Abstract
Endometriosis (EM) is defined as endometrial tissues found outside the uterus. Growth and development of endometriotic cells in ectopic sites can be promoted via multiple pathways, including MAPK/MEK/ERK, PI3K/Akt/mTOR, NF‐κB, Rho/ROCK, reactive oxidative stress, tumor necrosis factor, transforming growth factor‐β, Wnt/β‐catenin, vascular endothelial growth factor, estrogen, and cytokines. The underlying pathophysiological mechanisms include proliferation, apoptosis, autophagy, migration, invasion, fibrosis, angiogenesis, oxidative stress, inflammation, and immune escape. Current medical treatments for EM are mainly hormonal and symptomatic, and thus the development of new, effective, and safe pharmaceuticals targeting specific molecular and signaling pathways is needed. Here, we systematically reviewed the literature focused on pharmaceuticals that specifically target the molecular and signaling pathways involved in the pathophysiology of EM. Potential drug targets, their upstream and downstream molecules with key aberrant signaling, and the regulatory mechanisms promoting the growth and development of endometriotic cells and tissues were discussed. Hormonal pharmaceuticals, including melatonin, exerts proapoptotic via regulating matrix metallopeptidase activity while nonhormonal pharmaceutical sorafenib exerts antiproliferative effect via MAPK/ERK pathway and antiangiogenesis activity via VEGF/VEGFR pathway. N‐acetyl cysteine, curcumin, and ginsenoside exert antioxidant and anti‐inflammatory effects via radical scavenging activity. Natural products have high efficacy with minimal side effects; for example, resveratrol and epigallocatechin gallate have multiple targets and provide synergistic efficacy to resolve the complexity of the pathophysiology of EM, showing promising efficacy in treating EM. Although new medical treatments are currently being developed, more detailed pharmacological studies and large sample size clinical trials are needed to confirm the efficacy and safety of these treatments in the near future.
Keywords: endometriosis, pathophysiology, pathways, pharmaceuticals, targets, treatments
1. INTRODUCTION
1.1. Epidemiology and pathogenesis of endometriosis (EM)
EM is a disease caused by functional endometrial tissues growing in other areas outside the uterine cavity. It is a chronic disease that affects productivity and quality of life in women. 1 The typical presenting symptoms in women with EM include chronic pelvic pain, abnormal menstruation, and dyspareunia. EM occurs frequently in women of reproductive age, and the incidence is approximately 10%. 2 Approximately 40%–60% of women with EM experience dysmenorrhea, and 20%–30% are complicated with infertility. 3
Although EM presents as benign clinical and pathological manifestations, it has similar characteristics to cancers, including dissemination, invasion, and hyperplasia. It is generally accepted that EM is a hormone‐dependent disease. 4 Estrogen (E2) augmentation and progesterone resistance feature EM pathology, but the mechanism of how this occurs is unclear. Nevertheless, EM has been observed even in the absence of increased E2 production in postmenopausal women. 5 The pathogenesis of EM is dominated by the theory of ectopic implantation of the endometrium, along with multiple factors, such as endocrine, immunity, invasion, and angiogenesis. Retrograde menstruation theory suggests reflux of endometrial tissue through the fallopian tubes during menstruation and implantation into the peritoneal cavity.6, 7 Lymphatic and vascular dissemination theories suggest that endometrial cells disseminate via lymphatic or blood circulation. 8 Stem cell origin theory suggests that undifferentiated peritoneal tissue, ovarian surface epithelial tissue, and endometrium mesenchymal stem cells transform into endometrial‐like tissue in response to retrograde menstrual blood flow and stimulation from chronic inflammatory factors. 9
EM development is also associated with a combination of genetic variation and environmental factors. First‐degree relatives of women with EM have a seven fold greater risk of developing EM than those without a family history, and the risk of developing the disease in identical twins of women with EM is as high as 75%.10, 11 In recent years, the increased incidence of EM is also thought to be associated with exposure to environmental pollutants. Tetrachlorodibenzo‐p‐dioxin (TCDD) is the most prevalent air pollutant worldwide, and it promotes cytokine secretion. Endogenous E2 exacerbates the effects of TCDD and the interaction of the two chemicals provokes inflammatory responses, induces toxicity, and thus increases the severity of EM.12, 13, 14 Therefore, the pathophysiology of EM is complex, interrelated, and specific, thereby requiring multiple targeted therapies.
1.2. Dysregulated molecular and signaling pathways
Regardless of EM theories, endometrial cells must complete a serial process of immune escape, survival, adhesion, invasion, and angiogenesis to develop and grow in the ectopic sites. 15 Signaling pathway refers to a series of enzymatic reaction pathways that pass molecular signals into cells through the cell membrane to exert corresponding effects. EM‐related signaling pathways, together with their upstream and downstream regulatory factors, constitute a large and complex transduction system and play an important role in the occurrence and development of EM. Abnormalities in these pathways and their interactions can lead to abnormal proliferation, apoptosis, autophagy, adhesion, invasion, fibrosis, angiogenesis, reactive oxidative stress (ROS), immune system, and inflammatory responses of the ectopic endometrial tissues, thereby promoting its growth and development. Hormonal‐related enzymes, growth factors, inflammatory cytokines and chemokines, such as tumor necrosis factor (TNF)‐α, transforming growth factors (TGF)‐β, prostaglandin E2 (PGE2), prostaglandin‐endoperoxide synthase (COX)2 play important roles in these processes.16, 17 They induce local immune imbalance in the microenvironment to tolerate immune clearance and promote the survival of ectopic lesions. Downstream molecules, such as hypoxia‐inducible factors (HIF)‐1α, matrix metallopeptidase (MMPs), and vascular endothelial growth factors (VEGFs), are dysregulated and play roles in the angiogenesis and growth of EM lesions.2, 15, 16, 17
1.3. Current treatment of EM
Current treatments for EM include surgical and medical therapies. Conservative surgery removes the EM deposits but increases the risk of impairing ovarian reserve, harming other organs, and imposing postoperative recurrence. 18 Therefore, medical therapy (Table 1) always comes first into consideration, and the choices depend on multiple factors, such as symptom severity, conceive desire, and comorbidities. Generic classes of medical therapies for EM include hormonal therapy, including oral contraceptives (COC), progesterone and gonadotropin‐releasing hormone (GnRH) agonist and antagonist, and nonhormonal therapies such as nonsteroidal anti‐inflammatory drugs (NSAIDs).
Table 1.
Current FDA‐approved medication for endometriosis treatment
| Medication | Generic name | Rank | Market name a | Price range a b | Administration a | Mechanism of action | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Combinations of ethinyl estradiol + norgestimate (3rd‐generation progestin) | Estrogen and progestin (COC) | 1st linec | Previfem | $ | Oral tablet | Suppresses ovarian activity, and reduces estrogen‐induced production of prostaglandins and inflammation. |
|
|
[19, 20] |
| Tri‐Previfem | |||||||||
| Sprintec | |||||||||
| Tri‐Sprintec | |||||||||
| Estarylla | |||||||||
| Mono‐Linyah | |||||||||
| Tri‐Lo‐Sprintec | |||||||||
| Tri‐Estarylla | |||||||||
| Tri‐Linyah | |||||||||
| Tri‐Lo‐Marzia | |||||||||
| Combinations of ethinyl estradiol + norethindrone (1st‐generation progestin) | Femhrt (Jinteli) | $$ | |||||||
| Jevantique Lo (Fyavolv) | |||||||||
| Combinations of estrogen + ethynodiol diacetate (1st‐generation progestin) | Zovia 1/35E | $ | |||||||
| Zovia 1/50E | |||||||||
| Kelnor 1/50 | |||||||||
| Kelnor 1/35 | |||||||||
| Progestin | NETA (1st‐generation progestin) | 2nd line | Camila | $$ | Oral tablet | Multiple mechanisms of actions that include 1. suppress ovulation and E2 level results in endometrial thinning, 2. Induce endometrium decidualization and inhibit estrogen, results in atrophy of lesions. |
|
|
[21, 22] |
| Nora‐Be | |||||||||
| Ortho Micronor | |||||||||
| Errin | |||||||||
| Jolivette | |||||||||
| Sharobel | |||||||||
| Jencycla | |||||||||
| Deblitane | |||||||||
| Incassia | |||||||||
| Norlyda | |||||||||
| Norlyroc | |||||||||
| Heather | |||||||||
| Lyza | |||||||||
| Aygestin | $$$ | ||||||||
| Medroxyprogesterone acetate | 2nd line | Depo‐Provera | $$ | Intramuscular injection | |||||
| Provera | $$ | Oral tablet | |||||||
| Dienogest (4th‐generation progestin) | 2nd line | Visanne | $$ | Oral tablet | |||||
| Levonorgestrel‐Releasing Intrauterine Device | 2nd or 3rd line | Mirena | $$$ | Intrauterine system | |||||
| GnRH agonist | Nafarelin acetate | 2nd or 3rd line | Synarel | $$$$$ | Nasal Spray | Initial pituitary flare effect results in stimulation of pituitary LH and FSH, that deregulates pituitary GnRH receptor, suppresses pituitary secretion of LH and FSH, suppresses ovulation, mimic menopause sate and results in low circulating E2 and P4, leads to shrinkage of endometrium. |
|
|
[22] |
| Goserelin | Zoladex | $$$ | subcutaneous injection | ||||||
| 17‐ethinyl testosterone derivative | Danazol | NA | Danocrine | $$$$ | Oral tablet | Increases testosterone levels to inhibit pituitary gonadotropin secretion and E2 production by P450AROM and other enzymes. |
|
|
[22, 23] |
| GnRH antagonist | Elagolix | NA | Orilissa | $$$$ | Oral tablet | Directly blocks GnRH receptor on the pituitary gland to rapidly suppresses FSH, LH, and gonadal sex steroid production. |
|
|
[24, 34] |
| LHRH agonist | leuprolide | NA | Lupron Depot | $$$$$ | Intramuscular injection | Overstimulates production of LH and disrupt endogenous hormonal feedback systems to reduce LH and gonadal sex steroid production. |
|
|
[25] |
| Combinations of LHRH agonist and progesterone (1st‐generation progestin) | Leuprolide and Norethindrone | NA | Lupaneta Pack | $$$$ | Intramuscular injection | Suppresses Gonadotrope secretion of LH and FSH to reduce gonadal sex steroid production. |
|
|
[25] |
Abbreviations: COC, combined oral contraceptive; E2, estradiol; FDA, Food and Drug Administration; FSH, follicle‐stimulating hormone; GnRH, gonadotropin‐releasing hormone; LH, luteinizing hormone; LHRH, luteinizing hormone‐releasing hormone; NETA, norethindrone acetate; NSAID, nonsteroidal anti‐inflammatory drug; P4, progesterone; P450AROM, aromatase.
Price range was justified based on 3‐months therapy. $ denotes the approximate price range and are labelled as follows, $ (<$100); $$ ($100–$499); $$$ ($500–$1999); $$$$ ($2000–$4999); $$$$$ (>$5000).
Medication is usually prescribed together with NSAIDs.
The available reports on the effectiveness of NSAIDs on pain relief in EM are very limited, and there is no strong evidence to support a conclusion. 1 Among all medical treatments, combined COC and progestin monotherapy represent the first‐line therapy, which can be applied to most women clinically diagnosed with EM with or without a surgical diagnosis. 26 Continuous COC effectively reduces the recurrence of dysmenorrhea, 27 and progestin suppresses ovulation by maintaining a hypoestrogenic state. Women with risk factors such as thrombosis and myocardial infarction may tolerate the side effects of progestin better than those of COC. 28 To date, few derivatives of progesterone, namely, depot medroxyprogesterone acetate and norethindrone acetate, have been approved by the US Food and Drug Administration (FDA) as the sole therapy for EM.29, 30
Although GnRH is an effective hormonal treatment for EM, severe hypoestrogenic symptoms limit long‐term compliance.31, 32 GnRH agonists are second‐line hormonal therapies that exert strong action on the GnRH receptor, leading to an initial short stimulation and subsequent suppression of gonadotropin secretion. Decreased hormone levels result in the dormancy of endometriotic lesions. Owing to its long‐term adverse effects, especially osteoporosis, an add‐back therapy is recommended. 33 Recently, the FDA approved elagolix, a nonpeptide small molecule GnRH receptor antagonist that suppresses luteinizing hormone and follicle‐stimulating hormone and correspondingly reduces E2 and progesterone, as a treatment for moderate to severe EM‐associated pain. Its efficacy was shown after a 6‐month treatment, but it also caused a significant decrease in bone mineral density as the main side effect. 34 To overcome EM refractory to current hormonal treatments and NSAIDs, there have been extensive research of new medicines in recent years. Other than therapeutic efficacy, the potential use of a drug as a preventive treatment after surgery is also desirable. The recurrence of EM and the associated symptoms within 5 years after laparoscopy is approximately 19% in patients with endometrioma, 35 and up to 10% of women require secondary surgery after 1 year, 36 emphasizing the need for new medical treatments to prevent a recurrence.
In summary, to identify and develop new pharmaceuticals for EM treatment, understanding the dysregulated molecular and signaling pathways in EM development is essential (Figure 1). Numerous studies have focused on the antiproliferation mechanism and related targeted therapies in EM models and/or endometrial cells.37, 38 Owing to the interaction of different signaling pathways, the efficacy of potential pharmaceuticals in promoting or inhibiting a single signaling pathway is often very limited. Therefore, pharmaceutical targeting multisignaling pathways in EM has become important in the medical treatment of EM. An overview of the molecular pathways involved in the pathophysiology of EM has been reported by various publications,39, 40, 41 which provides a high quality evidence of the underlying pathophysiology of EM. However, previous publications only focused on currently available pharmaceuticals. In this review, we aimed to present an updated summary of studies focusing on new potential pharmaceuticals, including preclinical studies, clinical trials, as well as studies on marketed pharmaceuticals. In‐depth studies of signaling pathways targeted by pharmaceuticals are currently an emerging research direction, which will open up broad prospects for the new generation of EM treatment.
Figure 1.
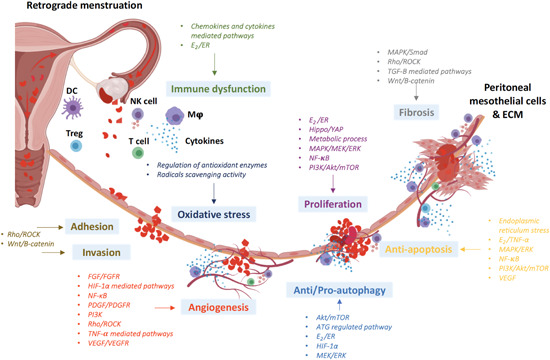
Pathophysiology of endometriosis. The schematic diagram was created using BioRender.com. Akt, protein kinase B; ATG, autophagy‐related genes; DC, dendritic cells; E2, estrogen; ECM, extracellular matrix; ER, estrogen eceptor; ERK extracellular signal‐regulated kinase; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptors; HIF, hypoxia‐inducible factors; MΦ, macrophages; MAPK, mitogen‐activated protein kinase; MEK, ERK kinase; mTOR, mammalian target of rapamycin; NF‐κB, nuclear factor κB; NK, natural killer; PDGF, platelet‐derived growth factor; PDGFR, platelet‐derived growth factor receptor; PI3K, phosphoinositide 3‐kinases; Rho, Ras homolog family; ROCK, Rho‐associated coiled‐coil kinase; VEGF, vascular endothelial growth factor; TGF, transforming growth factor; TNF, tumor necrosis factor; Treg, regulatory T cells; Wnt, wingless‐type mouse mammary tumor virus integration site family; YAP, Yes‐associated protein
2. DEFINITION OF POTENTIAL PHARMACEUTICALS FOR EM TREATMENT
2.1. Experimental evidence in vitro and in vivo
The choice of investigation models considerably influences the translational potential of preclinical research. Endometriotic and endometrial tissue cells with specific cell characteristics, defined by their morphology and phenotypes, confirmed by immunocytochemistry allow in vitro investigations of the mechanism of hormonal expression, cytokine secretion, cell proliferation, and differentiation. 42 Romano et al. 43 critically analyzed different EM culture models of samples from peritoneal, ovarian, and deep infiltration EM and recommended a guideline for assessing the quality of both primary endometriotic cells and immortalized endometriotic cell lines. Culture conditions can imitate EM in situ; for example, endometrium undergoing menstruation, 44 macrophage activation, 45 epithelium mesothelium transformation, 46 and cell–cell interactions.47, 48 In addition, in vivo animal experiments provide a biological system with an integrative environment and complete cellular and molecular network for lesion development and growth in vivo. It mimics the conditions in humans in the hopes that the results can be translated from bench to clinic. The application and limitations of various EM animal models, including autotransplantation of uterine tissues and xenotransplantation of human endometrial tissues into the peritoneal cavity or subcutaneous pocket in ectopic sites of rodent models, as well as in the primate model have been assessed, and the choice of the appropriate model for studies depends on the research questions. 49
Apart from the appropriate model, positive control of current pharmaceuticals should be included for comparison, which will serve as experimental evidence of the efficacy of new drugs. When choosing a positive control, pharmaceuticals with relevant actions to the examined molecular and signaling pathways should be considered. For example, dienogest can be used as a positive control to compare the inhibition of NF‐κB activation, enhancement of apoptosis, or inhibition of MMP‐2/‐9,50, 51 leuprolide acetate to compare the inhibition of promitogenic cytokines, 52 and celecoxib to compare the proliferation‐inhibitory and apoptosis‐enhancing effects. 53
2.2. Pharmacokinetics, pharmacodynamics, and safety profile
In addition to the efficacy, the pharmacokinetic profile of a drug with respect to absorption, distribution, metabolism, and excretion should be available to support its clinical use. 54 The bioavailability of a drug and its active metabolites in systematic circulation and local tissues should be quantified to justify the therapeutic dosage for clinical application. 55 The relationship between drug potency and pharmacological effects on the body and action site should be evaluated to prevent off‐target toxicities. 56 The possible adverse effects on other tissues also need to be determined. Medications with specific efficacy on the ectopic endometrium and minimal side effects on the eutopic endometrium are preferable for EM treatment, as these medications will affect reproductive cycles the least. In animal experiments, adverse effects on reproductive tissues and functions should be carefully monitored. As a short‐term measure, no significant change in body weight should occur in the test animals, and as a long‐term measure, the animals should be able to conceive and deliver. For women with EM who prefer symptomatic medical therapy, such side effects should be limited and well‐tolerated. Medicines that regulate E2 levels usually result in hypoestrogenism and are associated with side effects such as hot flushes and vaginal dryness, which are acceptable, but not preferable. 57 Other common adverse effects, such as osteoporosis and venous thromboembolism, should be avoided. The effect of the medications on fertility should also be monitored; however, the current data are very limited.
2.3. Cost‐effectiveness analysis
Several studies have systematically recorded the direct and indirect costs of EM treatment and highlighted its long‐term economic burden on the society, healthcare system, and affected women. 58 This has raised awareness of the disease and increased the demand for cost‐effective EM drugs. However, the choice of treatment depends not only on patients’ desired outcomes but also on treatment affordability. A cost‐effective medication is that with equivalent monetary value and efficiency. Therefore, as the most cost‐effective treatment for EM is considered for use as a standard hormonal treatment, 59 a potential new pharmaceutical should be affordable and easily accessible to the market, in addition to showing good efficacy with fewer side effects.
In summary, a potential new pharmaceutical should be well‐studied in terms of not only action mechanism and efficacy in vitro and in vivo, but also safety, efficiency, and cost‐effectiveness. Progress in this area is expected to provide clear and effective insights for policy‐making and for decision‐making in the individualized treatment of EM.
3. POTENTIAL NEW PHARMACEUTICALS AND THEIR TARGET‐SIGNALING PATHWAYS
Medications investigated in ongoing or completed clinical trials on EM are summarized in Table 2. Most drugs are mainly symptomatic. Outcome measures used in these studies are pain score, levels of dysmenorrhea and dyspareunia, and quality of life, except for epigallocatechin gallate (EGCG) and quinagolide, whose efficacy in reducing endometriotic lesions will be determined. To the best of our knowledge, there is limited clinical trial to examine the pathophysiology or signaling pathways targeted by the drugs. Moreover, heterogeneous pathophysiology among patients affects their responsiveness to drug treatment; therefore, the development of personalized medicines to specific patients based on EM pathophysiology is desirable. 39 These further emphasizes the demand for new pharmaceutical that is for symptomatic management, as well as targets specific pathophysiology and signaling pathways to eliminate the endometriotic lesions.
Table 2.
Pharmaceuticals under clinical trials within 2015–2025 for endometriosis (EM) treatmenta
| NCT number | Study completion | Phase | Study locations or centers | Medication | Control | Study aim | Outcome measuresb |
|---|---|---|---|---|---|---|---|
| NCT01769781 | Jul‐15 | Phase 4 | Italy | Anastrazole plus GnRH‐agonist | GnRH analog | Efficacy of drug for EM recurrence | Pelvic pain |
| NCT01767090 | Jul‐15 | Phase 2 | Belgium, Japan, United Kingdom, and so forth | ASP1707 | Placebo and Leuprorelin acetate | Safety and efficacy of drug in different doses for EM‐associated symptoms | Pelvic pain, dysmenorrhea, dyspareunia, adverse events, bleeding pattern |
| NCT01779232 | Sep‐15 | Phase 4 | Italy | Danazol | Placebo | Efficacy of drug for EM‐related infertility | Fertilization outcome |
| NCT01822080 | Nov‐15 | Phase 3 | China | Dienogest | Placebo | Efficacy of drug for EM‐related symptoms in Chinese Patients | Pelvic pain, dysmenorrhea, adverse events, bleeding pattern |
| NCT02475564 | Nov‐15 | Phase 4 | Brazil | Resveratrol | Placebo | Efficacy of drug for EM‐related pain | Pelvic pain, CA125, and prolactin marker |
| NCT01712763 | Mar‐16 | Phase 3 | Italy | Degarelix | Goserelin | Efficacy of drug for EM recurrence | Pelvic pain |
| NCT01760954 | Apr‐16 | Phase 3 | AbbVie Inc. | Elagolix | N/A | Long‐term safety and efficacy of drug for EM‐related symptoms | Pelvic pain, dysmenorrhea, dyspareunia, quality of life, adverse Events |
| NCT02534688 | May‐16 | Phase 4 | Thailand | LNG‐IUS and DMPA | N/A | Efficacy of drug for EM‐related symptoms | Pelvic pain, quality of life, hormone profile |
| NCT02387931 | Jul‐16 | Phase 4 | United States | Vitamin D3 and Fish Oil | Placebo | Efficacy of drug for adolescent girls with EM‐related symptoms | Pelvic pain, quality of life |
| NCT02427386 | Dec‐16 | Phase 4 | University of Sao Paulo General Hospital | Dynamized estrogen | Placebo | Efficacy of drug for EM‐related symptoms | Pelvic pain |
| NCT01931670 | Dec‐16 | Phase 3 | AbbVie Inc. | Elagolix | Placebo | Safety and efficacy of drug for EM‐related symptoms | Pelvic pain, dysmenorrhea, dyspareunia, quality of life |
| NCT01728454 | Mar‐17 | Phase 2 | United States | Telapristone acetate | Placebo | Safety and efficacy of drug for EM‐related symptoms in premenopausal women | Pelvic pain, dysmenorrhea, dyspareunia |
| NCT02143713 | May‐17 | Phase 3 | AbbVie Inc. | Elagolix | Placebo | Long‐term safety and efficacy of drug for EM‐related symptoms | Pelvic pain, dysmenorrhea, dyspareunia, quality of life, adverse Events |
| NCT02480647 | Aug‐17 | Phase 4 | Brazil | Levonorgestrel and Etonogestrel | N/A | Efficacy of drug for EM‐related symptoms | Pelvic pain, bleeding pattern |
| NCT02542410 | Sep‐18 | Phase 2 | United States | Cabergoline | Norethindrone acetate | Efficacy of drug for EM‐related symptoms | Pelvic pain |
| NCT02778399 | Jul‐19 | Phase 2 | United States, Poland, Russian Federation, Ukraine, and so forth | OBE2109 | Placebo | Efficacy and safety for EM‐related symptoms | Pelvic pain, dysmenorrhea, dyspareunia, dyschezia, quality of life, adverse events |
| NCT01553201 | Jul‐19 | Phase 1|Phase 2 | United States | Botulinum Toxin | Placebo | Efficacy of drug for EM‐related symptoms | Pelvic pain |
| NCT03232281 | Nov‐19 | Phase 3 | China | Triptorelin Pamoate PR 3‐month and Triptorelin Acetate PR 1‐month | N/A | Efficacy and safety of drug for EM‐related symptoms in Chinese patients | Pelvic pain, percentage of subjects castrated, hormones profile |
| NCT03340324 | Dec‐19 | Phase 2 | Mongolia | V‐Endo | N/A | Efficacy of drug for EM‐related symptoms | Pelvic pain, complete blood count, liver and kidney function |
| NCT03352076 | May‐20 | Phase 2 | Italy | Vaginal danazol and oral danatrol | N/A | Concentration of drug for EM‐related symptoms | Danazol concentration |
| NCT03654326 | Jun‐20 | Phase 2 | United States, Australia, Chile, New Zealand, and so forth | Gefapixant | Naproxen and Placebo | Efficacy and safety of drug for EM‐related symptoms | Pelvic pain, adverse events |
| NCT03931915 | Sep‐20 | Phase 3 | Japan | TAK‐385 and leuprorelin acetate | N/A | Efficacy and safety of drug for EM‐related symptoms | Pelvic pain, dyspareunia, adverse events, serum concentrations, menstrual pain |
| NCT03573336 | Oct‐20 | Phase 2 | United States, Austria, Canada, Japan, and so forth | Vilaprisan | Placebo | Efficacy and safety of drug for EM‐related symptoms | Pelvic pain, adverse events, clinical imaging assessments |
| NCT02832271 | Dec‐20 | Phase 2 | Hong Kong | SUNPHENON EGCG | Placebo | Efficacy and safety of drug for EM‐related symptoms and lesion size | Pelvic pain, lesion size, quality of life, adverse events |
| NCT03782740 | Feb‐21 | Phase 2 | Sweden | Melatonin | Placebo | Efficacy and safety of drug for EM‐related symptoms | Pelvic pain, quality of life, adverse events, acceptance of melatonin |
| NCT03204331 | Mar‐21 | Phase 3 | United States, Australia, Brazil, Chile, and so forth | Relugolix | Estradiol/norethindrone acetate, Placebo | Efficacy and safety of drug for EM‐related symptoms | Pelvic pain, dysmenorrhea, dyspareunia, quality of life, adverse events, hormone profiles |
| NCT03204318 | |||||||
| NCT03749109 | May‐21 | Phase 2 | Denmark, Germany, Italy, Poland, and so forth | Quinagolide | Placebo | Efficacy and safety of drug for EM‐related symptoms and lesion size | Lesion size, dysmenorrhea, quality of life, adverse events, clinical imaging assessments |
| NCT01942122 | Jun‐21 | Phase 2|Phase 3 | Indonesia | DLBS1442 | Mefenamic acid | Efficacy of drug for EM‐related symptoms | Pelvic pain, quality of life, adverse events, inflammatory markers, hormone profile |
| NCT03840993 | Aug‐21 | Phase 2 | United States | MT‐2990 | Placebo | Efficacy and safety of drug for EM‐related symptoms | Pelvic pain |
| NCT04256200 | Dec‐21 | Phase 2|Phase 3 | Lebanon | Dienogest 2‐mg oral tablet | Oral Contraceptive Pills | Efficacy and safety of drug for EM‐related symptoms | Pelvic pain, quality of life, adverse events, patient tolerability |
| NCT03991520 | Jan‐22 | Early Phase 1 | United States | Anakinra 100‐mg/0.67‐ml Inj Syringe | Placebo | Efficacy for EM‐related symptoms (pilot study) | Pelvic pain, dysmenorrhea, dyspareunia, quality of life, serum inflammatory markers |
| NCT03992846 | Jul‐22 | Phase 3 | United States, Austria, Bulgaria, Czechia, and so forth | Linzagolix | Placebo | Efficacy of drug for EM‐related symptoms | Pelvic pain, dysmenorrhea, dyspareunia, dyschezia |
| NCT03986944 | |||||||
| NCT03654274 | Dec‐22 | Phase 3 | United States, Argentina, Australia, Belgium, and so forth | Relugolix | Estradiol/norethindrone acetate | Efficacy and safety of drug for EM‐related symptoms | Pelvic pain, dysmenorrhea, dyspareunia, adverse events, hormone profile |
| NCT03213457 | Jan‐23 | Phase 3 | United States, Canada, Puerto Rico | Elagolix, estradiol/norethindrone acetate | Placebo | Efficacy and safety of drug for EM‐related symptoms | Pelvic pain, dysmenorrhea, dyspareunia |
| NCT03928288 | Feb‐23 | Phase 2 | United States | Cabergoline | Placebo | Efficacy of drug for EM‐related symptoms | Pelvic pain, dysmenorrhea, serum angiogenesis and inflammatory biomarkers, adverse events |
| NCT03970330 | May‐23 | Phase 3 | United States | Naltrexone and norethindrone acetate | Placebo | Efficacy of drug for EM‐related symptoms | Pelvic pain |
| NCT03692403 | Aug‐23 | Phase 2 | United States | Quinagolide | Placebo | Efficacy of drug for EM‐related symptoms | Pelvic pain, dysmenorrhea, dyspareunia, quality of life, bleeding pattern, adverse events |
All information was taken from the US National Library of Medicine, ClinicalTrials.gov, only completed or active clinical trials, and EM treatment as the primary study purpose between 2015 and 2025 are included.
Selected outcome measures are shown.
Here, we discuss the pathophysiology and molecular targets that are directly or indirectly associated with the drugs, as well as their effects on the corresponding signal transduction pathways in the treatment of EM. In Table 3, we distinguished potential drugs as a repurposed or a de novo drug of EM. A new drug is defined as a chemical that has not been studied in clinical trials for other diseases before EM and a repurposed drug is defined as a chemical that has been studied in clinical trials for other diseases before EM. We provided sufficient scientific data of their efficacies in reducing endometriotic cell viability in vitro or lesions in vivo, as well as in regulating specific signaling pathways and molecules involved in the pathophysiology of EM. The advantages, side effects, and limitations of the drugs are also highlighted.
Table 3.
Pathways and molecular targets of current and potential pharmaceuticals for endometriosis treatment
| Pathways | Molecular targets | Pathophysiology | Medication | PK and toxicity profile (accession number if available)a | Drug development approachb | Original purpose of drugs before EM (phase, start/stated year)c | Type/Classd | Clinical stages as EM treatment |
|---|---|---|---|---|---|---|---|---|
| Current medication | ||||||||
| COX‐2 | COX‐1, COX‐2, PPAR‐γ | Proliferation | Indomethacin | PK/toxicity (DB00328)h | Repurpose | Anti‐inflammatory Agent (since 1963) | Nonhormone, NSAID | Preclinical, off‐label prescription |
| COX‐2/PGE2, COX‐2/VEGF | COX‐2 | Proliferation and apoptosis | Celecoxib | PK/toxicity (DB00482)h | Repurpose | Arthritis (IV, 2002) | Nonhormone, NSAID | Preclinical, off‐label prescription |
| NF‐κB | TNF‐α | Proliferation and inflammation | GnRH agonist | PK/toxicity (DB11979, DB00050)h | Repurpose | Contraceptive agents (since 1978) | Hormone, GnRH agonist | Phase 4, on‐label prescription |
| TNFα/NF‐κB | NF‐κB | Proliferation and inflammation | Progesterone or dienogest or danazol | PK/toxicity (DB00396, DB09123, DB01406)h | Repurpose | NA | Hormones, progestogen & contraceptives | Phase 4, on‐label prescription |
| VEGF and IL‐8 mediated apoptosis | GnRH | Apoptosis and inflammation | Leuprolide acetate | PK/toxicity (DB00007)h | Repurpose | Prostate cancer (since 1985) | Hormone, GnRH agonist | Phase 4, on‐label prescription |
| PI3K/Akt/mTOR and MEK1/2/ERK1/2 | AKT and ERK1/2 | Apoptosis and autophagy | Dienogest | PK/toxicity (DB09123)h | Repurpose | Oral contraceptive (III, 2003) | Hormone, progestogen | Phase 3, on‐label prescription |
| Antiproliferation and proapoptotic agents | ||||||||
| CASP and apoptotic proteins effects | NF‐κB, IκB, JNK, p38 MAPK | Proliferation and apoptosis | BAY11‐7085 | No information | New | NA | Nonhormones, NF‐κB inhibitor | Preclinical |
| CASP | MMP‐3 | Apoptosis | Melatonin | PK/toxicity (DB01065)h | Repurpose | Insomnia (IV, 2007), chemotherapy‐induced toxicity (III, 2007), prevention of lung cancer (III, 2007) | Hormone, miscellaneous anxiolytics, sedatives, and hypnotics | Phase 2 |
| E2/ER | ERβ | Proliferation, inflammation, angiogenesis, and apoptosis | Chloroindazole | No information | New | NA | Nonhormones, NA | Preclinical |
| E2/ER | ERα | Proliferation, inflammation, angiogenesis, and apoptosis | Oxabicycloheptene sulfonate | PK (DB04574)h | New | NA | Nonhormone, NA | Preclinical |
| E2/ER | ESR1 | Proliferation | Resveratrol | PK/toxicity (drugs.com/resveratrol.)i | Repurpose | Inflammation in type 2 diabetic patients (III, 2013), Anti‐inflammatory and antioxidant effects (III, 2011) | Natural products, phytoalexin | Phase 4 |
| E2/ER/VEGF | / | Proliferation and angiogenesis | EGCG | PK 67 /toxicity 68 | Repurpose | Multiple sclerosis (III, 2009), cervical cancer (II, 2005), prostate cancer (II, 2004) | Natural products, catechin | Phase 2 |
| ER stress | TRAIL | Apoptosis | Tunicamycin | Toxicity 71 | New | NA | Nonhormones, antibiotic | Preclinical |
| Hypoxia/LATS1/YAP1 | YAP1 | Proliferation, angiogenesis, and migration | Verteporfin | PK/toxicity (DB00460)h | Repurpose | Neovascular macular degeneration (IV, 2014), polypoidal choroidal vasculopathy (IV, 2008) | Nonhormone, photosensitizing agent | Preclinical |
| NF‐κB | TNF‐α‐induced effect | Apoptosis and angiogenesis | Ginsenoside Rg3 | PK 76 /Toxicity 77 | Repurpose | Endothelial Function (II, 2007) | Natural product, Steroid glycoside | Preclinical |
| p53/NF‐κB | MMP‐3 | Apoptosis | Curcumin | PK/toxicity (DB11672)h | Repurpose | Inflammation in endometrial carcinoma (II, 2013), irritable bowel syndrome (IV, 2008) | Natural products, curcuminoid | Recruiting |
| NF‐κB and COX‐2 | TGF‐β | Apoptosis | Genistein | PK 82 | Repurpose | Endothelial function (III, 2010), vascular and skeletal protective in menopause women (III, 2003), prostate cancer (III, 2003) | Natural product, isoflavone | Preclinical |
| RAF/MEK/ERK and VEGF/VEGFR | RAF and VEGFR | Proliferation, inflammation, and angiogenesis | Sorafenib | PK/toxicity (DB00398)h | Repurpose | Hepatocellular carcinoma (IV, 2010) carcinoma, renal cell (III, 2003) | Nonhormone, multikinase inhibitor | Preclinical |
| MAPK/ERK | BARF | Proliferation and apoptosis | Vemurafenib | PK/toxicity (DB08881)h | Repurpose | Malignant Melanoma (III, 2010) | Nonhormone, kinase inhibitor | Preclinical |
| MAPK/PR | MEK1/2 | Proliferation and apoptosis | U0126 | PK 90 | New | NA | Nonhormone, MAPK/ERK kinase | Preclinical |
| MAPK/ERK1/2 | ERs | Proliferation | Puerarin | PK 92 | Repurpose | Alcohol abuse (II, 2009) | Natural product, isoflavonoid | Preclinical |
| EGFR/ERK1/2, AKT, B‐catenin, NF‐κB | EP2 and EP4 receptors | Apoptosis | PGE2 inhibitors | PK/toxicity (DB00917)h | Repurpose | Cataracts (IV, 2007) | Nonhormone, PGE2 inhibitors | Preclinical |
| mTOR/Akt | CB1 or CB2 | Proliferation, fibrogenesis, and oxidation | WIN 55212‐2 | PK 96 | New | NA | Nonhormone, cannabinoid receptor agonist | Preclinical |
| Akt/PR | AKT | Proliferation and apoptosis | MK2206 | No information | Repurpose | Recurrent ovarian carcinoma (II, 2012), endometrial adenocarcinoma (II, 2012) | Nonhormone, AKT inhibitor | Preclinical |
| p53, p21, CASP, FOXO, inducing apoptosis | / | Proliferation and apoptosis | Propofol | PK/toxicity (DB00818)h | Repurpose | Anaesthesia (IV, 2001) | Nonhormone, aesthetic | Preclinical |
| Metabolic process | PDH kinase | Proliferation | Dichloroacetate | PK 101 /Toxicity (DB08809)h | Repurpose | Brain cancer (II, 2007), lactic acidosis (III, 1998) | Nonhormone, alpha‐halocarboxylic acid | Preclinical |
| Autophagy modulators | ||||||||
| ATG regulated autophagy | / | Autophagy, proliferation, and apoptosis | MK2206 and chloroquine | NA | NA | NA | Combination therapy; AKT inhibitor (MK2206) and amebicide (chloroquine | Preclinical |
| E2/ER and PR | ERα and PRα | Autophagy and inflammation | Ginsenoside PPD | PK 76 /Toxicity 77 | Repurpose | Endothelial Function (II, 2007) | Natural product, Steroid glycoside | Preclinical |
| ERK and Beclin1 inducing autophagy, CDK | Beclin‐1 and ERK autophagy‐promoting proteins, p27 | Proliferation, apoptosis, and autophagy | MIS | No information | Repurpose | PCOS (III, 2012) | Hormone, glycoprotein hormone | Preclinical |
| Antimigration, anti‐invasion, and antifibrosis agents | ||||||||
| CBP/β‐catenin | CBP/β‐catenin complex | Proliferation, migration, apoptosis, and fibrogenesis | C‐82 | PK 105 | Repurpose | Systemic scleroderma (II, 2015), psoriasis (II, 2015) | Nonhormone, β‐catenin inhibitor | Preclinical |
| CBP/β‐catenin | CBP/β‐catenin complex | Proliferation, migration, apoptosis, and fibrogenesis | ICG‐001 | No information | Repurpose | Myeloid Leukaemia (II, 2012) | Nonhormone, β‐catenin inhibitor | Preclinical |
| Wnt/β ‐catenin | Tcf/β‐cateini complex | Proliferation, migration, and invasion | PKF115‐584 | No information | New | NA | Natural product, NA | Preclinical |
| Wnt2/β‐catenin | / | Invasion | Metformin | PK/toxicity (DB00331)h | Repurpose | PCOS (IV, 2003), type 2 diabetes (IV, 2000) | Nonhormone, antidiabetics and biguanides | Preclinical |
| Wnt/β‐catenin | Tcf/β‐cateini complex | Proliferation, migration, invasion, and fibrogenesis | PKF115‐584/CGP049090 | No information | New | NA | Natural product, NA | Preclinical |
| TGF‐β1‐stimulated activation of MAPK and Smad pathway | / | Proliferation, migration, invasion, and fibrogenesis | EGCG | PK 67 /toxicity 68 | Repurpose | Multiple sclerosis (III, 2009), cervical cancer (II, 2005), prostate cancer (II, 2004) | Natural product, catechin | Phase 2 |
| NF‐κB/MMP‐2/MMP‐9 | NF‐κB | Invasion | Genistein | PK 82 | Repurpose | Endothelial Function (III, 2010), vascular and skeletal protective in menopause women (III, 2003), prostate cancer (III, 2003) | Natural product, isoflavone | Preclinical |
| Rho/ROCK | ROCK | Proliferation, apoptosis, contractility, and differentiation | Fasudil | PK 113 | Repurpose | Raynaud, scleroderma (III, 2007) | Nonhormone, Rho‐kinase inhibitor, and vasodilator | Preclinical |
| Rho/ROCK | / | Fibrogenesis and differentiation | Heparin | PK/toxicity (DB01109)h | Repurpose | Thrombosis (IV, 1997), inflammation (IV, 2008), anticoagulation (IV, 2009), Cancer (IV, 2009), IVF‐ET failure, and thrombophilia (IV, 2009) | Nonhormone, anti‐inflammatory agent | Preclinical |
| Antiangiogenesis agents | ||||||||
| Multikinase | VEGFR, PDGFR | Apoptosis and angiogenesis | Sunitinib (SU11248) | PK/toxicity (DB01268)h | Repurpose | Carcinoma, renal cell (IV, 2008), gastrointestinal stromal tumors (IV, 2008) | Nonhormone, multikinase inhibitor | Preclinical |
| Multikinase | VEGFR‐2, FGFR‐1 and PDGFR‐β | Angiogenesis | SU6668 | PK/toxicity 121 | Repurpose | Hepatocellular carcinoma (II, 2003) | Nonhormone, multikinase inhibitor | Preclinical |
| VEGF/VEGFR | VEGFR2 | Angiogenesis | SU5416 | PK/toxicity 124 | Repurpose | Melanoma (II, 2001), malignant mesothelioma (II, 2000), gastrointestinal stromal tumour, sarcoma (II, 2000) | Nonhormone, VEGFR inhibitor | Preclinical |
| VEGF/VEGFR | VEGF | Angiogenesis and proliferation | Pazopanib (P), sunitinib (SU) and sorafenib (SO) | (P) PK(DB06589)h, others as mentioned | (P) repurpose, others as mentioned | (P) Cancer (IV, 2010), ovarian cancer (III, 2009), carcinoma, renal cell (II, 2006) others as mentioned | (P) Nonhormone, multikinase inhibitor, others as mentioned | Preclinical |
| VEGFC mediated c‐JUN, IFN‐γ, CXCL3, and MMP‐9 pathway | VEGFC/VEGFR2 | Proliferation and angiogenesis | EGCG | PK 67 /toxicity 68 | Repurpose | Multiple sclerosis (III, 2009), cervical cancer (II, 2005), prostate cancer (II, 2004) | Natural product, catechin | Phase 2 |
| VEGF | VEGF | Proliferation, angiogenesis and oxidative stress | ProEGCG | No information, | New | NA | Natural product, prodrug | Preclinical |
| NF‐κB/TNF‐α/VEGF | NF‐κB | Angiogenesis | Pyrrolidine dithiocarbamate | PK/toxicity 131 | New | NA | Nonhormone, metal chelator | Preclinical |
| VEGFC and VEGFR2 | VEGFC and VEGFR2 | Angiogenesis | PTX | PK/toxicity (DB00806)h | Repurpose | Hemodialysis (IV, 2006), intermittent claudication (since 1982) | Nonhormone, hemorheological agent | Phase 3 |
| VEGF/VEGFR2 | Dopamine receptor 2 | Angiogenesis and inflammatory | Quinagolide | PK/toxicity (DB09097)h | Repurpose | Hyperprolactinaemia (since 1994) 134 | Nonhormone, dopamine agonist | Phase 4 |
| Antioxidative stress agents | ||||||||
| Radical scavenging activity/ERK | ROS | Oxidative stress and proliferation | NAC | PK/toxicity (DB06151)h | Repurpose | Cystic fibrosis (II, 2008), multiple sclerosis (II, 2004), pulmonary fibrosis (III, 2000), | Nonhormone, antidote | Preclinical |
| Regulation of antioxidant enzymes | ROS | Oxidative stress | Resveratrol | PK/toxicity (drugs.com/resveratrol.)i | Repurpose | Inflammation in type 2 diabetic patients (III, 2013), anti‐inflammatory and antioxidant effects (III, 2011) | Natural product, phytoalexin | Phase 4 |
| Regulation of antioxidant enzymes | ROS | Oxidative stress | Caffeic Acid | PK 139 | Repurpose | Immune thrombocytopenia (III, 2012) | Natural product, phenolic acid | Preclinical |
| Radical scavenging activity | ROS, MMP, VEGF | Oxidative stress, angiogenesis, and inflammation | Melatonin | PK/toxicity (DB01065) | Repurpose | Insomnia (IV, 2007), chemotherapy‐induced toxicity (III, 2007), prevention of lung cancer (III, 2007) | Hormone, miscellaneous anxiolytics, sedatives, and hypnotics | Phase 2 |
| Anti‐inflammation agents | ||||||||
| Cytokines | / | Proliferation, invasion and inflammation | NAC | PK/toxicity (DB06151)h | Repurpose | Pulmonary fibrosis (III, 2000), cystic fibrosis (II, 2008), multiple sclerosis (II, 2004) | Nonhormone, antidote | Preclinical |
| Cytokines | / | Proliferation and inflammation | Crocin | PK 145 /toxicity 146 | Repurpose | Metabolic syndrome (IV, 2010) | Natural product, diterpenoid | Preclinical |
| Cytokines | / | Inflammation | Metformin | PK/toxicity (DB00331)h | Repurpose | PCOS (IV, 2003), type 2 diabetes (IV, 2000) | Nonhormone, antidiabetics and biguanides | Preclinical |
| Cytokines | / | Angiogenesis and inflammation | Resveratrol | PK/toxicity (drugs.com/resveratrol.)i | Repurpose | Inflammation in type 2 diabetic patients (III, 2013), anti‐inflammatory and antioxidant effects (III, 2011) | Natural product, phytoalexin | Phase 4 |
| TNFα‐mediated cytokines | SIRT1 | Inflammation | Resveratrol | PK/toxicity (drugs.com/resveratrol.) | Repurpose | Inflammation in type 2 diabetic patients (III, 2013), anti‐inflammatory and antioxidant effects (III, 2011) | Natural product, phytoalexin | Phase 4 |
| Cytokines | MIF | Angiogenesis and inflammation | ISO‐1 | No information | New | NA | Nonhormone, MIF inhibitor | Preclinical |
| E2/ER | P450AROM | Inflammation | Puerarin | PK 92 | Repurpose | Alcohol abuse (II, 2009) | Natural product, isoflavonoid | Preclinical |
| MAPK, Wnt pathway | / | Proliferation, angiogenesis, and inflammation | Niclosamide | PK/toxicity (DB06803)h | Repurpose | Anthelmintic (since 1982) | Nonhormone, anthelmintic agent | Preclinical |
| IκKα/β, NF‐κB, STAT3, and JNK | Chemokine and cytokines | Angiogenesis and inflammation | Curcumin | PK/toxicity (DB11672)h | Repurpose | Inflammation in endometrial carcinoma (II, 2013), irritable bowel syndrome (IV, 2008) | Natural product, curcuminoid | Recruiting |
| NK cells cytotoxicity | / | Immune system | Ginsenoside PPD | PK 76 /Toxicity1, 77 | Repurpose | Endothelial function (II, 2007) | Natural product, steroid glycoside | Preclinical |
| VEGF/VEGFR, iNOS/NO, COX‐2/PGE2 | VEGF, iNOS, and COX‐2 | Angiogenesis and inflammation | Acai | PK/toxicity (drugs.com/acai)i | Repurpose | Antioxidant (2010, III), prostate cancer (2011, II) | Natural product, anthocyanin | Preclinical |
| Medication | Study models | Positive control group | Negative control group | Assessments | Efficacy (compared to untreated) e , f , g | Size effects or other comments | Reference |
|---|---|---|---|---|---|---|---|
| Current medication | |||||||
| Indomethacin | Animals (EM mice model) | NA | Vehicle | Lesions assessment | ↓~46% in area of all lesions | Stomach upset, headache, drowsiness, dizziness, and so forth. 1 | [60] |
| Celecoxib | Cells (Primary human endometriotic stromal cells) | NA | Vehicle | Proliferation and apoptosis assays, IHC, Western blot, ELISA | ↓~60% in proliferation, ~50% VEGF and ~70% PGE2, ↑ ~3.25‐fold in apoptosis and ~2‐fold COX‐2 expression with 100 µM of celecoxib | Stomach upset, headache, drowsiness, dizziness, and so forth. 1 | [53] |
| GnRH agonist | Cells (primary human endometriotic stromal cells) | NA | Untreated | Western blot, EMA | ↓~80% TNF‐α mediated IL‐8 level | Hypoestrogenic31, 32 | [31] |
| Progesterone or dienogest or danazol | Cells (primary human endometriotic stromal cells) | NA | Untreated | EMA, ELISA, Northern blot analysis | ↓~40% in TNF‐α mediated IL‐8 level | Hypoestrogenic31, 32 | [50] |
| Leuprolide acetate | Cells (primary human eutopic epithelial endometriotic cells) | NA | Basal conditions | Apoptosis assay, ELISA | ↑1.74‐fold in apoptosis level, ↓62.5% in IL‐8 level, and ↓52.6% in VEGF level | Hypoestrogenic31, 32 | [52] |
| Dienogest | Cells (primary human endometriotic stromal cells) | AKT inhibitor VIII and U0126 | Untreated | Western blot, TEM, IF, autophagy, and apoptosis assays | ↑~1.5‐fold of LC3‐II and SQSTM1 expression, ~25% in autophagy level, ↓~40% in p‐Akt and p‐ERK | Hypoestrogenic31, 32 | [61] |
| Antiproliferation and proapoptotic agents | |||||||
| BAY11‐7085 | Cells (primary human endometriotic and endometrial stromal cells) | NA | Untreated | MTT, ELISA, apoptosis assay, flow cytometry, Western blot | ↓66.1% cell viability and ↑725.1% in apoptosis ability with 10 µM of BAY11‐7085 in ECSCs | No information | [62] |
| Melatonin | Animals (EM rat model) | Vehicle | NA | H&E, Western blot, RT‐PCR, EMSA, Tunel assay | ↓~80% secreted proMMP‐3 and ↓ ~80% synthesized proMMP‐3 on 35th day | No side effects reported | [63] |
| Chloroindazole | Cells (primary human endometriotic stromal cell) and animals (EM mice model) | NA | Vehicle | Lesions assessment, WST‐1 assay, Tunel assay, qRT‐PCR, LC‐MS | ↓~88% in lesions weight, ↓~90% in Ki67 and p65 cells, ↓~88% in IL‐6 cells, in the therapeutic model, ↓~60% cell viability | No adverse effects on the reproductive tract or disturb estrous cycling or fertility | [64] |
| Oxabicycloheptene sulfonate | Cells (primary human endometriotic stromal cell) and animals (EM mice model) | NA | Vehicle | Lesions assessment, WST‐1 assay, Tunel assay, qRT‐PCR, LC‐MS | ↓~78% in lesions weight, ↓~85% in Ki67 and p65 cells, ↓~78% in IL‐6 cells, in the therapeutic model, ↓~60% cell viability | No adverse effects on the reproductive tract or disturb estrous cycling or fertility | [64] |
| Resveratrol | Cell line (Ishikawa cells) and animals (EM xenograft model) | Progesterone | Vehicle | Alkaline phosphatase assay, IHC, RT‐PCR | ↓~50% ESR1 and ~60% Ki67 expression in epithelium in high dose | Mild, mainly related to headache and somnolence 65 | [66] |
| EGCG | Cells (primary human endometrial stromal and glandular cells), and animals (EM Syrian golden hamsters model) | NA | DMSO in vehicle (animal) and vehicles (cells) | Lesions and microvessel assessment, WST‐1 assay, Western blot, Intravital fluorescence microscopy, H&E | ↓~28.5% of E2 induced activation and ~33.3% E2 induced VEGF in EGC; ↓~38.5% endometriotic lesions regression; ↓50% of volumetric blood flow in endometriotic lesions on Day 14 | Well tolerated, only mild headache and fatigue 69 | [70] |
| Tunicamycin | Cells (primary human endometriotic and endometrial stromal cells) | TRAIL | Vehicle | qRT‐PCR, Flow cytometry |
↑59.1% in apoptosis (−TRAIL) ↑1.35‐fold in apoptosis (+TRAIL) in women with EM |
Major neurotoxicity and death in animals 72 | [73] |
| Verteporfin | Cells (primary human endometriotic stromal cells) and animals (EM mice model) | NA | Vehicle | Western blot, IHC, ChIP assay, MTS assay, GSEA, Lesions assessment, | ↓Proliferation in a dose‐dependent manner, ↓~50% in migration and tube formation, ↓~57% in lesions weight | Visual disturbances 74 | [75] |
| Ginsenoside Rg3 | Cells (primary human endometriotic stromal cells) | NA | Untreated | CCK8, Western blot, RT‐PCR | ↓~40% cells after 72 h with 150 µg/ml Rg3, ↓ TNF‐α induced effect of NF‐κB p65 (~20%), VEGF (~25%) and ↑~25% TNFα induced effect of CASP3 | No side effect reported from RCTs 78 | [79] |
| Curcumin | Animals (EM mice model) | Celecoxib | PBS | Lesions assessment, H&E, Western blot, AFM, electrophoresis | ↓~80% lesions glands, ↓~60% of p65/NF‐κB expression, ↑~6‐fold of Bax/bcl2 ratio on Day15 | Safe and well‐tolerated even at high dose 80 | [81] |
| Genistein | Animals (EM mice model) | Dienogest and Leuprolide acetate | Untreated | Lesions assessment, IHC | ↓4% of TGF‐β and 4.5% NF‐κB, 3.4% Bcl‐2, 1.35% COX2, 2% PGE, ↑1.35% Bax expression levels with 1.30 mg/day of Genistein | No side effect reported83, 84 | [85] |
| Sorafenib | Cells (primary Human endometriotic stromal cell), and animals (EM mice xenograft model) | NA | Untreated (cell) or placebo (animal) | Lesions assessment, crystal violet assay, Western Blot, H&E | ↓99.7% decreased in ectopic stromal cell, ↓64% in pERK–ERK ratio, ~33.3% in pVEGFR‐VEGFR ratio, ~35% implants size | Weight loss, skin, and gastrointestinal toxicities 86 | [87] |
| Vemurafenib | Cells (primary human stromal/epithelial; endometriotic/endometrial cells) and animals (EM mice xenograft model) | NA | vehicle (1% DMSO in media) | Western blot, IHC, lesions assessment, viability, apoptosis, and crystal violet assay assays | ↓viability (69%, 66.7%), ↓optic density of pERK/ERK (62%, 61%), BRAF/B‐actin (61%, 66%) in stromal, epithelial cells, ↓ 37% implants size | Arthralgias, rash, and hyperkeratosis 88 | [89] |
| U0126 | Cells (primary human endometriotic and endometrial stromal cells) | Progestin (R5020) | DMSO | Immunoblotting, qRT‐PCR, Viability assay, IHC | ↑~10% PRAB, ↓~20% viability in OSIS with 10uM of U0126 | No information | [91] |
| Puerarin | Cells (primary human endometriotic stromal cells) | U0126 | Vehicle | Binding assay, Western Blot, CCK‐8, qRT‐PCR, and phosphate kinase arrays | Relative binding affinity of 32.2% of ERs and puerarin complex, ↓~30% proliferation, and ERK related proteins: ↓~46% cyclin D1, ↓~73% COX‐2, and ↓~46% cyp19 | Can be used for long periods without severe side effects | [93] |
| PGE2 inhibitors | Cell line (12Z and 22B), Cells (primary human endometriotic and endometrial stromal cells) | NA | Untreated | Western blot, IP, IF, TUNEL, RT‐PCR, IHC | ↑Apoptosis in 12Z (~8‐fold) and 22B (~7‐fold), ↓ p‐EGFR/EGFR level in 12Z (~85%) and 22B (~63%), ↓ pERK/ERK level in 12Z (~50%) and 22B (~15%), ↓pAkt/Akt level in 12Z (~85%) and 22B (~72%), ↓B‐cate/B‐actin level in 12Z (~67%) and 22B (~43%), | GI upset, edema, and skin rash 94 | [95] |
| WIN 55212‐2 | Cells (primary human stromal/epithelial; endometriotic/endometrial cells) and animals (EM mice xenograft model) | NA | PBS | Proliferation and viability assays, Western blot, lesions assessment | ↓65% Akt level in endometriotic stromal cell and 50% that in endometrial stromal cell, ↓43% in lesions volume | Dizziness, drowsiness, sedation, dry mouth and cognitive impairment 97 | [98] |
| MK2206 | Cells (primary human endometriotic and endometrial stromal cells) and animals (EM xenograft mice model) | Progestin (R5020) | DMSO in vehicle (cell) and PBS (animal) | Lesions assessment, Western blot, qRT‐PCR, viability assay, IHC | ↑~20% PRA and 30% PRAB, ↓~30% viability in OSIS with 10 µM of MK2206, ↓ ~20% tumor volume | No information | [91] |
| Propofol | Cell line (CRL‐7566) | NA | 0.2% DMSO in vehicle | MTT, Flow cytometry, RT‐qPCR, Western blot | ↑~35% in apoptosis level with 10 µg/ml propofol, ↑ FOXO1, FOXO3, Bim, p21, p53, CASP3 expression levels in a dose‐dependent manner | Hemodynamic instability, pain on injection, dystonic movements, hypertriglyceridemia, pancreatitis, allergic reactions 99 | [100] |
| Dichloroacetate | Cells (primary human peritoneal mesothelial cells), cell line (SHT290), animals (EM mice model) | NA | Vehicle (water) | ECAR and OCR measurement, Enzymatic colorimetric kit, Lesions assessment | ↓~40% TGF‐β1–stimulated HPMC lactate secretion, ~25% cell proliferation, ↑ ~3‐fold PDH activity, ↓~20% lactate concentrations in PF, and ~50% lesions size | Peripheral neuropathy | [46] |
| Autophagy modulators | |||||||
| MK2206 and chloroquine | Cells (primary human stromal/epithelial; endometriotic/endometrial cells) and animals (EM mice xenograft model) | NA | Vehicle | MTS, flow cytometry, transfection, Western Blot, IF, clonogenic assay, IHC | ↓>80% proliferation in both cell growth and regrowth model in all cells and, >90% colony formation in both DES and EES, ↓>80% lesions volume, ↑>50% apoptosis in mice | Chloroquine alone might result in gastrointestinal symptoms but no information of adverse effects with combination treatment | [102] |
| Ginsenoside PPD | Cells (primary human endometriotic and endometrial stromal cells) and animals (EM mice model) | Esculentoside A | 0.1% DMSO in vehicle | CCK8, flow cytometry, RT 2 profiler™ PCR, Western blot, IHC, IF | >3‐fold difference in autophagy‐related genes ATG2, ATG3, and ATG5, ESR1, SQSTM1, and TGF‐B1, ↓~90% lesions weight and ~85% of lesions numbers with high dose PPD | Safe, low doses had no influence on growth of nESCs or the eutopic endometrium | [103] |
| MIS | Cell line (CRL‐7566) | NA | PBS | MTT, FACS analysis, flow cytometric analysis, Western blot | ↓50% viability, ↑1.8‐fold proptosis with 10 µg/ml MIS | No information | [104] |
| Antimigration, anti‐invasion, and antifibrosis agents | |||||||
| C‐82 | Cells (primary human endometriotic and endometrial stromal cells) | NA | Vehicle | IHC, MTT, Western blot, ELISA, qRT‐PCR, scratch assay | ↓51.8% cell viability, 91.9% cell proliferation, ↑200% apoptosis and 234.2% CASP3/7, ↓54% cell migration, all with 20 µM of C‐82 | Mild, e.g reaction at the injection site, nausea, and constipation | [106] |
| ICG‐001 | Cells (primary human endometriotic and endometrial stromal cells) and animals (EM mice model) | NA | Vehicle | IHC, MTT, Western blot, ELISA, qRT‐PCR, scratch assay, lesions assessment | ↓20.8% cell viability, 86.1% cell proliferation, ↑56.4% apoptosis, 128.9% CASP3/7, ↓64%cell migration, all with 20 µM of ICG‐001, ↓~87.5% number of lesions | High dose was required for preclinical study and might not be comparable to clinical study | [106] |
| PKF115‐584 | Cells (primary human stromal/epithelial; endometriotic/endometrial cells) | NA | Vehicle | qRT‐PCR, proliferation and migration assay, Western blot | ↓73% invasion in endometriotic epithelial cells and 75% invasion, 85% MMP‐9 in stromal cells | Wnt/B‐catenin is needed for stem cell maintenance and tissue homeostasis 107 | [108] |
| Metformin | Cells (primary human endometriotic and endometrial stromal cells, endometrial epithelial cell) | NA | Untreated | MTT, Western blot | ↓36.9% growth effects on epithelial cells by stromal factors, ↓~50% Wnt2 expression and secretion | Nausea and vomiting, diarrhea, abdominal pain 109 | [48] |
| PKF115‐584/CGP049090 | Cells (primary human endometriotic and endometrial stromal cells) | NA | Vehicle | qRT‐PCR, MTS, migration assay, ICC, collagen gel contraction assay | ↓>70% mRNA levels of αSMA, COL‐I, FN, CTGF in endometriotic stromal cell, collagen gel contraction | Wnt/B‐catenin is needed for stem cell maintenance and tissue homeostasis 107 | [110, 111] |
| EGCG | Cells (primary human endometriotic and endometrial stromal cells) and animals (EM mice model) | NAC | Vehicle | qRT‐PCR, MTS, migration and invasion assays, Collagen gel contraction assay, ICC, Western blot, H&E | ↓>90% mRNA levels of αSMA, COL‐I, FN, CTGF in endometriotic stromal cell, ↓ > 90% migrated and invasive cells | Well tolerated, only mild headache and fatigue 69 | [110] |
| Genistein | Animals (EM mice model) and In silico | NA | Untreated | Lesions assessment, ELISA, docking | ↑21.91 kJ/mol and 63.14 kJ/mol of NF‐κB to MMP‐2/‐9 binding energy, ↓~50% MMP‐2, ~30% MMP‐9 expression level with 100 mg/day genistein | No side effect reported83, 84 | [112] |
| Fasudil | Cells (Primary human endometriotic stromal cells) | NA | Untreated | Cell viability assay, ELISA, flow cytometry, Collagen gel contraction assay, Western blot, Morphology assessment | ↓43.7% proliferation, ~50% cell density, ~25% Bcl‐2, ~50% Bcl‐xl, αSMA, ROCKI, and ROCKII protein expressions, ~↑2‐fold apoptosis, 50% contractility, all with 100 µM Fasudil | Systemic vasodilation and hypotension 114 | [115] |
| Heparin | Cells (Primary human endometriotic stromal cells) | NA | Untreated | Morphology assessment, collagen gel contraction assay, Western blot | ↓55.7% gel contraction with 100 mg/ml heparin sodium solution, ↓ αSMA, RhoA, ROCKI, and ROCKII expressions levels | Thrombocytopenia 116 | [117] |
| Antiangiogenesis agents | |||||||
| Sunitinib (SU11248) | Animals (EM rat model) | NA | Saline | Lesions assessment, H&E, and Tunel assay | ↓78.8% in cross‐sectional area of the cyst, and 50% complete cyst disappearance in animal | fatigue, diarrhea, skin discoloration, and nausea 118 | [119, 120] |
| SU6668 | Animals (EM golden hamster model) | NA | DMSO | Lesions assessment, H&E, IHC | ↓~30% vascularized area of endometrial grafts, ↓~50% microvessel density, ↓~25% size of endometrial grafts | Fatigue, nausea, vomiting, diarrhea, pain, flu‐like complaints, anorexia, change of taste 122 | [123] |
| SU5416 | Animals (EM golden hamster model) | NA | DMSO | Lesions assessment, H&E, IHC | ↓~20% microvessel density, ↓~5% size of endometrial grafts | Headache, pain at injection site 125 | [123, 126] |
| Pazopanib (P), sunitinib (SU) and sorafenib (SO) | Animals (EM rat model) | NA | Saline | Lesions assessment, H&E, IHC | ↓~16% (SU), ~45% (P) in EM score; ↓~83%(SO), ~66% (SU), ~50% (P) in VEGF; ↓~56% (SU), ~40% (P) in CD117 | Adverse effects on reproductive functions in animal models, including ovulation inhibition, embryotoxicity 127 | [128] |
| EGCG | Cells (human microvascular endothelial cells), and animals (EM mice xenograft model) | Vitamin E | Saline | Lesions and microvessel imaging, microarray, qRT‐PCR, Western blot, IHC | ↓85% size and total vessel area of lesions with human endometrium tissue, ↓48% VEGFC mRNA, ↓~50% VEGFC expression level | Well tolerated, only mild headache and fatigue 69 | [70, 129] |
| ProEGCG | Animals (EM mice xenograft model) | Vitamin E | PBS | Lesions and Microvessel imaging, H&E, Tunel assay, ELISA, ORAC assay | ↓>90% in lesions weight and size, ↓>70% in VEGF concentration, and ↑>4‐fold ORAC capacity in plasma and lesions during intervention | No sign of side effects | [130] |
| Pyrrolidine dithiocarbamate | Cells (primary human endometriotic and endometrial stromal cells) | NA | NA | EMSA, RT‐PCR, and Western blot | ↓63.5% in TNFα–induced NF‐κB‐DNA binding activity, 53.9% CD44s, 55.2% MMP‐9, 68.5% VEGF expression in endometriotic stromal cells with 50 umol/L PD | No information | [132] |
| PTX | Animals (EM Wistar rats model) | NA | 0.9% NaCl in vehicle | Lesions assessment, H&E, IHC | ↓37.9% mean implant volume per animal, ↓47% VEGFC, and 40.1% Flk‐1 in glandular cells | No sign of side effects | [133] |
| Quinagolide | Human (hyperprolactinemic patients with EM) | NA | NA | Lesions morphology assessment, IHC, IF, and superarray | ↓69.5% lesions size and 35% of lesions vanished completely, ↓VEGF (5.8‐fold), CCL2 (6.1‐fold), ↑CCL10 (6.8‐fold) | Dizziness, nausea, and vomiting | [135] |
| Antioxidative stress agents | |||||||
| NAC | Cells (primary human stromal/epithelial; endometriotic/endometrial cells), animals (EM mice xenograft model) | Danazol and Mifepristone | PBS or sesame oil | UV spectroscopy, viability assays, H&E, Western blot, antioxidant enzyme activities | ↓40%–60% H2O2 concentrations and 30%–60% proliferation in mice, ↓88% ratio pERK/ERK in human OSIS | High amount cause nausea, vomiting 136 | [137] |
| Resveratrol | Animals (EM rat model) | NA | Vehicle | Lesions assessment, H&E, PCNA‐IHC, antioxidant enzyme activities | ↓41.5% of implants, ↑~50% (serum) and ↑ ~2‐fold (tissues) in SOD and GSH‐px activities, ↓46% (serum) and 77% (tissue) MDA, all with high dose resveratrol | Mild, mainly related to headache and somnolence 65 | [138] |
| Caffeic Acid | Cells (primary human endometriotic and endometrial stromal cells) | NA | Untreated | IHC, MTT, antioxidant activity assays | ↓48.8% MDA ↑90.7% GSH, 56% CAT, 81% GPx, 59.5% GR | Excessive consumption can have insomnia 140 | [141] |
| Melatonin | Animals (EM rat model) | Vehicle | NA | Antioxidant activity assays, H&E, IHC | ↓68.1% weight of implants, ↓ 66.8% in volume weight and ↓ 75.2% histologic score, ↓ 24% MDA, ↓45.6% CAT and ↑ 1.77‐fold SOD activities level, ↓ 61% VEGF ↓ 70% MMP‐9 | No negative effect on fertility | [142, 143] |
| Anti‐inflammation agents | |||||||
| NAC | Animals (EM mice model) | NA | Vehicle | Lesions assessment, IHC, qRT‐PCR | ↓60% lesions, ↓54% Ki67, ~50% COX2, >60% MMP‐9 levels | High amount cause nausea, vomiting 136 | [144] |
| Crocin | Animals (EM mice model), and cell line (HUVEC and THP‐1) | NA | Untreated and saline | Lesions assessment, IHC, qRT‐PCR, ELISA, FC | ↓~44% lesion size and ~64% of lesion weight, ~80% of VEGF and ~60% PCNA mRNA levels, ↓>50% of INF‐γ, TNF‐α, VEGF, and IL‐6 in serum | Mild effects like headache, insomnia, nausea, and dyspnea 147 | [148] |
| Metformin | Human (Stage1–2 EM patients) | NA | Placebo | ELISA | ↓~35% IL6, ~33% IL‐8 and ~38% VEGF | Nausea and vomiting, diarrhea, abdominal pain 109 | [149] |
| Resveratrol | Animals (EM rat model) | NA | Vehicle | Lesions assessment, ELIZA, H&E, IHC | ↓84.8% of implants volume, ↓VEGF level in Peritoneal fluid (54.8%), plasma (55.5%) and lesions (80.5%), ↓MCP‐1 in peritoneal fluid (48.3%) | Mild, mainly related to headache and somnolence 65 | [150] |
| Resveratrol | Cells (primary human endometriotic and endometrial stromal cells) | NA | Untreated | qRT‐PCR, CCK‐8, IHC, ELIZA | ↓>60% TNFα induced IL‐8 mRNA expression and concentrations in ESC | Mild, mainly related to headache and somnolence 65 | [151] |
| ISO‐1 | Animals (EM mice model) | NA | 5% DMSO in vehicle | Lesions assessment, qRT‐PCR, Western blot | ↓~70% in implant size, ~60% Flk‐1 expression, ~50% MIF activity | No information | [151] |
| Puerarin | Animals (EM rat model) | Raloxifene hydrochloride | Sodium carboxymethyl cellulose | Lesions and blood assessment, RT‐PCR, IHC | ↓35.3% weight, ↓21.6% E2, 69.4% P450AROM, 41.5% COX‐2, 59.2%, 17β‐hsd‐1m, and ↑2.3‐fold 17β‐hsd‐2m mRNA of ectopic endometrium with M‐SI group | Can be used for long periods without severe side effects 93 | [152] |
| Niclosamide | Animals (EM mice model) | NA | Vehicle | Lesions assessment, IHC, IF, TUNEL, qPCR, GSEA | ↓63.6% implant weight, 78.1% growth, 38.8% epithelial cell proliferation with 200 mg/kg niclosamid, ↓MAPK, Wnt, inflammation signaling‐related genes mRNA expression levels | Mild nausea and abdominal pain 153 | [154] |
| Curcumin | Cells (primary human endometriotic and endometrial stromal cells) | NA | 0.1% DMSO in vehicle | Morphology assessment, Western blot, magnetic bead‐based assays | ↑apoptosis, ↓phosphorylated form of IKKα/β, NF‐κB, STAT3, and JNK signaling, ↓10–15‐fold IL6, IL‐8, IP10, G‐CSF, MCP‐1 and RANTES, ↑ IL10, IL12, all in a dose‐dependent manner | Safe and well‐tolerated even at high dose 80 | [155] |
| Ginsenoside PPD | Cells (primary human endometriotic and endometrial stromal cells) and Animals (EM mice model) | Esculentoside A | 0.1% DMSO in vehicle | CCK8, Flow cytometry, RT 2 profiler™ PCR, Western blot, IHC, IF | ↓~33.3% Bcl‐2, ~15% Bcl‐xL, and ~17% Ki‐67, ↑~28% CD82 in NK cells, ↓~67% E2 induced lesion, and ~70% E2 induced lesions weight | Safe, low doses had no influence on growth of nESCs or the eutopic endometrium | [103] |
| Acai | Cell line (J774.G8) and animals (Sprague‐Dawley rats) | NA | Vehicle | Lesions assessment, H&E, IHC, RT‐qPCR, ELIZA, flow cytometry, MTT | ↓~92% lesions, ~33.3% VEGF conc., ~80% MMP‐9, 57% PGE2, ~50% viability with 40 µg/ml acai for 72 h | No information | [156] |
Abbreviations: 17β‐hsd, 17β‐hydroxysteroid dehydrogenase; ‐SMA, ‐smooth muscle actin; AFM, atomic force microscopy; AKT, protein kinase B; P450AROM, aromatase; ASK1, apoptosis signal‐regulating kinase 1; ATF4, activating transcription factor 4; ATG, autophagy‐related protein; BRAF, serine/threonine‐protein kinase B‐Raf; CASP, caspases; CAT, catalase; CB, cannabinoid receptor; CCK8, cell counting kit‐8; CDK, cyclin‐dependent kinases; CHOP, CCAAT/enhancer‐binding protein homologous 10 protein; COL, collagen; COX, cyclooxygenase; CTGF, connective tissue growth factor; CXCL3, chemokine ligand 3; CYPs, cytochromes P450; DMSO, dimethyl sulfoxide; DVT, deep vein thrombosis; E2, estrogen; ECAR, extracellular acidification rate; EGCG, epigallocatechin gallate; EGFR, epidermal growth factor receptor; eIF2α, eukaryotic initiation factor 2 alpha; ELISA, enzyme‐linked immunosorbent assay; EM, endometriosis; ECSCs, endometriotic cyst stromal cells; EMSA, electrophoretic mobility shift assay; ER, estrogen receptor; ER stress, endoplasmic reticulum stress; ERK, extracellular signal‐regulated kinase; ESR1, estrogen receptor 1; FGFR, fibroblast growth factor receptors; Flk‐1, vascular endothelial growth factor receptor 2; FN, fibronectin; GnRH, gonadotropin‐releasing hormone; GI, gastrointestinal; GSHPx, glutathione peroxidase; GR, glutathione reductase; GRP, G protein‐coupled receptor; GSH, glutathione; H&E, haemotoxylin and eosin; ICC, immunocytochemistry; IF, immunofluorescence; IHC, immunohistochemistry; IFN‐γ, interferon‐γ; IL, interleukin; iNOS, inducible nitric oxide synthase; IRE1, inositol‐requiring enzyme 1; IκB, stimulate inhibitor of NF‐κB; IκK, IκB kinase; JNK, c‐Jun N‐terminal kinase; LATS1, large tumor suppressor kinase 1; LC, lapidated microtubule‐associated proteins 1 A/1B light chain; MAPK, mitogen‐activated protein kinase; MDA, malondialdehyde; MEK, ERK kinase; MIF, macrophage migration inhibitory factor; MIS, Mullerian‐inhibiting substance; MMP, matrix metallopeptidases; mTOR, mammalian target of rapamycin; mRNA, messenger RNA; MTT/MTS, cell proliferation assay; NAC, N‐acetyl cysteine; nESCs, normal endometrial stromal cells; NF‐κB, nuclear factor κB; NK cells, natural killer cells; NO, nitrogen oxide; OCR, oxygen consumption rate; ORAC, oxygen radical absorbance capacity; OSIS, endometriotic stromal cells; PCNA, proliferating cell nuclear antigen; PDGF, platelet‐derived growth factor; PDGFR, platelet‐derived growth factor receptor; PDH, pyruvate dehydrogenase kinase; PERK, endoplasmic reticulum kinase; PGE2, prostaglandin E2; PI3K, phosphoinositide 3‐kinases; PK, pharmacokinetics; PPAR, peroxisome proliferator‐activated receptor; PPD, protopanaxadiol; PR, progesterone receptor; ProEGCG, prodrug of EGCG; PTX, pentoxifylline; RAF, RAF proto‐oncogene serine/threonine‐protein kinase; RT‐qPCR, real‐time reverse‐transcription polymerase chain reaction; Rho, Ras homolog family; ROCK, Rho‐associated coiled‐coil kinase; ROS, reactive oxidative stress; SIRT1, sirtuin 1; SOD, superoxide dismutase; STAT, signal transducer and activator of transcription; SQSTM1, sequestosome 1; TCF, T‐cell factor; TCM, traditional Chinese medicine; TGF, transforming growth factors; TNF, tumor necrosis factor; TRAIL, TNF‐related apoptosis inducing ligand; TRAF2, TNF receptor‐associated factor 2; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; Wnt, wingless‐type mouse mammary tumor virus integration site family; WST‐1, cell proliferation assay; YAP, Hippo/Yes‐associated protein.
Pk and toxicity profile of drugs can be found on The Drugs.com Database, drugs.com, or on DrugBank Online, go.drugbank.com, or otherwise as stated.
A new drug is defined as a chemical that has not been studied in clinical trials for other diseases before EM and a repurposed drug is defined as a chemical that has been studied in clinical trials for other diseases before EM.
Representative clinical indications of drugs shows the original purpose before it was studied on EM. Information was taken from US National Library of Medicine, ClinicalTrials.gov, or otherwise as stated.
Representative parameters were selected to show efficacy of drugs under corresponding pathophysiology.
Parameters of treated groups with a statistical difference of p < 0.05, compared to controls groups.
Data were extracted from tables or read from graphs.
Drug accession number is the ID of each drug entry on Drug bank.
Drug entry on the drug.com can be accessed via the URL.
3.1. Antiproliferative mechanism
3.1.1. E2‐mediated pathway
Increased levels of E2 reduce progesterone and inhibit endoplasmic reticulum stress in endometrial cells. 157 Increased expression of estrogen receptor (ER) isoforms has been observed in endometriotic lesions,158, 159 suggesting their contribution in regulating proliferation of the lesions. E2 is mediated by ERα and ERβ as well as by G protein‐coupled receptor 30 (GRP30), which is a seven‐transmembrane receptor. It activates phosphoinositide 3‐kinases (PI3K) and mitogen‐activated protein kinase (MAPK) through the transactivation of the epidermal growth factor receptor (EGFR) in the plasma membrane.160, 161 Chloroindazole and oxabicycloheptene sulfonate are two new chemicals bound to ERα and ERβ, respectively, and both inhibited E2‐driven proliferative and inflammatory activities in a dual action manner in ectopic lesions. This experimental study demonstrated great potential owing to their high potency and efficacy as preventive and therapeutic treatments. In addition, they do not exert any undesirable effects on the reproductive system. Co‐treatment of either ligands with letrozole enhanced the regression of ectopic endometrium, but it did not affect eutopic uterine tissues as with only letrozole. 64 Increased COX‐2 and aromatase (P450AROM) levels stimulate E2 synthesis.162, 163, 164 P450AROM inhibitor maintained a low E2 level and reduced EM lesion size. 57 COX‐2‐targeted treatment with celecoxib and indomethacin, which are two available NSAIDs, showed multiple effects on EM.60, 165 The drugs inhibited COX‐2‐mediated prostaglandin E2 (PGE2), which regulates E2 formation, 53 but caused side effects including reproductive failures and cardiac adverse conditions. 166
EGCG and resveratrol are both natural products that have been studied for EM treatment in clinical trials. They act as anti‐E2 agents, but with reduced side effects compared with hormonal drugs.65, 69 High doses of resveratrol reduced proliferation by interacting with ERα, and its expression in the endometrium epithelium was reduced to a profound level similar to that achieved with progesterone treatment. Nevertheless, progestogen did not reduce Ki‐67 expression in the endometrium stroma, whereas resveratrol reduced its expression in both the epithelium and stroma. 66 EGCG inhibited E2‐stimulated proliferation and VEGF expression in cultured endometriotic glandular cells as well as angiogenesis and lesion growth via VEGF in mouse models.70, 129, 167
3.1.2. NF‐κB pathway
NF‐κB is a protein that promotes cell proliferation and inhibits apoptosis in endometrial and endometriotic cells.168, 169, 170, 171 It is activated by cytokines, including TNF‐α, interleukins (IL)‐1β, and lipopolysaccharide. These stimulate inhibitors of NF‐κB (IκB) to be phosphorylated by IκB kinase (IκK). 171 NF‐κB binds to DNA and transcripts the genes of angiogenic and adhesion factors, cytokines, growth factors, and inducible enzymes such as nitric oxide synthase and COX. 172 Dienogest is a pregestational steroid of NF‐κB inhibitor, and it inhibits IL‐8 production to attenuate NF‐κB activation in endometriotic stromal cells in vitro. 50
3.1.3. MAPK/MEK/ERK
In EM, MAPK is activated to mediate the intracellular transmission of extracellular signals and induce cellular processes, 127 as shown by a high phosphorylated extracellular signal‐regulated kinase (ERK) level.173, 174 RAS binds to RAF and activates ERK kinase (MEK1/2) to phosphorylate ERK, which is a major MARK signaling cascade. 127 ERK1/2 regulates c‐fos and c‐jun expression to regulate mitosis and cell viability in endometrial cells under EM. 174 E2, IL‐1β, and TNF‐α stimulated the phosphorylation of ERK1/2 in endometriotic stromal cells, but not in normal endometrial cells. 175 Protease‐activated receptor 2 also activated ERK1/2 in cultured ectopic endometrial stromal cells. 176
Sorafenib has completed phase IV clinical trials in several types of carcinoma, and it significantly abrogated the phosphorylation of RAF kinase by 64% via the MAPK/ERK pathway in stromal cells of EM patients. However, weight loss was observed in the xenograft EM mouse models. 87 Vemurafenib is FDA‐approved for the treatment of metastatic melanoma and significantly inhibits ERK phosphorylation by over 60% in both endometriotic stromal cells and epithelial cells. 89 U0126 is an MEK1/2 inhibitor that increases progesterone receptor (PR)‐αβ levels in endometriotic stromal cells. 91 However, although the above treatments were shown to have significant efficacy, treatment of EM with MAPK inhibitors induced adverse effects on reproductive functions in animal models, including ovulation inhibition, embryotoxicity, and teratogenicity. 127 Puerarin is a natural product that strongly binds to ERs; its binding affinity to ERs is one‐third that of E2, and it suppresses E2‐induced endometriotic stromal cells by 30% via the ERK pathway in vitro, 93 and results in reduced adverse effects.
3.1.4. PI3K/Akt/mTOR
PI3K phosphorylates phosphatidylinositol 4,5‐bisphosphate (PiP2) into phosphatidylinositol 3,4,5‐trisphosphate (PiP3) and activates protein kinase B (Akt). 177 Mammalian target of rapamycin (mTOR), a downstream protein kinase of Akt, is overexpressed in ectopic lesions.178, 179 Reduction of the phosphatase and tensin homolog deleted from chromosome 10 (PTEN) by mutation 180 enhanced the phosphorylation of Akt, thus promoting proliferation, inhibiting apoptosis, and reducing PR expression in EM. 91 MK2206, an Akt inhibitor, is a drug candidate for cancer treatment that acts by increasing PRβ and PRαβ levels and decreasing the viability of endometriotic stromal cells, without affecting normal cells. 91 WIN 55212–2 is a nonselective cannabinoid agonist that binds to cannabinoid receptor (CB)1 or CB2 to inhibit Akt levels and Akt phosphorylation, suggesting the inactivation of the Akt pathway. However, although it reduced the proliferation rate of endometriotic cells, it also reduced that of eutopic endometrial stromal cells. 98
3.1.5. Hippo/Yes‐associated protein (YAP)
The Hippo/YAP pathway is important for balancing cell proliferation and apoptosis. Upregulation of this pathway increased the viability of endometriotic cells, whereas knockdown of YAP increased apoptosis and decreased B‐cell/B‐cell lymphoma 2 (Bcl‐2) expressions. 181 Verteporfin, a YAP1 inhibitor, inhibited the proliferation of endometriotic stromal cells, production of E2, and infiltration of immune cells. 75 It is an FDA‐approved drug for the treatment of subfoveal choroidal neovascularization. EM mouse models showed decreased vessel tube formation and cell migration, with no reported effects on reproductive organs, infertility, or transgenerational influence. 75 YAP1 is a potential target protein but has not been widely studied in EM.
3.1.6. Metabolic process
The metabolic pathways toward increased lactate and dysregulation of glycolysis were shown as contributing factors for cancer progression. 182 Lactate induces angiogenesis and supplies nutrients to proliferate tumor cells.182, 183 In EM peritoneal mesothelial cells, increased glycolysis, decreased mitochondrial respiration, decreased pyruvate dehydrogenase activity, and increased lactate was also observed. 46 Dichloroacetate, a nonhormonal treatment or recurrence prevention of EM, reversed the pathophysiology of EM by inhibiting pyruvate dehydrogenase kinase to activate pyruvate dehydrogenase. 46 Although dichloroacetate has completed a phase III clinical trial for lactic acidosis in 1998 and has widely studied in cancer, it has not been approved by the FDA for therapeutic use in cancer.
3.2. Proapoptotic mechanism
Apoptosis is a programmed cell death process that maintains the balance between the growth and differentiation of cells for tissue renewal. It is regulated by selective chromatin internucleosomal cleavage to shrink the cells. 184 Apoptosis is important in the normal endometrium to remove dysfunctional cells and repair tissues during the menstrual cycle. 185 Apoptotic cells were found to be more predominant in the endometrial epithelium glands than in the stroma. 186 Cell apoptotic activity was found to be relatively low in EM. 187 This can be explained by the reduced expression of proapoptotic factors (e.g., Bcl‐2‐associated X [Bax] and Bcl‐2 associated agonist of cell death [Bad]), overexpression of antiapoptotic factors (e.g., Bcl‐2), and dysregulation of cell cycle.188, 189 Endometriotic cells could not express surface receptors to trigger proapoptotic proteins; neither apoptotic signals were appropriately transduced, leading to proliferation being triggered instead. 188 GnRH agonists, such as leuprolide acetate or the preclinical nonhormonal drug propofol, increased the levels of proapoptotic proteins, as well as decreased the levels of antiapoptotic proteins and promitogenic cytokines.52, 100 Melatonin is highly effective in amplification of apoptotic activity via regulating MMP‐3 signal, was able to regress EM at either early or late stage. Melatonin in high dose and long‐term treatment shows no adverse effects in EM rodents. 63
3.2.1. MAPK, Akt, and NF‐κB pathways
The signaling pathways MAPK/ERK, PI3K/Akt, and NF‐κB also regulated apoptosis in endometriotic cells. ERK1/2 was activated as an antiapoptotic protein in eutopic and ectopic endometrial glands throughout the menstrual cycle. 174 Non‐E2 targeted treatments, such as selective PGE2 inhibitors, inhibited PGE2 receptor (EP) 2 and EP4 to induce apoptosis via multiple pathways including ERK1/2, AKT, and NF‐κB. They enhanced apoptosis by approximately 50% in both epithelial and stromal cells; thus, owing to their efficiency as selective or combination inhibitors, they are ideally used to treat stage I and II EM. 95 PGE2 inhibitors are preferred to COX‐2 inhibitors due to their fewer adverse effects and lack of hypoestrogenic effects. 95
BAY11‐7085 is an NF‐κB inhibitor that inhibits cell viability and enhances apoptosis in endometriotic cells by seven fold but induced less profound results in normal endometrial cells. 62 The activity of Bay11‐7085 has been shown in in vitro studies, but there has been no clinical study of its therapeutic potential. Ginsenoside Rg3, genistein, and curcumin are found in natural products, and they enhanced apoptosis via regulation of the NF‐κB pathway.79, 85 In addition, ginsenoside Rg3 neutralized the effect of TNF‐α to regulate proliferation. 79 Genistein had a similar capability as a GnRH agonist, that is, inhibiting transforming growth factor (TGF)‐β to regulate NF‐κB. Expression of Bcl‐2 was suppressed, whereas that of Bax was enriched. It also reduced COX‐2 and PGE2 expression to levels comparable to those in the control group. 85 Curcumin inhibited MMP‐3 and increased the Bax/Bcl‐2 ratio by upregulating tumor protein p53 (p53). 81 These natural products are highly attractive owing to their potential efficacies and minimal side effects reported in in vitro and in vivo studies of EM and clinical studies of other diseases.
Akt is a pleiotropic regulator of apoptosis that increases endometriotic cell survival and decreases apoptosis. 190 Akt/mTOR can be inhibited by endoplasmic reticulum stress. 51 Tunicamycin enhanced TNF‐related apoptosis‐inducing ligand‐induced apoptosis by inducing endoplasmic reticulum stress in endometriotic stromal cells. The effect was more potent in endometriotic cells than in eutopic endometrial cells 73 ; however, its action mechanism and pharmacokinetics profile have yet to be elucidated.
3.2.2. E2/TNF‐α
Transcription of antiapoptotic Bcl‐2 protein was increased by E2 through the promotion of thymic stromal lymphopoietin. 191 Extranuclear kinases are activated by an elevated ER complex to trigger a rapid nongenomic signaling cascade and inhibit apoptosis in stromal and epithelial cells.192, 193 Genistein interfered with the E2/ER pathway to induce apoptosis and apoptotic proteins in endometrial hyperplasia. 194 Moreover, the activity of E2 strongly regulates TNF‐α‐induced effects. In a healthy endometrium, TNF‐α stimulated apoptosis. In eutopic and ectopic endometriotic cells, TNF‐α stimulated proliferation and inhibited apoptosis instead. 195 In endometriotic lesions, apoptosis signal‐regulating kinase (ASK‐1), a TNF‐α induced apoptosis complex I, interacted with ERβ as well as serine/threonine kinase receptor‐associated protein (STRAP) and 14‐3‐3 proteins. 62 Formation of this complex disrupted the association of TNF‐receptor‐associated factor 2 (TRAF2) and ASK‐1 for TNF‐α‐induced apoptosis.196, 197 TNF‐α‐induced apoptosis complex I, complex II, and apoptosome were all inhibited, which dysregulated apoptosis and activated the invasiveness of lesions for survival. In addition, in endometriotic tissue, TNF‐α from macrophages and natural killer (NK) cells induced the generation of the steroid receptor coactivator (SRC)‐1 isoform from cleaved MMP‐9. 198 ERβ interacted with the caspase (CASP)‐8 and SRC‐1 isoforms to prevent activation of TNF‐α‐induced apoptosis complex II in endometriotic lesions in ectopic sites. 62 Therefore, inhibition of ASK1/ERβ/STRAP‐14‐3‐3 and ERβ/CASP8/SRC‐1 protein complex are potential therapeutic targets to regulate apoptosis via the E2/ER/TNF‐α pathway.
3.3. Autophagy mechanism
Autophagy is a process related to nonapoptotic cell death and is defined as self‐degradation. It balances the energy sources by removing misfolded proteins, damaging organelles, and eliminating intracellular pathogens. It promotes the proteolytic degradation of cytosolic components at the lysosome. 199 Recently, there have been more studies on the role of autophagy in both accelerating and decelerating the pathogenesis of EM. To examine the pathophysiology of autophagy in regulating EM, we divided the section into antiautophagy and proautophagy to discuss the controversies in the progression of EM.
3.3.1. Antiautophagy, anti‐EM
Downregulation of apoptosis favors the stimulation of autophagy, thus promotes EM growth. 200 A significant reduction in apoptosis inducer p53 mediated by Akt, an increased lapidated microtubule‐associated protein 1A/1B light chain 3 (LC3)‐II, and a significant decrease in sequestosome 1 (SQSTM1) were observed in ovarian endometrioma. 200 LC3‐II is a standard autophagy marker while SQSTM1 is an autophagy adaptor protein that transfers ubiquitinated proteins to the autophagic machinery and is degraded via autophagy to indicate the activation of autophagic flux.197, 198, 199, 200, 201 Hypoxia upregulated autophagy in endometriotic cells to induce HIF‐1α. 202 Overexpression of HIF‐1α under normoxic conditions also induced autophagy. 203 Decreased expression of homeobox A10 (HOXA10) induced autophagy in EM, which was attributed to excessive inflammation. 204 It contributed to mitochondrial damage, increased PGE2, and increased mitochondrial ROS,203, 204 all stimulated autophagic processes. An increased oxidant heme oxygenase (HO)‐1 was observed in ovarian endometrioma to activate an adaptive defense mechanism and negatively modulate inflammation and apoptosis, and positively stimulated autophagy. 200 A combination therapy with MK2206 and chloroquine was found to be more effective than with either MK2206 or chloroquine in reducing endometriotic cell viability and preventing regrowth by inhibiting autophagy. 102 SQSTM1 expression was significantly upregulated. 102
3.3.2. Proautophagy, anti‐EM
Upregulation of autophagy promoted apoptosis and suppressed cell growth and invasion in EM.205, 206 Endometriotic stromal cells have an abnormal response to progesterone, which suppresses PTEN expression, suppresses autophagy, and reduces apoptosis in the menstrual cycle via the AKT/mTOR pathway. 207 Increased expression of YAP significantly decreased autophagy through the mTOR pathway in eutopic endometrium stromal cells. 208 Mullerian‐inhibiting substance (MIS) induced autophagy and apoptotic cell death and inhibited proliferation in vitro. 104 MIS, also known as anti‐Müllerian hormone, however, was found to be increased in EM lesions and in the serum of women with ovarian endometrioma and promoted inflammation. More preclinical data are required before the clinical application of this agent in EM treatment.209, 210 A ginsenoside metabolite, protopanaxadiol (PPD), reduced ERα expression and induced PRα expression in vitro and in vivo, which then induced autophagy and suppressed lesion growth, resulting in a significantly different expression of autophagy‐related genes, including downregulated estrogen receptor (ESR1), SQSTM1, and TGF‐β levels as well as upregulated CASP‐3, ATG ‐3/‐5/‐12 after treatment. 103
3.4. Anti‐Cell migration and invasion mechanism
Cell migration and invasion are critical processes for EM establishment according to the implantation theory. EM is believed to occur due to the shedding of endometrial cells and then migration to ectopic sites.15, 211 Migration of endothelial cells mediates angiogenesis and plays a role in the pathophysiology of EM. 212
3.4.1. Wnt/β‐catenin
The wingless‐type mouse mammary tumor virus integration site family (Wnt) plays a role in developmental processes and homeostasis. β‐catenin is crucial in regulating the cell cycle, which includes proliferation, differentiation, and migration in ectopic lesions. 213 In the presence of Wnt ligands, an accumulation of β‐catenin translocates to the nucleus and interacts with T‐cell factor/lymphoid enhancer‐binding factor (Tcf/LEF) transcription factors to activate the Wnt/β‐catenin signaling pathway. 107 Wnt/β‐catenin was found to be abnormally activated in EM. 214 Multi‐drug resistance protein 4 (MRP4) regulates Wnt/β‐catenin signaling by stabilizing β‐catenin activity. It was involved in the pathogenic transformation of EM endometrium, confirmed in the ectopic lesion. In MRP4‐knockdown endometrial epithelial cells, reduced activity of β‐catenin was found to downregulate Wnt/β‐catenin signaling. 215
Overexpression of T‐cadherin inhibited the invasion and migration of cells in EM, and the phosphorylation of heat shock protein (HSP)‐ 27 and c‐Jun N‐terminal kinase (JNK)‐1/2/3 was promoted. MMP‐2/‐9 and vimentin expression was lowered in endometriotic cells. 216 MMP‐2/‐9 and Cyclin D1 are targets of Tcf/β‐catenin genes and were found to be upregulated in endometrial epithelial or stromal cells of EM, 107 MMPs are responsible for regulating migration, invasion, and angiogenesis by balancing growth factors and cytokines, and high expression of MMPs in EM favors lesions. 217 PKF115‐584 and CGP049090 are fungal derivatives and were screened through high‐throughput assay to disrupt Tcf/β‐catenin complex, 218 significantly inhibiting MMP‐9 activity and cell invasiveness in epithelial and stromal cells to a level close to that of normal endometrium. 108 However, they have not been widely studied in EM. Genistein regulates invasion and migration through downregulation of MMP‐2/‐9 by targeting NF‐B, as shown in an in silico study and in an EM mouse model. 112 In addition, Wnt/β‐catenin is important for stem cell maintenance and tissue homeostasis 107 ; thus, an antagonist of Wnt/β‐catenin may have potential side effects. Moreover, Wnt2 is secreted by ectopic stromal cells, which induces β‐catenin signaling activity in ectopic endometrial epithelial cells, as well as expressions of the growth‐associated proteins in endometrial epithelial cells. 48 Wnt2/β‐catenin is a pathway involved in the communication of stromal and epithelial cells in EM, which can be modified by metformin. 48
3.4.2. Rho/ROCK
Ras homolog family member A/Rho‐associated coiled‐coil kinase (RhoA/ROCK) plays a major role in cell migration via phosphorylation of cytoskeletal regulatory proteins and results in actin depolymerization, actomyosin contraction, endothelial cell adhesion, and migration. 219 ROCK in the endothelial cytoskeleton is activated by proangiogenic stimuli. 219 VEGF stimulates RhoA/ROCK and mediates endothelial cell migration. 220 ROCKII regulates cell body contraction during migration. 221 It is a positive regulator of p27 to further activate cell migration in endometrial stromal cells and regulate RhoA in the cells. 222 Fasudil is a ROCK inhibitor used clinically and has the potential for treating EM by reducing endometriotic cell viability and inducing apoptosis by targeting Rho/ROCK. 115
3.5. Anti‐Fibrotic mechanism
Recently, EM was defined as a profibrotic condition, as a new concept by Vigano et. al.223, 224 The crucial role of fibrosis and differentiation of myofibroblasts in the progression of EM lesions have been reported. Fibrosis is the pathological activity when activated myofibroblasts accumulate leading to contraction of the collagenous extracellular matrix and anatomical structure disruption. The event of fibrosis justifies EM‐associated morbidity and adhesiveness and is considered a potential EM therapeutic target.223, 224
3.5.1. TGF‐β mediated pathways
TGF‐β1 is a stimulating factor that triggers the production of collagen and transition of epithelial to mesenchymal phenotype, leading to fibrosis. TGF‐β1 was found to be significantly increased in the peritoneal fluid of women with EM. 225 Wnt signaling is required for TGF‐β1‐mediated fibrosis. 226 Matsuzaki and Darcha 110 also showed that the Wnt/β‐catenin pathway was involved in fibrogenesis in EM. PKF1150‐584, CGP049090, and EGCG significantly inhibited TGF‐β1‐induced fibrotic markers, including α‐smooth muscle actin (α‐SMA), type I collagen, fibronectin, and connective tissue growth factor in stromal cells via Wnt/β‐catenin signaling, with EGCG showing the greatest effects.110, 111 ICG‐001 and C‐82 are CBP inhibitors. They are also metabolites of PRI‐724, which is under clinical trials for its antitumor activity, 227 they inhibited fibrosis via Wnt/β‐Catenin signaling, with ICG‐001 showing great efficacy in upregulating apoptosis and inhibiting migration as well as C‐82 showing potent inhibition of proliferation and cell viability. 106
3.5.2. Rho/ROCK
Activation of the Rho/ROCK signaling pathway was associated with fibrosis in EM. 228 Heparin has completed phase IV clinical trials for several medical indications, including anticoagulation and cancer. It inhibited RhoA, ROCKI, ROCKII, and α‐SMA expression and activated the Rho/ROCK pathway to attenuate endometriotic stromal cell contractility, differentiation, and fibrosis. 117
3.6. Antiangiogenesis mechanism
Angiogenesis is highly regulated in the female reproductive system, and it is a process that results in the formation of new blood vessels from existing ones. Three mechanisms of angiogenesis have been described as sprouting, elongation, and intussusception. 229 It provides neovascularization to deliver essential nutrients and oxygen supply for the growth of endometriotic lesions. 230
3.6.1. VEGF
VEGF regulates angiogenic, endothelial cell‐specific mitogenic, and vascular permeability activities of endometrial and endometriotic cells through vascular endothelial growth factor receptor (VEGFR) 1–3 on the microvascular endothelial cell surface. The VEGF family helps in establishing and maintaining endometriotic foci.231, 232, 233 VEGFR2 is a highly active kinase that plays a major role in angiogenesis. VEGF binds to two proximal VEGFR2 receptors, promotes vascular permeability, increases the migration and proliferation of endothelial cells, and contributes to the formation of new blood vessels.234, 235 Sunitinib, SU6668, SU5416, sorafenib, and pazopanib were originally indicated as anticancer drugs but were further repurposed and tested for efficacy against EM.119, 120, 123, 126, 128 SU5416 selectively bound to VEGFR, and only reduced graft size by 5%.123, 126, 236 SU6668, a multi‐kinase inhibitor, reduced the endometrial graft by 25% by blocking VEFFR‐2, fibroblast growth factor receptors (FGFR)‐1, and platelet‐derived growth factor receptors (PDGFR)‐β.123, 236 Sunitinib regulates angiogenesis and apoptosis through multi‐kinase inhibition, regressing 50% cyst in an EM rat model. 120 The effects of pazopanib, sunitinib, and sorafenib on VEGF/VEGFR protein kinase pathways and their actions in EM were compared by Yildiz et. al., 128 which showed that pazopanib had better efficacy than the control and other treatments, reducing EM lesions by at least 45%, but Sorafenib was better in regulating VEGF. 128 However, tyrosine kinase inhibitors that regulate VEGFR are associated with a significant risk of treatment toxicities. 237 In sarcoma, pazopanib treatment led to a higher incidence of adverse effects, including fatigue and hypertension, compared with sunitinib or sorafenib. 237 An alternative VEGF regulator, EGCG, significantly inhibited lesion growth by suppressing VEGFC/VEGFR2 signaling. Overexpression of VEGFC induces migration of endothelial cells, increases vascular permeability, and induces angiogenesis and endometriotic lesions growth.70, 129 Prodrug of EGCG (ProEGCG), reduced lesion size, weight, and VEGF concentrations in plasma to a greater extent than the parent EGCG molecule. More importantly, there were no signs of side effects on reproductive tissues. 130 Quinagolide, a dopamine receptor 2 agonist, is under phase II clinical trial to examine its efficacy in reducing EM lesion size and related pain. It can completely reverse the size of lesions by downregulating the VEGF/VEGFR2 pathway in EM. 135 It has an acceptable safety profile and does not stimulate serotonin receptor subtype 2b to proliferate fibroblasts in cardiac valve tissues, and thus holds great potential as an alternative of tyrosine kinase inhibitor for EM. 135
Under normoxic conditions, HIF‐1α is regulated via proteasome‐mediated degradation. 238 However, under hypoxic conditions, HIF‐1α escapes ubiquitination and binds to hypoxia‐responsive enhancer on VEGF genes to upregulate their expression. 239 HIF‐1α was upregulated in lesions, thus promoting VEGF expression in an EM mouse model. 240 Increased VEGF secretion was observed in hypoxia‐induced endometrial stromal and glandular cells compared with that under normoxic conditions. 241 Oxidative stress also increased VEGF secretion, as shown by the results obtained after incubating endometrial epithelial cells with oxidized low‐density lipoprotein. 242 In the EM peritoneal environment, PGE2 was upregulated to elicit cell signals through upregulation of VEGF and FGFR. It induced the expression of COX‐2 and synthesis of E2 in ectopic endometrial cells to increase the production of MMP, thus enhancing VEGF expression and inducing angiogenesis.229, 243 TNF‐α mediates the angiogenic activity of macrophages, which stimulates endothelial cell migration and induces the release of VEGF and the formation of bloodvessel. 244 Pyrrolidine dithiocarbamate inhibited NF‐κB activation and attenuated TNF‐α‐mediated VEGF and MMP‐9 expressions. 132 Pentoxifylline attenuated TNF‐α mediated effects in other diseases, requires further investigation of this in EM, but it suppressed angiogenesis by reducing VEGFC and VEGFR2 (Flk‐1) expression levels in glandular cells of endometriotic lesions. 133 Pentoxifylline is an immunomodulatory agent and has completed a phase III clinical trial of EM‐associated infertility. The clinical trial did not present any data on lesion progression or recurrence, only on pregnancy rate. 245
3.6.2. Rho/ROCK
The Rho/ROCK pathway regulates VEGF‐mediated endothelial cell activation and vessel stability.233, 246 RhoB mitigates VEGF‐induced vessel sprouting via the RhoA/ROCK signaling pathway. 247 RhoA/ROCK activity can be blocked by inhibiting protein prenylation in endothelial cells to reduce migration and adhesion. 248 Avian myelocytomatosis virus oncogene cellular homolog (C‐Myc) is a target of the PI3K/Rho/ROCK signaling pathway and regulates VEGF expression. Under hypoxic conditions in EM, guanosine triphosphate (GTP)‐bound Rho was regulated in a PI3K‐dependent manner to induce VEGF by binding to C‐Myc without suppressing the induction of HIF‐1α. 249 ROCK activation initiates E2‐induced angiogenesis. 219 Inhibitors that target the Rho/ROCK pathway should be further investigated for their ability to regulate angiogenesis, migration, invasion, and fibrosis of endometriotic cells.
3.7. Antioxidative stress mechanism
Oxidative stress is the imbalance between ROS production and antioxidant function, which plays a main role in EM progression. 250 ROS are molecules that have unpaired electrons and can damage lipids, nucleic acids, and proteins.251, 252 Apoptotic endometrial tissues and macrophages induced oxidative stress in EM through retrograde menstruation. 253 Oxidative stress causes DNA hypermethylation and histone modification, which are linked to aberrant endometrium development in EM. 254
3.7.1. ROS‐mediated pathway
The production of ROS in the progression EM could be achieved through several pathways, including activation of inflammatory cytokines, MMPs, and transcriptional factors, such as NF‐κB. 255 Environmental factors, such as reproductive toxins, can also increase oxidative stress and decrease the expression of antioxidant enzymes. 256 Di‐2‐ethylexyl phthalate is used as a plasticizer and solvent in cosmetic and consumer products, and they altered the NF‐κB signaling pathway and expression levels of ER and PR in human endometrial stromal cells via activation of the MAPK/ERK and PI3K/Akt signaling pathways. 257
Balancing ROS production and antioxidant function by inhibiting free radicals or increasing antioxidant levels is important to regulate oxidative stress. Antioxidants include the enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx), HO, and catalase, as well as nonenzymatic molecules such as vitamins A, C, and E.250, 252, 255 Significantly lower levels of antioxidants were found in the peritoneal fluid of EM, which indicated that women with EM had a low free radical‐scavenging ability.255, 258, 259 N‐Acetyl cysteine (NAC) is an antioxidant that inhibits ROS as well as abrogates ERK activation and proliferation. 137 EGCG and resveratrol are both well‐known natural antioxidant supplements for the treatment of EM and other diseases, including cancer.130, 138, 260 Resveratrol acts as a radical scavenger, and it significantly regressed endometriotic implants, decreased lipid peroxidation, and increased at least 50% endogenous antioxidant capacity in tissues and serum in EM. 138 The antioxidant effects of ProEGCG against EM were significantly greater than those of EGCG and at least four fold that of the control. 130 Melatonin reduced the level of oxidative stress markers and increased level of antioxidants in EM. EM implants in rat were significantly regressed.142, 143 Caffeic acid found in plants exerted similar antioxidant effects in EM and an enhanced nuclear translocation of nuclear factor erythroid 2–related factor 2 (Nrf2) regulated antioxidant enzymes in EM.141, 261
3.7.2. NO‐mediated pathway
NO is a vasodilator that mediates endothelium‐dependent vasodilation and angiogenesis. 262 NO has an unpaired electron and is a highly reactive free radical. The formation of NO requires NO synthase (NOS), l‐arginine, oxygen, and a number of cofactors, including nicotinamide adenine dinucleotide phosphate, flavin mononucleotide (FMN), and flavin adenine dinucleotide. 263 Macrophages increased IL‐10 in EM and stimulated NO 264 ; NO and NOS levels were increased in endometrial tissues in EM. 265 Increased E2 level activated the formation of NO, 266 implying that macrophages and E2 regulate NO‐mediated oxidative stress in EM.
3.7.3. Iron‐mediated pathway
Iron carries hemoglobin throughout the body, an overproduction of it not only enhances epithelial cell proliferation but also induces oxidative stress.267, 268 Ferritin is a cellular iron storage that generates hydroxyl radical via Fenton reaction to initiate a free radical chain reaction, namely lipid peroxidation.37, 269 McKinnon et al. 37 reviewed studies on the implication of mTOR on iron homeostasis and suggested the dysregulation of mTOR in EM could overload iron levels to stimulate oxidative stress. Currently, there is lack of pharmaceuticals targeting iron‐mediated oxidative stress in EM and regulating the mTOR signaling pathway might reduce oxidative stress.
3.8. Immune system and inflammation
Immune system dysregulation and chronic inflammatory response are characterized in EM.270, 271 The adaptive immune system with increased quantity of regulatory T (Treg) cells and a shift towards type 2 immune response fail to recognize the endometriotic cells in the peritoneal cavity. 272 On the contrary, the innate immune system in EM is characterized by an enhanced activation state of macrophages, along with upregulated cytokines, but downregulated phagocytosis, 273 as well as a reduced cytotoxicity of natural killer (NK) cells, 274 and an altered population of dendritic cells. 275 These promote inflammation and contribute to the implantation process in EM and new drugs that modulate these specialized cells hold promise as a novel immunotherapy for EM.
3.8.1. Macrophages and cytokines
Macrophages exert their inflammatory effects against tumors via host defense mechanisms. 276 IL‐1 is a proinflammatory cytokine secreted by activated monocytes, macrophages, or NK cells, and is responsible for activating lymphocytes to reduce immune surveillance and stimulate PGE2 via COX‐2 in EM stromal cells.271, 277, 278 IL‐6 is responsible for stimulating B‐cell activity and T‐cell differentiation. The levels of IL6 in serum and peritoneal fluid are high, but its receptor is reduced in EM. However, endometriotic cells are resistant to its growth‐inhibitory effects.271, 279, 280 VEGF and TNF‐α are proinflammatory cytokines secreted by activated lymphocytes, neutrophils, NK cells, and macrophages to initiate the inflammatory cascade. 271
Niclosamide is an FDA‐approved nonsteroidal therapy for antihelminth 281 and was found to inhibit the proliferation and growth of endometriotic lesions. It reduced MAPK, WNT, and inflammation signaling‐related genes, such as NF‐κB and signal transducer and activator of transcription 3, in an EM mouse model. No disruption to reproductive function was observed, indicating potential therapeutic efficacy and safety for EM treatment. 154 NAC regressed lesions by suppressing COX‐2 and MMP‐9 expression. Its side effect is mild and seemed to not interfere with fertility in vivo. 144 Crocin, curcumin, and metformin inhibit proinflammatory cytokines and chemokines, including TNF‐α, IL‐1β, IL‐6, VEGF, and so forth.148, 149, 155 These are responsible for recruiting and activating macrophages, neutrophils, and NK cells to the EM site and further enhancing angiogenesis and inflammation.155, 282 Acai, a natural product found in plants from the Amazon region, has completed phase III clinical trials as an antioxidant agent. It reduces EM lesions by targeting active macrophages, VEGF, and COX‐2. 156 Resveratrol inhibited inflammatory responses by reducing peritoneal and serum cytokines, 150 as well as activating sirtuin 1 (SIRT1) to significantly suppress IL‐8 in TNF‐α‐induced endometriotic stromal cells via NF‐κB. 151 SIRT1 has a dual function as a tumor suppressor or promoter, 283 and it is a potential target protein, considering that it is a strong regulator of the inflammatory responses, apoptosis, and oxidative stress in EM. 284 Macrophage migration inhibitory factor (MIF) is a proinflammatory cytokine that is upregulated in peritoneal fluid in women with EM. It activates the MAPK/ERK pathway, stimulates COX‐2, and produces PGE2 in ectopic endometrial cells. MIF also contributes to angiogenesis via its effect on endothelial cell proliferation. 285 ISO‐1 is a MIF antagonist and a leading molecule discovered to treat sepsis. 286 It inhibited angiogenic and proinflammatory pathways via VEGF/VEGFR in peritoneal EM in vivo, without interrupting the reproductive cycle. 287
3.8.2. Estrogen
In EM, increased ESR2/E2 induces COX‐2 and PGE2, upregulates macrophages and NF‐κB,288, 289 and leads to oxidative stress. 255 ERβ modulates macrophage infiltration via NF‐κB in EM 290 and induces IL‐1β by interacting with the inflammasome complex to evade immune surveillance and promote the attachment of lesions at the endometriotic sites. 196 E2 activates thymic stromal lymphopoietin and induces the secretion of endometrial stromal cells‐associated growth‐promoting cytokines, including monocyte chemoattractant protein 1 and IL‐8, via the JNK and NF‐κB pathways. 291 IL‐6 reduces E2 production in human granulosa tumor cells via the MAPK signaling pathway, 292 implying its possible targeting of E2 biosynthesis in EM. Puerarin reduced the level of ERβ, but not ERα, by inhibiting P450AROM. In a rat model, simultaneous reduction of E2, COX‐2, and PGE2 expression levels, as well as enhancement of the metabolism of E2 into estrone, 152 led to the inhibition of lesion growth in the ectopic endometrium tissues. In another study, ginsenoside PPD inhibited the E2 signal, thus activating the cytotoxicity of NK cells against ectopic endometrial stromal cells to regulate cell death. This was also confirmed in peritoneal fluids of the EM mouse model. 103
4. POTENTIAL PHARMACEUTICALS WITH MULTIPLE TARGETS
In the treatment of EM, targeting a specific pathway, or multiple pathways alleviate the lesions. Targeting a single molecule can lead to several anti‐EM effects, as downstream transduction elements are usually connected to a series of molecular events as secondary responses. However, owing to synergistic effects, a multiple target therapy may have a greater suppressive effect on lesions compared with a single targeted therapy. 196 Table 4 summarizes several single pharmaceuticals with multiple molecular targets, which affect multiple signaling pathways in a complex disease such as EM.
Table 4.
Pharmaceuticals that hold multiple molecular targets to different pathophysiology for endometriosis treatment
| Pharmaceuticals | Classification | Proliferation | Apoptosis | Autophagy | Cell migration | Cell Invasion | Fibrosis | Angiogenesis | Oxidative stress | Immune and inflammation |
|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin | Anxiolytic agent | ‐ | MMP‐3 63 | ‐ | ‐ | ‐ | ‐ | VEGF, MMP‐9 142 | ROS 143 | COX‐2 143 |
| Metformin | Antidiabetic agent | ‐ | ‐ | ‐ | ‐ | Wnt2 48 | ‐ | ‐ | ‐ | Cytokines 149 |
| NAC | Amino acid cysteine | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ROS 137 | Cytokines 144 |
| Curcumin | Curcuminoid | ‐ | MMP‐3 81 | ‐ | ‐ | ‐ | ‐ | Chemokine and cytokines 253 | ‐ | Chemokine and cytokines 253 |
| EGCG | Catechin | E2 167 | ‐ | ‐ | TGF β1 110 | TGF β1 110 | TGF β1 110 | VEGFR270, 129 | ROS 130 | ‐ |
| TGF β1 110 | ||||||||||
| VEGFR270, 129 | ||||||||||
| Genistein | Isoflavone | ‐ | TGF‐β 85 | ‐ | NF‐κB 196 | NF‐κB 196 | ‐ | ‐ | ‐ | ‐ |
| Ginsenoside | Steroid glycoside | TNF‐α 79 | TNF‐α 79 | ERα and PRα 103 | ‐ | ‐ | ‐ | TNF‐α 79 | ‐ | Induce NK cells toxicity 103 |
| Puerarin | Isoflavone | ERs 93 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | P450AROM 152 |
| Resveratrol | Phytoalexin | ESR1 66 | ‐ | ‐ | ‐ | ‐ | ‐ | Cytokines 150 | ROS 138 | Cytokines, 150 SIRT1 151 |
Abbreviations: AKT, protein kinase B; AMPK, adenosine monophosphate‐activated protein kinase; CASP, caspases; CHOP, CCAAT/enhancer‐binding protein homologous 10 protein; COX, cyclooxygenase; DPPH, 2,2‐diphenyl‐1‐picrylhydrazyl; E2, Estrogen; EGCG, epigallocatechin gallate; ER, estrogen receptor; ERK, extracellular signal‐regulated kinase; ESR1, estrogen receptor 1; HMGB1, high mobility group box 1; H2O2, hydrogen peroxide; IKKB, IκB kinase beta; MAPK, mitogen‐activated protein kinase; MMP, matrix metallopeptidases; NAC, N‐acetyl cysteine; NF‐κB, nuclear factor κB; NK cells, natural killer cells; NOD2, nucleotide‐binding oligomerization domain‐containing protein 2; Nrf2, nuclear factor erythroid 2–related factor 2; O2, oxygen; OH, hydroxide; P450AROM, aromatase; PI3K, phosphoinositide 3‐kinases; PR, progesterone receptor; REDD1, protein regulated in development and DNA damage response 1; ROS, reactive oxidative stress; SIRT1, sirtuin 1; TCM, traditional Chinese medicine; TGF, transforming growth factors; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; Wnt, wingless‐type mouse mammary tumor virus integration site family.
4.1. Hormonal pharmaceuticals
Melatonin is a natural substance produced by plants. It is also a hormone produced in the pineal gland to regulate neuroendocrine functions and inhibits LH and FSH secretion from the anterior pituitary gland. 293 Melatonin acts as an antioxidant and anti‐inflammatory agent and is currently under phase 2 clinical trial for reducing EM‐related pain. Another randomized, double‐blind, and placebo‐controlled clinical trial of melatonin was completed in 2013. The results of the study showed that melatonin acts as an analgesic and can relieve EM‐related chronic pain. 294 Melatonin receptor (MR)1A and MR1B are significantly upregulated in peritoneal EM lesions compared with those in the eutopic tissue. Melatonin has been shown to reduce EM lesions in various studies. It inhibits cell proliferation and modulates endometrial epithelial cell function. 295 Melatonin also inhibits angiogenesis via VEGF and oxidative stress via regulating radical scavenging activity and amplifies apoptotic activity via CASP3 mediated pathway in vivo and in vitro in EM.63, 142, 143 Melatonin has no adverse effects on reproductive functions, instead, it can improve ovarian functions, and thus has potential to treat EM‐related infertility.296, 297 High‐dose intravenous treatment of pain and sepsis with melatonin showed no adverse effects. 298 Its bioavailability is 15%. 299 Long‐term therapeutic investigation of melatonin in EM should be conducted to elucidate its ability to regulate E2 functions in EM.
4.2. Nonhormone pharmaceuticals
Metformin was shown to target multiple pathways 300 by regulating stromal‐epithelial cell communication in EM via Wnt2‐mediated signaling 48 and exerted an anti‐inflammatory effect through regulating cytokines. 149 Although a mild side effect was implied, 149 metformin regulated reproductive functions, 301 and improved conception in EM patients by inhibiting serum cytokine production. 149 Metformin is available in the market as a treatment for type 2 diabetes and PCOS in women. Considering its low cost, metformin was advocated to be used as a long‐term treatment. 302 NAC, an acetylated form of cysteine, has been prescribed as an antidote since the 1960s. It replenishes intracellular glutathione levels and modulates the redox environment; therefore, NAC is a strong antioxidant. 303 In EM, it acts as an antioxidant, antiproliferative, anti‐inflammatory, and anti‐invasiveness agent via ROS‐scavenging mechanism or through regulating cytokines in vitro and in vivo.137, 144 NAC is highly efficacious at low doses, and with no adverse effects in EM. 144 Long‐term adverse effects are also limited, including no effect on fertility.137, 144 NAC is considered to have a good safety profile and has been evaluated in phase 4 clinical trials for treating gastrointestinal and metabolic diseases.303, 304 Its pharmacokinetics and toxicity profiles are available; its terminal half‐life is 6.25 h after oral administration and bioavailability is 9.1%. 305 NAC is commercially available and cost‐effective as a dietary supplement in the market; however, studies on its efficiency in EM are limited, requiring more preclinical evidence.
4.3. Natural products
Natural products have a long history of use in the management of medical conditions. Research advances in analytical and synthetic chemistry have improved the identification and isolation of active compounds from natural products. EGCG is a polyphenol catechin from green tea and a well‐known antioxidant. It exerts efficacy against diseases including cancer, diabetes, and inflammation. 306 In EM, it exerts ant antiangiogenetic effect via the VEGFC/VEGFR2 pathways,70, 129 antioxidant effects via ROS‐scavenging mechanism, 130 antiproliferative effect via reduction of E2 production, and anti‐migration and anti‐invasion effects via TGF‐β1‐induced phosphorylation of ERK1/2 and MAPK pathways, 167 thus inhibiting the development and growth of lesions. Promising evidence of its high potency and efficacy, and without major side effects in reproductive functions were reported.130, 167 EGCG is currently under phase 2 clinical trial for reducing lesion size and pain as well as an evaluation of its safety profile in EM. On the contrary, EGCG act as an adjuvant that brings synergistic effects, as well as reduces adverse effects in cancer treatment. 307 This suggests a potential role of EGCG in combination therapy with current EM treatment. However, the low bioavailability of EGCG has limited its attractiveness in the market. 308 ProEGCG, is a prodrug of EGCG, shows higher bioavailability and greater efficiency than EGCG to reduce lesions in vivo. 130 More studies should be conducted to confirm the underlying mechanism of ProEGCG in the treatment of EM. Resveratrol is a polyphenol found in grapes. In EM, it reduces proliferation via an anti‐E2 mechanism targeting ESR1, 66 inhibits inflammatory responses via radical scavenging, 138 and inhibits angiogenesis by reducing the cytokines COX‐2 and VEGF 150 and activating SIRT1. 151 Resveratrol has completed a phase 4 clinical trial in EM and is safe and effective in relieving EM‐related pain, as well as in reducing serum CA125 and prolactin levels. Resveratrol is well‐known for its chemopreventive property. It also has a promising clinical profile in cancer treatment, nevertheless, the rapid metabolism rate of resveratrol has limited its efficacy in vivo. 309
Curcumin, genistein, ginsenoside, and puerarin are not under any EM clinical studies but have been clinically evaluated in breast cancers, endometrial carcinoma, endothelial functions, and so forth. They have been studied for their action mechanism against EM in primary cells, cell lines, and animal models. Curcumin, which is found in ginger and turmeric, enhances apoptosis by increasing the Bax/Bcl2 ratio through targeting of MMP‐3 via NF‐κB 81 and regulates angiogenesis and inflammation by targeting chemokines and cytokines 155 in EM. It has multiple biological effects in different diseases, including cancer and inflammatory diseases. It establishes a good safety profile with no acute toxicity. 310 Genistein is an isoflavone that acts as an E2 agonist or antagonist to manage postmenopausal symptoms. In EM animal models, genistein downregulated MMP‐2/‐9 and regulated cell invasion and migration by targeting NF‐κB. 311 It also regulated NF‐κB by inhibiting TGF‐β. 85 Long‐term treatment with genistein lower the incidence of endometrial hyperplasia and provided support for bone formation in postmenopausal women.83, 312 It acts as a chemopreventive and chemotherapeutic agent against cancers and has synergistic effects with other anticancer drugs. 313 Toxicity of high dose is minimal, so it needs more study to test the safe range. 314 Ginsenoside RG3, extracted from ginseng, restored TNF‐α‐induced effects by inhibiting NF‐κB, VEGF, and CASP‐3 in EM, which are responsible for cell proliferation, apoptosis, and angiogenesis. 79 Ginsenoside PPD regulated ERα and PRα expression to suppress autophagy and lesion growth in EM. 103 It also targeted E2‐induced NK cell cytotoxicity to regulate the immune system in EM. 103 Ginsenoside also possess synergistic effect with anticancer drugs, as well as prevents toxicity and morbidity from chemotherapy. 315 Puerarin is a phytoestrogen, binds to ERs via the ERK pathway to regulate proliferation in EM. 93 It also regulates inflammation in ectopic endometrium by inhibiting P450AROM and COX‐2 and promoting ERβ expression to facilitate E2 metabolism in EM. 152 Its therapeutic effects are studied extensively in diseases including cancer and cardiovascular disease. 316
Most of these products have known toxicity or pharmacokinetic profiles and act via multiple targets, making them beneficial as anti‐EM agents. However, their poor aqueous solubility and low oral bioavailability in vivo is the major challenge to be potential EM treatment.82, 92, 315 There are several approaches available currently to progress the bioavailability of drugs, which include prodrug approach, 130 solid dispersions approach, 317 lipid‐based formulation approach. 318 On the contrary, the long‐term safety of natural products in reproductive function and EM recurrence profiles should be further elaborated in future studies. Nevertheless, minimal side effects and available as an over‐the‐counter dietary supplement and routine remedies make them preferable to hormonal medicines.
EM is a complex clinical challenge, and recently, more signaling pathways have been identified to contribute to its pathophysiology. EM drugs that target only one receptor have inadequate therapeutic efficiency. However, although multitarget drugs present potent efficacy in suppressing the progression of EM lesions, they also pose a risk of side effects such as binding to undesirable drug targets and bringing off‐target toxicities. 56 Therefore, designing a drug that targets the appropriate pathways with high selectivity is highly desirable. For this purpose, it is essential to understand the compound‐target pathway‐disease relationships.
5. TRADITIONAL CHINESE MEDICINE (TCM)
In the theory of Chinese medicine, EM is defined as a blood stasis syndrome that leads to the formation of endometriotic lesion and other associated symptoms. Stagnation of Qi (energy) is believed to be one of the causes of EM. TCM aims to lessen the chronic pain experienced by women with EM. Therefore, studies on the action mechanism of TCM are focused mainly on the alleviation of inflammation and oxidation. TCM decoctions containing several herbs in different compositions, which are varied according to the condition of the patient, are a combinational approach that can target various pathophysiology. Fang et al. 319 and Tsai et al. 320 have identified the decoctions commonly used for treating EM in Taiwan, which included Gui‐Zhi‐Fu‐Ling‐Wan, Dang‐Gui‐Shao‐Yao‐San, Jia‐Wei‐Xiao‐Yao‐San, Shao‐Fu‐Zhu‐Yu‐Tang, and Wen‐Jing‐Tan. The therapeutic efficacy and pathophysiology of TCM in cancer and other diseases have been widely evaluated in vitro and in vivo; however, there are limited studies on the efficacy of TCM for EM.
Most of the herbs exert anti‐inflammatory effects by inhibiting the production of proinflammatory cytokines. Poria has been confirmed to exert antitumor activities against various cancers. It binds to cytokines and effector immune cells to regulate immunity and upregulate apoptosis. 321 Angelicae Sinensis Radix exerts anti‐inflammatory effects by reducing TNF‐α inflammatory cells. 322 Ligusticum Rhizoma inhibits inflammation and reduces PGE2 production. 323 Moutan Cortex, Glycyrrhizae Radix, Paeoniae Alba Radix, and Bupleuri Radix suppress proinflammatory cytokines via the NF‐κB signaling pathways.324, 325, 326, 327 Paeoniae Alba Radix and Bupleuri Radix also exert such effect via MAPK signaling pathways.
Atractylodis Ovatae Rhizoma exerts antioxidant effect by activating the MAPK cascades and inhibiting the production of radicals by 2,2‐diphenyl‐1‐picrylhydrazyl and catalases, thus inhibiting the activity of free radicals. 328 Glycyrrhizae Radix and Poria act as radical scavengers against superoxide and hydroxy radicals.328, 329, 330 Ligusticum Rhizoma acts as a reducing agent via the Nrf2 and NF‐κB pathways. 331
Angelicae Sinensis Radix exerts antiproliferative and proapoptotic effects; it induces mitochondrial‐dependent apoptosis and inhibits the Akt/mTOR pathway. 332 Atractylodis Ovatae Rhizoma induces apoptosis by upregulating ROS. 333 Moutan Cortex exerts proapoptotic effects by increasing Bax/Bcl‐2 expression and decreasing MMP via the formation of apoptosome and cytochrome c, activation of CASP, and the adenosine monophosphate‐activated protein kinase pathway. 323 It also induces apoptosis via activation of CASP‐3/‐8. 334 Paeoniae Alba Radix induces apoptosis via activation of CASP‐3/‐9 293 and exerts antiproliferative activity via cell cycle arrest and Fas/Fas ligand‐mediated apoptotic pathway. 334 It also downregulates the antiapoptotic protein Bcl and upregulates the apoptotic proteins Bax and CASP‐3. 335
TCMs have great potential as multitarget drugs. As TCMs consist of herbal formulas with various combinations of herbs, they have multiple mechanisms of action, which can be beneficial to reduce the concentration of each herb, thus, drug toxicity. 336 However, the costs and availability vary for different herbs, which limits its acceptability in Western countries at present. Furthermore, there is a lack of clinical management methods to evaluate their clinical effectiveness and standardized regulations of TCM practice.
6. CONCLUDING REMARKS AND PERSPECTIVES
This is the first review article combining medicinal research based on EM pathophysiology and the related signaling pathways. Our review revealed the challenges in EM management and the need for various available medical treatment options. Most of the medications prescribed by the FDA to treat EM are hormonal, such as contraceptives, progesterone, and GnRH. However, current hormonal medicines raise a major concern in the case of long‐term treatment. Therefore, new nonhormonal pharmaceuticals with relatively safer and few side effects are urgently needed.
Our aims in this review were to facilitate the research and development of novel treatments for EM based on an understanding of the pathological process. To compare new and old pharmaceuticals, an effective scale to evaluate parameters between different treatments as well as to align outcome measures from preclinical to clinical studies is needed. There is a lack of experimental and clinical evidence to support the effectiveness, pharmacokinetic, and pharmacodynamic profiles of potential drugs in alleviating the pathophysiology of EM, compared with that of drugs already available in the market. Good practices such as the Endometriosis Phenome and Biobanking Harmonization Project, derived by the World Endometriosis Society, can help facilitate a large‐scale collaboration project worldwide. 337 It is a platform to ensure that the protocol is sufficient and consistent enough to maintain high research quality, datasets are shared to ensure data reproducibility, and results can better support the development of translational medicine. Moreover, multicenter collaboration can increase research visibility and avoid data integrity issues.
The nonhormonal treatments reviewed in this paper were only studied in vitro or in animal models or are still under clinical trials. The drugs mentioned in this review article showed significant efficacy in reducing ectopic endometrium cell viability and endometriotic lesion size; however, severe adverse effects were not elaborated in‐depth. High efficacy and innovative approach do not guarantee final success. Data from legal regulation and patients’ demand for available resources are as important as the pharmacological profile of medicines. In many countries, a new drug must be regulated and approved by the relevant authority before it is launched in the market. 338 Thus, apart from efficacy and safety data, the medical and financial burden of EM to women have raised the awareness on EM and accelerated the scientific research on this disease, which are key factors considered by R&D investors. To maximize a drug's value and cost‐effectiveness in the market while maintaining its affordability, fulfilling the society's demand, and making scientific advances, modification of lead compound or bioactive compound derived from natural products holds great potential because only the functional groups are modified, whereas the original core structure is conserved.
Considering both the medicinal and commercial perspectives of drug development, there is a huge pressure in the development of a new drug, starting from the synthesis or discovery stage to clinical trial, to proceeding with legal regulations, and to launch in the market. A drug requires 10–17 years of development, with less than 10% success rate to pass clinical trial. 339
Taking advantage of big data mining, drug repurposing is a strategy to identify new therapeutic use of a drug that is approved or under clinical trial, which comprises 30% of newly FDA‐approved drugs and vaccines.340, 341 These drugs can bind to the same target owing to the similar pathophysiology of different diseases, or these drugs can have multiple targets and are thus relevant to other diseases. 342 A repurposed drug offers sufficient preclinical pharmacology profile and safety reports, leading to a greater potential for phase III and IV clinical trials, which can reduce the time of drug development and cost of investment. Nevertheless, some advantages of de novo drug development outweigh the benefits of drug repurposing. A constant influx of chemicals via synthesis or extraction from natural products offers novel medical options for patients. A growing understanding of the pathophysiology of EM favors structure‐based or ligand‐based drug designs, in which by modifying lead compounds based on structure–activity relationships, the efficacy, potency, and selectivity can be compromised. However, a more in‐depth research is needed to study the underlying mechanisms and drug targets to support their potential as new EM treatments.
In conclusion, this review provides an update on the pathophysiology of EM and shows the efficacy of various medicines in treating EM. Increasing attention has been focused on understanding the pathophysiology of EM and the action mechanisms of potential pharmaceuticals; however, many of these are still not completely understood. With this review, we hoped to raise awareness on the missing puzzle pieces and to promote related research that can further advance diagnosis and treatment for better management of EM and improve the quality of women's lives.
CONFLICT OF INTERESTS
Chi Chiu Wang is an active member of the World Endometriosis Society and an advisor of the Aptorum Group.
AUTHOR CONTRIBUTIONS
Sze Wan Hung, Ruizhe Zhang, and Chi Chiu Wang participated in research design. Sze Wan Hung participated in data evaluation, extraction and interpretation. Sze Wan Hung, Ruizhe Zhang, and Zhouyurong Tan participated in data validation and in drafting the manuscript. Tao Zhang participated in designing figure 1. Sze Wan Hung, Tao Zhang, Jacqueline Pui Wah Chung and Chi Chiu Wang critically revised the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
This study was supported by General Research Fund from Research Grants Council, Hong Kong (475012); Health and Medical Research Fund from Food and Health Bureau, Hong Kong (03141386); Innovation and Technology Fund from Innovation and Technology Commission, Hong Kong (ITS/209/12); UGC Direct Grant from The Chinese University of Hong Kong, Hong Kong (2011.2012); HKOG Trust Fund, Hong Kong (2011, 2014, 2019); and National Natural Science Fund from National Natural Science Foundation of China, China (81974225).
Biographies
Sze Wan Hung is currently a PhD student of the Department of Obstetrics & Gynaecology at The Chinese University of Hong Kong. She got her MChem degree with honors in Medicinal and Biological Chemistry at The University of Edinburgh, UK, in 2017 and is currently an active member of The Royal Society of Chemistry (MRSC). Her research interest focuses on drug development for the treatment of endometriosis and she has received one research funding in the area.
Ruizhe Zhang is currently a PhD student of the Department of Obstetrics & Gynaecology at The Chinese University of Hong Kong. She got her bachelor's and master's degree in clinical medicine from Zhengzhou University, China respectively in the years 2015 and 2017. She has received clinical training in the department of O&G in the First Affiliated Hospital of Zhengzhou University. She has passed the examination of Attending Doctor of O&G in mainland China. Her main research interests include the treatment of endometriosis in artificial reproductive technology, DNA methylation modification in reproductive failure, endometrium imaging, and granulosa cell signaling pathway. She has published five articles in international peer‐reviewed journals and applied for three funding in these areas.
Zhouyurong Tan is currently a Mphil student of the Department of Obstetrics & Gynaecology at The Chinese University of Hong Kong. She got her BSc. degree of biomedical sciences in the Queen Mary University of London (UK) in 2019, and BM degree in clinical medicine in the University of Nanchang (China) in 2020. Her research interest focuses on endometriosis and human reproduction.
Tao Zhang is currently a Research Assistant Professor of the Department of Obstetrics & Gynaecology at The Chinese University of Hong Kong. She got her bachelor of clinical medicine from Changzhi Medical College in 2007 and master's degree in Obstetrics & Gynaecology from Shanghai Jiaotong University in 2010. She then received her PhD degree in 2013 and had postdoctoral training in 2015 at The Chinese University of Hong Kong. In addition, she gained her clinical practice in Reproductive Medicine Center as an IVF specialist for 3 years in China. Her research interests include the pathology of endometriosis, immunology in reproductive failures, and pathophysiology of polycystic ovarian syndrome. Dr. Zhang has obtained several external competitive grants as well as international and local awards in the field of reproductive immunology. She has published more than 20 articles in peer‐reviewed journals, including the European Journal of Immunology, Autoimmunity Reviews, Angiogenesis, Fertility and Sterility, and the American Journal of Reproductive Immunology. Dr. Zhang is also an active member of numerous societies and reviewer for selected peer‐reviewed journals, such as the American Journal of Reproductive Immunology and the International Journal of Molecular Sciences.
Jacqueline Pui Wah Chung is currently Associate Professor of Department of Obstetrics and Gynaecology at The Chinese University of Hong Kong and as an Honorary Associate Consultant at the Prince of Wales Hospital. She is the Deputy Director of the Assisted Reproductive Technology Unit and the Deputy Director of the Preimplantation Genetic Diagnosis Laboratory in the Chinese University of Hong Kong. She graduated from the Faculty of Medicine at the Chinese University of Hong Kong and received training in the field of Obstetrics and Gynaecology at the Prince of Wales Hospital. She obtained her fellowship at the Hong Kong College of Obstetricians and Gynaecologists and the Hong Kong Academy of Medicine (Obstetrics and Gynaecology) in 2013. Thereafter, she developed a special interest in Reproductive Medicine and completed her subspecialty training in 2016. Dr. Chung published more than 40 peer‐reviewed journals and four book chapters on these areas. She obtained several internal and external competitive grants as principal investigator and co‐investigator and was awarded the Distinguished Young Fellow from the Hong Kong Academy of Medicine in 2018 and the Inga Award for Excellence in Obstetrics and Gynaecology. Dr. Chung is a Council Member of the Hong Kong College of Obstetricians and Gynaecologists and the Honorary Secretary of the Reproductive Medicine Subspecialty Board of the Hong Kong College of Obstetricians and Gynaecologists. She is also the Honorary Treasurer of the Hong Kong Society for Reproductive Medicine. Moreover, she is an active member of the International Society for Fertility Preservation.
Chi Chiu Wang is currently Division Head and Professor of Department of Obstetrics & Gynaecology at The Chinese University of Hong Kong, Principal Investigator of Li ka Shing Institute of Health Sciences, In‐charge of Research Laboratory of the department, and Deputy Director of Prenatal Genetic Diagnosis Centre. Dr. Wang joined the department after his MBBS degree with honors in 1994, and in 1998 and 2011 he received his first PhD in Surgical Sciences from the Chinese University of Hong Kong and his second PhD in Genetics from Japan National Institute of Genetics. Dr. Wang has major research focus in studying pathophysiology and pharmaceutical research in endometriosis. With a wide range of experience in clinical diagnosis, medical research, and teaching, Dr. Wang was appointed to the editorial boards and review panels of many important research funding agents and journals. He is also an active member of the World Endometriosis Society and the European Society of Human Reproduction & Embryology. Dr. Wang has worked on many research projects received a total of over 70 million research grants and owned four patents. He has published over 180 ISI articles on many acclaimed journals including JAMA, Nat Commun, Gene Dev, Cell Research, PNAS, Hum Reprod Update, Angiogenesis, J Biol Chem, FRBM, Mol Hum Reprod, Hum Reprod, and Fertil Steril. His success in O&G research has earned him multiple local and international scholarships and awards. He is also a reviewer of BMJ, PNAS, Hum Reprod, Fertil Steril, BJOG, FRBM, FASEB, J Path, Development, Physiol Genomics, and so forth.
Hung SW, Zhang R, Tan Z, Chung JPW, Zhang T, Wang CC. Pharmaceuticals targeting signaling pathways of endometriosis as potential new medical treatment: a review. Med Res Rev. 2021;41:2489‐2564. 10.1002/med.21802
Contributor Information
Tao Zhang, Email: taozhang@cuhk.edu.hk.
Chi Chiu Wang, Email: ccwang@cuhk.edu.hk.
REFERENCES
- 1. Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2014;3:CD009590. 10.1002/14651858.CD009590.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244‐1256. 10.1056/NEJMra1810764 [DOI] [PubMed] [Google Scholar]
- 3. Deguara CS, Liu B, Davis C. Measured symptomatic and psychological outcomes in women undergoing laparoscopic surgery for endometriosis: a prospective study. Curr Opin Obstet Gynecol. 2013;25(4):299‐301. 10.1097/GCO.0b013e3283630da7 [DOI] [PubMed] [Google Scholar]
- 4. Velasco I, Rueda J, Acien P. Aromatase expression in endometriotic tissues and cell cultures of patients with endometriosis. Mol Hum Reprod. 2006;12(6):377‐381. 10.1093/molehr/gal041 [DOI] [PubMed] [Google Scholar]
- 5. de Almeida Asencio F, Ribeiro HA, Ayrosa Ribeiro P, et al. Symptomatic endometriosis developing several years after menopause in the absence of increased circulating estrogen concentrations: a systematic review and seven case reports. Gynecol Surg. 2019;16(1):1‐11. 10.1186/s10397-019-1056-x [DOI] [Google Scholar]
- 6. Javert CT. Pathogenesis of endometriosis based on endometrial homeoplasia, direct extension, exfoliation and implantation, lymphatic and hematogenous metastasis. Including five case reports of endometrial tissue in pelvic lymph nodes. Cancer. 1949;2(3):399‐410. [DOI] [PubMed] [Google Scholar]
- 7. Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422‐469. [Google Scholar]
- 8. Jerman LF, Hey‐Cunningham AJ. The role of the lymphatic system in endometriosis: a comprehensive review of the literature. Biol Reprod. 2015;92(3):64. 10.1095/biolreprod.114.124313 [DOI] [PubMed] [Google Scholar]
- 9. Lu Z, Zhang W, Jiang S, Zou J, Li Y. Effect of oxygen tensions on the proliferation and angiogenesis of endometriosis heterograft in severe combined immunodeficiency mice. Fertil Steril. 2014;101(2):568‐576. 10.1016/j.fertnstert.2013.10.039 [DOI] [PubMed] [Google Scholar]
- 10. Moen MH, Magnus P. The familial risk of endometriosis. Acta Obstet Gynecol Scand. 1993;72(7):560‐564. 10.3109/00016349309058164 [DOI] [PubMed] [Google Scholar]
- 11. Nouri K, Ott J, Krupitz B, Huber JC, Wenzl R. Family incidence of endometriosis in first‐, second‐, and third‐degree relatives: case‐control study. Reprod Biol Endocrinol. 2010;8:85. 10.1186/1477-7827-8-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi YL, Luo XZ, Zhu XY, Hua KQ, Zhu Y, Li DJ. Effects of combined 17beta‐estradiol with TCDD on secretion of chemokine IL‐8 and expression of its receptor CXCR1 in endometriotic focus‐associated cells in co‐culture. Hum Reprod. 2006;21(4):870‐879. 10.1093/humrep/dei414 [DOI] [PubMed] [Google Scholar]
- 13. Shi YL, Luo XZ, Zhu XY, Li DJ. Combination of 17beta‐estradiol with the environmental pollutant TCDD is involved in pathogenesis of endometriosis via up‐regulating the chemokine I‐309‐CCR8. Fertil Steril. 2007;88(2):317‐325. 10.1016/j.fertnstert.2006.11.129 [DOI] [PubMed] [Google Scholar]
- 14. Eskenazi B. Serum dioxin concentrations and menstrual cycle characteristics. Am J Epidemiol. 2002;156(4):383‐392. 10.1093/aje/kwf046 [DOI] [PubMed] [Google Scholar]
- 15. Sampson JA. The development of the implantation theory for the origin of peritoneal endometriosis. Am J Obstet Gynecol. 1940;40:549‐557. [Google Scholar]
- 16. Bersinger NA, Dechaud H, McKinnon B, Mueller MD. Analysis of cytokines in the peritoneal fluid of endometriosis patients as a function of the menstrual cycle stage using the Bio‐Plex(R) platform. Arch Physiol Biochem. 2012;118(4):210‐218. 10.3109/13813455.2012.687003 [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Nicholes K, Shih IM. The origin and pathogenesis of endometriosis. Annu Rev Pathol. 2020;15:71‐95. 10.1146/annurev-pathmechdis-012419-032654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bozdag G. Recurrence of endometriosis: risk factors, mechanisms and biomarkers. Womens Health (Lond). 2015;11(5):693‐699. 10.2217/whe.15.56 [DOI] [PubMed] [Google Scholar]
- 19. Schindler AE. Hormonal contraceptives and endometriosis/adenomyosis. Gynecol Endocrinol. 2010;26(12):851‐854. 10.3109/09513590.2010.534612 [DOI] [PubMed] [Google Scholar]
- 20. Lidegaard O, Nielsen LH, Skovlund CW, Skjeldestad FE, Lokkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001‐9. BMJ. 2011;343:d6423. 10.1136/bmj.d6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrero S, Barra F, Leone Roberti Maggiore U. Current and emerging therapeutics for the management of endometriosis. Drugs. 2018;78(10):995‐1012. 10.1007/s40265-018-0928-0 [DOI] [PubMed] [Google Scholar]
- 22. Bedaiwy MA, Allaire C, Alfaraj S. Long‐term medical management of endometriosis with dienogest and with a gonadotropin‐releasing hormone agonist and add‐back hormone therapy. Fertil Steril. 2017;107(3):537‐548. 10.1016/j.fertnstert.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 23. Cottreau CM, Ness RB, Modugno F, Allen GO, Goodman MT. Endometriosis and its treatment with danazol or lupron in relation to ovarian cancer. Clin Cancer Res. 2003;9(14):5142‐5144. [PubMed] [Google Scholar]
- 24. Ng J, Chwalisz K, Carter DC, Klein CE. Dose‐dependent suppression of gonadotropins and ovarian hormones by elagolix in healthy premenopausal women. J Clin Endocrinol Metab. 2017;102(5):1683‐1691. 10.1210/jc.2016-3845 [DOI] [PubMed] [Google Scholar]
- 25. Shaw RW. LHRH analogues in the treatment of endometriosis—comparative results with other treatments. Baillieres Clin Obstet Gynaecol. 1988;2(3):659‐675. 10.1016/s0950-3552(88)80051-8 [DOI] [PubMed] [Google Scholar]
- 26. Grandi G, Barra F, Ferrero S, et al. Hormonal contraception in women with endometriosis: a systematic review. Eur J Contracept Reprod Health Care. 2019;24(1):61‐70. 10.1080/13625187.2018.1550576 [DOI] [PubMed] [Google Scholar]
- 27. Muzii L, Di Tucci C, Achilli C, et al. Continuous versus cyclic oral contraceptives after laparoscopic excision of ovarian endometriomas: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(2):203‐211. 10.1016/j.ajog.2015.08.074 [DOI] [PubMed] [Google Scholar]
- 28. Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131(3):557‐571. 10.1097/AOG.0000000000002469 [DOI] [PubMed] [Google Scholar]
- 29. Barra F, Scala C, Ferrero S. Current understanding on pharmacokinetics, clinical efficacy and safety of progestins for treating pain associated to endometriosis. Expert Opin Drug Metab Toxicol. 2018;14(4):399‐415. 10.1080/17425255.2018.1461840 [DOI] [PubMed] [Google Scholar]
- 30. Simon MA, Shulman LP. Subcutaneous versus intramuscular depot methoxyprogesterone acetate: a comparative review. Womens Health (Lond). 2006;2(2):191‐197. 10.2217/17455057.2.2.191 [DOI] [PubMed] [Google Scholar]
- 31. Sakamoto Y, Harada T, Horie S, et al. Tumor necrosis factor‐α‐induced interleukin‐8 (IL‐8) expression in endometriotic stromal cells, probably through nuclear factor‐κB activation: gonadotropin‐releasing hormone agonist treatment reduced IL‐8 expression. J Clin Endocrinol Metab. 2003;88(2):730‐735. 10.1210/jc.2002-020666 [DOI] [PubMed] [Google Scholar]
- 32. Andres M, Lopes L, Baracat E, Podgaec S. Dienogest in the treatment of endometriosis: systematic review. Arch Gynecol Obstet. 2015;292(3):523‐529. 10.1007/s00404-015-3681-6 [DOI] [PubMed] [Google Scholar]
- 33. Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. Feb 2005;159(2):139‐144. 10.1001/archpedi.159.2.139 [DOI] [PubMed] [Google Scholar]
- 34. Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis‐associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28‐40. 10.1056/nejmoa1700089 [DOI] [PubMed] [Google Scholar]
- 35. Li XY, Chao XP, Leng JH, et al. Risk factors for postoperative recurrence of ovarian endometriosis: long‐term follow‐up of 358 women. J Ovarian Res. 2019;12(1):79. 10.1186/s13048-019-0552-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long‐term follow‐up, and fertility outcomes. Fertil Steril. 2014;102(1):160‐166. 10.1016/j.fertnstert.2014.03.055 [DOI] [PubMed] [Google Scholar]
- 37. McKinnon BD, Kocbek V, Nirgianakis K, Bersinger NA, Mueller MD. Kinase signalling pathways in endometriosis: potential targets for non‐hormonal therapeutics. Hum Reprod Update. 2016;22(3):382‐403. 10.1093/humupd/dmv060 [DOI] [PubMed] [Google Scholar]
- 38. Ferrero S, Evangelisti G, Barra F. Current and emerging treatment options for endometriosis. Expert Opin Pharmacother. 2018;19(10):1109‐1125. 10.1080/14656566.2018.1494154 [DOI] [PubMed] [Google Scholar]
- 39. Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. May 2014;10(5):261‐275. 10.1038/nrendo.2013.255 [DOI] [PubMed] [Google Scholar]
- 40. Baranov V, Malysheva O, Yarmolinskaya M. Pathogenomics of endometriosis development. Int J Mol Sci. 2018;19(7):1852. 10.3390/ijms19071852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laganà AS, Garzon S, Götte M, et al. The pathogenesis of endometriosis: molecular and cell biology insights. Int J Mol Sci. 2019;20(22):5615. 10.3390/ijms20225615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bouquet de Jolinière J, Ayoubi JMB, Gianaroli L, Dubuisson JB, Gogusev J, Feki A. Endometriosis: a new cellular and molecular genetic approach for understanding the pathogenesis and evolutivity. Front Surg. 2014;1:16. 10.3389/fsurg.2014.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Romano A, Xanthoulea S, Giacomini E, Delvoux B, Alleva E, Vigano P. Endometriotic cell culture contamination and authenticity: a source of bias in in vitro research? Hum Reprod. 2020;35(2):364‐376. 10.1093/humrep/dez266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Evron A, Goldman S, Shalev E. Effect of primary human endometrial stromal cells on epithelial cell receptivity and protein expression is dependent on menstrual cycle stage. Hum Reprod. 2011;26(1):176‐190. 10.1093/humrep/deq.296 [DOI] [PubMed] [Google Scholar]
- 45. Chan RWS, Lee C, Ng EHY, Yeung WSB. Co‐culture with macrophages enhances the clonogenic and invasion activity of endometriotic stromal cells. Cell Prolif. 2017;50(3):e12330. 10.1111/cpr.12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Horne AW, Ahmad SF, Carter R, et al. Repurposing dichloroacetate for the treatment of women with endometriosis. Proc Natl Acad Sci. 2019;116(51):25389‐25391. 10.1073/pnas.1916144116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu W, Niu W, Wang S, et al. Co‐culture with endometrial stromal cells enhances the differentiation of human embryonic stem cells into endometrium‐like cells. Exp Ther Med. 2015;10(1):43‐50. 10.3892/etm.2015.2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang H, Xue J, Li M, Zhao X, Wei D, Li C. Metformin regulates stromal‐epithelial cells communication via Wnt2/beta‐catenin signaling in endometriosis. Mol Cell Endocrinol. 2015;413:61‐65. 10.1016/j.mce.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 49. Grümmer R. Animal models in endometriosis research. Hum Reprod Update. 2006;12(5):641‐649. 10.1093/humupd/dml026 [DOI] [PubMed] [Google Scholar]
- 50. Horie S, Harada T, Mitsunari M, Taniguchi F, Iwabe T, Terakawa N. Progesterone and progestational compounds attenuate tumor necrosis factor alpha‐induced interleukin‐8 production via nuclear factor‐kappa B inactivation in endometriotic stromal cells. Fertil Steril. 2005;83(5):1530‐1535. 10.1016/j.fertnstert.2004.11.042 [DOI] [PubMed] [Google Scholar]
- 51. Choi J, Jo M, Lee E, Lee DY, Choi D. Involvement of endoplasmic reticulum stress in regulation of endometrial stromal cell invasiveness: possible role in pathogenesis of endometriosis. Mol Hum Reprod. 2019;25(3):101‐110. 10.1093/molehr/gaz002 [DOI] [PubMed] [Google Scholar]
- 52. Meresman GF, Bilotas MA, Lombardi E, Tesone M, Sueldo C, Baranao RI. Effect of GnRH analogues on apoptosis and release of interleukin‐1beta and vascular endothelial growth factor in endometrial cell cultures from patients with endometriosis. Hum Reprod. 2003;18(9):1767‐1771. 10.1093/humrep/deg356 [DOI] [PubMed] [Google Scholar]
- 53. Olivares C, Bilotas M, Buquet R, et al. Effects of a selective cyclooxygenase‐2 inhibitor on endometrial epithelial cells from patients with endometriosis. Hum Reprod. 2008;23(12):2701‐2708. 10.1093/humrep/den315 [DOI] [PubMed] [Google Scholar]
- 54. Prescott LF. Pharmacokinetic drug interactions. Lancet. 1969;294(7632):1239‐1243. 10.1016/s0140-6736(69)90766-1 [DOI] [PubMed] [Google Scholar]
- 55. Ufer M, Juif PE, Boof ML, Muehlan C, Dingemanse J. Metabolite profiling in early clinical drug development: current status and future prospects. Expert Opin Drug Metab Toxicol. 2017;13(8):803‐806. 10.1080/17425255.2017.1351944 [DOI] [PubMed] [Google Scholar]
- 56. Lin A, Giuliano CJ, Palladino A, et al. Off‐target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci Transl Med. 2019;11(509):eaaw8412. 10.1126/scitranslmed.aaw8412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mori T, Ito F, Koshiba A, et al. Aromatase as a target for treating endometriosis. J Obstet Gynaecol Res. 2018;44(9):1673‐1681. 10.1111/jog.13743 [DOI] [PubMed] [Google Scholar]
- 58. Fourquet J, Gao X, Zavala D, et al. Patients' report on how endometriosis affects health, work, and daily life. Fertil Steril. 2010;93(7):2424‐2428. 10.1016/j.fertnstert.2009.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vercellini P, Frattaruolo MP, Buggio L. Toward minimally disruptive management of symptomatic endometriosis: reducing low‐value care and the burden of treatment. Expert Rev Pharmacoecon Outcomes Res. Feb 2018;18(1):1‐4. 10.1080/14737167.2018.1411803 [DOI] [PubMed] [Google Scholar]
- 60. Efstathiou J, Sampson D, Levine Z, et al. Nonsteroidal antiinflammatory drugs differentially suppress endometriosis in a murine model. Fertil Steril. 2005;83(1):171‐181. 10.1016/j.fertnstert.2004.06.058 [DOI] [PubMed] [Google Scholar]
- 61. Choi J, Jo M, Lee E, Lee DY, Choi D. Dienogest enhances autophagy induction in endometriotic cells by impairing activation of AKT, ERK1/2, and mTOR. Fertil Steril. 2015;104(3):655‐664. 10.1016/j.fertnstert.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 62. Nasu K, Nishida M, Ueda T, Yuge A, Takai N, Narahara H. Application of the nuclear factor‐kappaB inhibitor BAY 11‐7085 for the treatment of endometriosis: an in vitro study. Am J Physiol Endocrinol Metab. 2007;293(1):E16‐E23. 10.1152/ajpendo.00135.2006 [DOI] [PubMed] [Google Scholar]
- 63. Paul S, Bhattacharya P, Das Mahapatra P, Swarnakar S. Melatonin protects against endometriosis via regulation of matrix metalloproteinase‐3 and an apoptotic pathway. J Pineal Res. 2010;49(2):156‐168. 10.1111/j.1600-079X.2010.00780.x [DOI] [PubMed] [Google Scholar]
- 64. Zhao Y, Gong P, Chen Y, et al. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci Transl Med. 2015;7(271):271ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. da Silva DM, Gross LA, de Paula Guedes Neto E, Lessey BA, Savaris RF. The use of resveratrol as an adjuvant treatment of pain in endometriosis: a randomized clinical trial. J Endocr Soc. 2017;1(4):359‐369. 10.1210/js.2017-00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Amaya SC, Savaris RF, Filipovic CJ, et al. Resveratrol and endometrium: a closer look at an active ingredient of red wine using in vivo and in vitro models. Reprod Sci. 2014;21(11):1362‐1369. 10.1177/1933719114525271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Janle EM, Morre DM, Morre DJ, Zhou Q, Zhu Y. Pharmacokinetics of green tea catechins in extract and sustained‐release preparations. J Diet Suppl. 2008;5(3):248‐263. 10.1080/19390210802414279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 3: teratogenicity and reproductive toxicity studies in rats. Food Chem Toxicol. 2006;44(5):651‐661. 10.1016/j.fct.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 69. Chow HH, Cai Y, Alberts DS, et al. Phase I pharmacokinetic study of tea polyphenols following single‐dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10(1):53‐58. [PubMed] [Google Scholar]
- 70. Xu H, Becker CM, Lui WT, et al. Green tea epigallocatechin‐3‐gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil Steril. 2011;96(4):1021‐1028. 10.1016/j.fertnstert.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 71. Morin MJ, Morin MJ, Bernacki RJ, Bernacki RJ. Biochemical effects and therapeutic potential of tunicamycin in murine L1210 leukemia. Cancer Res (Chicago, Ill). 1983;43(4):1669‐1674. [PubMed] [Google Scholar]
- 72. Foufelle F, Fromenty B. Role of endoplasmic reticulum stress in drug‐induced toxicity. Pharmacol Res Perspect. 2016;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hasegawa A, Osuga Y, Hirota Y, et al. Tunicamycin enhances the apoptosis induced by tumor necrosis factor‐related apoptosis‐inducing ligand in endometriotic stromal cells. Hum Reprod. 2009;24(2):408‐414. 10.1093/humrep/den385 [DOI] [PubMed] [Google Scholar]
- 74. Aronson JK. Side Effects of Drugs Annual: A Worldwide Yearly Survey of New Data and Trends in Adverse Drug Reactions. Vol 30. The Netherlands: Elsevier; 2010. https://www.sciencedirect.com/bookseries/side-effects-of-drugs-annual/vol/30/suppl/C [Google Scholar]
- 75. Lin SC, Lee HC, Hou PC, Fu JL, Wu MH, Tsai SJ. Targeting hypoxia‐mediated YAP1 nuclear translocation ameliorates pathogenesis of endometriosis without compromising maternal fertility. J Pathol. 2017;242(4):476‐487. 10.1002/path.4922 [DOI] [PubMed] [Google Scholar]
- 76. Choi MK, Jin S, Jeon JH, et al. Tolerability and pharmacokinetics of ginsenosides Rb1, Rb2, Rc, Rd, and compound K after single or multiple administration of red ginseng extract in human beings. J Ginseng Res. 2020;44(2):229‐237. 10.1016/j.jgr.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li C, Wang Z, Li G, et al. Acute and repeated dose 26‐week oral toxicity study of 20(S)‐ginsenoside Rg3 in Kunming mice and Sprague–Dawley rats. J Ginseng Res. 2020;44(2):222‐228. 10.1016/j.jgr.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee N‐H, Son C‐G. Systematic review of randomized controlled trials evaluating the efficacy and safety of ginseng. J Acupunct Meridian Studies. 2011;4(2):85‐97. [DOI] [PubMed] [Google Scholar]
- 79. Huang R, Chen S, Zhao M, Li Z, Zhu L. Ginsenoside Rg3 attenuates endometriosis by inhibiting the viability of human ectopic endometrial stromal cells through the nuclear factor‐kappaB signaling pathway. J Gynecol Obstet Human Reprod. 2020;49(1):101642. 10.1016/j.jogoh.2019.101642 [DOI] [PubMed] [Google Scholar]
- 80. Arablou T, Kolahdouz‐Mohammadi R. Curcumin and endometriosis: review on potential roles and molecular mechanisms. Biomed Pharmacother. 2018;97:91‐97. [DOI] [PubMed] [Google Scholar]
- 81. Jana S, Paul S, Swarnakar S. Curcumin as anti‐endometriotic agent: implication of MMP‐3 and intrinsic apoptotic pathway. Biochem Pharmacol. 2012;83(6):797‐804. 10.1016/j.bcp.2011.12.030 [DOI] [PubMed] [Google Scholar]
- 82. Takimoto CH, Glover K, Huang X, et al. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol, Biomarkers Prev. 2003;12(11):1213‐1221. [PubMed] [Google Scholar]
- 83. Malloy KM, Wang J, Clark LH, et al. Novasoy and genistein inhibit endometrial cancer cell proliferation through disruption of the AKT/mTOR and MAPK signaling pathways. Am J Transl Res. 2018;10(3):784. [PMC free article] [PubMed] [Google Scholar]
- 84. Liu J, Yuan F, Gao J, et al. Oral isoflavone supplementation on endometrial thickness: a meta‐analysis of randomized placebo‐controlled trials. Oncotarget. 2016;7(14):17369‐17379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sutrisno S, Sulistyorini C, Manungkalit EM, Winarsih L, Noorhamdani N, Winarsih S. The effect of genistein on TGF‐β signal, dysregulation of apoptosis, cyclooxygenase‐2 pathway, and NF‐kB pathway in mice peritoneum of endometriosis model. Middle East Fertility Society Journal. 2017;22(4):295‐299. 10.1016/j.mefs.2017.05.002 [DOI] [Google Scholar]
- 86. Krajewska J, Handkiewicz‐Junak D, Jarzab B. Sorafenib for the treatment of thyroid cancer: an updated review. Expert Opin Pharmacother. 2015;16(4):573‐583. 10.1517/14656566.2015.1005601 [DOI] [PubMed] [Google Scholar]
- 87. Leconte M, Santulli P, Chouzenoux S, et al. Inhibition of MAPK and VEGFR by sorafenib controls the progression of endometriosis. Reprod Sci. 2015;22(9):1171‐1180. 10.1177/1933719115592708 [DOI] [PubMed] [Google Scholar]
- 88. Lau P, Kee D. Optimal Selection of Targeted Therapies for Melanoma Patients: Role of the Mitogen‐Activated Protein Kinase Pathway. Vol 3. USA: Academic Press, Elsevier Inc; 2016:169‐183. https://www.sciencedirect.com/science/article/pii/B9780128035085000123 [Google Scholar]
- 89. Santulli P, Marcellin L, Chouzenoux S, et al. Role of the protein kinase BRAF in the pathogenesis of endometriosis. Expert Opin Ther Targets. 2016;20(8):1017‐1029. 10.1080/14728222.2016.1180367 [DOI] [PubMed] [Google Scholar]
- 90. Bae SH, Hwang JW, Shin SJ, Park GH, Yoon KD, Bae SK. Quantitation and pharmacokinetics of 1,4‐diamino‐2,3‐dicyano‐1,4‐bis (2‐aminophenylthio) butadiene (U0126) in rat plasma by liquid chromatography‐tandem mass spectrometry. J Sep Sci. 2013;36(2):239‐245. 10.1002/jssc.201200779 [DOI] [PubMed] [Google Scholar]
- 91. Eaton JL, Unno K, Caraveo M, Lu Z, Kim JJ. Increased AKT or MEK1/2 activity influences progesterone receptor levels and localization in endometriosis. J Clin Endocrinol Metab. 2013;98(12):E1871‐E1879. 10.1210/jc.2013-1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang L. Pharmacokinetics and drug delivery systems for puerarin, a bioactive flavone from traditional Chinese medicine. Drug Deliv. 2019;26(1):860‐869. 10.1080/10717544.2019.1660732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cheng W, Chen L, Yang S, et al. Puerarin suppresses proliferation of endometriotic stromal cells partly via the MAPK signaling pathway induced by 17ss‐estradiol‐BSA. PLOS One. 2012;7(9):e45529. 10.1371/journal.pone.0045529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Romm A, Clare B, Stansbury JE, et al. Menstrual wellness and menstrual problems. Botanic Med Women's Health. 2010:97‐185. [Google Scholar]
- 95. Banu SK, Lee J, Speights VO, Jr , Starzinski‐Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFkappaB, and beta‐catenin pathways and activation of intrinsic apoptotic mechanisms. Mol Endocrinol. 2009;23(8):1291‐1305. 10.1210/me.2009-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tai S, Fantegrossi WE. Synthetic cannabinoids: pharmacology, behavioral effects, and abuse potential. Curr Addict Rep. 2014;1(2):129‐136. 10.1007/s40429-014-0014-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kraft B. Is there any clinically relevant cannabinoid‐induced analgesia? Pharmacology. 2012;89(5–6):237‐246. 10.1159/000337376 [DOI] [PubMed] [Google Scholar]
- 98. Leconte M, Nicco C, Ngô C, et al. Antiproliferative effects of cannabinoid agonists on deep infiltrating endometriosis. Am J Pathol. 2010;177(6):2963‐2970. 10.2353/ajpath.2010.100375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Marik PE. Propofol: therapeutic indications and side‐effects. Curr Pharm Des. 2004;10(29):3639‐3649. [DOI] [PubMed] [Google Scholar]
- 100. Feng S, Sun Y. Protective role of propofol in endometriosis and its mechanism. Exp Ther Med. 2018;16(4):3646. 10.3892/etm.2018.6648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chu QSC, Sangha R, Spratlin J, et al. A phase I open‐labeled, single‐arm, dose‐escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Invest New Drugs. 2015;33(3):603‐610. 10.1007/s10637-015-0221-y [DOI] [PubMed] [Google Scholar]
- 102. Matsuzaki S, Pouly JL, Canis M. In vitro and in vivo effects of MK2206 and chloroquine combination therapy on endometriosis: autophagy may be required for regrowth of endometriosis. Br J Pharmacol. 2018;175(10):1637‐1653. 10.1111/bph.14170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang B, Zhou WJ, Gu CJ, et al. The ginsenoside PPD exerts anti‐endometriosis effects by suppressing estrogen receptor‐mediated inhibition of endometrial stromal cell autophagy and NK cell cytotoxicity. Cell Death Dis. 2018;9(5):574. 10.1038/s41419-018-0581-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Borahay MA, Lu F, Ozpolat B, et al. Mullerian inhibiting substance suppresses proliferation and induces apoptosis and autophagy in endometriosis cells in vitro. ISRN Obstet Gynecol. 2013;2013:361489‐6. 10.1155/2013/361489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kimura K, Ikoma A, Shibakawa M, et al. Safety, tolerability, and preliminary efficacy of the anti‐fibrotic small molecule PRI‐724, a CBP/β‐catenin inhibitor, in patients with hepatitis C virus‐related cirrhosis: a single‐center, open‐label, dose‐escalation phase 1 trial. EBioMedicine. 2017;23:79‐87. 10.1016/j.ebiom.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hirakawa T, Nasu K, Miyabe S, et al. beta‐catenin signaling inhibitors ICG‐001 and C‐82 improve fibrosis in preclinical models of endometriosis. Sci Rep. 2019;9(1):20056. 10.1038/s41598-019-56302-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Matsuzaki S, Botchorishvili R, Pouly JL, Canis M. Targeting the Wnt/β‐catenin pathway in endometriosis: a potentially effective approach for treatment and prevention. Mol Cell Ther. 2014;2(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Matsuzaki S, Darcha C. In vitro effects of a small‐molecule antagonist of the Tcf/ss‐catenin complex on endometrial and endometriotic cells of patients with endometriosis. PLOS One. 2013;8(4):e61690. 10.1371/journal.pone.0061690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Clement NS, Oliver TRW, Shiwani H, Sanner JRF, Mulvaney CA, Atiomo W. Metformin for endometrial hyperplasia. Cochrane Database Syst Rev. 2017:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Matsuzaki S, Darcha C. Antifibrotic properties of epigallocatechin‐3‐gallate in endometriosis. Hum Reprod. 2014;29(8):1677‐1687. 10.1093/humrep/deu123 [DOI] [PubMed] [Google Scholar]
- 111. Matsuzaki S, Darcha C. Involvement of the Wnt/beta‐catenin signaling pathway in the cellular and molecular mechanisms of fibrosis in endometriosis. PLOS One. 2013;8(10):e76808. 10.1371/journal.pone.0076808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Maharani M, Wahyuni ES, Sutrisno S. Effect of genistein on endometriosis lesion matrix metalloproteinase 2 and 9 level of endometriosis in silico and in vivo study. J Clinic Mol Endocrinol. 2016;1(1.4):1‐6. 10.21767/2572-5432.10004 [DOI] [Google Scholar]
- 113. Liu W, Gao J, Yi X, Li Y, Zeng Y. Absorption, tissue disposition, and excretion of fasudil hydrochloride, a RHO kinase inhibitor, in rats and dogs. Biopharm Drug Dispos. 2020;41(4–5):206‐220. 10.1002/bdd.2231 [DOI] [PubMed] [Google Scholar]
- 114. Chan SY, Loscalzo J. Pulmonary arterial hypertension. Vascular Medicine: A Companion to Braunwald's Heart Disease. The Netherlands: Elsevier; 2013:667‐686. [Google Scholar]
- 115. Tsuno A, Nasu K, Kawano Y, et al. Fasudil inhibits the proliferation and contractility and induces cell cycle arrest and apoptosis of human endometriotic stromal cells: a promising agent for the treatment of endometriosis. J Clin Endocrinol Metab. 2011;96(12):E1944‐E1952. 10.1210/jc.2011-1503 [DOI] [PubMed] [Google Scholar]
- 116. Alban S. Adverse Effects of Heparin. Vol 207, 2012 ed. Berlin Heidelberg: Springer; 2012:211‐263. https://pubmed.ncbi.nlm.nih.gov/22566227/ [DOI] [PubMed] [Google Scholar]
- 117. Nasu K, Tsuno A, Hirao M, Kobayashi H, Yuge A, Narahara H. Heparin is a promising agent for the treatment of endometriosis‐associated fibrosis. Fertil Steril. Jun 2010;94(1):46‐51. 10.1016/j.fertnstert.2009.02.057 [DOI] [PubMed] [Google Scholar]
- 118. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. The Lancet. 2006;368(9544):1329‐1338. 10.1016/S0140-6736(06)69446-4 [DOI] [PubMed] [Google Scholar]
- 119. Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet‐derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327‐337. [PubMed] [Google Scholar]
- 120. Abbas MA, Disi AM, Taha MO. Sunitinib as an anti‐endometriotic agent. Eur J Pharm Sci. 2013;49(4):732‐736. 10.1016/j.ejps.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 121. Xiong HQ, Herbst R, Faria SC, et al. A phase I surrogate endpoint study of SU6668 in patients with solid tumors. Invest New Drugs. 2004;22(4):459‐466. 10.1023/B:DRUG.0000036688.96453.8d [DOI] [PubMed] [Google Scholar]
- 122. Kuenen BC, Giaccone G, Ruijter R, et al. Dose‐finding study of the multitargeted tyrosine kinase inhibitor SU6668 in patients with advanced malignancies. Clin Cancer Res. 2005;11(17):6240‐6246. [DOI] [PubMed] [Google Scholar]
- 123. Laschke MW, Elitzsch A, Vollmar B, Vajkoczy P, Menger MD. Combined inhibition of vascular endothelial growth factor (VEGF), fibroblast growth factor and platelet‐derived growth factor, but not inhibition of VEGF alone, effectively suppresses angiogenesis and vessel maturation in endometriotic lesions. Hum Reprod. 2006;21(1):262‐268. 10.1093/humrep/dei308 [DOI] [PubMed] [Google Scholar]
- 124. Davis DW, Takamori R, Raut CP, et al. Pharmacodynamic analysis of target inhibition and endothelial cell death in tumors treated with the vascular endothelial growth factor receptor antagonists SU5416 or SU6668. Clin Cancer Res. 2005;11(2 pt 1):678‐689. [PubMed] [Google Scholar]
- 125. Haddad JJ. The immunopharmacologic potential of semaxanib and new generation directed therapeutic drugs: receptor tyrosine kinase regulation with anti‐tumorigenensis/angiogenesis properties. Saudi Pharm J. 2012;20(2):103‐123. 10.1016/j.jsps.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fong TA, Shawver LK, Sun L, et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk‐1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59(1):99. [PubMed] [Google Scholar]
- 127. Santulli P, Marcellin L, Tosti C, et al. MAP kinases and the inflammatory signaling cascade as targets for the treatment of endometriosis? Expert Opin Ther Targets. 2015;19(11):1465‐1483. 10.1517/14728222.2015.1090974 [DOI] [PubMed] [Google Scholar]
- 128. Yildiz C, Kacan T, Akkar OB, et al. Effects of pazopanib, sunitinib, and sorafenib, anti‐VEGF agents, on the growth of experimental endometriosis in rats. Reprod Sci. 2015;22(11):1445‐1451. 10.1177/1933719115584448 [DOI] [PubMed] [Google Scholar]
- 129. Xu H, Lui WT, Chu CY, Ng PS, Wang CC, Rogers MS. Anti‐angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum Reprod. 2009;24(3):608‐618. 10.1093/humrep/den417 [DOI] [PubMed] [Google Scholar]
- 130. Wang CC, Xu H, Man GCW, et al. Prodrug of green tea epigallocatechin‐3‐gallate (Pro‐EGCG) as a potent anti‐angiogenesis agent for endometriosis in mice. Angiogenesis. 2013;16(1):59‐69. 10.1007/s10456-012-9299-4 [DOI] [PubMed] [Google Scholar]
- 131. Chabicovsky M, Prieschl‐Grassauer E, Seipelt J, et al. Pre‐clinical safety evaluation of pyrrolidine dithiocarbamate: pre‐clinical safety of pyrrolidine dithiocarbamate. Basic Clin Pharmacol Toxicol. 2010;107(3):758‐767. 10.1111/j.1742-7843.2010.00573.x [DOI] [PubMed] [Google Scholar]
- 132. Zhang J, Xu Z, Dai H, et al. Application of the nuclear factor‐kappaB inhibitor pyrrolidine dithiocarbamate for the treatment of endometriosis: an in vitro study. Fertil Steril. 2010;94(7):2942‐2944. 10.1016/j.fertnstert.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 133. Vlahos NF, Gregoriou O, Deliveliotou A, et al. Effect of pentoxifylline on vascular endothelial growth factor C and FLK‐1 expression on endometrial implants in the rat endometriosis model. Fertil Steril. 2010;93(4):1316‐1323. 10.1016/j.fertnstert.2008.10.056 [DOI] [PubMed] [Google Scholar]
- 134. Giusti M, Porcella E, Carraro A, Cuttica M, Valenti S, Giordano G. A cross‐over study with the two novel dopaminergic drugs cabergoline and quinagolide in hyperprolactinemic patients. J Endocrinol Invest. 1994;17(1):51‐57. 10.1007/BF03344963 [DOI] [PubMed] [Google Scholar]
- 135. Gomez R, Abad A, Delgado F, Tamarit S, Simon C, Pellicer A. Effects of hyperprolactinemia treatment with the dopamine agonist quinagolide on endometriotic lesions in patients with endometriosis‐associated hyperprolactinemia. Fertil Steril. 2011;95(3):882‐888. 10.1016/j.fertnstert.2010.10.024 [DOI] [PubMed] [Google Scholar]
- 136. Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clin Toxicol. 2009;47(2):81‐88. 10.1080/15563650802665587 [DOI] [PubMed] [Google Scholar]
- 137. Ngô C, Chéreau C, Nicco C, Weill B, Chapron C, Batteux F. Reactive oxygen species controls endometriosis progression. Am J Pathol. 2009;175(1):225‐234. 10.2353/ajpath.2009.080804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yavuz S, Aydin N, Celik O, Yilmaz E, Ozerol E, Tanbek K. Resveratrol successfully treats experimental endometriosis through modulation of oxidative stress and lipid peroxidation. J Cancer Res Ther. 2014;10(2):324. 10.4103/0973-1482.136619 [DOI] [PubMed] [Google Scholar]
- 139. Li J, Bai Y, Bai Y, et al. Pharmacokinetics of caffeic acid, ferulic acid, formononetin, cryptotanshinone, and tanshinone IIA after oral administration of naoxintong capsule in rat by HPLC‐MS/MS. Evid‐Based Complement Alternat Med. 2017;2017:9057238‐12. 10.1155/2017/9057238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Butt MS, Sultan MT. Coffee and its consumption: benefits and risks. Crit Rev Food Sci Nutr. 2011;51(4):363‐373. 10.1080/10408390903586412 [DOI] [PubMed] [Google Scholar]
- 141. Jamali N, Mostafavi‐Pour Z, Zal F, et al. Combination effect of caffeine and caffeic acid treatment on the oxidant status of ectopic endometrial cells separated from patients with endometriosis. Iran J Med Sci. 2019;44(4):315‐324. 10.30476/IJMS.2019.44970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yilmaz B, Kilic S, Aksakal O, et al. Melatonin causes regression of endometriotic implants in rats by modulating angiogenesis, tissue levels of antioxidants and matrix metalloproteinases. Arch Gynecol Obstet. 2015;292(1):209‐216. 10.1007/s00404-014-3599-4 [DOI] [PubMed] [Google Scholar]
- 143. Guney M, Oral B, Karahan N, Mungan T. Regression of endometrial explants in a rat model of endometriosis treated with melatonin. Fertil Steril. 2008;89(4):934‐942. 10.1016/j.fertnstert.2007.04.023 [DOI] [PubMed] [Google Scholar]
- 144. Pittaluga E, Costa G, Krasnowska E, et al. More than antioxidant: N‐acetyl‐L‐cysteine in a murine model of endometriosis. Fertil Steril. 2010;94(7):2905‐2908. 10.1016/j.fertnstert.2010.06.038 [DOI] [PubMed] [Google Scholar]
- 145. Almodovar P, Briskey D, Rao A, Prodanov M, Inarejos‐Garcia AM. Bioaccessibility and pharmacokinetics of a commercial saffron (Crocus sativus L.) extract. Evid‐Based Complement Alternat Med. 2020;2020:1575730‐1575738. 10.1155/2020/1575730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bostan HB, Mehri S, Hosseinzadeh H. Toxicology effects of saffron and its constituents: a review. Iran J Basic Med Sci. 2017;20(2):110‐121. 10.22038/ijbms.2017.8230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Khalatbari‐mohseni A, Banafshe HR, Mirhosseini N, Asemi Z, Ghaderi A, Omidi A. The effects of crocin on psychological parameters in patients under methadone maintenance treatment: a randomized clinical trial. Subst Abuse Treat Prev Policy. 2019;14(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Liu Y, Qin X, Lu X. Crocin improves endometriosis by inhibiting cell proliferation and the release of inflammatory factors. Biomed Pharmacother. 2018;106:1678‐1685. 10.1016/j.biopha.2018.07.108 [DOI] [PubMed] [Google Scholar]
- 149. Foda AA, Aal IAA. Metformin as a new therapy for endometriosis, its effects on both clinical picture and cytokines profile. Middle East Fertil Soc J. 2012;17(4):262‐267. 10.1016/j.mefs.2012.09.001 [DOI] [Google Scholar]
- 150. Ergenoğlu AM, Yeniel AÖ, Erbaş O, et al. Regression of endometrial implants by resveratrol in an experimentally induced endometriosis model in rats. Reprod Sci. 2013;20(10):1230‐1236. 10.1177/1933719113483014 [DOI] [PubMed] [Google Scholar]
- 151. Taguchi A, Wada‐Hiraike O, Kawana K, et al. Resveratrol suppresses inflammatory responses in endometrial stromal cells derived from endometriosis: a possible role of the sirtuin 1 pathway. J Obstet Gynaecol Res. 2014;40(3):770‐778. 10.1111/jog.12252 [DOI] [PubMed] [Google Scholar]
- 152. Yu J, Zhao L, Zhang D, et al. The effects and possible mechanisms of puerarin to treat endometriosis model rats. Evid‐Based Complement Alternat Med. 2015;2015:1‐11. 10.1155/2015/269138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ryan ET. Antiparasitic agents. Principles and Practice of Pediatric Infectious Diseases. The Netherlands: Elsevier; 2018:1567‐1587. [Google Scholar]
- 154. Prather GR, MacLean JA, Shi M, Boadu DK, Paquet M, Hayashi K. Niclosamide as a potential nonsteroidal therapy for endometriosis that preserves reproductive function in an experimental mouse model 1. Biol Reprod. 2016;95(4):74. 10.1095/biolreprod.116.140236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Chowdhury I, Banerjee S, Driss A, et al. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF‐κB signaling pathway. J Cell Physiol. 2019;234(5):6298‐6312. 10.1002/jcp.27360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Machado DE, Rodrigues‐Baptista KC, Alessandra‐Perini J, et al. Euterpe oleracea extract (Açaí) is a promising novel pharmacological therapeutic treatment for experimental endometriosis. PLOS One. 2016;11(11):e0166059. 10.1371/journal.pone.0166059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Choi JY, Jo MW, Lee EY, Lee DY, Choi DS. Ovarian steroid dependence of endoplasmic reticulum stress involvement in endometrial cell apoptosis during the human endometrial cycle. Reproduction. 2018;155(6):493‐503. 10.1530/REP-17-0713 [DOI] [PubMed] [Google Scholar]
- 158. Brandenberger AW. Oestrogen receptor (ER)‐alpha and ER‐beta isoforms in normal endometrial and endometriosis‐derived stromal cells. Mol Hum Reprod. 1999;5(7):651‐655. 10.1093/molehr/5.7.651 [DOI] [PubMed] [Google Scholar]
- 159. Matsuzaki S, Murakami T, Uehara S, Canis M, Sasano H, Okamura K. Expression of estrogen receptor alpha and beta in peritoneal and ovarian endometriosis. Fertil Steril. 2001;75(6):1198‐1205. 10.1016/s0015-0282(01)01783-6 [DOI] [PubMed] [Google Scholar]
- 160. Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G‐protein‐coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80(2):231‐238. 10.1016/s0960-0760(01)00190-x [DOI] [PubMed] [Google Scholar]
- 161. Lin BC, Suzawa M, Blind RD, et al. Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF‐1 and promotes endometrial cell proliferation. Cancer Res. 2009;69(13):5415‐5423. 10.1158/0008-5472.CAN-08-1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Simpson ER, Mahendroo MS, Means GD, et al. Aromatase cytochrome p450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15(3):342‐355. 10.1210/edrv-15-3-342 [DOI] [PubMed] [Google Scholar]
- 163. Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase‐2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 2001;16(3):561‐566. [DOI] [PubMed] [Google Scholar]
- 164. Ebert AD, Bartley J, David M. Aromatase inhibitors and cyclooxygenase‐2 (COX‐2) inhibitors in endometriosis: new questions—old answers? Eur J Obstet Gynecol. 2005;122(2):144‐150. 10.1016/j.ejogrb.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 165. Lai ZZ, Yang HL, Ha SY, et al. Cyclooxygenase‐2 in endometriosis. Int J Biol Sci. 2019;15(13):2783‐2797. 10.7150/ijbs.35128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX‐2. Annu Rev Med. 2007;58:239‐252. 10.1146/annurev.med.57.121304.131253 [DOI] [PubMed] [Google Scholar]
- 167. Laschke MW, Schwender C, Scheuer C, Vollmar B, Menger MD. Epigallocatechin‐3‐gallate inhibits estrogen‐induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum Reprod. 2008;23(10):2308‐2318. 10.1093/humrep/den245 [DOI] [PubMed] [Google Scholar]
- 168. Laird SM, Tuckerman EM, Cork BA, Li TC. Expression of nuclear factor kappa B in human endometrium; role in the control of interleukin 6 and leukaemia inhibitory factor production. Mol Hum Reprod. 2000;6(1):34‐40. 10.1093/molehr/6.1.34 [DOI] [PubMed] [Google Scholar]
- 169. González‐Ramos R, Donnez J, Defrère S, et al. Nuclear factor‐kappa B is constitutively activated in peritoneal endometriosis. Mol Hum Reprod. 2007;13(7):503‐509. 10.1093/molehr/gam033 [DOI] [PubMed] [Google Scholar]
- 170. Ponce C, Torres M, Galleguillos C, et al. Nuclear factor kappaB pathway and interleukin‐6 are affected in eutopic endometrium of women with endometriosis. Reproduction. 2009;137(4):727‐737. 10.1530/REP-08-0407 [DOI] [PubMed] [Google Scholar]
- 171. Gonzalez‐Ramos R, Defrere S, Devoto L. Nuclear factor‐kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril. 2012;98(3):520‐528. 10.1016/j.fertnstert.2012.06.021 [DOI] [PubMed] [Google Scholar]
- 172. Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF‐kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30(1):43‐52. 10.1016/j.tibs.2004.11.009 [DOI] [PubMed] [Google Scholar]
- 173. Ngô C, Nicco C, Leconte M, et al. Protein kinase inhibitors can control the progression of endometriosis in vitro and in vivo. J Pathol. 2010;222(2):148‐157. 10.1002/path.2756 [DOI] [PubMed] [Google Scholar]
- 174. Murk W, Atabekoglu CS, Cakmak H, et al. Extracellularly signal‐regulated kinase activity in the human endometrium: possible roles in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2008;93(9):3532‐3540. 10.1210/jc.2007-2051 [DOI] [PubMed] [Google Scholar]
- 175. Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol. 2002;47(4):213‐221. 10.1034/j.1600-0897.2002.01075.x [DOI] [PubMed] [Google Scholar]
- 176. Hirota Y, Osuga Y, Hirata T, et al. Activation of protease‐activated receptor 2 stimulates proliferation and interleukin (IL)‐6 and IL‐8 secretion of endometriotic stromal cells. Hum Reprod. 2005;20(12):3547‐3553. 10.1093/humrep/dei255 [DOI] [PubMed] [Google Scholar]
- 177. Cantley LC. The phosphoinositide 3‐kinase pathway. Science. 2002;296(5573):1655‐1657. 10.1126/science.296.5573.1655 [DOI] [PubMed] [Google Scholar]
- 178. Guo J, Gao J, Yu X, Luo H, Xiong X, Huang O. Expression of DJ‐1 and mTOR in eutopic and ectopic endometria of patients with endometriosis and adenomyosis. Gynecol Obstet Invest. 2015;79(3):195‐200. 10.1159/000365569 [DOI] [PubMed] [Google Scholar]
- 179. Rogers‑Broadway KR, Kumar J, Sisu C, et al. Differential expression of mTOR components in endometriosis and ovarian cancer: effects of rapalogues and dual kinase inhibitors on mTORC1 and mTORC2 stoichiometry. Int J Mol Med. 2019;43(1):47‐56. 10.3892/ijmm.2018.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Sato N, Tsunoda H, Nishida M, et al. Loss of heterozygosity on 10q23. 3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60(24):7052‐7056. [PubMed] [Google Scholar]
- 181. Song Y, Fu J, Zhou M, et al. Activated Hippo/Yes‐associated protein pathway promotes cell proliferation and anti‐apoptosis in endometrial stromal cells of endometriosis. J Clin Endocrinol Metab. 2016;101(4):1552‐1561. 10.1210/jc.2016-1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Jiang B. Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes Dis. 2017;4(1):25‐27. 10.1016/j.gendis.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Hirschhaeuser F, Sattler UG, Mueller‐Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71(22):6921‐6925. 10.1158/0008-5472.CAN-11-1457 [DOI] [PubMed] [Google Scholar]
- 184. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide‐ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239‐257. 10.1038/bjc.1972.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Kokawa K, Shikone T, Nakano R. Apoptosis in the human uterine endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1996;81(11):4144‐4147. 10.1210/jcem.81.11.8923873 [DOI] [PubMed] [Google Scholar]
- 186. Vaskivuo TE, Stenback F, Karhumaa P, Risteli J, Dunkel L, Tapanainen JS. Apoptosis and apoptosis‐related proteins in human endometrium. Mol Cell Endocrinol. 2000;165(1–2):75‐83. 10.1016/s0303-7207(00)00261-6 [DOI] [PubMed] [Google Scholar]
- 187. Dmowski WP, Gebel H, Braun DP. Decreased apoptosis and sensitivity to macrophage mediated cytolysis of endometrial cells in endometriosis. Hum Reprod Update. 1998;4(5):696‐701. 10.1093/humupd/4.5.696 [DOI] [PubMed] [Google Scholar]
- 188. Gebel HM, Braun DP, Tambur A, Frame D, Rana N, Dmowski WP. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil Steril. 1998;69(6):1042‐1047. 10.1016/s0015-0282(98)00073-9 [DOI] [PubMed] [Google Scholar]
- 189. Nasu K, Nishida M, Kawano Y, et al. Aberrant expression of apoptosis‐related molecules in endometriosis: a possible mechanism underlying the pathogenesis of endometriosis. Reprod Sci. 2011;18(3):206‐218. 10.1177/1933719110392059 [DOI] [PubMed] [Google Scholar]
- 190. Cinar O, Seval Y, Uz YH, et al. Differential regulation of Akt phosphorylation in endometriosis. Reprod Biomed Online. 2009;19(6):864‐871. [DOI] [PubMed] [Google Scholar]
- 191. Yang HL, Chang KK, Mei J, et al. Estrogen restricts the apoptosis of endometrial stromal cells by promoting TSLP secretion. Mol Med Rep. 2018;18(5):4410‐4416. 10.3892/mmr.2018.9428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Jones RK, Bulmer JN, Searle RF. Immunohistochemical characterization of proliferation, oestrogen receptor and progesterone receptor expression in endometriosis: comparison of eutopic and ectopic endometrium with normal cycling endometrium. Hum Reprod. 1995;10(12):3272‐3279. 10.1093/oxfordjournals.humrep.a135901 [DOI] [PubMed] [Google Scholar]
- 193. Amaral JD, Sola S, Steer CJ, Rodrigues CM. Role of nuclear steroid receptors in apoptosis. Curr Med Chem. 2009;16(29):3886‐3902. 10.2174/092986709789178028 [DOI] [PubMed] [Google Scholar]
- 194. Granese R, Perino A, Calagna G, et al. Gonadotrophin‐releasing hormone analogue or dienogest plus estradiol valerate to prevent pain recurrence after laparoscopic surgery for endometriosis: a multi‐center randomized trial. Acta Obstet Gynecol Scand. 2015;94(6):637‐645. 10.1111/aogs.12633 [DOI] [PubMed] [Google Scholar]
- 195. Braun DP, Ding J, Dmowski WP. Peritoneal fluid‐mediated enhancement of eutopic and ectopic endometrial cell proliferation is dependent on tumor necrosis factor‐alpha in women with endometriosis. Fertil Steril. 2002;78(4):727‐732. 10.1016/s0015-0282(02)03318-6 [DOI] [PubMed] [Google Scholar]
- 196. Han SJ, Jung SY, Wu SP, et al. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163(4):960‐974. 10.1016/j.cell.2015.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Hatai T, Matsuzawa A, Inoshita S, et al. Execution of apoptosis signal‐regulating kinase 1 (ASK1)‐induced apoptosis by the mitochondria‐dependent caspase activation. J Biol Chem. 2000;275(34):26576‐26581. 10.1074/jbc.M003412200 [DOI] [PubMed] [Google Scholar]
- 198. Han SJ, Hawkins SM, Begum K, et al. A new isoform of steroid receptor coactivator‐1 is crucial for pathogenic progression of endometriosis. Nat Med. 2012;18(7):1102‐1111. 10.1038/nm.2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. The Journal of Pathology. 2010;221(1):3‐12. 10.1002/path.2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Allavena G, Carrarelli P, Del Bello B, Luisi S, Petraglia F, Maellaro E. Autophagy is upregulated in ovarian endometriosis: a possible interplay with p53 and heme oxygenase‐1. Fertil Steril. 2015;103(5):1244‐1251. 10.1016/j.fertnstert.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 201. Pankiv S, Clausen TH, Lamark T, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282(33):24131‐24145. 10.1074/jbc.M702824200 [DOI] [PubMed] [Google Scholar]
- 202. Xu TX, Zhao SZ, Dong M, Yu XR. Hypoxia responsive miR‐210 promotes cell survival and autophagy of endometriotic cells in hypoxia. Eur Rev Med Pharmacol Sci. 2016;20(3):399‐406. [PubMed] [Google Scholar]
- 203. Liu H, Zhang Z, Xiong W, et al. Hypoxia‐inducible factor‐1alpha promotes endometrial stromal cells migration and invasion by upregulating autophagy in endometriosis. Reproduction. 2017;153(6):809‐820. 10.1530/REP-16-0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Zheng J, Luo X, Bao J, et al. Decreased expression of HOXA10 may activate the autophagic process in ovarian endometriosis. Reprod Sci. 2018;25(9):1446‐1454. 10.1177/1933719118768704 [DOI] [PubMed] [Google Scholar]
- 205. Choi J, Jo M, Lee E, Oh YK, Choi D. The role of autophagy in human endometrium. Biol Reprod. 2012;86(3):70. 10.1095/biolreprod.111.096206 [DOI] [PubMed] [Google Scholar]
- 206. Choi J, Jo M, Lee E, Kim HJ, Choi D. Differential induction of autophagy by mTOR is associated with abnormal apoptosis in ovarian endometriotic cysts. Mol Hum Reprod. 2014;20(4):309‐317. 10.1093/molehr/gat091 [DOI] [PubMed] [Google Scholar]
- 207. Choi J, Jo M, Lee E, Hwang S, Choi D. Aberrant PTEN expression in response to progesterone reduces endometriotic stromal cell apoptosis. Reproduction. 2017;153(1):11‐21. 10.1530/REP-16-0322 [DOI] [PubMed] [Google Scholar]
- 208. Pei T, Huang X, Long Y, et al. Increased expression of YAP is associated with decreased cell autophagy in the eutopic endometrial stromal cells of endometriosis. Mol Cell Endocrinol. 2019;491:110432. 10.1016/j.mce.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 209. Marcellin L, Santulli P, Bourdon M, et al. Serum antimГјllerian hormone concentration increases with ovarian endometrioma size. Fertil Steril. 2019;111(5):944‐952. 10.1016/j.fertnstert.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 210. Carrarelli P, Rocha ALL, Belmonte G, et al. Increased expression of antimГјllerian hormone and its receptor in endometriosis. Fertil Steril. 2014;101(5):1353‐1358. 10.1016/j.fertnstert.2014.01.052 [DOI] [PubMed] [Google Scholar]
- 211. Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515‐179519. 10.1155/2014/179515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Laschke MW, Menger MD. Basic mechanisms of vascularization in endometriosis and their clinical implications. Hum Reprod Update. 2018;24(2):207‐224. [DOI] [PubMed] [Google Scholar]
- 213. van der Horst PH, Wang Y, van der Zee M, Burger CW, Blok LJ. Interaction between sex hormones and WNT/β‐catenin signal transduction in endometrial physiology and disease. Mol Cell Endocrinol. 2012;358(2):176‐184. [DOI] [PubMed] [Google Scholar]
- 214. Matsuzaki S, Darcha C, Maleysson E, Canis M, Mage G. Impaired down‐regulation of E‐cadherin and β‐catenin protein expression in endometrial epithelial cells in the mid‐secretory endometrium of infertile patients with endometriosis. J Clin Endocrinol Metabol. 2010;95(7):3437‐3445. 10.1210/jc.2009-2713 [DOI] [PubMed] [Google Scholar]
- 215. Chen JJ, Xiao ZJ, Meng X, et al. MRP4 sustains Wnt/beta‐catenin signaling for pregnancy, endometriosis and endometrial cancer. Theranostics. 2019;9(17):5049‐5064. 10.7150/thno.32097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Lu Q, Huang Y, Wu J, et al. T‐cadherin inhibits invasion and migration of endometrial stromal cells in endometriosis. Hum Reprod. 2020;35(1):145‐156. 10.1093/humrep/dez252 [DOI] [PubMed] [Google Scholar]
- 217. Nap AW, Dunselman GA, de Goeij AF, Evers JL, Groothuis PG. Inhibiting MMP activity prevents the development of endometriosis in the chicken chorioallantoic membrane model. Hum Reprod. 2004;19(10):2180‐2187. 10.1093/humrep/deh408 [DOI] [PubMed] [Google Scholar]
- 218. Lepourcelet M, Chen Y‐NP, France DS, et al. Small‐molecule antagonists of the oncogenic Tcf/ОІ‐catenin protein complex. Cancer Cell. 2004;5(1):91‐102. 10.1016/s1535-6108(03)00334-9 [DOI] [PubMed] [Google Scholar]
- 219. Liu J, Wada Y, Katsura M, et al. Rho‐associated coiled‐coil kinase (ROCK) in molecular regulation of angiogenesis. Theranostics. 2018;8(21):6053‐6069. 10.7150/thno.30305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. van Nieuw Amerongen GP, Koolwijk P, Versteilen A, van Hinsbergh VWM. Involvement of RhoA/Rho kinase signaling in VEGF‐induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol. 2003;23(2):211‐217. [DOI] [PubMed] [Google Scholar]
- 221. Fukata Y, Oshiro N, Kinoshita N, et al. Phosphorylation of adducin by Rho‐kinase plays a crucial role in cell motility. J Cell Biol. 1999;145(2):347‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Yotova IY, Quan P, Leditznig N, Beer U, Wenzl R, Tschugguel W. Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Hum Reprod. 2011;26(4):885‐897. [DOI] [PubMed] [Google Scholar]
- 223. Vigano P, Candiani M, Monno A, Giacomini E, Vercellini P, Somigliana E. Time to redefine endometriosis including its pro‐fibrotic nature. Hum Reprod. 2018;33(3):347‐352. 10.1093/humrep/dex354 [DOI] [PubMed] [Google Scholar]
- 224. Viganò P, Ottolina J, Bartiromo L, et al. Cellular components contributing to fibrosis in endometriosis: a literature review. J Minim Invasive Gynecol. 2020;27(2):287‐295. [DOI] [PubMed] [Google Scholar]
- 225. Young VJ, Brown JK, Saunders PTK, Duncan WC, Horne AW. The peritoneum is both a source and target of TGF‐b in women with endometriosis. PLOS One. 2014;9(9):e106773. 10.1371/journal.pone.0106773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Akhmetshina A, Palumbo K, Dees C, et al. Activation of canonical Wnt signalling is required for TGF‐beta‐mediated fibrosis. Nat Commun. 2012;3:735. 10.1038/ncomms1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Martinez‐Font E, Pérez‐Capó M, Ramos R, et al. Impact of Wnt/beta‐catenin inhibition on cell proliferation through CDC25A downregulation in soft tissue sarcomas. Cancers. 2020;12(9):2556. 10.3390/cancers12092556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Yuge A, Nasu K, Matsumoto H, Nishida M, Narahara H. Collagen gel contractility is enhanced in human endometriotic stromal cells: a possible mechanism underlying the pathogenesis of endometriosis‐associated fibrosis. Hum Reprod. 2007;22(4):938‐944. 10.1093/humrep/del485 [DOI] [PubMed] [Google Scholar]
- 229. Taylor RN, Jie Yu G, Torres PB, et al. Mechanistic and therapeutic implications of angiogenesis in endometriosis. Reprod Sci. 2009;16(2):140‐146. 10.1177/1933719108324893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Nap AW, Griffioen AW, Dunselman GAJ, et al. Antiangiogenesis therapy for endometriosis. J Clin Endocrinol Metab. 2004;89(3):1089‐1095. 10.1210/jc.2003-031406 [DOI] [PubMed] [Google Scholar]
- 231. Donnez J, Smoes P, Gillerot S, Casanas‐Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13(6):1686‐1690. [DOI] [PubMed] [Google Scholar]
- 232. Smith SK. Regulation of angiogenesis in the endometrium. Trends in Endocrinology & Metabolism. 2001;12(4):147‐151. [DOI] [PubMed] [Google Scholar]
- 233. Liu S, Xin X, Hua T, et al. Efficacy of anti‐VEGF/VEGFR agents on animal models of endometriosis: a systematic review and meta‐analysis. PLOS One. 2016;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Gille H, Kowalski J, Li B, et al. Analysis of biological effects and signaling properties of Flt‐1 (VEGFR‐1) and KDR (VEGFR‐2) A reassessment using novel receptor‐specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276(5):3222‐3230. [DOI] [PubMed] [Google Scholar]
- 235. Zhang Z, Neiva KG, Lingen MW, Ellis LM, Nör JE. VEGF‐dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ. 2010;17(3):499‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Hoekman K. SU6668, a multitargeted angiogenesis inhibitor. Cancer J. 2001;7:S134‐S138. [PubMed] [Google Scholar]
- 237. Que Y, Liang Y, Zhao J, et al. Treatment‐related adverse effects with pazopanib, sorafenib and sunitinib in patients with advanced soft tissue sarcoma: a pooled analysis. Cancer Manag Res. 2018;10:2141‐2150. 10.2147/cmar.s164535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Kallio PJ, Wilson WJ, O'Brien S, Makino Y, Poellinger L. Regulation of the hypoxia‐inducible transcription factor 1alpha by the ubiquitin‐proteasome pathway. J Biol Chem. 1999;274(10):6519‐6525. 10.1074/jbc.274.10.6519[doi] [DOI] [PubMed] [Google Scholar]
- 239. Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells: identification of a 5′ enhancer. Circ Res. 1995;77(3):638‐643. [DOI] [PubMed] [Google Scholar]
- 240. Becker CM, Rohwer N, Funakoshi T, et al. 2‐methoxyestradiol inhibits hypoxia‐inducible factor‐1{alpha} and suppresses growth of lesions in a mouse model of endometriosis. Am J Pathol. 2008;172(2):534‐544. 10.2353/ajpath.2008.061244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Sharkey AM, Sharkey AM, Day K, et al. Vascular endothelial growth factor expression in human endometrium is regulated by hypoxia. J Clin Endocrinol Metabol. 2000;85(1):402‐409. [DOI] [PubMed] [Google Scholar]
- 242. Park JK, Song M, Dominguez CE, et al. Glycodelin mediates the increase in vascular endothelial growth factor in response to oxidative stress in the endometrium. Am J Obstet Gynecol. 2006;195(6):1772‐1777. [DOI] [PubMed] [Google Scholar]
- 243. Sacco K, Portelli M, Pollacco J, Schembri‐Wismayer P, Calleja‐Agius J. The role of prostaglandin E2 in endometriosis. Gynecol Endocrinol. 2012;28(2):134‐138. 10.3109/09513590.2011.588753 [DOI] [PubMed] [Google Scholar]
- 244. Leibovich SJ, Polverini PJ, Shepard HM et al. Macrophage‐induced angiogenesis is mediated by tumour necrosis factor‐α. Nature. 1987;329(6140):630‐632. 10.1038/329630a0 [DOI] [PubMed] [Google Scholar]
- 245. Creus M, Fabregues F, Carmona F, del Pino M, Manau D, Balasch J. Combined laparoscopic surgery and pentoxifylline therapy for treatment of endometriosis‐associated infertility: a preliminary trial. Hum Reprod. 2008;23(8):1910‐1916. 10.1093/humrep/den167 [DOI] [PubMed] [Google Scholar]
- 246. Bryan BA, Dennstedt E, Mitchell DC, et al. RhoA/ROCK signaling is essential for multiple aspects of VEGF‐mediated angiogenesis. FASEB J. 2010;24(9):3186‐3195. 10.1096/fj.09-145102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Howe GA, Addison CL. RhoB controls endothelial cell morphogenesis in part via negative regulation of RhoA. Vasc Cell. 2012;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Hasmim M, Bieler G, Rüegg C. Zoledronate inhibits endothelial cell adhesion, migration and survival through the suppression of multiple, prenylation‐dependent signaling pathways. J Thromb Haemostasis. 2007;5(1):166‐173. [DOI] [PubMed] [Google Scholar]
- 249. Mizukami Y, Fujiki K, Duerr E‐M, et al. Hypoxic regulation of vascular endothelial growth factor through the induction of phosphatidylinositol 3‐kinase/Rho/ROCK and c‐Myc. J Biol Chem. 2006;281(20):13957‐13963. 10.1074/jbc.M511763200 [DOI] [PubMed] [Google Scholar]
- 250. Jackson LW, Schisterman EF, Dey‐Rao R, Browne R, Armstrong D. Oxidative stress and endometriosis. Hum Reprod. 2005;20(7):2014‐2020. 10.1093/humrep/dei001 [DOI] [PubMed] [Google Scholar]
- 251. Slater TF. Free‐radical mechanisms in tissue injury. Biochemical J. 1984;222(1):1–15. 10.1042/bj2220001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Phcog Rev. 2010;4(8):118‐126. 10.4103/0973-7847.70902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Scutiero G, Iannone P, Bernardi G, et al. Oxidative stress and endometriosis: a systematic review of the literature. Oxid Med Cell Longev. 2017;2017:7265238. 10.1155/2017/7265238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Ito Fuminori, Yamada Yuki, Shigemitsu Aiko, Akinishi Mika, Kaniwa Hiroko, Miyake Ryuta, Yamanaka Shoichiro, Kobayashi Hiroshi. Role of Oxidative Stress in Epigenetic Modification in Endometriosis. Reprod Sci. 2017;24(11):1493–1502. 10.1177/1933719117704909 [DOI] [PubMed] [Google Scholar]
- 255. Harlev A, Gupta S, Agarwal A. Targeting oxidative stress to treat endometriosis. Expert Opin Ther Targets. 2015;19(11):1447‐1464. 10.1517/14728222.2015.1077226 [DOI] [PubMed] [Google Scholar]
- 256. Al‐Gubory KH. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod Biomed Online. 2014;29(1):17‐31. 10.1016/j.rbmo.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 257. Cho YJ, Park SB, Han M. Di‐(2‐ethylhexyl)‐phthalate induces oxidative stress in human endometrial stromal cells in vitro. Mol Cell Endocrinol. 2015;407:9‐17. 10.1016/j.mce.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 258. Iwabuchi T, Yoshimoto C, Shigetomi H, Kobayashi H. Oxidative stress and antioxidant defense in endometriosis and its malignant transformation. Oxid Med Cell Longev. 2015;2015:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Turkyilmaz E, Yildirim M, Cendek BD, et al. Evaluation of oxidative stress markers and intra‐extracellular antioxidant activities in patients with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2016;199:164‐168. [DOI] [PubMed] [Google Scholar]
- 260. Ricci AG, Olivares CN, Bilotas MA, et al. Natural therapies assessment for the treatment of endometriosis. Hum Reprod. 2013;28(1):178‐188. [DOI] [PubMed] [Google Scholar]
- 261. Hayes JD, Dinkova‐Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199‐218. 10.1016/j.tibs.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 262. Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium‐derived relaxing factor. Nature. 1987;327(6122):524‐526. 10.1038/327524a0 [DOI] [PubMed] [Google Scholar]
- 263. Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829‐837. 10.1093/eurheartj/ehr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Corradin SB, Fasel N, Buchmuller‐Rouiller Y, Ransijn A, Smith J, Mauel J. Induction of macrophage nitric oxide production by interferon‐gamma and tumor necrosis factor‐alpha is enhanced by interleukin‐10. Eur J Immunol. 1993;23(8):2045‐2048. 10.1002/eji.1830230851 [DOI] [PubMed] [Google Scholar]
- 265. Wu MY, Chao KH, Yang JH, Lee TH, Yang YS, Ho HN. Nitric oxide synthesis is increased in the endometrial tissue of women with endometriosis. Hum Reprod. 2003;18(12):2668‐2671. [DOI] [PubMed] [Google Scholar]
- 266. Nevzati E, Shafighi M, Bakhtian KD, Treiber H, Fandino J, Fathi AR. Estrogen induces nitric oxide production via nitric oxide synthase activation in endothelial cells. Acta Neurochir Suppl. 2015;120:141‐145. 10.1007/978-3-319-04981-6_24 [DOI] [PubMed] [Google Scholar]
- 267. van Langendonckt A, Casanas‐Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril. 2002;77(5):861‐870. 10.1016/S0015-0282(02)02959-X [DOI] [PubMed] [Google Scholar]
- 268. Defrère S, van Langendonckt A, Vaesen S, et al. Iron overload enhances epithelial cell proliferation in endometriotic lesions induced in a murine model. Hum Reprod. 2006;21(11):2810‐2816. [DOI] [PubMed] [Google Scholar]
- 269. Bresgen N, Eckl PM. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules. 2015;5(2):808‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Zhang T, de Carolis C, Man GCW, Wang CC. The link between immunity, autoimmunity and endometriosis: a literature update. Autoimmun Rev. Oct 2018;17(10):945‐955. 10.1016/j.autrev.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 271. Ahn SH, Monsanto SP, Miller C, Singh SS, Thomas R, Tayade C. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2015;2015:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. de Barros IBL, Malvezzi H, Gueuvoghlanian‐Silva BY, Piccinato CA, Rizzo LV, Podgaec S. What do we know about regulatory T cells and endometriosis? A systematic review. J Reprod Immunol. 2017;120:48‐55. 10.1016/j.jri.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 273. Zeller JM, Henig I, Radwanska EWA, Dmowski WP. Enhancement of human monocyte and peritoneal macrophage chemiluminescence activities in women with endometriosis. Am J Reprod Immunol Microbiol. 1987;13(3):78‐82. 10.1111/j.1600-0897.1987.tb00097.x [DOI] [PubMed] [Google Scholar]
- 274. Sikora J, Mielczarek‐Palacz A, Kondera‐Anasz Z. Role of natural killer cell activity in the pathogenesis of endometriosis. Curr Med Chem. 2011;18(2):200‐208. 10.2174/092986711794088416 [DOI] [PubMed] [Google Scholar]
- 275. Schulke L, Berbic M, Manconi F, Tokushige N, Markham R, Fraser IS. Dendritic cell populations in the eutopic and ectopic endometrium of women with endometriosis. Hum Reprod. 2009;24(7):1695‐1703. 10.1093/humrep/dep071 [DOI] [PubMed] [Google Scholar]
- 276. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958‐969. 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Tabibzadeh S, Kaffka KL, Satyaswaroop PG, Kilian PL. Interleukin‐1 (IL‐1) regulation of human endometrial function: presence of IL‐1 receptor correlates with IL‐1‐stimulated prostaglandin E2 production. J Clini Endocrinol Metabol. 1990;70(4):1000‐1006. [DOI] [PubMed] [Google Scholar]
- 278. Tanikawa M, Lee H‐Y, Watanabe K, et al. Regulation of prostaglandin biosynthesis by interleukin‐1 in cultured bovine endometrial cells. J Endocrinol. 2008;199(3):425‐434. [DOI] [PubMed] [Google Scholar]
- 279. Rier SE, Parsons AK, Becker JL. Altered interleukin‐6 production by peritoneal leukocytes from patients with endometriosis. Fertil Steril. 1994;61(2):294‐299. [DOI] [PubMed] [Google Scholar]
- 280. Rier SE, Zarmakoupis PN, Hu X, Becker JL. Dysregulation of interleukin‐6 responses in ectopic endometrial stromal cells: correlation with decreased soluble receptor levels in peritoneal fluid of women with endometriosis. J Clin Endocrinol Metabol. 1995;80(4):1431‐1437. [DOI] [PubMed] [Google Scholar]
- 281. Sano M, Terada M, Ishii A, Kino H, Anantaphruti M. Studies on chemotherapy of parasitic helminths (V). Effects of niclosamide on the motility of various parasitic helminths. Experientia. 1982;38(5):547‐549. 10.1007/BF02327042 [DOI] [PubMed] [Google Scholar]
- 282. Reis FM, Petraglia F, Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update. 2013;19(4):406‐418. 10.1093/humupd/dmt010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Carafa V, Altucci L, Nebbioso A. Dual tumor suppressor and tumor promoter action of sirtuins in determining malignant phenotype. Front Pharmacol. 2019;10:38. 10.3389/fphar.2019.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Tatone C, Di Emidio G, Barbonetti A, et al. Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility. Hum Reprod Update. 2018;24(3):267‐289. 10.1093/humupd/dmy003 [DOI] [PubMed] [Google Scholar]
- 285. Carli C, Metz CN, Al‐Abed Y, Naccache PH, Akoum A. Up‐regulation of cyclooxygenase‐2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: involvement of novel kinase signaling pathways. Endocrinology. 2009;150(7):3128‐3137. 10.1210/en.2008-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Al‐Abed Y, Dabideen D, Aljabari B, et al. ISO‐1 Binding to the tautomerase active site of MIF inhibits its pro‐inflammatory activity and increases survival in severe sepsis. J Biol Chem. 2005;280(44):36541‐36544. 10.1074/jbc.c500243200 [DOI] [PubMed] [Google Scholar]
- 287. Nothnick WB, Colvin A, Cheng KF, Al‐Abed Y. Inhibition of macrophage migration inhibitory factor reduces endometriotic implant size in mice with experimentally induced disease. J Endometr. 2011;3(3):135‐142. [PMC free article] [PubMed] [Google Scholar]
- 288. Tamura M, Deb S, Sebastian S, Okamura K, Bulun SE. Estrogen up‐regulates cyclooxygenase‐2 via estrogen receptor in human uterine microvascular endothelial cells. Fertil Steril. 2004;81(5):1351‐1356. [DOI] [PubMed] [Google Scholar]
- 289. González‐Ramos R, van Langendonckt A, Defrère S, et al. Involvement of the nuclear factor‐κB pathway in the pathogenesis of endometriosis. Fertil Steril. 2010;94(6):1985‐1994. 10.1016/j.fertnstert.2010.01.013 [DOI] [PubMed] [Google Scholar]
- 290. Gou Y, Li X, Li P, et al. Estrogen receptor β upregulates CCL2 via NF‐κB signaling in endometriotic stromal cells and recruits macrophages to promote the pathogenesis of endometriosis. Hum Reprod. 2019;34(4):646‐658. [DOI] [PubMed] [Google Scholar]
- 291. Chang KK, Liu LB, Li H, et al. TSLP induced by estrogen stimulates secretion of MCP‐1 and IL‐8 and growth of human endometrial stromal cells through JNK and NF‐kappaB signal pathways. Int J Clin Exp Pathol. 2014;7(5):1889‐1899. [PMC free article] [PubMed] [Google Scholar]
- 292. Deura I, Harada T, Taniguchi F, Iwabe T, Izawa M, Terakawa N. Reduction of estrogen production by interleukin‐6 in a human granulosa tumor cell line may have implications for endometriosis‐associated infertility. Fertil Steril. 2005;83(4):1086‐1092. [DOI] [PubMed] [Google Scholar]
- 293. Nabavi SM, Nabavi SF, Sureda A, et al. Anti‐inflammatory effects of melatonin: a mechanistic review. Crit Rev Food Sci Nutr. 2019;59(supply 1):S4‐S16. 10.1080/10408398.2018.1487927 [DOI] [PubMed] [Google Scholar]
- 294. Schwertner A, Conceição dos Santos CC, Costa GD, et al. Efficacy of melatonin in the treatment of endometriosis: a phase II, randomized, double‐blind, placebo‐controlled trial. Pain. 2013;154(6):874‐881. 10.1016/j.pain.2013.02.025 [DOI] [PubMed] [Google Scholar]
- 295. Mosher AA, Tsoulis MW, Lim J, et al. Melatonin activity and receptor expression in endometrial tissue and endometriosis. Hum Reprod. 2019;34(7):1215‐1224. 10.1093/humrep/dez082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Zhao X, Wang D, Wu Z, et al. Female reproductive performance in the mouse: effect of oral melatonin. Molecules. 2018;23(8):1845. 10.3390/molecules23081845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Mojaverrostami S, Asghari N, Khamisabadi M, Heidari Khoei H. The role of melatonin in polycystic ovary syndrome: A review. Int J Reprod Biomed. 2019;17(12):865‐882. 10.18502/ijrm.v17i12.5789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Meng X, Li Y, Li S, et al. Dietary sources and bioactivities of melatonin. Nutrients. 2017;9(4):367. 10.3390/nu9040367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Harpsoe NG, Andersen LP, Gogenur I, Rosenberg J. Clinical pharmacokinetics of melatonin: a systematic review. Eur J Clin Pharmacol. 2015;71(8):901‐909. 10.1007/s00228-015-1873-4 [DOI] [PubMed] [Google Scholar]
- 300. Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 2012;122(6):253‐270. 10.1042/CS20110386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Tao X, Cai L, Chen L, Ge S, Deng X. Effects of metformin and exenatide on insulin resistance and AMPKalpha‐SIRT1 molecular pathway in PCOS rats. J Ovarian Res. 2019;12(1):86. 10.1186/s13048-019-0555-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Shao R, Li X, Billig H. Promising clinical practices of metformin in women with PCOS and early‐stage endometrial cancer. BBA Clin. 2014;2:7‐9. 10.1016/j.bbacli.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Salamon S, Kramar B, Marolt TP, Poljsak B, Milisav I. Medical and dietary uses of N‐acetylcysteine. Antioxidants (Basel). 2019;8(5):111. 10.3390/antiox8050111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Rhodes K, Braakhuis A. Performance and side effects of supplementation with N‐acetylcysteine: a systematic review and meta‐analysis. Sports Med. 2017;47(8):1619‐1636. 10.1007/s40279-017-0677-3 [DOI] [PubMed] [Google Scholar]
- 305. Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N‐acetylcysteine. Eur J Clin Pharmacol. 1988;34(1):77‐82. 10.1007/BF01061422 [DOI] [PubMed] [Google Scholar]
- 306. Chu C, Deng J, Man Y, Qu Y. Green tea extracts epigallocatechin‐3‐gallate for different treatments. BioMed Res Int. 2017;2017:5615647‐5615649. 10.1155/2017/5615647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Lecumberri E, Dupertuis YM, Miralbell R, Pichard C. Green tea polyphenol epigallocatechin‐3‐gallate (EGCG) as adjuvant in cancer therapy. Clin Nutr. 2013;32(6):894‐903. 10.1016/j.clnu.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 308. Cai Z‐Y, Li X‐M, Liang J‐P, et al. Bioavailability of tea catechins and its improvement. Molecules. 2018;23(9):2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Singh CK, Ndiaye MA, Ahmad N. Resveratrol and cancer: challenges for clinical translation. Biochim Biophys Acta. 2015;1852(6):1178‐1185. 10.1016/j.bbadis.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Sharifi‐Rad J, Rayess YE, Rizk AA, et al. Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol. 2020;11:01021. 10.3389/fphar.2020.01021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Tsai YC, Leu SY, Peng YJ, et al. Genistein suppresses leptin‐induced proliferation and migration of vascular smooth muscle cells and neointima formation. J Cell Mol Med. 2017;21(3):422‐431. 10.1111/jcmm.12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Marini H, Bitto A, Altavilla D, et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow‐up study. J Clin Endocrinol Metab. 2008;93(12):4787‐4796. 10.1210/jc.2008-1087 [DOI] [PubMed] [Google Scholar]
- 313. Spagnuolo C, Russo GL, Orhan IE, et al. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015;6(4):408‐419. 10.3945/an.114.008052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Sirtori CR. Risks and benefits of soy phytoestrogens in cardiovascular diseases, cancer, climacteric symptoms and osteoporosis. Drug Saf. 2001;24(9):665‐682. 10.2165/00002018-200124090-00003 [DOI] [PubMed] [Google Scholar]
- 315. Sun M, Ye Y, Xiao L, Duan X, Zhang Y, Zhang H. Anticancer effects of ginsenoside Rg3 (Review). Int J Mol Med. 2017;39(3):507‐518. 10.3892/ijmm.2017.2857 [DOI] [PubMed] [Google Scholar]
- 316. Zhou YX, Zhang H, Peng C. Puerarin: a review of pharmacological effects. Phytother Res. 2014;28(7):961‐975. 10.1002/ptr.5083 [DOI] [PubMed] [Google Scholar]
- 317. Medarevic D, Cvijic S, Dobricic V, Mitric M, Djuris J, Ibric S. Assessing the potential of solid dispersions to improve dissolution rate and bioavailability of valsartan: in vitro‐in silico approach. Eur J Pharm Sci. 2018;124:188‐198. 10.1016/j.ejps.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 318. Sun J, Bi C, Chan HM, Sun S, Zhang Q, Zheng Y. Curcumin‐loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf B Biointerfaces. 2013;111:367‐375. 10.1016/j.colsurfb.2013.06.032 [DOI] [PubMed] [Google Scholar]
- 319. Fang R‐C, Tsai Y‐T, Lai J‐N, Yeh C‐H, Wu C‐T. The traditional chinese medicine prescription pattern of endometriosis patients in Taiwan: a population‐based study. Evid‐based Complement Alternat Med: eCAM. 2012;2012:591391‐591399. 10.1155/2012/591391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Tsai P‐J, Lin Y‐H, Chen J‐L, Yang S‐H, Chen Y‐C, Chen H‐Y. Identifying Chinese herbal medicine network for endometriosis: implications from a population‐based database in Taiwan. Evid‐Based Complement Alternat Med. 2017;2017:1‐10. 10.1155/2017/7501015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Li X, He Y, Zeng P, et al. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J Cell Mol Med. 2019;23(1):4‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Xie C‐H, Zhang M‐S, Zhou Y‐F, et al. Chinese medicine Angelica sinensis suppresses radiation‐induced expression of TNF‐α and TGF‐β1 in mice. Oncol Rep. 2006;15(6):1429‐1436. [PubMed] [Google Scholar]
- 323. Park C, Han M‐H, Park S‐H, et al. Induction of apoptosis by Moutan Cortex Radicis in human gastric cancer cells through the activation of caspases and the AMPK signaling pathway. Rev Bras Farmacogn. 2017;27(3):315‐323. [Google Scholar]
- 324. Chung H‐S, Kang M, Cho C, et al. Inhibition of nitric oxide and tumor necrosis factor‐alpha by moutan cortex in activated mouse peritoneal macrophages. Biol Pharm Bull. 2007;30(5):912‐916. [DOI] [PubMed] [Google Scholar]
- 325. Kim YW, Zhao RJ, Park SJ, et al. Anti‐inflammatory effects of liquiritigenin as a consequence of the inhibition of NF‐κB‐dependent iNOS and proinflammatory cytokines production. Br J Pharmacol. 2008;154(1):165‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Zhu J, Luo C, Wang P, He Q, Zhou J, Peng H. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF‐κB pathways in LPS‐stimulated RAW 264.7 cells. Exp Ther Med. 2013;5(5):1345‐1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Bi X, Han L, Qu T, et al. Anti‐inflammatory effects, SAR, and action mechanism of monoterpenoids from Radix paeoniae Alba on LPS‐stimulated RAW 264.7 cells. Molecules. 2017;22(5):715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Wang K‐T, Chen L‐G, Chou D‐S, Liang W‐L, Wang C‐C. Anti‐oxidative abilities of essential oils from Atractylodes ovata Rhizome. Evid‐Based Complement Alternat Med. 2011;2011:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Wang Y, Yu Y, Mao J. Carboxymethylated β‐glucan derived from Poria cocos with biological activities. J Agricult Food Chem. 2009;57(22):10913‐10915. [DOI] [PubMed] [Google Scholar]
- 330. Fu N, Liu Z. Antipromoting, antimutagenic and antioxidant action of glycyrrhizae flavonoids. Chin J Cancer Res. 1995;7(3):163‐167. 10.1007/BF03023467 [DOI] [Google Scholar]
- 331. Yang W‐J, Li Y‐R, Gao H, et al. Protective effect of the ethanol extract from Ligusticum chuanxiong rhizome against streptozotocin‐induced diabetic nephropathy in mice. J Ethnopharmacol. 2018;227:166‐175. [DOI] [PubMed] [Google Scholar]
- 332. Liao K‐F, Chiu T‐L, Huang S‐Y, et al. Anti‐cancer effects of Radix angelica sinensis (Danggui) and N‐butylidenephthalide on gastric cancer: implications for REDD1 activation and mTOR inhibition. Cell Physiol Biochem. 2018;48(6):2231‐2246. [DOI] [PubMed] [Google Scholar]
- 333. Huang H‐L, Chen C‐C, Yeh C‐Y, Huang R‐L. Reactive oxygen species mediation of Baizhu‐induced apoptosis in human leukemia cells. J Ethnopharmacol. 2005;97(1):21‐29. 10.1016/j.jep.2004.09.058 [DOI] [PubMed] [Google Scholar]
- 334. Lin M‐Y, Lee Y‐R, Chiang S‐Y, et al. Cortex Moutan induces bladder cancer cell death via apoptosis and retards tumor growth in mouse bladders. Evid‐Based Complement Alternat Med. 2013;2013:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Zhang L, Zhang S. Modulating Bcl‐2 family proteins and caspase‐3 in induction of apoptosis by paeoniflorin in human cervical cancer cells. Phytother Res. 2011;25(10):1551‐1557. [DOI] [PubMed] [Google Scholar]
- 336. Sun W, Sanderson PE, Zheng W. Drug combination therapy increases successful drug repositioning. Drug Discov Today. 2016;21(7):1189‐1195. 10.1016/j.drudis.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Becker CM, Laufer MR, Stratton P, et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: I. Surgical phenotype data collection in endometriosis research. Fertil Steril. 2014;102(5):1213‐1222. 10.1016/j.fertnstert.2014.07.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. van Norman GA. Drugs and devices: comparison of European and U.S. approval processes. JACC Basic Transl Sci. 2016;1(5):399‐412. 10.1016/j.jacbts.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3(8):673‐683. 10.1038/nrd1468 [DOI] [PubMed] [Google Scholar]
- 340. Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2018;18(1):41‐58. 10.1038/nrd.2018.168 [DOI] [PubMed] [Google Scholar]
- 341. Pillaiyar T, Meenakshisundaram S, Manickam M, Sankaranarayanan M. A medicinal chemistry perspective of drug repositioning: recent advances and challenges in drug discovery. Eur J Med Chem. 2020;195:112275. 10.1016/j.ejmech.2020.112275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Sleigh SH, Barton CL. Repurposing strategies for therapeutics. Pharm Med. 2010;24(3):151‐159. 10.1007/BF03256811 [DOI] [Google Scholar]


