Abstract
Aim
The aim of this single‐blind RCT was to evaluate the adjunctive clinical and microbiological effect of systemic amoxicillin (AMX) plus metronidazole (MTZ) to non‐surgical treatment of peri‐implantitis.
Material and methods
Patients (N = 62) with peri‐implantitis were randomly assigned to receive full‐mouth mechanical debridement and decontamination and use of chlorhexidine (control group) or combined with antibiotic therapy of AMX/MTZ (test group). Primary outcome was change in bleeding score from baseline (T 0) to 3‐month follow‐up (T 3). Secondary parameters were plaque, suppuration, PPD, CAL, bone level, microbiology, adverse events and need for additional surgery. Data were analysed with linear multiple regression analysis.
Results
57 patients with 122 implants completed 3‐month follow‐up. Both groups showed major clinical improvements at T 3 in both peri‐implant and periodontal parameters. However, no significant differences were observed between both groups for any of the primary or secondary parameters.
Conclusions
Systemic antibiotic therapy of AMX/MTZ does not improve clinical and microbiological outcomes of non‐surgical peri‐implantitis treatment and should not be routinely recommended. Although complete disease resolution may be difficult to achieve, meticulously performed full‐mouth non‐surgical treatment, achieving a high level of daily oral hygiene and healthy periodontal tissues, can significantly improve the starting position of the subsequent (surgical) peri‐implantitis treatment phase.
Keywords: dental implants, non‐surgical therapy, peri‐implantitis, periodontal disease, systemic antibiotics
Clinical relevance.
Scientific rationale for study: The beneficial clinical effects of adjunctive systemic antibiotics to non‐surgical periodontal therapy have been well documented. However, studies on the use of systemic antibiotics for treatment of peri‐implantitis are scarce and inconclusive.
Principal findings: Adjunctive systemic antibiotic therapy of amoxicillin and metronidazole does not improve clinical and microbiological outcomes of non‐surgical peri‐implantitis treatment.
Practical implications: Systemic amoxicillin plus metronidazole should not be routinely used for non‐surgical peri‐implantitis therapy.
1. INTRODUCTION
Peri‐implantitis is a plaque‐associated pathological condition, characterized by inflammation in the peri‐implant mucosa and subsequent progressive bone loss (Berglundh et al., 2018). Treatment of peri‐implantitis aims to reduce the infectious challenge posed by the biofilm on the implant surface, to allow resolution of inflammation and preservation of supporting bone. Progression of peri‐implantitis appears to be faster than periodontitis (Berglundh et al., 2018), and hence, treatment of peri‐implantitis may be considered more challenging. While mechanical debridement alone may be sufficient for treatment of peri‐implant mucositis, alternative or adjunctive measures seem indicated for treatment of peri‐implantitis (Schwarz et al., 2015). Increased probing depth, structure of the implant surface, its screw‐shaped design and unfavourable shape of the suprastructure may hinder adequate non‐surgical mechanical debridement (Renvert & Polyzois, 2018). Furthermore, peri‐implant pockets may be recolonized by pathogens from supra‐ and subgingival biofilms on teeth or other oral surfaces. This may advocate a treatment strategy aimed at reduction of total periodontal and peri‐implant bacterial load by full‐mouth cleaning and disinfection and use of antimicrobial agents.
In periodontitis, the concept that full‐mouth ecological change (suppression of periodontal pathogens and recolonization of the biofilm by host‐compatible species) is necessary to re‐establish periodontal health has led to widespread use of antimicrobial agents (Feres et al., 2015). Well‐documented evidence shows that beneficial changes in subgingival microbial composition achieved with scaling and root planing can be considerably improved by adjunctive use of systemic antibiotics, more specifically metronidazole or metronidazole plus amoxicillin and that these microbiological benefits are accompanied by important clinical improvements (Feres et al., 2015) However, in terms of disease progression, the role of systemic amoxicillin plus metronidazole may be less clear. It has shown to lead to a small absolute, although statistically significant, reduction in future attachment loss and may subordinate to the effect of proper mechanical debridement and modification of behavioural risk factors (Harks et al., 2015).
Unfortunately, evidence on adjunctive use of systemic antibiotics in non‐surgical treatment of peri‐implantitis is scarce and inconclusive. Few cohort studies and one RCT have been published, suggesting that non‐surgical treatment involving systemic antibiotics can improve microbiological and clinical parameters (Mombelli & Lang, 1992; Stein et al., 2017; Liñares et al., 2019; Nart et al., 2020), but maybe no better than mechanical non‐surgical treatment alone (Shibli et al., 2019).
The aim of the present study was to evaluate the adjunctive effect of systemic amoxicillin (AMX) plus metronidazole (MTZ) to full‐mouth non‐surgical peri‐implantitis treatment. The null hypothesis of no differences between treatment strategies was tested.
2. MATERIAL AND METHODS
2.1. Trial design
This study is a single‐centre, single‐blind, controlled, parallel‐group study with balanced randomization (1:1) and superiority design, evaluating adjunctive clinical and microbiological effects of systemic amoxicillin plus metronidazole to combined full‐mouth non‐surgical peri‐implantitis and periodontitis treatment (mechanical debridement and use of chlorhexidine (CHX)). Follow‐up time was three months.
2.2. Participants
Eligible participants were adults referred for treatment of peri‐implantitis to The Hague Clinic for Periodontology, the Netherlands. Peri‐implantitis was defined as marginal bone loss ≥2 mm combined with bleeding and/or suppuration on probing and peri‐implant probing depth ≥5 mm. Exclusion criteria were as follows:
-
‐
History of local radiotherapy to head and neck region;
-
‐
Pregnancy and lactation;
-
‐
Uncontrolled diabetes (HbA1c>7% or >53 mmol/mol);
-
‐
Mononucleosis infectiosa;
-
‐
Organic neurological disorders;
-
‐
Use of antibiotics during last 3 months;
-
‐
Known allergy to AMX/MTZ/CHX;
-
‐
Long‐term use of anti‐inflammatory drugs;
-
‐
Full edentulism;
-
‐
Incapability of performing basal oral hygiene measures due to physical or mental disorders;
-
‐
Implant function time <2 years
-
‐
Implants placed in augmented autogenous bone from the crista iliac region;
-
‐
Implants with bone loss exceeding 2/3 of the implant length;
-
‐
Implant mobility;
-
‐
Previous non‐surgical peri‐implantitis treatment (scaling or curettage) during the last 6 months or surgical treatment.
Written informed consent was obtained from all participants before entering the trial. The study took place between August 2012 and July 2019 and was approved by the Institutional Review Board of the University Medical Center Groningen, the Netherlands (METc2012.311). Clinical trial registration was done at www.ClinicalTrials.gov (NCT04149327), and CONSORT guidelines were followed (Schulz et al., 2010).
2.3. Intervention
All patients received full‐mouth mechanical cleansing of implants and teeth in one to five sessions by experienced dental hygienists. Implants were supra‐ and submucosally cleaned using an air polisher with subgingival tip (EMS Air‐flow® with erythritol‐based powder containing chlorhexidine (14 μm, Air‐Flow® Powder PLUS, EMS) and ultrasonic instruments (PL1 and PL2 instruments, EMS Piezon®; only on exposed screw threads, never on smooth implant surfaces). Teeth were supra‐ and subgingivally cleaned using ultrasonic instruments (EMS Piezon®) and hand instruments (Hu‐friedy).
During each treatment session, patients received individualized oral hygiene (re‐) instructions of tooth brushing with an electric toothbrush, use of interdental brushes (at implants in combination with 1% chlorhexidine gel (Corsodyl®, GlaxoSmithKline)) and use of floss (at implants only, Oral‐B® superfloss, Proctor & Gamble Company or Meridol® floss, Colgate‐Palmolive Company).
Prior to each session, patients rinsed their mouth with 0.12% CHX +0.05% cetylpyridinium chloride (CPC) mouthrinse during 30 s (Perioaid, Dentaid SL). At the final session, all previously cleaned areas were re‐examined and again cleaned. After the final session, all patients used 0.12% CHX +0.05% CPC mouthrinse (Perioaid, Dentaid SL) twice daily during 30 s for 2 weeks (Feres et al., 2012). Test group patients additionally used systemic amoxicillin and metronidazole (500/500 mg, 3 times daily for 7 days).
2.4. Outcomes
Primary outcome parameter was change in peri‐implant full‐mouth bleeding score (peri‐implantitis BS, %) from baseline (T 0) to 3‐month follow‐up (T 3).
Secondary clinical outcomes were changes from baseline to 3 months in peri‐implant and periodontal full‐mouth plaque scores (PS, %), suppuration scores (SS, %), mean probing pocket depths (PD), mean (relative) clinical attachment levels (CAL) and mean peri‐implant bone levels (BL).
Other secondary outcomes parameters included the following: change from T 0 to T 3 in detection frequency of 7 periodontal bacterial species, number of patients at T 3 with adverse events and number of patients in need for additional surgery at implants at T 3.
2.4.1. Clinical parameters
All clinical parameters were scored at six sites per implant/teeth at T 0 and T 3. Presence of plaque was assessed visually by using a dental probe and scored as either present or absent. PD was assessed in mm using a Hu‐Friedy® PCPUNC156 periodontal probe. Subsequent bleeding and/or suppuration on probing was scored as present or absent. Recession of the marginal gingiva at teeth was determined by measuring the distance from the top of the marginal gingiva to the cemento‐enamel junction with a periodontal probe. CAL at teeth was calculated by taking the sum of PD and recession. Location of the marginal mucosa at implants was determined by using a transparent template fabricated on a dental cast. The distance from the marginal mucosa to the transparent template was measured with a periodontal probe. The “relative” CAL at implants was calculated as the sum of PD and location of the mucosa.
Change in BL around implants was assessed on standardized digital radiographs (Planmeca Prostyle Intra) taken with long‐cone paralleling technique and individualized film holders (individualized with silicone bite blocks). BL measurements were done at the two approximal implant sites using DICOM software (Dicomworks 1.0). The distance from the first visible bone‐to‐implant contact to a fixed, reproducible reference point was measured. Radiographs were calibrated using the known implant dimensions as reference values. The mean of all measurements within one patient was calculated.
2.4.2. Microbiological parameters
For microbiological assessment, two pooled samples were taken at the four peri‐implant and periodontal sites with the least favourable conditions using sterile paperpoints. Microbial samples were analysed using quantitative polymerase chain reaction. Presence and numbers of Aggregatibacter actinomycetemcomitans, Porhyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, Parvimonas micra, Fusobacterium nucleatum and Treponema denticola were determined.
2.4.3. Other parameters
Adverse events related to the treatment were evaluated at T 3 using a questionnaire with visual analogue scales (VAS). The need for additional surgical peri‐implantitis treatment was scored at T 3 and was based on clinical judgement of the examiner.
All examinations were performed by one experienced periodontist (TV).
2.5. Sample size
No data were available for estimation of the effect size of non‐surgical treatment of peri‐implantitis combined with systemic antibiotics. Therefore, results from a comparable study on periodontitis (Winkel et al., 2001) were used (Cohen's d = 1, difference in change from baseline between control and test group in mean bleeding index = 0.2 (SD 0.2)). For the present study on peri‐implantitis, a medium effect size (Cohen's d = 0.5, f 2 = 0.33) was assumed. Sample size estimates (G*Power, Version 3.1; University of Kiel (Faul et al., 2009)) showed that 48 patients would give a power (β) of more than 80% with a significance level (α) of .05 using two‐sided linear multiple regression with 6 predictor variables (therapy, baseline value, number of treatment sessions, smoking (2 dummy variables) and full‐mouth plaque score at T 3). To compensate for patient withdrawal and losses to follow‐up, a minimum sample size of 60 patients was chosen, that is 30 per group.
2.6. Randomization
Patients were randomly assigned to the study groups using a computer generated randomization list with permuted block design (fixed block sizes of 4). No stratification was performed. A researcher not involved in patient enrolment, examination or treatment (YdW) transferred this randomization list to identical, sequentially numbered, non‐transparent envelopes by putting either one recipe (for mouthrinse) or two recipes (for mouthrinse and antibiotics) into the envelopes. The weight of the envelopes and the number of sheets in the envelopes were kept identical, thus ensuring that treatment allocation could not be revealed. The envelopes were irreversibly sealed, only to be opened by the dental hygienist who had performed the treatment, immediately after the last treatment session. The patients received the recipe(s) and were instructed accordingly by the dental hygienist. Thus, patients and dental hygienist were aware of treatment allocation (after completion of the mechanical treatment), but the outcome assessor (TV) and data analyst were kept blinded to allocation.
2.7. Statistical methods
For full‐mouth BS, PS and SS, the percentage of sites with positive scores of all included implants, respectively all teeth, were combined. For the outcome variables PD, CAL and BL, mean scores were calculated of all measurements of all included implants and all teeth respectively. Patient level response variables were calculated by taking the difference between T 0 and T 3 measurements. Normally distributed data were tested with linear multiple regression analyses. Baseline (T 0) values, number of treatment sessions, full‐mouth plaque score at T 3 (continuous variables) and smoking (dichotomous variables) were a priori identified as potential confounders. For each outcome variable, two analyses were performed. With the crude analysis, the effect of the intervention was determined, while controlling for baseline value. In the adjusted analysis, the other potential confounders were additionally included in the model.
Non‐normally distributed data (peri‐implant SS, periodontal SS and adverse event VAS scores) were analysed with the Mann–Whitney U test. Differences in PD reductions between control and test group for initially shallow (≤3 mm), moderate (4–6 mm) and deep (≥7 mm) peri‐implant and periodontal pockets were analysed with the independent‐samples t test. Within‐group differences in detection frequency of single bacterial species between T 0 and T 3 were analysed with the McNemar test, and between‐group differences at T 3 with the Fisher's exact test.
Data were analysed according to the intention‐to‐treat principal, with a significance level (α) of .05, using IBM® SPSS® Statistics 23 (version 23.0.0.3, IMB).
3. RESULTS
The flow of the participants throughout the different phases of the study is illustrated in Figure 1. In total, 62 patients with 143 implants with peri‐implantitis were allocated. Baseline characteristics of the included patients are shown in Table 1. Two patients allocated to the test group refused to take the prescribed antibiotics. However, according to the intention‐to‐treat analysis, these patients were still analysed in the test group. One patient in the test group suffered from hallucinations after using antibiotics for one day. This patient discontinued the intervention, but also withdrew consent and was therefore regarded as drop‐out. Test group patients were on average older than control group patients (60.0 ± 10.4 vs. 53.5 ± 11.2 years, p = .021) and had a higher proportion of implants in the mandibula (50.7% vs. 33.8%, p = .042). A small majority of the patients had mild/moderate periodontitis (stage I or II periodontitis, 53.2%) as opposed to severe periodontitis (stage III or IV, 46.8%), with no differences between control and test group.
FIGURE 1.
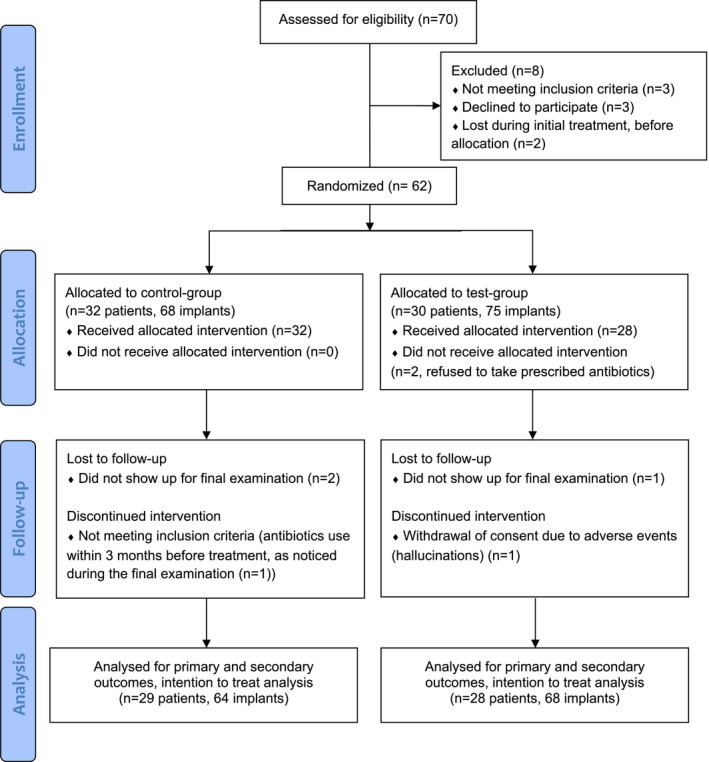
Flow‐diagram
TABLE 1.
Baseline characteristics of included patients/implants
| Characteristics | Control | Test |
|---|---|---|
| Number of patients | 32 | 30 |
| Age (years; mean (SD)) | 53.5 (11.2) | 60.0 (10.4) |
| Gender; M(male), F(female) | M 12, F 20 | M 15, F 15 |
| Smoking; n patients (%) | ||
| Never smoking or quit smoking >1 year ago | 19 (59.4) | 22 (73.3) |
| Quit smoking ≤1 year ago | 3 (9.4) | 1 (3.3) |
| Current smoking | 10 (31.3) | 7 (23.3) |
| Daily alcohol consumption; n patients (%) | 18 (56.3) | 15 (50.0) |
| Daily medication intake; n patients (%) | 15 (46.9) | 14 (46.7) |
| If yes, number of medications daily (mean (SD)) | 1.9 (1.1) | 1.8 (0.7) |
| Diabetes; n patients (%) | 2 (6.3) | 1 (3.3) |
| Periodontal classification; n patients (%) | ||
| Localized, stage I, grade A | 4 (12.5) | 1 (3.3) |
| Localized, stage II, grade A | 0 | 1 (3.3) |
| Generalized, stage I, grade A | 1 (3.1) | 0 |
| Generalized, stage II, grade A | 8 (25.0) | 10 (33.3) |
| Generalized, stage II, grade B | 5 (15.6) | 3 (10.0) |
| Generalized, stage III, grade B | 9 (28.1) | 9 (30.0) |
| Generalized, stage III, grade C | 2 (6.3) | 2 (6.7) |
| Generalized, stage IV, grade C | 3 (9.4) | 4 (13.3) |
| All implants | ||
| Total number of implants (range) | 75 (1–5) | 93 (1–10) |
| Clinical diagnosis; n implants (%) | ||
| Health (no BoP or suppuration) | 0 (0) | 4 (4.3) |
| Peri‐implant mucositis (BoP/suppuration, but no bone loss) | 4 (5.3) | 10 (10.8) |
| Peri‐implantitis | ||
| Excluded because placed in iliac crest bone | 0 (0) | 3 (3.2) |
| Explantation only possible treatment option | 3 (4.0) | 1 (1.1) |
| Implants allocated to treatment | 68 (90.7) | 75 (80.6) |
| Allocated implants | ||
| Implant function time (years; mean (SD)) | 8.9 (5.9) | 8.0 (4.3) |
| Range | 3.0–27.9 | 2.6–20.9 |
| Implant brand; n implants (%) | ||
| Alpha‐Bio Tec | 6 (8.8) | 0 (0) |
| Camlog | 1 (1.5) | 3 (4.0) |
| Dentium | 2 (2.9) | 0 (0) |
| Dentsply Sirona | 20 (29.4) | 24 (32.0) |
| MIS | 4 (5.9) | 0 (0) |
| Neobiotech | 0 (0) | 3 (4.0) |
| Nobel Biocare | 16 (23.5) | 24 (32.0) |
| Straumann | 14 (20.6) | 13 (17.3) |
| Zimmer Biomet | 5 (7.4) | 7 (9.3) |
| Type of restoration; n implants involved (%) | ||
| Single crown | 42 (61.8) | 39 (52.0) |
| Implant supported fixed partial denture | 22 (32.4) | 32 (42.7) |
| Implant‐teeth supported fixed partial denture | 0 (0) | 4 (5.3) |
| Overdenture | 4 (5.9) | 0 (0) |
| Screw‐ or cement‐retained restoration; n implants involved (%) | ||
| Screw‐retained | 18 (26.5) | 20 (26.7) |
| Cement‐retained | 50 (73.5) | 55 (73.3) |
| Implants placed in maxilla or mandible; n implants (%) | ||
| Maxilla | 45 (66.2) | 37 (49.3) |
| Mandible | 23 (33.8) | 38 (50.7) |
| Implants partially placed in augmented bone/bone substitute; n implants (%) | ||
| Yes | 17 (25.0) | 21 (28.0) |
| No | 43 (63.2) | 38 (50.7) |
| Unknown | 8 (11.8) | 16 (21.3) |
Descriptive statistics of clinical outcomes at T 0 and T 3 and mean differences are shown in Table 2. This table only includes patients with complete follow‐up (57 patients with 132 implants). Mean bone loss is based on 55 patients and 122 implants, since radiographs of 10 implants were qualitatively insufficient for appropriate measurements. Outcomes of the statistical analyses are depicted in Table 3. No significant differences between control and test group were observed for any of the clinical parameters, both for peri‐implant and periodontal parameters. Only for initially deep periodontal pockets (≥7 mm), a significantly greater reduction in pocket depths was seen between T 0 and T 3 for the test group versus the control group (see Table 4).
TABLE 2.
Descriptive statistics of clinical parameters
| N = 57 | Control group (n = 29) | Test group (n = 28) | |||||
|---|---|---|---|---|---|---|---|
| T 0 | T 3 | Δ | T 0 | T 3 | Δ | ||
| Peri‐implantitis parameters | |||||||
| BS | Mean % (SD) | 94.66 (9.42) | 55.47 (31.60) | −39.20 (32.31) | 85.96 (19.32) | 47.37 (30.43) | −38.59 (29.60) |
| PS | Mean % (SD) | 42.11 (30.89) | 6.88 (14.72) | −35.23 (32.67) | 42.35 (28.02) | 8.20 (13.28) | −34.15 (27.60) |
| SS | Median % (IQR) | 8.33 (16.67) | 0.00 (0.00) | 0.00 (16.67) | 8.33 (16.67) | 0.00 (5.56) | −6.90 (12.83) |
| PD | Mean (SD) | 5.82 (1.42) | 4.42 (1.38) | −1.40 (0.80) | 5.63 (1.24) | 3.96 (1.21) | −1.67 (0.82) |
| CAL (relative) | Mean (SD) | 12.45 (2.36) | 11.49 (2.01) | −0.96 (1.01) | 12.35 (1.68) | 11.39 (1.62) | −0.97 (0.93) |
| BL a | Mean (SD) | 3.03 (1.24) | 3.08 (1.32) | −0.04 (0.20) | 2.65 (1.61) | 2.70 (1.65) | −0.06 (0.17) |
| Periodontal parameters | |||||||
| BS | Mean % (SD) | 48.80 (21.53) | 20.51 (15.60) | −28.29 (16.36) | 44.93 (19.12) | 14.40 (9.28) | −30.53 (16.55) |
| PS | Mean % (SD) | 43.89 (23.94) | 17.69 (22.36) | −26.20 (24.97) | 36.40 (19.37) | 10.35 (9.80) | −26.05 (20.49) |
| SS | Median % (IQR) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | −1.06 (4.01) |
| PD | Mean (SD) | 3.22 (0.70) | 2.53 (0.68) | −0.70 (0.37) | 3.22 (0.64) | 2.39 (0.48) | −0.84 (0.44) |
| CAL | Mean (SD) | 3.80 (1.34) | 3.28 (1.50) | −0.52 (0.40) | 3.91 (0.99) | 3.32 (0.87) | −0.60 (0.45) |
Abbreviations: BL, bone level; BS, bleeding score; CAL, clinical attachment level; PD, probing pocket depth; PS, plaque score; S, suppuration score.
n = 28 in control group, n = 27 in test group.
TABLE 3.
Mean differences in clinical outcomes between control and test group
| Outcome variable | Crude model a | Adjusted model b | ||||
|---|---|---|---|---|---|---|
| β | 95%CI | p‐Value | β | 95%CI | p‐Value | |
| Peri‐implantitis parameters | ||||||
| BS | −3.724 | −20.531; 13.082 | .659 | .028 | −17.567; 17.622 | .997 |
| SS c | .509 | |||||
| PD | −.309 | −0.717; 0.100 | .136 | −.338 | −0.779; 0.103 | .130 |
| CAL (relative) | −.030 | −0.497; 0.436 | .896 | −.173 | −0.652; 0.305 | .470 |
| BL | −0.027 | −0.124; 0.071 | .586 | −.01 | −0.111; 0.091 | .841 |
| Periodontal parameters | ||||||
| BS | −4.665 | −10.262; 0.932 | .100 | −1.777 | −5.861; 2.307 | .386 |
| SS c | .746 | |||||
| PD | −.141 | −0.332; 0.049 | .142 | −.146 | −0.339; 0.046 | .133 |
| CAL | −.071 | −0.299; 0.157 | .534 | −.111 | −0.337; 0.116 | .332 |
The reference category for intervention effect is the control group.
Abbreviations: BL, bone level; BS, bleeding score; CAL, clinical attachment level; PD, probing pocket depth; SS, suppuration score.
Adjusted for baseline value.
Adjusted for baseline value, number of treatment sessions, smoking and full‐mouth plaque score at T 3.
Analysed with Mann–Whitney U test.
TABLE 4.
Mean change in probing pocket depth between T 0 and T 3 for initially shallow, moderate and deep peri‐implant and periodontal pockets
| N = 57 | Baseline pocket depth | Mean pocket depth reduction | p‐Value | |
|---|---|---|---|---|
| Control group (n = 29) | Test group (n = 28) | |||
| Peri‐implant pockets | ≤3 mm | −0.10 (0.86) | 0.37 (0.80) | .099 |
| [3 (2)]; n = 17 | [4 (3)]; n = 19 | |||
| 4–6 mm | 1.07 (1.00) | 1.29 (0.86) | .407 | |
| [7 (4)]; n = 26 | [8 (8)]; n = 26 | |||
| ≥7 mm | 2.42 (1.23) | 3.19 (1.53) | .054 | |
| [5 (4)]; n = 25 | [4 (3)]; n = 26 | |||
| Periodontal pockets | ≤3 mm | 0.36 (0.32) | 0.47 (0.23) | .135 |
| [94 (32)]; n = 29 | [92 (30)]; n = 28 | |||
| 4–6 mm | 1.24 (0.52) | 1.33 (0.45) | .513 | |
| [40 (20)]; n = 29 | [37 (15)]; n = 28 | |||
| ≥7 mm | 2.78 (1.37) | 3.75 (1.23) | .025 a | |
| [6 (8)]; n = 19 | [5 (7)]; n = 20 | |||
Significant difference between test and control group (Independent‐Samples T test); [..] = mean number of pockets (SD); n = number of patients.
Results from the microbiological analyses are depicted in Table 5. There were no significant differences between both groups at T 3, neither for implants nor for teeth. Detection frequencies of most bacterial species, both at implants and teeth and in the control and test group, were lower at T 3 than at T 0, except for 4 out of 7 species at implants in the control group (equal or higher detection frequency). However, the reductions between T 0 and T 3 were not significant, with the exception of number of patients positive for T. denticola in the test group.
TABLE 5.
Number of patients with selected periodontal pathogens and mean log‐transformed counts (SD) for the control and test group at T 0 and T 3 at IMPLANTS and TEETH
| N = 57 | IMPLANTS | TEETH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 29) | Test (n = 28) | Control (n = 29) | Test (n = 28) | ||||||
| T 0 | T 3 | T 0 | T 3 | T 0 | T 3 | T 0 | T 3 | ||
| Aa | n | 4 | 3 | 1 | 0 | 1 | 2 | 1 | 0 |
| Mean counts (SD) | 6.08 (0.36) | 6.07 (1.85) | 7.51 | 8.51 | 4.81 (0.14) | 6.80 | |||
| Pg | n | 13 | 15 | 15 | 12 | 15 | 13 | 16 | 12 |
| Mean counts (SD) | 6.29 (1.09) | 5.46 (1.88) | 6.98 (1.11) | 5.98 (2.22) | 6.03 (1.49) | 5.41 (1.66) | 6.84 (1.57) | 4.48 (1.43) | |
| Pi | n | 20 | 17 | 14 | 11 | 19 | 18 | 16 | 12 |
| Mean counts (SD) | 5.13 (1.26) | 5.23 (1.61) | 5.47 (1.22) | 3.99 (2.02) | 5.26 (1.55) | 4.72 (1.31) | 5.35 (1.38) | 3.34 (1.85) | |
| Tf | n | 26 | 28 | 28 | 25 | 28 | 26 | 28 | 25 |
| Mean counts (SD) | 5.82 (0.94) | 5.26 (1.71) | 6.22 (0.90) | 5.32 (0.93) | 5.67 (1.21) | 5.19 (1.05) | 6.33 (0.94) | 4.09 (1.60) | |
| Pm | n | 28 | 29 | 28 | 26 | 29 | 27 | 28 | 24 |
| Mean counts (SD) | 5.13 (0.94) | 4.71 (1.15) | 5.14 (0.79) | 4.55 (0.81) | 4.85 (0.86) | 4.48 (1.19) | 5.08 (0.66) | 4.31 (1.22) | |
| Fn | n | 29 | 28 | 28 | 27 | 29 | 28 | 28 | 27 |
| Mean counts (SD) | 5.65 (0.92) | 5.31 (1.23) | 5.78 (0.84) | 5.11 (0.86) | 5.58 (0.78) | 5.01 (1.30) | 5.81 (0.80) | 4.93 (1.06) | |
| Td | n | 13 | 14 | 19 | 11 a | 14 | 10 | 19 | 10 a |
| Mean counts (SD) | 4.96 (1.13) | 4.39 (1.57) | 5.19 (0.75) | 4.64 (0.80) | 4.68 (1.45) | 4.46 (1.20) | 5.27 (1.17) | 3.96 (1.44) | |
Abbreviations: Aa, A. actinomycetemcomitans; Fn, F. nucleatum; Pg, P. gingivalis; Pi, P. intermedia; Pm, P. micra; Td, T. denticola; Tf, T. forsythia.
Significant change from baseline p < .05 (McNemar test). There were no significant differences between both groups at T 3 neither for implants nor for teeth (Fisher's exact test).
Percentages of patients with no, mild, moderate or severe adverse events related to the use of antibiotics and/or mouthrinse and corresponding median VAS scores are depicted in Figure 2. No significant differences were observed between both groups. Other adverse events reported (not in questionnaire) were pain in the ears, toothaches and globus sensation (control group) and vaginal yeast infection and vaginal dryness (test group).
FIGURE 2.
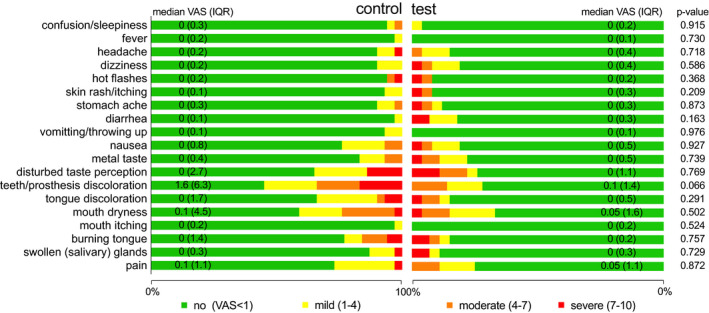
Percentage of patients with no, mild, moderate and severe adverse events related to the treatment and median VAS scores (IQR)
At 3‐month follow‐up, it was decided that 29 out of 57 patients (13 control group and 16 test group patients) required no immediate further treatment and could continue with the follow‐up programme (Supportive Periodontal Therapy, SPT, every 3–6 months). For 8 of these patients (3 control group and 5 test group), decisions on further (surgical) treatment were postponed to the re‐evaluation at 6 months because doubts existed regarding its necessity. Twenty patients (11 control group and 9 test group) were scheduled for a surgical treatment, including 2 patients who required explantation of 1 of their implants. Eight patients (5 control group and 3 test group) were scheduled for non‐surgical re‐treatment, mainly due to insufficient oral hygiene and/or compliance.
4. DISCUSSION
The present study shows that systemically administered AMX plus MTZ in conjunction with a meticulously performed full‐mouth non‐surgical peri‐implantitis treatment does not generally lead to better clinical and microbiological results than non‐surgical treatment alone. Thus, the null hypothesis of no difference has to be accepted. Only initially deep peri‐implant pockets may benefit form systemic AMX + MTZ (p = .054, borderline significance), but probably not to such an extent that surgical follow‐up treatment can be avoided.
Our peri‐implantitis treatment results are similar to Shibli et al. (2019) who also found no beneficial clinical and microbiological effects of adjunctive systemic AMX + MTZ (500 mg + 400 mg, three times daily for 14 days) to non‐surgical treatment of severe peri‐implantitis. However, our results for periodontitis are somewhat different than regularly seen for systemic antibiotics in periodontitis treatment (Feres et al., 2015; Jepsen & Jepsen, 2016). The clinical benefits of adjunctive MTZ or MTZ plus AMX in periodontitis have been well established in a large number of RCTs and systematic reviews. These benefits are especially true for aggressive compared to chronic periodontitis and in deep compared to moderately deep pockets (Feres et al., 2015; Harks et al., 2015; Jepsen & Jepsen, 2016). The benefits of systemic antibiotics on initially deep periodontal pockets could be confirmed in the present study, but were not generalizable to full‐mouth scores. This discrepancy may be explained by the fact that patients were selected on presence of peri‐implantitis and not on presence of periodontitis. Consequently, degrees of periodontitis varied between patients, from localized mild to generalized severe periodontitis, resulting in substantially lower full‐mouth baseline pocket depths (3.22 ± 0.7 mm) than generally observed in RCTs reporting specifically on periodontitis (range 3.6–4.4 mm) (Winkel et al., 2001; Matarazzo et al., 2008; Silva et al., 2011). In contrast, baseline peri‐implant pockets in the present study (5.71 ± 1.4 mm) were much deeper, reflecting severe grades of peri‐implantitis, comparable to Shibli et al. (2019).
One of the limitations of the present study is the short follow‐up period of 3 months, making it impossible to evaluate “true” end points of therapy, such as implant/tooth loss and prevention of disease progression (Tomasi & Wennström, 2017). However, it was deemed ethically unacceptable to withhold patients their necessary (surgical) follow‐up treatment if problems persisted at the 3 month evaluation. In addition, one may question the added value of the outcome parameter bone level change, as measured on radiographs three months after treatment. Although no large changes in bone levels were expected and observed, these radiographs were deemed useful for identification of cases with rapidly progressive bone loss and for decision‐making on the subsequent treatment phase.
Sample size calculation for the present study was based on data from a study on periodontal and not peri‐implant treatment. Although wide margins were applied, one may argue whether there was enough power to detect relevant differences. To facilitate a correct interpretation of the results, a post hoc power analysis was performed. Based on the explained variance (R squared), the power of the “crude” regression model (2 predictors) for the primary outcome variable peri‐implantitis BS was 46% and for the “adjusted” regression model (6 predictors) 78%. For peri‐implantitis PPD, the power was 86% for the “crude” model and 87% for the “adjusted” model. Although post hoc power analyses have to be interpreted with caution, the present study appears to have sufficient power for detecting relevant differences, underlining the outcome of no beneficial effects of systemic AMX + MTZ in non‐surgical peri‐implantitis treatment.
The reason for this lack of beneficial effects is as of yet not clear. It could be speculated that this is due to specific histopathological and immunological characteristics of peri‐implantitis (Berglundh et al., 2011), such as absence of a periodontal ligament, limited vascularization and lack of dento (“implanto”)‐gingival fibres. These characteristics may explain why implant tissues seem less capable in controlling inflammation than periodontal tissues (Buser et al., 1992; Lindhe et al., 1992; Berglundh et al., 1994, 2011). The poor vascular supply, especially in the supra‐alveolar area apical of the junctional epithelium, may also be responsible for an antibiotic concentration too low to be effective and significantly affect the peri‐implant microbiota.
In addition, controversy exists regarding the specific role of bacteria in peri‐implant disease initiation. Instead of primarily being the result of a true infection, it has been speculated that (initial) marginal peri‐implant bone loss is merely the result of a dis‐balanced foreign body reaction related to compromised implant, prosthodontics and patient factors (Qian et al., 2012; Albrektsson et al., 2017, 2014. Secondary to an already compromised situation (marginal bone loss, induced immune response), an infection may then develop. Open‐ended microbiome studies have shown that the microbiomes associated with periodontitis and peri‐implantitis show major differences (Kumar et al., 2012; Dabdoub et al., 2013; Lafaurie et al., 2017; Sahrmann et al., 2020). The microbiome in peri‐implantitis seems associated with predominantly non‐cultivable Gram‐negative species and is not associated with a uniform microbial profile. A limitation of the present study is that targeted periodontal microbiological investigations were used, both for teeth and implants, providing an incomplete picture of the potential changes in composition of the peri‐implant and periodontal microbiome. Although the investigated periodontal species may be considered marker species for periodontitis (Griffen et al., 2012), their role in peri‐implantitis onset, progression and/or treatment outcome is not clear.
However, regardless of the composition and complexity of the peri‐implant microbiome and the discussion whether a microbial infection is the primary etiological factor or a secondary complication, it is generally accepted that anti‐infective measures aimed at disturbance/removal of the implant biofilm are mandatory for achieving treatment success. Yet, adequate subgingival biofilm removal is more challenging for implants than for teeth, due the screw‐shaped implant design, rough surface and/or unfavourable shape of the suprastructure. Bacteria in (undisturbed) biofilms, as compared to planktonic bacteria, display an increased tolerance of antimicrobial agents (Marsh, 2005), which may cause adjunctive systemic antibiotics to be less effective. Additionally, in trying to remove the implant biofilm there is the risk of damaging the implant surface. In vitro studies have shown that released titanium particles have the potential to induce severe inflammatory responses in macrophages and stimulate osteoclastogenesis (Eger et al., 2017). In addition, greater levels of dissolved titanium have been detected in submucosal plaque around implants with peri‐implantitis compared with healthy implants (Safioti et al., 2017) and titanium dissolution products have been shown to act as a modifier in the peri‐implant microbiome structure and diversity (Daubert et al., 2018), both indicating an association between titanium dissolution products and peri‐implantitis. A limitation of the present study is the fact that, amongst other methods and instruments, ultrasonic metal tips were used for debridement of rough implant parts, causing a high risk for implant surface abrasion. Although treatment was always concluded by using an air polisher and no exacerbations were seen immediately after treatment, there is the possibility that remaining titanium particles could have negatively influenced the results.
One may speculate that, since adequate implant biofilm removal/disruption is more easily achieved during a surgical procedure, systemic antibiotics may be of value then. However, the two available RCTs on this topic have either shown no clinical benefits (azithromycin, 250 mg × 2 at day of surgery +250 mg × 1 per day during 4 additional days) (Hallström et al., 2017) or only a positive effect in a subgroup of implants with modified surfaces (AMX 750 mg × 2 per day, during 10 days) (Carcuac et al., 2016). In the present study, many different implant types and surfaces were included, making it impossible to determine the influence of implant surface topography on treatment outcomes. This heterogeneity may be considered a limitation of the study, but on the other hand it may reflect the complexitiy of the implant patient population in general.
Since peri‐implantitis is difficult to treat successfully, one may argue that all available means should be incorporated in the treatment plan. However, the phrase “there's no harm in trying” does not hold true for antimicrobial agents. Systemic antibiotics may cause (serious) adverse events, may interact with other drugs leading to comorbidity, may increase proliferation of antimicrobial resistance and may cause superinfections and overgrowth of opportunistic pathogens difficult to eradicate (Verdugo et al., 2016).
The risk for bacterial resistance is increased when sufficient disruption of the submucosal biofilm cannot be achieved. In vitro analysis of submucosal peri‐implantitis biofilm specimens has shown that peri‐implantitis patients indeed frequently yield submucosal bacterial pathogens resistant to individual therapeutic concentrations of clindamycin, amoxicillin, doxycycline or metronidazole, however, only rarely to both amoxicillin and metronidazole (Rams et al., 2014).
The negative impact of antimicrobial agents on the normal protective microflora may cause a shift from a symbiotic ecosystem into a dysbiotic ecosystem, allowing overgrowth of superinfecting opportunistic bacteria, viruses and yeast, such as Staphylococcus aureus or Epstein–Barr virus (EBV) (Verdugo et al., 2016). Indiscriminative antibiotic administration may thus induce a cascade of microbiological shifts that could support escalation of peri‐implantitis. Antibiotic susceptibility testing of bacterial pathogens and identification of opportunistic microorganisms could help to minimize these risks and may aid in selection of antimicrobial therapy for peri‐implantitis patients (Rams et al., 2014; Verdugo et al., 2016).
Despite the fact that adjunctive systemic AMX + MTZ does not seem to enhance outcomes of non‐surgical peri‐implantitis therapy, meticulously performed full‐mouth mechanical therapy itself, including a thorough control of self‐performed oral hygiene, does significantly improve clinical peri‐implant parameters. Complete disease resolution (no BoP, PPD<5 mm) was rarely achieved, but more than half of the patients required no immediate further treatment and continued with the aftercare programme. This observation supports the notion that treatment of peri‐implantitis should start with a non‐surgical treatment phase. Since peri‐implantitis is rarely a solitary disease, but virtually always accompanied by some form of periodontal disease, a full‐mouth approach is preferred, combining treatment of implants and remaining dentition.
5. CONCLUSION
Within the limitations of this study, it can be concluded that adjunctive systemic antibiotic therapy of AMX and MTZ does not improve clinical and microbiological outcomes of non‐surgical peri‐implantitis treatment and should therefore not be routinely recommended. Complete disease resolution may be difficult to achieve, but a meticulously performed full‐mouth non‐surgical treatment, achieving a high level of daily oral hygiene and healthy periodontal tissues, can significantly improve the starting position of the subsequent (surgical) treatment phase.
CONFLICT OF INTEREST
Prof. Van Winkelhoff reports co‐ownership of LabOral Diagnostics (until June 1, 2020), service laboratory for clinical microbiology in dentistry. Dr. Vangsted and Dr. De Waal have nothing to disclose.
ETHICAL APPROVAL
All authors were involved in conceiving the study; T.V. collected the data. Y.D.W. processed and analysed the data and led the writing; A.J.V.W. and T.V. critically revised the manuscript. All authors read and approved the final manuscript.
Funding information
The study was self‐funded by the authors and their institutions
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Albrektsson, T. , Chrcanovic, B. , Östman, P. O. , & Sennerby, L. (2017). Initial and long‐term crystal bone responses to modern dental implants. Periodontology 2000, 73, 41–50. 10.1111/prd.12176 [DOI] [PubMed] [Google Scholar]
- Albrektsson, T. , Dahlin, C. , Jemt, T. , Sennerby, L. , Turri, A. , & Wennerberg, A. (2014). Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clinical Implant Dentistry and Related Research, 16, 155–165. 10.1111/cid.12142 [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Armitage, G. , Araujo, M. G. , Avila‐Ortiz, G. , Blanco, J. , Camargo, P. M. , Chen, S. , Cochran, D. , Derks, J. , Figuero, E. , Hämmerle, C. H. F. , Heitz‐Mayfield, L. J. A. , Huynh‐Ba, G. , Iacono, V. , Koo, K. T. , Lambert, F. , McCauley, L. , Quirynen, M. , Renvert, S. , … Zitzmann, N. (2018). Peri‐implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and conditions. Journal of Periodontology, 89(Suppl. 1), S313–S318. 10.1002/JPER.17-0739 [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Lindhe, J. , Jonsson, K. , & Ericsson, I. (1994). The topography of the vascular systems in the periodontal and peri‐implant tissues in the dog. Journal of Clinical Periodontology, 21, 189–193. 10.1111/j.1600-051x.1994.tb00302.x [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Zitzmann, N. U. , & Donati, M. (2011). Are peri‐implantitis lesions different from periodontitis lesions? Journal of Clinical Periodontology, 38(Suppl. 11), 188–202. 10.1111/j.1600-051X.2010.01672.x [DOI] [PubMed] [Google Scholar]
- Buser, D. , Weber, H. P. , Donath, K. , Fiorellini, J. P. , Paquette, D. W. , & Williams, R. C. (1992). Soft tissue reactions to non‐submerged unloaded titanium implants in beagle dogs. Journal of Periodontology, 63, 225–235. 10.1902/jop.1992.63.3.225 [DOI] [PubMed] [Google Scholar]
- Carcuac, O. , Derks, J. , Charalampakis, G. , Abrahamsson, I. , Wennström, J. , & Berglundh, T. (2016). Adjunctive systemic and local antimicrobial therapy in the surgical treatment of peri‐implantitis: A randomized controlled clinical trial. Journal of Dental Research, 95, 50–57. 10.1177/0022034515601961 [DOI] [PubMed] [Google Scholar]
- Dabdoub, S. M. , Tsigarida, A. A. , & Kumar, P. S. (2013). Patient‐specific analysis of periodontal and peri‐implant microbiomes. Journal of Dental Research, 92(Suppl. 2), 168–175. 10.1177/0022034513504950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert, D. M. , Pozhitkov, A. E. , McLean, J. , & Kotsakis, G. A. (2018). Titanium as a modifier of the peri‐implant microbiome structure. Clinical Implant Dentistry and Related Research, 20, 945–953. 10.1111/cid/12676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger, M. , Sterer, N. , Liron, T. , Kohavi, D. , & Gabet, Y. (2017). Scaling of titanium implants entrains inflammation‐induced osteolysis. Scientific Reports, 7, 39612. 10.1038/srep39612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul, F. , Erdfelder, E. , Buchner, A. , & Lang, A. G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behaviour Research Methods, 41, 1149–1160. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- Feres, M. , Figueiredo, L. C. , Soares, G. M. , & Faveri, M. (2015). Systemic antibiotics in the treatment of periodontitis. Periodontology 2000, 67, 131–186. 10.1111/prd.12075 [DOI] [PubMed] [Google Scholar]
- Feres, M. , Soares, G. M. , Mendes, J. A. , Silva, M. P. , Faveri, M. , Teles, R. , Socransky, S. S. , & Figueiredo, L. C. (2012). Metronidazole alone or with amoxicillin as adjuncts to non‐surgical treatment of chronic periodontitis: A 1‐year double‐blinded, placebo‐controlled, randomized clinical trial. Journal of Clinical Periodontology, 39, 1149–1158. 10.1111/jcpe.12004 [DOI] [PubMed] [Google Scholar]
- Griffen, A. L. , Beall, C. J. , Campbell, J. H. , Firestone, N. D. , Kumar, P. S. , Yang, Z. K. , Podar, M. , & Leys, E. J. (2012). Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. The International Society for Microbial Ecology Journal, 6, 1176–1185. 10.1038/ismej.2011.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallström, H. , Persson, G. R. , Lindgren, S. , & Renvert, S. (2017). Open flap debridement of peri‐implantitis with or without adjunctive systemic antibiotics: A randomized clinical trial. Journal of Clinical Periodontology, 44, 1285–1293. 10.1111/jcpe.12805 [DOI] [PubMed] [Google Scholar]
- Harks, I. , Koch, R. , Eickholz, P. , Hoffmann, T. , Kim, T.‐S. , Kocher, T. , Meyle, J. , Kaner, D. , Schlagenhauf, U. , Doering, S. , Holtfreter, B. , Gravemeier, M. , Harmsen, D. , & Ehmke, B. (2015). Is progression of periodontitis relevantly influenced by systemic antibiotics? Journal of Clinical Periodontology, 42, 832–842. 10.1111/jcpe.12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen, K. , & Jepsen, S. (2016). Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontology 2000, 71, 82–112. 10.1111/prd.12121 [DOI] [PubMed] [Google Scholar]
- Kumar, P. S. , Mason, M. R. , Brooker, M. R. , & O'Brien, K. (2012). Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. Journal of Clinical Periodontology, 39, 425–433. 10.1111/j.1600-051X.2012.01856.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaurie, G. I. , Sabogal, M. A. , Castillo, D. M. , Rincón, M. V. , Gómez, L. A. , Lesmes, Y. A. , & Chambrone, L. (2017). Microbiome and microbial biofilm profiles of peri‐implantitis: A systematic review. Journal of Periodontology, 88, 1066–1089. 10.1902/jop.2017.170123 [DOI] [PubMed] [Google Scholar]
- Liñares, A. , Pico, A. , Blanco, C. , & Blanco, J. (2019). Adjunctive systemic metronidazole to nonsurgical therapy of peri‐implantitis with intrabony defects: A retrospective case series study. The International Journal of Oral & Maxillofacial Implants, 34, 1237–1245. 10.11607/jomi.7343 [DOI] [PubMed] [Google Scholar]
- Lindhe, J. , Berglundh, T. , Ericsson, I. , Liljenberg, B. , & Marinello, C. (1992). Experimental breakdown of peri‐implant and periodontal tissues. A study in the beagle dog. Clinical Oral Implants Research, 3, 9–16. [DOI] [PubMed] [Google Scholar]
- Marsh, P. D. (2005). Dental plaque: Biological significance of a biofilm and community life‐style. Journal of Clinical Periodontology, 32(Suppl. 6), 7–15. [DOI] [PubMed] [Google Scholar]
- Matarazzo, F. , Figueiredo, L. C. , Cruz, S. E. , Faveri, M. , & Feres, M. (2008). Clinical and microbiological benefits of systemic metronidazole and amoxicillin in the treatment of smokers with chronic periodontitis: A randomized placebo‐controlled study. Journal of Clinical Periodontology, 35, 885–896. 10.1111/j.1600-051X.2008.01304.x [DOI] [PubMed] [Google Scholar]
- Mombelli, A. , & Lang, N. P. (1992). Antimicrobial treatment of peri‐implant infections. Clinical Oral Implants Research, 3, 162–168. [DOI] [PubMed] [Google Scholar]
- Nart, J. , Pons, R. , Valles, C. , Esmatges, A. , Sanz‐Martín, I. , & Monje, A. (2020). Non‐surgical therapeutic outcomes of peri‐implantitis: 12‐Month results. Clinical Oral Investigations, 24, 675–682. 10.1007/s00784-019-02943-8 [DOI] [PubMed] [Google Scholar]
- Qian, J. , Wennerberg, A. , & Albrektsson, T. (2012). Reasons for marginal bone loss around oral implants. Clinical Implant Dentistry and Related Research, 14, 792–807. 10.1111/cid.12014 [DOI] [PubMed] [Google Scholar]
- Rams, T. E. , Degener, J. E. , & van Winkelhoff, A. J. (2014). Antibiotic resistance in human peri‐implantitis microbiota. Clinical Oral Implants Research, 25, 82–90. 10.1111/clr.12160 [DOI] [PubMed] [Google Scholar]
- Renvert, S. , & Polyzois, I. (2018). Treatment of pathologic peri‐implant pockets. Periodontology 2000, 76(1), 180–190. 10.1111/prd.12149 [DOI] [PubMed] [Google Scholar]
- Safioti, L. M. , Kotsakis, G. A. , Pozhitkov, A. E. , Chung, W. O. , & Daubert, D. M. (2017). Increased levels of dissolved titanium are associated with peri‐implantitis‐A cross‐sectional study. Journal of Periodontology, 88, 436–442. 10.1902/jop.2016.160524 [DOI] [PubMed] [Google Scholar]
- Sahrmann, P. , Gilli, F. , Wiedemeier, D. B. , Attin, T. , Schmidlin, P. R. , & Karygianni, L. (2020). The microbiome of peri‐implantitis: A systematic review and meta‐analysis. Microorganisms, 8, 661. 10.3390/microorganisms8050661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, K. F. , Altman, D. G. , & Moher, D. , & CONSORT Group (2010). CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology, 63, 834–840. 10.1016/j.jclinepi.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Schmucker, A. , & Becker, J. (2015). Efficacy of alternative or adjunctive measures to conventional treatment of peri‐implant mucositis and peri‐implantitis: A systematic review and meta‐analysis. International Journal of Implant Dentistry, 1(1), 22. 10.1186/s40729-015-0023-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibli, J. A. , Ferrari, D. S. , Siroma, R. S. , Figueiredo, L. C. , Faveri, M. , & Feres, M. (2019). Microbiological and clinical effects of adjunctive systemic metronidazole and amoxicillin in the non‐surgical treatment of peri‐implantitis: 1 Year follow‐up. Brazilian Oral Research, 33, e080. 10.1590/1807-3107bor-2019.vol33.0080 [DOI] [PubMed] [Google Scholar]
- Silva, M. P. , Feres, M. , Sirotto, T. A. , Soares, G. M. , Mendes, J. A. , Faveri, M. , & Figueiredo, L. C. (2011). Clinical and microbiological benefits of metronidazole alone or with amoxicillin as adjuncts in the treatment of chronic periodontitis: A randomized placebo‐controlled clinical trial. Journal of Clinical Periodontology, 38, 828–837. 10.1111/j.1600-051X.2011.01763.x [DOI] [PubMed] [Google Scholar]
- Stein, J. M. , Hammächer, C. , & Michael, S. S. (2017). Combination of ultrasonic decontamination, soft tissue curettage, and submucosal air polishing with povidone‐iodine application for non‐surgical therapy of peri‐implantitis: 12 Month clinical outcomes. Journal of Periodontology, 89, 139–147. 10.1902/jop.2017.170362 [DOI] [PubMed] [Google Scholar]
- Tomasi, C. , & Wennström, J. L. (2017). Is the use of differences in the magnitude of CAL gain appropriate for making conclusion on the efficacy of non‐surgical therapeutic means? Journal of Clinical Periodontology, 44, 601–602. 10.1111/jcpe.12733 [DOI] [PubMed] [Google Scholar]
- Verdugo, F. , Laksmana, T. , & Uribarri, A. (2016). Systemic antibiotics and the risk of superinfection in peri‐implantitis. Archives of Oral Biology, 64, 39–50. 10.1016/j.archoralbio.2015.12.007 [DOI] [PubMed] [Google Scholar]
- Winkel, E. G. , van Winkelhoff, A. J. , Timmerman, M. F. , van der Velden, U. , & van der Weijden, G. A. (2001). Amoxicillin plus metronidazole in the treatment of adult periodontitis patients. A double‐blind placebo‐controlled study. Journal of Clinical Periodontology, 28, 296–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


