Abstract
Background
Pulmonary embolism (PE) is a potentially fatal disease, but data on the incidence of fatal PE in cancer patients are scant.
Objective
We sought to estimate the proportion of cancer patients with PE at autopsy.
Methods
For this retrospective cohort study, all autopsy reports of cancer patients were retrieved from PALGA: Dutch Pathology Registry and used for data extraction. The primary outcome was PE at time of autopsy, defined as any clot obstructing a pulmonary artery. The secondary outcome was venous thromboembolism, defined as the composite of thrombotic PE, deep vein thrombosis, splanchnic vein thrombosis, or internal jugular vein thrombosis.
Results
A total of 9571 cancer patients were included. In 1191 (12.4%; 95% confidence interval [CI], 11.8‐13.1) patients, one or more PE events were observed at autopsy, of whom 1074 (90.2%) had a thrombotic embolism, 168 (14.1%) a tumor embolism, 9 (0.8%) a septic embolism, 7 (0.6%) a fat tissue embolism, and 3 (0.3%) a bone marrow embolism. Among patients with PE for whom the cause of death was specified in the autopsy report, death was considered PE‐related in 642 patients (66.7%), which was 6.7% of the total study population. Venous thromboembolism was observed in 1223 (12.8%; 95% CI, 12.1‐13.5) patients.
Conclusion
The proportion of PE in cancer patients at autopsy is substantial. Although the study population is not representative for the total cancer population, it suggests that PE is an important disease complication in cancer patients.
Keywords: autopsy, neoplasms, pulmonary embolism, thrombosis, venous thromboembolism
Essentials.
PE (Pulmonary Embolism) is a frequent complication in cancer patients, but data on fatal PE in cancer patients are scant.
We performed an autopsy‐based cohort study of all autopsies in the Netherlands between 2008 and 2020.
Of the 9571 cancer patients, 1191 (12.4%; 95% CI, 11.8–13.1) patients had PE at autopsy.
The study underscores that PE is an important disease complication in cancer patients.
1. INTRODUCTION
Venous thromboembolism (VTE), manifesting primarily as deep vein thrombosis (DVT) and pulmonary embolism (PE), is a frequent complication in cancer patients. Recent evidence shows that the risk of VTE in cancer patients is currently 12‐fold higher than in the general population and even 23‐fold higher when receiving chemotherapy or targeted therapy. 1 The increased VTE risk is related to a combination of the intrinsic prothrombotic activity of cancer cells, prothrombotic effects of chemotherapy treatment, and other cancer‐related risk factors such as surgery and immobility. 2 VTE in cancer patients is associated with mortality, of which the risk appeared approximately twofold higher in cancer patients with VTE compared to cancer patients without VTE in an observational cohort study of 668 cancer patients from the Danish Cancer Registry. 3
Two recent randomized clinical trials showed that primary thromboprophylaxis with direct oral anticoagulants for 6 months substantially reduced the risk of VTE in ambulatory patients compared to placebo (relative risk, 0.56; 95% confidence interval [CI], 0.35‐0.89). However, the tradeoff between safety and efficacy of primary thromboprophylaxis in cancer patients is challenging because a concurrent increased bleeding risk was observed in the intervention groups (relative risk, 1.96; 95% CI, 0.80‐4.82). 4 , 5 , 6 Therefore, the decision on initiation of primary thromboprophylaxis should be based on patient preference and the carefully balanced benefit–risk ratio of such an intervention.
For this decision, information on PE‐related death in cancer patients is relevant, but contemporary data on this topic are scant. 7 The nationwide network and registry of histo‐ and cytopathology in the Netherlands (PALGA) maintains a database with all Dutch pathology reports, including autopsy reports. 8 Although deceased patients undergoing autopsy form a selected population, this registry provides a unique opportunity to assess how common (fatal) PE is in cancer patients. By using data from autopsy reports, we aimed to estimate the proportion of deceased cancer patients with PE or any VTE, and to evaluate how often PE was considered a cause of death in these patients.
2. METHODS
2.1. Study design and patient selection
PALGA was used for present analysis, which contains data on all histological biopsies and autopsies in the Netherlands. 8 All autopsy reports between January 1, 2008, and April 30, 2020, with a cancer diagnosis code were used for the current study. Patients 18 years and older with a cancer autopsy code at time of autopsy were considered eligible. Patients with skin cancer other than primary melanoma, Merkel cell carcinoma, or sebaceous gland carcinoma were excluded. This study was registered at the Netherlands Trial Register (NL8670).
2.2. Data collection and study outcomes
Clinical data on patient characteristics, cancer type, cancer stage at autopsy, and study outcomes were retrospectively collected from the autopsy reports. Time between cancer diagnosis and autopsy was measured by subtracting the date of biopsy (either collection of a cytological, histological, or resection specimen) on which cancer was diagnosed from the autopsy date. Data on annual cancer deaths were obtained from Statistics Netherlands (CBS; 2008‐2019). 9 The primary outcome was the proportion of patients with PE at time of autopsy, which was defined as a sudden obstruction of a pulmonary artery or one of its branches caused by a bloodborne clot or other material. 10 Hence, this definition includes thrombotic PE, tumor PE, septic PE, fat tissue PE, and bone marrow PE. Tumor PE was defined as an obstruction of a pulmonary artery or one of its branches caused by individual tumor cells, clusters of tumor cells, or large tumor fragments. 11 Cases of bone marrow embolism after cardiac massage were excluded because, in these patients, the embolism developed postmortem. The secondary outcome was the proportion with VTE, including thrombotic PE, deep vein thrombosis of the upper or lower extremities, splanchnic vein thrombosis, and internal jugular vein thrombosis. Study outcomes were systematically identified from the autopsy records by an automated electronic text search for which several search terms for these events were combined. The search strategy was tested on a subset of 300 cases, which were manually verified and yielded a sensitivity of 100%, indicating a low risk of missing events with this method. The identified potential cases, yielded by the search, were manually confirmed (I.A.G.). All events were centrally reviewed by two of the authors (F.I.M. and F.T.M.B.) to minimize observer bias. In case of disagreement, a third author was consulted (J.E.F.). Data on autopsy date, age, sex, location of VTE, cancer site, metastatic disease, and cause of death for PE cases were manually collected from the autopsy reports. PE was considered the primary cause of death if it was described as such or if named the most probable cause of death. PE was considered a contributing factor to death if it was described as such or if named as one of the causes of death. In both cases, death was considered PE‐related. Pulmonary embolic events were categorized according to the origin of the obstructing material. PE was defined as central when it was observed in the pulmonary trunk or in the main pulmonary arteries and as peripheral when observed in segmental, subsegmental, or more peripheral arteries.
2.3. Statistical analysis
Patient and tumor characteristics were summarized using standard descriptive statistics. Study outcomes were presented as proportions with 95% CIs calculated using the Clopper‐Pearson method. 12 To explore a potential time trend of PE at death, the annual number of autopsy reports of cancer patients, total cancer‐related deaths in the Netherlands, and the proportion of cancer patients with PE were plotted against calendar year. The time trend of the proportion of cancer patients with PE was formally tested with the Jonckheere‐Terpstra test. 13 , 14 The year 2020 was excluded from these explorative analyses because these data were only available until April 2020. Cases with missing reports were excluded prior analysis. Statistical analyses and the automated text search in autopsy reports were performed using R software, version 3.5.1 (The R Project for Statistical Computing, www.R‐project.org), specifically using the “stringr,” “ggplot2,” and “dplyr” packages.
3. RESULTS
A total of 9571 cancer patients with autopsy between January 2008 and April 2020 were included. The median age was 70 years (interquartile range, 62‐78) and 60.5% were male. The most frequent cancer types were lung cancer (26.2%), hematological cancer (12.4%), and colorectal cancer (10.3%). The median time between cancer diagnosis (date of tumor cytology/biopsy/resection) and autopsy was 404 days (interquartile range, 49‐2271). For 3215 (33.6%) patients, the cancer was not diagnosed by tumor biopsy before death and hence potentially undiscovered before autopsy. The number of autopsies decreased over the years; a total of 4324 autopsies were performed between 2008 and 2011, 3038 between 2012 and 2015, and 2209 between 2016 and April 2020. All patient characteristics are summarized in Table 1.
TABLE 1.
Patient characteristics at autopsy
| — |
All Cancer Patients (N = 9571) |
Cancer Patients with pulmonary embolism at autopsy (N = 1191) |
|---|---|---|
| Male, (%) | 5786 (60.5%) | 640 (53.9%) |
| Median age, y (IQR) | 70 (62–78) | 68 (60–76) |
| Median time between diagnostic cancer diagnosis and autopsy, days (IQR) | 404 (49–2271) | 270 (30–1763) |
| Cancer not pathologically confirmed prior to autopsy (%) | 3215 (33.6%) | 398 (33.4%) |
| Year of autopsy (%) | — | — |
| 2008‐2011 | 4324 (45.2) | 514 (43.2) |
| 2012‐2015 | 3038 (31.7) | 391 (32.8) |
| 2016‐2020 a | 2209 (23.1) | 286 (24.0) |
| Primary cancer site, (%) | — | — |
| Biliary | 161 (1.7) | 27 (2.3) |
| Brain | 122 (1.3) | 10 (0.8) |
| Breast | 415 (4.3) | 56 (4.7) |
| Colorectal | 988 (10.3) | 129 (10.8) |
| Gastroesophageal | 585 (6.1) | 87 (7.3) |
| Gynecological | 251 (2.6) | 43 (3.6) |
| Head and neck | 120 (1.3) | 10 (0.8) |
| Hematological cancer | 1191 (12.4) | 80 (6.7) |
| Kidney | 468 (4.9) | 55 (4.6) |
| Liver | 276 (2.9) | 39 (3.3) |
| Lung | 2509 (26.2) | 324 (27.2) |
| Melanoma | 89 (0.9) | 6 (0.5) |
| Pancreas | 611 (6.4) | 118 (9.9) |
| Prostate | 641 (6.7) | 58 (4.9) |
| Sarcoma | 145 (1.5) | 18 (1.5) |
| Small bowel cancer | 155 (1.6) | 14 (1.2) |
| Thyroid | 114 (1.2) | 16 (1.3) |
| Urinary bladder | 396 (4.1) | 57 (4.8) |
| Unknown primary tumor | 242 (2.5) | 36 (3.0) |
| Other | 92 (1.0) | 8 (0.7) |
| Metastatic disease at autopsy, (%) b | — | 811 (68.1) |
Abbreviations: IQR, interquartile range.
Autopsies from 2016 to April 2020.
Cancer stage only extracted for patients with pulmonary embolism at death.
3.1. Pulmonary embolism
Of all 9571 cancer patients, 1191 (12.4%; 95% CI, 11.8‐13.1) patients had one or more types of PE at time of autopsy, of which 1074 (90.2%) had thrombotic embolism, 168 (14.1%) tumor embolism, 9 (0.8%) septic embolism, 7 (0.6%) fat tissue embolism, and 3 (0.3%) bone marrow embolism (Table 2). The location of PE was central in 280 (23.5%) patients, peripheral in 263 (22.1%) patients, and was not formally described in 648 (54.4%) patients. The proportion with PE at death was highest in patients with pancreatic cancer (19.3%; 95% CI, 16.3‐22.7), gynecological cancers (17.1%; 95% CI, 12.7‐22.4), and biliary cancer (16.8%; 95% CI, 11.4‐23.5). Conversely, the proportion with PE was lower in those with hematological malignancies (6.7%; 95% CI, 5.4‐8.3) or melanoma (6.7%; 95% CI, 2.5‐14.1) (Figure 1).
TABLE 2.
Pulmonary embolism outcomes at autopsy
| — |
Patients with PE (N = 1191) |
|---|---|
| Type of pulmonary embolism, (%) a | — |
| Thrombotic embolism | 1074 (90.2) |
| Tumor embolism | 168 (14.1) |
| Septic embolism | 9 (0.8) |
| Fat tissue embolism | 7 (0.6) |
| Bone marrow embolism | 3 (0.3) |
| Location PE, (%) | — |
| Central | 280 (51.6) |
| Peripheral | 263 (48.4) |
| Cause of death mentioned, (%) | 960 (80.6) |
| Cause of death PE‐related | 642 (66.9) |
| PE primary cause of death | 361 (56.2) |
| PE contributing factor to death | 281 (43.8) |
Abbreviation: PE, pulmonary embolism.
Events are not mutually exclusive.
FIGURE 1.
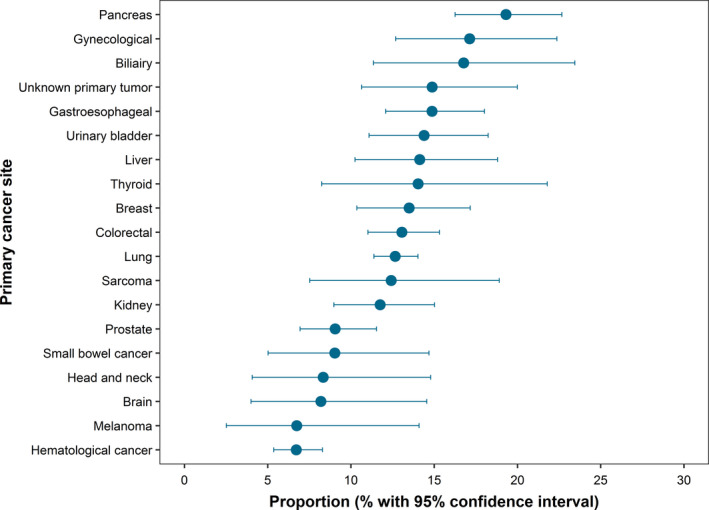
Proportion of cancer patients with pulmonary embolism at autopsy by cancer site
For 960 of the 1191 (80.6%) patients with a PE at autopsy, a cause of death was explicitly described by the pathologist. In 642 (66.9%) of these patients, death was considered to be PE‐related; PE was considered the primary cause of death for 361 (37.6%) of these patients and a contributing factor to death for 281 (29.3%) (Table 2). The anatomical location of the PE event was central in 71.6% of all patients with a PE‐related death, 89.6% in the patients in which PE was considered the primary cause of death, and 37.7% in the patients in which PE was a considered a contributing factor to death.
Compared with the total cancer population of 9571 cancer patients in our study, the 642 patients with a PE‐related death formed 6.7% of the study population, the 361 patients of which PE was considered primary cause of death 2.9%, and the 281 patients of which PE was considered a contributing factor to death 3.8% (Table 2).
3.2. Venous thromboembolic events
Of all 9571 patients, 1223 (12.8%; 95% CI 12.1‐13.5) had VTE at time of autopsy, of whom 1074 (87.8%) had thrombotic PE, 140 (11.4%) splanchnic vein thrombosis, 117 (9.6%) deep vein thrombosis of the lower or upper extremity and 12 (1.0%) internal jugular vein thrombosis, and 31 (2.5%) thrombosis at other sites (Table 3).
TABLE 3.
Venous thromboembolism outcomes a
| — |
Patients with Venous Thromboembolism (N = 1223) |
|---|---|
| Thrombotic PE, b (%) | 1074 (87.8) |
| Splanchnic vein thrombosis, (%) | 140 (11.4) |
| Deep‐vein thrombosis lower extremity, (%) | 108 (8.8) |
| Internal jugular vein thrombosis, (%) | 12 (1.0) |
| Deep vein thrombosis upper extremity, (%) | 9 (0.7) |
| Other, (%) | 31 (2.5) |
Abbreviations: PE, pulmonary embolism; VTE, venous thromboembolism.
Patients can have multiple events.
Only thrombotic pulmonary embolism; hence, tumor embolism, septic embolism, fat tissue embolism, and bone marrow embolism are not included in this variable.
3.3. Time trend
From 2008 to 2019, the annual absolute number of autopsies of cancer patients in the Netherlands decreased from 1124 to 452, whereas the number of cancer‐related deaths increased from 41 874 to 46 864. The proportion of cancer patients with PE at autopsy did not significantly change over time; 11.7% (95% CI, 10.0‐13.7) in 2008 and 15.1% (95% CI, 11.9‐18.7) in 2019 (p value for increasing trend, 0.22). Figure 2 shows the number of annual autopsies of cancer patients, total number of cancer‐related deaths, and the proportion of cancer patients with PE at autopsy between 2008 and 2019.
FIGURE 2.

Number of autopsies, cancer‐related deaths, and proportion with pulmonary embolism at autopsy between 2008 and 2019. (A) Number of annual autopsies of cancer patients. (B) Total number of cancer‐related deaths (CBS: Statistics Netherlands). (C) Proportion of cancer patients with PE at autopsy
4. DISCUSSION
In this nationwide study of almost 10 000 cancer patients who underwent autopsy, 12% had PE at the time of death. Two‐thirds of PE were considered fatal among patients for whom the cause of death was recorded by the pathologist. These findings suggest that PE is a relatively frequent cause of death in cancer patients.
It should be acknowledged that the group of cancer patients undergoing autopsy studied here does not represent the total cancer population. Unfortunately, no information on indication for autopsy was available in our data. This complicates the understanding of the background of the study population. Reasons for autopsy are to investigate the cause of (sudden) death, evaluate cancer stage at death, collecting data for research, evaluate medical practice, train physicians, or to investigate an incompletely understood disease course. Consequently, the proportion of patients with complicated disease courses and unexplained deaths is higher than in the general cancer population. In addition, autopsies may be performed more frequently in patients who were hospitalized before death, which is a risk factor for VTE. Hence, the proportion of patients with PE in this study is likely to be higher than in the general cancer population.
Identifying PE‐related deaths in cancer patients has proven to be challenging in cohort studies and trials because these patients often do not undergo radiologic imaging for PE in the terminal disease phase. Additionally, PE‐related symptoms, such as fatigue, pain, and dyspnea, are often attributed to cancer itself. 15 , 16 Therefore, the incidence of fatal PE could be underestimated in these studies. Conversely, a definition of fatal PE including “sudden death for which PE cannot be ruled out,” which is used in several trials, may lead to an overestimated risk of PE‐related death. In contrast, autopsy is considered the gold standard for disease diagnosis and yields a higher sensitivity and specificity for study outcomes than radiologic modalities. 17
Few other studies evaluated the proportion of PE in cancer patients at autopsy. Sakumu et al. 18 evaluated 65 181 autopsy reports of cancer patients in Japan in 2006. The proportion with PE was 3%, which is substantially lower than identified in the current study. It is unclear whether this study used diagnostic codes to identify potential PE cases, which could be an explanation for the lower incidence: in the current study, we would have only identified 551 PE cases (46%) if we solely used the diagnostic codes to identify potential PE, instead of the 1191 by our text mining approach. The difference may also be contributed to ethnic differences as the incidence of PE is estimated to be five to seven times lower in Asians than in blacks or whites. 19 , 20 Ögren et al. 21 used 23 796 autopsy reports of cancer patients from Sweden and identified an overall PE prevalence of 23% and an overall fatal PE prevalence of 10%. No distinction was made between PE that contributed to death and fatal PE, in contrast to our study, possibly explaining the high fatal PE prevalence in this study. In contrast to our study, this study did not include certain prevalent cancer types that are associated with a lower risk of VTE, such as melanoma. Other reasons for differences between studies may be caused by variations in autopsy protocols, hospital populations, and national autopsy rates among cancer patients.
In the current study, a substantial number of PE events were tumor embolisms (14%), defined as a sudden obstruction of a pulmonary artery by tumor cells. The identified prevalence of 2% in the total study population was lower than in previous studies, which reported a prevalence between 3% and 26%. 22 In the present study, cancer sites with the highest proportion of tumor PE were liver (5%), sarcoma (5%), and breast (4%) (Table S1), which is in line with reported cancer sites related to tumor embolism in literature. 18 , 23 Notably, the presence of limited numbers of tumor cells in the embolus may lead to false‐negative results on light microscopy. The prevalence of tumor emboli in our study might therefore be underestimated by sampling error and assessment error of light microscopy. Tumor embolism may affect both microvascular as main pulmonary arteries. Despite the considerable prevalence of tumor PE at autopsy, diagnosis before death is rarely made. 24 Clinical presentation of tumor PE is indistinguishable from VTE, with similar symptoms such as dyspnea and chest pain. 25 Tumor PE of the larger pulmonary arteries can be detected on computed tomography; however, no differentiation between tumor and thrombotic PE can be made because both appear as focal hypodense filling defects. 23 Theoretically, 18‐fluorodeoxyglucose positron emission tomography is able to distinguish both entities because tumor emboli are characterized by a high fluorodeoxyglucose uptake, whereas venous emboli are not. 25 To the best of our knowledge, this technique has not been used systematically in clinical practice for this purpose. Because the distinction between thrombotic PE and tumor PE may not be easily made based on clinical or radiological findings, tumor PE might need to be considered in patients with cancer not responding to anticoagulant treatment.
Besides the notion that we studied a selected group of cancer patients undergoing autopsy mostly within the hospital setting, several other limitations need to be considered. Although autopsies are performed accordingly to a general procedure, heterogeneity in experience of pathologists, focus on the clinical question, and the procedure itself could result in missed cases, thus possibly leading to an underestimation of the study outcomes. The reported cause of death in autopsy records was based on the interpretations of individual pathologists and did not follow standardized definitions. Additionally, cause of death was not described in all reports, possibly influencing the estimates of fatal PE. Assessment of DVT of the lower extremities at autopsy is difficult, which might result in an underestimation of the proportion of patients with lower extremity DVT. In a minor subset of patients with a brain tumor, only the brain was assessed at autopsy. This could lead to an underestimation of PE cases in patients with brain cancer. The current study showed declining autopsy rates over the years. Several causes for the decline have been identified, the most important being few physicians requesting for autopsy nowadays, 26 difficulty in obtaining consent from relatives of deceased patients, 27 and use of radiological imaging techniques as substitution for autopsy. 28 , 29 Theoretically, this could result in selection bias, because of a more predominant selection of patients with an unexpected death or without a clear cause of death. Although this could possibly inflate the proportion of cancer patients with PE over the years, the time trend analysis did not indicate a significant difference in the PE proportion between 2008 and 2019 (Figure 2).
Strengths of this study include the large study population, which comprises all cancer patients who have undergone autopsy in The Netherlands, irrespective of insurance status, ethnic background, or geographic region. The distribution of cancer types, age, and sex in our study population is comparable to that of the general cancer population in the Netherlands, with lung cancer, colorectal cancer, and hematological cancers as most frequent cancer types in both populations (CBS; 2008‐2019). 9 Autopsy is regarded as the “gold standard” for disease diagnosis, with a higher specificity than all radiologic modalities. 17 Furthermore, this study included the most recent data on autopsies available. In contrast to previous studies, 18 , 21 data on study outcomes and other clinical variables were extracted manually by examining complete pathological reports after electronic text mining, rather than relying on diagnostic codes. Study outcomes were reviewed by two of the authors. This strengthens the robustness of the estimated prevalence of PE.
In conclusion, PE was present in 12% of all cancer patients at autopsy in The Netherlands between 2008 and 2020, and two‐thirds of these events were considered fatal. Of all PE events at autopsy, 14% were tumor embolisms, indicating that this type of PE is more frequent in cancer patients than clinically recognized. Although the patients studied here possibly do not represent the entire cancer population, our findings underscore the importance of PE as potentially fatal complication in cancer patients.
CONFLICT OF INTEREST
Dr. van Es has received advisory board fees from Bayer, Daiichi Sankyo, and LEO Pharma, which were transferred to his institution. Dr. Kamphuisen received research funding from Daiichi Sankyo. Dr. Middeldorp received grants and fees paid to her institution from GlaxoSmithKline, BMS/Pfizer, Aspen, Daiichi Sankyo, Bayer, Boehringer Ingelheim, Sanofi, and Portola. The remaining authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Frits I. Mulder and Floris T. M. Bosch were responsible for the study concept and design. Inge A. Gimbel collected and analyzed the data. All authors drafted the paper, critically evaluated all versions, and approved the final manuscript for submission.
Supporting information
Table S1
Inge A. Gimbel and Frits I. Mulder: Contributed equally
Manuscript handled by: Marc Carrier
Final decision: Marc Carrier, 21 January 2021
Registration: Netherlands Trial Register; number NL8670
REFERENCES
- 1. Mulder F, Horvàth‐Puhó E, van Es N, et al. Venous thromboembolism in cancer patients: a population‐based cohort study. Blood. 2020. Advance online publication. 10.1182/blood.2020007338 [DOI] [PubMed] [Google Scholar]
- 2. Becattini C, Verso M, Muňoz A, Agnelli G. Updated meta‐analysis on prevention of venous thromboembolism in ambulatory cancer patients. Haematologica. 2019;105(3):838‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sørensen H, Mellemkjær L, Olsen J, Baron J. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846‐1850. [DOI] [PubMed] [Google Scholar]
- 4. Carrier M, Abou‐Nassar K, Mallick R, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380(8):711‐719. [DOI] [PubMed] [Google Scholar]
- 5. Khorana A, McNamara M, Kakkar A, et al. Assessing full benefit of rivaroxaban prophylaxis in high‐risk ambulatory patients with cancer: thromboembolic events in the randomized CASSINI trial. TH Open. 2020;04(02):e107‐e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li A, Kuderer N, Garcia D, et al. Direct oral anticoagulant for the prevention of thrombosis in ambulatory patients with cancer: a systematic review and meta‐analysis. J Thromb Haemost. 2019;17(12):2141‐2151. [DOI] [PubMed] [Google Scholar]
- 7. Mulder F, Bosch F, van Es N. Primary thromboprophylaxis in ambulatory cancer patients: where do we stand? Cancers. 2020;12(2):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casparie M, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in the Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29(1):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Statistics Netherlands . Deceased; major causes of death (short list), age, gender; 2020. Retrieved from https://opendata.cbs.nl/statline/#/CBS/nl/dataset/7052_95/table?fromstatweb [Google Scholar]
- 10. Stöppler M, Shiel W. Webster's new world medical dictionary, 3rd edn. Boston, MA: Houghton Mifflin Harcourt; 2009. [Google Scholar]
- 11. Spiro S, Silvestri G, Agustí A. Clinical respiratory medicine. Philadelphia, PA: Elsevier/Saunders; 2012. [Google Scholar]
- 12. Clopper C, Pearson E. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404‐413. [Google Scholar]
- 13. Jonckheere AR. A distribution‐free k‐sample test again ordered alternatives. Biometrika. 1954;41:133‐145. [Google Scholar]
- 14. Terpstra TJ. The asymptotic normality and consistency of Kendall's test against trend, when ties are present in one ranking. Indagationes Mathematicae. 1952;14:327‐333. [Google Scholar]
- 15. Van Es N, Bleker S, Di Nisio M. Cancer‐associated unsuspected pulmonary embolism. Thromb Res. 2014;133:S172‐S178. [DOI] [PubMed] [Google Scholar]
- 16. Noble S. The challenges of managing cancer related venous thromboembolism in the palliative care setting. Postgrad Med J. 2007;83(985):671‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldman L. Autopsy 2018. Circulation. 2018;137(25):2686‐2688. [DOI] [PubMed] [Google Scholar]
- 18. Sakuma M, Fukui S, Nakamura M, et al. Cancer and pulmonary embolism. Circ J. 2006;70(6):744‐749. [DOI] [PubMed] [Google Scholar]
- 19. Lee L, Gallus A, Jindal R, Wang C, Wu C. Incidence of venous thromboembolism in Asian populations: a systematic review. Thromb Haemost. 2017;117(12):2243‐2260. [DOI] [PubMed] [Google Scholar]
- 20. White R. The epidemiology of venous thromboembolism. Circulation. 2003;107(90231):I4‐I8. [DOI] [PubMed] [Google Scholar]
- 21. Ögren M, Bergqvist D, Wåhlander K, Eriksson H, Sternby N. Trousseau's syndrome – what is the evidence? Thromb Haemost. 2006;95(03):541‐545. [DOI] [PubMed] [Google Scholar]
- 22. Roberts K, Hamele‐Bena D, Saqi A, Stein C, Cole R. Pulmonary tumor embolism: a review of the literature. Am J Med. 2003;115(3):228‐232. [DOI] [PubMed] [Google Scholar]
- 23. Khashper A, Discepola F, Kosiuk J, Qanadli S, Mesurolle B. Nonthrombotic pulmonary embolism. Am J Roentgenol. 2012;198(2):W152‐W159. [DOI] [PubMed] [Google Scholar]
- 24. Rossi S, Goodman P, Franquet T. Nonthrombotic pulmonary emboli. Am J Roentgenol. 2000;174(6):1499‐1508. [DOI] [PubMed] [Google Scholar]
- 25. Restrepo C, Betancourt S, Martinez‐Jimenez S, Gutierrez F. Tumors of the pulmonary artery and veins. Semin Ultrasound CT MRI. 2012;33(6):580‐590. [DOI] [PubMed] [Google Scholar]
- 26. Loughrey MB, McCluggage WG, Toner PG. The declining autopsy rate and clinicians' attitudes. Ulster Medical J. 2000;69(2):83‐89. [PMC free article] [PubMed] [Google Scholar]
- 27. Oluwasola OA, Fawole OI, Otegbay AJ, Ogun GO, Clement A. The autopsy knowledge, attitude, and perceptions of doctors and relatives of the deceased. Arch Pathol Lab Med. 2009;133:78‐82. [DOI] [PubMed] [Google Scholar]
- 28. Turnbull A, Martin J, Osborn M. The death of autopsy? The Lancet. 2015;386(10009):2141. [DOI] [PubMed] [Google Scholar]
- 29. Friberg N, Ljungberg O, Berglund E, Berglund D, Ljungberg R. Cause of death and significant disease found at autopsy. Virchows Arch. 2019;475(6):781‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


