Abstract
Objectives
We aim to determine the prevalence and the course of anxiety and mood disorders in Dutch adolescents (12–18 years old) with type 1 diabetes, and to examine correlates of symptom severity, including parental emotional distress.
Methods
Participants were 171 adolescents and 149 parents. The Diagnostic Interview Schedule for Children‐IV was used to assess current, past year and lifetime anxiety and mood disorders in adolescents. Symptom severity and diabetes distress were measured with validated questionnaires. Correlates of these symptoms were examined using hierarchical regression analyses and included demographics (adolescent sex and age), clinical factors (diabetes duration, treatment modality, most recent glycated hemoglobin A1c; all extracted from medical charts), adolescent diabetes distress, and parent emotional distress.
Results
Twenty‐four (14%) adolescents met the criteria for ≥1 disorder(s) in the previous 12 months. Anxiety disorders were more prevalent than mood disorders (13% vs. 4%). Lifetime prevalence of anxiety and mood disorders was 29% (n = 49). The presence of any of these disorders earlier in life (from 5 years old up to 12 months prior to assessment) was associated with disorders in the past 12 months (OR = 4.88, p = 0.001). Higher adolescent diabetes distress was related to higher symptoms of anxiety (b = 0.07, p = 0.001) and depression (b = 0.13, p = 0.001), while demographics, clinical characteristics, and parental emotional distress were not related.
Conclusions
Anxiety and mood disorders are common among adolescents and related to earlier disorders. Higher diabetes distress was related to higher symptom severity. Clinicians are advised to address past psychological problems and remain vigilant of these problems.
Keywords: adolescents, anxiety, depression, type 1 diabetes
1. INTRODUCTION
Adolescence is a period of risk for the development of anxiety and depression. 1 , 2 Living with type 1 diabetes (T1D) may augment this vulnerability. Unfortunately, exact estimates are lacking. Systematic reviews addressing the prevalence of anxiety or depression in youth with T1D only included studies using self‐report questionnaires. 3 , 4 Meta‐analysis showed a pooled prevalence of 30% for questionnaire‐based depression and 32% for questionnaire‐based anxiety. 4 The few studies that used a diagnostic interview (presented in Table 1), the gold standard in assessing the presence of psychiatric disorders, had a small sample size, 5 , 6 , 7 , 8 were conducted over 20 years ago, 5 , 6 or failed to report adolescent specific rates. 6 , 8 , 9 , 10 As adolescents are developmentally distinct from other age groups, adolescent‐specific estimates are needed. Furthermore, current and previous disorders should be considered simultaneously to better understand the underlying vulnerability and course of these disorders. 11
TABLE 1.
Overview of studies assessing anxiety and mood disorders in adolescents with type 1 diabetes
| Authors (year) | Sample size | Interview | Timeframe | Prevalence rates |
|---|---|---|---|---|
| Blanz et al. (1993) 5 | N = 92 | Semi‐structured interview for the psychiatric assessment of children developed by Graham and Rutter | Within the last 6 months | Separate prevalence rates not reported |
| Kovacs et al. (1997) 6 | N = 92 | Interview Schedule for Children and Adolescents (ISCA) | From diagnosis up to 10 year follow‐up | Anxiety disorders: 19.6% (N = 18) |
| Major depression or dysthymic disorder: 26.1% (N = 24) | ||||
| Northam et al. (2004) 7 | N = 41 | Diagnostic Interview for Children and Adolescents—Version Four (DICA‐IV) | Current (after 10 years of diagnosis) | Anxiety disorders: 17.1% (N = 7) |
| Mood disorders: 17.1% (N = 7) | ||||
| Liakopoulou et al. (2001) 8 | N = 55 | Schedule for Affective Disorders and Schizophrenia for School‐Age Children (KSADS‐P) | Current | Prevalence rates not reported |
| Butwicka et al. (2016) 9 | N = 207 | Schedule for Affective Disorders and Schizophrenia for Children—Present and Lifetime version (KSADS‐PL) | Current | Anxiety disorders: 11.6% (N = 24) |
| Mood disorders: 3.9% (N = 8) | ||||
| Lifetime | Anxiety disorders: 15% (N = 31) | |||
| Mood disorders: 4.3% (N = 9) | ||||
| Berger et al. (2018) 10 | N = 241 | Children's Diagnostic Interview for Psychiatric Disorders (CDI‐MD) | Current and lifetime | Anxiety disorders*: 21.2% (N = 51) |
| Depression (all subtypes) and dysthymia: 12.4% (N = 30) |
Note: We summed the separate rates for disorder, agoraphobia, specific (isolated) phobias, social phobia, obsessive–compulsive disorder, generalized anxiety disorder, and posttraumatic stress disorder to obtain an overall prevalence rate of anxiety disorders.
Psychiatric disorders in young people with T1D have been related to suboptimal glycemic outcomes and lower quality of life, 9 and psychiatric disorders later in adulthood. 12 Factors related to higher anxiety or depressive symptoms include female gender, older age, and ethnic minority group status. 13 , 14 Insulin pump therapy, less frequent blood glucose monitoring, and higher HbA1c were associated with higher anxiety, 15 while longer diabetes duration and suboptimal glycemic control 7 , 16 were associated with increased depressive symptoms. As for psychological factors, higher adolescent diabetes distress has been related to increased depressive symptoms. 17 An important limitation of previous studies is the focus on adolescent‐centered correlates. The link between parental and adolescent psychological functioning is overlooked, even though studies in the general population have shown a bidirectional transmission of negative affect between parents and children. 18 , 19 In the context of T1D, both diabetes‐related and general parental emotional distress could be of importance, 20 , 21 , 22 , 23 but to our knowledge have rarely both been integrated in studies examining emotional problems in adolescents with T1D.
Therefore, we aim to: (1) determine the prevalence and course of anxiety and mood disorders in Dutch adolescents with T1D, and (2) examine whether demographic and clinical characteristics, adolescent diabetes distress, and parental distress are related to the severity of adolescent anxiety and depressive symptoms.
2. PATIENTS AND METHODS
2.1. Procedure and participants
Baseline data from the first pediatric diabetes clinics participating in the ongoing Longitudinal study of Emotional problems in Adolescents with T1D and their Parents/caregivers (Diabetes LEAP) were used. Adolescents with T1D aged 12–18 years were recruited at Diabeter (locations Deventer, Rotterdam, Schiphol, Veldhoven), Meander Medical Center Amersfoort, and Albert Schweitzer hospital Dordrecht, as was the parent/caregiver primarily involved in their diabetes care. Exclusion criteria were: (a) diabetes duration <6 months, (b) intellectual disability, (c) insufficient mastery of the Dutch language, or (d) severe circumstances interfering with participation. Eligible families were sent information about the study. Participation was discussed at the next regular clinic visit. Parent participation was only possible if the adolescent agreed to participate. Informed consent was obtained from all participants. The Medical Ethical Review Committee of Máxima Medical Center Veldhoven approved the study (NL48232.015.14).
2.2. Measures
2.2.1. Demographic and clinical characteristics
Adolescent sex, birthdate, diagnosis‐date, treatment modality (multiple daily injections [MDI] or continuous subcutaneous insulin infusion [CSII]), and HbA1c were extracted from medical records. HbA1c values ≥58 mmol/mol (7.5%) were considered suboptimal. 24 Age and sex of the primary caregiver were self‐reported.
2.2.2. Adolescent anxiety and mood disorders/symptoms
To systematically assess anxiety and mood disorders, the Dutch translation of the Diagnostic Interview Schedule for Children –IV (DISC‐IV) was employed. 25 An overview of the assessed disorders is presented in Figure 1. Parents were not present for the interview. The interviews were conducted by members of the research team (medical psychologists, MSc or PhD), by students (master's level Medical Psychology), and trained research assistants. While the interview is suitable to be used by laypersons, all students followed mandatory training prior to conducting the interviews. The training consisted of a workshop, observing interviews of a research team‐member, and conducting interviews under supervision of a research team‐member. The introductory module (demographic questions and the construction of a timeline based on life‐events) and the modules assessing the presence of anxiety and mood disorders in previous 4 weeks and the previous 12 months were used. A module concerning phobic fear of hypoglycemia was added to the interview, directly adapted from the Specific Phobia module of the DISC‐IV. We replaced the question segment describing a fear assessed in the Specific Phobia module, such as “…heights…” with “…hypoglycemia….” The Whole Life screening module was used to assess occurrence of anxiety and/or mood disorders from the age of 5 up to 12 months prior to assessment. Results were summed into separate binary variables for the previous 4 weeks, the previous 12 months, and in earlier life separately, all coded with values 0 (disorder not present) and 1 (disorder present). Based on these three variables, we computed the lifetime presence of disorders, coded with value 0 (did not experience a disorder during lifetime) and 1 (experienced [a] disorder[s] during lifetime).
FIGURE 1.

Overview of the assessed mood and anxiety disorders
Depressive symptoms in the past 2 weeks were assessed using the Children's Depression Inventory (CDI)‐2nd edition which consists of 28 items, each rated from 0 to 2. 26 The CDI has widely been used in pediatric diabetes studies. 4 Item scores were recoded if necessary and summed into a total score with higher scores indicating more depressive symptoms (range 0–56). 26 A maximum of three missing values was permitted, as a prorated score could be computed by multiplying the obtained raw score by 28, divided by the number of completed items of the total scale. The internal consistency was good in the present study (α = 0.83).
To assess anxiety severity, the Generalized Anxiety Disorder‐7 (GAD‐7) was used. 27 This short, validated self‐report questionnaire contains items about how often anxiety symptoms were experienced in the past 2 weeks. Response options ranged from 0 (not at all) to 3 (nearly every day). A total score was computed by summing the seven item responses (range 0–21). One missing response was permitted, in which case person mean imputation was applied. While the GAD‐7 was initially developed for adult samples, it has also been shown to detect clinically relevant anxiety in adolescents with a generalized anxiety disorder. 28 The internal consistency was acceptable (α = 0.72).
2.2.3. Parental general emotional distress
The GAD‐7 and the Patient Health Questionnaire–9 (PHQ‐9) were used to assess anxiety and depression in parents. 27 , 29 The internal consistencies were good (α = 0.87 and 0.81). The PHQ‐9 contains items describing how often depressive symptoms were present in the past 2 weeks, with response options ranging from 0 (not at all) to 3 (nearly every day). A total score was obtained by summing the item responses (range 0–27). Up to three missing values were permitted, in which case a person's own mean score was imputed for that person.
2.2.4. Diabetes‐specific distress
Diabetes‐specific distress in adolescents was measured using the Problem Areas in Diabetes – Teens (PAID‐T) questionnaire. 30 The PAID‐T consists of 26 items describing aspects of diabetes (self‐care) to which the adolescents rated whether they experienced (emotional) problems with these aspects, ranging from 1 (not a problem) to 6 (serious problem). A total score was computed by summing responses (range 26–156), with higher scores indicating higher levels of distress. Internal consistency was very good (α = 0.93).
Parental diabetes‐specific distress was assessed using the Problem Areas in Diabetes – Parent Revised (PAID‐PR). 31 This 18‐item validated self‐report questionnaire contains questions about the burden of having a child with type 1 diabetes. Response options ranged from 0 (agree) to 4 (disagree). Item scores were reverse scored so higher scores reflect higher levels of distress. A mean score of all items was computed and multiplied by 25 into a total score (range 0–100) as instructed in the PAID‐PR scoring manual. The internal consistency was very good (α = 0.91).
2.2.5. Power analysis and statistical analyses
G*Power was used to conduct a power analysis. To detect a small to medium effect size (0.02 < f 2 < 0.15) between the nine individual predictors and the dependent variables, with a power of 0.80, alpha at 0.05, a minimum sample size range of 55–395 is required.
Statistical analyses were performed using SPSS 24.0. Descriptive statistics were applied to examine the sample characteristics and the prevalence of anxiety and depression in adolescents. Pearson Chi‐square tests or Fisher's exact tests (expected cell counts <5%) were conducted to examine the association between past year emotional problems and earlier lifetime emotional problems. Two hierarchical regression analyses were conducted to examine correlates of adolescent anxiety (GAD‐7 score) and depression (CDI‐2 score) severity. Only adolescent‐parent dyads with complete data were included in these analyses (n = 147). The correlates were entered in the following blocks: (1) adolescent age and sex, (2) HbA1c (continuous), diabetes duration, and insulin pump therapy, (3) adolescent diabetes‐specific distress (PAID‐T score), and (4) parental distress: anxiety symptoms (GAD‐7 score), depressive symptoms (PHQ‐9 score), diabetes‐specific distress (PAID‐PR score). We inspected the assumptions for linearity and homoscedasticity (standardized residuals vs. standardized predicted values plots, histograms, P–P plots), normality of residuals (histograms, P–P plots), multicollinearity (correlations, variance inflation factor, and tolerance scores), and independent errors (Durbin‐Watson tests). Regarding the assumption of homoscedasticity there were some concerns for both models. Therefore, robust regression analyses were used by bootstrapping on 1000 subsamples. 32 Post‐hoc independent t‐tests were conducted to exploratively examine whether adolescents depressive and anxiety symptoms differed between parental education level groups (no, low and medium education vs. high), and whether adjustment for adolescent depressive symptoms in the anxiety severity model and vice versa yielded different results. Statistical significance was set at p < 0.05.
3. RESULTS
Characteristics of the adolescents (N = 171) and parents (N = 149) are presented in Table 2. Adolescent anxiety symptoms and depressive symptoms did not differ by parental education level (no, low, medium vs. high; data not shown).
TABLE 2.
Sample characteristics of adolescents (n = 171) with type 1 diabetes and their parents (n = 149)
| Missing values | % (n) | Mean ± SD (median; interquartile range) | Range | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age adolescent (years) | 0 | 15 ± 2 (15; 13–17) | 12–19 | |
| Female sex adolescent | 0 | 47% (81) | ||
| Education level adolescent | 1 | |||
| Primary school | 11% (19) | |||
| High school | 76% (129) | |||
| Secondary vocational education | 12% (20) | |||
| Secondary professional education | 1% (2) | |||
| University | 0% (0) | |||
| Being an only child, yes | 22 | 12% (18) | ||
| Age parent (years) | 2 | 46 ± 5 (46; 43–49) | 34–59 | |
| Sex parent, women | 1 | 89% (132) | ||
| Parent from (non‐Dutch) ethnic minority group | 1 | 3% (5) | ||
| Relationship status parent, being single | 1 | 12% (17) | ||
| Education level parent a | 2 | |||
| Low | 5% (8) | |||
| Moderate | 36% (53) | |||
| High | 57% (83) | |||
| Other | 2% (3) | |||
| Clinical characteristics | ||||
| Diabetes duration (years) | 0 | 7 ± 4 (7; 3–11) | 0–16 | |
| Insulin treatment modality, CSII | 0 | 78% (134) | ||
| Most recent HbA1c, % | 0 | 7.7 ± 1 (7.8; 6.9–8.3) | 5.1–11.5 | |
| Most recent HbA1c, mmol/mol | 0 | 61 ± 12 (62; 52–67) | 33–102 | |
| Optimal glycemic control (<7.5%/58 mmol/mol) b | 42% (71) | |||
| Psychosocial characteristics | ||||
| Anxiety symptoms (GAD‐7) | 4 | 2.9 ± 2.7 (3.0; 1.0–4.0) | 0–13 | |
| Depressive symptoms adolescents (CDI‐2) | 4 | 6.2 ± 5.0 (5.0; 3.0–8.0) | 0–26 | |
| Diabetes distress adolescent (PAID‐T) | 4 | 54.3 ± 19.3 (52.0; 38.0–68.0) | 26–105 | |
| Anxiety symptoms parent (GAD‐7) | 0 | 2.4 ± 3.0 (2.0; 0.0–4.0) | 0–14 | |
| Depressive symptoms parent (PHQ‐9) | 0 | 2.3 ± 3.0 (1.0; 0.0–3.0) | 0–16 | |
| Diabetes distress parent (PAID‐PR) | 0 | 21.7 ± 15.9 (18.1; 9.72–29.2) | 0–75 | |
Note: Reported are valid percentages. Due to scheduling issues, one adolescent was 11.99 years old at assessment and two adolescents had a diabetes duration of less than 6 months (0.55 and 0.56 years).
Abbreviations: CDI‐2, children's depression inventory ‐ 2nd edition; CSII, Continuous subcutaneous insulin infusion; DISC‐IV, diagnostic interview schedule for children – 4th edition; GAD‐7, generalized anxiety disorder – 7 item anxiety scale; HbA1c, glycated hemoglobin A1c; PAID‐PR, problem areas in diabetes –parents revised; PAID‐T, problem areas in diabetes – teens; PHQ‐9, patient health questionnaire – 9 item depression scale.
Low: no education, primary school, lower vocational education; Moderate: (Secondary) Vocational education; High: (Secondary) Higher professional education and university.
When a cut‐off of 7.0% or 53 mmol/mol was used, 26% (n = 44) adolescents were in optimal control.
3.1. Prevalence and course of anxiety and mood disorders
Forty‐nine adolescents (29%) have had an anxiety or mood disorder during their lifetime. Anxiety disorders were more prevalent than mood disorders during lifetime (26% vs. 7%, Table 3). Twenty‐four adolescents (14%) appeared to have had (an) anxiety and/or mood disorder(s) in the past year.
TABLE 3.
The prevalence of anxiety and/or mood disorders in adolescents (n = 171) in the past 4 weeks, the past year, and during lifetime (≥5 years old)
| Past 4 weeks | Past year | Lifetime | ||
|---|---|---|---|---|
| Anxiety disorders | Social phobia | 2% (3) | 4% (6) | 7% (12) |
| Separation anxiety disorder | 0% (0) | 0.6% (1) | 5% (8) | |
| Specific phobia | 7% (12) | 9% (15) | 15% (26) | |
| Phobic fear of hypoglycemia | 1% (2) | 2% (3) | 5% (8) | |
| Panic disorder | 0% (0) | 0% (0) | 2% (3) | |
| Agoraphobia | 0% (0) | 0% (0) | 2% (3) | |
| Generalized anxiety disorder | 0.6% (1) | 2% (3) | 4% (6) | |
| Selective mutism | 0% (0) | 0.6% (1) | 0.6% (1) | |
| Obsessive compulsive disorder | 2% (3) | 3% (5) | 3% (5) | |
| Post‐traumatic stress disorder | 0% (0) | 0.6% (1) | 0.6% (1) a | |
| Prevalence of anxiety disorder(s) | 9% (16) | 13% (23) | 26% (44) | |
| Mood disorders | Major depression | 1% (2) | 2% (4) | 4.7% (8) |
| Dysthymia | 0.6% (1) | 1% (2) | 1.2% (2) b | |
| Mania | 0% (0) | 0% (0) | 0.6% (1) | |
| Hypomania | 0% (0) | 1% (2) | 1.2% (2) c | |
| Prevalence of mood disorder(s) | 2% (3) | 4% (7) | 7% (12) | |
| Total | 9% (16) | 14% (24) | 29% (49) |
Note: Reported are percentages (n). Past year prevalence also includes 4 week prevalence. Lifetime prevalence also includes past year‐prevalence.
Lifetime occurrence of post‐traumatic stress disorder was embedded in the diagnostic module.
Lifetime occurrence of dysthymia was not assessed in the whole life module. Lifetime occurrence of decreased mood was assessed in the whole life Major depression screening.
Lifetime occurrence of hypomania was not assessed in the whole life module. Lifetime occurrence of mania was assessed in the whole life screening.
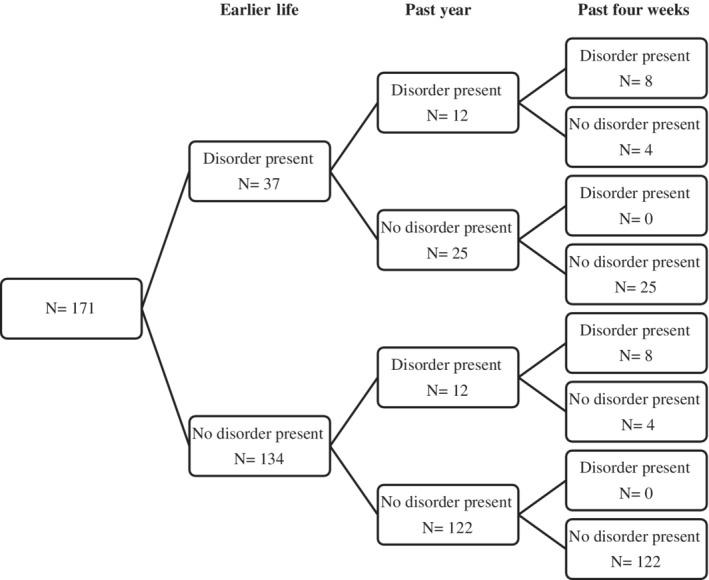
Twenty‐three adolescents (13%) had an anxiety disorder in the past year, of whom 16 reported the presence of an anxiety disorder in the past 4 weeks. Specific phobias (n = 15) were most common, followed by social phobia (n = 6), and obsessive–compulsive disorder (n = 5). The most common phobia endorsed was fear of animals (n = 7), while fear of needles (n = 1) and fear of blood and injury (n = 1) were less prevalent. Mood disorders (past year) were present in 4% (n = 7) of the adolescents, with major depression being the most common disorder (n = 4). Three adolescents had a mood disorder in the past 4 weeks. Of the 171 adolescents, 6% (n = 10) had more than one disorder in the past year.
Of the 147 adolescents who did not have emotional problems in the past year, 25 (17%) had a disorder earlier in life. Of this group, 21 adolescents reported earlier anxiety disorders, while four reported earlier mood disorders. Ten of the 25 adolescents (40%) had contacted a (mental) health care professional for these problems.
Out of the adolescents who did not have a history of anxiety or mood disorders (n = 134), 12 (9%) had an anxiety and/or mood disorder in the past year. Of the 24 adolescents who had an anxiety and/or mood disorder in the past year, 50% (12) also appeared to have had earlier psychological problems. Eleven adolescents had an anxiety disorders and one adolescent had a mood disorder earlier in life. Four (33%) had contacted a (mental) health care professional for these problems.
An overview of the number of disorders per timeframe is presented in Figure 2.
FIGURE 2.
Overview of the number of disorders per timeframe among adolescents with type 1 diabetes
The presence of an emotional problem in earlier life was strongly and significantly associated with the presence of an emotional problem in the past year (Χ 2 (1) = 13, p < 0.001, odds ratio [OR] = 4.88, 95% CI 1.97–12.11). Earlier life anxiety disorders were also strongly associated with past year presence of anxiety disorders (p = 0.001, OR = 5.54, 95% CI 2.17–14.18) and mood disorders (p = 0.003, OR = 12.69, 95% CI 2.34–68.81). However, earlier life mood disorders were not associated with past year anxiety disorders (p = 0.52) or mood disorders (p > 0.99).
3.2. Correlates of anxiety severity
In the first step of bootstrapped hierarchical regression analysis (Table 4), demographic characteristics accounted for 7% of the variance in anxiety severity and the model significantly fit the data (p = 0.002). After adding clinical characteristics, 9% of the variance in anxiety severity was explained (p = 0.08). Adding adolescent diabetes distress to the model led to a significant improvement of explained variance (27%, p < 0.001). Lastly, parental general emotional and diabetes‐specific distress were added, but did not significantly improve the model (p = 0.49). In this final model, accounting for 27% of the variance in anxiety severity, higher adolescent diabetes distress was the only factor significantly related to higher anxiety severity (p = 0.001). After including adolescent depressive symptoms (CDI‐2 score) (post hoc analysis), a total of 39% of the variance in anxiety severity was explained. In this fully adjusted model, higher diabetes distress (b = 0.04, 95% BCa = 0.02–0.06, p = 0.002) and higher depression severity (b = 0.23, 95% BCa = 0.14–0.34, p = 0.001) were related to higher anxiety severity.
TABLE 4.
The association of adolescent anxiety severity (GAD‐7 total score) with adolescent demographical characteristics, clinical characteristics, adolescent diabetes‐specific distress, and parental distress (n = 147)
| Step 1 B (95% BCa) | Step 2 B (95% BCa) | Step 3 B (95% BCa) | Step 4 B (95% BCa) | ||
|---|---|---|---|---|---|
| Demographic characteristics | Age adolescent (years) | 0.21 (0.03–0.40)* | 0.26 (0.04–0.47)* | 0.10 (−0.10 to 0.29) | 0.09 (−0.10 to 0.27) |
| Female sex adolescent | 1.29 (0.47–2.19)** | 1.25 (0.43–2.09)** | 0.41 (−0.25 to 1.15) | 0.39 (−0.30 to 1.12) | |
| Clinical characteristics | Diabetes duration (years) | −0.08 (−0.20 to 0.04) | −0.08 (−0.20 to 0.03) | −0.08 (−0.19 to 0.03) | |
| Insulin treatment modality, CSII | −1.01 (−2.32 to 0.24) | −0.91 (−2.02 to 0.22) | −0.99 (−2.10 to 0.08) | ||
| Most recent HbA1c (mmol/mol) | 0.01 (−0.02 to 0.04) | −0.01 (−0.04 to 0.02) | −0.01 (−0.04 to 0.02) | ||
| Adolescent diabetes distress | Diabetes distress (PAID‐T) | 0.07 (0.05–0.09)** | 0.07 (0.05–0.09)** | ||
| Parental distress | Anxiety symptoms (GAD‐7) | −0.07 (−0.30 to 0.13) | |||
| Depressive symptoms (PHQ‐9) | 0.12 (−0.09 to 0.34) | ||||
| Diabetes distress (PAID‐PR) | −0.01 (−0.05 to 0.02) | ||||
| Adjusted R 2 | 0.07** | 0.09** | 0.27** | 0.27** | |
| R 2 change | 0.08** | 0.04 | 0.18** | 0.01 |
Note: Reported are the bootstrapped unstandardized regression coefficients and the 95% bias corrected accelerated confidence interval, and the proportion of explained variance. Results from post‐hoc analyses were not included in this table. Sex adolescent was binary coded as boy = 0 versus girl = 1; insulin treatment modality as multiple daily self‐injections = 0 versus continuous subcutaneous insulin infusion = 1. Adjusted R 2 reported the fit of the regression model adjusted for the number of predictor variables. R 2 change signified whether each hierarchical block significantly improved the fit of the regression model.
p < 0.05;
p < 0.01; ***p < 0.001.
3.3. Correlates of depression severity
Demographic characteristics explained 6% of the variance in depression severity (p = 0.003, Table 5). Adding clinical characteristics added 8% explained variance, with no significant improvement (p = 0.14). Adding adolescent diabetes‐distress led to a significant improvement to the model explaining a total of 31% variance in depression severity (p < 0.001). Lastly, adding parental general emotional and diabetes‐specific distress did not significantly improve the model (p = 0.08). In this final model, higher adolescent diabetes distress was related to more severe depression (p = 0.001), but the other factors were not. After including adolescent anxiety symptoms (GAD‐7 score)(post hoc analysis), 44% of the variance in depression severity was explained, with higher adolescent diabetes distress (b = 0.08, 95% BCa = 0.04–0.12, p = 0.002) and anxiety severity (b = 0.75, 95% BCa 0.43–1.09, p = 0.001) being related to higher depression severity.
TABLE 5.
The association between adolescent demographical characteristics, clinical characteristics, adolescent diabetes‐specific distress, and parental distress with adolescent depression severity (n = 147)
| Step 1 B (95% BCa) | Step 2 B (95% BCa) | Step 3 B (95% BCa) | Step 4 B (95% BCa) | ||
|---|---|---|---|---|---|
| Demographic characteristics | Age adolescent (years) | 0.21 (−0.15 to 0.59) | 0.21 (−0.21 to 0.67) | −0.14(−0.52 to 0.27) | −0.09 (−0.46 to 0.32) |
| Female sex adolescent | 2.66 (1.11–4.19)** | 2.53 (0.99–3.99)** | 0.76 (−0.57 to 2.11) | 0.73 (−0.77 to 2.33) | |
| Clinical characteristics | Diabetes duration (years) | −0.10 (−0.29 to 0.10) | −0.11 (−0.27 to 0.07) | −0.14 (−0.30 to 0.03) | |
| Insulin treatment modality, CSII | −1.23 (−3.39 to 0.95) | −1.01 (−2.89 to 0.75) | −1.09 (−2.80 to 0.62) | ||
| Most recent HbA1c (mmol/mol) | 0.05 (−0.01 to 0.12) | 0.01 (−0.04 to 0.07) | 0.01 (−0.04 to 0.08) | ||
| Adolescent diabetes distress | Diabetes distress (PAID‐T) | 0.14 (0.10–0.19)** | 0.13 (0.09–0.17)** | ||
| Parental distress | Anxiety symptoms (GAD‐7) | −0.29 (−0.70 to 0.14) | |||
| Depressive symptoms (PHQ‐9) | 0.36 (−0.04 to 0.76) | ||||
| Diabetes distress (PAID‐PR) | 0.04 (−0.02 to 0.10) | ||||
| Adjusted R 2 | 0.06** | 0.08** | 0.31** | 0.33** | |
| R 2 change | 0.08** | 0.03 | 0.23** | 0.03 |
Note: Reported are the bootstrapped regression coefficients and the 95% bias corrected accelerated confidence interval, and the proportion of explained variance. Results from post‐hoc analyses were not included in this table. Sex adolescent was binary coded as boy = 0 versus girl = 1; insulin treatment modality as multiple daily self‐injections = 0 versus continuous subcutaneous insulin infusion = 1. Adjusted R 2 reported the fit of the regression model adjusted for the number of predictor variables. R 2 change signified whether each hierarchical block significantly improved the fit of the regression model.
p < 0.05; **p < 0.01; ***p < 0.001.
4. DISCUSSION
Almost one‐in‐three adolescents with T1D reported a lifetime anxiety or mood disorder and 14% had a past year anxiety or mood disorder. Anxiety disorders were more prevalent than mood disorders, consistent with earlier studies 33 and most studies in adolescents with T1D. 5 , 8 , 10 Due to the different time frames used (e.g., lifetime prevalence, 9 , 10 10‐year prevalence of newly diagnosed children, 6 current prevalence 9 ), it is difficult to directly compare rates between studies. The four‐week prevalence of anxiety disorders in our study (9%) appears to be lower than the current rates reported in previous studies (Table 1). Similarly, our prevalence rate of mood disorders in the past 4 weeks (2%) appears to be lower than rates reported in previous studies (4%–12%). 4 , 9
The lifetime prevalence of anxiety disorders does seem higher in our sample (26%) compared with other studies in adolescents with T1D (15%–21%), 9 , 10 perhaps due to our adding phobic fear of hypoglycemia. After excluding fear of hypoglycemia, the lifetime prevalence of anxiety disorders in our (21%) and other studies in adolescents with T1D is lower than the 28% reported for the general Dutch adolescent population. 33 The same trend is found for lifetime prevalence of mood disorders, where the estimates in our (7%) and previous studies in adolescents with type 1 diabetes (4%–12%) 9 , 10 are lower than the 17% reported in the general Dutch adolescent population. 33 There are several potential explanations for this difference. Firstly, these general population estimates are based on adolescents from one Dutch region, while we included young people from across the country. Secondly, youth with T1D regularly consult with their diabetes teams, with more opportunities for early detection of (sub‐clinical) emotional problems and to offer psychosocial support, compared to adolescents without diabetes. 34 Thirdly, the possibility of developing emotional problems is normalized in diabetes care. Without this explicit attention to emotional problems, youth (and their parents) may find it more difficult to recognize symptoms 35 or perceive more related stigma, delaying help‐seeking. 35
Though overall rates of anxiety and mood disorders appear lower in the present sample of adolescents with T1D compared with rates in the general population, 33 it is important recognize and treat these conditions in this group, as these problems have a strong negative impact on quality of life, contribute to suboptimal diabetes management and impact diabetes outcomes. Adolescents who had emotional problems earlier in life were more likely to have emotional problems in the past year, corroborating evidence that earlier disorders increase risk for future disorders. 6 Coupled with the finding that less than half of the adolescents who had anxiety or mood disorders earlier in life had contacted a (mental) health care professional for these problems, this stresses the value of continued vigilance of health care professionals concerning emotional symptoms and the importance of addressing attitudes toward mental health care. Similar to the general population, families may not always feel the need for specialized treatment, for example when symptoms are considered transient or when self‐reliance is preferred over formal treatment. 35 The final decision about whether or not to initiate professional psychological treatment generally rests with adolescents and their families, but health care professionals do have a responsibility to provide them with all the information necessary (e.g., about the chronicity of symptoms) to make a decision and to address any potentially harmful barriers.
With respect to correlates of symptom severity, we expected older age, female gender, longer diabetes duration, insulin pump therapy, and suboptimal glycemic control to be related to anxiety and depression severity, 7 , 13 , 14 , 15 , 16 but these associations were not replicated in the present study. The relatively mild severity of anxiety and depressive symptoms could have undermined the power to detect these hypothesized associations. Our results did show that higher adolescent diabetes‐specific distress is related to higher adolescent anxiety and depression severity, in accordance with Powers et al. (2017). 17 Diabetes‐specific distress could be an important contributor to more general emotional distress. 17 which in its most severe form could manifest as major depression, 36 but confusing diabetes distress with psychiatric disorders can lead to ineffective treatment.
We did not find parental distress to be associated with adolescent anxiety or depression severity, contrasting earlier findings. 20 , 21 , 22 While other factors could counteract the negative effects of parental problems on children (e.g., relying on and spending time with other people), adolescents themselves may also be more resilient to parental distress than previously reported. It should be noted that these earlier studies only included mothers 20 and did not focus on adolescents specifically. 22 Furthermore, our results could be result of developmental processes, in which adolescents spend less time and rely less on parents as they progress through the developmental stage. It could also be the case that parental distress is not directly associated with adolescent emotional distress, but rather indirectly through the way parents interact with their child. In a previous study, parental depressive symptoms were associated with lower parental warmth, and in turn lower parental warmth was associated with higher severity of depressive symptoms in youth with type 1 diabetes. 37 Furthermore, parental depressive symptoms were indirectly associated with higher HbA1c, through lower parental monitoring. 37
While depression and anxiety have been a topic of previous research interest, we have addressed important methodological limitations and gaps in knowledge of existing studies. Thus, strengths of the current study include the large sample size, the use of a structured diagnostic psychiatric interview, the use of validated questionnaires, and the inclusion of both adolescent and parental correlates of anxiety/depression severity. However, there are also several limitations. First, as we did not collect (mental health) data of non‐participants, selection bias cannot be ruled out, possibly threatening the generalizability of our results. Secondly, ethnic minorities were underrepresented in the sample. Thirdly, the upper limit of the HbA1c values in the present study does not represent the highest risk category. Underrepresentation of adolescents with high HbA1c could have led to an underestimation of cases, and limits the generalizability of our results. Fourthly, the presence of disorders was determined retrospectively at one measurement occasion, introducing the possibility of recall bias. Specifically, the lifetime‐module, which includes early childhood experiences (from the age of 5 years old), may be susceptible for recall bias Including parent reports could provide more detailed information, especially for the early years. Fifthly, the Phobic fear of hypoglycemia‐module was adapted from the Specific phobia‐module specifically for the present study and therefore lacks previous validation. While we kept the wording as close to the original phrasing as possible, a validation study is needed to establish the reliability and validity of this module. Sixthly, not all possibly relevant correlates of anxiety/depression could be included in the model, as the sample size did not permit the addition of more predictors increasing the risk of overfitting the models. Socioeconomic status, 38 for example, is a likely contributor to distress and should be addressed in future research. Further recommendations for future studies are to examine whether interview‐based symptom counts are related to diabetes‐specific distress, whether there is a moderation effect of development stage (i.e., early vs. late adolescence) in the relation between parental distress and adolescent distress, and to examine anxiety and mood disorders in this group relate to overall quality of life and diabetes self‐management. Finally, the cross‐sectional observational data on anxiety/depression severity and diabetes‐specific distress preclude inferences about causality.
Within pediatric diabetes care, clinical practice guidelines recognize that emotional wellbeing of adolescents with type 1 diabetes warrants attention. Our results show that adolescents with a history of emotional problems are particularly at risk for subsequent emotional problems, hence health‐care providers are advised to not only inquire about current symptoms, but also to pay attention to symptoms experienced in the past for a more comprehensive evaluation of risk. A periodic stepped screening procedure using a validated mental health screener could be valuable to track current emotional symptoms, but only when there are resources available to follow‐up with psychosocial support and/or treatment if necessary. While screening instruments are useful in assessing the severity of emotional distress, the context in which the symptoms are experienced is at least as important. Persistent feelings of sadness, for example, could be attributed to adjustment disorder, but also a mood or an anxiety disorder, or diabetes‐specific distress. An interpretation of screening‐results by a mental health professional is therefore warranted. Furthermore, the addition of an optional check‐in with a mental health professional to the annual review appointments could improve the recognition of psychological problems. As diabetes‐specific distress is highly prevalent in adolescents with T1D 39 and appeared to be uniquely related to higher severity of anxiety and depression, psychologists specialized in diabetes play an important role in care support. Understanding how these factors interplay is crucial for effective treatment 37 and attention to diabetes‐specific topics should be part of the therapy content when aiming to improve psychological wellbeing through evidence‐based techniques (e.g., cognitive behavioral therapy). 40
5. CONCLUSIONS
Mood disorders and particularly anxiety disorders are common in adolescents with T1D and related to disorders earlier in life, warranting continued vigilance by health‐care providers. Diabetes‐specific distress was uniquely related to higher symptom severity. Prospective longitudinal studies are needed to provide insight into the directionality of this association and into the further course of disorders across adolescence and into adulthood.
ETHICS APPROVAL
The study protocol was approved by the Medical Research Ethics Committee of Máxima Medical Centre in Veldhoven (NL48232.015.14).
CONFLICT OF INTEREST
The authors have no conflicts of interest relevant to this article to disclose.
AUTHOR CONTRIBUTIONS
Giesje Nefs, Frans Pouwer, Henk‐Jan Aanstoot, and Linh Anh Nguyen, provided substantial contributions to the conception and design of the study, the analysis and interpretation of data, and the drafting of the initial manuscript, and revised the manuscript for important intellectual content.
Per Winterdijk, Esther Hartman, Theo Sas, Roos Nuboer, Willie Bakker‐Van Waarde, Ineke de Kruijff provided substantial contributions to the data collection, the interpretation of data, and critically revised the manuscript for important intellectual content.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
6.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.13174.
ACKNOWLEDGMENTS
We thank all participants of Diabetes LEAP.
This study was supported by a Dutch Diabetes Research Foundation Junior Fellowship granted to Giesje Nefs, with Frans Pouwer as senior researcher (2013.81.1645). The sponsor had no role in the study design, the collection and analysis and interpretation of data, the writing of the report, and the decision to submit the manuscript for publication.
Nguyen LA, Pouwer F, Winterdijk P, et al. Prevalence and course of mood and anxiety disorders, and correlates of symptom severity in adolescents with type 1 diabetes: Results from diabetes LEAP . Pediatr Diabetes. 2021;22:638–648. 10.1111/pedi.13174
Funding information Diabetes Fonds, Grant/Award Number: 2013.81.1645
REFERENCES
- 1. Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM‐V. Psychiatr Clin North Am. 2009;32(3):483‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet. 2012;379(9820):1056‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson B, Eiser C, Young V, Brierley S, Heller S. Prevalence of depression among young people with type 1 diabetes: a systematic review. Diabet Med. 2013;30(2):199‐208. [DOI] [PubMed] [Google Scholar]
- 4. Buchberger B, Huppertz H, Krabbe L, Lux B, Mattivi JT, Siafarikas A. Symptoms of depression and anxiety in youth with type 1 diabetes: a systematic review and meta‐analysis. Psychoneuroendocrinology. 2016;70:70‐84. [DOI] [PubMed] [Google Scholar]
- 5. Blanz BJ, Rensch‐Riemann BS, Fritz‐Sigmund DI, Schmidt MH. IDDM is a risk factor for adolescent psychiatric disorders. Diabetes Care. 1993;16(12):1579‐1587. [DOI] [PubMed] [Google Scholar]
- 6. Kovacs M, Goldston D, Obrosky DS, Bonar LK. Psychiatric disorders in youths with IDDM: rates and risk factors. Diabetes Care. 1997;20(1):36‐44. [DOI] [PubMed] [Google Scholar]
- 7. Northam EA, Matthews LK, Anderson PJ, Cameron FJ, Werther GA. Psychiatric morbidity and health outcome in type 1 diabetes‐perspectives from a prospective longitudinal study. Diabet Med. 2005;22(2):152‐157. [DOI] [PubMed] [Google Scholar]
- 8. Liakopoulou M, Alifieraki T, Katideniou A, et al. Maternal expressed emotion and metabolic control of children and adolescents with diabetes mellitus. Psychother Psychosom. 2001;70(2):78‐85. [DOI] [PubMed] [Google Scholar]
- 9. Butwicka A, Fendler W, Zalepa A, et al. Psychiatric disorders and health‐related quality of life in children with type 1 diabetes mellitus. Psychosomatics. 2016;57(2):185‐193. [DOI] [PubMed] [Google Scholar]
- 10. Berger G, Waldhoer T, Barrientos I, et al. Association of insulin‐manipulation and psychiatric disorders: a systematic epidemiological evaluation of adolescents with type 1 diabetes in Austria. Pediatr Diabetes. 2018;20(1):127‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen JR, Andrews AR, Davis MM, Rudolph KD. Anxiety and depression during childhood and adolescence: testing theoretical models of continuity and discontinuity. J Abnorm Child Psychol. 2018;46(6):1295‐1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bryden KS, Dunger DB, Mayou RA, Peveler RC, Neil HAW. Poor prognosis of Young adults with type 1 diabetes: a longitudinal study. Diabetes Care. 2003;26(4):1052‐1057. [DOI] [PubMed] [Google Scholar]
- 13. Lawrence JM, Standiford DA, Loots B, et al. Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for diabetes in youth study. Pediatrics. 2006;117(4):1348‐1358. [DOI] [PubMed] [Google Scholar]
- 14. Adal E, Onal Z, Ersen A, Yalcin K, Onal H, Aydin A. Recognizing the psychosocial aspects of type 1 diabetes in adolescents. J Clin Res Pediatr Endocrinol. 2015;7(1):57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knight A, Weiss P, Morales K, et al. Identifying differences in risk factors for depression and anxiety in pediatric chronic disease: a matched cross‐sectional study of youth with lupus/mixed connective tissue disease and their peers with diabetes. J. Pediatr. 2015;167(6):1397‐1403.e1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herzer M, Hood KK. Anxiety symptoms in adolescents with type 1 diabetes: association with blood glucose monitoring and glycemic control. J Pediatr Psychol. 2010;35(4):415‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Powers MA, Richter SA, Ackard DM, Craft C. Diabetes distress among persons with type 1 diabetes. Diabetes Educ. 2017;43(1):105‐113. [DOI] [PubMed] [Google Scholar]
- 18. Forbes EE, Shaw DS, Silk JS, et al. Children's affect expression and frontal EEG asymmetry: transactional associations with mothers depressive symptoms. J Abnorm Child Psychol. 2008;36(2):207‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beardslee WR, Gladstone TRG, O'Connor EE. Transmission and prevention of mood disorders among children of affectively ill parents: a review. J Am Acad Child Adolesc Psychiatry. 2011;50(11):1098‐1109. [DOI] [PubMed] [Google Scholar]
- 20. Duru NS, Civilibal M, Elevli M. Quality of life and psychological screening in children with type 1 diabetes and their mothers. Exp Clin Endocrinol Diabetes. 2016;124(2):105‐110. [DOI] [PubMed] [Google Scholar]
- 21. Eilander MMA, Snoek FJ, Rotteveel J, et al. Parental diabetes behaviors and distress are related to glycemic control in youth with type 1 diabetes: longitudinal data from the DINO study. J Diabetes Res. 2017;2017:1462064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Helgeson VS, Becker D, Escobar O, Siminerio L. Families with children with diabetes: implications of parent stress for parent and child health. J Pediatr Psychol. 2012;37(4):467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whittemore R, Jaser S, Chao A, Jang M, Grey M. Psychological experience of parents of children with type 1 diabetes: a systematic mixed‐studies review. Diabetes Educ. 2012;38(4):562‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rewers MJ, Pillay K, de Beaufort C, et al. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. 2014;15(S20):102‐114. [DOI] [PubMed] [Google Scholar]
- 25. Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab‐Stone ME. NIMH diagnostic interview schedule for children version IV (NIMH DISC‐IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28‐38. [DOI] [PubMed] [Google Scholar]
- 26. Kovacs M. Children's Depression Inventory (CDI and CDI 2). The Encyclopedia of Clinical Psychology. Hoboken: John Wiley & Sons, Inc.; 2014. [Google Scholar]
- 27. Spitzer RL, Kroenke K, Williams JW, Löwe B, et al. A brief measure for assessing generalized anxiety disorder: the Gad‐7. Ann Clin Psychiatry. 2006;166(10):227–234A. 1092–1097. [DOI] [PubMed] [Google Scholar]
- 28. Mossman SA, Luft MJ, Schroeder HK. The generalized anxiety disorder 7‐item scale in adolescents with generalized anxiety disorder: signal detection and validation. Ann Clin Psychiatry. 2017;29(4):227‐234A. [PMC free article] [PubMed] [Google Scholar]
- 29. Kroenke K, Spitzer RL, Williams JBW. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weissberg‐Benchell J, Antisdel‐Lomaglio J. Diabetes‐specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes‐teen version. Pediatr Diabetes. 2011;12(4pt1):341‐344. [DOI] [PubMed] [Google Scholar]
- 31. Markowitz JT, Volkening LK, Butler DA, Antisdel‐Lomaglio J, Anderson BJ, Laffel LMB. Re‐examining a measure of diabetes‐related burden in parents of young people with type 1 diabetes: the problem areas in diabetes survey – parent revised version (PAID‐PR). Diabet Med. 2012;29(4):526‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Field A. Discovering statistics using IBM statistics. 4th ed. London: SAGE publications Ltd; 2013. [Google Scholar]
- 33. Ormel J, Raven D, van Oort F, et al. Mental health in Dutch adolescents: a TRAILS report on prevalence, severity, age of onset, continuity and co‐morbidity of DSM disorders. Psychol Med. 2015;45(2):345‐360. [DOI] [PubMed] [Google Scholar]
- 34. Zwaanswijk M, Verhaak PFM, van der Ende J, Bensing JM, Verhulst FC. Consultation for and identification of child and adolescent psychological problems in Dutch general practice. Fam Pract. 2005;22(5):498‐506. [DOI] [PubMed] [Google Scholar]
- 35. Gulliver A, Griffiths KM, Christensen H. Perceived barriers and facilitators to mental health help‐seeking in young people: a systematic review. BMC Psychiatry. 2010;10:113‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31(7):764‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eckshtain D, Ellis DA, Kolmodin K, Naar‐King S. The effects of parental depression and parenting practices on depressive symptoms and metabolic control in urban youth with insulin dependent diabetes. J Pediatr Psychol. 2010;35(4):426‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodman E. The role of socioeconomic status gradients in explaining differences in US adolescents' health. Am J Public Health. 1999;89(10):1522‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hagger V, Hendrieckx C, Cameron F, Pouwer F, Skinner TC, Speight J. Cut points for identifying clinically significant diabetes distress in adolescents with type 1 diabetes using the PAID‐T: results from diabetes MILES youth‐Australia. Diabetes Care. 2017;40(11):1462‐1468. [DOI] [PubMed] [Google Scholar]
- 40. Serlachius AS, Scratch SE, Northam EA, Frydenberg E, Lee KJ, Cameron FJ. A randomized controlled trial of cognitive behaviour therapy to improve glycaemic control and psychosocial wellbeing in adolescents with type 1 diabetes. J Health Psychol. 2016;21(6):1157‐1169. [DOI] [PubMed] [Google Scholar]


