Abstract
Background and Aims
Intrahepatic cholangiocarcinoma (iCCA) with liver metastases is perceived to have a poor prognosis, but the American Joint Committee on Cancer (AJCC) classifies them as early stage in the absence of lymph nodes or extrahepatic spread.
Approach and Results
Patients with iCCA from the European Network for the Study of Cholangiocarcinoma (ENS‐CCA) and Surveillance, Epidemiology, and End Results (SEER) registries with survival/staging (AJCC v.7) data were eligible. Modified staging was used (mAJCC v.7): group A: stages I‐III (excluding T2bN0); group B: stage IVa (excluding T2bN1M0); group C: liver metastases (T2bN0/1); and group D: stage IVb (extrahepatic metastases). Survival analysis (Kaplan‐Meier and Cox regression) was performed in an ENS‐CCA training cohort (TC) and findings internally (ENS‐CCA iVC) and externally (SEER) validated. The aim was to assess whether liver metastases (group C) had a shorter survival compared to other early stages (group A) to propose a modified version of AJCC v.8 (mAJCC v.8). A total of 574 and 4,171 patients from the ENS‐CCA and SEER registries were included. Following the new classification, 19.86% and 17.31% of patients from the ENS‐CCA and SEER registries were reclassified into group C, respectively. In the ENS‐CCA TC, multivariable Cox regression was adjusted for obesity (p = 0.026) and performance status (P < 0.001); patients in group C (HR, 2.53; 95% CI, 1.18‐5.42; P = 0.017) had a higher risk of death (vs. group A). Findings were validated in the ENS‐CCA iVC (HR, 2.93; 95% CI, 2.04‐4.19; P < 0.001) and in the SEER registry (HR, 1.88; 95% CI, 1.68‐2.09; P < 0.001).
Conclusions
iCCA with liver metastases has a worse outcome than other early stages of iCCA. Given that AJCC v.8 does not take this into consideration, a modification of AJCC v.8 (mAJCC v.8), including “liver metastases: multiple liver lesions, with or without vascular invasion” as an “M1a stage,” is suggested.
Abbreviations
- AJCC
American Joint Committee on Cancer
- CCA
cholangiocarcinoma
- ECOG‐PS
Eastern Cooperative Oncology Group performance status
- ENS‐CCA
European Network for the Study of Cholangiocarcinoma
- iCCA
intrahepatic cholangiocarcinoma
- IQR
interquartile range
- M
metastasis
- mAJCC
modified American Joint Committee on Cancer
- mAJCC v.7
modified version of the AJCC v.7 staging criteria
- mAJCC v.8
modified AJCC v.8
- N
node
- OS
overall survival
- SEER
Surveillance, Epidemiology, and End Results
- T
tumor
Biliary tract cancer (BTC) includes gallbladder cancer (GBC), cholangiocarcinoma (CCA), and ampullary tumors (AMPs). CCAs are subdivided according to location into intrahepatic (iCCA) and extrahepatic CCA (eCCA). iCCA represents the second‐most common primary liver cancer after HCC.( 1 ) iCCA has received substantial attention in recent years given the progressive worldwide increase in incidence. Prognosis is poor because of the fact that BTCs usually present in advanced stages attributable to their asymptomatic nature in early stages.( 2 , 3 ) Thus, there is an urgent need to develop better diagnostic and therapeutic strategies for patients affected by these cancers.( 4 , 5 , 6 )
For many years, iCCAs were joined together with other BTCs, and clinical trials have traditionally recruited all subgroups of BTC. In fact, current standard‐of‐care adjuvant( 7 , 8 ) and palliative( 9 , 10 , 11 , 12 ) chemotherapy for BTC does not distinguish between BTC subtypes. In contrast, there is increasing evidence suggesting that iCCA, eCCA, GBC, and AMPs have different etiological, clinical, genomic, and molecular characteristics.( 5 )
Among all BTCs, iCCAs are gaining lots of attention for a variety of reasons. First, patients diagnosed with iCCA rarely present with biliary obstruction and jaundice, which may lead to an increased percentage of patients diagnosed with advanced disease, when no curative options are available.( 6 ) In addition, chronic liver diseases (NAFLD, viral hepatitis [B or C], and cirrhosis) are well‐known risk factors for both iCCA and HCC, making radiological differentiation of iCCA from HCC in patients with underlying liver disease challenging. However, most iCCAs develop in the absence of underlying liver disease, thus making screening programs challenging.( 6 ) Second, a proportion of iCCAs may develop multiple liver lesions (“liver metastases”), with no evidence of extrahepatic disease; therefore, liver‐directed therapies( 13 , 14 ) and external beam radiotherapy( 15 ) could be used as potential treatment options. Third, there is evidence suggesting that patients diagnosed with iCCA have a better prognosis compared to other advanced BTCs.( 16 ) Finally, there is strong evidence indicating that genomic and molecular aberrations in iCCA differ from other BTCs,( 17 ) mainly represented by increased presence of fibroblast growth factor receptor 2 fusion rearrangements and isocitrate dehydrogenase‐1 and ‐2 mutations.( 18 , 19 ) Identification of these molecular alterations has direct implications for access to targeted therapies and precision medicine strategies, whose success is currently almost limited to iCCA among the BTCs.( 20 )
The most commonly used staging classification in oncology is the one developed by the American Joint Committee on Cancer (AJCC).( 21 ) Staging criteria for CCA were not introduced until the 2nd Edition (published in 1983 and made effective in 1984).( 22 ) In addition, iCCA was staged together with other primary liver tumors, such as HCC, and it was not until the 7th Edition (published in 2009 and effective between 2010 and 2017; AJCC v.7; Table 1)( 23 ) that a specific staging system for iCCA was provided. The latest update was published in 2016 and made effective in 2018 (8th Edition).( 23 ) Several changes were applied in this latest version, involving, in particular, the tumor (T) staging (AJCC v.8; Table 1). The T1 category was subdivided according to tumor size, whereas the T3 and T4 definitions were slightly modified and assigned to stage III disease (if M0). Moreover, the previously defined T2a (solitary lesion with vascular invasion) and T2b (multiple tumors; so‐called liver metastases for the purpose of this study) categories were merged.
TABLE 1.
Current Staging of CCA (AJCC 7th and 8th Editions and Corresponding Modified Versions)
| iCCA | ||||
|---|---|---|---|---|
| AJCC v.7 | mAJCC v.7 Modified version used to assess impact of liver metastases | AJCC v.8 | mAJCC v.8 Proposed modified version of the current AJCC v.8 | |
| Primary tumor (T) | ||||
| TX | Primary tumor cannot be assessed. | Primary tumor cannot be assessed. | Primary tumor cannot be assessed. | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor | No evidence of primary tumor | No evidence of primary tumor | No evidence of primary tumor |
| Tis | Carcinoma in situ (intraductal tumor) | Carcinoma in situ (intraductal tumor) | Carcinoma in situ (intraductal tumor) | Carcinoma in situ (intraductal tumor) |
| T1 | Solitary tumor without vascular invasion | Solitary tumor without vascular invasion | — | — |
| T1a | — | — | Solitary tumor ≤5 cm without vascular invasion | Solitary tumor ≤5 cm without vascular invasion |
| T1b | — | — | Solitary tumor >5 cm without vascular invasion | Solitary tumor >5 cm without vascular invasion |
| T2 | — | — | Solitary tumor with intrahepatic vascular invasion or multiple tumors with or without vascular invasion | Solitary tumor with intrahepatic vascular invasion |
| T2a | Solitary tumor with vascular invasion | Solitary tumor with vascular invasion | — | — |
| T2b | Multiple tumors, with or without vascular invasion | Multiple tumors, with or without vascular invasion | — | — |
| T3 | Tumor perforating the visceral peritoneum or involving local hepatic structures by direct invasion | Tumor perforating the visceral peritoneum or involving local hepatic structures by direct invasion | Tumor perforating the visceral peritoneum | Solitary tumor perforating the visceral peritoneum |
| T4 | Tumor with periductal invasion | Tumor with periductal invasion | Tumor involving local extrahepatic structures by direct invasion | Solitary tumor involving local extrahepatic structures by direct invasion |
| Regional lymph nodes (N) | ||||
| NX | Regional lymph nodes cannot be assessed. | Regional lymph nodes cannot be assessed. | Regional lymph nodes cannot be assessed. | Regional lymph nodes cannot be assessed. |
| N0 | No regional lymph node metastasis | No regional lymph node metastasis | No regional lymph node metastasis | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis present | Regional lymph node metastasis present | Regional lymph node metastasis present | Regional lymph node metastasis present |
| Distant metastasis (M) | ||||
| M0 | No distant metastasis | No distant metastasis | No distant metastasis | No distant metastasis |
| M1 | M1a: liver metastases: multiple liver lesions, with or without vascular invasion | |||
| Distant metastasis present | Distant metastasis present | Distant metastasis present | M1b: Distant (extrahepatic) metastasis present | |
| Prognostic stage groups | ||||
| 0 | Tis, N0, M0 | Tis, N0, M0 | Tis, N0, M0 | Tis, N0, M0 |
| I | T1, N0, M0 | T1, N0, M0 (group A) | — | T1, N0, M0 |
| Ia | — | — | T1a, N0, M0 | T1a, N0, M0 |
| Ib | — | — | T1b, N0, M0 | T1b, N0, M0 |
| II | T2a/b, N0, M0 | T2a, N0, M0 (group A) | T2, N0, M0 | T2, N0, M0 |
| III | T3, N0, M0 | T3, N0, M0 (group A) | — | — |
| IIIa | — | — | T3, N0, M0 | T3, N0, M0 |
| IIIb | — | — | T4, Any N, M0 or any T, N1, M0 | T4, N0, M0 or any T, N1, M0 |
| IV | — | Any T, any N, M1 | — | |
| IVa | T4, N0, M0 or any T, N1, M0 | T4, N0, M0 or any T (except T2b), N1, M0 (group B) | — | Any T, any N, M1a |
| — | — | T2b, any N, M0 (group C) | — | — |
| IVb | Any T, any N, M1 | Any T, any N, M1 (group D) | — | Any T, any N, M1b |
As stated above, a significant proportion of patients (up to 48%) with iCCA may develop multiple liver lesions in the absence of other extrahepatic metastases.( 16 ) This liver‐only pattern is not unique to iCCA, but can also be observed in other primary liver cancers, such as HCC. In HCC, the scenario of multiple liver lesions may represent multiple primary tumors (multifocal disease) arising in the background of cirrhosis. However, this phenomenon is still elusive in iCCA, especially when detected in the absence of a preexisting liver disease such as cirrhosis, primary sclerosing cholangitis, or exposure to specific chemicals.( 24 , 25 , 26 ) In the majority of patients diagnosed with iCCA, presence of multiple liver lesions usually reflects hematogenous intrahepatic dissemination (liver metastases) from a primary predominant tumoral liver lesion and is clinically expected to feature worse prognosis, more similar to metastatic disease than to earlier stages. In fact, these patients are usually managed with palliative strategies, similar to conditions where other distant metastases have been identified.( 27 ) In contrast, current staging systems, such as the AJCC, do not take account for this issue and, in the absence of other sites of distant metastases, the AJCC still classifies liver metastases in iCCA as early stage in the absence of lymph node or extrahepatic spread( 23 , 28 ) (Table 1).
The European Network for the Study of Cholangiocarcinoma (ENS‐CCA) represents an open, multidisciplinary group of clinical, translational, and basic researchers aiming to improve the knowledge on CCA and promote translational activities; as part of the ENS‐CCA initiatives, a multicenter pan‐European clinical registry of patients with CCA has been in development since 2016, including both retrospective (from 2010) and prospective data.( 4 ) This study aimed to describe the outcomes of patients with iCCA complicated by liver metastases and compare them to other earlier stages, in order to ascertain whether current staging accurately reflects natural behavior and aggressiveness, or whether any changes to the current staging system should be considered.
Patients and Methods
Study Design
A modified version of the AJCC v.7 staging criteria (mAJCC v.7; Table 1), where patients diagnosed with liver metastases (T2b) were classified as a separate group (group C) regardless of node (N) status (any N) and in the absence of other sites of distant metastases (M0), was generated. Groups were defined as follows: group A (stages I‐III [excluding T2bN0M0]); group B (stage IVa [excluding T2bN1M0]); group C (liver metastases: multiple liver lesions, with or without vascular invasion [T2b, any N, M0], group of interest); and group D (stage IVb [M1 extrahepatic disease]).
The primary objective of this study was to apply both the AJCC v.7 and mAJCC v.7 classification to assess whether patients with liver metastases (regardless of lymph node status; group C) had a different prognosis compared to other early‐stage disease (stages I‐III; group A), using overall survival (OS) as the primary end‐point (defined as the time from first diagnosis to death/last visit). Given that the AJCC v.8 classification does not differentiate between number of liver lesions (T2 stage includes solitary lesions with vascular invasion and multiple liver lesions within the same group), the analysis was performed using the AJCC v.7 (which provides the distinction between T2a and T2b subgroups).
The secondary objective was to create a modified AJCC v.8 (mAJCC v.8) that could be used for the development of future AJCC versions.
In order to generate the above‐mentioned staging groups, individual patient data on the T, N, and metastasis (M) stage was individually reviewed and staging groups defined for individual patients. As a quality control, patients with mismatching information were reviewed (if required) or excluded (if unable to satisfactorily reply to issued queries).
Definition Of A Modified Staging System: Training And Internal Validation Cohorts
Data from patients included in the ENS‐CCA registry up to February 2019 were retrospectively analyzed. In order for data to be included in the registry, individual sites involved had obtained appropriate ethical approval; ethical guidelines of the 1975 Declaration of Helsinki were met. Eligible patients were those with a diagnosis of iCCA with available data of survival and staging (according to AJCC v.7). Patients diagnosed with mixed HCC‐iCCA or with different subtypes of CCA were excluded. Two cohorts of patients were analyzed: (1) the training cohort, including consecutive patients in the ENS‐CCA registry from The Christie NHS Foundation Trust, who had been diagnosed between 2013 and 2017; (2) the internal validation cohort, including consecutive patients in the ENS‐CCA registry from The Christie NHS Foundation Trust, who had been diagnosed between 2017 and 2018, along with all patients included in the ENS‐CCA registry by other contributing centers (2013‐2018). The aim was to use the training cohort for building a modified staging system and for these results to be validated in the internal validation cohort. By doing so, lessons learned from the training cohort could be used to improve the modified staging system before its application in the internal validation cohort, should this be required.
All eligible patients included in this study were staged as per AJCC v.7 and mAJCC v.7 classification. The following assumptions were made at time of data interpretation: if metastatic sites were not reported, M stage was assumed to be M0; when presence/absence of lymph node metastases were unknown (Nx), these were assumed to be N0 disease; if two separate T or N stages were reported, the highest stage was used for analysis purposes. Patients with M0 disease with reported T2 tumors were excluded from the analysis if no further specification regarding T2a/T2b was provided.
Statistical Analysis
The last update of clinical data was in February 2019. Patients who were alive at the time of the last follow‐up were censored. Survival analysis was first performed in the training cohort, and then findings were validated in the internal validation cohort. Chi‐square, Fisher’s exact‐test, and t test were used whenever appropriate. The Kaplan‐Meier method was used to estimate median OS. Additional survival analyses with univariate Cox regression and a log‐rank test were also performed. Step‐wise Cox regression, including all baseline characteristics collected as part of the ENS‐CCA registry (including staging), was used for identification of variables of interest to be included in the multivariable Cox regression model (P value cutoff, 0.05). In order to adjust the multivariable Cox regression model to potential confounding factors impacting on patients’ outcome for which relying on statistical significance in the step‐wise Cox regression model alone would not be fully appropriate, a few variables with a well‐defined prognostic impact were preselected to be included in the multivariable Cox regression model regardless of the step‐wise Cox regression findings. These factors included: stage and performance status, in view of previous evidence suggesting their impact on OS in this disease group.( 16 )
Two‐sided P values of <0.05 were considered statistically significant. Stata software (version 12.0; StataCorp LP, College Station, TX) was used for the statistical analysis.
Sensitivity Analysis
In order to confirm that our originally proposed definition of group C was adequate, a sensitivity analysis was performed. Given that the impact of lymph node positivity (N1) in the presence of liver metastases (T2b) is uncertain, a sensitivity analysis in the whole ENS‐CCA series by limiting group C to patients with N0 disease only (patients with N1 disease would be classified as group B) was completed. In the sensitivity analysis, group B was defined as “stage IVa, including T2b, N1, M0”) and group C as “T2b, N0, M0” (instead of “T2b, any N, M0” as defined for the rest of the analysis in this article). A decision was made to keep patients with N1 in group B instead of removing them from the analysis to explore whether prognosis was driven by liver metastases (T2b) on their own and independent from the presence of N1 disease.
External Validation: Surveillance, Epidemiology, and End Results Registry
Data extracted from the Surveillance, Epidemiology, and End Results (SEER) registry were used for external validation.( 29 ) Cases reported from 1975 through 2016 with available data on survival and stage (as per AJCC v.7) were deemed eligible. The staging assumptions used for the ENS‐CCA cohort were applied for the SEER registry. External validation with SEER data was aimed at confirming whether patients with liver metastases (regardless of lymph node status; group C) had indeed a different outcome compared to other “early‐stage” disease (stages I‐III; group A). Survival analysis with SEER data was performed using Cox regression analysis (multivariable analysis performed with ENS‐CCA data was planned to be reproduced, if variables of interest were available in the SEER dataset; otherwise, only a univariate analysis would be conducted).
Definition of the Proposed Updated AJCC Classification
Based on the information derived from mAJCC v.7 and the outcome of the current staging group, changes to the current AJCC v.8 in the form of an mAJCC v.8 were proposed, and outcomes of each specifically defined group were assessed in the joined ENS‐CCA and SEER cohort. Survival analysis was performed according to the previously indicated methodology.
Results
Patient Characteristics: ENS‐CCA Registry
Of the 1,820 patients included in the ENS‐CCA registry, 810 had been diagnosed with iCCA and were assessed for eligibility. A total of 574 patients were eligible (141 within the training cohort [24.56%] and 433 in the internal validation cohort [75.44%]). Figure 1A summarizes patient flow for the ENS‐CCA cohort.
FIG. 1.
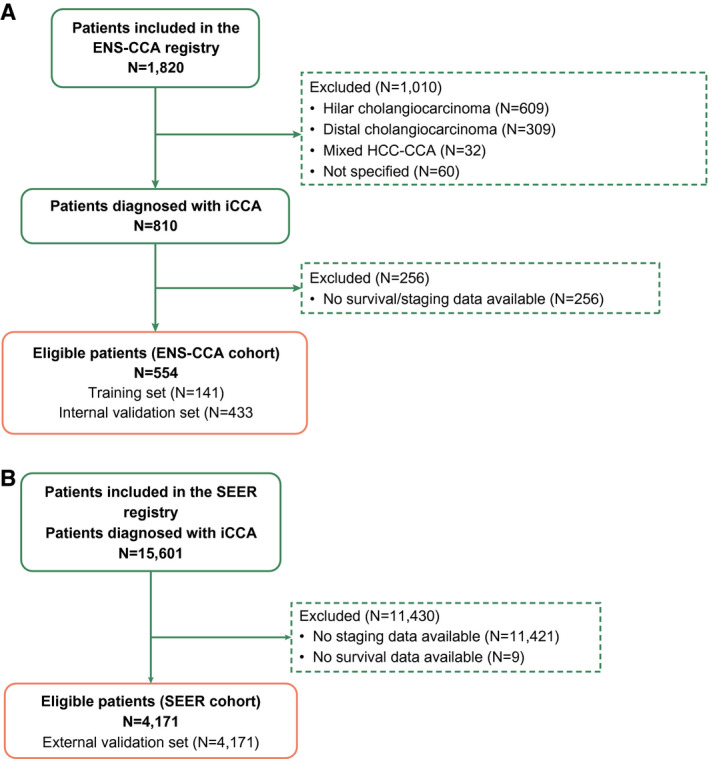
Patient flow. N refers to number of patients. (A) Patient flow for patients included in the ENS‐CCA registry. (B) Patient flow for patients included in the SEER registry.
Patient baseline characteristics, including treatment, for both the training and internal validation cohorts, are summarized in Table 2. Median follow‐up time was 11.01 months (range, 0.00‐183.12; interquartile range [IQR], 4.03‐23.89) for the whole cohort; 8.99 (range, 0.00‐57.96; IQR, 3.48‐18.01) for the training cohort; and 11.39 (range, 0.00‐183.12; IQR, 4.31‐25.93) for the internal validation cohort (P = 0.0017). For the whole cohort, median age at diagnosis was 66.16 years (range, 26‐92); the majority of patients were Eastern Cooperative Oncology Group performance status (ECOG‐PS) 0 (41.99%) or 1 (35.89%). Obesity and diabetes were present in 20.21% and 20.21% of patients, respectively. With respect to treatments, 47.21% of patients underwent previous surgical resection (5.67% and 60.74% in the training and internal validation cohorts, respectively; P < 0.001), whereas palliative chemotherapy was offered to 39.37% of patients (60.28% and 32.56% in the training and internal validation cohorts, respectively; P < 0.001).
TABLE 2.
Patient Baseline Characteristics and Summary of Treatments Received (ENS‐CCA Registry)
| Patient Characteristics | Whole ENS‐CCA Series (N = 574) | Training Cohort (N = 141) | Internal Validation Cohort (N = 433) | P Value (Training vs. Internal Validation) | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Sex | Female | 279 | 48.61 | 88 | 62.41 | 191 | 44.11 | <0.001 |
| Male | 295 | 51.39 | 53 | 37.59 | 242 | 55.89 | ||
| Age (years) | Median (range) | 66.16 (26‐92) | 65.83 (28‐90) | 66.15 (26‐92) | 0.4462 | |||
| Ethnicity | Caucasian | 539 | 93.90 | 122 | 86.52 | 417 | 96.30 | <0.001 |
| Other | 20 | 3.49 | 7 | 4.97 | 13 | 3.01 | ||
| Not reported | 15 | 2.61 | 12 | 8.51 | 3 | 0.69 | ||
| Obesity | Yes | 116 | 20.21 | 28 | 19.86 | 88 | 20.32 | 0.693 |
| Diabetes mellitus | Yes | 116 | 20.21 | 27 | 19.15 | 89 | 20.55 | 0.709 |
| Liver cirrhosis | Yes | 53 | 9.23 | 3 | 2.13 | 50 | 11.55 | <0.001 |
| Primary sclerosing cholangitis | Yes | 10 | 1.74 | 0 | 0.00 | 10 | 2.31 | 0.130 |
| ECOG‐PS | 0 | 241 | 41.99 | 22 | 15.60 | 219 | 50.58 | <0.001 |
| 1 | 206 | 35.89 | 58 | 41.13 | 148 | 34.18 | ||
| 2 | 78 | 13.59 | 35 | 24.82 | 43 | 9.93 | ||
| 3 | 43 | 7.49 | 24 | 17.02 | 19 | 4.39 | ||
| 4 | 5 | 0.87 | 2 | 1.42 | 3 | 0.69 | ||
| Not reported | 1 | 0.17 | 0 | 0 | 1 | 0.23 | ||
| Patient treatment | N | % | N | % | N | % | ||
| Previous surgery | Yes | 271 | 47.21 | 8 | 5.67 | 263 | 60.74 | <0.001 |
| Adjuvant treatment* | Yes | 49 | 8.54 | 1 | 0.71 | 48 | 11.09 | 0.314 |
| Tumor recurrence | Yes | 113 | 19.69 | 7 | 4.96 | 106 | 24.48 | <0.001 |
| Clinical trials | Yes | 37 | 6.45 | 21 | 14.89 | 16 | 3.70 | <0.001 |
| Palliative chemotherapy | Yes | 226 | 39.37 | 85 | 60.28 | 141 | 32.56 | <0.001 |
N refers to number, % to percentage.
Adjuvant treatment was not standard of care at the time these patients were treated. Chi‐square, Fisher’s exact test, and t test P values are provided (as appropriate).
Staging of iCCA: ENS‐CCA Registry
Staging groups in the ENS‐CCA registry, according to the AJCC v.7, mAJCC v.7, and mAJCC v.7 adjusted for sensitivity analysis are shown in Table 3. When the whole population was staged based on the AJCC v.7 classification, 46.52%, 22.3%, and 31.18% of patients were stages I‐III, IVa, and IVb disease, respectively. When applying the mAJCC v.7 classification, a total of 114 of the 395 (28.9%) patients previously staged as I‐IVa were reclassified into group C (75 N0; 39 N1), with 33.45%, 15.51%, 19.86%, and 31.18% of patients staged within groups A, B, C, and D, respectively.
TABLE 3.
Staging of Patients: AJCC v.7 and Proposed mAJCC Classification (ENS‐CCA Registry)
| Staging of Patients | Whole ENS‐CCA Series (N = 574) | Training Cohort (N = 141) | Internal Validation Cohort (N = 433) | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| AJCCv.7 | Stages I‐III (including T2bN0M0; liver satellite lesions) | 267 | 46.52 | 43 | 30.50 | 224 | 51.73 |
| Stage IVa (including T2bN1M0) | 128 | 22.30 | 28 | 19.86 | 100 | 23.09 | |
| Stage IVb (distant mts) | 179 | 31.18 | 70 | 49.65 | 109 | 25.17 | |
| mAJCC | Group A: stages I‐III (excluding T2bN0M0) | 192 | 33.45 | 13 | 9.22 | 179 | 41.34 |
| Group B: stage IVa (excluding T2bN1M0) | 89 | 15.51 | 20 | 14.18 | 69 | 15.94 | |
| Group C: stage T2bN0/1M0: liver mts | 114 | 19.86 | 38 | 26.95 | 76 | 17.55 | |
| N0 | 75 | 30 | 45 | ||||
| N1 | 39 | 8 | 31 | ||||
| Group D: stage IVb | 179 | 31.18 | 70 | 49.65 | 109 | 25.17 | |
| mAJCC (sensitivity analysis) | Group A: stages I‐III (excluding T2bN0M0) | 192 | 33.45 | 13 | 9.22 | 179 | 41.34 |
| Group B: stage IVa (including T2bN1M0) | 128 | 22.30 | 28 | 19.86 | 100 | 23.09 | |
| Group C: stage T2bN0M0: liver mts | 75 | 22.30 | 30 | 21.28 | 45 | 10.39 | |
| Group D: stage IVb | 179 | 31.18 | 70 | 49.65 | 109 | 25.17 | |
N refers to number, % to percentage.
Abbreviation: mts, metastases.
Survival Analysis: ENS‐CCA Registry
Estimated median OS was 9.98 (95% CI, 6.96‐11.99; 135 events; 95.74% of patients), 18.52 (95% CI, 15.44‐22.07; 280 events; 64.67% of patients), and 15.01 months (95% CI, 12.64‐16.95; 415 events; 75.30% of patients) for the training, internal validation, and whole ENS‐CCA cohorts, respectively.
In the training cohort, univariate survival analysis confirmed that both staging systems (AJCCv.7 and mAJCC v.7) had an impact on OS (Supporting Information S1A), with patients with distant metastases (stage IVb) demonstrating shorter OS. Similar findings were obtained in the univariate analysis using the internal validation cohort (Supporting Information S1B).
Step‐wise multivariable Cox regression analysis performed in the training cohort (Supporting Information S2) identified stage (HR, 1.37; 95% CI, 1.10‐1.69; P = 0.004), obesity (HR, 0.56; 95% CI, 0.34‐0.91‐1.69; P = 0.018), and ECOG‐PS (HR, 1.89; 95% CI, 1.51‐2.39; P < 0.001) as prognostic variables of interest to be included in the multivariable analysis. Presence of background liver cirrhosis did not impact on OS (P = 0.917). The multivariable Cox regression model, adjusted for these variables in the training cohort, confirmed that patients classified in group C (liver metastases) as per the mAJCC v.7 had a worse outcome than patients with early stage (group C [vs. group A] HR, 2.53; 95% CI, 1.18‐5.42; P = 0.017). These findings were validated in the internal validation cohort (group C [vs. group A] HR, 2.93; 95% CI, 2.04‐4.19; P < 0.001; Supporting Information S3).
Sensitivity Analysis: ENS‐CCA Registry
For the sensitivity analysis, only the 75 patients with pT2bN0 were included in group C (Table 3), whereas patients with T2bN1 disease were classified as group B (128 patients). When taking this approach, multivariable Cox regression analysis applied to the whole series confirmed that liver metastases had a prognostic effect, which was independent from lymph node status (group C [vs. group A] HR, 2.51; 95% CI, 1.76‐3.56; P < 0.001; Supporting Information S4). When the outcome of patients with T2bM0 disease were analyzed according to N status (total of 114 patients; 75 T2bN0 and 39 T2bN1), N1 disease was shown to be associated with worse OS (median OS for patients with T2bN0 was 11.82 months [95% CI, 7.89‐20.19], median OS for patients with T2bN1 was 8.99 months [95% CI, 5.02‐14.61]; T2bN1 [vs. T2bN0] HR, 1.68; 95% CI, 1.10‐2.57; P = 0.015). These two observations supported our analysis to continue with the originally proposed definition of group C (T2b, any N, M0).
External Validation: SEER Registry
Among 15,601 records provided by the SEER database, 4,171 were deemed eligible (Fig. 1B); their baseline and staging characteristics are summarized in Supporting Information S5. All eligible patients were diagnosed between 2010 and 2015. Median follow‐up was 8 months (range, 0‐83; IQR, 2‐18). Median OS was 10 months (95% CI, 9‐10; 3,434 events; 82.33% of patients), with 17.31% of patients classified in group C (liver metastases).
Univariate survival analysis confirmed that patients classified in group C (liver metastases) as per the mAJCC v.7 had a poorer outcome than patients with early stage (group C [vs. group A] HR, 1.88; 95% CI, 1.68‐2.09; P < 0.001; Supporting Information S1D). Since obesity and ECOG‐PS data was not available, multivariable analysis could not be performed.
Outcomes for Each Stage Group
Figure 2 summarizes patients’ outcome for each disease stage, following both the standard AJCC v.7 (Fig. 2A) and the mAJCC v.7 (Fig. 2B) for ENS‐CCA (whole population). Outcomes of mAJCC v.7 in the SEER cohort are also shown (Fig. 2C). The updated mAJCC v.7 classification in the ENS‐CCA cohort (Fig. 2B) and SEER registry (Fig. 2C) showed that compared to stage I disease, risk of death steadily increased through stage progression (stage I > stage II [excluding T2bN0M0] > stage III > stage IVa [excluding T2bN1M0] > liver metastases [T2bN0/1M0] > stage IVb).
FIG. 2.
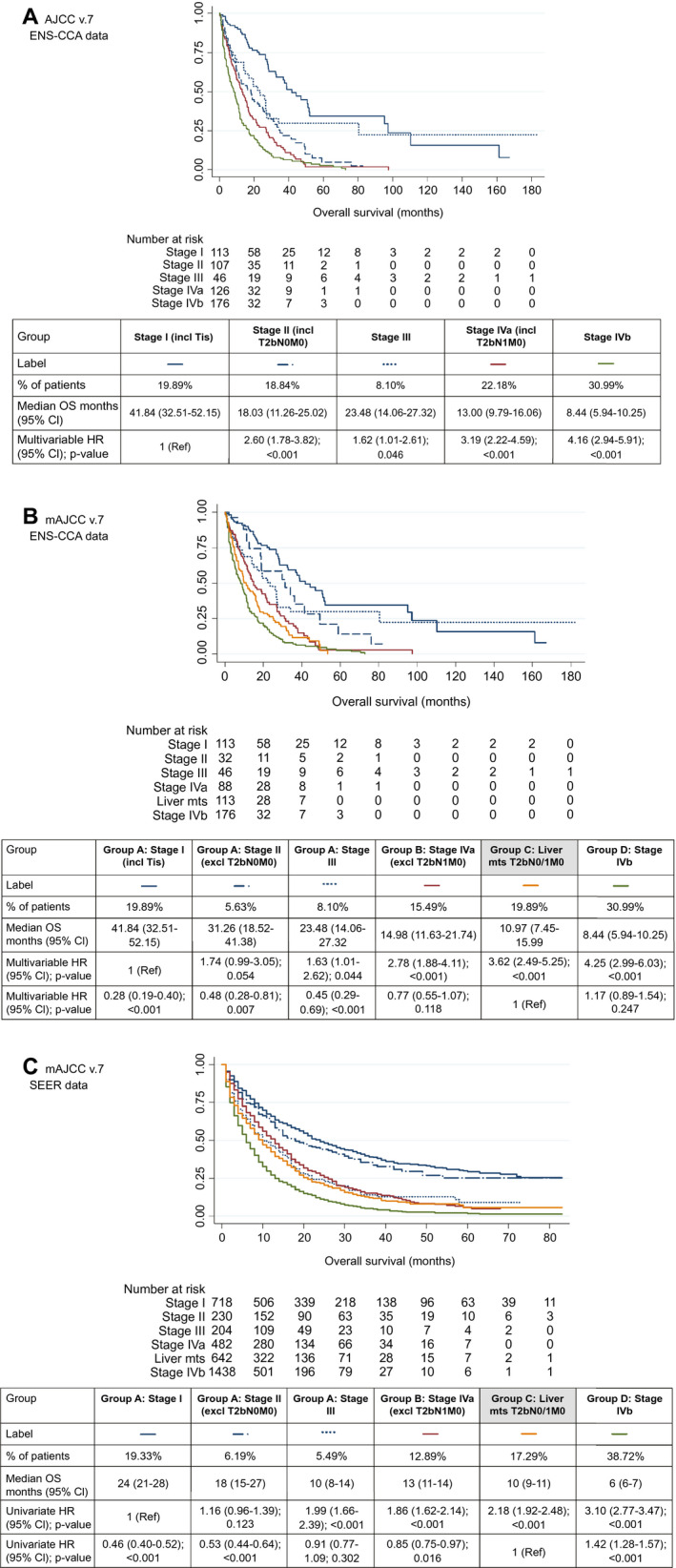
OS for each stage groups. (A) Kaplan‐Meier for the ENS‐CCA cohort using AJCC v.7 is shown; multivariable Cox regression HRs are shown for the stage variable (with stage I as the reference category); the multivariable HR for obesity was 0.79 (95% CI, 0.62‐1.03; P = 0.083), and the multivariable HR for ECOG‐PS (continuous variable) was 1.69 (95% CI, 1.51‐1.89; P < 0.001). (B) Kaplan‐Meier for the ENS‐CCA cohort using mAJCC v.7 is shown; multivariable Cox regression HRs are shown for the stage variable (separately for analysis with stage I and group C as reference categories); the multivariable HR for obesity was 0.81 (95% CI, 0.63‐1.05; P = 0.113), and the multivariable HR for ECOG‐PS (continuous variable) was 1.68 (95% CI, 1.49‐1.89; P < 0.001). (C) Kaplan‐Meier for the SEER cohort using mAJCC v.7 is shown; univariate Cox regression HRs are shown for the stage variable (separately for analysis with stage I and group C as reference categories). Abbreviations: excl, excluding; incl, including; mts, metastases; Tis: tumour in situ (1 case only).
Data from the ENS‐CCA registry (Fig. 2B) showed that when group C was used as the reference group, patients diagnosed with early‐stage disease group A (stages I‐III) had a lower risk of death than group C (stage I [vs. liver metastases]; HR, 0.28; 95% CI, 0.19‐0.40; P < 0.001; stage II [vs. liver metastases]; HR, 0.48; 95% CI, 0.28‐0.81; P = 0.007). Compared to group C, groups B and D showed a trend toward longer and shorter survival, respectively, but differences did not reach statistical significance (P = 0.118 and P = 0.247, respectively).
Survival analysis confirmed similar trends in the SEER registry (Fig. 2C). Patients diagnosed with stage I and stage II had a lower risk of death compared to group C. Differences between group A/stage III and group C (reference category) did not reach statistical significance (P = 0.302). Similar to what had been identified in the ENS‐CCA cohort, group B (P = 0.016) and group D (P < 0.001) showed longer and shorter survival compared to group C, respectively.
Proposed Changes to the Current Staging System: mAJCC v.8
Based on these findings, we proposed some changes to the current AJCC v.8 (mAJCC v.8; Table 1). Stage IV were divided into two groups: stage IVa (M1a disease; liver metastases regardless of N status) and stage IVb (M1b disease; extrahepatic metastases, regardless of T and N status). The updated M1a included liver metastases (defined as the presence of multiple liver lesions, with or without vascular invasion). In this respect, stage II was restricted to patients with solitary tumors with vascular invasion and in the absence of lymph node or other distant metastatic localizations (T2N0M0). Stage III encompassed T3N0M0, T4N0M0, and AnyTN1M0 patients according to the latest AJCC v8 classification; our findings from the mAJCC v.7 supported this approach given that these subpopulations shared a similar prognosis (Fig. 2B,C).
When these criteria were applied to the whole cohort of joined ENS‐CCA and SEER patients, differences between all these staging groups reached statistical significance, when compared with either stage I or stage IVa as the reference category (Fig. 3B). Multivariable survival analysis was not performed given the lack of data from the SEER registry (no data on ECOG‐PS available for the SEER registry). In order to allow comparison with the preceding staging system (AJCC v.7), Fig. 3A provides survival outcome using this classification in the whole cohort of joined ENS‐CCA and SEER patients. Harrell’s C index was slightly higher for the mAJCC v.8 (Fig. 3B; C index, 0.624) than for the AJCC v.7 (Fig. 3A; C index, 0.614).
FIG. 3.
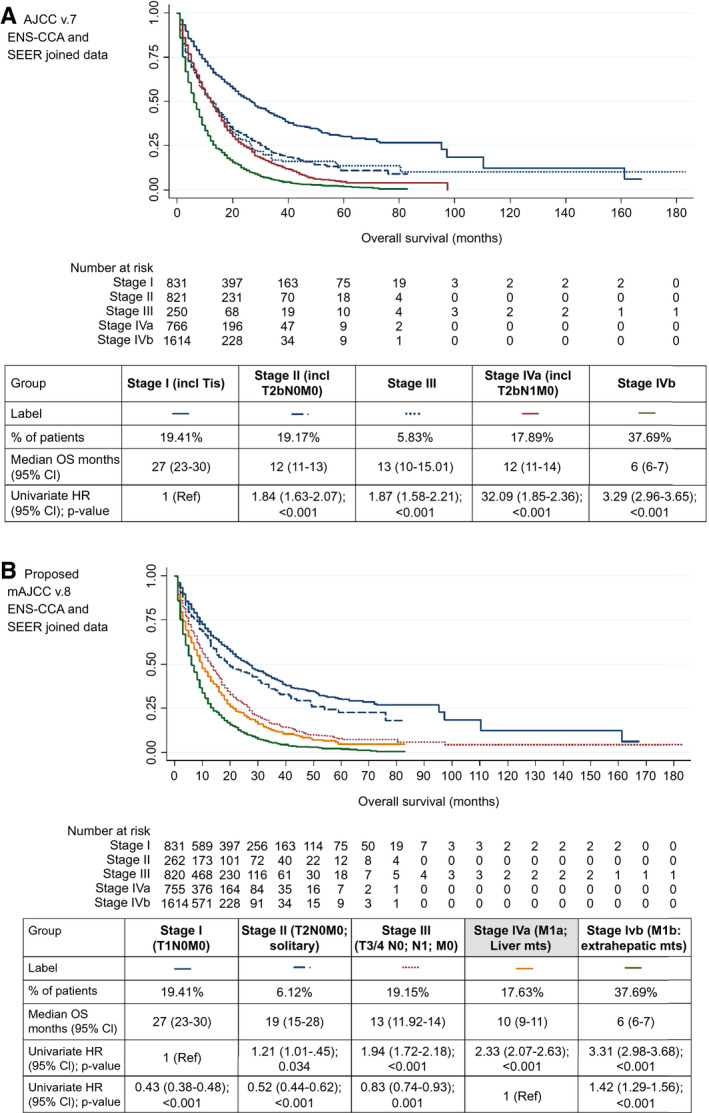
OS for each stage groups: ENS‐CCA and SEER joined data. Using the proposed updated staging system, mAJCCv.8, provided a slightly higher Harrell’s C index (mAJCC v.8 (FIG. 3.B); C index, 0.624) compared to the AJCC v.7 (FIG. 3.A); C index, 0.614). In addition, the mAJCC v.8 allowed for a more clinically relevant separation of survival curves (while there was significant overlapping in AJCC v.7). (A) Kaplan‐Meier for the ENS‐CCA and SEER joined cohort using the proposed AJCC v.7 is shown; univariate Cox regression HRs are shown for the stage variable (with stage I as the reference category). (B) Kaplan‐Meier for the ENS‐CCA and SEER joined cohort using the proposed updated mAJCC v.8 is shown; univariate Cox regression HRs are shown for the stage variable (separately for analysis with stage I and stage IVa [liver metastases] as reference categories). Of the 820, 755, and 1,614 patients with stage III, IVa, and IVb disease, 418 (50.1%), 194 (25.7%), and 671 (41.6%), respectively, were N1. Abbreviations: excl, excluding; incl, including; mts, metastases; Tis, tumor in situ (1 case only).
Discussion
Staging classifications are in constant evolution and require frequent review.( 30 , 31 ) The present study confirms that the presence of liver metastases is a feature of poor prognosis in patients with iCCA independent of lymph node status. Based on these findings, future staging classifications should be adjusted to classify iCCA patients with liver metastases as a separate group (M1a), regardless of N status and in the absence of other sites of distant metastases (mAJCC v.8; Table 1). Our proposed modification of the current AJCC v.8 confirms that patients in this group have a worse prognosis compared to stage I‐III disease and a better prognosis compared to patients with extrahepatic metastases (M1b). This was also suggested back in 2009 by Nathan et al., who showed that patients with liver metastases from iCCA had an increased risk of death (HR, 1.42; 95% CI, 1.01‐2.10; P < 0.005).( 32 ) Other studies have also shown similar findings, with liver metastases exerting a negative impact on prognosis.( 33 ) For his reason, presence of liver metastasis in iCCA is regarded, by many, as a contraindication to surgery.( 34 ) Moreover, identifying the subset of iCCAs with liver metastases also has translational relevance. This finding is consistent with a defining biological feature of the tumor, that is, the early intrahepatic dissemination promoted by perineural invasion, portal encasement, and intraductal growth that frequently occurs before lymph node and hematogenous spread.( 35 )
These changes in staging classifications may affect a significant number of patients given that ~20% of patients diagnosed with iCCA fall into this group. The implementation of the proposed reclassification is likely to have clinical implications at the time of treatment decision, allowing for a clearer patient selection given that patients with M1a disease would be suitable for strategies for advanced disease, including liver‐directed therapies and locoregional strategies.( 16 ) Based on our results, patients with liver metastases should not be offered therapeutic strategies suitable for early stages, in view of poor survival. In addition, it would optimize terminology given that clinicians have been referring to this scenario as liver metastases for decades, which, in contrast with the strict definition based on AJCC v.7 and v.8 (M0), implies M1 disease. That said, it is tempting to speculate that stage may have been reported incorrectly in previous clinical trials. As an example, a post hoc analysis of ABC01/02/03 clinical trials exploring the role of palliative chemotherapy in biliary tract malignancies was recently published.( 16 ) In this study, 21.1% of patients diagnosed with iCCA were classified as “locally advanced” (defined as nonresectable disease in the absence of extrahepatic metastases), whereas no evidence of extrahepatic disease was found in 47.7%. Therefore, it could be argued that in these studies, a relevant proportion of patients were misclassified as “metastastic” in the absence of extrahepatic distant metastases.
One of the challenges of the proposed mAJCC v.8 is the fact that in some countries, surgery is still pursued even with evidence of liver metastases if disease is confined to the same hepatic lobe. However, this is not a widely adopted practice, and benefit of surgery in this context is not proven( 36 ); therefore, individual decisions based on discussion in multidisciplinary teams are required.
Our study has some limitations. First, data were collected retrospectively, with the intrinsic drawbacks of every retrospective analysis. Multivariable survival analysis was performed, adjusted to available clinical variables; in view of missing data, other variables, such as presence of cirrhosis, baseline tumor markers, and bilirubin level, among others, which could have been of interest, were not been included. In addition, the SEER registry was lacking some of the clinical information required (i.e., ECOG‐PS), thereby limiting the applicability of a multivariable analysis. There were clear differences between the ENS‐CCA training cohort and the internal validation cohort, with a bias toward a higher percentage of patients with advanced and noncurable disease in the training cohort, attributable to the center of origin of this cohort (cancer center with expertise in medical oncology for management of advanced disease). In addition, follow‐up of patients included in the SEER registry was shorter than in the ENS‐CCA registry. Given that the AJCC v.8 classification removed the T2a/T2b subgroups, it was not possible to assess the prognostic implications of liver metastases in the most recent series, therefore relying on series related to the use of AJCC v.7. Given that both adjuvant and targeted therapies were rarely used therapeutic strategies during the years when the AJCC v.7 staging system was used, we did not have sufficient observations to explore the prognostic impact of our staging system in patients treated with these treatment strategies. Thus, outcome was evaluated without accounting for the potential impact of the latest advances in treatments, including both adjuvant and targeted therapies,( 8 , 20 ) which could have produced (specially adjuvant therapies) an even more marked prognostic difference between M1a (liver metastases) and early‐stage patients.
Even though the TNM stage provides a clinically meaningful classification for healthcare professionals, and adequately correlates with prognosis,( 37 ) the staging of iCCA gained significant attention given that it was the latest to be incorporated.( 38 ) In this regard, AJCC v.8 changes have been greatly welcomed, even though further observations have been sadly lacking.( 30 ) In fact, in addition to our proposed mAJCC v.8 classification, other studies have shown that T2 and T3 iCCA tumors seem to have similar outcomes.( 37 , 39 ) Finally, identification of other factors affecting prognosis, such as ECOG‐PS (as shown this study), are also to be taken into account and adjusted for in future studies.
In conclusion, our study shows that patients diagnosed with iCCA and liver metastases have a worse prognosis compared to other early stages of disease and a better outcome compared to patients with extrahepatic metastases. On this basis, we propose that the latest AJCC v.8 needs to be revised by reclassifying the condition of “liver metastases: multiple liver lesions, with or without vascular invasion” from T2 into M1a disease, as part of a modified mAJCC v.8 (reserving M1b for distant metastases). These changes are urgently needed to allow capturing of data regarding presence of liver metastases, recently removed for the AJCC staging system for iCCA, in future studies thereby stratifying patients with liver metastases appropriately to evaluate the efficacy of these treatments in clinical trials.
Author Contributions
A.L. and J.W.V. designed the study; All authors contributed to data collection within ENS‐CCA registry; A.L. extracted data to be employed from both ENS‐CCA ans SEER registries, performed data analysis and drafted the manuscript; All authors were involved in manuscript reviewing process and approved the final version.
Supporting information
Supplementary Material
Acknowledgment
The authors of this article are members of the European Network for the Study of Cholangiocarcinoma (ENS‐CCA) and participate in the initiative European H2020 COST Action EURO‐CHOLANGIO‐NET granted by the COST Association (CA18122). The ENS‐CCA registry is supported by the European Association for the Study of the Liver (EASL: Registry Grant Awards 2016 and 2019), the Spanish Association of Gastroenterology (AEG: RedCap access) and Incyte® (grant 2020). This article/publication is based upon work from COST Action European Cholangiocarcinoma Network, supported by COST (European Cooperation in Science and Technology). COST (European Cooperation in Science and Technology: www.cost.eu) is a funding agency for research and innovation networks. Drs Angela Lamarca, Juan Valle and Jesus M. Banales also received funding from The Christie Charity and the European Union's Horizon 2020 Research and Innovation Programme [grant number 825510, ESCALON]. Some of the authors of this manuscript are members of the European Reference Network (ERN)‐Liver (Liver Tumor Working Group) (European H2020 project).
Potential conflict of interest: Dr. Lamarca advises for and received grants from Roche and Ipsen. She is on the speakers’ bureau for and received grants from AAA and Pfizer. She advises for Eisai and Nutricia. She is on the speakers’ bureau for Merck and Incyte. She received grants from Bayer, Sirtex, Novartis, Mylan, and Delcath. Dr. Forner consults for, is on the speakers’ bureau for, and received grants from Bayer. He consults for Guerbet and AstraZeneca. He is on the speakers’ bureau for MSD and Gilead. Dr. Braconi is on the speakers’ bureau for Bayer, Eli Lilly, Pfizer, Merck, and Serono. Dr. Valle advises for and is on the speakers’ bureau for Ipsen and Novartis. He is on the speakers’ bureau for and received grants from Nucana. He advises for Agios, AstraZeneca, Delcath, Keocyt, Genoscience, Incyte, Merck, Mundipharma, PCT Biotech, Pfizer, and Qed. He is on the speakers’ bureau for AAA. He received grants from Celgene. Dr LaCasta received grants from Roche, Amgen and Pierre‐Fabré outside the scope of this work. Dr. Banales reports grants from INCYTE, personal fees for lecturer from BAYER and INTERCEPT, and consulting for QED Therapeutics, Albireo Pharma and OWL METABOLOMICS, outside the submitted work.
Contributor Information
Angela Lamarca, Email: angela.lamarca@nhs.net.
Juan W. Valle, Email: juan.valle@nhs.net.
References
- 1. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353‐1357. [DOI] [PubMed] [Google Scholar]
- 2. Anderson C, Kim R. Adjuvant therapy for resected extrahepatic cholangiocarcinoma: a review of the literature and future directions. Cancer Treat Rev 2009;35:322‐327. [DOI] [PubMed] [Google Scholar]
- 3. DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty‐one‐year experience with 564 patients at a single institution. Ann Surg 2007;245:755‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS‐CCA). Nat Rev Gastroenterol Hepatol 2016;13:261‐280. [DOI] [PubMed] [Google Scholar]
- 5. Lamarca A, Frizziero M, McNamara MG, Valle JW. Clinical and translational research challenges in biliary tract cancers. Curr Med Chem 2020;27:4756‐4777. [DOI] [PubMed] [Google Scholar]
- 6. Forner A, Vidili G, Rengo M, Bujanda L, Ponz‐Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int 2019;39(Suppl. 1):98‐107. [DOI] [PubMed] [Google Scholar]
- 7. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al.; on behalf of the BILCAP Study Group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:P663‐P673. [DOI] [PubMed] [Google Scholar]
- 8. Lamarca A, Edeline J, McNamara MG, Hubner RA, Nagino M, Bridgewater J, et al. Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer Treat Rev 2020;84:101936. [DOI] [PubMed] [Google Scholar]
- 9. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273‐1281. [DOI] [PubMed] [Google Scholar]
- 10. Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ting Ma Y, Arora A, et al. ABC‐06 | A randomised phase III, multi‐centre, open‐label study of Active Symptom Control (ASC) alone or ASC with oxaliplatin/5‐FU chemotherapy (ASC + mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously‐treated with cisplatin/gemcitabine (CisGem) chemotherapy [abstract no. 4003]. J Clin Oncol 2019;37(15 Suppl.). [Google Scholar]
- 11. Shroff RT, Borad MJ, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. A phase II trial of gemcitabine (G), cisplatin (C), and nab‐paclitaxel (N) in advanced biliary tract cancers (aBTCs). J Clin Oncol 2017;35:4018. [Google Scholar]
- 12. Sakai D, Kanai M, Kobayashi S, Eguchi H, Baba H, Seo S, et al. Randomized phase III study of gemcitabine, cisplatin plus S‐1 (GCS) versus gemcitabine, cisplatin (GC) for advanced biliary tract cancer (KHBO1401‐MITSUBA). Ann Oncol 2018;29(Suppl. 8):viii205‐viii270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al‐Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau S‐S. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium‐90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol 2015;41:120‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edeline J, Touchefeu Y, Guiu B, Farge O, Tougeron D, Baumgaertner I, et al. Radioembolization plus chemotherapy for first‐line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol 2019;6:51‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hong TS, Wo JY, Yeap BY, Ben‐Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi‐institutional phase II study of high‐dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2016;34:460‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lamarca A, Ross P, Wasan HS, et al. Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC‐01, ‐02, and ‐03 clinical trials. J Natl Cancer Inst 2020;112:200‐210. [DOI] [PubMed] [Google Scholar]
- 17. Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New horizons for precision medicine in biliary tract cancers. Cancer Discov 2017;7:943‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Javle M, Kelley RK, Roychowdhury S, Weiss KH, Abou‐Alfa GK, Macarulla T, et al. Updated results from a phase II study of infigratinib (BGJ398), a selective pan‐FGFR kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma containing FGFR2 fusions. Ann Oncol 2018;29(Suppl. 8):viii720. [Google Scholar]
- 19. Abou‐Alfa GK, Macarulla Mercade T, Javle M, Kelley RK, Lubner S, Adeva J, et al. Ivosidenib in IDH1‐mutant, chemotherapy‐refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 study. ESMO 2019 Congress. Ann Oncol 2019;30(Suppl. 5):v851‐v934. 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: ready for “prime time” in biliary tract cancer. J Hepatol 2020;73:170‐185. [DOI] [PubMed] [Google Scholar]
- 21. American Joint Committee on Cancer . Cancer Staging System. What is Cancer Staging? http://cancerstaging.org/references‐tools/Pages/What‐is‐Cancer‐Staging.aspx. Published 2020. Accessed October 2018.
- 22. Bearhs OH, Meyers MH, eds. AJCC Manual for Staging of Cancer, 2nd ed. Philadelphia, PA: Lippincott; 1983. [Google Scholar]
- 23. Edge S, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A, eds. AJCC Cancer Staging Manual. New York, NY: Springer‐Verlag; 2015. [Google Scholar]
- 24. Kubo S, Nakanuma Y, Takemura S, Sakata C, Urata Y, Nozawa A, et al. Case series of 17 patients with cholangiocarcinoma among young adult workers of a printing company in Japan. J Hepatobiliary Pancreat Sci 2014;21:479‐488. [DOI] [PubMed] [Google Scholar]
- 25. Mimaki S, Watanabe M, Kinoshita M, Yamashita R, Haeno H, Takemura S,, et al. Multifocal origin of occupational cholangiocarcinoma revealed by comparison of multilesion mutational profiles. Carcinogenesis 2020;41:368‐376. [DOI] [PubMed] [Google Scholar]
- 26. Wu TT, Levy M, Correa AM, Rosen CB, Abraham SC. Biliary intraepithelial neoplasia in patients without chronic biliary disease: analysis of liver explants with alcoholic cirrhosis, hepatitis C infection, and noncirrhotic liver diseases. Cancer 2009;115:4564‐4575. [DOI] [PubMed] [Google Scholar]
- 27. Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016;27:v28‐v37. [DOI] [PubMed] [Google Scholar]
- 28. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al., eds. AJCC Cancer Staging Manual, 8th ed. New York, NY: Springer International; 2017. [Google Scholar]
- 29. Surveillance, Epidemiology, and End Results (SEER) Program . Research Data (1975‐2016), National Cancer Institute, DCCPS, Surveillance Research Program. www.seer.cancer.gov. Published 2020. Accessed March 30, 2020, based on the November 2018 submission. Published 2020.
- 30. Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol 2018;7:52. [DOI] [PubMed] [Google Scholar]
- 31. RuiYang W, ZhiMing Y, Jiao F, Liang Z, Gang Z. Evaluation and Recommendation of the 8th Edition of American Joint Committee on Cancer (AJCC) Staging System for Intrahepatic Cholangiocarcinoma (ICC) in 820 Patients from the Surveillance, Epidemiology, and End Results (SEER) Database. J Gastrointest Surg 2020. Mar 19. 10.1007/s11605-020-04557-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32. Nathan H, Aloia TA, Vauthey JN, Abdalla EK, Zhu AX, Schulick RD, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol 2009;16:14‐22. [DOI] [PubMed] [Google Scholar]
- 33. Raoof M, Dumitra S, Ituarte PHG, Melstrom L, Warner SG, Fong Y, et al. Development and validation of a prognostic score for intrahepatic cholangiocarcinoma. JAMA Surg 2017;152:e170117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM, et al. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li J, Moustafa M, Linecker M, Lurje G, Capobianco I, Baumgart J, et al. ALPPS for locally advanced intrahepatic cholangiocarcinoma: did aggressive surgery lead to the oncological benefit? An international multi‐center study. Ann Surg Oncol 2020;27:1372‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spolverato G, Bagante F, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Comparative performances of the 7th and the 8th editions of the American Joint Committee on Cancer staging systems for intrahepatic cholangiocarcinoma. J Surg Oncol 2017;115:696‐703. [DOI] [PubMed] [Google Scholar]
- 38. Ronnekleiv‐Kelly SM, Pawlik TM. Staging of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:35‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kang SH, Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, et al. Prognostic comparison of the 7th and 8th editions of the American Joint Committee on Cancer staging system for intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2018;25:240‐248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


