the target pathogen, often established by in vitro microbiological methods (e.g. the MIC of a representative panel of isolate of the target pathogen).
Very few PK/PD studies are available to re‐examine the dosage regimens of currently approved antimicrobial agents, although the PK/PD profile of tiamulin in an experimental intratracheal infection model of Mycoplasma gallisepticum in young chickens has been described. 71 Although valuable information was obtained in this study, tiamulin is not widely used in Australia, as Mycoplasma gallisepticum is very effectively controlled by vaccination. Application of the mutant selection window approach to the evaluation of the killing of Mycoplasma gallisepticum has been investigated for danofloxacin, doxycycline, tilmicosin, tylvalosin and valnemulin. 72 However, none of these antimicrobial agents are registered for use in Australia and the efficacy of vaccination in control of mycoplasmoses in chickens obviates any need for their use.
Water and feed administration
The most practical and common route of administration of antimicrobial agents in poultry in Australia is per os, with drugs being mixed in water or feed. There is only a single class of antimicrobial agent registered for injection in poultry (lincomycin–spectinomycin) and, although in ovo injection commonly used outside Australia, 73 , 74 , 75 no antimicrobial agents are registered for this route in Australia.
Effective use of antimicrobial agents in water requires an understanding of the drug and its formulation, especially its stability and solubility, as well as knowledge of factors influencing water intake and thereby exposure of birds to the treatment. Inconsistent antimicrobial administration has been observed after intravenous infusion of drugs into individual patients, 76 so it can be assumed that drug delivery in water or feed to populations of birds will have many challenges, both in the medication and consumption of water and feed, and the systemic availability of administered drugs. At best, administration by the oral route to a population of birds can be expected to be associated with significant imprecision. 77
Key considerations about feed and water medication have been described by a number of authors 11 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 and include a range of important factors affecting water consumption, including bird age (absolute water consumption increases with age, but consumption per kg live weight decreases), environmental temperature and heat stress, water temperature, electrolyte composition of the water, the feeding regimen and the lighting program (during dark periods birds do not usually drink and a peak of water consumption can occur just after lights are turned on).
Other factors affecting water and feed consumption and drug availability are presented as follows in the sections on interactions and sources of variability.
Interactions contributing to pharmacokinetic variability
Avian metabolism
The metabolism of foreign compounds or xenobiotics, including antimicrobial agents, in birds has received some attention, 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 but is not nearly as well understood as the metabolism of drugs in mammalian species.
One notable observation in birds is the ability of chickens to metabolise monensin and other ionophores, allowing them to be used with caution, but greater safety than in many mammalian species. 88 When the metabolism of monensin is impaired by coadministration of tiamulin, an inhibitor of Cytochrome P450 family 3 subfamily A (CYP3A) enzymes, monensin biotransformation is reduced, monensin accumulates, the margin of safety is eroded and toxicity can be observed. Not all ionophores are equally susceptible to the consequences of concurrent tiamulin exposure – for example, the safety of lasalocid 100 does not appear to be affected.
Other impacts of drugs on the CYPs of poultry have been described and include effects associated with sulfadimidine, 101 sanguinarine, 102 and the interaction of butyrate and erythromycin. 103
It is clear that there are some unique features of avian metabolism and that there are important differences in drug metabolism within species of birds and, importantly, between species. 89 For this reason, caution is required when using a new drug or a well‐established one in a new bird species.
Transport proteins
Transport proteins play an essential role in the absorption, distribution and excretion of drugs and toxins 104 , 105 , 106 , 107 and are located throughout the body in the cytoplasmic membranes of cells of the gastrointestinal tract, liver, kidney and brain. It is likely, just as observed in mammals, that there are important differences within and between species of birds in the rate and extent of drug transport across membranes and consequent PK.
Adsorption
Adsorption of drugs to the surface of chemical substances with particular properties can lead to reduced local and systemic availability. Examples include the interaction of bentonite and tylosin, 108 , 109 mycotoxin binders and tetracyclines, 110 tylosin and salinomycin 111 and, potentially, biochar immobilisation of lipophilic substances. 112
Tetracycline solubility and chelation
The bioavailability of chlortetracycline can be reduced by the presence of high concentrations of calcium and NaSO4 113 and increased in a low pH environment, as may occur following administration of citric acid to chickens 114 or turkeys. 115
Drug–drug interactions
A number of drug–drug interactions (DDIs) have been described in poultry between drugs not registered for use in birds in Australia, for example between doxycycline and diclazuril or halofuginone, 116 flunixin and doxycycline 117 and ionophores and florfenicol, 118 as well as between registered drugs, for example between monensin and sulphonamides. 119 The potential for DDIs should always be considered when more than one drug is used.
As described above, the best known DDI is between tiamulin and the ionophores, 100 and has been seen with monensin 120 and salinomycin. 121
Drug–herb interactions
A number of plants contain bioactive substances that can lead to interactions, such as that seen between silymarin and doxycycline in quail. 122
Hard water
Hard water can interfere with absorption, leading to decreased plasma concentrations of enrofloxacin 123 (not registered for use in poultry in Australia) and reduced availability of oxytetracycline. 55 , 124
Microbial degradation
Lactobacillus species in the crop of birds have been associated with the degradation of orally administered erythromycin. 125 , 126
Prandial status
Although not registered for use in poultry in Australia, the bioavailability of doxycycline is substantially reduced in the presence of feed, 127 highlighting prandial status as a potential source of variation. However, it is usually neither practical nor desirable to administer oral treatments to birds that have been fasted.
Water sanitisers
Water sanitisers can adversely affect the stability of antimicrobial agents, such as amoxicillin 128 and other antimicrobial agents. 129
Other sources of variability
A large number of pharmaceutical, physiological, pathological and pharmacological factors have been described as having an impact on the PK and clinical outcomes of antimicrobial use, particularly in mammals. 130 , 131 , 132 , 133 However, there are a growing number of examples of factors influencing PK and clinical outcome in poultry, with representative examples presented below. It should be recognised that most of the examples on sources of PK variation have been reported in studies of antimicrobial agents not registered for use in birds in Australia (all registered antimicrobial agents are set out in Appendix 2). However, the findings of these studies do highlight the diversity of sources of variation that need to be considered when designing dosage regimens or investigating poor responses to treatment.
Dose imprecision
Delivery of drugs in water or feed to populations of birds of variable weight and health makes delivering a predictable, accurate and intended dose impossible. 77 Measures can be introduced to reduce the degree of imprecision, but there will always be birds receiving less than or more than the target dose.
Age
The age of birds can have an impact on PK 134 and has been shown to influence the bioavailability of enrofloxacin, which was increased by 15.9% in 8‐week‐old broilers compared with that in 4‐week‐old birds. 104 In contract, plasma concentrations of sulfaquinoxaline and sulfadimidine were higher in younger broilers than in older birds. 135 Age and growth of broilers has also been shown to have a significant impact on the PK of florfenicol. 136
Bacterial isolate variation
When multiple isolates of Gallibacterium anatis were taken from various organs of layers, significant variation in antimicrobial resistance was observed. 137 This clearly can have an impact on clinical success if dose regimens are inadequate to control the full spectrum of resistances present.
Circadian variation
When monitored throughout the day, tylosin concentrations in plasma from broilers were subtherapeutic at night, an unfavourable finding for a time‐dependant antibacterial agent. 138 It is likely that there was no water and feed consumption during the night.
Sulfadimidine given orally to chicks was found to have dramatic differences in PK throughout the day, 139 sufficient to question the reliability of dosage regimens.
Fatty liver
Induced fatty liver in chickens led to significant changes in the PK of erythromycin, lincomycin and oxytetracycline. 140
Taste
Chickens have a small repertoire of bitter taste receptors (T2R) and the umami receptor (T1R1/T1R3) responds to amino acids such as alanine and serine. They lack a counterpart of the mammalian sweet sensing T1R2, so T1R2‐independent mechanisms for glucose sensing might be particularly important in chickens. The avian nutrient chemosensory system is present in the gastrointestinal tract and hypothalamus and is related to the enteroendocrine system, which mediates the gut–brain dialogue relevant to the control of feed intake. 141
It may not necessarily be related to taste, but water intake has been shown to increase in birds fed lasalocid. 142
Formulation
Modified formulations of doxycycline have been shown (not unexpectedly) to be associated with differing PK profiles in treated broilers. 143
Gender
Differences in the PK of antibacterial drugs (including the sulphonamides) have been shown when comparing hens and cockerels. 144 Tobramycin was eliminated more rapidly in ducks than in drakes, 145 similar to observations with apramycin. 144
Disease
Generally, antimicrobial agents are administered to birds that are affected by infection, from early subtle clinical stages to more obvious florid disease. While PK studies are frequently undertaken in normal birds, not surprisingly, the presence of disease can have a significant impact on PK and between and within bird variability in PK. The following examples illustrate the complexity and unpredictability of the effects of disease on the PK of various antibacterial agents. Most of the examples describe the use of antibacterial agents not registered for use in birds in Australia. However, the cases remain important as they demonstrate the importance of the impacts of disease on drug PK.
Amoxicillin administered to chickens with caecal coccidiosis was associated with a lower Cmax, a reduced AUC and lower bioavailability. 146
Endotoxaemia in turkeys had dramatic effects on cardiovascular function, but the PK of amoxicillin was not influenced, though PK was impacted by the rapid growth of the birds. 147
Infection of turkeys with Pasteurella multocida resulted in higher plasma levels of chlortetracycline (15 mg/kg) than in uninfected turkeys, and citric acid (150 mg/kg), a chelating agent of divalent cations such as calcium and magnesium, led to higher plasma levels in birds whether or not infected with Pasteurella multocida. 115 , 148
Danofloxacin (not registered) had a reduced Cmax in chickens infected with Pasteurella multocida, but the concentrations achieved adequately controlled infection. 149 However, with increasing pathogen MIC this may not always be the case.
In contrast, in ducks infected with Pasteurella multocida, danofloxacin (not registered) had a higher AUC. 150
Difloxacin (not registered) had increased clearance in broilers infected with Escherichia coli. 151
Doxycycline (not registered) had reduced plasma concentrations and a shorter elimination half‐life in chickens infected with Mycoplasma gallisepticum. 152
Enrofloxacin (not registered) had a reduced Cmax in broilers infected with Escherichia coli. 153
Enrofloxacin (not registered) was absorbed more slowly and had a shorter elimination half‐life in broilers infected with Escherichia coli. 154
Infection of broilers with Escherichia coli was associated with a decrease in the Vd and the elimination half‐life of florfenicol (not registered). 155
Florfenicol (not registered) had reduced Cmax and AUC0‐12 h values in lung tissue in Gaoyou ducks infected with Pasteurella multocida. 156
Florfenicol (not registered) had a reduced Cmax after administration by IM or IV in Muscovy ducks infected with Pasteurella multocida. 157
Infection of broilers with Salmonella gallinarum was associated with reduced clearance of kitasamycin (not registered). 158
Muscovy ducks with induced renal dysfunction had increased plasma concentrations of levofloxacin (not registered). 159
Infection of ducks with Pasteurella multocida was associated with increased plasma concentrations and slower elimination of orbifloxacin (not registered). 160
Chickens with infectious coryza had higher plasma concentrations, and reduced clearance (and possibly reduced residue elimination) of sulphachloropyridazine (not registered)–trimethoprim. 161
Conclusion
The effective treatment of birds with antimicrobial agents requires an understanding of the multitude of factors that influence selection of the appropriate drug, administration according to a route and dose regimen that increases the likelihood of adequate drug exposure of treated birds, and minimisation of those factors that are associated with PK variability.
The choice of antimicrobial agents is from a small formulary for treatment of birds with pathogens with evolving antimicrobial resistance status.
In many respects, it is amazing that drugs from the 1980s, and before, continue to provide clinical benefit. However, in the absence of monitoring of the PK and pathogen status of individual birds, the vigilance of farm personnel and the veterinarian in assessing the response to treatment is critical.
Disease investigation – general approach
Production records
Most commercial poultry farming operations have production records. These are useful indicators of the recent history of the flock. There are often also husbandry records that may provide clues about any recent husbandry or management factors that could influence the incidence and/or outcomes of disease. Vaccination programmes are also valuable sources of information. While some records may not be immediately available, a little time spent requesting and assessing further information is often well worth the effort. 162
An important consideration when investigating infectious diseases is to review the farm location and the placement of nearby farms. A quick view on Google Earth prior to your visit may assist in identifying potential risks, including nearby farms and dams on which wild waterfowl may reside. The other important records to review are the recent visitor entries, feed/gas deliveries, water sources and water sanitation.
Prior to arrival, ask the farmer to keep recently deceased or currently affected birds for you, to maximise your chance of a rapid diagnosis.
Ask if there have been recent disease outbreaks in the area, or previously on the farm.
If there have been severe clinical signs or mortalities, recommend that the flock/farm be quarantined until the visit.
Depending on the body system involved, there may be more specific details to be gathered. These will be covered, where appropriate, in each of the following chapters.
Flock examination
Farm and shed conditions should be the first part of flock examination. Observing the general farm conditions, biosecurity standards, rodent management and wild bird activity can greatly inform the general assessment of the husbandry and management standards employed by the farmer.
Inside the shed, indicators such as litter condition, air quality, temperature, humidity, lighting, and the availability of feed and water, are all important factors in disease investigation.
Flock behaviour is a good indicator of its general health status. Observations include bird distribution (huddling), general flock activity levels, noise levels, and eating and drinking behaviours.
In production systems where birds are not fed ad libitum, observing birds at feeding time is very useful.
Clinical examination – Signs of disease
The examination progresses to considering individual animals, looking for typical cases within the flock. Individual birds are very adept at disguising signs of illness and injury, so it is prudent to take the time to examine several birds to look for consistent clinical signs.
Post‐mortem examination
Once typical cases have been selected, 5–10 individuals can be selected for necropsy. Ideally, use cull chickens or recently deceased birds to reduce the risk of decomposition interfering with the gross and/or histological assessment, as well as microbiological diagnoses. On commercial farms, if the pathological signs of disease are not easily distinguished, the owner may allow some healthy birds to be euthanased as well to enable direct comparisons.
At this point, appropriate samples can be taken for laboratory investigation.
For advice on conducting a necropsy on a chicken, go to one of the following links:
https://www.agric.wa.gov.au/sites/gateway/files/A%20visual%20guide%20to%20a%20chicken%20necropsy.pdf
http://www.poultryhub.org/resources/poultry-videos/
Personal biosecurity, hygiene and the use of personal protective equipment should always be adopted when handling potentially infectious or zoonotic birds or samples from them. For advice on these matters, refer to the Australian Veterinary Association guidelines:
https://www.ava.com.au/library-resources/other-resources/veterinary-personal-biosecurity/
Treatment options
If a diagnosis can be made based on clinical signs and gross pathology, a treatment regimen can be commenced immediately. A presumptive bacterial infection would indicate the commencement of antimicrobial therapy only if there is enough time for treatment and the WHP can be complied with. The choice of drug is likely to be influenced by time constraints and food safety considerations as much as by susceptibility considerations.
Prevention advice
A good rule of thumb is that the recurrence of an identified problem is unsatisfactory! In commercial poultry medicine, preventive medicine is the ultimate goal. There is a wealth of knowledge and there are many tools available to assist a veterinarian in providing advice on disease prevention. Biosecurity, vaccinations, husbandry, nutrition and hygiene practices should all be discussed with a farmer in conjunction with treatment advice in the event of a disease outbreak.
Field veterinarian's kit [162]
| Disposable overalls | Bottles/tubes for blood collection (20) |
| Masks | Swabs and transport media (bacterial/viral) |
| Hairnets | Esky ice brick |
| Disposable gloves | Plain swabs |
| Biohazard bags | Sterile 100 mL jars |
| Rubbish bags | Tissue collection jars with 10% formalin solution |
| Scissors | Ammonia strips/metre |
| Knife | Thermometer/humidity metre, preferably with an anemometer (such as a Kestrel 3000) |
| Bucket/sanitiser | Camera/phone (washable case) |
| Water sanitation measurement device (strips measuring free chlorine/meter/test kit) and/or oxidation‐reduction potential meter | |
Diseases of the digestive tract
The digestive tract of birds has a significant number of differences from that of mammals, primarily to allow rapid food consumption and storage, and simple digestion (Figure 2).
| Oropharynx | Contains salivary glands, very few taste buds |
| Crop | Temporary food storage |
| Proventriculus | ‘True’ glandular stomach – acidification, enzyme addition, mixing of food |
| Ventriculus (gizzard) | ‘Mechanical stomach’, grinding and mixing of food |
| Duodenum | Pancreatic and hepatic enzyme and bile addition |
| Jejunum | Enzymatic digestion, nutrient absorption |
| Ileum | Further digestion, nutrient absorption |
| Caecum (plural: caeca) | Anaerobic fermentation of indigestible nutrients |
| Colon | Faecal accumulation and water absorption |
| Cloaca | Defecation and uric acid excretion |
Figure 2.
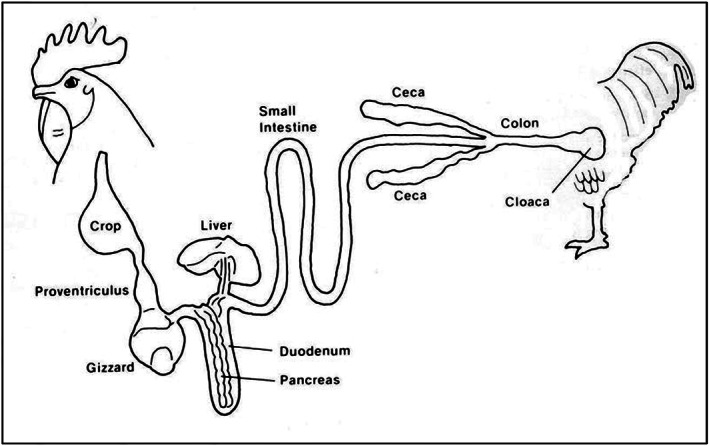
Schematic anatomy of the avian digestive system 163 (ErikBeyersdorf/CC BY‐SA; https://creativecommons.org/licenses/by‐sa/3.0).
Each of the organs of the digestive tract of chickens has a specific role in the digestion and absorption of nutrients. The highly refined nature of commercial feedstuffs alters the functional homeostasis of the digestive tract of commercial chickens, leading to slight anatomical differences in organ size and shape from those of the backyard chicken.
The composition of the gastrointestinal microbiota (the community of commensal, symbiotic and pathogenic microorganisms) is a key functional component of general and gastrointestinal tract health and productivity in poultry.
The digestive tract has historically been the target of non‐specific antimicrobial treatments aimed at improving the productivity of flocks, through manipulation of the microbial population. However, increasing awareness of the need for improved antimicrobial stewardship has seen this practice disappear. Many non‐antimicrobial interventions (enzymes, organic acids, probiotics, prebiotics, essential oil extracts, yeast extracts) are now available to assist in the maintenance of a healthy gut microbiota, thus removing the need for antimicrobial therapies under normal growing conditions. 164 , 165 , 166 , 167 However, 168 imbalances in the microbiota can and do occur, leading to both clinical disease and subclinical, production‐limiting infections.
| General approach | |
|---|---|
| Specific considerations for investigations of digestive tract disease |
Gastrointestinal tract health is such an important component of bird health and productivity that even subtle non‐specific changes to gut health and physiology can have a significant bearing on flock health and performance. It is important for the clinician to have a very good understanding of normal gut morphology and physiology in order to detect mild pathological changes or altered intestinal contents. Wet droppings can be due to either digestive or urinary tract problems. It is important to differentiate between the two early in the case investigation. |
| Before farm entry | Look at mortality and production records. Review other farm records. Review current coccidiostat and worming programs. |
| On farm | Observe:
|
| Disease presentations/differential diagnosis 164 | |
|---|---|
| Diseases of the oropharynx | Epithelial lesions – necrotic, erosive, inflammatory, hyperkeratotic |
| Differential diagnosis |
Viral Fowlpox virus Fungal Candida albicans Toxic Mycotoxins Nutritional Vitamin A deficiency Protozoal Canker (trichomoniasis) |
| Diseases of the crop | Pendulous crop, sour crop, crop impaction |
| Differential diagnosis |
Fungal Candida albicans Physical Overeating, grass eating |
| Diseases of the proventriculus | Erosion, dilatation, inflammation |
| Differential diagnosis |
Viral Infectious proventriculitis Newcastle disease virus Avian influenza virus Unknown/nutritional Flaccid proventriculus (proventricular dilatation disease) Toxic Mycotoxins Biogenic amines |
| Diseases of the ventriculus (gizzard) | Erosion, flaccidity, atrophy |
| Differential diagnosis |
Viral Adenoviruses Toxic Mycotoxins Biogenic amines Unknown/nutritional Atrophy (linked to flaccid proventriculus) Low fibre diet Bacterial Clostridium perfringens |
| Diseases of the small and large intestines | Diarrhoea, depression, lethargy, runting/stunting, mortality |
| Differential diagnosis |
Bacterial Necrotic enteritis (Clostridium perfringens) Dysbacteriosis Spirochaetosis Protozoal Coccidiosis (Eimeria tenella/Eimeria brunetti/Eimeria necatrix/Eimeria maxima) Viral Adenoviruses, enteroviruses, rotaviruses, coronavirus, astrovirus, reoviruses, parvovirus Parasitic Intestinal nematodes, cestodes Nutritional Nutritional imbalances |
| Diseases of the caeca | Abnormal caecal droppings |
| Differential diagnosis |
Protozoal Coccidiosis Blackhead (Histomonas meleagridis) Trichomonads Bacterial Salmonella enterica serovar Typhimurium (Salmonella Typhimurium) Parasitic Caecal worms (Heterakis gallinarum) Nutritional Excess or undigestible nutrients in the diet |
| Diseases of the liver | Liver pathology |
| Differential diagnosis |
Viral Marek's disease Lymphoid leukosis Inclusion body hepatitis Big liver‐spleen disease (hepatitis E virus) Bacterial Spotty liver disease (Campylobacter hepaticus) Salmonellosis (Salmonella spp.) Fowl cholera (Pasteurella multocida) Colibacillosis (Escherichia coli) Cholangiohepatitis (Clostridium perfringens) Staphylococcal infections Other septicaemic infections Protozoal Histomoniasis/Blackhead (Histomonas meleagridis) |
| Necropsy and sampling |
|---|
Necropsy 5–10 birds that have typical clinical signs or 5–10 birds that have recently died and note the findings. It is important to conduct a full examination of the digestive tract from mouth to cloaca.
|
Key issues.
The history will be important for determining the differential diagnosis. This will include vaccination and flock history, along with overall flock and necropsy signs.
It can be prudent to delay treatment until a diagnosis and antimicrobial susceptibility has been established, but this can depend on the level of mortality, the prognosis and the time until slaughter.
Treatment is not warranted for viral infections.
Coccidiosis is very common in backyard flocks and young chicks will almost invariably be challenged at some point. Older birds will develop immunity and will sporadically shed coccidial oocysts into the environment, thus perpetuating the infection cycle.
Intestinal worms are also very common in backyard flocks and a regular treatment program should be encouraged.
Coccidiosis
Background/nature of infection/organisms involved
Coccidiosis results from infection with members of the genus Eimeria. In the chicken, there are four common species, with a couple of less common species. Disease is generally seen in birds around 4–5 weeks of age, but can be seen in older flocks if exposure has been delayed, or if vaccinal immunity has waned. Secondary involvement of Clostridium perfringens can lead to necrotic enteritis.
With each diagnosis of coccidiosis, particularly if it is caused by Eimeria maxima and Eimeria necatrix, it is worthwhile performing a direct smear of the intestinal mucosa to look for an overgrowth of Clostridium perfringens, using a gram stain to identify the organism.
Treatment
The presence of a few coccidial lesions is a normal occurrence and does not indicate disease or warrant treatment.
If coccidiosis is strongly suspected, it is often appropriate to commence a course of anti‐coccidial medication based on pathology alone, as delaying treatment could result in high mortality rates because of the explosive course of the disease in intensively raised flocks.
Treatment choice is not affected by species of Eimeria, although the response to the treatment can be impacted. Eimeria necatrix infections tend to take longer to respond to treatment due to the severity of the lesions.
Anti‐coccidials used
Amprolium combined with ethopabate is the treatment of choice for short‐lived flocks such as broilers. Toltrazuril is suitable for longer‐lived or more valuable birds. Note that there are label restraints for both treatment options that must be followed.
Specific details on diseases, prevention and specific treatment choices can be found in Appendix 1. In food‐producing species, it is critical that contraindications and WHPs are reviewed as described in the label requirements and guidance in Appendix 2.
| Situation | First choice treatment | Second choice treatment |
|---|---|---|
| Short‐lived flock (e.g. broiler flock) | Amprolium/ethopabate. When in a concentration of amprolium 216 g/L and ethopabate 14 g/L, a dose rate of 500–1000 mL/900 L drinking water may be required for 5–7 days, depending on the severity of the disease. | Toltrazuril is administered at a dose rate of 3 L/1000 L for 2 consecutive days. Note that there is a 14‐day WHP for meat. |
| Long‐lived flock (e.g. layer flock, breeder flock, backyard flock, fancy breeds) up to 8 weeks before commencement of lay | Toltrazuril is administered at a dose rate of 3 L/1000 L for 2 consecutive days. This drug cannot be used in birds that will be laying eggs within 8 weeks of treatment. | Amprolium can be used at 250 mg/L of drinking water for 5–7 days, followed by a reduced dose rate of 150 mg/L of drinking water for 5–7 days to treat an outbreak. |
| Long‐lived flock (e.g. layer flock, breeder flock, backyard flock, fancy breeds) within 8 weeks of lay, or birds in lay | Amprolium can be used at 250 mg/L of drinking water for 5–7 days, followed by a reduced dose rate of 150 mg/L of drinking water for 5–7 days to treat an outbreak. | No alternative treatment. |
Necrotic enteritis
Background/nature of infection/organisms involved
Necrotic enteritis is caused by Clostridium perfringens. Necrotic enteritis is often found in association with coccidiosis and should be investigated in any suspect coccidiosis outbreak. Clostridium perfringens is a commensal in the chicken digestive tract under normal conditions, but it tends to overgrow and cause clinical disease when there is an excess of nutrients in the jejunum and ileum, which results in changes in the intestinal micro‐environment.
Treatment
If necrotic enteritis is suspected, then the treatment of choice until the diagnosis is confirmed would be amoxicillin at 20 mg/kg/day for three to five days, depending on the speed of recovery, whilst being aware of withholding periods as it will have good efficacy against Clostridium perfringens, has a short WHP and, since water soluble, can be applied immediately.
Another treatment option is Zinc bacitracin in feed at 200ppm active ingredient for 5‐7 days. However, as zinc bacitracin is not water soluble and requires in feed treatment this approach may not be practical in a sudden disease outbreak situation such as occurs with necrotic enteritis.
Where previous flock history suggests that necrotic enteritis is not able to be controlled with other measures as outlined in Appendix 1 (eg dietary) then preventative treatment with either zinc bacitracin in feed at a rate of 40 ppm (active ingredient) or avilamycin at a rate of 10‐15ppm (active ingredient) in feed may be required. The preventative treatment period will usually coincide with the times of coccidiosis challenge on the farm and is fed continuously through this risk period. Probiotics could also be considered as a potential alternative to antibiotics in these situations.
The choice of preventative treatment option will depend on applicable poultry species and production type, along with previous successful prevention regimes.
Zinc bacitracin can be used as per label directions in poultry with a nil withholding period for meat and egg production.
Avilamycin can only be used in broiler chickens.
Antibiotic treatment may be useful for necrotic enteritis prevention, but it is not a replacement for poor management, use of aggravating feed ingredients or inadequate coccidiosis control.
NOTE: Virginiamycin is also registered for use as a preventative treatment for necrotic enteritis. As it has a ‘HIGH’ ASTAG rating this antibiotic should only be used as a treatment of last resort and used strictly according to label directions.
Antimicrobials used
Specific details on diseases, prevention and specific treatment choices are shown in Appendix 1. In food‐producing species, it is critical that contraindications and WHPs are reviewed as described in the label requirements and guidance in Appendix 2.
| Situation | First choice treatment | Second choice treatment |
|---|---|---|
| Short‐lived flock not producing eggs (e.g. broiler flock) | Amoxicillin a in the drinking water is the first line treatment. Use at 20 mg/kg for 3 days. | Chlortetracycline can be used at a dose rate of 60 mg/kg bodyweight in drinking water for 3–5 days. |
| Long‐lived flock (e.g. layer flock, breeder flock, backyard flock, fancy breeds) up to 8 days before commencement of lay | Amoxicillin a in the drinking water is the first line treatment. Use at 20 mg/kg for 3 days. | Chlortetracycline can be used at a dose rate of 60 mg/kg bodyweight in drinking water for 3–5 days. |
| Long‐lived flock (e.g. layer flock, breeder flock, backyard flock, fancy breeds) in lay | If affected birds are producing eggs for human consumption, chlortetracycline can be used at 60 mg/kg bodyweight in drinking water for 3–5 days. | CCD amoxicillin trihydrate for poultry (APVMA # 36443) is currently the only amoxicillin formulation with a NIL WHP for eggs. However, it does have a 14‐day export egg WHP. Medicate at 20 mg/kg for 3–5 days in drinking water. |
CCD amoxicillin trihydrate for poultry (APVMA # 36443) is currently the only amoxicillin formulation with a NIL WHP for eggs. However, it does have a 14‐day export egg WHP.
Dysbacteriosis
Background/nature of infection/organisms involved
Dysbacteriosis is an imbalance of the normal bacterial flora, causing mild enteritis with wet droppings, leading to wet floors and dirty feathering, and potentially poor performance. It is mainly seen in broiler flocks. Lesions at necropsy include undigested feed, watery intestinal contents, flaccid intestines with a poor tone and excess caecal volume with gassy contents.
Treatment
Antimicrobial treatment is not recommended for dysbacteriosis. It is important to address the underlying cause.
Avian intestinal spirochaetosis
Background/nature of infection/organisms involved
Avian intestinal spirochaetosis (AIS) is caused by Brachyspira spp. (most commonly Brachyspira pilosicoli or Brachyspira intermedia). The typical presentation of AIS is a chronic diarrhoea causing stained vents and manure‐stained eggs. It is a disease of long‐lived floor‐based flocks. As the presentation is chronic, it is generally not reported in broiler flocks.
Specific details on diseases, prevention and specific treatment choices are shown in Appendix 1. In food‐producing species, it is critical that contraindications and WHPs are reviewed as described in the label requirements and guidance in Appendix 2.
| Situation | First choice treatment | Second choice treatment |
|---|---|---|
| Breeder and layer flocks | Chlortetracycline as an in‐feed treatment at 400 ppm for 7 days, followed, if necessary, by in‐feed treatment at 200 ppm for up to 28 days. | No alternative treatments. |
Salmonellosis
Background/nature of infection/organisms involved
Salmonella species do not usually cause clinical disease in poultry, unless there is an overwhelming infectious dose or concomitant immunosuppressive disease. Treatment of commercial broiler flocks is not recommended because of the food safety implications of clinical salmonellosis.
Treatment
Specific details on diseases, prevention and specific treatment choices are shown in Appendix 1. In food‐producing species, it is critical that contraindications and WHPs are reviewed as described in the label requirements and guidance in Appendix 2.
| Situation | First choice treatment (chicks under 2 weeks of age) | Second choice treatment |
|---|---|---|
| Flocks not producing eggs for consumption | Trimethoprim/sulphadiazine at a dose rate of 25 mg sulphadiazine/kg and 5 mg trimethoprim/kg per day for 3–5 days if the birds are less than 2 weeks old, or 12.5 mg sulphadiazine/kg and 2.5 mg trimethoprim/kg per day for 3–5 days if the birds are older than 2 weeks of age. | Amoxicillin a in the drinking water at 20 mg/kg for 3 days. |
CCD amoxycillin trihydrate for poultry (APVMA # 36443) is currently the only amoxicillin formulation with a NIL WHP for eggs. However, it does have a 14 days export egg WHP.
Spotty liver disease
Background/nature of infection/organism involved
Spotty liver disease is caused by Campylobacter hepaticus. It is a disease of longer‐lived floor‐living layer and breeder flocks and is rarely seen in caged birds or broilers. Clinical disease is almost invariably associated with a drop in egg production. The disease can occur throughout the year but tends to result in higher mortalities and greater drops in egg production in summer.
Antimicrobial treatment, although effective, should not be relied upon for long‐term control, as resistance to commonly used antimicrobials occurs rapidly.
Specific details on diseases, prevention and specific treatment choices are shown in Appendix 1. In food‐producing species, it is critical that contraindications and WHPs are reviewed as described in the label requirements and guidance in Appendix 2.
| Situation | First choice treatment | Second choice treatment |
|---|---|---|
| All situations | Chlortetracycline at 60 mg/kg bodyweight in drinking water for 5 days. | Lincomycin–spectinomycin at 100 g combined antibiotic activity/200 L in drinking water for 3–5 days. |
Histomoniasis
Background/nature of infection/organisms involved
Histomoniasis (or blackhead) is caused by a protozoan parasite, Histomonas meleagridis. Turkeys are highly susceptible, but disease is also seen in chickens. It is very rare in broilers. Lesions are commonly found in both the caeca (large caseous casts) and the liver (discrete circular lesions). It is often transmitted by the nematode Heterakis gallinae, so control of Heterakis gallinae will assist in control of histomoniasis in chickens. However, direct transmission occurs readily in turkeys.
Treatment
There is no currently registered treatment for histomoniasis. Consider control of the vector (Heterakis gallinae) and earthworms to reduce the incidence of disease.
Intestinal worms
Background/nature of infection/organisms involved
There is a wide range of nematodes and cestodes that can affect poultry, some of which are almost invisible to the naked eye. Intestinal worms should always be considered as a differential diagnosis, particularly in free‐range flocks. Faecal flotation can be used to detect eggs or tapeworm segments and assess the severity of an intestinal worm burden.
Treatment
Specific details on diseases, prevention and specific treatment choices are shown in Appendix 1. In food‐producing species, it is critical that contraindications and WHPs are reviewed as described in the label requirements and guidance in Appendix 2.
| Situation | First choice anthelmintic | Second choice anthelmintic |
|---|---|---|
| Ascaridia galli | Levamisole at 28 mg/kg live weight. As a guide, assuming a medicated water intake of 35 mL/bird over the treatment period, use 800 g levamisole per 900–1000 L drinking water, or 8 g per 10 L water for a small number of birds. The amount of solution prepared should be the volume that will be consumed over 12 h. Remove other sources of water during the treatment period. Note there is a 7‐day WHP for meat. | Piperazine (adult worms only). The recommended dose for poultry is 200 mg/kg (1 g per 5 kg bodyweight). Use 1 kg of Piperazine Wormer to treat 2500 birds with a bodyweight of 2 kg. The volume of medicated water provided should be able to be consumed by the birds over a 6 to 8 h period. Discard any remaining medicated water after 6–8 h. Add the amount required to a small quantity of water first. When it is completely dissolved, add it to the medication tank, mixing thoroughly. When treating a severe worm infestation, repeat the dose 17–21 days later. |
| All other species of immature and mature nematodes | Levamisole at 28 mg/kg live weight. As a guide, assuming a medicated water intake of 35 mL/bird over the treatment period, use 800 g levamisole per 900–1000 L drinking water, or 8 g per 10 L water for a small flock. The amount of solution prepared should be the volume that will be consumed over 12 h. Remove other sources of water during the treatment period. Note there is a 7‐day WHP for meat. | Flubenol (flubendazole) in feed at 600 g/tonne of feed, equivalent to 30 g flubendazole (30 ppm) for 7 days. Note there is a 7‐day WHP for meat. Do not use in pigeons or parrots. |
| Cestodes (tapeworms) | Flubenol (flubendazole) in feed at 1200 g/tonne of feed, equivalent to 60 g flubendazole (60 ppm) for 7 days. Note there is a 7‐day WHP for meat. Do not use in pigeons or parrots | No alternative treatments |
Diseases of the respiratory system
John Glisson wrote ‘Although much is known about the individual agents responsible for respiratory diseases in poultry, uncomplicated infections with single agents are the exception. Under commercial conditions, complicated infections with multiple aetiologies, with viruses, mycoplasmas and other bacteria, immunosuppressive agents, and unfavourable environmental conditions, are more commonly observed than simple infections’.
This combination makes antimicrobial treatment in the face of a disease outbreak both challenging and often unrewarding.
It is important to systematically step through all potential predisposing factors including:
Interactions between respiratory pathogens
Effects of immunosuppressive factors
Environmental factors
Management of vaccination (including adverse reactions)
The respiratory system relies on cilia, mucus and phagocytic cells to protect against infections. High levels of dust and/or high ammonia reduce cilial motility and thus clearance of pathogens trapped in mucus, as well as the function of phagocytes.
As a result, disease presentations can be complex, but can be subdivided into the following categories:
Conjunctivitis
Sinusitis/rhinitis
Tracheitis
Pneumonia
Airsacculitis
Functions and unique features of the avian respiratory system
As in mammals, the respiratory system in birds is involved in:
absorption of oxygen (O2)
release of carbon dioxide (CO2)
release of heat (temperature regulation)
vocalisation
In contrast to mammalian species, the lungs in birds do not expand. On inspiration air passes through the lungs and into the air sacs, and then on expiration returns through the lungs, taking excess heat and CO2 and exchanging it with O2. The transfer of heat in the air sacs is responsible for a considerable proportion of a bird's heat loss under high temperature conditions. As a result, birds with respiratory disease are much more susceptible to mortality in hot, humid environments.
Another unique feature is the intimate association of the air sacs with the some of the bird's bones. Consequently, respiratory infection may also result in a related osteomyelitis (Figures 3 and 4).
Figure 3.
Schematic anatomy of the avian respiratory system (Cruithne9/CC BY‐SA; https://creativecommons.org/licenses/by‐sa/4.0).
Figure 4.
Path of airflow through a bird's respiratory system during inhalation and exhalation (Wikimedes/CC BY‐SA; https://creativecommons.org/licenses/by‐sa/3.0 ).
| General approach | |
|---|---|
| Specific considerations for respiratory tract disease investigations | Assessment of ventilation is very important. Overnight ventilation, especially in winter, can be compromised, leading to high levels of dust and ammonia. If visiting during the day, try to assess the capacity of the farm to ventilate effectively without chilling the birds during night hours. Records of minimum temperatures will assist the investigation. |
| Disease presentations/differential diagnosis | |
|---|---|
| Conjunctivitis | Conjunctivitis, keratitis, photophobia, excess lacrimation |
| Differential diagnosis |
Viral Infectious laryngotracheitis virus Infectious bronchitis virus Avian influenza virus Turkey rhinotracheitis virus Bacterial Chlamydiosis (Chlamydia psittaci) Mycoplasma gallisepticum Fungal Aspergillus species Toxic/irritant High levels of ammonia Nutritional Vitamin A toxicity |
| Rhinitis and sinusitis | Sneezing and nasal discharge, facial swelling, periorbital swelling and epiphora |
| Differential diagnosis |
Viral Infectious laryngotracheitis virus Infectious bronchitis virus Avian influenza virus Turkey rhinotracheitis virus Bacterial Chlamydiosis (Chlamydia psittaci) Mycoplasma gallisepticum Infectious coryza (Avibacterium paragallinarum) Fowl cholera (Pasteurella multocida) Ornithobacterium rhinotracheale Riemerella anatipestifer |
| Tracheitis | Coughing, gasping |
| Differential diagnosis |
Viral Infectious laryngotracheitis virus Infectious bronchitis virus Avian influenza virus Turkey rhinotracheitis virus Newcastle disease virus Bacterial Mycoplasma gallisepticum Escherichia coli Bordetella avium Ornithobacterium rhinotracheale Fowl cholera (Pasteurella multocida) Toxic/irritant High levels of ammonia and/or dust |
| Pneumonia | Coughing |
| Differential diagnosis |
Viral Avian influenza virus Turkey rhinotracheitis virus Bacterial Escherichia coli Ornithobacterium rhinotracheale Fowl cholera (Pasteurella multocida) Fungal Aspergillus species |
| Airsacculitis | Gasping, coughing |
| Differential diagnosis |
Viral Infectious bronchitis virus Avian influenza virus Turkey rhinotracheitis virus Newcastle disease virus Bacterial Mycoplasma gallisepticum/Mycoplasma synoviae/Mycoplasma meleagridis Escherichia coli Bordetella avium Ornithobacterium rhinotracheale Fowl cholera (Pasteurella multocida) Chlamydiosis (Chlamydia psittaci) Infectious serositis (Riemerella anatipestifer) Fungal Aspergillus species |
| Necropsy and Sampling |
|---|
| Necropsy 5–10 birds with typical clinical signs or 5–10 birds that have recently died and note findings. If birds that have recently died cannot be submitted to the laboratory, sample the choanal cleft/trachea/affected tissue of 5–10 affected birds with swabs for viral and bacterial isolation, as well as plain swabs. Alternatively, collect fresh affected tissue in a sterile jar. If Chlamydia psittaci is suspected also collect the spleen. Collect a minimum of 10 blood samples from live affected birds for serology. |
Key issues.
The history will be important in determining a differential diagnosis. This will include the vaccination and flock history, along with overall flock and necropsy signs.
Most causes of respiratory disease are highly contagious, so quarantine of the affected flock is critical.
As mortality can be exacerbated by stress and poor ventilation, these adverse management factors should be minimised.
Many causes of respiratory disease can be prevented by vaccination, so vaccination should be considered as a key control strategy for future flocks, along with thorough cleanout and disinfection, strict biosecurity and improved management to ensure high air quality and lower stress.
Respiratory disease is very common in backyard poultry flocks. Outbreaks are most commonly attributable to poor biosecurity.
Treatment
It would be prudent to delay treatment until a microbiological diagnosis and antimicrobial susceptibility can be established, but this can be affected by concerns for bird welfare, WHPs, economic considerations, the level of mortalities, and the time until slaughter.
If treatment is required before a diagnosis can be established, then the treatment of choice would be a tetracycline, as it will have a broad spectrum of activity against the bacterial agents that are most likely to be involved.
Treatment is not warranted for any viral infection.
Treatment will not eliminate most bacterial respiratory pathogens. Birds will generally remain carriers, so measures to minimise the risk of spread should be considered.
Antimicrobials used
Specific details on diseases, prevention and specific treatment choices are shown in Appendix 1. In food‐producing species, it is critical that contraindications and WHPs are reviewed as described in the label requirements and guidance in Appendix 2.
| Organism | First Choice Treatment | Second Choice Treatment |
|---|---|---|
| Chlamydia psittaci | Chlortetracycline at 60 mg/kg for 5–7 days in drinking water, followed by chlortetracycline at 400–750 ppm in feed for a minimum of 2 weeks, depending on the severity of the disease. | Oxytetracycline at 70 mg/kg for 5–7 days, followed by in‐feed medication. Note that this is NOT a suitable treatment for birds producing eggs for human consumption. |
| Mycoplasma gallisepticum/Mycoplasma synoviae | Tylosin tartrate at 100 g/200 L of drinking water for 3–6 days depending on the severity of the disease (not registered for birds producing eggs for human consumption). | In the case of food‐producing egg layers and where secondary infection complicates the disease picture, use chlortetracycline at 60 mg/kg bodyweight for 3–5 days, depending on the severity of the disease. |
| Infectious coryza (Avibacterium paragallinarum) |
Chlortetracycline at 60 mg/kg live weight can be used for 3–5 days, depending on the severity of the clinical signs. Relapse often occurs after treatment is discontinued and treatment with chlortetracycline at 100 ppm in feed for up to 28 days may be required. |
Amoxicillin a can be used at 20 mg/kg if there is resistance to tetracyclines and sensitivity to amoxicillin has been conformed in vitro. Prior to antimicrobial treatment, collect samples for culture and susceptibility testing. |
| Fowl cholera (Pasteurella multocida) |
Tetracycline–oxytetracycline at 70 mg/kg for 5–7 days, or chlortetracycline at 60 mg/kg for 5–7 days. Note that oxytetracycline is NOT suitable for treatment of birds producing eggs for human consumption. Fowl cholera outbreaks can recur after cessation of treatment, so in the case of severe disease, chlortetracycline may be required in‐feed at 100 ppm for up to 28 days |
Amoxicillin a at 20 mg/kg for 3–5 days |
| Ornithobacterium rhinotracheale | Amoxicillin a at 20 mg/kg for 3–5 days. | Chlortetracycline at 60 mg/kg for 5–7 days. |
| Riemerella anatipestifer | Culture and susceptibility testing are necessary to determine an appropriate antimicrobial for treatment because of variation in patterns of resistance. However, the most consistently effective treatment in ducks has been amoxicillin a at 20 mg/kg for 3–5 days. | |
| Escherichia coli | Amoxicillin a at 20 mg/kg live weight for 3–5 days can be used in broilers with respiratory colibacillosis. | Chlortetracycline can be used at 60 mg/kg live weight for 3–5 days, depending on the severity of the clinical signs. |
| Bordetella avium | Non‐responsive to antibiotics | |
CCD Amoxycillin Trihydrate for poultry (APVMA # 36443) is currently the only amoxicillin formulation with a NIL WHP for eggs. However, it does have a 14 days export egg WHP.
Diseases of the locomotory system
Functions and unique features of the avian musculoskeletal system
The avian skeletal system is similar to that of mammals but must balance the requirement for reduced weight to enable flight and the tensile strength needed for structural support. Consequently, the skeleton of a bird has some unique features.
The bones of birds are lighter in weight than those of mammals. Some bones are hollow and are part of the avian respiratory system. These bones, called pneumatic bones, include the humerus, clavicle, keel, pelvic girdle, and lumbar and sacral vertebrae.
Other important bones in the avian skeleton are the medullary bones. These include the tibia, femur, pubic bone, ribs, ulna, phalanges and scapula. Medullary bones are an important source of calcium when hens are laying eggs. Eggshells are primarily composed of calcium salts, and a hen's body mobilises approximately 47% of its body calcium to make an eggshell. When in production, a commercial laying hen cannot obtain enough dietary calcium to support daily egg production. Without medullary bones from which to draw calcium, the hen would produce eggs with very thin and weak shells (Figure 5).
Figure 5.
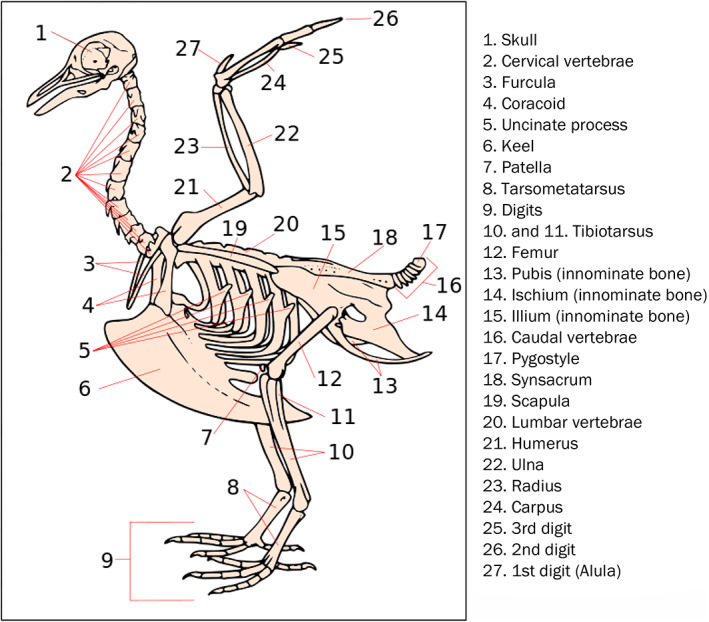
The skeleton of the Fowl. 169 1. Skull; 2. Cervical vertebrae; 3. Furcula; 4. Coracoid; 5. Uncinate process; 6. Keel; 7. Patella; 8. Tarsometatarsus; 9. Digits; 10. and 11. Tibiotarsus; 12. Femur; 13. Pubis (innominate bone); 14. Ischium (innominate bone); 15. Illium (innominate bone); 16. Caudal vertebrae; 17. Pygostyle; 18. Synsacrum; 19. Scapula; 20. Lumbar vertebrae; 21. Humerus; 22. Ulna; 23. Radius; 24. Carpus; 25. 3rd digit; 26. 2nd digit; 27. 1st digit (Alula) (Squelette_oiseau.JPG: BIODIDACderivative work: mario modesto/CC BY; https://creativecommons.org/licenses/by/2.5).
Key issues.
Lameness can have a nutritional, viral, bacterial or traumatic aetiology. 170 Therefore, it is important to ask questions about the feed source, access to feed, and changes in feed and its formulation. This applies to commercial and backyard flocks.
As bacteria (particularly Staphylococcus species) can enter the birds well before the onset of clinical lameness, a full history, including early chick quality, the donor source, scratching injuries, respiratory insults, gut health issues (including the quality of the water) and traumatic tendon damage should be recorded.
Donor flock information is important for assessing potential viral aetiologies and genetic predispositions.
Understanding the rapidity of the growth rate (particularly in broilers) and modifications (such as light programs) is important information.
It is important to determine the root cause of infection if Staphylococcus aureus or Escherichia coli are involved. They can enter the blood stream through the skin, the respiratory tract, the intestinal tract or during incubation or hatching.
| Disease presentations/differential diagnosis | |
|---|---|
| Lameness/reluctance to walk | |
| Differential diagnosis |
Viral Reoviruses Marek's disease virus Bacterial Escherichia coli Fowl cholera (Pasteurella multocida) Mycoplasma synoviae/Mycoplasma iowae/Mycoplasma meleagridis Staphylococcus aureus Enterococcus species Toxic Botulism Developmental/nutritional Dyschondroplasia Rickets Vitamin deficiency Tendon strain Cage layer fatigue Other Amyloid arthropathy Ionophore toxicity |
| Dog sitting posture | |
| Differential diagnosis |
Bacterial Enterococcus caecorum Developmental Kinky back |
| Necropsy and sampling |
|---|
|
Birds with lameness can often present with varying signs. At least 15 birds with typical clinical signs should be necropsied. Starting at the feet, note the condition of the footpads, any joint swelling (pus or serous fluid), the thickness and firmness of the gastrocnemius tendon, and any erosions in the hips. Slice the top of the hock from the medial side to inspect cartilage formation for dyschondroplasia. Bend the tibia to detect reduced bone strength, which will be affected in rickets. Open the abdomen and check for lesions, especially around the air sacs. Check the keel for breast blisters. Check the sciatic nerve if lameness caused by Marek's disease is suspected. With a sharp knife slice ventrally through the spinal column to look for abscesses, which can be found on the free thoracic vertebrae. Check other joints, such as those of the wing, for swelling or abnormal fluid. Note the findings in each bird to determine the predominant cause. If a bacterial aetiology is suspected, swab the affected joints and place the swabs in bacterial transport medium. Collect blood samples from 10 birds for serology. Collect feed and water samples. If feed retention samples are kept by the farm, collect samples from the time when leg problems were first noted. |
Treatment
As lameness due to bacterial infection can often be chronic, antimicrobial treatment will often not resolve the problem. Infection will often be secondary to other causes and the penetration of antimicrobials to the sites of infection is often poor. When individual birds are of high value or are considered pets, long‐term antimicrobial therapy may improve some less severe cases. Label directions for food‐producing animal usage must still be taken into consideration.
The exception to this will be when Mycoplasma synoviae or Pasteurella multocida are involved. The vaccination history and other signs in the birds should help differentiate these from other causes, such as Staphylococcus aureus.
Use of antimicrobials can often wait until culture and susceptibility are performed, so appropriate sampling is important.
Nutritional stress can also trigger bacterial infections. This stress may be due to an inadequate diet, but any factor that inhibits feed intake in some or all birds in the flock can be responsible.
Non‐bacterial causes of lameness (e.g. nutritional/developmental) should not be treated with antimicrobials. Correcting the nutritional cause should be the priority.
Antimicrobials used
Specific details on diseases, prevention and specific treatment choices are shown in Appendix 1. In food‐producing species, it is critical that contraindications and WHPs are reviewed as described in the label requirements and guidance in Appendix 2.
| Organism | First choice treatment | Second choice treatment |
|---|---|---|
| Mycoplasma synoviae | Refer to Section 9 | |
| Staphylococcus aureus |
Antimicrobial susceptibility testing should be performed to ensure that the most efficacious antimicrobial is used. A number of antimicrobials, including amoxicillin a , erythromycin, tylosin, oxytetracycline, and chlortetracycline have been used to treat acute and sub‐acute staphylococcal infections. Clinically affected birds respond well early in the course of the disease, but once lameness is seen in birds, treatment efficacy decreases. |
|
| Enterococcus species |
Antimicrobial susceptibility testing should be performed to ensure that the most efficacious antimicrobial is used. A number of antimicrobials, including amoxicillin a , erythromycin, tylosin, oxytetracycline, and chlortetracycline have been used to treat acute and sub‐acute enterococcosis. Clinically affected birds respond well early in the course of the disease, but once lameness is seen in birds, treatment efficacy decreases. |
|
| Escherichia coli | Refer to Section 9 | |
| Fowl cholera (Pasteurella multocida) | Refer to Section 9 | |
CCD amoxycillin trihydrate for poultry (APVMA # 36443) is currently the only amoxicillin formulation with a NIL day WHP for eggs. However, it does have a 14 days export egg WHP.
Systemic diseases
Systemic diseases in poultry can be peracute, acute, sub‐acute or chronic.
In peracute and acute cases, the challenge when presented with a sudden increase in mortality is differentiation and recognition of exotic and new emerging diseases, so empirical treatment for suspected endemic bacterial pathogens should not be undertaken until exotic and new emerging diseases have been considered. However, if the cause is a primary bacterial infection (such as fowl cholera or erysipelas), then treatment at this stage can be the most successful of any antimicrobial therapy in poultry in terms of reducing morbidity and mortality.
In chronic cases, the systemic infection can often be secondary to other factors, especially in the case of colibacillosis, and therefore treatment is often unrewarding until the primary factor is removed.
Key issues.
If high rates of mortality with a sudden onset are seen, quarantine should be implemented on the farm prior to the veterinary visit.
The veterinarian would be wise to inform government veterinarians of the situation to ensure that laboratory services are ready to perform exotic disease exclusion testing, if necessary.
If exotic or zoonotic disease is suspected, ensure that laboratory staff are aware and that birds are transported and submitted to the laboratory in biosecure containers.
| Disease presentations/differential diagnosis | |
|---|---|
| Peracute/acute | Sudden increase in mortality with or without clinical signs or post‐mortem lesions |
| Differential diagnosis |
Viral Avian influenza virus Newcastle disease virus Duck viral enteritis (duck plague) Bacterial Erysipelas (Erysipelothrix rhusiopathiae) Fowl cholera (Pasteurella multocida) Necrotic enteritis (Clostridium perfringens) Spotty liver disease (Campylobacter hepaticus) Protozoal Coccidiosis (Eimeria tenella/Eimeria brunetti/Eimeria necatrix/Eimeria maxima) Management Heat stress/anoxia Smothering Nutritional Calcium tetany in broiler breeders Metabolic Spiking mortality syndrome (hypoglycaemia) Sudden death syndrome in broiler breeders Acute death syndrome in broiler chickens Traumatic Aortic rupture in turkeys Peri‐renal haemorrhage in turkeys |
| Sub‐acute/chronic | Increase in mortality/depression with chronic signs of septicaemia, such as pericarditis/perihepatitis/focal liver necrosis |
| Differential diagnosis |
Bacterial Chlamydia psittaci Erysipelas (Erysipelothrix rhusiopathiae) Fowl cholera (Pasteurella multocida) Spotty liver disease (Campylobacter hepaticus) Escherichia coli Staphylococcus aureus Riemerella anatipestifer Salmonella species |
| Necropsy and sampling |
|---|
|
Necropsy 5–10 birds with typical clinical signs or 5–10 birds that have recently died and note findings. Depending on the findings, collect: • Swabs of heart blood from 5–10 affected birds and place into bacterial transport medium • The femur from 5–10 affected birds • Samples from affected tissues (e.g. lung/liver) from 5–10 affected birds If avian influenza or Newcastle disease are suspected, collect swabs from the palatine cleft or trachea and cloacal swabs from 10 affected birds (into viral transport medium). If sudden deaths are seen, collect a sample of feed and any retention samples from the previous week. |
Treatments
The history will be important for determining the differential diagnoses. This will include vaccination and flock history, as well as clinical signs in the flock and the necropsy findings.
In cases where there is a rapid onset of mortality and a primary bacterial disease is suspected, then treatment with antimicrobials prior to the return of laboratory results is justified on welfare grounds, as the antimicrobial therapy can effectively and fairly rapidly minimise mortalities. Refer to Appendix 1 for the preferred choice of antimicrobial.
However, laboratory samples must be taken prior to treatment to confirm the diagnosis and determine the susceptibility of the organism responsible.
Antimicrobials used
Specific details on diseases, prevention and specific treatment choices are shown in Appendix 1. In food‐producing species, it is critical that contraindications and WHPs are reviewed as described in the label requirements and guidance in Appendix 2.
| Organism | First choice treatment | Second choice treatment |
|---|---|---|
| Erysipelas (Erysipelothrix rhusiopathiae) | Amoxicillin a at 20 mg/kg live weight for 3–5 days can be used in chicken and turkey breeders and broilers. | Chlortetracycline at 60 mg/kg bodyweight for 3–5 days. |
| Spotty liver disease (Campylobacter hepaticus) | Refer to Section 8 | |
| Chlamydia psittaci | Refer to Section 9 | |
| Fowl cholera (Pasteurella multocida) | Refer to Section 9 | |
| Escherichia coli (colibacillosis) |
Do not treat with antibiotics in most cases of colibacillosis. Instead, try to investigate and correct the root cause. If treatment is undertaken, in young birds trimethoprim/sulphonamide combinations can occasionally have a beneficial impact on early omphalitis/yolk sac infection. Treat with trimethoprim/sulphadiazine at a dose rate of 25 mg sulphadiazine/kg and 5 mg trimethoprim/kg per day for 3–5 days if the birds are less than 2 weeks old, or 12.5 mg sulphadiazine/kg and 2.5 mg trimethoprim/kg per day for 3–5 days if the birds are older than 2 weeks of age. In older birds amoxicillin a can be used at 20 mg/kg live weight for 3–5 days in broilers with respiratory colibacillosis or birds with reproductive tract colibacillosis. |
Colibacillosis in young birds can be treated with lincomycin–spectinomycin at 100 g combined antibiotic activity/200 L of drinking water. Colibacillosis in older birds can be treated with chlortetracycline at 60 mg/kg live weight for 3–5 days, depending on the severity of the clinical signs. |
| Staphylococcus aureus | Refer to Section 10 | |
| Riemerella anatipestifer | Refer to Section 9 | |
| Salmonella species | Refer to Section 8 | |
CCD amoxicillin trihydrate for poultry (APVMA # 36443) is currently the only amoxicillin formulation with a NIL day WHP for eggs. However, it does have a 14 days export egg WHP.
Refer to Appendix 1 for further information, including dose rates, duration of treatment, preferred treatment choice/s and any contraindications.
Prognosis
Because of the potentially devastating impact of acute systemic bacterial disease, once an outbreak is controlled with antimicrobials, a future preventive control program must be discussed. This discussion should be held with the diagnosing veterinarian, and a government veterinarian may assist in development of future biosecurity plans.
These plans should include biosecurity measures, cleaning and disinfection, rodent control and possible vaccination strategies.
This is critical to ensure that antimicrobials are not relied upon as a future preventive strategy.
Diseases of the reproductive system
Reproductive tract disorders can have several sequelae, including loss of production, loss of egg quality (both external and internal), and reduced fertility and/or hatchability. A good understanding is needed of the development of both an egg and an embryo in order to gain insights into the location and timing of developmental abnormalities.
Records of production are usually readily available and are extremely useful tools when investigating egg production problems.
Specific records related to egg production include:
Hen‐day egg production rates
Hen‐housed egg production rates
Egg weights
Fertility (%)
Hatchability (%)
Egg recovery rates (percentage of first grade eggs)
Eggshell defects – thin shells, pale shells, other shell deformities
Shell‐less egg residues noticed in sheds
Request that the farm keep:
Dead birds aside for you
Deformed eggs aside for assessment
Structure and features of the female reproductive system
Ovary – consists of a cluster of developing ova or follicles, and is fully developed at birth, but follicles only start to develop at the commencement of sexual maturity. Follicles develop sequentially, usually one every 24 h, which allows for daily production of a single ovum, or egg.
Infundibulum – the infundibulum is like a patent funnel that engulfs the follicle and feeds it into the oviduct. Fertilisation of the ovum occurs in the infundibulum.
Magnum – this is the largest part of the oviduct, and it is here that thick albumen is laid down.
Isthmus – this is where inner and outer shell membranes form.
Tubular shell gland – this is where shell calcification commences.
Shell gland pouch – the majority of shell deposition and, finally, shell pigment is laid down in this section of the oviduct.
Vagina – the shell cuticle is deposited on the fully formed egg as it passes through the vagina during the process of laying.
Cloaca – is the single cavity receiving faeces, uric acid and eggs prior to discharge.
Vent – the external opening of the digestive and urogenital tracts.
Vestigial (persistent) right oviduct – this blind sac serves no functional purpose, but often fills with clear, water‐like fluid (Figure 6).
Figure 6.
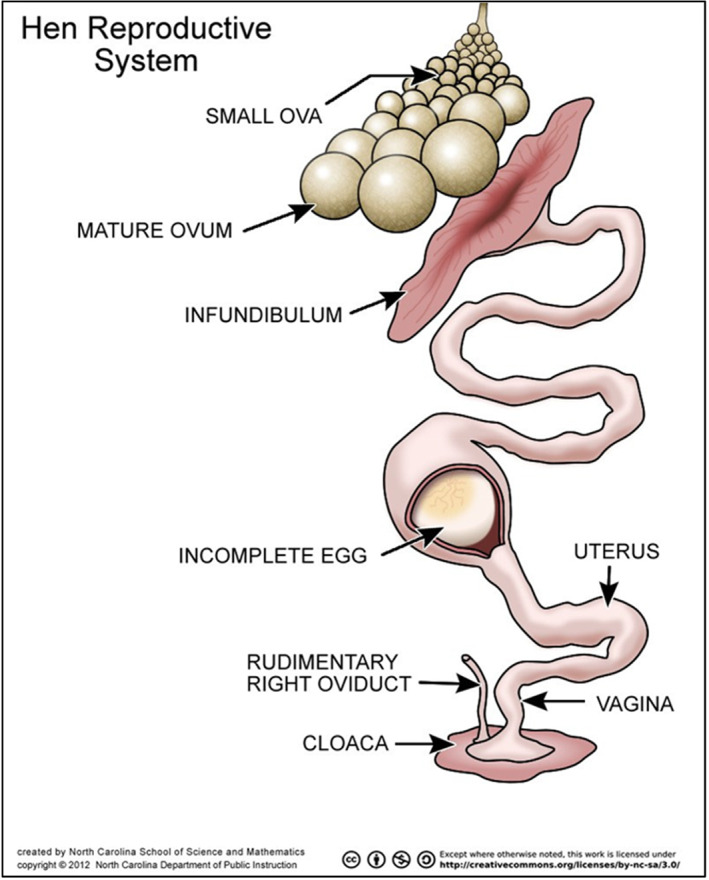
Schematic anatomy of the avian female reproductive tract.
The reproductive system of a layer or breeder hen is highly active, cycling daily to produce an egg as often as every 24 h.
Hens can store sperm for up to 10 days, so daily mating is not required. Semen is stored in sperm storage tubules in the oviduct. Fertilisation of the ovum occurs after ovulation, in the infundibulum.
Key issues.
Dietary and environmental changes can have significant effects on reproductive performance in hens and should always be considered when investigating egg production problems.
Reproductive disease is seen most frequently, but not exclusively, in high egg production commercial poultry breeds over 2 years of age.
Reproductive disease is common in backyard poultry.
Some of the most commonly seen reproductive diseases in clinical practice include egg yolk coelomitis, egg dystocia, pyometra, oviductal prolapse, and ovarian and oviductal neoplasia.
| Disease presentations/differential diagnosis | |
|---|---|
| Primary egg production drops | In all instances of egg production drops, husbandry, lighting, feed and water intake, nutrition and environmental stresses must be considered early in the investigation |
| Differential diagnosis |
Viral Egg drop syndrome virus (adenovirus) Infectious bronchitis virus Low pathogenic avian influenza virus Infectious laryngotracheitis virus Newcastle disease virus Avian encephalomyelitis virus Big liver and spleen virus (avian hepatitis E virus) Bacterial Non‐specific salpingitis (trauma, ascending infection) Fowl cholera (Pasteurella multocida) Mycoplasma gallisepticum Mycoplasma synoviae Brachyspira species Protozoal Coccidiosis (Eimeria tenella/Eimeria brunetti/Eimeria necatrix/Eimeria maxima) Histomonas meleagridis (histomoniasis) Nutritional Cage layer fatigue Fatty liver haemorrhagic syndrome Environmental/management Internal layers Broodiness Toxic Ionophores Nicarbazin Mycotoxins |
| Egg peritonitis | Internal laying can occur as a result of a sudden stress event/fright |
| Differential diagnosis |
Viral Newcastle disease virus Turkey rhinotracheitis virus Avian influenza virus Bacterial Fowl cholera (Pasteurella multocida) Escherichia coli Erysipelas (Erysipelothrix rhusiopathiae) Chlamydiosis (Chlamydia psittaci) Environmental Feather pecking leading to cannibalism |
| Shell deformities | Shell deformities are often early indicators of underlying issues |
| Differential diagnosis |
Viral Egg drop syndrome virus Infectious bronchitis virus Newcastle disease virus Bacterial Mycoplasma synoviae Nutritional Inadequate or surplus calcium Inadequate Vitamin D Calcium/Phosphorus imbalance |
| Internal quality | Albumen quality, yolk colour |
| Differential diagnosis |
Viral Egg drop syndrome virus Infectious bronchitis virus Infectious causes Yolk colour is artificially managed with feed additives, but changes in yolk colour can be indicative of disease and need investigation. Caecal infections with protozoa can cause a loss of yolk colour – Histomonas meleagridis (histomoniasis) or coccidiosis (Eimeria tenella/Eimeria brunetti/Eimeria necatrix/Eimeria maxima). Egg handling Old eggs Poor storage conditions Nutritional: Lack of artificial yolk colouring in diet |
| Pasty vent | |
| Differential diagnosis |
Visceral gout Ascending salpingitis due to cannibalism |
| Infertility | |
| Differential diagnosis |
Management/husbandry Excess weight |
| Necropsy and sampling |
|---|
|
Necropsy 5–10 birds with typical clinical signs or 5–10 birds that have recently died and note findings. Swab typical lesions and submit swabs for laboratory testing by polymerase chain reaction and/or culture and susceptibility testing. Collect blood samples from 10 birds for serology to detect evidence of viral or mycoplasma infection. Note, if an egg production drop is rapid and unexplained with no other signs, then extra samples (up to 30) may need to be taken from apparently normal birds for detection of pathogens such as low pathogenic avian influenza virus. |
Treatment
The history will be important for determining the differential diagnoses. This will include vaccination and flock history, as well as clinical signs in the flock and the necropsy findings. It would be prudent to delay antimicrobial treatment until a bacteriological diagnosis and susceptibility can be established.
Most causes of reproductive system disease are non‐infectious, so a thorough investigation of non‐infectious causes is warranted.
Primary bacterial causes of reproductive disease are very uncommon and a decision to use antimicrobials should only be made once a specific diagnosis has been made.
Depending on the underlying cause, treatment may consist of medical or surgical therapy. Euthanasia is often required for neoplastic causes of reproductive disease because of the frequent occurrence of metastasis.
Antimicrobials used
Specific details on diseases, prevention and specific treatment choices are shown in Appendix 1. In food‐producing species, it is critical that contraindications and WHPs are reviewed as described in the label requirements and guidance in Appendix 2.
| Organism | First choice treatment | Second choice treatment |
|---|---|---|
| Chlamydia psittaci | Refer to Section 9 | |
| Erysipelas rhusiopathiae | Refer to Section 11 | |
| Non‐specific salpingitis | Chlortetracycline at 60 mg/kg bodyweight for 3–5 days in drinking water | No alternative treatments |
| Mycoplasma synoviae | Refer to Section 9 | |
| Pasteurella multocida | Refer to Section 9 | |
Diseases of the nervous system
The avian nervous system is less complex than, but essentially identical in structure and function to, the mammalian nervous system. Consequently, diseases of the central and peripheral nervous systems have similar presentations to those seen in mammals.
Diseases causing neurological signs are many and varied. It is important when investigating neurological disease that exotic, transboundary diseases are considered in the differential diagnosis. The major presenting clinical signs for which neurological disease might be a diagnostic consideration are paresis, paralysis, leg misplacement, tonic tremors, incoordination, blindness, opisthotonos and depression.
Key issues.
The investigation of neurological disorders should always include exclusion of avian influenza, Newcastle disease and turkey rhinotracheitis. With that in mind, consideration needs to be given to placing the property under quarantine as a precautionary measure until a workup has been conducted.
Ask the farm to keep dead and affected birds aside for you – do not cull all affected birds as the clinical signs are a significant contributor to making a diagnosis.
Check the history of the parent flocks for vaccination against Marek's disease, Newcastle disease and avian encephalomyelitis.
The clinical picture is a very important contributor to making a diagnosis, but is not necessarily pathognomonic. It would be prudent to institute quarantine measures until the diagnosis is confirmed.
| Disease presentations/differential diagnosis | |
|---|---|
| Paresis/paralysis | It is important to confirm the paresis/paralysis by conducting proprioceptive tests to differentiate it from other causes of immobility |
| Differential diagnosis |
Viral Marek's disease virus Toxic Ionophores Botulism |
| Leg misplacement | |
| Differential diagnosis |
Nutritional/management Perosis (slipped tendon) Spraddle leg Spondylolisthesis (kinky back) Valgus/varus deformity |
| Tonic Tremoring | |
| Differential diagnosis |
Viral Avian encephalomyelitis virus Nutritional Epidemic tremor (Vitamin E deficiency) |
| Incoordination | |
| Differential diagnosis |
Viral Avian influenza virus Newcastle disease virus |
| Blindness | |
| Differential diagnosis |
Viral Marek's disease virus Fungal Aspergillus species (ocular aspergillosis) Toxic Ammonia blindness |
| Opisthotonus/Torticollis | |
| Differential diagnosis |
Viral Avian influenza virus Newcastle disease virus Turkey rhinotracheitis virus Bacterial Fowl cholera (Pasteurella multocida) Riemerella anatipestifer Middle ear infection Nutritional Vitamin B deficiencies Dinitolmide, 3,5‐dinitro‐o‐toluamide (DOT) toxicity |
| Depression | |
| Differential diagnosis |
Viral Avian influenza virus Newcastle disease virus Other Any late stage disease that causes depression and moribundity |
| Necropsy and sampling |
|---|
|
Necropsy 5–10 birds with typical clinical signs or 5–10 birds that have recently died and note findings. If no clear gross lesions are identified, or confirmation of diagnosis is required, then collect samples of the brain and/or affected nerves for histopathology. Swab typical lesions and submit swabs for laboratory testing by polymerase chain reaction and/or culture and susceptibility testing. Collect swabs of the heart blood from 5–10 affected birds and place into bacterial transport medium. Collect blood samples from 10 birds for serology to detect evidence of viral infection. Collect feed samples for testing if vitamin deficiencies are suspected. |
Treatment
Specific details on diseases, prevention and specific treatment choices are shown in Appendix 1. In food‐producing species, it is critical that contraindications and WHPs are reviewed as described in the label requirements and guidance in Appendix 2.
| Condition | First choice treatment | Second choice treatment |
|---|---|---|
| Riemerella anatipestifer | Refer to Section 9 | |
Immunosuppressive diseases
The avian immune system shares many similarities with that of mammals, but also has some fundamental differences.
The avian system has a cell‐mediated and a humoral immune system, essentially as in mammalian systems. The thymus, bursa of Fabricius and bone marrow are primary lymphoid organs, while the spleen, mucosal associated lymphoid tissues germinal centres, and diffuse lymphoid tissues are secondary lymphoid organs. Birds do not have lymph nodes.
The thymus, where T cells develop, is a lobulated organ located in the neck, running parallel to the cervical artery and jugular vein. The bursa of Fabricius is an organ that is unique to birds and is the site of B cell development, differentiation and maturation. Located dorsal to the rectum, this organ contains stem cells and is highly active in young birds, but atrophies after about 6 weeks. There are diffuse lymphoid accumulations in the head and associated with the respiratory system and gastrointestinal tract, such as the Harderian gland, located behind the eyes, and the Peyer's patches, in each of the caeca, just proximal to the junction of the caecum with the colon.
A number of agents, including viruses, bacteria, parasites, toxins, mycotoxins, chemicals and drugs, can cause immunosuppression in birds. The most common immunosuppressive viruses encountered in Australia are infectious bursal disease virus, chicken anaemia virus, inclusion body hepatitis virus, avian lymphoid leukosis virus and Marek's disease virus. Primary immunosuppressive infections increase the susceptibility of birds to secondary bacterial, viral and fungal infections.
The presentation of a primary immunosuppressive disease is often complicated by secondary infections, making diagnosis of the primary disease more complex, but also more important. Treatment of secondary infections can be unrewarding if the primary cause of disease is not managed correctly. The secondary pathogen most frequently encountered because of immunosuppressive disease is Escherichia coli.
Persistence of immunosuppressive viruses in the environment will result in ongoing secondary infections in subsequent flocks. It is therefore imperative to implement preventive measures, such as thorough cleaning and disinfection of facilities, and vaccination, to minimise the impact of these viruses on subsequent flocks.
| Disease presentations/differential diagnosis | |
|---|---|
| Lameness with paralysis, paresis | |
| Differential diagnosis | Marek's disease virus |
| Chronic wasting, emaciation +/− visceral or lymphoproliferative tumours | |
| Differential diagnosis |
Marek's disease virus Avian lymphoid leukosis virus |
| Septicaemia, colibacillosis | |
| Differential diagnosis |
Secondary infections Escherichia coli (secondary to infection with infectious bursal disease virus, inclusion body hepatitis virus, chicken anaemia virus) |
| Runting out, ill‐thrift in young chicks | |
| Differential diagnosis |
Chicken anaemia virus Runting/stunting syndrome |
| Unresponsive to antimicrobial therapy for other conditions | |
| Differential diagnosis |
Infectious bursal disease virus Chicken anaemia virus Marek's disease virus Avian lymphoid leukosis virus |
| Anaemia, subcutaneous haemorrhaging | |
| Differential diagnosis | Chicken anaemia virus |
| Necropsy and sampling |
|---|
|
Necropsy 5–10 birds with typical clinical signs or 5–10 birds that have recently died and note findings. Collect samples of the lymphoid tissues for histopathology. Swab typical lesions and submit swabs for laboratory testing by polymerase chain reaction and/or culture and susceptibility testing. Collect blood samples from 10 birds for serology to detect evidence of viral infection. |
| Recommendations |
|---|
| Thorough cleaning, disinfection, biosecurity and vaccination, where appropriate, are required to eradicate viral immunosuppressive agents in order to manage the secondary clinical impacts of these infections. |
Diseases of the young chick
One of the more common disease entities encountered in poultry practice is that of poor chick health and vitality, and early chick mortality. Day‐old chicks are highly susceptible to environmental and infectious disease challenges and can succumb rapidly.
It is generally accepted that mortality issues in the first 3–4 days are more likely associated with the source hatchery, or the source breeder flock. In this instance, investigations should go beyond the individual affected flock to other flocks derived from the same breeder flocks or hatchery. This will often provide important information about the cause of the problem.
Brooding conditions are also critical to the successful early development of a chick. Mortalities starting after 4 days of age can often be attributed to brooding issues, primarily either environmental stresses or poor hygiene.
| General approach | |
|---|---|
| Specific considerations when investigating disease problems in young chicks |
Disease problems in young chicks are usually related to one of three issues – on‐farm brooding conditions, breeder flock problems or hatchery problems – so investigations should look beyond the immediate farm to other farms that may have received similar chicks, then back to the hatchery or breeder farm. If early bacterial infections are present, then potential points of infection will be associated with specific stages and areas – from the time the egg is laid, to the time the chick is placed onto the farm. At any point, high levels of contamination or poor barriers to infection (such as poor eggshell quality or poor navel healing of the chick after hatching) can result in mortality from bacterial infection. Early stress, especially chilling, can increase mortality caused by these bacteria significantly. |
| Before farm entry |
Look at the mortality and production records. Review other farm records, including those for temperature/ventilation/biosecurity. Review hatchery records. Review vaccination records of parent flocks. Review transport records. |
| On farm |
Observe: Biosecurity standards Ventilation, litter temperature, brooding conditions and air quality Bird behaviour Crop fill Clinical signs |
| Disease presentations/differential diagnosis | |
|---|---|
| High mortality 1–7 days | |
| Differential diagnosis |
Bacterial Yolk sac infection Omphalitis Contaminated vaccines Fungal Aspergillus species Management Non‐starters (poor brooding conditions) Dehydration (gout) |
| High mortality 7–14 days | |
| Differential diagnosis |
Bacterial Ongoing mortalities from poor brooding conditions Viral Inclusion body hepatitis Chicken anaemia virus Fungal Aspergillus species Management Poor weaning (temperature, ventilation, feed or water availability) |
| Diarrhoea/wet floors | |
| Differential diagnosis |
Viral Nephrosis caused by infectious bronchitis virus Runting/stunting complex Bacterial Salmonella species Escherichia coli Management Under or over heating High stocking density Poor drinker management |
| Swollen abdomen | |
| Differential diagnosis |
Bacterial Yolk sac infection |
| Respiratory/ocular signs | |
| Differential diagnosis |
Viral Infectious bronchitis virus (wild or vaccine strains) Fungal Aspergillus species Management Under or over heating Elevated ammonia levels (poor ventilation, poor litter management) |
| Poor growth rate | |
| Differential diagnosis |
Viral Enteric viruses Runting/stunting complex Management Brooding conditions Feed/water availability Nutritional Feed quality |
| Neurological signs | |
| Differential diagnosis |
Bacterial Meningitis, encephalitis Constaminated vaccines Viral Avian encephalomyelitis virus Newcastle disease virus Nutritional Vitamin deficiencies (B vitamins and vitamin E) |
| Lameness | |
| Differential diagnosis |
Bacterial Osteomyelitis, femoral head necrosis Deformities of the legs Hatchery‐related issues Rickets Vitamin deficiencies |
| Necropsy and sampling |
|---|
|
Necropsy 10–20 cull chicks with typical clinical signs or 10–20 chicks that have recently died and note findings. Submit whole live chicks to the diagnostic laboratory. Swab typical lesions or sample tissues and submit swabs and/or tissues for laboratory testing by polymerase chain reaction and/or culture and susceptibility testing. |
| Recommendations |
|---|
|
The history will be important for determining the differential diagnoses. This will include a total review of brooding conditions, vaccination history and the flock history, as well as clinical signs in the flock and the necropsy findings. It would be prudent to delay antimicrobial treatment until a bacteriological diagnosis and susceptibility can be established. |
Treatment
Specific details on diseases, prevention and specific treatment choices are shown in Appendix 1. In food‐producing species, it is critical that contraindications and WHPs are reviewed as described in the label requirements and guidance in Appendix 2.
Treatment of young chicks is often unrewarding as it is difficult to entice them to drink or eat, leading to rapid loss of vitality and unsatisfactory intake of medication. For this reason, the most humane option for the welfare of sick young chicks is often euthanasia.
| Condition | First choice treatment | Second choice treatment |
|---|---|---|
| Omphalitis (navel ill, yolk sac infection, mushy chick disease) | Refer to Section 11 | |
Diseases of turkeys, ducks and other poultry
When dealing with unfamiliar species or unfamiliar disease scenarios, it is prudent to use first principles, then investigate and treat based on the premise that all species of poultry are similar at a base level, but refer to Appendix 2 for a list of known or possible exceptions. From that base, knowledge can be acquired from the owner, from references and textbooks and from experienced poultry and avian veterinarians to assist with diagnosis and treatment.
However, it should be noted that products registered for chickens may not be registered for other species. Check the label for the registration status and contraindications in the species you are wishing to treat. There are some products used in one species that may be toxic for another. For example, salinomycin is toxic for turkeys.
There are several disease entities that occur in a wide range of species, so it is worthwhile referring to Appendix 1 and the specific system sections for references to diseases encountered.
| General approach | |
|---|---|
| Before the farm visit |
Ask: What are the species and breed of birds? About the history of the farm. About the history of the flock. What is the age of the flock? What was the source of the flock? Tell the farm manager to: Keep dead birds aside for you. Prepare: Swabs and transport media (viral and bacterial). Biohazard bags for bird collection. Esky and ice bricks. |
| On the farm |
Ask: What is the mortality rate? About the vaccination history. When were clinical signs first noticed? Have there been any management changes or problems (e.g. ventilation, brooding setup)? Have there been any introductions of stock to the farm recently? Have the same clinical signs been seen previously on the farm? Have there been associated outbreaks on other farms? Observe: Look at mortality and production records. Review other farm records, including those for temperatures/ventilation/biosecurity. Ventilation and brooding conditions, and air quality. The shed condition and the exclusion of pests. The birds and note the proportion affected. Clinical signs. |
| Species and specific diseases encountered | |
|---|---|
| Ducks | Differential diagnosis |
| Septicaemia (acute or chronic) |
Bacterial Riemerella anatipestifer Pasteurella multocida Erysipelothrix rhusiopathiae Escherichia coli |
| Turkeys | Differential diagnosis |
| Septicaemia (acute or chronic) |
Bacterial Chlamydia psittaci Erysipelothrix rhusiopathiae |
| Focal hepatitis |
Protozoal Histomonas meleagridis (histomoniasis) |
| Quail | Differential diagnosis |
| Enteritis |
Bacterial Ulcerative enteritis (Clostridium colinum) |
| Pigeons | Differential diagnosis |
| Septicaemia (acute or chronic) |
Bacterial Chlamydia psittaci Salmonella species Streptococcus species |
| Respiratory |
Bacterial Mycoplasma species Chlamydia psittaci Fungal Aspergillus |
| Enteric | Coccidiosis (Eimeria spp.) |
Antimicrobials used
Each disease is covered either in the specific system involved or in Appendix 1.
Considerations for choice of first priority antimicrobials
Antimicrobial decision tree
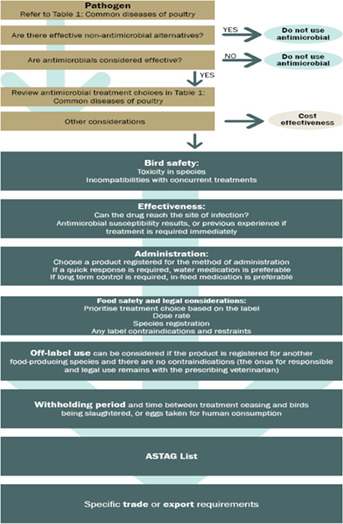
Funding and acknowledgments
Funding for these guidelines was provided by the Australian Veterinary Association (AVA), Animal Medicines Australia (AMA), and Agrifutures Australia as part of the Agrifutures Chicken Meat Program.
These guidelines would not have been possible without the considerable expertise and efforts of the Expert Panel authors: Dr Peter Gray, Dr Rod Jenner, Dr Stephen Page, Professor Jacqueline Norris, and Professor Glenn Browning.
Additional in‐kind contributions were made by the Australian Veterinary Association, Animal Medicines Australia, and NSW Department of Primary Industries.
The work of Project Manager Dr Amanda Black is gratefully acknowledged, as are the contributions of the project Steering Committee members Dr Phillip McDonagh, Dr John Messer, Professor James Gilkerson, and Dr Melanie Latter.
APPENDIX A1.
Appendix 1.
Common diseases of poultry
| Pathogen/disease | Age | Species | Clinical signs | Preventive elements | Suitable treatment choice (refer to decision tree) | Notes |
|---|---|---|---|---|---|---|
| Aspergillus species (and other fungal pneumonias) (brooder pneumonia) | Acute cases in young birds. |
All species, but especially chickens and turkeys. |
Gasping, eye lesions, neurological signs (torticollis/lack of equilibrium), stunting. White caseous nodules in lungs and air sacs. |
Infections acquired from environmental exposure, so focus on eliminating the source of infection. Avoid mouldy litter and feed. Practice good hatchery and hatchery transport vehicle sanitation. Improve ventilation – reduce humidity. Use of antifungal disinfectants such as enilconazole. Feed and litter antifungal additives may be helpful in control, but ensure they meet food safety requirements. |
Effective treatment for avian aspergillosis and other fungal infections is not available. | To investigate the source of fungal infections, microbiological monitoring of the hatchery and the litter source can be helpful, as long as results are interpreted in the light of normal source environmental levels. |
| Avian tuberculosis (Mycobacterium avium) | All ages susceptible, but more likely in older birds. | All species. | Weight loss. Irregular, discrete, greyish yellow or greyish white nodules in spleen, liver and intestine. |
Quarantine/biosecurity (infection risk for birds placed in previously contaminated premises or in‐contact with infected birds). Source clean stock (quarantine new additions to the aviary for 60 days and retest with avian tuberculin). |
Treatment unsuccessful. | More likely to be seen in backyard and zoo birds. |
| Crop mycosis (candidiasis; Candida albicans) | All ages, but young more severely affected. | Chickens, turkeys, geese, pigeons, guinea fowl, pheasants, quail. |
Poor growth, stunting. Crop/oral mucosal thickening with white to off‐white, raised lesions. May present as a pendulous crop. |
Opportunistic endogenous mycosis that results from disturbance of microflora or immunosuppression. Correct management factors, such as water and feed hygiene, husbandry and nutrition. |
Copper sulfate at a 1:2000 dilution in the drinking water may be helpful, but effectiveness is questionable. Antimicrobials are contra‐indicated. |
|
| Cannibalism (feather/vent pecking) | All ages, but especially young adults. | Chickens, turkeys (there is a genetic predisposition in some strains). | Vary from pecking without removal of feathers to plucking of feathers. Egg production may drop. Pecking of the vent can also be observed soon after birds come into lay and can be responsible for 80% of all prolapses and may trigger salpingitis and egg peritonitis. |
Balanced diet. Inclusion of fibre in the diet. Rearing on the floor rather than slats. Reducing light intensity. Provide perches as a refuge for pecked birds. Avoid overcrowding. Light intensity (keep shed free of glare and rays of light). Environmental enrichment. Adequate beak trimming. |
Medication is rarely effective even there are secondary bacterial infections (problem continues after medication if the underlying cause is not identified). |
Reduce stocking density, review diet, change light intensity and colour, cull flock early. Keep shed free of glare and minimise temperature variation in the shed. Investigate for primary insult/causes of cannibalism. Second beak trim may be warranted. |
| Cellulitis (most frequently Escherichia coli) | 4–8 weeks. | Chickens ‐broiler strains. |
Caseous plaques in subcutaneous tissue of skin over the abdomen or between thigh and midline. Lesions develop rapidly. More common in males. |
Predisposed by skin scratching. Good feather cover can protect from scratching. Higher stocking density can have an impact. Litter management and source (more likely on straw than shavings). Reduce husbandry/management issues, be aware of flock activity levels, lighting schedule, movement through flocks. Good sanitation between flocks to reduce bacterial loads. |
Unresponsive to antimicrobials. Address underlying causes, such as immunosuppressive disease and factors resulting in scratching. | Specific Escherichia coli strains are often involved in cellulitis and have a greater capacity to survive in deeper tissue layers. |
| Chlamydiosis (Chlamydia psittaci) | >1 week. | All (but particularly turkeys, ducks and pigeons). |
Nasal and ocular discharge, conjunctivitis, sinusitis, green to yellow‐green droppings, fever, inactivity, ruffled feathers, weakness, inappetence and weight loss. Necropsy findings in acute infections include serofibrinous polyserositis (airsacculitis, pericarditis, perihepatitis, peritonitis), pneumonia, hepatomegaly and splenomegaly. |
Biosecurity, especially from wild birds and rodents. Stressors (such as transport, crowding, breeding, cold or wet weather, dietary changes or reduced food availability) and concurrent infections, especially those causing immunosuppression, can initiate shedding in latently infected birds and cause recurrence of clinical disease. Carriers often shed the organism intermittently for extended periods in faeces. Organisms in dried excrement can remain infectious for many months. Very susceptible to sanitisers such as formaldehyde and phenolic disinfectants. No effective vaccine available for birds. |
Tetracyclines (oxytetracycline and chlortetracycline) are the antimicrobial treatment of choice. Tetracyclines are bacteriostatic and effective only against actively multiplying organisms, making extended treatment times necessary (from 2–8 weeks, throughout which minimum inhibitory concentrations must be consistently maintained in the blood). Recommendation: Chlortetracycline at 60 mg/kg for 5–7 days in drinking water followed by 400–750 ppm chlortetracycline in feed for a minimum of 2 weeks, depending on the severity of disease. Longer treatments maybe required for elimination of the organism and retesting maybe required prior to processing. |
Absorption of oxytetracycline and chlortetracycline may be reduced by calcium in the diet and the level of active drug may be reduced by heat treatment of feed. Zoonotic potential and notifiable in some states. Higher than labelled dose rates or duration may require extension of the withholding period and is the responsibility of the veterinarian. |
| Coccidiosis (Eimeria species) | Normally young birds, but all ages affected depending on time of exposure. | All, but coccidial species involved will vary between hosts. |
Mortality, diarrhoea, poor feed conversion and growth rate. Depending on the Eimeria species and the level of infection, gross lesions in the intestine will vary from haemorrhage and ballooning to inapparent. |
Vaccination, often at the hatchery using gel technology (used primarily in breeders and layers, but also an increasing number of broilers). Should be well controlled with coccidiostats if used at the correct levels in feed. |
Recommendation: Amprolium/ethopabate in water is the primary treatment of choice in chicken broilers when in‐feed medication control is insufficient. When in a concentration of 216 g amprolium/L and 14 g ethopabate/L, a rate of 500 mL‐1000 mL/900 L drinking water for 5–7 days may be required, depending on the severity of the disease. This product is registered for all poultry with a nil withholding period for meat, but cannot be used for egg layers where eggs are used for human consumption. Amprolium alone (without ethopabate) (200 g/kg) can be used at 5 g/4 L for 5–7 days followed by 3 g/4 L for 5–7 days to treat an outbreak. This product can be used in chicken egg and meat birds, as well as in ducks, turkeys and pigeons. There is a nil withholding period for eggs and meat. Toltrazuril (Baycox) in water is more effective in stopping an outbreak of mortality due to coccidiosis, but it is registered only for chickens, has a withholding period of 14 days for meat, and cannot be used for birds that will be laying eggs within 8 weeks of treatment. Treatment is 3 L/1000 L for 2 consecutive days. Sulphaquinoxaline can also be used, but also has a withholding period that may make it unsuitable for meat birds. There is also a risk of vitamin K deficiency. It is the least preferred treatment. |
|
| Colibacillosis (Escherichia coli) | Any age, but especially young birds. | All species. |
Primarily secondary opportunistic infection. Often occurs concurrently with other diseases. Birds often terminally moribund or very lethargic. Localised form • omphalitis/yolk sac infection • cellulitis • swollen head syndrome • salpingitis/peritonitis Systemic form • colisepticaemia (respiratory origin, enteric origin) • haemorrhagic septicaemia (turkeys) neonatal septicaemia Sequelae • meningitis/encephalitis, panophthalmitis, osteomyelitis, arthritis, synovitis, sternal bursitis, pericarditis, juvenile salpingitis, coligranuloma |
Keeping E. coli out of the flock is not a realistic goal because intestinal colonisation is universal. Reducing E. coli numbers in feed and water, ensuring good sanitation practices, optimising air quality, and protecting the flock from co‐factors, especially viral infections and suboptimal management, that decrease host resistance are important. Reduce faecal contamination of hatching eggs and early contamination at hatch. Reduce stress and ensure good litter management. Identify and correct primary causes. An E. coli live vaccine is now registered and may be considered as an alternative control. However, it is only recently available and industry experience is limited. Probiotics (but must start early), prebiotics, and essential oils have been used, but there is no reliable evidence of efficacy at this stage. |
Recommendation: Do not treat with antibiotics in most cases of colibacillosis. Rather try to investigate and correct the root cause. If treatment is undertaken: Trimethoprim/sulphonamide combinations have occasional beneficial impacts on early omphalitis/yolk sac infection. There are several combinations registered for use in poultry, including chickens, turkeys and pigeons. Used at labelled rates they can have some effect but cannot be used in birds that will lay eggs for human consumption, have a relatively long (14–15 day) meat withholding period and can interfere with coccidiosis vaccines if used. Amoxicillin a at 20 mg/kg live weight can be used for 3–5 days in broilers with respiratory colibacillosis or birds with reproductive colibacillosis. Chlortetracycline at 60 mg/kg live weight can be used for 3–5 days, depending on the severity of the clinical signs. The advantage of chlortetracycline is that there is a nil withholding period for eggs, and it is also effective against a range of other organisms that may be involved, including mycoplasmas. A 7‐day meat withholding period for chlortetracycline limits its use in shorter lived birds, such as chicken broilers. Oxytetracycline will also be effective and is less expensive, but it is not registered for birds laying eggs for human consumption. |
Treatment strategies include attempts to control predisposing infections or environmental factors. Benefits from antimicrobial therapy are variable, with most cases unresponsive (or responsive only in the short term), especially if the underlying cause is not (or cannot) be rectified. Antimicrobial susceptibility tests should be carried out. Often isolates are resistant to tetracyclines and sulphonamides. Even after antimicrobial susceptibility tests, a highly effective drug may not result in improvement in the flock if too little is used, or if it is incapable of reaching the site of infection. |
| Dysbacteriosis (non‐specific bacterial enteritis) | Most commonly seen in broiler chickens from 7–42 days of age. |
Chickens (broilers and layers). |
Diarrhoea. Water intake may be increased or irregular. Excessive fluid content throughout the small intestine. Wet faeces in the rectum. Voluminous caeca, often with gas bubbles. |
Dietary changes, feed interruptions and subclinical coccidiosis may be contributory factors. No single bacterial species appears to be responsible. Rather this problem results from a disruption of the normal flora of the gut. Competitive exclusion (introduction of ‘normal adult flora’) in day‐old chicks reduces the risk of this problem. Feed acidification may be helpful in some circumstances. Water hygiene and good litter quality are important. It can occur in free‐range layers after consuming water pooling on the range. Careful choice of any feed enzymes and matching them with local raw materials can have an impact on the substrates available to intestinal bacteria. Good control of coccidiosis. Good quality feed ingredients. Feed additives, such as probiotics, prebiotics, short chain and medium‐chain fatty acids and essential oils, have been used to support gut integrity. |
Generally, antimicrobials are not used. It is important to address the underlying cause. | |
| Enterococcus species | All ages. | Chickens. |
Enterococcus species isolated from birds with clinical disease include Enterococcus avium, Enterococcus cecorum, Enterococcus durans, Enterococcus faecalis, Enterococcus faecium and Enterococcus hirae. Infection may result in: • septicaemia • lameness, with spinal abscessation at free thoracic vertebrae, resulting in birds sitting with legs extended cranially • increased late losses in hatchability • splenomegaly, hepatomegaly • pericarditis, perihepatitis • valvular endocarditis |
Prevention of immunosuppressive diseases and conditions is required, because enterococcosis is often secondary to another disease. In addition, ensuring proper cleaning and disinfection of facilities can reduce environmental reservoirs of the bacteria. Water hygiene is also important. Enterococcal species are resistant to drying and can survive for prolonged periods in the environment. Hatchery and egg hygiene are important for control, especially if using injectable vaccines. |
See ‘Colibacillosis’. Antimicrobial susceptibility testing should be performed to ensure that the most efficacious antimicrobial is used. Several antimicrobials, including amoxicillin a , erythromycin, tylosin, oxytetracycline and chlortetracycline have been used to treat acute and sub‐acute enterococcosis. Clinically affected birds respond well early in the course of the disease, but treatment efficacy decreases as the disease progresses. |
Enterococcus species are part of the normal flora in poultry, but can cause secondary infections, so control of primary diseases can prevent enterococcal infections. Clinical signs of enterococcosis are related to septicaemia, and treatment is effective if provided in the early stages of the disease. If enterococcosis becomes chronic, skeletal diseases have been reported, and treatment efficacy decreases with chronicity. |
| Erysipelas (Erysipelothrix rhusiopathiae) | Any age, but most commonly older birds. | Chickens, turkeys, ducks, geese. |
Sporadic outbreaks. General weakness, depression, diarrhoea and sudden death. Egg production may be decreased. Signs of septicaemia, including petechial haemorrhages, vegetative endocarditis and dark crusty skin lesions. |
Strict biosecurity. The source of the organism may be contaminated feed or soil, infected carrier birds or infected rodents. Pigs and sheep can be a potential source. In an outbreak, thoroughly decontaminate equipment with disinfectants, promptly remove dead birds, encourage feed and water intake, handle birds as little and as gently as possible to minimise risk of scratching. Vaccinate with inactivated sheep bacterins (off‐label). |
Antimicrobial of choice is amoxicillin a at 20 mg/kg liveweight for 3–5 days. | |
| Femoral head necrosis (Escherichia coli, Staphylococcus aureus) | Up to 12 weeks of age. | Chicken broilers and breeders/duck broilers. | Lameness, often with ‘wing walking’ and reluctance to move. |
The root cause is bacterial entry into the blood stream and deposition in femoral growth plates, so focus on reducing exposure of the birds to the bacterial causes (e.g. good husbandry, optimal gut health, reduced skin lesions). Good chick quality (hatchery/breeder hygiene). Good air quality and good control of respiratory pathogens. Good gut health. Reduced stress factors (stocking density, optimal environment). Culling of affected birds. Preventive use of probiotics has been found to reduce levels of mortality. 171 (Wideman. Poultry Science 91: 870‐883. April 2012) |
Once clinical signs are seen medication with antimicrobials is not effective because of the advanced pathology. Affected birds should be culled on welfare grounds. |
|
| Fowl cholera (Pasteurella multocida) | Most commonly in mature birds, or birds approaching maturity, but all ages susceptible. | Turkeys and waterfowl are more susceptible than chickens; older chickens are more susceptible than young ones. |
Acute: Sudden death, fever, mucus excretion from mouth, diarrhoea. General hyperaemia, petechial and ecchymotic haemorrhages. Livers swollen and containing multiple small necrotic foci. Chronic: Localised infections in wattles, sinuses, leg or wing joints, footpads, sternal bursa. Pharyngeal lesions. Occasionally torticollis. Tracheal râles, dyspnoea. Localised suppurative lesions, often in the respiratory tract, but also the conjunctiva, foot pads, peritoneal cavity and oviduct. Pneumonia is an especially common lesion in turkeys. Facial oedema. |
Prevent introduction into farm by carrier birds (wild and introduced), farm animals (dogs, pigs, cats), feed/water contamination, rodents. A mixed flock age is a risk factor. Vaccination with live attenuated vaccine (not suitable for turkeys) or killed autogenous bacterins. Poor management/stress triggers outbreaks. Outbreaks may be caused by a new strain, so swabs should be collected for culture and typing. They can be added to a bacterin vaccine. All rearing birds going to that site in future can be vaccinated with the bacterin with the new strain in it. This is not necessary for the live vaccine, which is cross protective. |
If fowl cholera is suspected and mortality is elevated, antimicrobial treatment (oxytetracycline at 70 mg/kg for 5–7 days, chlortetracycline at 60 mg/kg for 5–7 days or amoxicillin a at 20 mg/kg for 3–5 days should commence immediately). First choice treatment should be tetracyclines on the understanding that oxytetracycline is not registered for birds producing eggs for human consumption. However, as fowl cholera often affects older birds, the choice may be limited by the registered withholding period available before processing of the flock or part of the flock. Samples should be taken to confirm the diagnosis, to enable antimicrobial susceptibility testing to be performed and to isolate the causative strain for potential inclusion in a bacterin vaccine. Fowl cholera outbreaks can recur after cessation of treatment, so in the case of severe infections chlortetracycline may be required in‐feed at 100 ppm for up to 28 days. In the event of an outbreak, increase the frequency of pickup of dead birds and introduce regular water sanitation, as dissemination of Pasteurella multocida within a flock and between houses is primarily by excretions from the mouth, nose and conjunctiva of diseased birds that contaminate their environment. Eradication of infection requires depopulation and cleaning and disinfection of buildings and equipment. The premises should then be kept free of poultry for a few weeks. |
|
| Histomoniasis (Histomonas meleagridis; blackhead) | > 4 weeks. | Chickens, turkeys, game birds. |
Depression, inappetence. Thickening of the caecal wall and development of a caseous core. Liver lesions can occur, especially in turkeys, seen as circular depressed areas of necrosis up to 1 cm in diameter. |
Primary reservoir of infection is the ova of the caecal nematode Heterakis gallinarum. As chickens are carriers, keep them separate from turkeys. Worming strategies are important. Ranges can become contaminated with eggs. Flubendazole (feed) or levamisole are suitable wormers. As the nematodes can also live inside earthworms, control of drainage around sheds is important. Caecal coccidiosis can interact with histomoniasis in chickens. Cleanout and possible worm egg control measures on the floor (such as salt or heat treatment) can be helpful. |
There are no chemotherapeutic products available for treatment of infections. Essential oils (oregano oil) have been used in a disease outbreak situation with minimal success. 172 Greater success seems to occur from preventive treatment with oregano oil on farms where challenge is expected. |
|
| Internal laying | Layers. | Chickens. | Partially or fully formed egg in abdominal cavity. The egg reaches the cavity by reverse peristalsis of the oviduct, frequently with the eggshell membrane left in the cavity. | No control. | No treatment. | |
| Internal parasites | All ages. | All species. | Unless infestations are heavy, clinical disease is usually not evident. |
Cleanout. Disinfection. Preventive worming programs. |
A faecal examination can be performed before treatment to assess levels of infestation (and monitor effectiveness of treatment). Piperazine is suitable for mature Ascaridia species only. Levamisole is effective against Heterakis gallinarum, Capillaria and Ascaridia species with a nil withholding period for eggs and a 7‐day withholding period before slaughter. In practical experience, the effectiveness of levamisole against Capillaria species has been variable and new products such as Flubenol may need to be considered. |
There is currently no registered product in Australia to use against poultry tapeworms. Flubendazole in‐feed wormer (Flubenol) is expected to be registered and available from mid‐2020. |
| Infectious coryza (Avibacterium paragallinarum) | All ages are susceptible, but susceptibility increases with age. | Chickens. | Nasal discharge, sneezing and peri‐orbital swelling of the face under the eyes. |
Biosecurity. Chronically ill or healthy carrier birds are the reservoir of infection. Therefore, there is a greater risk in multi‐age flocks. Obtain birds from clean flocks. Vaccination with killed bacterins. Because serovars A, B and C are not cross protective, it is essential that bacterins contain the serovars present in the target population. Vaccination should be completed ~4 weeks before infectious coryza usually occurs on the farm. |
Chlortetracycline at 60 mg/kg live weight can be used for 3–5 days, depending on severity of clinical signs. Relapse often occurs after treatment is discontinued and chlortetracycline in feed for up to 28 days at 100 ppm may be required. Amoxicillin a can be used at 20 mg/kg if there is resistance to chlortetracycline treatment and sensitivity to amoxicillin is determined in vitro. Prior to antimicrobial treatment, collect samples for culture and susceptibility testing. |
To alleviate effects, reduce other management/disease stressors such as poor ventilation. |
| Miscellaneous bacterial infections (e.g. Pseudomonas infection due to vaccine contamination) | < 1 week. | All species. |
High mortality with yolk sac infection, septicaemia. If related to injection, there will be serosanguinous fluid under the neck skin. |
Hatchery and egg hygiene. Perform cultures on infected chicks. Do not carry over partially used vaccine diluent unless refrigerated. Review vaccination procedures and hygiene in the hatchery. |
Antimicrobials can be useful in reducing losses, but Pseudomonas aeruginosa has high and variable levels of antimicrobial resistance, so culture and susceptibility testing are essential. | |
| Mycoplasma gallisepticum (infectious sinusitis; chronic respiratory disease) | > 4 weeks. | Chickens, turkeys, occasionally pheasants, partridge, quail. | Respiratory râles, coughing, nasal discharge, conjunctivitis, airsacculitis, infraorbital sinusitis (in turkeys). |
Obtain birds from Mycoplasma gallisepticum free breeders. Biosecurity from surrounding farms and wild birds. Move to single age flocks. Reduce other stressors, for example ensuring good air quality, in case of infection. Vaccination of parent flocks and at‐risk flocks. Preventive antimicrobial treatment of progeny from infected parents. |
Tylosin tartrate at 100 g/200 L of drinking water for 3–6 days, depending on the severity of the disease (not registered for birds producing eggs for human consumption). In the case of food‐producing egg layers and where secondary infection complicates the disease picture, use chlortetracycline at 60 mg/kg bodyweight for 3–5 days, depending on disease severity. |
Complete elimination of Mycoplasma gallisepticum from all birds in an infected flock by mass antimicrobial therapy is an unrealistic expectation, and treatment should be regarded as a method for short‐term amelioration of disease and economic effects, rather than a long‐term solution to the problem. 173 |
| Infectious synovitis (Mycoplasma synoviae) |
Chickens: 4–16 weeks. Turkeys: 10–24 weeks. |
Chickens, turkeys. |
Lameness, warm flocculent swelling of one or more joints. Occasional enlargement of sternal bursa (more common in chickens than turkeys). Failure to grow. Respiratory signs not usually observed (more common in chickens than turkeys). Occasional airsacculitis. Apical eggshell abnormalities in chickens and occasional transient egg production drops. |
Infection from vertical and horizontal transmission. Vaccination with temperature sensitive vaccine. Choose birds from Mycoplasma synoviae‐free flocks. Hatch Mycoplasma synoviae positive flocks separately from Mycoplasma synoviae negative flocks. Effective biosecurity measures, including single age flocks. |
As for Mycoplasma gallisepticum. | Complete elimination of Mycoplasma synoviae from all birds in an infected flock by mass antimicrobial therapy is an unrealistic expectation, and treatment should be regarded as a method for short‐term amelioration of disease and economic effects, rather than a long‐term solution to the problem. |
| Necrotic enteritis (Clostridium perfringens) |
Broiler chickens (2–5 weeks old). Turkey broilers (7–12 weeks old). Chicken breeders associated with coccidiosis outbreaks. |
Primarily broiler chickens and turkeys. |
Depressed, diarrhoea, peracute mortality. Jejunal and ileal diphtheritic membranes. Small intestine distended and friable. |
Exacerbated by co‐infection with coccidia, wheat or barley‐based diets, high fishmeal diets, diets with additional zinc. Reduce exposure to risk factors, control with coccidiostatic drugs, such as ionophores, or with coccidial vaccines. Need good husbandry/management practices and correct diet/nutritional profile +/− preventive alternatives, especially in the absence of antimicrobials. Feed additives such as probiotics, prebiotics. Short chain and medium‐chain fatty acids in feed. Water acidification and chlorination. Bacillus subtilis (administered as spores) can competitively exclude Clostridium perfringens from broiler chicks. |
Amoxicillin a in the drinking water is the first line treatment. Use at 20 mg/kg for 3 days. Chlortetracycline at 60 mg/kg bodyweight for 3–5 days can be used as a second line treatment Where previous flock history suggests that necrotic enteritis is not able to be controlled with other measures as outlined in Table 1 (eg dietary) then preventative treatment with either zinc bacitracin in feed at a rate of 40 ppm (active ingredient) or avilamycin at a rate of 10‐15ppm (active ingredient) in feed may be required. The preventative treatment period will usually coincide with the times of coccidiosis challenge on the farm and is fed continuously through this risk period. Probiotics could also be considered as a potential alternative to antibiotics in these situations. The choice of preventative treatment option will depend on applicable poultry species and production type, along with previous successful prevention regimes. NOTE: Virginiamycin is also registered for use as a preventative treatment for necrotic enteritis. As it has a ‘HIGH’ ASTAG rating this antibiotic should only be used as a treatment of last resort and used strictly according to label directions.. |
|
| Omphalitis (navel ill, yolk sac infection, mushy chick disease) | < 1 week. | All species. |
The navel may be inflamed and fail to close, producing a wet spot on the abdomen. A scab may be present. The yolk sac is not absorbed and is often highly congested or may contain solidified pieces of yolk material. Peritonitis may be extensive. Affected chicks or poults usually appear normal until a few hours before death. They have little interest in food or water and are often found severely dehydrated. Depression, drooping of the head and huddling near the heat source usually are the only signs. |
Breeder parent farm should have good floor litter and a clean nest environment. There should be regular egg collection and sanitation. If eggs are washed it must be under well controlled conditions. Egg storage and transport should minimise the risk of microbial growth on eggs. The hatchery should have careful control of temperature, humidity and sanitation in the incubator. Only clean, uncracked eggs should be set. If it is necessary to set dirty eggs, they should be segregated from clean eggs. The incubator should be cleaned and disinfected thoroughly between hatches. If young poultry are placed in contaminated transportation boxes before their navels have completely closed, bacteria can migrate up the patent yolk stalk and infect the yolk sac. Stressors in transport and on‐farm should be reduced to minimise early mortality. |
See ‘Colibacillosis’. | Investigation will often involve firstly data collection on affected breeder flocks or hatcheries, followed by a visit to the sites involved to check shed and nest conditions, egg handling and sanitation practices, including egg transport from the farm to the hatchery. The hatchery visit will look at incubation and sanitation procedures. |
| Ornithobacterium rhinotracheale | All ages susceptible, but more significant in older birds. | Chickens, partridge, ducks, geese, turkeys. |
Coughing, sneezing. Reduced weight gain. Reduced egg production. Unilateral or bilateral severe bronchopneumonia, airsacculitis, tracheitis. |
Highly contagious – strict biosecurity measures needed to prevent introduction. Causes minimal pathology in chickens and turkeys by itself. Severity of lesions enhanced with concurrent infection with respiratory viruses or bacteria and management stressors such as poor ventilation. Autogenous vaccines can be developed for farms with a history of infection. |
Treatment needs to be based on culture and susceptibility testing as resistance varies between strains. Treatment with amoxicillin a (20 mg/kg for 3–5 days) or chlortetracycline (60 mg/kg for 3–5 days) as a second line treatment. Chlortetracycline has a zero day withholding period for eggs. |
Cultures from the trachea of birds showing typical signs are preferred. |
| Pododermatitis/bumblefoot (Staphylococcus aureus) | Older birds. |
All species (heavier breeds). |
Lameness with swelling of one or both feet. |
Litter management. Bodyweight control. |
See ‘Staphylococcus aureus’. Unresponsive to medication. |
|
| Reproductive colibacillosis (salpingitis/egg peritonitis/oophoritis; Escherichia coli) | Adult. | All species. |
Sudden death. Birds in good condition, often febrile. May have damage around the vent. Caseous peritonitis, sometimes with egg in oviduct. |
Bodyweight control. Uniformity of flock – both under and overweight birds are prone to disease. Consider cannibalism triggers. Feeding programs – ensure diet is optimal to avoid very large egg size, leaky gut (too much calcium) and dysbacteriosis. Light control. Prevent overstimulation with lights or feed that could otherwise result in birds laying eggs before sufficiently mature. See ‘Cannibalism’. See ‘Colibacillosis’. |
Address primary cause first, or disease will recur as soon as medication stops. Culture and susceptibility testing are necessary to determine an appropriate antimicrobial for treatment. See ‘Colibacillosis’. |
In duck breeders, recent experience has found that salpingitis (salpingo‐peritonitis) is most often responsive to tylosin tartrate at 35 mg/kg for 5 days. Trueperella pyogenes and Gallibacterium anatis isolated from cases but exact cause is unknown at this stage. |
| Respiratory colibacillosis (Escherichia coli) | < 12 weeks. | Chickens, turkeys, ducks. |
Escherichia coli colonises the respiratory tract following damage to the respiratory mucosa by infectious and non‐infectious agents and from there can gain access to the circulation. Lesions in trachea, lungs, air sacs, pericardial sac, peritoneal cavities. Pneumonia and pleuropneumonia are more common in turkeys. |
Prevented with appropriate vaccination against respiratory pathogens (infectious bronchitis virus, Mycoplasma gallisepticum, Mycoplasma synoviae, Newcastle disease virus), but can occur in flocks experiencing adverse vaccine reactions. Good management, including ventilation. Hygiene. Biosecurity. See ‘Colibacillosis’. |
Response to antimicrobial treatment is variable unless underlying cause is controlled. Culture and susceptibility testing are necessary to determine an appropriate antimicrobial for treatment. See ‘Colibacillosis’. |
Important to review control of primary respiratory pathogens. |
| Riemerella anatipestifer | Primarily affects young birds. | Ducks and, less frequently, turkeys and geese. |
Affected ducks usually 1–7 weeks old, often have ocular and nasal discharges, mild coughing and sneezing, tremors of the head and neck, and incoordination. In typical cases, affected ducklings lie on their backs, paddling their legs. Stunting may occur. Fibrinous exudate in the pericardial cavity and over the surface of the liver is the most characteristic lesion. Also, fibrinous airsacculitis. Affected turkeys usually 5–15 weeks old, often exhibit dyspnoea, droopiness, hunched back, lameness, and a twisted neck. Fibrinous pericarditis and epicarditis are the most pronounced lesions. |
Biosecurity. Separation of flocks on multi‐age farms. Rigid sanitation and depopulation are required to remove infection from endemic farms. Transmission is mainly direct, bird‐to‐bird, via toenail scratches, especially of the duckling foot, or through the respiratory epithelium during respiratory disease. Has been controlled in breeders with autogenous killed vaccines. In broiler ducks the prevalence of outbreaks has been reduced by improving husbandry/management practices. |
Culture and susceptibility testing are necessary to determine an appropriate antimicrobial for treatment because of variation in patterns of resistance. However, most consistent effective treatment in ducks has been amoxicillin a (20 mg/kg for 3–5 days). |
|
| Salmonellosis (Salmonella species) | Usually clinical disease only seen in very young birds. | Chickens, turkeys. |
Embryo mortality, rapid death among newly hatched birds. Acute septicaemia. Occasionally, Salmonella Enteritidis has caused anorexia, diarrhoea and egg production drops in laying hens. Spleen and liver swollen and congested. Fibrinopurulent perihepatitis and pericarditis. |
To prevent infection: Eggs and chicks from Salmonella‐free breeding flocks. Hatching eggs disinfected and hatched under strict sanitation standards. Sheds cleaned and disinfected between flocks. Rodent and pest control measures. Strict biosecurity, including personnel visits. Only use heat‐pelleted feed from a clean source. May treat feeds with formaldehyde or organic acids to minimise contamination, but high levels of acids may have adverse effect on enzymes in feed and on machinery. Supply potable water. Vaccination may be used to reduce susceptibility to infection. Water acidification. |
General recommendation in cases of Salmonella infection is not to treat birds with antimicrobials. The exception may be when young birds have clinical disease associated with Salmonella infection. In those cases, amoxicillin a at 20 mg/kg for 3 days or trimethoprim/sulphonamide may be beneficial. Culture and susceptibility testing should be carried out to determine an appropriate antimicrobial for treatment because of variations in patterns of resistance. |
Review biosecurity. Check for immunosuppressive viruses. Check management practices. Review feed and water hygiene. Most Salmonella species infections in birds cause no pathology, mortality or illness and the concern relates more to food safety. |
| Spirochaete typhlitis (Brachyspira species) | Adult birds. | Chickens. | Delayed and/or reduced egg production and wet faeces. |
Biosecurity to prevent organism entry, especially to prevent wild bird contact, including via the water and feed supply. Avoid mixed farming enterprises. Good rodent control. |
Difficult to diagnose Brachyspira and assess whether antimicrobials have a positive impact on faecal moisture content. Treatment with antimicrobials should be based on confirmed diagnosis. Antimicrobials are rarely used for this condition in Australia, but if treatment is warranted, chlortetracycline as an in‐feed treatment at 400 ppm for 7 days, followed, if necessary, by inclusion in‐feed at 200 ppm for up to 28 days is a suitable option. Some evidence that essential oils have had a positive effect on infections of pigs with Brachyspira species. 174 |
|
| Spotty liver disease (Campylobacter hepaticus) | Adult birds around point of lay. | Chickens. | Spotty liver disease is an acute, randomly distributed, focal, necrotic hepatitis causing mortality in up to 10% of a flock and a 10%–15% fall in egg production. |
Biosecurity improvements appear to have had some success. These include measures such as: • using specific boots and clothing for the shed. • decreasing flies, rodents, mites, litter beetles and wild birds, which all carry the bacteria. • improving cleaning of sheds and ensuring good terminal disinfection. • water acidification and chlorination. Reducing management/stress factors, such as improving cooling of sheds, reduces incidence. Preventive medications are NOT appropriate. Feed additives such as probiotics, prebiotics, phytogenics, short chain and medium‐chain fatty acids in feed have not resulted in a significant improvement. |
Once sites are contaminated with the bacteria and they become endemic. Birds often develop disease early in lay and clinically affected flocks will require antimicrobial therapy with tetracyclines. The recommended antimicrobial of first choice is chlortetracycline at 60 mg/kg for 5 days. Use of a second choice antibiotic depends on resistance to chlortetracycline and in vitro antibiotic sensitivity. The second choice is lincomycin‐spectinomycin at 100 g combined antibiotic activity/200 L water for 3 days or amoxicillin a for 3–5 days at 20 mg/kg. These antimicrobials are usually successful when used to treat, and have nil withholding periods for eggs produced during and after the treatment period. |
An alternative approach to medication noted by some veterinarians is, when possible, to keep the shed 10°C cooler during an outbreak. This will reduce mortality and treatment will probably not be required. |
| Staphylococcus (usually Staphylococcus aureus) | All ages. |
Clinical signs vary with site of entry. Most frequent sites are bones, tendon sheaths and joints. Also, skin, sternal bursa, yolk sac, heart, vertebrae, eyelid and testes. Ruffled feathers and lameness followed by severe depression and death. If birds survive acute disease, they may have swollen joints and be reluctant to walk. Osteomyelitis, arthritis, peri‐arthritis, synovitis, spondylitis. Enlarged yolk sacs. Plantar abscesses. Green discoloured livers in turkeys. Septicaemia with necrosis and vascular congestion in many internal organs. |
Ubiquitous, normal skin inhabitants, and are common environmental organisms where poultry are hatched, reared and processed. Genetic influence on susceptibility, via the major histocompatibility complex. Any management procedure reducing damage to host defence mechanisms will help prevent staphylococcosis. Minimise injury, ensure optimal litter control and good hatchery management and sanitation. Reduce effects of immunosuppressive agents. Ensure good gut health (some evidence for benefits from probiotics). Stress management. Handling management, especially at vaccination and weighing. Water hygiene. Litter quality. Feeding programs and uniformity of feeding. |
Disease is usually chronic and responds poorly to antimicrobial therapy. Staphylococcus aureus can sometimes be treated successfully, but culture and susceptibility tests should always be performed because resistance is variable. Choice of antimicrobial must include the ability to reach the site of infection. |
Note: when tenosynovitis is seen in broiler breeders, reoviruses and Mycoplasma synoviae need to be excluded as potential causes. | |
| Ulcerative enteritis (Clostridium colinum) | No specific age. | Young quail, chickens, turkeys and game birds. |
Sudden onset of rapidly increasing flock mortality. Quail – watery, white droppings. Mucosal ulcers throughout intestine that can perforate into the peritoneum. |
Infectious organism is in faeces and remains viable indefinitely in litter. Therefore, remove contaminated litter. Reduce stress and coccidial challenge. Prevention – in‐feed bacitracin or probiotics. |
Amoxicillin a at 20 mg/kg for 3–5 days. | |
| Vent gleet/pasty vents (cloacitis) | Birds in lay. | Chickens, ducks. | Foul smelling white discharge from cloaca of laying birds. |
Pasty vents usually in breeder birds with peritonitis. Cull affected birds. Good gut health management. Feed additives such as probiotics, prebiotics, short chain and medium‐chain fatty acids. Water acidification and chlorination. |
Can be due to pecking. See ‘Cannibalism’. |
CCD amoxicillin trihydrate for poultry (APVMA # 36443) is currently the only amoxicillin formulation with a zero day withholding period for eggs. However, it does have a 14 days export egg withholding period.
Appendix 2.
Antimicrobial agents used in poultry in Australia
| Antimicrobial agent | Class | Importance ASTAG 2018 | Route of administration | Withholding period (WHP) (Meat and Eggs) (the WHP is product specific so always review product label carefully to confirm WHP) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Broilers a | Layer hens | Layer Pullets | Eggs b | Turkeys | Other | ||||
| Amoxicillin | Penicillin | Low | Water | 1‐2D | Nil or DNU | NIL or 8D | NIL or DNU* | 1‐2D | Ducks 1‐2D |
| Amprolium | Anticoccidial | nhu | Water | NIL | Hen NIL | NIL | NIL | NIL | Ducks NIL, Pigeons NIL |
| Apramycin | Aminoglycoside | Medium | Water | 14D | DNU | DNU | DNU | DNU | |
| Avilamycin | Orthosomycin | nhu | Feed | NIL | DNU | DNU | DNU | DNU | |
| Bacitracin | Polypeptide | Low | Feed | NIL | NIL | NIL | NIL | NIL | Ducks NIL |
| Chlortetracycline | Tetracycline | Low | Feed, Water | 2, 4 or 7D | 2, 4 or 7D | 2, 4 or 7D | NIL | 2, 4 or 7D | Ducks 2, 4 or 7D |
| Decoquinate | Anticoccidial | nhu | Feed | NIL | DNU | DNU | DNU | DNU | |
| Dinitolmide | Anticoccidial | nhu | Feed | NIL | DNU | DNU >14wk | DNU | NIL | |
| Erythromycin | Macrolide | Low | Water | 7D | DNU | DNU | DNU | 7D | Ducks 7D |
| Ethopabate + Amprolium | Anticoccidial | nhu | Feed, water | NIL | DNU | DNU | DNU | NIL | Ducks NIL |
| Flavophospholipol | Glycophospholipid | nhu | Feed | NIL | NIL | NIL | NIL | NIL | |
| Flubendazole | Anthelmintic | – | feed | 7D | eNIL | eNIL | NIL | DNU | |
| Lasalocid | Anticoccidial Ionophore | nhu | Feed | 3D | DNU | DNU <14D | DNU | NIL | |
| Levamisole | Anthelmintic | – | Water | 7D | Hens eNIL | eNIL | NIL | 7D | Ducks 7D |
| Maduramicin | Anticoccidial Ionophore | nhu | Feed | NIL | DNU | DNU | DNU | DNU | |
| Methylbenzoquate + clopidol | Anticoccidial [anthelmintic] | nhu | Feed | NIL | DNU | DNU | DNU | DNU | |
| Monensin | Anticoccidial Ionophore | nhu | Feed | NIL | NIL | DNU | DNU | ||
| Narasin | Anticoccidial Ionophore | nhu | Feed | NIL | DNU | DNU | DNY | DNU | |
| Neomycin (feed) | Aminoglycoside | Low |
Feed Water |
5D 5D |
14D DNU |
14D e>14D |
NIL DNU |
14D 5D |
Ducks 5D |
| Nicarbazin c | Anticoccidial | nhu | Feed | 1D | DNU | DNU | DNU | DNU | |
| Oxytetracycline | Tetracycline | Low | Feed, Water | 7or 21D | DNU | DNU | DNU | 7or 21D | Ducks 7 or 21D |
| Piperazine | Anthelmintic | – | water | NIL | NIL | NIL | NIL | NIL | Ducks NIL |
| Robenidine | Anticoccidial | nhu | Feed | 5D | DNU | DNU | DNU | DNU | |
| Salinomycin | Anticoccidial Ionophore | nhu | Feed | NIL | DNU | e>7d | DNU | DNU | |
| Semduramicin | Anticoccidial Ionophore | nhu | Feed | NIL | DNU | DNU | DNU | DNU | |
| Spectinomycin + Lincomycin | Aminocyclitol, Lincosamide | Medium | Water, Injection |
10D 10D |
10D DNU |
10D DNU |
NIL DNU |
10D DNU |
Ducks 10D DNU |
| Sulfadimidine | Sulphonamide | Low | Water | 15D | DNU | DNU | DNU | 15D | Ducks 15D |
| Sulfaquinoxaline c | Anticoccidial Sulphonamide | Low | Water | 14D | DNU | DNU | DNU | 14D | Ducks 14D |
| Tiamulin c | Pleuromutilin | Low | Feed, Water | 5D | DNU | DNU | DNU | 5D | Duck 5D |
| Toltrazuril | Anticoccidial | nhu | Water | 14D | DNU | E>8w | DNU | DNU | |
| Trimethoprim + Sulfadiazine | Diaminopyrimidine + Sulphonamide | Medium | Water | 14D | DNU | DNU or e>14D | DNU | 14D | |
| Trimethoprim + Sulfadimidine | Diaminopyrimidine + Sulphonamide | Medium | Water | 14D | DNU | e>14D | DNU | 14D | Ducks 14D |
| Tylosin | Macrolide | Low |
Feed Water |
NIL NIL, 2D |
NIL DNU |
NIL DNU |
NIL DNU, e>7D |
NIL 5D |
Ducks NIL |
| Virginiamycin | Streptogramin | High | Feed | NIL | DNU | DNU | DNU | DNU | |
The meat WHP for broilers is also applicable to meat chickens previously used as laying hens or broiler breeder.
Always read label carefully and follow label directions for use.
All actives can be associated with adverse effects, especially at higher than labelled dose rates. However, special note should be taken of label cautions for nicarbazin (use in hot weather), sulfaquinoxaline and tiamulin (drug interactions).
Antibacterial agents approved for use in non‐food‐producing avian species: carnidazole, dimetridazole, doxycycline, ronidazole. Use in food‐producing birds is extra‐label.
Note: not all registered antimicrobial agents are used or available for use.
IMPORTANCE (ASTAG 2018): importance for human medicine; nhu, no human use. 175
Target Bird: Pullets – rearing hens prior to point of lay; Pullets – check label, only some products can be used in pullets, Hens – hens in lay.
Withholding period for Eggs: DNU – do not use in egg laying birds; DNU* – withholding period in pullets is product specific.
Gray, P. , Jenner, R. , Norris, J. , Page, S. and Browning, G. , and the Australian Veterinary Association Ltd and Animal Medicines Australia . Antimicrobial prescribing guidelines for poultry. Aust Vet J. 2021;99:181–235. 10.1111/avj.13034
The copyright line for this article was changed on 15 April 2021 after original online publication.
References
- 1. Lloyd DH, Page SW. In: Aarestrup FM, Cavaco LM, Shen J, editors. Antimicrobial stewardship in veterinary medicine, in antimicrobial resistance in bacteria from livestock and companion animals. American Society for Microbiology, Washington, 2018;675–697. [Google Scholar]
- 2. Agunos A, Leger D, Carson C. Review of antimicrobial therapy of selected bacterial diseases in broiler chickens in Canada. Can Vet J 2012;53(12):1289–1300. [PMC free article] [PubMed] [Google Scholar]
- 3. Agunos A, Carson C, Leger D. Antimicrobial therapy of selected diseases in turkeys, laying hens, and minor poultry species in Canada. Can Vet J 2013;54(11):1041–1052. [PMC free article] [PubMed] [Google Scholar]
- 4. Dorrestein GM. Formulation and (bio)availability problems of drug formulations in birds. J Vet Pharmacol Ther 1992;15(2):143–150. [DOI] [PubMed] [Google Scholar]
- 5. Hofacre CL, Fricke JA, Inglis T. Antimicrobial drug use in poultry. In: Antimicrobial therapy in veterinary medicine, Oxford: John Wiley & Sons, Inc., 2013;569–587. [Google Scholar]
- 6. Hofacre CL, Singer RS, Johnson TJ. Antimicrobial therapy (including resistance). In: Swayne DE, Glisson JR, McDougald LR et al., editors. Diseases of poultry. 13th edn. John Wiley & Sons, Chichester, 2013;40. [Google Scholar]
- 7. Joosten P, Sarrazin S, van Gompel L et al. Quantitative and qualitative analysis of antimicrobial usage at farm and flock level on 181 broiler farms in nine European countries. J Antimicrob Chemother 2019;74(3):798–806. [DOI] [PubMed] [Google Scholar]
- 8. Landoni MF, Albarellos G. The use of antimicrobial agents in broiler chickens. Vet J 2015;205(1):21–27. [DOI] [PubMed] [Google Scholar]
- 9. Löhren U, Ricci A, Cummings TS. Guidelines for antimicrobial use in poultry. In: Guardabassi L Jensen L & Kruse H Guide to antimicrobial use in animals, Oxford: Blackwell Publishing, Ltd., 2008;126–142. [Google Scholar]
- 10. Sumano‐Lopez H, Negron‐Gonzalez G, Fernandez‐Surumay G. Practical and pharmacological considerations for the administration of antibacterial drugs in poultry. A review. Rev Cient‐Fac Cien Vet 2000;10(3):251–266. [Google Scholar]
- 11. Vermeulen B, De Backer P, Remon JP. Drug administration to poultry. Adv Drug Deliv Rev 2002;54(6):795–803. [DOI] [PubMed] [Google Scholar]
- 12. Cornejo J, Pokrant E, Carvallo C et al. Depletion of tylosin residues in feathers, muscle and liver from broiler chickens after completion of antimicrobial therapy. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2018;35(3):448–457. [DOI] [PubMed] [Google Scholar]
- 13. Donoghue DJ, Hairston H. Oxytetracycline transfer into chicken egg yolk or albumen. Poult Sci 1999;78(3):343–345. [DOI] [PubMed] [Google Scholar]
- 14. Furusawa N. Transference of dietary veterinary drugs into eggs. Vet Res Commun 2001;25(8):651–662. [DOI] [PubMed] [Google Scholar]
- 15. Furusawa N, Kishida K. Transfer and distribution profiles of dietary sulphonamides in the tissues of the laying hen. Food Addit Contam 2002;19(4):368–372. [DOI] [PubMed] [Google Scholar]
- 16. Goetting V, Lee KA, Tell LA. Pharmacokinetics of veterinary drugs in laying hens and residues in eggs: a review of the literature. J Vet Pharmacol Ther 2011;34(6):521–556. [DOI] [PubMed] [Google Scholar]
- 17. Kan CA, Petz M. Residues of veterinary drugs in eggs and their distribution between yolk and white. J Agric Food Chem 2000;48(12):6397–6403. [DOI] [PubMed] [Google Scholar]
- 18. Munoz R, Cornejo J, Maddaleno A et al. Withdrawal times of oxytetracycline and tylosin in eggs of laying hens after oral administration. J Food Prot 2014;77(6):1017–1021. [DOI] [PubMed] [Google Scholar]
- 19. Patel T, Marmulak T, Gehring R et al. Drug residues in poultry meat: a literature review of commonly used veterinary antibacterials and anthelmintics used in poultry. J Vet Pharmacol Ther 2018;41(6):761–789. [DOI] [PubMed] [Google Scholar]
- 20. Roudaut B. Residues of aminoglycoside antibiotics in eggs after medication of laying hens. Br Poultry Sci 1989;30(2):265–271. [DOI] [PubMed] [Google Scholar]
- 21. Roudaut B, Garnier M. Sulphonamide residues in eggs following drug administration via the drinking water. Food Addit Contam 2002;19(4):373–378. [DOI] [PubMed] [Google Scholar]
- 22. Roudaut B, Moretain JP. Residues of macrolide antibiotics in eggs following medication of laying hens. Br Poultry Sci 1990;31(3):661–675. [DOI] [PubMed] [Google Scholar]
- 23. Roudaut B, Moretain JP, Boisseau J. Excretion of oxytetracycline in eggs after medication of laying hens. Food Addit Contam 1987;4(3):297–307. [DOI] [PubMed] [Google Scholar]
- 24. Roudaut B, Moretain JP, Boisseau J. Excretion of tetracycline and chlortetracycline in eggs after oral medication of laying hens. Food Addit Contam 1989;6(1):71–78. [DOI] [PubMed] [Google Scholar]
- 25. Vandenberge V, Delezie E, Huyghebaert G et al. Residues of sulfadiazine and doxycycline in egg matrices due to cross‐contamination in the feed of laying hens and the possible correlation with physicochemical, pharmacokinetic and physiological parameters. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2012;29(6):908–917. [DOI] [PubMed] [Google Scholar]
- 26. Abo El‐Sooud K, Al‐Tarazi YH, Al‐Bataineh MM. Comparative pharmacokinetics and bioavailability of amoxycillin in chickens after intravenous, intramuscular and oral administrations. Vet Res Commun 2004;28(7):599–607. [DOI] [PubMed] [Google Scholar]
- 27. Abu‐Basha EA, Gehring R, Albwa'neh SJ. Pharmacokinetics and bioavailability of spectinomycin after i.v., i.m., s.c. and oral administration in broiler chickens. J Vet Pharmacol Ther 2007;30(2):139–144. [DOI] [PubMed] [Google Scholar]
- 28. Afifi NA, Ramadan A. Kinetic disposition, systemic bioavailability and tissue distribution of apramycin in broiler chickens. Res Vet Sci 1997;62(3):249–252. [DOI] [PubMed] [Google Scholar]
- 29. Anadon A, Martinez‐Larrañaga MR, Diaz MJ et al. Pharmacokinetics of amoxicillin in broiler chickens. Avian Pathol 1996;25(3):449–458. [DOI] [PubMed] [Google Scholar]
- 30. Anadon A, Gamboa F, Martínez MA et al. Plasma disposition and tissue depletion of chlortetracycline in the food producing animals, chickens for fattening. Food Chem Toxicol 2012;50(8):2714–2721. [DOI] [PubMed] [Google Scholar]
- 31. Baert K, De Baere S, Croubels S et al. Pharmacokinetics and oral bioavailability of sulfadiazine and trimethoprim in broiler chickens. Vet Res Commun 2003;27(4):301–309. [DOI] [PubMed] [Google Scholar]
- 32. Black WD. A study in the pharmacodynamics of oxytetracycline in the chicken. Poult Sci 1977;56(5):1430–1434. [DOI] [PubMed] [Google Scholar]
- 33. Dorrestein GM. The pharmacokinetics of avian therapeutics. Vet Clin North Am Small Anim Pract 1991;21(6):1241–1264. [DOI] [PubMed] [Google Scholar]
- 34. Dorrestein GM, van Miert AS. Pharmacotherapeutic aspects of medication of birds. J Vet Pharmacol Ther 1988;11(1):33–44. [DOI] [PubMed] [Google Scholar]
- 35. Dorrestein GM, van Gogh H, Rinzema JD. Pharmacokinetic aspects of penicillins, aminoglycosides and chloramphenicol in birds compared to mammals. A review. Vet Q 1984;6(4):216–224. [DOI] [PubMed] [Google Scholar]
- 36. el‐Sayed MG, el‐Aziz MI, el‐Komy AA et al. Serum concentrations and tissue residues of spectinomycin in chickens. Dtsch Tierarztl Wochenschr 1995;102(11):446–450. [PubMed] [Google Scholar]
- 37. Fajt VR. Introduction to food animal pharmacotherapy. In: Mealey KL, editor. Pharmacotherapeutics for veterinary dispensing. John Wiley & Sons, Inc, Hoboken, 2019;501–517. [Google Scholar]
- 38. Goren E, de Jong WA, Doornenbal P. Some pharmacokinetic aspects of four sulphonamides and trimethoprim, and their therapeutic efficacy in experimental Escherichia coli infection in poultry. Vet Q 1984;6(3):134–140. [DOI] [PubMed] [Google Scholar]
- 39. Goudah A, Abo K, Sooud E et al. Pharmacokinetics and tissue residue profiles of erythromycin in broiler chickens after different routes of administration. Dtsch Tierarztl Wochenschr 2004;111(4):162–165. [PubMed] [Google Scholar]
- 40. Haritova AM, Djeneva HA, Lashev LD et al. Pharmacokinetics of gentamicin and apramycin in turkeys roosters and hens in the context of pharmacokinetic‐pharmacodynamic relationships. J Vet Pharmacol Ther 2004;27(5):381–384. [DOI] [PubMed] [Google Scholar]
- 41. Hornish RE, Gosline RE, Nappier JM. Comparative metabolism of lincomycin in the swine, chicken, and rat. Drug Metab Rev 1987;18(2–3):177–214. [DOI] [PubMed] [Google Scholar]
- 42.Houben R, Antonissen G, Croubels S et al. Pharmacokinetics of drugs in avian species and the applications and limitations of dose extrapolation. Vlaams Diergen Tijds 2016;85(3):124–132. [Google Scholar]
- 43. Ji LW, Dong L‐L, Ji H et al. Comparative pharmacokinetics and bioavailability of tylosin tartrate and tylosin phosphate after a single oral and i.v. administration in chickens. J Vet Pharmacol Ther 2014;37(3):312–315. [DOI] [PubMed] [Google Scholar]
- 44. Koutoulis KC, Pappas I, Filioussis G et al. Pharmacokinetics and clinical assessment of amoxicillin for the control of necrotic enteritis in broiler‐breeders under field conditions. Avian Biol Res 2015;8(2):89–96. [Google Scholar]
- 45. Kowalski C, Roliński Z, Zań R et al. Pharmacokinetics of tylosin in broiler chickens. Pol J Vet Sci 2002;5(3):127–130. [PubMed] [Google Scholar]
- 46. Krasucka D, Kowalski CJ. Pharmacokinetic parameters of amoxicillin in pigs and poultry. Acta Pol Pharm 2010;67(6):729–732. [PubMed] [Google Scholar]
- 47. Kung K, Wanner M. Pharmacokinetics of doxycycline in turkeys and comparison between feed and water medication. Arch Geflugelkd 1994;58(2):84–88. [Google Scholar]
- 48. Laber G, Schutze E. Blood level studies in chickens, turkey poults and swine with tiamulin, a new antibiotic. J Antibiot (Tokyo) 1977;30(12):1119–1122. [DOI] [PubMed] [Google Scholar]
- 49. Loscher W, Fassbender CP, Weissing M et al. Drug plasma levels following administration of trimethoprim and sulphonamide combinations to broilers. J Vet Pharmacol Ther 1990;13(3):309–319. [DOI] [PubMed] [Google Scholar]
- 50. Nouws JF, Geertsma MF, Grondel JL et al. Plasma disposition and renal clearance of sulphadimidine and its metabolites in laying hens. Res Vet Sci 1988;44(2):202–207. [PubMed] [Google Scholar]
- 51. Queralt J, Castells I. Pharmacokinetics of sulfamethoxazole and trimethoprim association in hens. Poult Sci 1985;64(12):2362–2367. [DOI] [PubMed] [Google Scholar]
- 52. Santos MDF, Vermeersch H, Remon JP et al. Administration of doxycycline hydrochloride via drinking water to turkeys under laboratory and field conditions. Poult Sci 1997;76(10):1342–1348. [DOI] [PubMed] [Google Scholar]
- 53. Smith HW. Serum levels of penicillin, dihydrostreptomycin, chloramphenicol, aureomycin and terramycin in chickens. J Comp Pathol 1954;64(3):225–233. [DOI] [PubMed] [Google Scholar]
- 54. Vinothini P, Ramesh, S , Sooraj Nair, V et al. Pharmacokinetics and relative bioavailability of tiamulin in broiler chicken as influenced by different routes of administration. J Vet Pharmacol Ther, 2019;42(4):447–451. [DOI] [PubMed] [Google Scholar]
- 55. Ziolkowski H, Grabowski T, Jasiecka A et al. Pharmacokinetics of oxytetracycline in broiler chickens following different routes of administration. Vet J 2016;208:96–98. [DOI] [PubMed] [Google Scholar]
- 56. Ziv G. Preliminary clinical pharmacological investigations of tylosin and tiamulin in chickens. Vet Q 1980;2(4):206–210. [DOI] [PubMed] [Google Scholar]
- 57. Agunos A, Gow SP, Léger DF et al. Antimicrobial use and antimicrobial resistance indicators‐integration of farm‐level surveillance data from broiler chickens and Turkeys in British Columbia, Canada. Front Vet Sci 2019;6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burch DG, Harding C, Alvarez R et al. Treatment of a field case of avian intestinal spirochaetosis caused by Brachyspira pilosicoli with tiamulin. Avian Pathol 2006;35(3):211–216. [DOI] [PubMed] [Google Scholar]
- 59. Collier CT, van der Klis JD, Deplancke B et al. Effects of tylosin on bacterial mucolysis, Clostridium perfringens colonization, and intestinal barrier function in a chick model of necrotic enteritis. Antimicrob Agents Chemother 2003;47(10):3311–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garmyn A, Vereecken M, Degussem K et al. Efficacy of tiamulin alone or in combination with chlortetracycline against experimental Mycoplasma gallisepticum infection in chickens. Poult Sci 2017;96(9):3367–3374. [DOI] [PubMed] [Google Scholar]
- 61. Hamdy AH, Kleven SH, McCune EL. Efficacy of Linco‐Spectin water medication on Mycoplasma synoviae Airsacculitis in broilers. Avian Dis 1976;20(1):118–125. [PubMed] [Google Scholar]
- 62. Hamdy AH, Kratzer DD, Paxton LM et al. Effect of a single injection of lincomycin, spectinomycin, and linco‐spectin on early chick mortality caused by Escherichia coli and Staphylococcus aureus . Avian Dis 1979;23(1):164–173. [PubMed] [Google Scholar]
- 63. Hamdy AH, YM Saif, SH Kleven et al. Efficacy of Linco‐Spectin medication on mycoplasma meleagridis airsacculitis in turkey poults. Avian Dis 1979;23(3):670–681. [PubMed] [Google Scholar]
- 64. Hamdy AH, Saif YM, Kasson CW. Efficacy of lincomycin‐spectinomycin water medication on Mycoplasma meleagridis airsacculitis in commercially reared turkey poults. Avian Dis 1982;26(2):227–233. [PubMed] [Google Scholar]
- 65. Leitner G, Waiman R, Heller ED. The effect of apramycin on colonization of pathogenic Escherichia coli in the intestinal tract of chicks. Vet Q 2001;23(2):62–66. [DOI] [PubMed] [Google Scholar]
- 66. Marien M, Nauwynck H, Duchateau L et al. Comparison of the efficacy of four antimicrobial treatment schemes against experimental Ornithobacterium rhinotracheale infection in turkey poults pre‐infected with avian pneumovirus. Avian Pathol 2006;35(3):230–237. [DOI] [PubMed] [Google Scholar]
- 67. Marrett LE, Robb EJ, Frank RK. Efficacy of neomycin sulfate water medication on the control of mortality associated with colibacillosis in growing turkeys. Poult Sci 2000;79(1):12–17. [DOI] [PubMed] [Google Scholar]
- 68. Mosleh N, Shomali T, Namazi F et al. Comparative evaluation of therapeutic efficacy of sulfadiazine‐trimethoprim, oxytetracycline, enrofloxacin and florfenicol on Staphylococcus aureus‐induced arthritis in broilers. Br Poultry Sci 2016;57(2):179–184. [DOI] [PubMed] [Google Scholar]
- 69. Simoneit C, Burow E, Tenhagen B‐A et al. Oral administration of antimicrobials increase antimicrobial resistance in E. coli from chicken–a systematic review. Prev Vet Med 2015;118(1):1–7. [DOI] [PubMed] [Google Scholar]
- 70. Tang T, Wu Y, Lin H et al. The drug tolerant persisters of Riemerella anatipestifer can be eradicated by a combination of two or three antibiotics. BMC Microbiol 2018;18:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xiao X, Sun J, Yang T et al. Pharmacokinetic/pharmacodynamic profiles of tiamulin in an experimental intratracheal infection model of Mycoplasma gallisepticum . Front Vet Sci 2016;3:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang N, Ye X, Wu Y et al. Determination of the mutant selection window and evaluation of the killing of Mycoplasma gallisepticum by danofloxacin, doxycycline, tilmicosin, tylvalosin and valnemulin. PLos One 2017;12(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hofstad MS. The injection of turkey hatching eggs with tylosin to eliminate Mycoplasma meleagridis infection. Avian Dis 1974;18(1):134–138. [PubMed] [Google Scholar]
- 74. McCapes RH, Yamamoto R, Ortmayer HB et al. Injecting antibiotics into turkey hatching eggs to eliminate Mycoplasma meleagridis infection. Avian Dis 1975;19(3):506–514. [PubMed] [Google Scholar]
- 75. Peebles ED. In ovo applications in poultry: a review. Poult Sci 2018;97(7):2322–2338. [DOI] [PubMed] [Google Scholar]
- 76. Rout J, Essack S, Brysiewicz P. Are nursing infusion practices delivering full‐dose antimicrobial treatment? J Antimicrob Chemother 2019;74(12):3418–3422. [DOI] [PubMed] [Google Scholar]
- 77. Love DC, Davis MF, Bassett A et al. Dose imprecision and resistance: free‐choice medicated feeds in industrial food animal production in the United States. Environ Health Perspect 2011;119(3):279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fairchild BD, Ritz CW. Poultry drinking water primer. Bulletin No. 1301. Athens, University of Georgia, 2015. [Google Scholar]
- 79. Kietzmann M, Baumer W. Oral medication via feed and water – pharmacological aspects. Dtsch Tierarztl Wochenschr 2009;116(6):204–208. [PubMed] [Google Scholar]
- 80. Löhren U. Problems with oral administration of antimicrobially effective substances in animals–the situation with poultry. Dtsch Tierarztl Wochenschr 2008;115(8):312–316. [PubMed] [Google Scholar]
- 81. Maes S, Vackier, T , Huu, SN , et al. Occurrence and characterisation of biofilms in drinking water systems of broiler houses. BMC Microbiol 2019;19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Page SW, Gautier P. Use of antimicrobial agents in livestock. Rev Sci Tech 2012;31(1):145–188. [DOI] [PubMed] [Google Scholar]
- 83. Radu J, Van Dijk C, Wheelhouse RK et al. Feed and water consumption and performance of male and female broilers fed salinomycin and maduramicin followed by a withdrawal ration. Poult Sci 1987;66(11):1878–1881. [DOI] [PubMed] [Google Scholar]
- 84. Siegmann O, Luders H. Technical problems of drug therapy through drinking water. Zentralbl Veterinarmed B 1970;17(1):147–149. [PubMed] [Google Scholar]
- 85. Toutain P‐L, Ferran A, Bousquet‐Mélou A. Species differences in pharmacokinetics and pharmacodynamics. In: Cunningham F, Elliott J, Lees P, editors. Comparative and veterinary pharmacology. Springer Berlin Heidelberg, Berlin, Heidelberg, 2010;19–48. [DOI] [PubMed] [Google Scholar]
- 86. Williams RB. The ratio of the water and food consumption of chickens and its significance in the chemotherapy of coccidiosis. Vet Res Commun 1996;20(5):437–447. [DOI] [PubMed] [Google Scholar]
- 87. Dalvi RR, Nunn VA, Juskevich J. Studies on comparative drug metabolism by hepatic cytochrome P‐450‐containing microsomal enzymes in quail, ducks, geese, chickens, turkeys and rats. Comp Biochem Physiol C Comp Pharmacol 1987;87(2):421–424. [DOI] [PubMed] [Google Scholar]
- 88. Fink‐Gremmels J. Implications of hepatic cytochrome P450‐related biotransformation processes in veterinary sciences. Eur J Pharmacol 2008;585(2–3):502–509. [DOI] [PubMed] [Google Scholar]
- 89. Giorgi M, Marini S, Longo V et al. Cytochrome P450‐dependent monooxygenase activities and their inducibility by classic P450 inducers in the liver, kidney, and nasal mucosa of male adult ring‐necked pheasants. Toxicol Appl Pharmacol 2000;167(3):237–245. [DOI] [PubMed] [Google Scholar]
- 90. Gupta RP, Abou‐Donia MB. Cytochrome P450 enzymes in chickens: characteristics and induction by xenobiotics. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 1998;121(1–3):73–83. [DOI] [PubMed] [Google Scholar]
- 91. Hu SX. Effect of age on hepatic cytochrome P450 of Ross 708 broiler chickens. Poult Sci 2013;92(5):1283–1292. [DOI] [PubMed] [Google Scholar]
- 92. Kawalek JC, Myers MJ, Howard KD et al. Hepatic CYP isoforms and drug‐metabolizing enzyme activities in broiler chicks. Int J Poult Sci 2006;5(2):104–111. [Google Scholar]
- 93. Nebbia C, Ceppa L, Dacasto M et al. Oxidative monensin metabolism and cytochrome P450 3A content and functions in liver microsomes from horses, pigs, broiler chicks, cattle and rats. J Vet Pharmacol Ther 2001;24(6):399–403. [DOI] [PubMed] [Google Scholar]
- 94. Pan GP, Fouts JR. Drug metabolism in birds. Drug Metab Rev 1978;7(1):1–253. [DOI] [PubMed] [Google Scholar]
- 95. Pan HP, Fouts JR. Drug metabolism in birds. Pharmacology 1979;19(6):289–293. [DOI] [PubMed] [Google Scholar]
- 96. Sinclair JF, Sinclair PR. Avian cytochrome P450. In: Schenkman JB, Greim H, editors. Cytochrome P450. Springer Berlin Heidelberg, Berlin, Heidelberg, 1993;259–277. [Google Scholar]
- 97. Sobhakumari A, Poppenga RH, Tawde S. In: Gupta RC Chapter 53 ‐ Avian Toxicology. editor. Veterinary Toxicology. London: Academic Press, 2018;711–731. [Google Scholar]
- 98. Walker CH. Avian forms of cytochrome P450. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 1998;121(1–3):65–72. [DOI] [PubMed] [Google Scholar]
- 99. Walker CH, Ronis MJ. The monooxygenases of birds, reptiles and amphibians. Xenobiotica 1989;19(10):1111–1121. [DOI] [PubMed] [Google Scholar]
- 100. Islam KM, Klein U, Burch DG. The activity and compatibility of the antibiotic tiamulin with other drugs in poultry medicine–a review. Poult Sci 2009;88(11):2353–2359. [DOI] [PubMed] [Google Scholar]
- 101. Kodam KM, Govindwar SP. Effect of sulfamethazine on mixed function oxidase in chickens. Vet Hum Toxicol 1995;37(4):340–342. [PubMed] [Google Scholar]
- 102. Palócz O, Szita G, Csikó G. Alteration of avian hepatic cytochrome P450 gene expression and activity by certain feed additives. Acta Vet Hung 2019;67(3):418–429. [DOI] [PubMed] [Google Scholar]
- 103. Csiko G, Nagy G, Mátis G et al. Effects of dietary sodium butyrate on hepatic biotransformation and pharmacokinetics of erythromycin in chickens. J Vet Pharmacol Ther 2014;37(4):406–412. [DOI] [PubMed] [Google Scholar]
- 104. Guo M, Bughio S, Sun Y et al. Age‐related P‐glycoprotein expression in the intestine and affecting the pharmacokinetics of orally administered enrofloxacin in broilers. PLoS One 2013;8(9):e74150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Haritova AM, Schrickx J, Lashev LD et al. Expression of MDR1, MRP2 and BCRP mRNA in tissues of turkeys. J Vet Pharmacol Ther 2008;31(4):378–385. [DOI] [PubMed] [Google Scholar]
- 106. Haritova AM, Schrickx J, Fink‐Gremmels J. Expression of drug efflux transporters in poultry tissues. Res Vet Sci 2010;89(1):104–107. [DOI] [PubMed] [Google Scholar]
- 107. Schrickx JA, Fink‐Gremmels J. Implications of ABC transporters on the disposition of typical veterinary medicinal products. Eur J Pharmacol 2008;585(2–3):510–519. [DOI] [PubMed] [Google Scholar]
- 108. De Baere S, De Mil T, Antonissen G et al. In vitro model to assess the adsorption of oral veterinary drugs to mycotoxin binders in a feed‐ and aflatoxin B1‐containing buffered matrix. Food Addit Contam A Chem Anal Control Expo Risk Assess 2018;35(9):1728–1738. [DOI] [PubMed] [Google Scholar]
- 109. Devreese M, Osselaere A, Goossens J et al. Interaction between tylosin and bentonite clay from a pharmacokinetic perspective. Vet J 2012;194(3):437–439. [DOI] [PubMed] [Google Scholar]
- 110. De Mil T, Devreese M, Broekaert N et al. In vitro adsorption and in vivo pharmacokinetic interaction between doxycycline and frequently used mycotoxin binders in broiler chickens. J Agric Food Chem 2015;63(17):4370–4375. [DOI] [PubMed] [Google Scholar]
- 111. De Mil T, Devreese M, Maes A et al. Influence of mycotoxin binders on the oral bioavailability of tylosin, doxycycline, diclazuril, and salinomycin in fed broiler chickens. Poult Sci 2017;96(7):2137–2144. [DOI] [PubMed] [Google Scholar]
- 112. Schmidt HP, Hagemann N, Draper K et al. The use of biochar in animal feeding. Peerj 2019;7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Waldroup PW, Owen JA, Blackman JR et al. Comparison of low dietary calcium and sodium sulfate for the potentiation of tetracycline antibiotics in broiler diets. Avian Dis 1981;25(4):857–865. [PubMed] [Google Scholar]
- 114. Pollet RA, Glatz CE, Dyer DC et al. Pharmacokinetics of chlortetracycline potentiation with citric acid in the chicken. Am J Vet Res 1983;44(9):1718–1721. [PubMed] [Google Scholar]
- 115. Pollet RA, Glatz CE, Dyer DC. The pharmacokinetics of chlortetracycline orally administered to turkeys: influence of citric acid and Pasteurella multocida infection. J Pharmacokinet Biopharm 1985;13(3):243–264. [DOI] [PubMed] [Google Scholar]
- 116. El‐Gendi AYI, Atef M, Amer AMM et al. Pharmacokinetic and tissue distribution of doxycycline in broiler chickens pretreated with either: diclazuril or halofuginone. Food Chem Toxicol 2010;48(11):3209–3214. [DOI] [PubMed] [Google Scholar]
- 117. Yang F, Li GH, Meng XB et al. Pharmacokinetic interactions of flunixin meglumine and doxycycline in broiler chickens. J Vet Pharmacol Ther 2013;36(1):85–88. [DOI] [PubMed] [Google Scholar]
- 118. Wang GY, Tu P, Chen X et al. Effect of three polyether ionophores on pharmacokinetics of florfenicol in male broilers. J Vet Pharmacol Ther 2013;36(5):494–501. [DOI] [PubMed] [Google Scholar]
- 119. Ershov E, Bellaiche M, Hanji V et al. The effect of hepatic microsomal cytochrome P450 monooxygenases on monensin‐sulfadimidine interactions in broilers. J Vet Pharmacol Ther 2001;24(1):73–76. [DOI] [PubMed] [Google Scholar]
- 120. Szucs G, Tamási V, Laczay P et al. Biochemical background of toxic interaction between tiamulin and monensin. Chem Biol Interact 2004;147(2):151–161. [DOI] [PubMed] [Google Scholar]
- 121. Islam KM, Afrin S, Das PM et al. Compatibility of a combination of tiamulin and chlortetracycline with salinomycin in feed during a pulsed medication program coadministration in broilers. Poult Sci 2008;87(12):2528–2534. [DOI] [PubMed] [Google Scholar]
- 122. Pavlova I, Lukanov H, Ivanov V et al. Simultaneous administration of silymarin and doxycycline in Japanese quails suggests probable herb‐drug interaction. Bulgarian J Agr Sci 2018;24(1):126–131. [Google Scholar]
- 123. Sumano LH, Gutierrez OL, Aguilera R et al. Influence of hard water on the bioavailability of enrofloxacin in broilers. Poult Sci 2004;83(5):726–731. [DOI] [PubMed] [Google Scholar]
- 124. Ziolkowski H, Madej‐Śmiechowska H, Grabowski T et al. Hard water may increase the inhibitory effect of feed on the oral bioavailability of oxytetracycline in broiler chickens. Pol J Vet Sci 2019;22(2):251–258. [DOI] [PubMed] [Google Scholar]
- 125. Devriese LA, Dutta GN. Effects of erythromycin‐inactivating Lactobacillus crop flora on blood levels of erythromycin given orally to chicks. J Vet Pharmacol Ther 1984;7(1):49–53. [DOI] [PubMed] [Google Scholar]
- 126. Dutta GN, Devriese LA. Degradation of macrolide‐lincosamide‐streptogramin antibiotics by Lactobacillus strains from animals. Ann Microbiol (Paris) 1981;132a(1):51–57. [PubMed] [Google Scholar]
- 127. Laczay P, Semjén G, Lehel J et al. Pharmacokinetics and bioavailability of doxycycline in fasted and nonfasted broiler chickens. Acta Vet Hung 2001;49(1):31–37. [DOI] [PubMed] [Google Scholar]
- 128. Ledesma C, Rosario C, Gracia‐Mora J et al. Antibacterial activity of amoxicillin in vitro and its oral bioavailability in broiler chickens under the influence of 3 water sanitizers. Poult Sci 2018;97(7):2391–2399. [DOI] [PubMed] [Google Scholar]
- 129. Ledesma C, Rosario C, Gracia‐Mora J et al. Influence of chlorine, iodine, and citrate‐based water sanitizers on the oral bioavailability of enrofloxacin in broiler chickens. J Appl Poultry Res 2018;27(1):71–80. [Google Scholar]
- 130. Martinez M, Modric S. Patient variation in veterinary medicine: part I. Influence of altered physiological states. J Vet Pharmacol Ther 2010;33(3):213–226. [DOI] [PubMed] [Google Scholar]
- 131. Martinez MN, Court MH, Fink‐Gremmels J et al. Population variability in animal health: influence on dose–exposure–response relationships: part I: drug metabolism and transporter systems. J Vet Pharmacol Ther 2018;41(4):E57–E67. [DOI] [PubMed] [Google Scholar]
- 132. Martinez MN, Gehring R, Mochel JP et al. Population variability in animal health: influence on dose‐exposure‐response relationships: part II: modelling and simulation. J Vet Pharmacol Ther 2018;41(4):E68–E76. [DOI] [PubMed] [Google Scholar]
- 133. Modric S, Martinez M. Patient variation in veterinary medicine–part II–influence of physiological variables. J Vet Pharmacol Ther 2011;34(3):209–223. [DOI] [PubMed] [Google Scholar]
- 134. Lashev L, Milanova A. Impact of age and growth of chickens and turkeys on pharmacokinetics of anti‐bacterial drugs: a brief review. Berl Munch Tierarztl Wochenschr 2017;130(11–12):523–528. [Google Scholar]
- 135. Luders H, Lai KW, Hinz KH. Blood and tissue content of sulfamethazine and sulfaquineoxaline in broilers following medication with drinking water. A contribution to mass medication in poultry. Zentralbl Veterinarmed B 1974;21(1):110–118. [PubMed] [Google Scholar]
- 136. Pozniak B, Pawłowski P, Pasławska U et al. The influence of rapid growth in broilers on florfenicol pharmacokinetics ‐ allometric modelling of the pharmacokinetic and haemodynamic parameters. Br Poultry Sci 2017;58(2):184–191. [DOI] [PubMed] [Google Scholar]
- 137. Hess C, Grafl, B , Bagheri, S et al. Antimicrobial resistance profiling of Gallibacterium anatis from layers reveals high number of multiresistant strains and substantial variability even between isolates from the same organ. Microb Drug Resist 2019;26(2):169–177. [DOI] [PubMed] [Google Scholar]
- 138. Lilia G, Aguilera R, Cortés‐Cuevas A et al. Circadian serum concentrations of tylosin in broilers after feed or water medication. Br Poultry Sci 2008;49(5):619–624. [DOI] [PubMed] [Google Scholar]
- 139. Kietzmann M. Effect of the period of the day on the pharmacodynamics of sulfadimidine in chickens. 4. Studies on sulfonamides. Zentralbl Veterinarmed A 1982;29(9):653–657. [PubMed] [Google Scholar]
- 140. Soback S, Ziv G, Bogin E et al. Pharmacokinetic changes of several antibiotics in chickens during induced fatty liver. Res Vet Sci 1987;43(1):49–54. [PubMed] [Google Scholar]
- 141. Niknafs S, Roura E. Nutrient sensing, taste and feed intake in avian species. Nutr Res Rev 2018;31(2):256–266. [DOI] [PubMed] [Google Scholar]
- 142. Wheelhouse RK, Groves BI, Hammant CA et al. Effects of coccidiostats and dietary protein on performance and water consumption in broiler chickens. Poult Sci 1985;64(5):979–985. [DOI] [PubMed] [Google Scholar]
- 143. Gutierrez L, Vargas D, Ocampo L et al. Plasma concentrations resulting from florfenicol preparations given to pigs in their drinking water. J Anim Sci 2011;89(9):2926–2931. [DOI] [PubMed] [Google Scholar]
- 144. Lashev L, Semerdziev V, Boichev G. Pharmacokinetics of antibiotics and sulphonamides in hens and cocks. Sex‐related differences. Acta Vet Scand 1991;87:288–291. [Google Scholar]
- 145. Dimitrova D, Moutafchieva R, Kanelov I et al. Pharmacokinetics of tobramycin in ducks and sex‐related differences. Vet J 2009;179(3):462–464. [DOI] [PubMed] [Google Scholar]
- 146. Kandeel M. Pharmacokinetics and oral bioavailability of amoxicillin in chicken infected with caecal coccidiosis. J Vet Pharmacol Ther 2015;38(5):504–507. [DOI] [PubMed] [Google Scholar]
- 147. Pozniak B, Pasławska U, Motykiewicz‐Pers K et al. The influence of growth and E. coli endotoxaemia on amoxicillin pharmacokinetics in turkeys. Br Poultry Sci 2017;58(4):462–468. [DOI] [PubMed] [Google Scholar]
- 148. Pollet RA, Glatz CE, Dyer DC. Oral absorption of chlortetracycline in turkeys: influence of citric acid and Pasteurella multocida infection. Poult Sci 1984;63(6):1110–1114. [DOI] [PubMed] [Google Scholar]
- 149. Zeng Z, Deng G, Shen X et al. Plasma and tissue pharmacokinetics of danofloxacin in healthy and in experimentally infected chickens with Pasteurella multocida . J Vet Pharmacol Ther 2011;34(1):101–104. [DOI] [PubMed] [Google Scholar]
- 150. Xiao X, Lan W, Wang Y et al. Comparative pharmacokinetics of danofloxacin in healthy and Pasteurella multocida infected ducks. J Vet Pharmacol Ther 2018;41(6):912–918. [DOI] [PubMed] [Google Scholar]
- 151. Abo El‐Ela FI, Radi AM, El‐Banna HA et al. Pharmacokinetics of difloxacin in healthy and E. coli‐infected broiler chickens. Br Poultry Sci 2014;55(6):830–836. [DOI] [PubMed] [Google Scholar]
- 152. Ismail MM, El‐Kattan YA. Disposition kinetics of doxycycline in chickens naturally infected with Mycoplasma gallisepticum . Br Poultry Sci 2004;45(4):550–556. [DOI] [PubMed] [Google Scholar]
- 153. Guo M, Sun Y, Zhang Y et al. E. coli infection modulates the pharmacokinetics of oral enrofloxacin by targeting P‐glycoprotein in small intestine and CYP450 3A in liver and kidney of broilers. PLoS One 2014;9(1):e87781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Soliman GA. Tissue distribution and disposition kinetics of enrofloxacin in healthy and E. coli infected broilers. Dtsch Tierarztl Wochenschr 2000;107(1):23–27. [PubMed] [Google Scholar]
- 155. Shen J, Wu X, Hu D et al. Pharmacokinetics of florfenicol in healthy and Escherichia coli‐infected broiler chickens. Res Vet Sci 2002;73(2):137–140. [DOI] [PubMed] [Google Scholar]
- 156. Lan W, Xiao X, Jiang Y et al. Comparative pharmacokinetics of florfenicol in healthy and Pasteurella multocida‐infected Gaoyou ducks. J Vet Pharmacol Ther 2019;42(3):355–360. [DOI] [PubMed] [Google Scholar]
- 157. el‐Banna HA. Pharmacokinetics of florfenicol in normal and Pasteurella‐infected Muscovy ducks. Br Poultry Sci 1998;39(4):492–496. [DOI] [PubMed] [Google Scholar]
- 158. Hassan AB, Atta AH, Soliman ZI. Pharmacokinetics and tissue residues of kitasamycin in healthy and diseased broilers. Dtsch Tierarztl Wochenschr 1990;97(8):315–317. [PubMed] [Google Scholar]
- 159. Aboubakr M, Soliman A. Comparative pharmacokinetics of levofloxacin in healthy and renal damaged muscovy ducks following intravenous and oral administration. Vet Med Int 2014;2014:986806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Tohamy MA. Comparative pharmacokinetics of orbifloxacin in healthy and Pasteurella multocida infected ducks. Br Poultry Sci 2011;52(5):639–644. [DOI] [PubMed] [Google Scholar]
- 161. Sumano H, Fuentes V, Ocampo L. Pharmacokinetic aspects of a sulphachloropyridazine trimethoprim preparation in normal and diseased fowl. Br Poultry Sci 1990;31(3):627–634. [DOI] [PubMed] [Google Scholar]
- 162. Bell I. Rational use of chemotherapeutics. In: Poultry health, 92, (429–467). Sydney, Australia: Post Grad Committee in Veterinary Science in association with Australian Veterinary Poultry Association, 1986. [Google Scholar]
- 163. ErikBeyersdorf/CC BY‐SA. Available at: https://creativecommons.org/licenses/by-sa/3.0. Accessed 01/03/2020.
- 164. Roberts T, Wilson J, Guthrie A et al. New issues and science in broiler chicken intestinal health: emerging technology and alternative interventions. J Appl Poultry Res 2015;24(2):257–266. [Google Scholar]
- 165. Mehdi Y, Létourneau‐Montminy M‐P, Gaucher M‐L et al. Use of antibiotics in broiler production: global impacts and alternatives. Anim Nutr 2018;4(2):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Gadde U, Kim WH, Oh ST et al. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev 2017;18(1):26–45. [DOI] [PubMed] [Google Scholar]
- 167. Murphy D, Ricci A, Auce Z et al. EMA and EFSA joint scientific opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA J 2017;15(1):4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Diaz‐Sanchez S, D'Souza D, Biswas D et al. Botanical alternatives to antibiotics for use in organic poultry production. Poult Sci 2015;94(6):1419–1430. [DOI] [PubMed] [Google Scholar]
- 169. Squelette_oiseau.JPG: BIODIDACderivative work: mario modesto/CC BY. Available at: https://creativecommons.org/licenses/by/2.5. Accessed 01/03/20.
- 170. Van Wettere AJ. Infectious skeletal disorders in poultry, 2020. Available at: https://www.msdvetmanual.com/poultry/disorders-of-the-skeletal-system/infectious-skeletal-disorders-in-poultry. Accessed 01/03/20.
- 171. Wideman RF, Hamal KR, Stark JM et al. A wire‐flooring model for inducing lameness in broilers: evaluation of probiotics as a prophylactic treatment. Poult Sci 2012;91(4):870–883. [DOI] [PubMed] [Google Scholar]
- 172. Clark S, Kimminau E. Critical review: future control of blackhead disease (histomoniasis) in poultry. Avian Dis 2017;61(3):281–288. [DOI] [PubMed] [Google Scholar]
- 173. Raviv Z, Ley DH. Mycoplasma gallisepticum infection. In: Swayne DE, Glisson JR, McDougald LR, editors. Diseases of poultry. John Wiley & Sons, Chichester, 2013;913–928. [Google Scholar]
- 174. Verlinden M, Pasmans F, Mahu M et al. In vitro sensitivity of poultry Brachyspira intermedia isolates to essential oil components and in vivo reduction of Brachyspira intermedia in rearing pullets with cinnamaldehyde feed supplementation. Poult Sci 2013;92(5):1202–1207. [DOI] [PubMed] [Google Scholar]
- 175. ASTAG . Importance ratings and summary of antibacterial uses in human and animal health in Australia, version 1.0 2018. Available at: https://www.amr.gov.au/resources/importance-ratings-and-summary-antibacterial-uses-human-and-animal-health-australia. Accessed 27 October 2020.


