Abstract
This study of real‐world data from the Maccabi database in Israel compared the risk of heart failure hospitalization (HHF) or death in patients with type 2 diabetes (T2D) initiating sodium‐glucose cotransporter‐2 (SGLT2) inhibitors versus other glucose‐lowering drugs (OGLDs) according to baseline left ventricular (LV) ejection fraction (EF). After propensity‐matching patients by baseline EF there were 10 614 episodes of treatment initiation; 57% had diabetes for >10 years, the mean glycated haemoglobin level was 66 mmol/mol (8.2%), ∼43% had cardiovascular disease, ∼7% had heart failure and ∼ 20% had chronic kidney disease. A total of 2876 patients (∼9%) had reduced EF (<50%). Over a mean follow‐up of 1.5 years there were 371 HHFs or deaths, 88 (23.7%) in patients with reduced EF. Initiation of SGLT2 inhibitors versus OGLDs was associated with lower risk of HHF or death overall (hazard ratio [HR] 0.57, 95% confidence interval [CI] 0.46‐0.70]; P < 0.001) and in patients with both reduced EF (HR 0.61, 95% CI 0.40‐0.93) and preserved EF (HR 0.55, 95% CI 0.43‐0.70), with no significant heterogeneity (P interaction = 0.72). Our findings from real‐world clinical practice show that the lower risk of HHF and death associated with use of SGLT2 inhibitors versus OGLDs is consistent in T2D patients with both reduced and preserved EF.
Keywords: diabetes, ejection fraction, heart failure hospitalization, mortality, real‐world evidence
1. INTRODUCTION
The term “diabetic cardiomyopathy” introduced in 1972 referred to left ventricular (LV) systolic dysfunction in the absence of coronary artery disease and hypertension, leading to heart failure (HF) in patients with diabetes. 1 We now recognize that HF may occur with preserved ejection fraction (HFpEF), without passing through a stage of reduced ejection fraction (HFrEF). Type 2 diabetes (T2D) is a known risk factor for all types of HF, including HFpEF. 2 Since there is, as yet, no therapy proven to improve outcomes in HFpEF, its prevention is of utmost importance.
Recently, large outcomes trials in patients with established HF, with or without diabetes, have demonstrated that sodium‐glucose cotransporter‐2 (SGLT2) inhibitors reduce the composite of HF hospitalization (HHF) or cardiovascular (CV) death. 3 , 4 However these completed HF trials exclusively included HFrEF (not HFpEF). In earlier CV outcomes trials in T2D, secondary analyses suggested that SGLT2 inhibitor treatment may reduce the risk of both HFrEF and HFpEF events, 5 and that the magnitude of effects on preventing HHFs and death among patients with pre‐existing HF may depend on baseline left ventricular ejection fraction (LVEF). 6 Yet the numbers of patients with LVEF assessed in these secondary analyses were relatively modest, and real‐world evidence on this topic has thus far been lacking.
Using data from routine clinical practice, we aimed to compare the risk of HHF or death in T2D patients starting SGLT2 inhibitors versus other glucose lowering drugs (OGLDs) according to baseline LVEF.
2. METHODS
Data were obtained from the Maccabi Health Management Organization (HMO) which was one of the contributors to the CVD‐REAL 2 database. The Maccabi HMO is the second largest health management organization in Israel and includes complete medical data for over 100 000 patients with T2D. 7 We included individuals who initiated treatment based on the first date medication was dispensed (index date) from April 2015 to June 2018, without a previously issued or filled prescription for that medication class during the preceding year, and who had documented LVEF measurements. LVEF was categorized as reduced (<50%) or preserved (≥50%) using the last measurement prior to the index date. Propensity scores for SGLT2 inhibitor initiation were developed separately for each baseline LVEF strata with 1:1 matching, based on age, gender, frailty, glycated haemoglobin (HbA1c), diabetes duration, estimated glomerular filtration rate, index year, history of CV disease (CVD) and CV risk factors, neuropathy, diabetic eye complications, bariatric surgery and baseline medications. Patients were followed from index date to the last date of data collection, or death. Hazard ratios (HRs) were assessed using Cox proportional hazards models, with an intention‐to‐treat approach.
3. RESULTS
Of 89 993 eligible patients, 32 365 (36%) had documented LVEF measurements. Patients with and without LVEF measurements had similar baseline characteristics. The median duration between LVEF measurement and index date was 2.6 (25th‐75th percentile: 1.0‐4.9) years. Overall, 2876 patients (∼9%) had EF <50%, and 1354 (∼4%) had EF <40%. After propensity‐matching, there were 10 614 episodes of treatment initiation; 57% had diabetes for >10 years, mean HbA1c was 66 mmol/mol (8.2%), ∼43% had established CVD, ∼7% had documented HF, and ∼20% had chronic kidney disease (stage 3 or worse; Table 1). Baseline characteristics were well balanced; none of the variables had a standardized difference >10% except socioeconomic status, which was slightly higher among patients receiving SGLT2 inhibitors. In the SGLT2 inhibitor group, ∼38% of treatment episodes were with dapagliflozin, and ∼62% with empagliflozin. In the OGLD group, 23% of treatment episodes were with dipeptidyl peptidase‐4 inhibitors, 18% with glucagon‐like peptide‐1 receptor agonists, 5% with metformin, 15% with insulin, 13% with sulphonylureas, 9% with thiazolidinediones, and the remainder with other agents.
TABLE 1.
Baseline characteristics after propensity‐score matching
| Characteristic | SGLT2 inhibitors N = 5307 | OGLDs N = 5307 | Standardized difference (%) a |
|---|---|---|---|
| Age, years | 64.4 (9.4) | 64.4 (10.7) | −0.3 |
| Years in diabetes registry | |||
| Mean (SD) | 11.1 (5.5) | 11.1 (5.5) | 0.9 |
| >10 years, n (%) | 3041 (57.3) | 3062 (57.7) | |
| Socioeconomic status b | 6.1 (1.8) | 5.9 (1.7) | 14.5 |
| Frailty c , n (%) | 707 (13.3) | 709 (13.4) | −0.1 |
| BMI, kg/m2 | 31.7 (5.4) | 31.6 (5.4) | 1.0 |
| Systolic blood pressure, mmHg | 133.3 (15.3) | 132.6 (15.4) | 4.7 |
|
HbA1c, mmol/mol HbA1c, % |
66 (16.4) 8.2 (1.5) |
66 (17.5) 8.2 (1.6) |
−19.7 −1.8 |
| eGFR, mL/min/1.73 m2 | 86.5 (24.3) | 87.0 (25.5) | −1.9 |
| eGFR <60 mL/min/1.73 m2, n (%) | 675 (12.7) | 697 (13.1) | −1.2 |
| Ejection fraction <50%, n (%) | 473 (8.9) | 473 (8.9) | 0 |
| Established CVD history, n (%) | 2322 (43.8) | 2190 (41.3) | 5.0 |
| Baseline glucose‐lowering medications, n (%) | |||
| Metformin | 4848 (91.4) | 4904 (92.4) | −3.9 |
| DPP‐4 inhibitors | 2689 (50.7) | 2666 (50.2) | 0.9 |
| Sulphonylureas | 1428 (26.9) | 1397 (26.3) | 1.3 |
| Insulin | 1488 (28.0) | 1424 (26.8) | 2.7 |
| GLP‐1RAs | 682 (12.9) | 603 (11.4) | 4.6 |
| Metiglinides | 613 (11.6) | 597 (11.2) | 0.9 |
| Thiazolidinediones | 276 (5.2) | 294 (5.5) | −1.5 |
| Acarbose | 133 (2.5) | 132 (2.5) | 0.1 |
| Antihypertensives, n (%) | 4521 (85.2) | 4523 (85.2) | −0.1 |
| ACE inhibitors | 2208 (41.6) | 2329 (43.9) | −4.6 |
| ARBs | 2233 (42.1) | 2065 (38.9) | 6.5 |
| Dihydropyridines (calcium channel blockers) | 1378 (26.0) | 1424 (26.8) | −2.0 |
| Low ceiling diuretics (thiazides) | 341 (6.4) | 376 (7.1) | −2.6 |
| Non‐hydropyridines (calcium channel blockers) | 83 (1.6) | 101 (1.9) | −2.6 |
| High ceiling diuretics (loop diuretics) | 525 (9.9) | 526 (9.9) | −0.1 |
| Aldosterone antagonists, n (%) | 363 (6.8) | 358 (6.7) | 0.4 |
| Beta blockers, n (%) | 2826 (53.3) | 2800 (52.8) | 1.0 |
| Statins, n (%) | 4495 (84.7) | 4475 (84.3) | 1.0 |
Note: Data are mean (SD), unless otherwise indicated.
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CVD, cardiovascular disease; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; OGLD, other glucose‐lowering drug; SGLT2, sodium‐glucose cotransporter‐2.
Standardized difference >10% is considered a statistically significant difference.
Based on a score ranked from 1 (lowest) to 10 (highest) derived for commercial purposes by Points Location Intelligence using geographic information systems and data such as expenditure related to retail chains, credit cards and housing; score is highly correlated with socioeconomic status as measured by the Central Bureau of Statistics in Israel.
≥1 hospitalization of ≥3 consecutive days during the year prior to index.
Over a mean follow‐up of 1.5 years, there were 371 HHFs or deaths, of which 88 (23.7%) occurred in patients with reduced EF. Initiation of SGLT2 inhibitors versus OGLDs was associated with a lower risk of HHF or death overall (HR 0.57, 95% confidence interval [CI] 0.46‐0.70; P < 0.001) and in patients with both reduced EF (HR 0.61, 95% CI 0.40‐0.93) and preserved EF (HR 0.55, 95% CI 0.43‐0.70; Figure 1), with no significant heterogeneity between LVEF strata (P interaction = 0.72). Use of SGLT2 inhibitors versus OGLDs was also associated with lower risk of HHF (HR 0.72, 95% CI 0.53‐0.98; P = 0.036) and death (HR 0.50, 95% CI 0.39‐0.66; P ≤ 0.001), with no significant heterogeneity between LVEF strata (P interaction = 0.97 for HHF and 0.34 for death, respectively). Effects were consistent when EF was dichotomized using the 40% threshold, as well as when restricting analyses to those with a history of HF or using EF measurements within 2 or 4 years of the index date.
FIGURE 1.
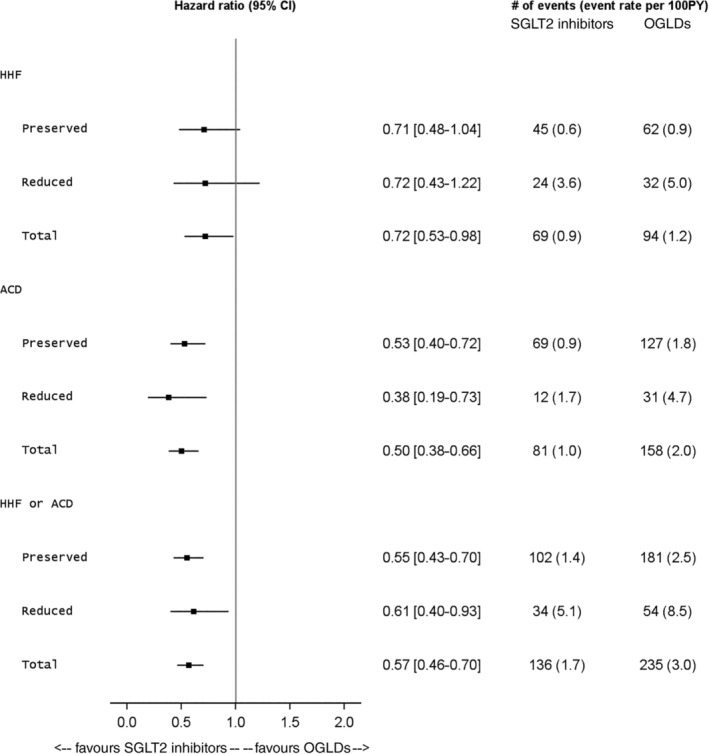
Forest plot of hazard ratios and incident rates demonstrating lower risks of heart failure hospitalizations (HHFs) and all‐cause death (ACD) with sodium‐glucose cotransporter‐2 (SGLT2) inhibitors versus other glucose‐lowering drugs (OGLDs) across those with either preserved (≥50%) or reduced (<50%) ejection fraction at index drug initiation. CI, confidence interval
4. DISCUSSION
Our findings from real‐world clinical practice demonstrate lower risk of HHF and death associated with the use of SGLT2 inhibitors versus OGLDs that is consistent in T2D patients with both reduced and preserved EF, and suggest that the HHF and mortality benefits of SGLT2 inhibitors may extend across the range of baseline EF. These results are consistent with recent HFrEF clinical trials in patients with and without diabetes, 3 , 4 , 8 including evidence of improvement in LV structure and function with SGLT2 inhibitors independent of diabetes. 8 Our results further suggest that the benefit may extend to HFpEF, at least in patients with diabetes, as the recent SOLOIST trial of sotagliflozin in HF also suggested. 9 Moreover, our results support and extend upon secondary analyses from the DECLARE‐TIMI 58 trial,6 showing that dapagliflozin reduced the primary composite endpoint of CV death or HHF to a greater extent in patients with HFrEF than in those without—a difference driven by significant reductions in CV death in patients with HFrEF but not in those without—yet with consistently lower risk of HHF irrespective of baseline EF, which is overall consistent with the present findings.
In CVD‐REAL, we did not have assessment of EF at the time of presentation of HHF; however, a subanalysis of the CANVAS trial retrospectively assessed the type of HF events (HFrEF or HFpEF) in the study using patients' medical records. 5 In CANVAS, 101 patients had a first HF event considered as HFpEF (EF ≥50%), 122 patients had a first event considered as HFrEF (EF <50%) and 61 patients had a first event with unknown EF. The overall risk of HF events was shown to be reduced with canagliflozin versus placebo. The HR for HFrEF events was 0.69 (95% CI 0.48‐1.00), for HFpEF events it was 0.83 (95% CI 0.55‐1.25), and for HF events with unknown EF it was 0.54 (95% CI 0.32‐0.89). In the sensitivity analysis where unknown EF events were assumed to be HFpEF, the updated HR for HFpEF events was 0.71 (95% CI 0.52‐0.97), and if the unknown EF events were assumed to be HFrEF events, the updated HR for HFrEF events was 0.64 (95% CI 0.48‐0.86). The authors therefore concluded that canagliflozin reduced the overall risk of HF events in patients with T2D and high CV risk, with no clear difference in effects on HFrEF versus HFpEF events. EF at baseline was unfortunately not available in that study.
The present study used de‐identified patient records from a large health plan, and included over 32 000 patients with LVEF measurements. However, it has some limitations. Following propensity‐score matching, 10 614 participants were included, and fewer than 10% of these patients had an EF <50% (some of whom had an intermediate range EF), resulting in a small overall number of patients with truly reduced EF. Additionally, as in all observational studies, despite using robust statistical techniques including propensity‐score matching, the possibility of residual unmeasured confounding cannot be excluded.
In conclusion, our findings from routine clinical practice suggest that the HHF and mortality benefits of SGLT2 inhibitors may extend across the range of baseline EF in patients with T2D. While both clinical trial and real‐world data support the efficacy of SGLT2 inhibitors for the prevention and treatment of HFrEF, the case with HFpEF regardless of T2D status remains unclear, and the results of ongoing clinical trials evaluating the use of SGLT2 inhibitors for treatment of manifest HFpEF are eagerly anticipated.
CONFLICTS OF INTEREST
C.S.P.L. reports research grants from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic and Vifor Pharma, has served as consultant or on the Advisory Board/ Steering Committee/ Executive Committee for Abbott Diagnostics, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Biofourmis, Boehringer Ingelheim, Boston Scientific, Corvia Medical, Cytokinetics, Darma Inc., Us2.ai, JanaCare, Janssen Research & Development LLC, Medtronic, Menarini Group, Merck, MyoKardia, Novartis, Novo Nordisk, Radcliffe Group Ltd., Roche Diagnostics, Sanofi, Stealth BioTherapeutics, The Corpus, Vifor Pharma and WebMD Global LLC, and is co‐founder and non‐executive director of Us2.ai. A.K. reports research grants from AstraZeneca, NovoNordisk and Boehringer Ingelheim, and honoraria from AstraZeneca, NovoNordisk and Boehringer Ingelheim. C.M.C. has nothing to disclose. M.A.C. reports research support (non‐salary) from AstraZeneca, Bristol Myers Squibb, Chiesi, GlaxoSmithKline, Novartis, NovoNordisk, Takeda and The Medicines Company, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Edwards Lifesciences, Janssen, Merck and Sanofi. S.K. reports honoraria from Bayer, Bristol Myers Squibb and AstraZeneca, other research support from Bayer and Daiichi Sankyo. A.N. reports honoraria from AstraZeneca, Boehringer Ingelheim, and MSD Sweden. M.T. is employed at Statisticon AB, a statistical consultant company, for which AstraZeneca is a client. H.C. is an employee of and has stock ownership in AstraZeneca. E.W. and P.F. are employees and have stock ownership in AstraZeneca. M.K. reports research grants from Boehringer Ingelheim and AstraZeneca, other research support from AstraZeneca, honoraria from Boehringer Ingelheim, AstraZeneca, Sanofi, Amgen, NovoNordisk, Merck (Diabetes), Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia, Novartis and Applied Therapeutics.
AUTHOR CONTRIBUTIONS
C.S.P.L., M.T., E.W., P.F. and M.K. were involved in the concept and design of the study. C.S.P.L., A.K., C.M.‐C., M.A.C., S.K., A.N. and M.L. were involved in data collection. All authors provided analysis and interpretation of data. C.M.‐C., M.T. and H.C. conducted the statistical analyses. C.S.P.L. drafted the manuscripts. All authors provided critical review of the draft and provided final approval for submission.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14356.
ACKNOWLEDGMENTS
Róisín O'Connor, inScience Communications, assisted with manuscript styling and submission, which was funded by AstraZeneca.
Lam CSP, Karasik A, Melzer‐Cohen C, et al. Association of sodium‐glucose cotransporter‐2 inhibitors with outcomes in type 2 diabetes with reduced and preserved left ventricular ejection fraction: Analysis from the CVD‐REAL 2 study. Diabetes Obes Metab. 2021;23:1431–1435. 10.1111/dom.14356
Funding information AstraZeneca, Grant/Award Number: N/A
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
REFERENCES
- 1. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595‐602. 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 2. Paulus WJ, Dal Canto E. Distinct myocardial targets for diabetes therapy in heart failure with preserved or reduced ejection fraction. JACC Heart Fail. 2018;6:1‐7. 10.1016/j.jchf.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995‐2008. 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 4. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413‐1424. 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 5. Figtree GA, Radholm K, Barrett TD, et al. Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes: results from the CANVAS program. Circulation. 2019;139:2591‐2593. 10.1161/CIRCULATIONAHA.119.040057. [DOI] [PubMed] [Google Scholar]
- 6. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528‐2536. 10.1161/CIRCULATIONAHA.119.040130. [DOI] [PubMed] [Google Scholar]
- 7. Chodick G, Heymann AD, Shalev V, Kookia E. The epidemiology of diabetes in a large Israeli HMO. Eur J Epidemiol. 2003;18:1143‐1146. [DOI] [PubMed] [Google Scholar]
- 8. Santos‐Gallego CG, Vargas‐Delgado AP, Requena‐Ibanez JA, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77:243‐255. 10.1016/j.jacc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 9. Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2020;384:117‐128. 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure


