Background: The antibody response after 2 doses of an mRNA vaccine against SARS-CoV-2 is excellent in the general population (1), but the response is different in recipients of solid organ transplants. For example, we have found markedly attenuated antibody responses in transplant recipients after 2 doses of an mRNA vaccine against SARS-CoV-2 (2). In addition, reports of COVID-19 breakthrough infections in vaccinated transplant recipients (3) have prompted interest in administering additional doses of vaccine.
Objective: To describe antibody responses and vaccine reactions in recipients of solid organ transplants who had a suboptimal response to standard vaccination and subsequently received a third dose of vaccine between 20 March 2021 and 10 May 2021.
Case Series: This study was approved by the Johns Hopkins institutional review board, and participants provided informed consent.
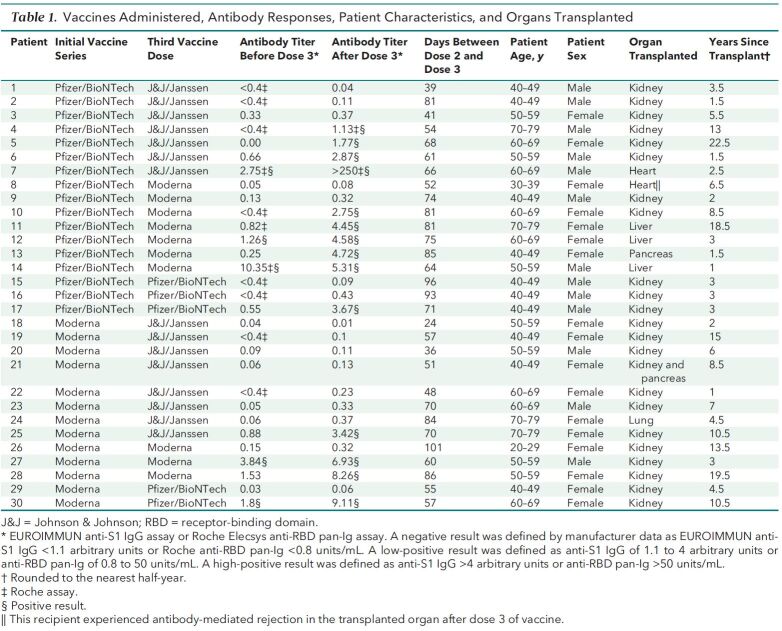
Thirty patients reported receiving a third dose of vaccine (Table 1). Their median age was 57 years (interquartile range [IQR], 44 to 62 years), 17 were women, and 1 identified as non-White. None of the patients reported an illness before vaccination that was consistent with COVID-19 or a positive result on polymerase chain reaction (PCR) assay. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine plus mycophenolate. In addition, corticosteroids were used for 24 patients, sirolimus for 1, and belatacept for 1. The median time between transplantation and initial vaccination was 4.5 years (IQR, 2.3 to 10.5 years). During the initial vaccination, 57% of the 30 patients received 2 doses of the 162b2 vaccine (Pfizer/BioNTech), and 43% received 2 doses of the mRNA-1273 vaccine (Moderna).
Table 1.
Vaccines Administered, Antibody Responses, Patient Characteristics, and Organs Transplanted
We tested all patients for antibodies against the spike protein at a median of 9 days (IQR, 2 to 33 days) before they received their third dose of vaccine; 24 patients had negative antibody titers, and 6 patients had low-positive antibody titers. Patients received the third dose of vaccine a median of 67 days (IQR, 54 to 81 days) after the second dose of their initial vaccine series; 15 patients received the Ad26.COV2.S vaccine (Johnson & Johnson/Janssen), 9 received the mRNA-1273 vaccine (Moderna), and 6 received the 162b2 vaccine (Pfizer/BioNTech).
We repeated antibody testing a median of 14 days (IQR, 14 to 17 days) after the third dose of vaccine. Of the 6 patients with low-positive antibody titers before the third dose, all had high-positive antibody titers after the third dose. In contrast, of the 24 patients with negative antibody titers before the third dose, only 6 (25%) had high-positive antibody titers after the third dose. Two (8%) had low-positive antibody titers, and 16 (67%) remained negative.
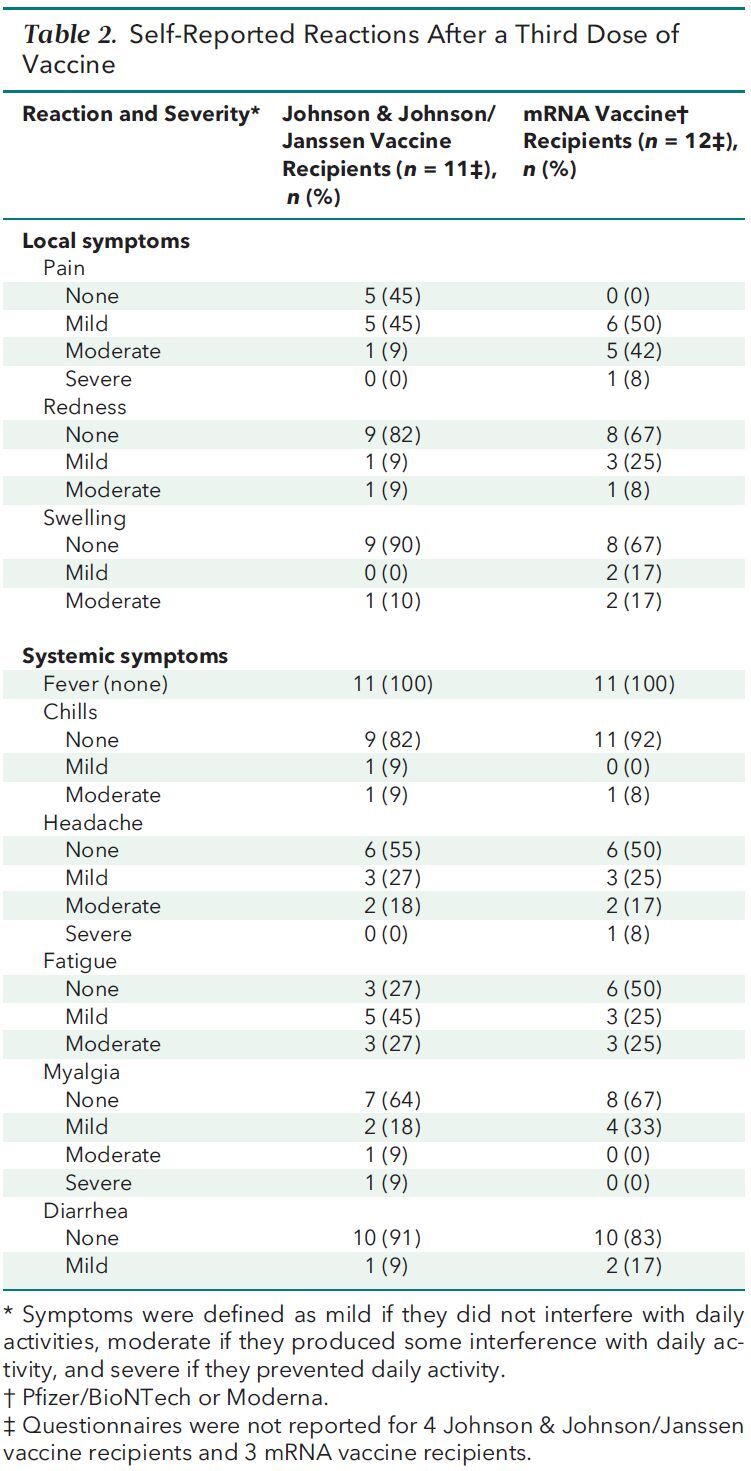
Twenty-three patients completed a questionnaire 7 days after receiving their third dose that asked about specific vaccine reactions (Table 2). Fifteen patients reported mild or moderate local reactions, and 1 reported severe arm pain. The most frequent systemic reaction was mild or moderate fatigue in 14 participants; 1 patient reported severe headache, and 1 patient reported severe myalgia. No patient reported fever, and we did not observe any anaphylactoid reactions or neurologic complications. One heart transplant recipient had biopsy-proven, antibody-mediated rejection 7 days after her third dose of vaccine in the setting of acute volume overload. She did not experience an increase in her titer of antibodies against the spike protein, heart function remained normal, and immunosuppressive intensification was not initiated. In addition, no patient reported PCR-confirmed COVID-19 during additional follow-up, although the duration of this follow-up was limited.
Table 2.
Self-Reported Reactions After a Third Dose of Vaccine
Discussion: To our knowledge, this is the first report of patients with solid organ transplants receiving a third dose of vaccine directed against SARS-CoV-2. It is encouraging that antibody titers increased after the third dose in one third of patients who had negative antibody titers and in all patients who had low-positive antibody titers. In addition, the vaccine reactions seem acceptable, given the benefits that these vaccines can confer. Antibody responses, however, appear to vary, and potential risks, such as organ rejection, should be evaluated on an individual basis.
Limitations of this study include a small and heterogeneous convenience sample and the absence of assays for neutralizing antibody, B-cell memory, and T-cell responses.
We believe that these observations support the use of clinical trials to determine whether booster doses to prevent COVID-19 in transplant patients can be incorporated into clinical practice, as they have been for hepatitis B and influenza vaccination (4).
Footnotes
This article was published at Annals.org on 15 June 2021.
References
- 1. Jackson LA , Anderson EJ , Rouphael NG , et al; mRNA-1273 Study Group. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-31. [PMID: ] doi: 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyarsky BJ , Werbel WA , Avery RK , et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204-6. [PMID: ] doi: 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ali NM , Alnazari N , Mehta SA , et al. Development of COVID-19 infection in transplant recipients after SARS-CoV-2 vaccination. Transplantation. 2021. [PMID: ] doi: 10.1097/TP.0000000000003836 [DOI] [PubMed] [Google Scholar]
- 4. Danziger-Isakov L , Kumar D ; AST ID Community of Practice. Vaccination of solid organ transplant candidates and recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13563. [PMID: ] doi: 10.1111/ctr.13563 [DOI] [PubMed] [Google Scholar]